Abstract
The aim of this study was the investigation of analgesic and anti-inflammatory activity of naproxen and pioglitazone following intra-plantar injection of carrageenan and assessment of the PPAR-γ receptor involvement in these effects. Rats were intra-plantarly injected with carrageenan (1%, 100 μl) to induce thermal hyperalgesia and paw inflammation. Different groups of rats were pre-treated intraperitoneally with naproxen (1 and 10 mg/kg) or pioglitazone (3 and 10 mg/kg) or GW9662 (a selective PPAR-γ antagonist, 100 μl/paw). The volume of the paw was evaluated using a plethysmometer, and the hot plate test was employed to assess the pain threshold in the animals. Finally, TNF-α, IL-1ß, IL-6, and myeloperoxidase (MPO) activity status were evaluated in the hind paw tissue. Naproxen and pioglitazone demonstrated analgesic and anti-inflammatory activity. Concurrent injection of an ineffective dose of naproxen (1 mg/kg) with an ineffective dose of pioglitazone (3 mg/kg) caused augmented analgesic and anti-inflammatory activity, significantly (p≤0.001 and p≤0.01, respectively). Additionally, intra-plantar injection of GW-9662 before naproxen or pioglitazone significantly suppressed their analgesic (p≤0.001) and anti-inflammatory activity (p≤0.01). Also, naproxen and pioglitazone (10 mg/kg) significantly (p≤0.001) reduced carrageenan-induced MPO activity and TNF-α, IL-6, and IL-1ß releasing. Furthermore, PPAR-γ blockade significantly prevented suppressive effects of naproxen and pioglitazone on the MPO activity and inflammatory cytokines. Pioglitazone significantly increased analgesic and anti-inflammatory effects of naproxen. This study proposes that concurrent treatment with naproxen and pioglitazone may be a substitute for overcome pain and inflammation clinically, in the future, particularly in patients with cardiovascular disorders and diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammation is a critical innate defense and the body’s response against different pathogens and harmful stimuli. The five prominent signs of inflammation are redness, warmth, swelling, pain, and altered function of the affected site (Zhang and An, 2007). Different studies have been reported that inflammation participates in the pathogenesis of several diseases, such as rheumatoid arthritis (Choy and Panayi, 2001), cardiovascular diseases (Berg and Scherer, 2005), neurodegenerative disorders (Kheradmand et al., 2016, Haddadi et al., 2018), and cancer (Coussens and Werb, 2002). Many anti-inflammatory drugs are used to relieve inflammation. On the other hand, due to the cardiovascular and gastrointestinal adverse effects of the current anti-inflammatory-drugs (Gabriel et al., 1991, Gunter et al., 2017), there is concern about suppression of the inflammatory reactions.
Pain as an unpleasant sensory experience is one of the most important symptoms of the inflammation. During inflammatory pain, the sensitivity of the primary nociceptors increases following inflammation, which cause an enhancement in the nociception. This hypersensitivity is created by the direct effect of proinflammatory mediator’s (e.g., prostaglandins) and cytokines on pain receptors (Verri Jr et al., 2006).
Several reports have demonstrated that inflammatory cytokines are increased in insulin resistance and diabetes. It has also been proven that rheumatoid arthritis, a systemic inflammatory disease, increases the risk of diabetes (Wellen and Hotamisligil, 2005, Solomon et al., 2011). Pioglitazone, a thiazolidinedione (TZD) antidiabetic agent, is a strong PPAR-γ activator and augments lipid and glucose metabolism via decreasing resistance to insulin (Wang et al., 2014). Furthermore, several studies propose that PPAR-γ agonists are useful in the decrease of inflammatory response in cardiovascular cells (Hamblin et al., 2009) and treatment of inflammatory diseases, including colitis, stroke, and Parkinson disease probably via suppressing of macrophage activation and secretion of inflammatory cytokines (Murphy and Holder, 2000, Culman et al., 2007, Carta, 2013). Also, it has been reported that TZDs decrease allodynia and hyperalgesia in neuropathic pain model through reducing of TNF-α and IL-6 in the pain pathway (Maeda and Kishioka, 2009).
Non-steroidal anti-inflammatory drugs (NSAIDs), e.g., naproxen, are extensively applied in the control of various types of pain and inflammatory disorders. Naproxen has a lower risk of cardiovascular events like myocardial infarction than other NSAIDs, such as ibuprofen and diclofenac (Kearney et al., 2006). Although the main molecular mechanism of analgesic and anti-inflammatory action of NSAIDs is the cyclooxygenase (COX) inhibition, nevertheless, there are some reports indicated that PPAR-γ receptor activates by some NSAIDs drugs such as indomethacin (Maruyama et al., 2022). On the other hand, the cardiovascular safety of naproxen cannot simply be described as the inhibition of COX. Previous studies have shown the relationship between COX-2 regulation and PPAR-γ activity in animal and in vitro models (Bonazzi et al., 2000, Konturek et al., 2003, Wang et al., 2019). Furthermore, the therapeutic effects of PPARγ agonist in inhibiting cardiovascular diseases and vascular endothelial disorder have been reported previously. PPARγ ligand improve ventricular contractility and regulate heart rate and blood pressure through adjustment of vascular homeostasis, endothelial nitric oxide synthase, and renin-angiotensin system (Kvandova et al., 2016).
According to the literature, the anti-inflammatory activity of PPAR-γ agonists and naproxen was proven when administrated alone, but there is no information about their analgesic and anti-inflammatory effect in the animal model when used simultaneously. Also, there is currently no report about the involvement of PPAR-γ in the analgesic and anti-inflammatory effect of naproxen. So, it is valuable to investigate the interaction between naproxen and pioglitazone on nociception and inflammation. Hence, in the current study for the first time, the role of PPAR-γ on the analgesic and anti-inflammatory effect of naproxen and pioglitazone has been investigated when injected alone or concurrently, in the carrageenan intra-plantarly injected rat.
Material and methods
Animals
Male Wistar rats (200–240 g) were used for the current study. Every 3 of them kept in a standard polypropylene cage with free access to food and water under 12-h light, 12-h dark cycle, and a temperature-controlled room (23±1 °C). Animals were adapted to the testing conditions for 2 days before the behavioral tests. All procedures used in the present study were done in accordance with the National Institutes of Health ethical guidelines for the Care and Use of Laboratory Animals and approved by the University of Medical Sciences of Hamadan (UMSHA) Ethical Committee, Hamadan, Iran (ID: IR.UMSHA.REC.1396.588).
