Abstract
Fungal infections impose a significant impact on global health and encompass major expenditures in medical treatments. Human mycoses, a fungal co-infection associated with SARS-CoV-2, is caused by opportunistic fungal pathogens and is often overlooked or misdiagnosed. Recently, there is increasing threat about spread of antimicrobial resistance in fungus, mostly in hospitals and other healthcare facilities. The diagnosis and treatment of fungal infections are associated with several issues, including tedious and non-selective detection methods, the growth of drug-resistant bacteria, severe side effects, and ineffective drug delivery. Thus, a rapid and sensitive diagnostic method and a high-efficacy and low-toxicity therapeutic approach are needed. Nanomedicine has emerged as a viable option for overcoming these limitations. Due to the unique physicochemical and optical properties of nanomaterials and newer biosensing techniques, nanodiagnostics play an important role in the accurate and prompt differentiation and detection of fungal diseases. Additionally, nano-based drug delivery techniques can increase drug permeability, reduce adverse effects, and extend systemic circulation time and drug half-life. This review paper is aimed at highlighting recent, promising, and unique trends in nanotechnology to design and develop diagnostics and treatment methods for fungal diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidences of fungal infections are increasing, and one leading risk factor for invasive fungal infection is modulation in the immune system (Lockhart and Guarner 2019). Each year, there is a global incidence of more than 150 million severe cases of fungal infections, contributing to an estimated 1.7 million annual fatalities (Kainz et al. 2020b).
The coronavirus disease 2019 (COVID-19) outbreak was a global emergency, with its rapid expansion and high-mortality rate wreaking havoc (Fauci et al. 2020; Yang et al. 2020a). People who suffer from severe COVID-19, such as those who are in an intensive care unit (ICU), are especially prone to fungal infections. These viral and fungal co-infections are becoming more frequent, and they have been linked to serious sickness and death (Sanguinetti et al. 2019; Gangneux et al. 2020). Such rapid and accurate diagnosis becomes very important. Some of the most frequent fungal infections include aspergillosis, candidiasis, and mucormycosis (Ghosh et al. 2021a).
COVID-19-associated candidiasis (CAC) is characterized by the occurrence of Candida fungal infections in individuals who have contracted the SARS-CoV-2 virus, leading to COVID-19 (Tsai et al. 2022). Candida albicans is the most prevalent species in infections; however, Candida auris has recently started generating drug-resistant infections, particularly in COVID-19 patients (Arastehfar et al. 2020a). COVID-19 patients may be at an increased risk of developing candidiasis due to factors such as prolonged hospitalization, the use of broad-spectrum antibiotics, mechanical ventilation, and the presence of indwelling medical devices (Arastehfar et al. 2020a). The exact prevalence and impact of COVID-19-associated candidiasis are still being studied, but it is recognized as an important secondary infection in critically ill COVID-19 patients.
COVID-19-associated pulmonary aspergillosis (CAPA) is one of the causes that has contributed to the elevated mortality rates of COVID19 as per numerous studies (Arastehfar et al. 2020b). Invasive pulmonary aspergillosis (IPA) is becoming more common in patients as a potential co-infection alongside COVID19 and acute respiratory distress syndrome (ARDS) (Maiese et al. 2021). The significance of SARS-CoV-2 in CAPA is yet unknown, and other additional risk factors, such as corticosteroid medication, may be responsible for the disease's progression (Ghosh et al. 2021a).
COVID-19-associated mucormycosis (CAM), also known as black fungus or mucormycosis, is caused by a group of fungi called mucormycetes. Mucormycosis primarily affects individuals with weakened immune systems or underlying health conditions, and it often emerges as a secondary infection in COVID-19 patients treated with steroids and having uncontrolled diabetes (Selarka et al. 2021).
With the widespread use of antibacterial drugs and the increasing number of immunocompromised patients, the risk of drug resistance increases in the absence of a complete understanding of fungal and bacterial co-infections. Also, the limited diagnostic methods and time-consuming detection methods have made it difficult to distinguish fungal and bacterial co-infections from other pathogenic infections (Zhao et al. 2021). Such constraints have repercussions; delayed diagnosis leads to delayed treatment. Rapid diagnosis is a major risk factor in patient outcomes, as early diagnosis of fungal infection is critical to effective treatment and reducing morbidity and mortality (Pappas et al. 2018).
The toxicity of antifungals and their interactions with other medications make fungal infections notoriously difficult to treat (Bicanic and Harrison 2014; Brunet et al. 2018). Amphotericin B, for example, is commonly used in treatments because of its wide spectrum of activity, but it can produce significant nephrotoxicity and infusion-related complications (Roemer and Krysan 2014). Patients treated with the conventional antifungal medicines have been seen to fail to react due to drug resistance, resulting in infection spread, negative social, psychological, and occupational health impacts and a reduction in the patient’s quality of life (Rai et al. 2017). Overall, when new or drug-resistant fungi evolve, drug permeability, adverse effects, and non-targeting abilities of drugs are all factors that limit their effectiveness.
Nanotechnology-based approaches have the potential to provide highly sensitive and specific methods for detecting and treating fungal infection (Muhammad et al. 2020). The advantage of using NP for targeted drug delivery is depicted in Fig. 1. It has been used for pathogen detection and treatment to overcome the shortcomings of traditional antifungal treatment and detection methods (Abd-Elsalam 2021). One of the advancements in nanotechnology that proves promising for efficient fungal detection is the use of biosensors. Nanotechnology is extensively used in the research and development of biosensors. The fact of the matter is that biosensors can be a worthy replacement for integrated sensors for fast and efficient point-of-care detection (Lee and Jun 2019). The discovery of innovative antifungal medications has already been a focus of nanomedicine. Over the years, mycology and nanotechnology have developed a bidirectional interaction (myco-nanotechnology) (Asghari et al. 2016). Nanotechnology had already proven to be a promising strategy for increasing the potential and efficiency of conventional antifungals, allowing for a reduction in toxicity and cost, avoiding expected degradation, improving drug distribution by increasing circulation time and improving pharmacokinetics, and improving drug targeting, with promising in vitro and in vivo results (Souza and Amaral 2017a). In the field of nanomedicine, the antifungal principle is analogous to the antibacterial principle since fungi and bacteria have many similar cell structures. Antimicrobial action of NP is mediated by a variety of ways. Because of their interaction with proteins, nanometric particles can traverse the cell interstitium and release metal ions from the surface of the NP inside the cell, enhancing antibacterial activity or causing damage to the cell wall, resulting to pathogen death (Oberdörster et al. 2005; Reddy et al. 2012).
In short, the use of nanomedicine as a method offers enormous potential to significantly advance the detection and treatment of fungal infections. Thus, the objective of this review article is to comprehensively gather and analyze existing and recent information on the utilization of nanotechnology for timely detection and treatment of above mentioned three common fungal infections. By establishing a knowledge base through the relevant research findings, this review is aimed at facilitating the design and development of diagnostic and treatment strategies for COVID-19-associated fungal infections.
Fugal detection using nanomedicine
The term “nano diagnostics” refers to the use of nanotechnology in diagnosis. Nanodiagnostics has made important contributions to accurate and timely diagnosis of infectious diseases in recent decades; the unique physio-chemical and optical properties of nanomaterials have a significant role to play. Because of their high surface-to-volume ratio, nanoparticles (NP) in biosensing design resulted in a considerable improvement in sensor performance with a reduced limit of detection (LOD) (Malhotra and Ali 2018).
Sensors are analytical tools that may convert chemical, physical, or biological data into usable analytical signals. A biosensor, according to the IUPAC, is “a device that detects chemical compounds using specialized biochemical reactions mediated by isolated enzymes, immunosystems, tissues, organelles, or whole cells, usually via electrical, thermal, or optical signals” (Jurado-Sánchez 2018). It is a self-contained integrated device that uses a biological recognition element (biochemical receptor) that is kept in direct spatial contact with a transduction element to provide specific quantitative or semi-quantitative analytical information (Bhattarai and Hameed 2020). A biosensor is a device that detects and produces electrical signals proportional to the concentration of an analyte in the reaction using a biomolecule. The signal transducer attaches to the receptor molecule after the target analyte. This physically and chemically active transducer element transforms biological event into quantifiable signal. Different types of transducers used biosensors are depicted in Fig. 2. Throughout the years, numerous matrices have been used to immobilize biomolecules in order to facilitate recognition, including metallic electrodes, nanomaterials, biopolymers, self-assembled monolayers, and conducting polymers (Tang et al. 2018).
Currently, most fungal diagnostic approaches necessitate invasive samples, are time intensive, and/or have specificity or sensitivity limitations. Nanoscale biosensors have been successfully used as a biological tool for large-scale infectious disease (including fungal diseases) detection, and this sector is quickly developing. NPs can detect dangerous microorganisms quickly, precisely, and at a minimal cost due to their unique physicochemical features (Wang et al. 2013; Sangiao et al. 2019).
NP’s optical and electrochemical qualities make them ideal candidates for detecting fungi. Electrochemical biosensors play a vital role in fungal detection. Metal NP can increase electron transfer and act as a small conduction center, resulting in signal amplification and improved electrochemical biosensor sensitivity and accuracy (Lu et al. 2007). There are numerous forms of metal and metal oxide nanoparticles (NPs) that exhibit antimicrobial action, including titanium dioxide (TiO2), silver (Ag), silver oxide (Ag2O), zinc oxide (ZnO), calcium oxide (CaO), gold (Au), copper oxide (CuO), silica (Si), and magnesium oxide (MgO) (Shaikh et al. 2019). For example, Lee et al. created a colorimetric sensor to find Aspergillus niger spores. This sensor contains AuNPs that had been altered to bind to these spores. When the spores of this fungus underwent sedimentation during the reaction, a change in the intensity of the color of the suspension created by AuNPs-fungus was measured using spectroscopy (Lee et al. 2021). Various magnetic nanomaterials are considered to be a candidate for the development of biosensors as they can be functionalized with dextran, polyethylene glycol, and polyvinyl alcohol, as well as functional groups like thiols, amines, and carboxyl (Ghosh et al. 2021b).
Nanomaterials such as carbon nanotubes, graphene, quantum dots, metal nanoparticles, 2D layered metal oxides, magnetic nanoparticles, and transition metal nitride have been employed to create biosensors with exceptional properties. For example, graphene is compatible with antibodies, nucleic acids, aptamers, enzymes, organisms, peptides, and bacteriophages which have been included into the surface graphene to develop the biosensor. The covalent and non-covalent interactions formed between biomolecules and graphene improve its biocompatibility and solubility, shielding it from interference from the surrounding environment and improving pathogen identification and analysis (Jiang et al. 2020). Apart from graphene, carbon quantum dots are also utilized for detecting microbes. This quantum dots illuminate when exposed to light. High water solubility, exceptional optical and fluorescence capabilities, and a high emission quantum yield are all characteristics of carbon quantum dots (Malavika et al. 2021). Through cell tracking and DNA labelling, these fluorescent carbon dots have been extensively studied and employed in biomedicine, biosensing, and bioimaging. They can be used to identify phytopathogens (Shi et al. 2014).