Drugs and chemicals
λ-Carrageenan, GW-9662, and naproxen obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). Pioglitazone purchased from Iran Daru Pharmaceutical Co. (Tehran, Iran). Other materials obtained from Merck Company (Germany). λ-Carrageenan was dissolved in sterile normal saline. Naproxen, GW-9662, and pioglitazone dissolved in DMSO 10%. Carrageenan and GW- 9662 injected intra-plantarly with 100 μL/paw and other drugs injected intraperitoneally (i.p.).
Experimental design
Animals (n=120) randomly divided into 20 groups (6 rats): (1) carrageenan (1%; 100 μL/paw), (2) carrageenan + vehicle (normal saline containing 10% DMSO), (3) carrageenan + GW-9662, (4 and 5) carrageenan + naproxen (1 and 10 mg/kg), (6 and 7) carrageenan + pioglitazone (3 and 10 mg/kg), (8 and 9) carrageenan + pioglitazone (3 mg/kg) + naproxen (1 and 10 mg/kg), (10) carrageenan + naproxen (1 mg/kg) + pioglitazone (10 mg/kg), (11 and 12) carrageenan + GW-9662 + naproxen (1 and 10 mg/kg), (13 and 14) carrageenan + GW-9662 + pioglitazone (3 and 10 mg/kg), (15) carrageenan + GW-9662 + naproxen (1 mg/kg) + pioglitazone (3 mg/kg), (16 and 17) carrageenan + vehicle + naproxen (1 and 10 mg/kg), (18 and 19) carrageenan + vehicle + pioglitazone (3 and 10 mg/kg), (20) carrageenan + vehicle + naproxen (1 mg/kg) + pioglitazone (3 mg/kg). To detect the ineffective and effective doses of the drugs, a pilot study was carried out.
Naproxen (1 or 10 mg/kg), pioglitazone (3 or 10 mg/kg), and their combination were injected i.p half an hour before carrageenan intra-plantar injection. Combination therapy was done, to appraise the interaction between analgesic and anti-inflammatory activity of naproxen and pioglitazone.
To determine the possible participation of peroxisome proliferator activated receptor-gamma (PPARγ) in the analgesic and anti-inflammatory effect of naproxen (1 and 10 mg/kg; i.p), pioglitazone (3 and 10 mg/kg; i.p) and their ineffective doses combination, PPARγ selective antagonist, GW-9662 (3 μg/paw) (Ghavimi et al., 2014a), has been intra-plantarly injected 45 min before carrageenan injection (Fig. 1).
Carrageenan-induced rat paw edema
Intra-plantar administration of 100 μL of carrageenan (1%) into the right hind paw of the rats caused paw edema, and the inflammation level was evaluated by calculating of the paw volume, using a plethysmometer (Ugo Basile Co., Italy). Paw edema volume was measured immediately prior to and 1st, 2nd, 3th, 4th, and 5th h after injection of carrageenan. The mean volume difference of the edema was assessed by Vt-V0 and was shown as ΔV. Additionally, percentage of inflammation was calculated using the following formula (Muhammad et al., 2012):
where Vt = the volume (mL) of the right hind paw after injection of carrageenan in the different time, V0 = the volume (mL) of the right hind paw immediately prior to injection of carrageenan (i.e., initial paw volume), and t=test.
Hot plate test
Carrageenan intra-plantar administration in free-motility rats causes acute limited paw edema and thermal hyper-nociception. The original hot plate method was used to assess the animal pain threshold in this study. Every rat was individually placed on the hot plate with a temperature of 55 ± 1 °C and 30 s cut off time to avoid tissue damage. The latency time for paw licking and jumping on the hot plate was considered as an animal’s sensitivity to pain. This test for all groups (n=6 each) was done in 5 repeated times with 1 h interval (immediately prior to (0th) and 1st, 2nd, 3th, 4th, and 5th h after injection of carrageenan). Analgesia was quantified as percentage of maximum possible effect (MPE%), to equalize the bases latency time in different animals, according to the following formula (YAKSH, 1981):
where MPE = maximum possible effect, BL = base latency time (i.e., initial (0th) hot plate latency time), TL = test latency time, CT = cut off time.
Sampling
One hour after the last test (6 h after carrageenan injection), the rats were euthanized by decapitation under ketamine-xylazine deeply anesthesia, and then swollen feet were removed and stored at −80 °C for measuring myeloperoxidase (MPO) activity and cytokines IL-1ß, IL-6 and TNF-α levels. Pads tissue from the right hind paw of the animal was harvested and tissue homogenate prepared as reported previously (Haddadi et al., 2020, Malaekehpoor et al., 2020). Briefly, RIPA buffer containing protease inhibitor cocktail was used to homogenate tissue and then centrifuged at 10,000 g for 10 min at 4 °C; and the supernatant was gathered and used for the assessment of cytokines.
Myeloperoxidase activity assessment
MPO activity as an indication of neutrophil aggregation was assessed in the paw tissue as explained before (Abdollahi et al., 2021, Sadeghian et al., 2022). Briefly, paw tissue homogenized in phosphate buffer (PBS with 0.5% hexadecyl-trimethyl-ammonium bromide; pH 6) and centrifuged and then supernatant was mixed with PBS containing O-dianisidine and hydrogen peroxide. Finally, hydrochloric acid was used to stop reaction and absorption was read at 460 nm, spectrophotometrically.
IL-1ß, IL-6, and TNF-α assessment by ELISA
Paw tissue levels of IL-1ß, IL-6, and TNF-α were measured by enzyme-linked immunosorbent assay kits as explained before (Kheradmand et al., 2016, Haddadi and Rashtiani, 2020). All protocols are executed according to the manufacturer’s instructions (BioLegend, Germany).
Statistical analysis
Statistical analysis was carried out using Graph Pad Prism Software (version 6, Graph Pad software Inc., San Diego, CA). Data are presented as means ± S.E.M for six rats in every group. Graphs were drawn by plotting the MPE% for analgesia and Δ paw volume for inflammation as a function of time. The percentage of inflammation was evaluated, as mentioned above. The area under the percentage of inflammation curves (AUC) was evaluated. Differences between groups were calculated by two-way ANOVA or one-way ANOVA with Tukey’s post hoc test, as appropriate. Statistical difference was regarded as significant at p≤0.05.
Results
The effect of naproxen and pioglitazone on the nociception threshold following intra-plantar injection of carrageenan in the hot plate test
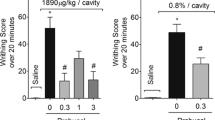
Intra-plantar injection of carrageenan in rats induced a time-dependent slow increase in pain (Fig. 2A), so that latency time for nociception throughout the course of the test record was decreased significantly (p≤0.05 after 4 h and p≤0.01 after 5 h), as shown as percentage of maximum possible effect (MPE%) in Fig. 2A. The results of the current study showed that i.p injection of naproxen and pioglitazone significantly reduced nociception following carrageenan injection (Fig. 2A, B). Naproxen at the dose of (10 mg/kg) demonstrated a significant (p≤0.001) anti-nociceptive effect, as shown as elevation in MPE% in Fig. 2, while at a lower dose (1 mg/kg) did not show significant analgesic effect (Fig. 2A). Likewise, pioglitazone at the dose of (3 mg/kg) did not show significant anti-nociceptive effect, but at the dose of (10 mg/kg) demonstrated a significant (p≤0.001) analgesic effect, so that at the higher dose, naproxen and pioglitazone increased MPE% in a significant manner (p≤0.001) (Fig. 2A, B).
The analgesic effect of A naproxen 1 and 10 mg/kg (Nap), and B pioglitazone 3 and 10 mg/kg following intra-plantar injection of carrageenan (Carr) in the hot plate test in rats. Data are shown as mean ± S.E.M. of the percentage of maximum possible effect (MPE%) (two-way ANOVA with repeated measurement followed by Tukey’s test, n = 6 rat). *p≤0.05, *** p≤0.001 versus carrageenan group in the same time group in the same time, &p≤0.05 versus base line (time=0) of carrageenan, +p≤0.05, ++p≤0.01 versus control group in the same time
No significant change was seen on the nociceptive effect of carrageenan in the vehicle-treated group (results are not shown in the figures).
The effect of combined injection of naproxen and pioglitazone on the nociception threshold following intra-plantar injection of carrageenan in the hot plate test
As illustrated in Fig. 3, the ineffective dose of naproxen (1 mg/kg) was concurrently injected with pioglitazone (3 and 10 mg/kg), and also an ineffective dose of pioglitazone (3 mg/kg) was concurrently injected with the naproxen (1 and 10 mg/kg). Concurrent treatment with an ineffective dose of naproxen with pioglitazone led to a significant (p≤0.001) increase in MPE% on the hot plate test (Fig. 3A). So that, combined injection of naproxen 1 mg/kg with pioglitazone 3 or 10 mg/kg resulted in a significant increase (p≤0.001, p≤0.05 (1 and 2 h after carrageenan injection); respectively) in the latency time to nociception in the hot plate test when compared with their use alone. Similarly, combined treatment with an ineffective dose of pioglitazone (3 mg/kg) with the naproxen1or 10 mg/kg demonstrated a significant increase in the analgesic activity of naproxen (1 mg/kg; p≤0.001 and 10 mg/kg; p≤0.05) in the hot plate test when compared with their use alone (Fig. 3B). Interestingly, the combination of two ineffective doses of naproxen (1 mg/kg) and pioglitazone (3 mg/kg) produced a significant (p≤0.001) analgesic effect and enhanced MPE% in the hot plate test (Fig. 3A and B).
The analgesic effect of combined injection of naproxen (Nap, A) and pioglitazone (B) following intra-plantar injection of carrageenan (Carr) in the hot plate test in rats. Data are shown as mean ± S.E.M. of the percentage of maximum possible effect (MPE%) (two-way ANOVA with repeated measurement followed by Tukey’s test, n = 6 rat). *; p≤0.05 versus carrageenan group in the same time, #; p≤0.05 versus Carr+Nap10 group in the same time, φ; p≤0.05 versus Carr+Pgl 10 group in the same time, ***; p≤0.001 versus carrageenan, Carr+Nap 1 or Carr+Pgl3 group in the same time
The effect of PPARγ blockade on the analgesic effect of naproxen and pioglitazone in single or mixed injection
In the current study, in order to the blockade of the PPARγ, GW-9662 (3 μg/paw) as an antagonist of this receptor was injected into the right hind paw before carrageenan injection. The results showed that injection of GW-9662 alone, before carrageenan, did not produce any significant effect on nociception induced by hot plate when compared with carrageenan group (Fig. 4A). However, injection of GW-9662 into the right hind paw before naproxen and pioglitazone significantly (p≤0.001) antagonized the analgesic effect produced by naproxen (10 mg/kg) (Fig. 4A) and pioglitazone (10 mg/kg) (Fig. 4B). So that, percentage of maximal possible effect (MPE%) of naproxen and pioglitazone decreased after blockade of PPARγ in a significant manner throughout the all times of the test (T1; p≤0.001 and p≤0.01, T2; p≤0.001 and p≤0.001, T3; p≤0.001 and p≤0.01, T4; p≤0.001 and p≤0.01 and T5; p≤0.05 and p≤0.05, respectively for naproxen and pioglitazone) (Fig. 4A and B). Additionally, concurrent injection of GW-9662 with the combination of ineffective doses of naproxen (1 mg/kg) and pioglitazone (3 mg/kg) significantly (p≤0.001) prevented from increasing effect of pioglitazone on analgesic effect of naproxen and significantly (p≤0.001) decreased MPE% of pioglitazone plus naproxen combination in the hot plate test (Fig. 4C).