To detect aflatoxin B1, Althagafi et al. developed new and fast electrochemical biosensors based on gold/graphene nanostructure-modified ITO electrodes (detection limit of 6.9 pg/mL) (Althagafi et al. 2019). Also, Geleta et al. developed an electrochemical aptasensor for detecting aflatoxin B1 using the conductivity and surface modification of gold NP (detection limit: 0.002 fg/mL) (Geleta et al. 2018). The optical properties of NP, particularly those of metal NP that can transition from the ultraviolet to the near-infrared range, are extremely valuable for biosensors and biological imaging. Sojinrin et al. created unique gold NP that changed shape when exposed to spore-forming fungus, resulting in a color shift from red to blue. With 80% sensitivity and 95% specificity, this naked-eye approach may identify beriberi efficiently and promptly (2 min) (detection limit: 10 CFU/mL) (Sojinrin et al. 2017). Hu et al. used nanotechniques to gather positively charged silver NP on the surface of a negatively charged fungal cell wall, boosting the surface-enhanced Raman scattering (SERS) signal to identify and differentiate Cryptococcus neoformans from Cryptococcus gattii, and the method was a hundred percent accurate (Hu et al. 2020). Common types of biosensors used in fungal detection are depicted in Table 1. These biosensors are further divided into various subtypes. For instance, optical biosensors which are used for fungal detection are classified into fluorescence resonance energy biosensors and chemiluminescence-based biosensors. Fluorescence resonance energy biosensors have been employed for multiple analytes label-free biodetection with great sensitivity in a number of medicinal applications. In this method, a fluorescent bio-receptor is coupled to an optical transducer, and non-radioactive energy is transferred from the donor fluorophore to the nearby acceptor molecule (Shi et al. 2015). The design of fluorescent biosensors involves attaching whole cells to the biosensor film. Typically, this film is placed at the tip of optical filaments to produce a fluorescent indicator. Carlson et al. developed a fluorometric biosensor for measuring and detecting aflatoxins (Carlson et al. 2000). Additionally, fluorescent dye was used on 228 oral candidiasis specimens, which were identified by the blue fluorescence that surrounded their tubular or annular formations (Yao et al. 2019). This method provides a quick way to detect Candida. Apart from fluorescence biosensors, using light emission based on chemical reaction, chemiluminescence-based sensors are a developing technique for the detection and diagnosis of fungi. They possess excellent sensitivity, quick dynamic response, and a wide calibration range and are low-cost devices (Ray et al. 2017).
In recent years, nanoparticle-based biosensors have been devised for the diagnosis of Candidiasis. Membrane-based electrochemical impedance spectroscopy was used to identify C. albicans directly (Kwasny et al. 2018). For detecting Candida albicans, Villamizar et al. created a field-effect transistor (FET) biosensor based on single-walled carbon nanotubes (SWCNTs) in which monoclonal anti-Candida antibodies functionalized SWCNTs work as particular binding sites for the adsorption of fungal antigens. In the presence of Cryptococcus albidus and Saccharomyces cerevisiae, the results demonstrated that this sensor could selectively detect Candida albicans at low concentrations. Furthermore, this sensor could aid in the early detection of unwell people, allowing for better drug and treatment administration (Villamizar et al. 2009). According to Asghar et al., a novel immuno-based microfluidic device for the fast detection of Candida albicans in human whole blood has been developed. However, due to low sensitivity, the microchip technology was only able to catch C. albicans in phosphate buffer solution with an efficiency of 61–78% for cell densities ranging from 10 to 105 CFU/mL. The principle, on the other hand, is portable, adaptable and has the potential to be modified to increase specificity and sensitivity (Asghar et al. 2019). Additionally, biofunctionalized upconverting CaF2:Yb, Tm nanoparticles were employed for Candida albicans luminescence detection. A multistep production process was used to uniformly distribute the doping ions within the nanoparticle’s volume, reducing luminescence quenching. The usefulness of the produced nanoparticles as bio-labels for Candida albicans was also demonstrated, and because of the existence of the linked molecules, this approach showed significant potential because of its high specificity (Misiak et al. 2017).
In miniaturized settings, detecting invasive aspergillosis (IA) caused by Aspergillus fumigatus is difficult. In a miniaturized experimental setup, Bhatnagar et al. created an electrochemical nanobiosensor for the sensitive detection of IA by sensing the lethal glip target gene (glip-T). UV–visible spectroscopy, electrochemical impedance spectroscopy, and cyclic voltammetry are used to describe the biosensor. The sensor probe was made on a gold electrode using 1,6-hexanedithiol, and glip probes (glip-P) were self-assembled utilizing chitosan-stabilized gold NP. The results showed that the created sensor is simple, quick, cost-effective, generic, and reusable, making it a promising candidate for fabricating a tiny hand-held device for onsite glip-T detection for IA patients (Bhatnagar et al. 2018). Another study developed a plasmonic gold nanoparticle-based method for diagnosing Aspergillus fungal infections. It generated colored solutions with distinct tones by measuring the shape change of gold nanoparticles (Sojinrin et al. 2017). Aspergillus species have also been detected using SWCNT-based sensors. Jin et al. used SWCNT-integrated FETs functionalized with pentameric antibodies that specifically bind to Aspergillus species to establish a biosensor for real-time detection of Aspergillus species. With a low concentration of 0.3 pg/mL, a detection dynamic range of 0.5 pg/mL to 10 g/mL, and excellent sensitivity, the sensor showed good selectivity towards Aspergillus niger with no response to other fungus species Alternaria alternata (Jin et al. 2015).
Very few biosensors have been developed to diagnose mucormycosis. One study proposed using breath-based biosensors to diagnose invasive mucormycosis. The volatile sesquiterpene metabolite profiles from murine breath were analyzed using thermal desorption gas chromatography/tandem mass spectrometry (GC–MS/MS), and it was discovered that each Mucorales species produced a consistent profile of sesquiterpene secondary metabolite that can be distinguished from aspergillosis (Koshy et al. 2017). In contrast to other fungal infections, where a wide range of fungal biomarkers such as mannan, galactomannan, mannan, beta-glucan, and cryptococcal antigen have been investigated for the development of diagnostic methods, only one biomarker (cell wall fucomannan) has been used for the rapid detection of mucormycosis. The investigation involved the development of a lateral flow immunoassay (LIFA) technique utilising a monoclonal antibody (mAb 2DA6) that targets the cell wall fucomannan of Mucorales. The monoclonal antibody, designated as mAb 2DA6, exhibits remarkable sensitivity towards fucomannan, which can be attributed to its apparent low degree of side chain substitution. Subsequently, it was employed as a biomarker to facilitate prompt diagnosis of invasive murine mucormycosis (Orne et al. 2018). Nevertheless, additional investigations were not conducted utilising this biomarker to cultivate sophisticated methodologies for prompt identification of mucormycosis. Hence, given its sensitivity, it is plausible to develop and utilize biosensors based on mAb 2DA6 for prompt detection and premature diagnosis of CAM (Samson and Dharne 2022).
The field of fungal diagnostics has experienced notable progress with the utilization of Matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) for the identification of fungal peptides that are specific to particular species. Marketing of two commercially available MALDI-TOF MS platforms that have been approved by the Food and Drug Administration (FDA); the Bruker Biotyper, which is manufactured in Germany, and the Vitek MS, which is manufactured in France. The technique’s benefit is its ability to quickly identify fungal isolates or clinical specimens in approximately 30 min (Lau et al. 2019).
The utilization of quantitative polymerase chain reaction (qPCR) is a highly sensitive and expeditious approach for the detection of mucormycosis in individuals who are unable to undergo a biopsy or those who have hematological malignancies with severe thrombocytopenia (Gurunathan et al. 2022). In a study conducted by Millon et al., the efficacy of three qPCR assays targeting Absidia corymbifera (Lichtheimia), Mucor/Rhizopus, and Rhizomucor 18S ribosomal RNA genes was evaluated for the detection of mucormycosis in patient sera. The results indicated that this approach exhibited high sensitivity, a low detection threshold, and the ability to identify infections up to 68 days earlier than conventional methods (Millon et al. 2013). The aforementioned results were validated in a subsequent investigation of said cohort in conjunction with ten additional establishments affiliated with the French network for monitoring invasive fungal infections (Millon et al. 2016). Legrand et al. conducted a study utilising a qPCR method and discovered that the identification of circulating Mucorales DNA (cmDNA) facilitated the prompt diagnosis of invasive wound mucormycosis (IWM) in burn patients who were severely ill, thereby enabling early initiation of treatment (Legrand et al. 2016). Another study conducted by Springer et al. discovered that hematological patients with invasive mucormycosis can be diagnosed earlier (by 21 days) than with conventional methods by utilising a rapid (4-h turnaround time) probe-based Mucorales-specific real-time PCR assay (Muc18S) to detect Mucorales in blood and tissue samples (Springer et al. 2016).
Drawing upon the detection methods outlined above, employing a multidisciplinary approach to promptly detect and treat COVID-19-associated fungal infections in patients may prove pivotal in mitigating the mortality rate associated with these infections (Fig. 3).
Although there is less research on nano-based detection of fungal infection than there is on bacteria and viruses, nanomedicine nevertheless enhances fungal detection.
The current treatment
Azole, polyene, and echinocandin are the three main categories of antifungals used to treat fungal infections. Azole antifungals impede the 14-αdemethylation of lanosterol by blocking cytochrome P450, a crucial enzyme in the pathway that leads to the synthesis of ergosterol. Further, fungal growth is hindered because of a drop in the level of ergosterol and the build-up of harmful sterol intermediates in the cytoplasmic membrane (Shirvani and Fattahi 2021). Polyenes bind to ergosterol in fungal cell membranes, forming aqueous pores that enhance intracellular ion leakage and obstruct active transport mechanisms dependent on membrane potential, resulting in changes in cell membrane permeability and cell death (Ahmed et al. 2022). Echinocandins induce cell death in fungi by inhibiting the transmembrane glucan synthase complex and thus disrupts β-(1,3)-D-glucan synthesis which is necessary for the synthesis of essential components of fungal cell wall (Gonzalez-Lara and Ostrosky-Zeichner 2020).
The preferred antifungal depends on factors such as disease severity, the presence of coexisting conditions, and prior fungal exposure (Gonzalez-Lara and Ostrosky-Zeichner 2020).