The role of peroxisome proliferator activated receptor-gamma (PPARγ) on the analgesic effect of ineffective and effective doses of naproxen (Nap 1 and 10 mg/kg) and pioglitazone (Pgl 3 and 10 mg/kg) on the nociception induced by hot plate in male rats. The effect of intra-plantar injection of GW-9662 (3 μg/paw) alone or before naproxen (A) and pioglitazone (B), and before combination of ineffective doses of naproxen (1 mg/kg) and pioglitazone (3 mg/kg) (C) on MPE%, following intra-plantar injection of carrageenan (Carr) in the hot plate test in male rats. GW-9662 (GW) was injected 15 min prior to naproxen or pioglitazone (45 min before carrageenan). Data are shown as mean ± S.E.M. of percentage of maximum possible effect (MPE%) (two-way ANOVA with repeated measurement followed by Tukey’s test, n = 6 rat). *; p≤0.05 versus carrageenan group in the same time, ***; p≤0.001 versus carrageenan, Nap 1 or Pgl 3 group in the same time, †††; p≤0.001 versus Carr+Pgl 10 or Carr+Nap10 or Carr+Pgl3 +Nap1 group in the same time
The effect of pioglitazone and naproxen alone on the inflammation induced by intra-plantar injection of carrageenan in male rats
Intra-plantar injection of carrageenan in rats induced a time-dependent slow enhancement in paw volume that maximum size was seen after 5 h (Fig. 5). The results of the current study showed that i.p injection of naproxen and pioglitazone meaningfully decreased (p≤0.001) paw edema volume subsequent to carrageenan injection (Fig. 5A–D). Naproxen at the dose of (10 mg/kg) showed a significant (p≤0.001) anti-inflammatory effect, but at lower dose (1 mg/kg) did not demonstrate significant anti-inflammatory effect (Fig. 5A, B). Likewise, pioglitazone at the dose of (3 mg/kg) did not show significant anti-inflammatory effect, while at the dose of (10 mg/kg) produced a significant (p≤0.001) anti-inflammatory effect (Fig. 5C-D), so that at the higher dose, naproxen and pioglitazone decreased paw edema volume in a significant manner (p≤0.001) (Fig. 5A–D).
Anti-inflammatory effect of naproxen 1 and 10 mg/kg (A and B) and pioglitazone 3 and 10 mg/kg (C and D) following carrageenan (Carr)-induced paw edema in rats. Data are shown as mean ± S.E.M. of paw volume (ΔV) in Fig. 4A and C and percentage of inflammation in Fig. 4B and D (one-way (B and D) and two-way (A and C) ANOVA with repeated measurement followed by Tukey’s test, n = 6 rat). *; p≤0.05, **; p≤0.01, ***; p≤0.001 versus carrageenan group in the same time, ##; p≤0.01, ###; p≤0.001
No significant change was seen on the inflammation-induced by carrageenan in the vehicle-treated group (results are not shown in the figures).
The effect of combined injection of pioglitazone and naproxen on the inflammation induced by intra-plantar injection of carrageenan in male rats
As shown in Fig. 6, the ineffective dose of naproxen (1 mg/kg) was concurrently injected with pioglitazone (3 and 10 mg/kg), and also an ineffective dose of pioglitazone (3 mg/kg) was concurrently injected with the naproxen (1 and 10 mg/kg). Concurrent treatment with an ineffective dose of naproxen with pioglitazone resulted in a significant (p≤0.01) decrease in paw edema volume (Fig. 6A). So that, the combined injection of naproxen 1 mg/kg with pioglitazone 3 or 10 mg/kg resulted in a significant (p≤0.01, p≤0.001; respectively) decrease in the percentage of inflammation when compared with carrageenan group (Fig. 6A). Similarly, concurrent treatment with an ineffective dose of pioglitazone (3 mg/kg) with the naproxen 1 or 10 mg/kg produced a significant enhancement in the anti-inflammatory activity of naproxen (p≤0.05 and p≤0.01, respectively) (Fig. 6C and D). It is interesting that the combination of two ineffective doses of naproxen (1 mg/kg) and pioglitazone (3 mg/kg) produced a significant (p≤0.01) anti-inflammatory effect and decreased paw edema volume following carrageenan injection (Fig. 6C and D).
Anti-inflammatory effect of combined injection of ineffective doses of naproxen (Nap 1 mg/kg; A and B) and pioglitazone (Pgl 3 mg/kg; C and D) following carrageenan (Carr)-induced paw edema in rats. Data are shown as mean ± S.E.M. of paw volume (ΔV; A and C) and percentage of inflammation (B and D) (one-way (B and D) and two-way (A and C) ANOVA with repeated measurement followed by Tukey’s test, n = 6 rats). *; p≤0.05, **; p≤0.01, ***; p≤0.001 versus carrageenan group in the same time, #; p≤0.05, ##; p≤0.01, ###; p≤0.001
The effect of PPAR-γ blockade on the anti-inflammatory activity of naproxen and pioglitazone alone and in combination
To specify the involvement of PPAR-γ in the anti-inflammatory effect of naproxen and pioglitazone, the irreversible selective antagonist of PPAR-γ, GW-9662 (3 μg/paw), was administrated into the right hind paw before carrageenan injection. The results demonstrated that inhibition of PPARγ via administration of GW-9662 alone, before carrageenan, did not generate a considerable effect on paw edema volume and percentage of inflammation induced by carrageenan (Fig. 7). Nevertheless, intra-plantar injection of GW-9662 prior to naproxen and pioglitazone significantly (p≤0.01) inhibited the anti-inflammatory activity of naproxen at the dose 10 mg/kg (Fig. 7A and B) and pioglitazone at the dose 10 mg/kg (Fig. 7C and D). So that, paw edema volume of naproxen and pioglitazone groups increased after blockade of PPAR-γ and percentage of inflammation enhanced (p≤0.01) in a significant manner (Fig. 7D). Also, concurrent administration of GW-9662 with the combination of ineffective doses of naproxen (1 mg/kg) and pioglitazone (3 mg/kg) significantly (p≤0.05) prevented from augmentation effect of pioglitazone on anti-inflammatory activity of naproxen (Fig. 7E and F).
The role of peroxisome proliferator activated receptor-gamma (PPAR-γ) on the anti-inflammatory activity of ineffective and effective doses of naproxen (Nap 1 and 10 mg/kg) and pioglitazone (Pgl 3 and 10 mg/kg) alone and in co-administration, on the paw edema volume and inflammation induced by carrageenan in male rats. The effect of intra-plantar injection of GW-9662 (3 μg/paw) alone or before naproxen (A and B) and pioglitazone (C and D), and before co-administration of ineffective doses (E and F) of naproxen (1 mg/kg) and pioglitazone (3 mg/kg) on paw edema volume and percentage of inflammation, following intra-plantar injection of carrageenan (Carr) in the male rats. GW-9662 (GW) was injected 15 min prior to naproxen or pioglitazone (45 min before carrageenan). Data are shown as mean ± S.E.M. of paw edema volume (ΔV; A, C, and E) and percentage of inflammation (B, D, and F) (one-way (B, D, and F) and two-way (A, C, and E) ANOVA with repeated measurement followed by Tukey’s test, n = 6 rat). *; p≤0.05, **; p≤0.01, ***; p≤0.001 versus carrageenan group at the same time, †; p≤0.05 versus carr+Nap10+GW group at the same time. #; p≤0.05, ##; p≤0.01, ###; p≤0.001
The effect of pioglitazone, naproxen and PPAR-γ blockade on the carrageenan induced MPO activity and inflammatory cytokines levels
Intra-plantar carrageenan injection significantly enhanced (p≤0.001) the paw tissue activity of MPO in comparison with intact animals. Also, acute treatment with naproxen (10 mg/kg; i.p), pioglitazone (10 mg/kg; i.p) and combination of the non-effective doses of naproxen (1 mg/kg) plus pioglitazone (3 mg/kg) after carrageenan markedly decreased MPO activity (p≤0.001, p≤0.01 and p≤0.01; respectively). Additionally, pretreatment with GW-9662 (3 μg/paw, intra-plantar) meaningfully abolished (p≤0.05) the decreasing effect of naproxen (10 mg/kg), pioglitazone (10 mg/kg) and their non-effective doses combination on the MPO activity in the paw tissue of rats (Fig. 8A). Intra-plantar injection of GW-9662 alone did not show any effect on the MPO activity.
Involvement of peroxisome proliferator activated receptor-gamma (PPAR-γ) on the paw tissue levels of A MPO activity, B TNF-α, C IL-6, and D IL-1ß following intra-plantar injection of carrageenan (Carr) in the rats treated with naproxen (Nap 10 mg/kg), pioglitazone (Pgl 10 mg/kg), and their non effective doses combination. GW-9662 (GW) was injected 15 min prior to naproxen or pioglitazone (45 min before carrageenan). Data are shown as mean ± S.E.M. (two-way ANOVA with repeated measurement followed by Tukey’s test, n = 6 rat). *; p≤0.05, **; p≤0.01, ***; p≤0.001 versus carrageenan group, †; p≤0.05, ††; p≤0.01, †††; p≤0.001. ###; p≤0.001 versus intact group
On the other hand, the release of inflammatory cytokines such as TNF-α, IL-6, and IL-1ß following intra-plantar injection of carrageenan significantly augmented (p≤0.001) in the paw tissue of inflamed rats when compared with intact animals. Acute treatment with naproxen (10 mg/kg; i.p), pioglitazone (10 mg/kg; i.p) and combination of the non-effective doses of naproxen (1 mg/kg) plus pioglitazone (3 mg/kg) after carrageenan considerably attenuated release of TNF-α (p≤0.001, p≤0.001, and p≤0.01; respectively), IL-6 (p≤0.001, p≤0.001, and p≤0.001; respectively), and IL-1ß (p≤0.001, p≤0.001, and p≤0.01; respectively). Furthermore, pretreatment with GW-9662 (3 μg/paw, intra-plantar) significantly suppressed (p≤0.05) the attenuating effect of naproxen (10 mg/kg), pioglitazone (10 mg/kg), and their non-effective doses combination on the levels of TNF-α (p≤0.001, p≤0.01, and p≤0.05; respectively), IL-6 (p≤0.001, p≤0.001, and p≤0.01; respectively), and IL-1ß (p≤0.001, p≤0.01, and p≤0.01; respectively) in the paw tissue of rats (Fig. 8B–D). Intra-plantar injection of GW-9662 alone did not show any effect on the TNF-α, IL-6, and IL-1ß levels.
Discussion
The current study indicated that naproxen (COX-1and2 inhibitor) and pioglitazone (PPAR-γ activator) decreased nociception and paw edema volume in a time-dependent manner following carrageenan intra-plantar injection in rats.
Carrageenan-induced hyperalgesia has been mainly used as a model to investigate pain sensory mechanisms, particularly inflammatory pain (Bach-Rojecky and Lackovic, 2005, Kwon et al., 2014). Results of the present study showed that intra-plantar injection of carrageenan caused a time dependent decrease in the paw withdrawal latency time as well as MPE% in the rat on hotplate. So, after 5 h, the perception of the pain significantly increased when compared to the beginning of the test. This is totally in accordance with previous study reported carrageenan-induced hyperalgesia (Bach-Rojecky and Lackovic, 2005, Kwon et al., 2014). The findings of the current study demonstrated that naproxen and pioglitazone significantly and time-dependently decreased carrageenan-induced hyperalgesia. These results are in line with previous studies reported the analgesic effect of naproxen and pioglitazone (Derry et al., 2009, Morgenweck et al., 2013, Ghavimi et al., 2014b). According to literature, the present study is the first report to demonstrate that concurrent injection of naproxen and pioglitazone at small doses, which illustrated no analgesic effect when injected alone, resulted in additive analgesic activity in the carrageenan-induced hyperalgesia in rats.
Furthermore, in the current study, the possible involvement of PPAR-γ was investigated in the analgesic effect of naproxen and pioglitazone or their combination. Previously, it has been shown that COX inhibitors such as indomethacin can activate PPAR-γ transcription factor (Maruyama et al., 2022). Earlier reports have indicated that reduction of COX-2 and TNF-α and also increasing of PPAR-γ and IL-10 are participated in the analgesic activity of NSAIDs and several agonists of PPAR-γ in different pain models (Maeda and Kishioka, 2009, Maruyama et al., 2022). According to the findings of this study, PPAR-γ involved in the analgesic activity of naproxen and pioglitazone. The analgesic effect of pioglitazone and naproxen were suppressed by GW-9662, the selective PPAR-γ inhibitor. Also, the increased analgesic activity of the combination of ineffective doses of them was prevented by GW-9662. This is feasible just if there is a connection among COX and PPAR-γ. Similar effect has already been reported in other inflammatory pain models (Churi et al., 2008, Hasegawa-Moriyama et al., 2013, Alsalem et al., 2016). In accordance with the results of the present study, it has been shown that single injection of pioglitazone prevented hyperalgesia which was suppressed by GW-9662 (Morgenweck et al., 2013, Griggs et al., 2015). In this regard, in agree with our results, it has been already demonstrated that intrathecal injection of rosiglitazone (PPAR-γ agonist) and 15d-PGJ2 (15-deoxy-prostaglandin J2; natural PPAR-γ agonist) reduced cold hypersensitivity in a neuropathic pain model in rat, which was also inhibited by BADGE (PPAR-γ antagonist) (Churi et al., 2008). Also, Hasegawa et al. reported that rosiglitazone decreased inflammatory pain via up-regulation of heme oxygenase-1 in macrophage (Hasegawa-Moriyama et al., 2013). In another study, Alsalem et al. presented that ibuprofen (COX inhibitor) reduced thermal hyper-nociception via PPAR-γ activation and GW9662 meaningfully decreased the analgesic effects of ibuprofen (Alsalem et al., 2016), which is totally in line with the finding of the current study.