Candidiasis
Patients receiving antibacterial, immunomodulating therapy, undergoing hemodialysis, parenteral nutrition, having central venous catheters, mechanical ventilation, having renal insufficiency, or having diabetes mellitus—all of which are common in COVID-19 critical-care patients—are at significant risk of developing invasive candidiasis (IC) (Riche et al. 2020; White 2021). Additionally, a couple of studies suggest that the use of immunomodulating medications, such as corticosteroids and tocilizumab, has been proposed as a risk factor for candidemia (Antinori et al. 2020; Riche et al. 2020; Mo et al. 2021).
The most common pathogens linked with CAC are Candida albicans and Candida glabrata, presenting as candidemia (Arastehfar et al. 2020a; Seagle et al. 2022). However, the developing pathogen and multidrug-resistant species C. auris predominated in specific geographic locations, most prevalent in India, which has taken over from C. albicans as the most common Candida species in at least one hospital in Spain (Mulet Bayona et al. 2021). C. auris is difficult to differentiate from other species of candida and is resistant to all available classes of antifungal drugs (Spivak and Hanson 2018; Koehler et al. 2021). The most effective method of combating C. auris is not yet known. Echinocandins are recommended as the first line of treatment since they are effective against the majority of C. auris isolates in the United States (Chakrabarti et al. 2015; Larkin et al. 2017). Additionally, despite being resistant to azoles, isavuconazole was reported to be effective against several C. auris isolates (Larkin et al. 2017).
Aspergillosis
Early in 2020, China reported the first cases of CAPA. Multiple case series and cohort studies have since emphasized the significance of this potentially fatal secondary infection (Yang et al. 2020b; Arastehfar et al. 2020b). Patients with acute respiratory failure (severe lung damage) caused by COVID-19, particularly those taking systemic corticosteroid or tocilizumab therapy, are at high risk of developing CAPA (Hoenigl et al. 2022c). Additionally, according to a couple of studies, independent predictors of the development of CAPA are thrombocytopenia, vasopressor usage before CAPA diagnosis, receiving invasive ventilation, and widespread use of broad-spectrum antibiotics in intensive care units (Cox et al. 2020; Xu et al. 2021; Prattes et al. 2022).
Aspergillus fumigatus is known to be the most common pathogen that causes invasive aspergillosis (IA). It is one of the Aspergillus species that has been studied the most. On the other hand, it was found that Aspergillus flavus is more virulent than Aspergillus fumigatus, even though they both cause the same clinical syndromes (Machado et al. 2021). Based on existing data, there may be differences in where these pathogens are found. For example, most cases of Aspergillus fumigatus infections have been reported in Western Europe, while Aspergillus flavus infections are more common in Asia, the Middle East, and Africa (Rudramurthy et al. 2019). To combat Aspergillus spp., voriconazole or isavuconazole is used as first-line treatments and liposomal amphotericin B, posaconazole, or echinocandins as second-line treatments for potential, likely, and proven CAPA (Koehler et al. 2021). Also, itraconazole, along with posaconazole and echinocandins, is used in patients who are unable to tolerate primary antifungal treatment (Vahedi-Shahandashti and Lass-Flörl 2020).
Mucormycosis
The burden CAM in India is estimated to be 70 times higher than the global rate, with an annual case count of more than 200,000 (Prakash and Chakrabarti 2021). The prevalence of CAM has been connected to environmental factors such as fungal spore exposure and chronic illnesses such as poorly managed diabetic mellitus (Jeong et al. 2019; Prakash et al. 2020). Additionally, widespread use of steroids, and broad-spectrum antibiotics, contaminated oxygen, and burns are the most common risk factors of CAM (Bhanuprasad et al. 2021; Palanisamy and Elango 2022).
Rhizopus and Mucor are the most prevalent agents of CAM (Jeong et al. 2019). Among COVID-19 patients with diabetes, rhino-cerebral mucormycosis manifests most frequently (Palanisamy and Elango 2022). Several methods are used to treat mucormycosis, including surgical removal of infected tissue or organs, early administration of effective antifungal medication, and the implementation of other adjuvant therapy (Hassan and Voigt 2019). The first-choice treatment for mucormycosis is liposomal amphotericin B (Hoenigl et al. 2022a). However, ideal dosage, side effects associated with infusion, and increased risk of nephrotoxicity are all major, potentially therapy-limiting factors (León-Buitimea et al. 2021). Additionally, posaconazole and isavuconazole have demonstrated efficacy in cases of renal failure (Cornely et al. 2019a). Various fungal infections, their incidence, risk factors, current line of treatment, and its limitations are depicted in Table 2.
Fungal treatment using nanotechnology
Despite the constant progress in antifungal treatment, the efficacy of fungal therapeutics is hampered by a variety of therapy-related hurdles, such as poor drug penetration and the emergence of drug-resistant strains. Additionally, when new or drug-resistant fungi evolve, drug permeability, adverse effects, and non-targeting abilities of drugs are factors that limit their effectiveness. It has been seen that patients treated with conventional antifungal medications fail to respond owing to drug resistance, resulting in the spread of infection, poor social, psychological, and occupational health effects, and a decrease in the patient’s quality of life (Kaur et al. 2021). Moreover, the toxicity of antifungals and their interactions with other medications make fungal infections notoriously difficult to treat (Brunet et al. 2018). The ever-growing number of research indicates that novel nanotechnology-based agents and approaches may help overcome these limitations (Khezri et al. 2021).
Nanoparticles are currently gaining attention in the scientific community due to their numerous potential therapeutic and diagnostic uses, including drug delivery and detection of biological and chemical agents, among others (Barrak et al. 2019). Furthermore, nanostructures are regarded as promising therapeutic alternatives due to their low toxicity, ability to cross many biological barriers, and capacity to be covalently conjugated with hydrophobic or hydrophilic drugs and macromolecules, enhancing solubility and stability (Khan et al. 2019; Mitchell et al. 2021).
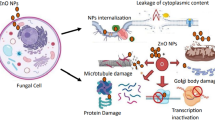
Several types of nanostructures are being studied for the administration of antifungal drugs and to improve their ability to function as adjuvants (Souza and Amaral 2017b). The antifungal activity of various types of nanoparticles is believed to involve a mechanism consisting of five distinct steps. The process of internalizing nanoparticles involves endocytosis and diffusion. This is followed by the disassembly of the membrane and the release of nanoparticles into the cytoplasm. The induction of oxidative stress is a subsequent step, which leads to ROS-induced cell death. This is achieved through the activation of pro-apoptotic proteins and ROS production, resulting in mitochondrial dysfunction and the endoplasmic reticulum stress-mediated apoptosis pathway. The detailed mechanism of antifungal activity of NP is depicted in Fig. 4.
These nanostructures can be classified according to their composition into (Soliman 2017): polymeric NPs, phospholipid-based vesicles, nanostructured lipid carriers (NLCs), dendrimers, nanoemulsions (NE), and metallic and magnetic NPs.
Considering the shape, size, and chemical and physical properties, nanoparticles can be classified as organic or polymeric, inorganic (metallic), and carbon nanoparticles. Various nanotechnology based antifungal treatments are depicted in Table 3.
Candidiasis
The microbiota of humans includes species from the genus Candida. But when the immune system is compromised, or when biological barriers are lowered, these microbes can cause life-threatening infections. As discussed earlier, CAC has emerged as a major threat due to its multi-drug resistance nature. Thus, several antifungal medications based on nanoparticles have been developed and are currently being tested against Candida spp.
Recent research indicates that AgNPs are effective against nosocomial infections and multidrug-resistant pathogens (Lara et al. 2020). For example, the effectiveness of AgNPs combined with propolis extract (PE) against mature biofilms of Candida species and other fungi was assessed. It was discovered that the concentration needed for the formulation to have fungicidal activity was lower than the cytotoxic concentration (Kischkel et al. 2020). Researchers also showed that AgNPs were effective against C. auris biofilm on colonized catheters and hospital fabrics. Importantly, the antifungal activity of AgNP-interlaced fabric fibers (such as elastic bandage wraps) was maintained even after multiple washes(Lara et al. 2020). Apart from this, fluconazole-resistant Candida albicans and Candida glabrata were considerably inhibited by the curcumin—AgNPs, and the degree of inhibition varied with the curcumin concentration used (Paul et al. 2018). In addition, it was discovered that curcumin-AgNPs were hemolytic; yet, the investigators proposed that putting such nanoparticles into bacterial cellulose hydrogel might be an appealing method for exhibiting antimicrobial activity against three common wound-infecting pathogenic microbes (Staphylococcus aureus, Pseudomonas aeruginosa, and Candida auris) in chronic wound infections (Gupta et al. 2020). AgNPs incorporated with fluconazole were able to reduce the biological activity of C. albicans biofilms, which was attributed to the ability of the NPs to promote the penetration of the azole, thereby disrupting the cell membrane (Różalska et al. 2018).
Trimetallic composite nanoparticles, comprised of silver, copper, and cobalt (Ag–Cu-Co), have been found to have more potent antimicrobial effects than their monometallic and bimetallic counterparts (Kamli et al. 2021). As per the recent study, these trimetallic nanoparticles exhibited higher stability, strong fungicidal activity, higher catalytic activity, and enhanced drug encapsulation efficacy (Kamli et al. 2021). In addition, the same study suggested that these nanoparticles exert their effect on C. auris by inducing cellular apoptosis and stopping its cell cycle (Kamli et al. 2021).
Elemental bismuth is well known for its anti-fungal effects, particularly against Candida albicans. The same research found that bismuth nanoparticles (BiNPs) showed promising action against all clades of planktonic C. auris. The IC50 values for biofilms showed that BiNPs have a moderate inhibitory effect on the biofilm phenotype (Vazquez-Munoz et al. 2020). Further studies of BiNPs are still required. Apart from bismuth, high doses of gallium (gallium nitrate) have been suggested to be used as a fungistatic agent due to its proven antimicrobial activity against various pathogens (Bastos et al. 2019). However, the toxicity of such high doses of gallium has not been investigated.
Nitric oxide (NO), a crucial element of the mammalian innate immune system, can be both cytotoxic and cytostatic against a variety of pathogens. NO has previously been shown to have antifungal effects on C. albicans (Bandara and Samaranayake 2022). These nanoparticles that induce a sustained release of NO inhibited both biofilm and planktonic growth of the C. auris isolates. This provides further evidence that nitric oxide nanoparticles are a promising antifungal treatment option for treating this drug-resistant fungal infection (Cleare et al. 2020).