Another important finding of the present study was that naproxen and pioglitazone decreased carrageenan-induced paw edema and inflammation in a time-dependent manner. The inflammation caused by carrageenan has been defined as a biphasic phenomenon. The first stage is seen about 1 h, which is associated with the secretion of histamine, bradykinin, serotonin, and, in a lower level, prostaglandins released by COX. On the other hand, the second stage (after 1 h) is related to infiltration of neutrophils, and more prostaglandin secretion (Vinegar et al., 1969).
Carrageenan-induced inflammation is very susceptible to NSAIDs and serves as a practical test to detect novel anti-inflammatory drugs (Wallace et al., 1999). Results of the current study demonstrated that intra-plantar injection of carrageenan caused a time-dependent increase in the paw edema volume as well as inflammation% in the rat. This is totally in line with previous studies (Wallace et al., 1999, Li et al., 2015). The findings of the present study illustrated that acute pre-treatment with naproxen or pioglitazone significantly and time-dependently attenuated carrageenan-induced paw edema volume. Also, our results demonstrated that MPO activity and levels of TNF-α, IL-6, and IL-1ß as the pro-inflammatory mediators significantly increased following carrageenan injection in the inflamed paw tissue. On the other hand, naproxen and pioglitazone markedly reduced carrageenan-induced MPO activity and the releasing of inflammatory cytokines. These findings agree with earlier studies reported the anti-inflammatory activity of COX inhibitors and PPAR-γ agonists (Jiang et al., 1998, Wallace et al., 1999, Charkhpour et al., 2015). Based on our knowledge, no reports have yet been provided on the additive anti-inflammatory activity of concurrent injection of the low doses of these drugs and the current study is the first report in this area.
Additionally, the possible interaction of PPAR-γ was evaluated in the anti-inflammatory activity of naproxen and pioglitazone or their combination. Based on the results of the current investigation, PPAR-γ participated in the anti-inflammatory activity of naproxen and pioglitazone. The anti-inflammatory activity of pioglitazone and naproxen was prevented by GW-9662. Also, the additive anti-inflammatory activity of the combination of ineffective doses of these drugs was suppressed by GW-9662. Furthermore, PPAR-γ blockade by GW-9662 significantly prevented suppressive effects of naproxen and pioglitazone on the MPO activity and TNF-α, IL-6, and IL-1ß as inflammatory cytokines. Similar effect has already been reported in other inflammatory models by other researchers. In relating PPAR possible role in inflammation, a study has shown some evidences for anti-inflammatory function of 15d-PGJ2 and PGD2 in a rat model of pleural inflammation induced by carrageenan (Gilroy et al., 1999). Also, it has been reported that indomethacin upregulate peroxisome proliferator-activated receptors -γ (Maruyama et al., 2022). Macrophages express a large amount of PPARs, which has been proven to have an important role in the activation and differentiation of monocytes and in the adjustment of inflammatory activities (Jiang et al., 1998, Tontonoz et al., 1998). Some reports have shown that PPAR agonists prevent inflammatory responses of macrophage (Jiang et al., 1998, Ricote et al., 1998). Also, thiazolidinediones and 15d-PGJ2 suppress release of several inflammatory cytokines (such as IL-1ß, IL-6, and TNF) (Jiang et al., 1998, Ricote et al., 1998). In agree with the results of the current study, it has been illustrated that injection of rosiglitazone attenuated inflammation induced by zymosan, but this anti-inflammatory activity was suppressed by GW-9662 (Cuzzocrea et al., 2004). Similarly, it has been reported that rosiglitazone decreased lipopolysaccharide-induced pulmonary inflammation in an endotoxemia model through reduction of myeloperoxidase, TNF-α, and nuclear factor-kappa B, but GW9662 abrogated these effects (Liu et al., 2005). Also, 15d-PGJ2 decreased organ injury following hemorrhage shock, while GW-9662 abolished its protective effects (Abdelrahman et al., 2004). In another study, it has been indicated that 15d-PGJ2 decreased the progress of chronic and acute inflammation via down regulation of COX-2 in the joints of carrageenan-injected mice (Cuzzocrea et al., 2002). Furthermore, in a recent study, it has been reported that indomethacin decreased carrageenan-induced paw edema and GW9662 attenuated the anti-inflammatory activity of indomethacin (Houshmand et al., 2016), which is totally in agree with the result of the current study.
Finally, according to the results, it can be suggested that PPAR-γ has an important role in the analgesic and anti-inflammatory activity of naproxen. Also, based on the above mentioned and pivotal role of cytokines in the inflammation and inflammatory pain, it can be postulated that the analgesic and anti-inflammatory activity of naproxen-pioglitazone are associated to the capability to decrease the production of cytokine. The authors suppose that cardiovascular safety of naproxen seems to be mediated possibly through PPAR-γ activation pathway.
Conclusion
The results of the current study demonstrated that intra-plantar injection of carrageenan caused hyperalgesia and inflammation in the hind paw of the rat. Naproxen and pioglitazone showed analgesic and anti-inflammatory activity following carrageenan injection. Also, combination therapy with low-doses of naproxen and pioglitazone produced additive analgesic and anti-inflammatory activity. Interestingly, GW9662, selective antagonist of PPAR-γ, attenuated analgesic and, anti-inflammatory activity of these drugs and their combination. Therefore, these effects appear to be evoked through a PPARγ-dependent pathway. The findings demonstrate that pioglitazone can increase the effects of naproxen and permit the dose of NSAIDs to be decreased for overcome pain and inflammation clinically, particularly in patients with cardiovascular disorders and diabetes. Pioglitazone may be developed as a beneficial drug candidate for control of pain and inflammation in the future, although more clinical evaluation should be carried out to prove these effects.
Data availability
All datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abdelrahman M, Collin M, Thiemermann C (2004) The peroxisome proliferator-activated receptor-γ ligand 15-deoxyd12, 14 prostaglandin J2 reduces the organ injury in hemorrhagic shock. Shock 22:555–561
Abdollahi AR, Firouzian F, Haddadi R, Nourian A (2021) Indomethacin loaded dextran stearate polymeric micelles improve adjuvant-induced arthritis in rats: design and in vivo evaluation. Inflammopharmacology 29:107–121
Alsalem M, Altarifi A, Kalbouneh H, Al-Zer H, Azab B, El-Salem K (2016) Role of PPARα and PPARγ in mediating the analgesic properties of ibuprofen in vivo and the effects of dual PPARα/γ activation in inflammatory pain model in the rat. Int J Pharmacol 12:812–820
Bach-Rojecky L, Lackovic Z (2005) Antinociceptive effect of botulinum toxin type a in rat model of carrageenan and capsaicin induced pain. Croat Med J 46:201–208
Berg AH, Scherer PE (2005) Adipose tissue, inflammation, and cardiovascular disease. Circ Res 96:939–949
Bonazzi A, Mastyugin V, Mieyal PA, Dunn MW, Laniado-Schwartzman M (2000) Regulation of cyclooxygenase-2 by hypoxia and peroxisome proliferators in the corneal epithelium. J Biol Chem 275:2837–2844
Carta AR (2013) PPAR-γ: therapeutic prospects in Parkinson’s disease. Curr Drug Targets 14:743–751
Charkhpour M, Ghavimi H, Ghanbarzadeh S, Yousefi B, Khorrami A, Mesgari M, Hassanzadeh K (2015) Protective effect of pioglitazone on morphine-induced neuroinflammation in the rat lumbar spinal cord. J Biomed Sci 22:1–6
Choy EH, Panayi GS (2001) Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med 344:907–916
Churi SB, Abdel-Aleem OS, Tumber KK, Scuderi-Porter H, Taylor BK (2008) Intrathecal rosiglitazone acts at peroxisome proliferator–activated receptor-γ to rapidly inhibit neuropathic pain in rats. J Pain 9:639–649
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420:860
Culman J, Zhao Y, Gohlke P, Herdegen T (2007) PPAR-γ: therapeutic target for ischemic stroke. Trends Pharmacol Sci 28:244–249
Cuzzocrea S, Pisano B, Dugo L, Ianaro A, Patel NS, Di Paola R, Genovese T, Chatterjee PK, Fulia F, Cuzzocrea E (2004) Rosiglitazone, a ligand of the peroxisome proliferator-activated receptor-γ, reduces the development of nonseptic shock induced by zymosan in mice. Crit Care Med 32:457–466
Cuzzocrea S, Wayman NS, Mazzon E, Dugo L, Di Paola R, Serraino I, Britti D, Chatterjee PK, Caputi AP, Thiemermann C (2002) The cyclopentenone prostaglandin 15-deoxy-Δ12, 14-prostaglandin J2attenuates the development of acute and chronic inflammation. Mol Pharmacol 61:997–1007
Derry CJ, Derry S, Moore RA, McQuay HJ (2009) Single dose oral naproxen and naproxen sodium for acute postoperative pain in adults. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD004234.pub3
Gabriel SE, Jaakkimainen L, Bombardier C (1991) Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs: a meta-analysis. Ann Intern Med 115:787–796
Ghavimi H, Azarfardian A, Maleki-Dizaji N, Hassanzadeh K, Ghanbarzadeh S, Charkhpour M (2014a) Acute administration of pioglitazone attenuates morphine withdrawal syndrome in rat: a novel role of pioglitazone. Drug Res:113–118
Ghavimi H, Hassanzadeh K, Maleki-Dizaji N, Azarfardian A, Ghasami S, Zolali E, Charkhpour M (2014b) Pioglitazone prevents morphine antinociception tolerance and withdrawal symptoms in rats. Naunyn Schmiedeberg's Arch Pharmacol 387:811–821
Gilroy DW, Colville-Nash P, Willis D, Chivers J, Paul-Clark M, Willoughby D (1999) Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med 5:698
Griggs RB, Donahue RR, Morgenweck J, Grace PM, Sutton A, Watkins LR, Taylor BK (2015) Pioglitazone rapidly reduces neuropathic pain through astrocyte and non-genomic PPARγ mechanisms. Pain 156:469
Gunter B, Butler K, Wallace R, Smith S, Harirforoosh S (2017) Non-steroidal anti-inflammatory drug-induced cardiovascular adverse events: a meta-analysis. J Clin Pharm Ther 42:27–38
Haddadi R, Eyvari-Brooshghalan S, Nayebi AM, Sabahi M, Ahmadi SA (2020) Neuronal degeneration and oxidative stress in the SNc of 6-OHDA intoxicated rats; improving role of silymarin long-term treatment. Naunyn-Schmiedeberg's Archives of Pharmacology: 1-11
Haddadi R, Poursina M, Zeraati F, Nadi F (2018) Gastrodin microinjection suppresses 6-OHDA-induced motor impairments in parkinsonian rats: insights into oxidative balance and microglial activation in SNc. Inflammopharmacology: 1-12
Haddadi R, Rashtiani R (2020) Anti-inflammatory and anti-hyperalgesic effects of milnacipran in inflamed rats: involvement of myeloperoxidase activity, cytokines and oxidative/nitrosative stress. Inflammopharmacology 28:903–913
Hamblin M, Chang L, Fan Y, Zhang J, Chen YE (2009) PPARs and the cardiovascular system. Antioxid Redox Signal 11:1415–1452
Hasegawa-Moriyama M, Kurimoto T, Nakama M, Godai K, Kojima M, Kuwaki T, Kanmura Y (2013) Peroxisome proliferator-activated receptor-gamma agonist rosiglitazone attenuates inflammatory pain through the induction of heme oxygenase-1 in macrophages. PAIN® 154: 1402-1412
Houshmand G, Mansouri MT, Naghizadeh B, Hemmati AA, Hashemitabar M (2016) Potentiation of indomethacin-induced anti-inflammatory response by pioglitazone in carrageenan-induced acute inflammation in rats: role of PPARγ receptors. Int Immunopharmacol 38:434–442
Jiang C, Ting AT, Seed B (1998) PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature 391:82
Kearney PM, Baigent C, Godwin J, Halls H, Emberson JR, Patrono C (2006) Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. Bmj 332:1302–1308
Kheradmand A, Nayebi AM, Jorjani M, Khalifeh S, Haddadi R (2016) Effects of WR1065 on 6-hydroxydopamine-induced motor imbalance: possible involvement of oxidative stress and inflammatory cytokines. Neurosci Lett 627:7–12