The transformation of C. albicans from yeast to hyphae is one of the virulence elements that contributes significantly to its pathogenicity. A molecule made by Candida albicans called farnesol is a crucial quorum-sensing molecule (QSM) that prevents hyphae from growing. A recent study conducted on farnesol loaded niosomes comprising gel formulation revealed that niosomal gel has higher antifungal activity against Candida albicans. Additionally, it showed higher ex vivo skin permeation and no irritation when tested on rabbit skin (Barot et al. 2021). Additionally, a mouse model of vulvovaginal candidiasis was used to assess the efficacy of chitosan NPs encapsulating farnesol and miconazole. Miconazole with farnesol did not show synergism in-vitro. However, chitosan nanoparticles (NPs) containing farnesol were found to be beneficial in lowering pathogenicity in mice, and they also suppressed hyphal development in C. albicans (Costa et al. 2019). The precise mechanisms by which farnesol affects the planktonic and biofilm phenotypes of candida are not yet known, but it has been hypothesised that the decreased activity of drug efflux pumps and the downregulation of the genes encoding them (CDR1, CDR2, and SNQ2) may be the cause of such observations (Srivastava and Ahmad 2020).
Amphotericin B (AmB) is commonly used in antifungal treatments because of its wide spectrum of activity, but it can produce significant nephrotoxicity (Roemer and Krysan 2014). Using AmB encapsulated in PLGA-PEG NPs (PLGA-PEGAmB), the in vivo and in vitro efficacy, toxicity, and oral bioavailability of these formulations were studied. Compared to free AmB, PLGA-PEG-AmB nanoparticles lowered C. albicans MIC. Additionally, using a hemolysis assay, NP formulations exhibited less toxicity than Fungizone®. After a week of oral treatment of PLGA-PEG-AmB NPs to rats, blood urea nitrogen and plasma creatinine values remained normal in vivo. The addition of glycyrrhizin acid increased the bioavailability of PLGA-PEG-AmB nanoparticles considerably (Radwan et al. 2017). Additionally, SLNs and NLCs that were easily loaded with AmB were synthesized, and the NPs demonstrated decreased hemolytic activity when compared to Fungizone®. Also, these formulations were more effective against C. albicans than free AmB or Fungizone®. According to the research, these formulations may boost antifungal activity, increase AmB solubility, and reduce treatment toxicity (Jansook et al. 2018).
In short, the use of a nanotechnology-based approach in the treatment of CAC can be beneficial. However, further research is warranted on the modes of action, toxicity, and dosage of these nanonovel formulations.
Aspergillosis
Aspergillosis members are characterized as opportunistic pathogens that can cause allergic reactions and systemic infections in humans. During the initial months of the epidemic, CAPA cases began to surface. However, incidence rates varied substantially, likely because CAPA is challenging to diagnose in COVID-19-associated acute respiratory distress syndrome (ARDS) patients (Hoenigl et al. 2022c). The need for newer treatment options is necessary as many challenges are arising while treating COVID-19-associated fungal infections. For example, the emergence of azole resistance in A. fumigatus has led to treatment failures.
Metal nanoparticles have great potential in treatment of fungal infections. Various studies based on marketed metallic nanoparticles are carried out. As per study conducted on commercial AgNPs against Aspergillus fumigatus showed that treatment with 100 mg/L of AgNPs resulted in 54% growth of A. fumigatus when compared to 100% growth in control on potato dextrose agar (Ogar et al. 2015). Similarly, study focusing on antifungal activity of commercial AgNPs in comparison with natamycin against Aspergillosis spp. showed that, AgNPs (MIC50: 0.5 µg/mL) exhibited potent in vitro activity when compared to that of natamycin (MIC50: 32 µg/mL) (Xu et al. 2013). In one study, cross-linked chitosan biguanidine (CChG) that had been loaded with AgNPs (CChG/AgNPs) and Chitosan biguanidine hydrochloride (ChG) was tested for antimicrobial activity. The results showed that The ChG and CChG/AgNPs shown superior antimicrobial activity against A. fumigatus in comparison to chitosan and CChG. Additionally, CChG/AgNPs showed lower cytotoxicity to breast cancer cells (MCF-7) along with improved thermal stability (Salama et al. 2016).
Various polymers can be enhanced or given new capabilities by the addition of metal nanoparticles, such as the ability to inhibit the development or adhesion of potentially hazardous microbes (Vazquez-Rodriguez et al. 2020). In a related work, a nanosponge composite made of polyurethane cyclodextrin co-polymerized phosphorylated multiwalled carbon nanotube-doped Ag-TiO2 nanoparticle (pMWCNT-CD/Ag-TiO2) was created, and its antifungal properties were tested against Aspergillus ochraceus and Aspergillus fumigatus. The MIC of this nanocomposite was 437.5 g/mL, which is lower than the MIC of the undoped nanosponge, which was 1750 g/mL. The TiO2 and Ag NPs dopped onto the material are thought to have caused the antifungal action by engaging with the functional groups of the fungus membrane, which caused ROS generation and breakdown of the cell wall membrane (Leudjo Taka et al. 2020). Another study investigated the antifungal activity of two core–shell bimetallic nanoparticles (Cu-Ag and Ag–Cu) synthesized in a polyvinyl alcohol (PVA) matrix. This study reported that Cu-Ag core–shell NPs outperformed Ag–Cu NPs, creating an inhibition zone of 23 and 16 mm, respectively, at 0.1 M. In contrast, Ag–Cu had a better fungicidal impact than Cu-Ag, with MFCs of 15 and 25 g/mL, respectively, causing destruction to the fungal cell wall as revealed by scanning electron microscope (SEM) images (Sabira et al. 2020).
The nano-formulation of conventional antifungal agents allows for greater control of active ingredient release by altering the outer shell of the nanocapsules, resulting in better treatment of diverse ailments. The most research has been done on nanoformulations for AmB delivery because it is a first-line antifungal for treating fungal infections. AmBisome® is one of the most frequently cited AmB formulations in publications comparing the efficacy of different nanoformulations. In one study, the toxicity and efficacy of AmBisome® and Lambin® were examined in mice. Both formulations considerably reduced the fungal burden in the lungs of mice treated after A. fumigatus infection. However, a single 50 mg/kg dose of the Lambin® resulted in 80% mortality and a similar dose of the AmBisome® resulted in 0% mortality (Olson et al. 2015). Another study developed AmB-entrapping plain, anionic and PEG (polyethylene glycol)-Lipid nanoparticles. The antifungal activity of this nanoscale drug carrier was compared with Fungizone® and AmBisome® both in vivo and in vitro. The in vitro antifungal activity of the AmB-entrapping LNPs was higher than that of Fungizone® but not AmBisome®, with MICs of 0.025, 0.25, and 1 g/mL for LNPs, AmBisome®, and Fungizone®, respectively. However, in an in vivo model (mice infection-A. fumigatus) AmB-entrapped LNPs showed higher survival rates than AmBisome® (Fukui et al. 2003). One study evaluated the kinetics of AmBisome® and Abelcet® accumulation in the lungs of immunocompromised mice with invasive pulmonary aspergillosis. This study reported that Abelcet® transports active AmB to the lung more quickly than AmBisome® at 5 mg/kg/day, resulting in a quicker reduction in fungal load (Lewis et al. 2007). Another study examined the antifungal activity of poly(D, L-lactide-co-glycolide (PLGA) NPs loaded with AmB against different fungal infections, including A. fumigatus. In comparison to AmB, Fungizone®, and AmBisome®, AmB-loaded PLGA NPs had much stronger antifungal activity against A. fumigatus in vitro. They also shown decreased cytotoxicity towards MRC-5 cells and hemolytic activity towards red blood cells. Similarly, when applied in vivo at a dosage of 5 mg/kg, the AmB-loaded PLGA NPs generated a considerable reduction of A. fumigatus in comparison to Fungizone® and a 2-times more effective reduction than AmBisome® (Van De Ven et al. 2012). According to a study conducted on mice, the recommended doses of AmBisome® for neutropenic patients and non-neutropenic patients with isolates of A. fumigatus with MICs between 0.5 and 1 mg/L are 7.5 to 10 mg/kg and 1 to 3 mg/kg, respectively (Siopi et al. 2019a). Another study evaluated the clinical response and renal toxicity of AmBisome® in 71 patients for chronic pulmonary aspergillosis therapy. This study showed that, in 48 patients (73.8%), the first AmBisome® treatment was effective. Improvements in quality of life (QOL) were seen in 37 (92.5%) of the 40 patients. However, acute kidney injury occurred in 25% of the patients, indicating that these drugs should be used with caution (Newton et al. 2016).
In short, the implementation of nanotechnology in the treatment of fungal infections, such as aspergillosis, has great potential since it allows for the targeted and efficient delivery of antifungal agents, enhanced drug stability, and improved therapeutic outcomes.
Mucormycosis
Mucormycosis is caused by various species of fungi belonging to the order Mucorales and is considered an opportunistic pathogen. Various types of fungi, such as Rhizopus spp., Mucor spp., Rhizomucor spp., Syncephalastrum spp., Cunninghamella bertholletiae, Apophysomyces spp., and Lichtheimia spp., have been identified as causative agents of mucormycosis (Skiada et al. 2020). COVID-19 pandemic has paved way for this secondary fungal infection (CAM) in many countries. This infectious condition has a significant mortality rate. Additionally, the Mucorales have developed multi-resistance to existing antifungals used to treat the infection, including amphotericin B, posaconazole, and isavuconazole. Thus, there is need for more advanced treatment options (Dogra et al. 2022).
Various metal nanoparticles have demonstrated their antimicrobial activity against Mucorales. Silver has been employed as a nanomaterial in the treatment of antifungal and antibacterial. Silver nanoparticles have long been known for their biocidal effects, which include antibacterial, antifungal, antiviral, and anticancer properties (Chatterjee et al. 2023). In one study, it was observed that β-cyclodextrin-encapsulated silver nanoparticles exhibited the capability to combat Mucorales, resulting in a decrease in the growth of M. ramosissimus (George et al. 2011). Considering this data, silver nanoparticles can be a viable antifungal strategy to prevent the progression of mucormycosis. Another new antifungal treatment that has emerged uses zirconium oxide (ZrO2NPs) nanoparticles. Only a few studies have been conducted so far on the antifungal properties of zirconium oxide nanoparticles. However, it has been feasible to show that they can suppress a number of Mucor and Rhizopus spp. (Dogra et al. 2022). Apart from ZrO2NPs, the antifungal activity of Saussurea lappa plant root-mediated zinc oxide nanoparticles was observed against various fungal strains including Rhizopus oryzae (Kolahalam et al. 2021). A study focusing on copper and nickel nanoparticles reports that, copper nanoparticles (DPMMCuNPs) and nickel nanoparticles, stabilized by Schiff base ligand 2-(4,6-dimethoxypyrimidin-2-ylimino)methyl)-6-methoxyphenol (DPMM), demonstrated antibacterial and antifungal effects that were dependent on the administered dose. Research has been conducted on the efficacy of DPMM-CuNPs and DPMM-NiNPs in inhibiting the growth of Mucor indicus and Rhizopus spp. (Adwin Jose et al. 2018).
Another therapeutic option for treatment is the utilization of nanoemulsion NB-201. NB-201 incorporates a surfactant known as benzalkonium chloride (BZK), which exerts a fungicidal effect by disrupting the cell membrane of the fungal organism (Brunet and Rammaert 2020). According to in vitro susceptibility studies, NB-201 significantly inhibits the growth of Mucorales, including several Rhizopus and Mucor isolates (M. circinelloides, R. microsporus, and R. delemar). This finding implies that mucormycosis may be treated topically with nanoemulsion NB-201 (León-Buitimea et al. 2021).
In short, the integration of nanotechnology in the treatment of mucormycosis signifies a notable advancement. However, more research is warranted on medications based on nanoparticles to treat this infection.
Toxicity of NP
Nanoparticles have gained significant attention in various biomedical applications, including the field of infectious disease management. However, alongside their potential benefits, concerns regarding the potential toxicity of nanoparticles have also emerged. When nanoparticles enter the human body, they can pass through multiple cellular barriers and reach the most sensitive organs, such as the liver, lung, and kidney, causing mitochondrial damage, deoxyribonucleic acid (DNA) alterations, and finally cell apoptosis or death (Tan et al. 2018). One of the main mechanisms of NP toxicity is the generation of reactive oxygen species (ROS), which can result in oxidative stress, inflammation, and subsequent damage to proteins, cell membranes, and DNA (Fu et al. 2014; Fard et al. 2015). The amount of ROS produced by nanoparticles is determined by various factors, including particle size, surface, shape, composition, solubility, particle absorption, aggregation/agglomeration, and the presence of mutagens and transition metals associated with the particles (He et al. 2018; Jeevanandam et al. 2018). For example, a study conducted by Rizk et al. showed that the genetic disturbance began at a high dose of exposure (500 mg/kg body weight) and for a lengthy period of time (45 days), while the liver function enzymes, oxidative stress markers, and liver histological pattern were all significantly influenced by the dose and time of titanium oxide nanoparticles (21 nm) (Rizk et al. 2017). Furthermore, Steckiewicz et al. showed that the geometry of gold nanoparticles affected their cytotoxicity. The most cytotoxic gold nanoparticles were discovered to be gold nanostars with the highest anticancer potential, while gold nanospheres with low anticancer potential were found to be less harmful (Steckiewicz et al. 2019).
Nanoparticles are often introduced into the human body through ingestion (gastrointestinal tract), breathing (respiratory tract), injection (blood circulation), and skin contact (Wu and Tang 2018). Conditions associated with these entry points are depicted in Table 4.
Conclusion
Prevalence and spread of invasive fungal infections and co-infections with viruses are increasing at alarming rate. These infections are extremely difficult to treat and pose great challenges to clinicians. In addition, like in bacteria, antimicrobial resistance is spreading in fungi which can make existing treatments ineffective. Additionally, the number of active agents that can be used for treating pathogenic fungi is limited. Rapid diagnosis and effective treatments of fungal infections are the need of an hour. Nanotechnology has offered advanced technologies and tools to diagnose and treat fungal infections. On an account of its nanosize and unique optical and physicochemical properties, nanomaterials developed from lipids, polymers, and different metals have great promise to offer a wide range of solutions to current problems associated with fungal diagnosis and treatments. However, several factors including nanotechnology-explicit risks must be taken into account while developing strategies for diagnosis and treatment of fungal infections using these materials and as a future work, more research is warranted to evaluate the safety of complex and hybrid nanomaterials.
Data availability
Not applicable.
References
Abd-Elsalam KA (2021) Special issue: fungal nanotechnology. J fungi (Basel, Switzerland) 7. https://doi.org/10.3390/JOF7080583
Adwin Jose P, Dhaveethu Raja J, Sankarganesh M, Rajesh J (2018) Evaluation of antioxidant, DNA targeting, antimicrobial and cytotoxic studies of imine capped copper and nickel nanoparticles. J Photochem Photobiol B 178:143–151. https://doi.org/10.1016/J.JPHOTOBIOL.2017.11.005
Aguilar-Pérez KM, Medina DI, Narayanan J et al (2021) Synthesis and nano-sized characterization of bioactive oregano essential oil molecule-loaded small unilamellar nanoliposomes with antifungal potentialities. Molecules 26. https://doi.org/10.3390/MOLECULES26102880
Ahmed N, Mahmood MS, Ullah MA et al (2022) COVID-19-associated candidiasis: possible patho-mechanism, predisposing factors, and prevention strategies. Curr Microbiol 79. https://doi.org/10.1007/S00284-022-02824-6
Alhakamy NA, Hosny KM, Rizg WY et al (2022) Development and optimization of hyaluronic acid-poloxamer in-situ gel loaded with voriconazole cubosomes for enhancement of activity against ocular fungal infection. Gels (Basel, Switzerland) 8. https://doi.org/10.3390/GELS8040241
Alhowyan AA, Altamimi MA, Kalam MA et al (2019) Antifungal efficacy of Itraconazole loaded PLGA-nanoparticles stabilized by vitamin-E TPGS: in vitro and ex vivo studies. J Microbiol Methods 161:87–95. https://doi.org/10.1016/J.MIMET.2019.01.020
Ali M, Afzal M, Bhattacharya SM et al (2013) Nanopharmaceuticals to target antifilarials: a comprehensive review. Expert Opin Drug Deliv 10:665–678. https://doi.org/10.1517/17425247.2013.771630
Althagafi II, Ahmed SA, El-Said WA (2019) Fabrication of gold/graphene nanostructures modified ITO electrode as highly sensitive electrochemical detection of Aflatoxin B1. PLoS One 14:e0210652. https://doi.org/10.1371/JOURNAL.PONE.0210652
Alunda BO, Lee YJ (2020) Review: cantilever-based sensors for high speed atomic force microscopy. Sensors (Basel) 20:1–39. https://doi.org/10.3390/S20174784
Alzayyat ST, Almutiri GA, Aljandan JK et al (2021) Antifungal efficacy and physical properties of poly(methylmethacrylate) denture base material reinforced with SiO 2 nanoparticles. J Prosthodont 30:500–508. https://doi.org/10.1111/JOPR.13271
Ambati S, Ferarro AR, Kang SE et al (2019) Dectin-1-targeted antifungal liposomes exhibit enhanced efficacy. mSphere 4. https://doi.org/10.1128/MSPHERE.00025-19
Antinori S, Bonazzetti C, Gubertini G et al (2020) Tocilizumab for cytokine storm syndrome in COVID-19 pneumonia: an increased risk for candidemia? Autoimmun Rev 19. https://doi.org/10.1016/J.AUTREV.2020.102564
Arastehfar A, Carvalho A, Hong Nguyen M et al (2020a) COVID-19-associated candidiasis (CAC): an underestimated complication in the absence of immunological predispositions? J Fungi (Basel, Switzerland) 6:1–13. https://doi.org/10.3390/JOF6040211
Arastehfar A, Carvalho A, van de Veerdonk FL et al (2020b) COVID-19 associated pulmonary aspergillosis (CAPA)-from immunology to treatment. J Fungi (Basel, Switzerland) 6:1–17. https://doi.org/10.3390/JOF6020091
Asghar W, Sher M, Khan NS et al (2019) Microfluidic chip for detection of fungal infections. ACS Omega 4:7474–7481. https://doi.org/10.1021/ACSOMEGA.9B00499
Asghari F, Jahanshiri Z, Imani M et al (2016) Antifungal nanomaterials: synthesis, properties, and applications. Nanobiomaterials Antimicrob Ther Appl Nanobiomaterials 343–383. https://doi.org/10.1016/B978-0-323-42864-4.00010-5
Bandara N, Samaranayake L (2022) Emerging and future strategies in the management of recalcitrant Candida auris. Med Mycol 60:8. https://doi.org/10.1093/MMY/MYAC008
Barot T, Rawtani D, Kulkarni P (2021) Development, characterization and in vitro-in vivo evaluation of Farnesol loaded niosomal gel for applications in oral candidiasis treatment. Heliyon 7. https://doi.org/10.1016/J.HELIYON.2021.E07968
Barrak H, Saied T, Chevallier P et al (2019) Synthesis, characterization, and functionalization of ZnO nanoparticles by N-(trimethoxysilylpropyl) ethylenediamine triacetic acid (TMSEDTA): investigation of the interactions between Phloroglucinol and ZnO@TMSEDTA. Arab J Chem 12:4340–4347. https://doi.org/10.1016/J.ARABJC.2016.04.019
Barreau F, Tisseyre C, Ménard S et al (2021) Titanium dioxide particles from the diet: involvement in the genesis of inflammatory bowel diseases and colorectal cancer. Part Fibre Toxicol 18. https://doi.org/10.1186/S12989-021-00421-2
Bastos RW, Rossato L, Valero C et al (2019) Potential of gallium as an antifungal agent. Front Cell Infect Microbiol 9. https://doi.org/10.3389/FCIMB.2019.00414
Bergeron A, Porcher R, Sulahian A et al (2012) The strategy for the diagnosis of invasive pulmonary aspergillosis should depend on both the underlying condition and the leukocyte count of patients with hematologic malignancies. Blood 119:1831–1837. https://doi.org/10.1182/BLOOD-2011-04-351601
Bhanuprasad K, Manesh A, Devasagayam E et al (2021) Risk factors associated with the mucormycosis epidemic during the COVID-19 pandemic. Int J Infect Dis 111:267. https://doi.org/10.1016/J.IJID.2021.08.037
Bhatnagar I, Mahato K, Ealla KKR et al (2018) Chitosan stabilized gold nanoparticle mediated self-assembled gliP nanobiosensor for diagnosis of Invasive Aspergillosis. Int J Biol Macromol 110:449–456. https://doi.org/10.1016/J.IJBIOMAC.2017.12.084