Konturek P, Brzozowski T, Kania J, Kukharsky V, Bazela K, Kwiecien S, Harsch I, Konturek S, Hahn E (2003) Pioglitazone, a specific ligand of the peroxisome proliferator-activated receptor gamma reduces gastric mucosal injury induced by ischaemia/reperfusion in rat. Scand J Gastroenterol 38:468–476
Kvandova M, Majzúnová M, Dovinová I (2016) The role of PPAR [gamma] in cardiovascular diseases. Physiol Res 65:S343
Kwon S-G, Roh D-H, Yoon S-Y, Moon J-Y, Choi S-R, Choi H-S, Kang S-Y, Han H-J, Beitz AJ, Lee J-H (2014) Blockade of peripheral P2Y1 receptors prevents the induction of thermal hyperalgesia via modulation of TRPV1 expression in carrageenan-induced inflammatory pain rats: involvement of p38 MAPK phosphorylation in DRGs. Neuropharmacology 79:368–379
Li Y-Y, Huang S-S, Lee M-M, Deng J-S, Huang G-J (2015) Anti-inflammatory activities of cardamonin from Alpinia katsumadai through heme oxygenase-1 induction and inhibition of NF-κB and MAPK signaling pathway in the carrageenan-induced paw edema. Int Immunopharmacol 25:332–339
Liu D, Zeng B, Zhang S, Yao S (2005) Rosiglitazone, an agonist of peroxisome proliferator-activated receptor γ, reduces pulmonary inflammatory response in a rat model of endotoxemia. Inflamm Res 54:464–470
Maeda T, Kishioka S (2009) PPAR and pain. Int Rev Neurobiol 85:165–177
Malaekehpoor SM, Derakhshandeh K, Haddadi R, Nourian A, Ghorbani-Vaghei R (2020) A polymer coated MNP scaffold for targeted drug delivery and improvement of rheumatoid arthritis. Polym Chem 11:2408–2417
Maruyama K, Goto K, Hiramoto K, Tanaka S, Ooi K (2022) Indomethacin, a non-steroidal anti-inflammatory drug, induces skin dryness via PPARγ in mice. Biol Pharm Bull 45:77–85
Morgenweck J, Griggs R, Donahue R, Zadina JE, Taylor BK (2013) PPARγ activation blocks development and reduces established neuropathic pain in rats. Neuropharmacology 70:236–246
Muhammad N, Saeed M, Khan H (2012) Antipyretic, analgesic and anti-inflammatory activity of Viola betonicifolia whole plant. BMC Complement Altern Med 12:59
Murphy GJ, Holder JC (2000) PPAR-γ agonists: therapeutic role in diabetes, inflammation and cancer. Trends Pharmacol Sci 21:469–474
Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK (1998) The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature 391:79
Sadeghian Z, Eyvari-Brooshghalan S, Sabahi M, Nourouzi N, Haddadi R (2022) Post treatment with Gastrodin suppresses oxidative stress and attenuates motor disorders following 6-OHDA induced Parkinson disease. Neurosci Lett 790:136884
Solomon DH, Massarotti E, Garg R, Liu J, Canning C, Schneeweiss S (2011) Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. Jama 305:2525–2531
Tontonoz P, Nagy L, Alvarez JG, Thomazy VA, Evans RM (1998) PPARγ promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell 93:241–252
Verri WA Jr, Cunha TM, Parada CA, Poole S, Cunha FQ, Ferreira SH (2006) Hypernociceptive role of cytokines and chemokines: targets for analgesic drug development? Pharmacol Ther 112:116–138
Vinegar R, Schreiber W, Hugo R (1969) Biphasic development of carrageenin edema in rats. J Pharmacol Exp Ther 166:96–103
Wallace JL, Chapman K, McKnight W (1999) Limited anti-inflammatory efficacy of cyclo-oxygenase-2 inhibition in carrageenan-airpouch inflammation. Br J Pharmacol 126:1200–1204
Wang L, Waltenberger B, Pferschy-Wenzig E-M, Blunder M, Liu X, Malainer C, Blazevic T, Schwaiger S, Rollinger JM, Heiss EH (2014) Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARγ): a review. Biochem Pharmacol 92:73–89
Wang Z, Li F, Quan Y, Shen J (2019) Avicularin ameliorates human hepatocellular carcinoma via the regulation of NF-κB/COX-2/PPAR-γ activities. Mol Med Rep 19:5417–5423
Wellen KE, Hotamisligil GS (2005) Inflammation, stress, and diabetes. J Clin Invest 115:1111–1119
Yaksh TL (1981) The antinociceptive effects of intrathecally administered levonantradol and desacetyllevonantradol in the rat. J Clin Pharmacol 21:334S–340S. https://doi.org/10.1002/j.1552-4604.1981.tb02612.x
Zhang J-M, An J (2007) Cytokines, inflammation and pain. Int Anesthesiol Clin 45:27
Acknowledgements
This data was adopted from the Pharm D. thesis of Dr. Mohammad Cheraghi-poor. The authors would like to thank Research and Technology Vice-Chancellor of Hamadan University of Medical Sciences (Hamadan, Iran) for supporting this study.
Funding
This work was supported by a grant from Research and Technology Vice-Chancellor of Hamadan University of Medical Sciences, Hamadan, Iran (code: 9604132335).
Author information
Authors and Affiliations
Contributions
RH the supervisor of the study participated and was involved in the concept, design, support of the study, interpretation of data, statistical analyses, drafting, and a final check of the draft. MC doing behavioral experiments and biochemical assessment. All authors read and approved the final manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethics approval
All procedures used in the present study were done in accordance with the National Institutes of Health ethical guidelines for the Care and Use of Laboratory Animals and approved by the University of Medical Sciences of Hamadan (UMSHA) Ethical Committee, Hamadan, Iran (ID: IR.UMSHA.REC.1396.588).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Haddadi, R., Cheraghi-poor, M. Peroxisome proliferator activated receptor-gamma (PPAR-γ) ligand, pioglitazone, increases analgesic and anti-inflammatory effects of naproxen. Naunyn-Schmiedeberg's Arch Pharmacol 397, 1633–1646 (2024). https://doi.org/10.1007/s00210-023-02715-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02715-y