Bhattarai P, Hameed S (2020) Basics of biosensors and nanobiosensors. https://doi.org/10.1002/9783527345137.ch1
Bicanic TA, Harrison TS (2014) Systemic fungal infections. Medicine (Baltimore) 42:26–30. https://doi.org/10.1016/J.MPMED.2013.10.006
Brunet K, Alanio A, Lortholary O, Rammaert B (2018) Reactivation of dormant/latent fungal infection. J Infect 77:463–468. https://doi.org/10.1016/J.JINF.2018.06.016
Brunet K, Rammaert B (2020) Mucormycosis treatment: recommendations, latest advances, and perspectives. J Mycol Med 30. https://doi.org/10.1016/J.MYCMED.2020.101007
Buzea C, Pacheco II, Robbie K (2007) Nanomaterials and nanoparticles: sources and toxicity. Biointerphases 2:MR17–MR71. https://doi.org/10.1116/1.2815690
Carlson MA, Bargeron CB, Benson RC et al (2000) An automated, handheld biosensor for aflatoxin. Biosens Bioelectron 14:841–848. https://doi.org/10.1016/S0956-5663(99)00057-3
Chakrabarti A, Sood P, Rudramurthy SM et al (2015) Incidence, characteristics and outcome of ICU-acquired candidemia in India. Intensive Care Med 41:285–295. https://doi.org/10.1007/S00134-014-3603-2
Chatterjee K, Taneja J, Khullar S, Pandey AK (2023) Antifungal activity of silver nanoparticles on fungal isolates from patients of suspected mucormycosis. Int Microbiol 26:143–147. https://doi.org/10.1007/S10123-022-00280-7
Cheong YK, Arce MP, Benito A et al (2020) Synergistic antifungal study of PEGylated graphene oxides and copper nanoparticles against Candida albicans. Nanomater (Basel, Switzerland) 10. https://doi.org/10.3390/NANO10050819
Cleare LG, Li KL, Abuzeid WM et al (2020) NO Candida auris: nitric oxide in nanotherapeutics to combat emerging fungal pathogen Candida auris. J Fungi (Basel, Switzerland) 6:1–13. https://doi.org/10.3390/JOF6020085
Cornely OA, Alastruey-Izquierdo A, Arenz D et al (2019) Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis 19:e405. https://doi.org/10.1016/S1473-3099(19)30312-3
Cornely OA, Alastruey-Izquierdo A, Arenz D et al (2019b) Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis 19:e405–e421. https://doi.org/10.1016/S1473-3099(19)30312-3
Costa AF, Araujo DE, Cabral MS et al (2019) Development, characterization, and in vitro-in vivo evaluation of polymeric nanoparticles containing miconazole and farnesol for treatment of vulvovaginal candidiasis. Med Mycol 57:52–62. https://doi.org/10.1093/MMY/MYX155
Cox MJ, Loman N, Bogaert D, O’Grady J (2020) Co-infections: potentially lethal and unexplored in COVID-19. Lancet Microbe 1:e11. https://doi.org/10.1016/S2666-5247(20)30009-4
Cui F, Zhou Z, Zhou HS (2020) Molecularly imprinted polymers and surface imprinted polymers based electrochemical biosensor for infectious diseases. Sensors (Basel) 20. https://doi.org/10.3390/S20040996
Das S, Devireddy R, Gartia MR (2023) Surface plasmon resonance (SPR) sensor for cancer biomarker detection. Biosensors 13. https://doi.org/10.3390/BIOS13030396
Dogra S, Arora A, Aggarwal A et al (2022) Mucormycosis amid COVID-19 crisis: pathogenesis, diagnosis, and novel treatment strategies to combat the spread. Front Microbiol 12. https://doi.org/10.3389/FMICB.2021.794176
Dyussembayev K, Sambasivam P, Bar I et al (2021) Biosensor technologies for early detection and quantification of plant pathogens. Front Chem 9. https://doi.org/10.3389/FCHEM.2021.636245
Fard JK, Jafari S, Eghbal MA (2015) A review of molecular mechanisms involved in toxicity of nanoparticles. Adv Pharm Bull 5:447–454. https://doi.org/10.15171/APB.2015.061
Farooq M, Usman F, Zaib S et al (2022) Fabrication and evaluation of voriconazole loaded transethosomal gel for enhanced antifungal and antileishmanial activity. Molecules 27:3347. https://doi.org/10.3390/MOLECULES27103347
Fauci AS, Lane HC, Redfield RR (2020) Covid-19 — navigating the uncharted. N Engl J Med 382:1268–1269. https://doi.org/10.1056/NEJME2002387/SUPPL_FILE/NEJME2002387_DISCLOSURES.PDF
Fu PP, Xia Q, Hwang HM et al (2014) Mechanisms of nanotoxicity: generation of reactive oxygen species. J Food Drug Anal 22:64–75. https://doi.org/10.1016/J.JFDA.2014.01.005
Fukui H, Koike T, Nakagawa T et al (2003) Comparison of LNS-AmB, a novel low-dose formulation of amphotericin B with lipid nano-sphere (LNS®), with commercial lipid-based formulations. Int J Pharm 267:101–112. https://doi.org/10.1016/j.ijpharm.2003.08.002
Gangneux JP, Bougnoux ME, Dannaoui E et al (2020) Invasive fungal diseases during COVID-19: we should be prepared. J Mycol Med 30:100971. https://doi.org/10.1016/J.MYCMED.2020.100971
Geleta GS, Zhao Z, Wang Z (2018) A novel reduced graphene oxide/molybdenum disulfide/polyaniline nanocomposite-based electrochemical aptasensor for detection of aflatoxin B1. Analyst 143:1644–1649. https://doi.org/10.1039/C7AN02050C
George C, Kuriakose S, George S, Mathew T (2011) Antifungal activity of silver nanoparticle-encapsulated β-cyclodextrin against human opportunistic pathogens. 23:593–597. https://doi.org/10.1080/10610278.2011.575471
Ghosh A, Sarkar A, Paul P, Patel P (2021a) The rise in cases of mucormycosis, candidiasis and aspergillosis amidst COVID19. Fungal Biol Rev 38:67–91. https://doi.org/10.1016/J.FBR.2021.09.003
Ghosh S, Ahmad R, Zeyaullah M, Khare SK (2021b) Microbial nano-factories: synthesis and biomedical applications. Front Chem 9. https://doi.org/10.3389/FCHEM.2021.626834
Gonzalez-Lara MF, Ostrosky-Zeichner L (2020) Invasive candidiasis. Semin Respir Crit Care Med 41:3–12. https://doi.org/10.1055/S-0040-1701215
Gupta A, Briffa SM, Swingler S et al (2020) Synthesis of silver nanoparticles using curcumin-cyclodextrins loaded into bacterial cellulose-based hydrogels for wound dressing applications. Biomacromol 21:1802–1811. https://doi.org/10.1021/ACS.BIOMAC.9B01724
Gurunathan S, Lee AR, Kim JH (2022) Antifungal effect of nanoparticles against COVID-19 linked black fungus: a perspective on biomedical applications. Int J Mol Sci 23. https://doi.org/10.3390/IJMS232012526
Hasheminejad N, Khodaiyan F, Safari M (2019) Improving the antifungal activity of clove essential oil encapsulated by chitosan nanoparticles. Food Chem 275:113–122. https://doi.org/10.1016/J.FOODCHEM.2018.09.085
Hassan MIA, Voigt K (2019) Pathogenicity patterns of mucormycosis: epidemiology, interaction with immune cells and virulence factors. Med Mycol 57:S245–S256. https://doi.org/10.1093/MMY/MYZ011
Hassanpour P, Hamishehkar H, Bahari Baroughi B et al (2021) Antifungal effects of voriconazole-loaded nano-liposome on fluconazole - resistant clinical isolates of Candida albicans, biological activity and ERG11, CDR1, and CDR2 gene expression. Assay Drug Dev Technol 19:453–462. https://doi.org/10.1089/ADT.2020.1057
He X, Fu P, Aker WG, Hwang HM (2018) Toxicity of engineered nanomaterials mediated by nano-bio-eco interactions. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 36:21–42. https://doi.org/10.1080/10590501.2017.1418793
Hoenigl M, Seidel D, Carvalho A et al (2022b) The emergence of COVID-19 associated mucormycosis: a review of cases from 18 countries. Lancet Microbe 3:e543–e552. https://doi.org/10.1016/S2666-5247(21)00237-8
Hoenigl M, Seidel D, Sprute R et al (2022c) COVID-19-associated fungal infections. Nat Microbiol 7:1127–1140. https://doi.org/10.1038/S41564-022-01172-2
Hoenigl M, Seidel D, Carvalho A et al (2022a) The emergence of COVID-19 associated mucormycosis: a review of cases from 18 countries. Lancet Microbe 3. https://doi.org/10.1016/S2666-5247(21)00237-8
Hu S, Gu F, Chen M et al (2020) (2020) A novel method for identifying and distinguishing Cryptococcus neoformans and Cryptococcus gattii by surface-enhanced Raman scattering using positively charged silver nanoparticles. Sci Reports 101(10):1–9. https://doi.org/10.1038/s41598-020-68978-0
Ilinskaya AN, Dobrovolskaia MA (2013) Nanoparticles and the blood coagulation system. Part II: Safety Concerns. Nanomedicine (Lond) 8:969–981. https://doi.org/10.2217/NNM.13.49
Jansook P, Pichayakorn W, Ritthidej GC (2018) Amphotericin B-loaded solid lipid nanoparticles (SLNs) and nanostructured lipid carrier (NLCs): effect of drug loading and biopharmaceutical characterizations. Drug Dev Ind Pharm 44:1693–1700. https://doi.org/10.1080/03639045.2018.1492606
Jatana S, Palmer BC, Phelan SJ, Delouise LA (2017) Immunomodulatory effects of nanoparticles on skin allergy. Sci Rep 7. https://doi.org/10.1038/S41598-017-03729-2
Jeevanandam J, Barhoum A, Chan YS et al (2018) Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol 9:1050–1074. https://doi.org/10.3762/BJNANO.9.98
Jeong W, Keighley C, Wolfe R et al (2019) The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect 25:26–34. https://doi.org/10.1016/J.CMI.2018.07.011
Jiang Z, Feng B, Xu J et al (2020) Graphene biosensors for bacterial and viral pathogens. Biosens Bioelectron 166. https://doi.org/10.1016/J.BIOS.2020.112471
Jin J-H, Kim J, Jeon T et al (2015) Real-time selective monitoring of allergenic Aspergillus molds using pentameric antibody-immobilized single-walled carbon nanotube-field effect transistors. RSC Adv 5:15728–15735. https://doi.org/10.1039/C4RA15815F
Jin Z, Dong Y-T, Liu S et al (2022) Potential of polyethyleneimine as an adjuvant to prepare long-term and potent antifungal nanovaccine. Front Immunol 13. https://doi.org/10.3389/FIMMU.2022.843684
Jurado-Sánchez B (2018) Nanoscale biosensors based on self-propelled objects. Biosens 8:59. https://doi.org/10.3390/BIOS8030059
Kainz K, Bauer MA, Madeo F, Carmona-Gutierrez D (2020) Biosensors and diagnostics for fungal detection. J Fungi (Basel, Switzerland) 6:1–26. https://doi.org/10.3390/JOF6040349
Kainz K, Bauer MA, Madeo F, Carmona-Gutierrez D (2020) Fungal infections in humans: the silent crisis. Microb Cell (Graz, Austria) 7:143–145. https://doi.org/10.15698/MIC2020.06.718
Kakkar S, Singh M, Mohan Karuppayil S et al (2021) Lipo-PEG nano-ocular formulation successfully encapsulates hydrophilic fluconazole and traverses corneal and non-corneal path to reach posterior eye segment. J Drug Target 29:631–650. https://doi.org/10.1080/1061186X.2020.1871483
Kamli MR, Srivastava V, Hajrah NH et al (2021) Facile bio-fabrication of Ag-Cu-Co trimetallic nanoparticles and its fungicidal activity against Candida auris. J Fungi (Basel, Switzerland) 7:1–21. https://doi.org/10.3390/JOF7010062
Kammoun AK, Khedr A, Hegazy MA et al (2021) Formulation, optimization, and nephrotoxicity evaluation of an antifungal in situ nasal gel loaded with voriconazole-clove oil transferosomal nanoparticles. Drug Deliv 28:2229–2240. https://doi.org/10.1080/10717544.2021.1992040
Kaur M, Singh K, Jain SK (2020) Luliconazole vesicular based gel formulations for its enhanced topical delivery. J Liposome Res 30:388–406. https://doi.org/10.1080/08982104.2019.1682602
Kaur N, Bains A, Kaushik R et al (2021) A review on antifungal efficiency of plant extracts entrenched polysaccharide-based nanohydrogels. Nutrients 13. https://doi.org/10.3390/NU13062055
Khames A, Khaleel MA, El-Badawy MF, El-Nezhawy AOH (2019) Natamycin solid lipid nanoparticles - sustained ocular delivery system of higher corneal penetration against deep fungal keratitis: preparation and optimization. Int J Nanomedicine 14:2515–2531. https://doi.org/10.2147/IJN.S190502
Khan I, Saeed K, Khan I (2019) Nanoparticles: properties, applications and toxicities. Arab J Chem 12:908–931. https://doi.org/10.1016/J.ARABJC.2017.05.011
Khezri K, Saeedi M, Mohammadamini H, Zakaryaei AS (2021) A comprehensive review of the therapeutic potential of curcumin nanoformulations. Phytother Res 35:5527–5563. https://doi.org/10.1002/PTR.7190
Kischkel B, De Castilho PFD, De Oliveira KMP et al (2020) Silver nanoparticles stabilized with propolis show reduced toxicity and potential activity against fungal infections. Future Microbiol 15:521–539. https://doi.org/10.2217/FMB-2019-0173
Koehler P, Bassetti M, Chakrabarti A et al (2021) Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis 21:e149–e162. https://doi.org/10.1016/S1473-3099(20)30847-1
Kolahalam LA, Prasad KRS, Murali Krishna P, Supraja N (2021) Saussurea lappa plant rhizome extract-based zinc oxide nanoparticles: synthesis, characterization and its antibacterial, antifungal activities and cytotoxic studies against Chinese Hamster Ovary (CHO) cell lines. Heliyon 7. https://doi.org/10.1016/J.HELIYON.2021.E07265
Koshy S, Ismail N, Astudillo CL et al (2017) Breath-based diagnosis of invasive mucormycosis (IM). Open Forum Infect Dis 4:S53–S54. https://doi.org/10.1093/OFID/OFX162.124
Kumar M, Tiwari A, Asdaq SMB et al (2022) Itraconazole loaded nano-structured lipid carrier for topical ocular delivery: Optimization and evaluation. Saudi J Biol Sci 29:1–10. https://doi.org/10.1016/J.SJBS.2021.11.006
Kwasny D, Tehrani SE, Almeida C et al (2018) Direct detection of Candida albicans with a membrane based electrochemical impedance spectroscopy sensor. Sensors 18:2214. https://doi.org/10.3390/S18072214
Lara HH, Ixtepan-Turrent L, Jose Yacaman M, Lopez-Ribot J (2020) Inhibition of Candida auris biofilm formation on medical and environmental surfaces by silver nanoparticles. ACS Appl Mater Interfaces 12:21183–21191. https://doi.org/10.1021/ACSAMI.9B20708
Larkin E, Hager C, Chandra J et al (2017) The emerging pathogen Candida auris: growth phenotype, virulence factors, activity of antifungals, and effect of SCY-078, a novel glucan synthesis inhibitor, on growth morphology and biofilm formation. Antimicrob Agents Chemother 61. https://doi.org/10.1128/AAC.02396-16
Lau AF, Walchak RC, Miller HB et al (2019) Multicenter study demonstrates standardization requirements for mold identification by MALDI-TOF MS. Front Microbiol 10. https://doi.org/10.3389/FMICB.2019.02098
Laurent A, Pantet O, Laurent L et al (2020) Potency and stability of liposomal amphotericin B formulated for topical management of Aspergillus spp. infections in burn patients. Burn Open 4:110–116. https://doi.org/10.1016/J.BURNSO.2019.09.001
Lee SH, Jun BH (2019) Silver nanoparticles: synthesis and application for nanomedicine. Int J Mol Sci 20. https://doi.org/10.3390/IJMS20040865
Lee JI, Jang SC, Chung J et al (2021) Colorimetric allergenic fungal spore detection using peptide-modified gold nanoparticles. Sensors Actuators B Chem 327. https://doi.org/10.1016/J.SNB.2020.128894
Legrand M, Gits-Muselli M, Boutin L et al (2016) Detection of circulating mucorales DNA in critically ill burn patients: preliminary report of a screening strategy for early diagnosis and treatment. Clin Infect Dis 63:1312–1317. https://doi.org/10.1093/CID/CIW563
Lei R, Wu P, Li L et al (2021) Ultrasensitive isothermal detection of a plant pathogen by using a gold nanoparticle-enhanced microcantilever sensor. Sensors Actuators B Chem 338:129874. https://doi.org/10.1016/J.SNB.2021.129874
León-Buitimea A, Garza-Cervantes JA, Gallegos-Alvarado DY, et al (2021) Nanomaterial-based antifungal therapies to combat fungal diseases Aspergillosis, Coccidioidomycosis, Mucormycosis, and Candidiasis. Pathog (Basel, Switzerland) 10. https://doi.org/10.3390/PATHOGENS10101303
Leudjo Taka A, Doyle BP, Carleschi E et al (2020) Spectroscopic characterization and antimicrobial activity of nanoparticle doped cyclodextrin polyurethane bionanosponge. Mater Sci Eng C Mater Biol Appl 115. https://doi.org/10.1016/J.MSEC.2020.111092
Lewis RE, Liao G, Hou J et al (2007) Comparative analysis of amphotericin B lipid complex and liposomal amphotericin B kinetics of lung accumulation and fungal clearance in a murine model of acute invasive pulmonary aspergillosis. Antimicrob Agents Chemother 51:1253–1258. https://doi.org/10.1128/AAC.01449-06
Lockhart SR, Guarner J (2019) Emerging and reemerging fungal infections. Semin Diagn Pathol 36:177–181. https://doi.org/10.1053/J.SEMDP.2019.04.010
Lu Y, Yang M, Qu F et al (2007) Enzyme-functionalized gold nanowires for the fabrication of biosensors. Bioelectrochemistry 71:211–216. https://doi.org/10.1016/J.BIOELECHEM.2007.05.003
Machado M, Valerio M, Álvarez-Uría A et al (2021) Invasive pulmonary aspergillosis in the COVID-19 era: An expected new entity. Mycoses 64:132–143. https://doi.org/10.1111/MYC.13213
Mahdi WA, Bukhari SI, Imam SS et al (2021) Formulation and optimization of butenafine-loaded topical nano lipid carrier-based gel: Characterization, Irritation Study, and Anti-Fungal Activity. Pharmaceutics 13. https://doi.org/10.3390/PHARMACEUTICS13071087
Maiese A, Manetti AC, La Russa R et al (2021) Autopsy findings in COVID-19-related deaths: a literature review. Forensic Sci Med Pathol 17:279–296. https://doi.org/10.1007/S12024-020-00310-8
Malavika JP, Shobana C, Ragupathi M et al (2021) A sustainable green synthesis of functionalized biocompatible carbon quantum dots from Aloe barbadensis Miller and its multifunctional applications. Environ Res 200. https://doi.org/10.1016/J.ENVRES.2021.111414
Malhotra BD, Ali MA (2018) Nanomaterials in biosensors: fundamentals and applications. Nanomater Biosens 1. https://doi.org/10.1016/B978-0-323-44923-6.00001-7
Marques SM, Chavan DU, Bhide PJ et al (2022) Novel luliconazole spanlastic nanocarriers: development and characterisation. Curr Drug Deliv 19. https://doi.org/10.2174/1567201819666220516155048
Martínez-Montelongo JH, Medina-Ramírez IE, Romo-Lozano Y et al (2021) Development of nano-antifungal therapy for systemic and endemic mycoses. J Fungi (Basel, Switzerland) 7:1–23. https://doi.org/10.3390/JOF7020158
Millon L, Herbrecht R, Grenouillet F et al (2016) Early diagnosis and monitoring of mucormycosis by detection of circulating DNA in serum: retrospective analysis of 44 cases collected through the French Surveillance Network of Invasive Fungal Infections (RESSIF). Clin Microbiol Infect 22:810.e1-810.e8. https://doi.org/10.1016/J.CMI.2015.12.006
Millon L, Larosa F, Lepiller Q et al (2013) Quantitative polymerase chain reaction detection of circulating DNA in serum for early diagnosis of mucormycosis in immunocompromised patients. Clin Infect Dis 56. https://doi.org/10.1093/CID/CIT094
Misiak M, Skowicki M, Lipiński T et al (2017) Biofunctionalized upconverting CaF2:Yb,Tm nanoparticles for Candida albicans detection and imaging. Nano Res 2017 1010 10:3333–3345. https://doi.org/10.1007/S12274-017-1546-Y
Mitchell MJ, Billingsley MM, Haley RM et al (2021) Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov 20:101–124. https://doi.org/10.1038/S41573-020-0090-8
Mo Y, Adarkwah O, Zeibeq J et al (2021) Treatment with tocilizumab for patients with COVID-19 infections: a case-series study. J Clin Pharmacol 61:406–411. https://doi.org/10.1002/JCPH.1787
Muhammad W, Zhai Z, Gao C (2020) Antiviral activity of nanomaterials against coronaviruses. Macromol Biosci 20. https://doi.org/10.1002/MABI.202000196
Mulet Bayona JV, Tormo Palop N, Salvador García C et al (2021) Impact of the SARS-CoV-2 pandemic in Candidaemia, invasive aspergillosis and antifungal consumption in a tertiary hospital. J Fungi (Basel, Switzerland) 7. https://doi.org/10.3390/JOF7060440
Nasseri M, Golmohammadzadeh S, Arouiee H et al (2016) Antifungal activity of Zataria multiflora essential oil-loaded solid lipid nanoparticles in-vitro condition. Iran J Basic Med Sci 19:1231. https://doi.org/10.22038/ijbms.2016.7824
Nemmar A, Beegam S, Yuvaraju P et al (2016) Ultrasmall superparamagnetic iron oxide nanoparticles acutely promote thrombosis and cardiac oxidative stress and DNA damage in mice. Part Fibre Toxicol 13. https://doi.org/10.1186/S12989-016-0132-X
Newton PJ, Harris C, Morris J, Denning DW (2016) Impact of liposomal amphotericin B therapy on chronic pulmonary aspergillosis. J Infect 73:485–495. https://doi.org/10.1016/J.JINF.2016.06.001
Oberdörster G, Oberdörster E, Oberdörster J (2005) Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 113:823–839. https://doi.org/10.1289/EHP.7339
Ogar A, Tylko G, Turnau K (2015) Antifungal properties of silver nanoparticles against indoor mould growth. Sci Total Environ 521–522:305–314. https://doi.org/10.1016/J.SCITOTENV.2015.03.101
Olson JA, Schwartz JA, Hahka D et al (2015) Toxicity and efficacy differences between liposomal amphotericin B formulations in uninfected and Aspergillus fumigatus infected mice. Med Mycol 53:107–118. https://doi.org/10.1093/MMY/MYU070
Orne C, Burnham-Marusich A, Baldin C, Gebremariam T, Ibrahim A, Kvam A, Kozel T (2018) Cell wall fucomannan is a biomarker for diagnosis of invasive murine mucormycosis. Proceedings of the 28th ECCMID, Madrid, Spain. 2018 Apr:21–4
Palanisamy PR, Elango D (2022) COVID19 associated mucormycosis: a review. J Fam Med Prim Care 11:418. https://doi.org/10.4103/JFMPC.JFMPC_1186_21
Pappas PG, Lionakis MS, Arendrup MC et al (2018) Invasive candidiasis. Nat Rev Dis Prim 4. https://doi.org/10.1038/NRDP.2018.26
Paul S, Mohanram K, Kannan I (2018) Antifungal activity of curcumin-silver nanoparticles against fluconazole-resistant clinical isolates of Candida species. Ayu 39:182. https://doi.org/10.4103/AYU.AYU_24_18
Pinilla CMB, Thys RCS, Brandelli A (2019) Antifungal properties of phosphatidylcholine-oleic acid liposomes encapsulating garlic against environmental fungal in wheat bread. Int J Food Microbiol 293:72–78. https://doi.org/10.1016/J.IJFOODMICRO.2019.01.006
Pohanka M (2018) Overview of piezoelectric biosensors, immunosensors and DNA sensors and their applications. Mater (Basel, Switzerland) 11. https://doi.org/10.3390/MA11030448
Prakash H, Chakrabarti A (2021) Epidemiology of Mucormycosis in India. Microorganisms 9:1–12. https://doi.org/10.3390/MICROORGANISMS9030523
Prakash H, Singh S, Rudramurthy SM et al (2020) An aero mycological analysis of mucormycetes in indoor and outdoor environments of northern India. Med Mycol 58:118–123. https://doi.org/10.1093/MMY/MYZ031
Prattes J, Wauters J, Giacobbe DR et al (2022) Risk factors and outcome of pulmonary aspergillosis in critically ill coronavirus disease 2019 patients-a multinational observational study by the European Confederation of Medical Mycology. Clin Microbiol Infect 28:580–587. https://doi.org/10.1016/J.CMI.2021.08.014
Prestel C, Anderson E, Forsberg K et al (2021) Candida auris outbreak in a COVID-19 specialty care unit - Florida, July-August 2020. MMWR Morb Mortal Wkly Rep 70:56–57. https://doi.org/10.15585/MMWR.MM7002E3
Radwan MA, AlQuadeib BT, Šiller L et al (2017) Oral administration of amphotericin B nanoparticles: antifungal activity, bioavailability and toxicity in rats. Drug Deliv 24:40–50. https://doi.org/10.1080/10717544.2016.1228715
Rai M, Ingle AP, Pandit R et al (2017) Nanotechnology for the treatment of fungal infections on human skin. https://doi.org/10.1016/B978-0-12-811079-9.00019-7
Ray M, Ray A, Dash S et al (2017) Fungal disease detection in plants: traditional assays, novel diagnostic techniques and biosensors. Biosens Bioelectron 87:708–723. https://doi.org/10.1016/J.BIOS.2016.09.032
Reddy LH, Arias JL, Nicolas J, Couvreur P (2012) Magnetic nanoparticles: design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem Rev 112:5818–5878. https://doi.org/10.1021/CR300068P
Riche CVW, Cassol R, Pasqualotto AC (2020) Is the frequency of candidemia increasing in COVID-19 patients receiving corticosteroids? J Fungi (Basel, Switzerland) 6:1–4. https://doi.org/10.3390/JOF6040286
Rizk MZ, Ali SA, Hamed MA et al (2017) Toxicity of titanium dioxide nanoparticles: effect of dose and time on biochemical disturbance, oxidative stress and genotoxicity in mice. Biomed Pharmacother 90:466–472. https://doi.org/10.1016/J.BIOPHA.2017.03.089
Roemer T, Krysan DJ (2014) Antifungal drug development: challenges, unmet clinical needs, and new approaches. Cold Spring Harb Perspect Med 4:a019703. https://doi.org/10.1101/CSHPERSPECT.A019703
Różalska B, Sadowska B, Budzyńska A et al (2018) Biogenic nanosilver synthesized in Metarhizium robertsii waste mycelium extract - as a modulator of Candida albicans morphogenesis, membrane lipidome and biofilm. PLoS One 13. https://doi.org/10.1371/JOURNAL.PONE.0194254
Rudramurthy SM, Paul RA, Chakrabarti A et al (2019) Invasive aspergillosis by Aspergillus flavus: epidemiology, diagnosis, antifungal resistance, and management. J Fungi (Basel, Switzerland) 5. https://doi.org/10.3390/JOF5030055
Sabira SF, Kasabe AM, Mane PC et al (2020) Selective antifungal and antibacterial activities of Ag-Cu and Cu-Ag core-shell nanostructures synthesized in-situ PVA. Nanotechnology 31. https://doi.org/10.1088/1361-6528/AB9DA5
Salama HE, Saad GR, Sabaa MW (2016) Synthesis, characterization, and biological activity of cross-linked chitosan biguanidine loaded with silver nanoparticles. J Biomater Sci Polym Ed 27:1880–1898. https://doi.org/10.1080/09205063.2016.1239950
Samson R, Dharne M (2022) COVID-19 associated mucormycosis: evolving technologies for early and rapid diagnosis. 3 Biotech 12:6. https://doi.org/10.1007/S13205-021-03080-4
Sangiao ET, Holban AM, Gestal MC (2019) Applications of nanodiamonds in the detection and therapy of Iinfectious diseases. Mater 12:1639. https://doi.org/10.3390/MA12101639
Sanguinetti M, Posteraro B, Beigelman-Aubry C et al (2019) Diagnosis and treatment of invasive fungal infections: looking ahead. J Antimicrob Chemother 74:ii27–ii37. https://doi.org/10.1093/JAC/DKZ041
Seagle EE, Jackson BR, Lockhart SR et al (2022) The landscape of candidemia during the coronavirus disease 2019 (COVID-19) pandemic. Clin Infect Dis 74:802–811. https://doi.org/10.1093/CID/CIAB562
Selarka L, Sharma S, Saini D et al (2021) Mucormycosis and COVID-19: an epidemic within a pandemic in India. Mycoses 64:1253–1260. https://doi.org/10.1111/MYC.13353
Shah MKA, Azad AK, Nawaz A et al (2021) Formulation development, characterization and antifungal evaluation of chitosan NPs for topical delivery of voriconazole in vitro and ex vivo. Polymers (Basel) 14. https://doi.org/10.3390/POLYM14010135
Shaikh S, Nazam N, Rizvi SMD et al (2019) Mechanistic insights into the antimicrobial actions of metallic nanoparticles and their implications for multidrug resistance. Int J Mol Sci 20. https://doi.org/10.3390/IJMS20102468
Shi H, Wei J, Qiang L et al (2014) Fluorescent carbon dots for biolmaging and biosensing applications. J Biomed Nanotechnol 10:2677–2699. https://doi.org/10.1166/JBN.2014.1881
Shi J, Tian F, Lyu J, Yang M (2015) Nanoparticle based fluorescence resonance energy transfer (FRET) for biosensing applications. J Mater Chem B 3:6989–7005. https://doi.org/10.1039/C5TB00885A
Shirvani F, Fattahi A (2021) Pulmonary candidiasis associated with COVID-19: evaluation of causative agents and their antifungal susceptibility patterns. Tanaffos 20:29
Singh A, Sharma A, Ahmed A et al (2021) Recent advances in electrochemical biosensors: applications, challenges, and future scope. Biosensors 11. https://doi.org/10.3390/BIOS11090336
Siopi M, Mouton JW, Pournaras S, Meletiadis J (2019a) In vitro and in vivo exposure-effect relationship of liposomal amphotericin B against Aspergillus fumigatus. Antimicrob Agents Chemother 63. https://doi.org/10.1128/AAC.02673-18
Siopi M, Mouton JW, Pournaras S, Meletiadis J (2019b) In vitro and in vivo exposure-effect relationship of liposomal amphotericin B against Aspergillus fumigatus. Antimicrob Agents Chemother 63. https://doi.org/10.1128/AAC.02673-18
Skiada A, Pavleas I, Drogari-Apiranthitou M (2020) Epidemiology and diagnosis of mucormycosis: an update. J Fungi (Basel, Switzerland) 6:1–20. https://doi.org/10.3390/JOF6040265
Skottrup P, Hearty S, Frøkiær H et al (2007) Detection of fungal spores using a generic surface plasmon resonance immunoassay. Biosens Bioelectron 22:2724–2729. https://doi.org/10.1016/J.BIOS.2006.11.017
Smallcombe CC, Harford TJ, Linfield DT et al (2020) Titanium dioxide nanoparticles exaggerate respiratory syncytial virus-induced airway epithelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 319:L481–L496. https://doi.org/10.1152/AJPLUNG.00104.2020
Sojinrin T, Conde J, Liu K et al (2017) (2017) Plasmonic gold nanoparticles for detection of fungi and human cutaneous fungal infections. Anal Bioanal Chem 40919(409):4647–4658. https://doi.org/10.1007/S00216-017-0414-7
Soliman KA, Zedan AF, Khalifa A et al (2017) Silver nanoparticles-decorated titanium oxynitride nanotube arrays for enhanced solar fuel generation. Sci Rep 7:1913. https://doi.org/10.1038/s41598-017-02124-1
Souza ACO, Amaral AC (2017) Antifungal therapy for systemic mycosis and the nanobiotechnology era: improving efficacy, biodistribution and toxicity. Front Microbiol 0:336. https://doi.org/10.3389/FMICB.2017.00336
Souza ACO, Amaral AC (2017b) Antifungal therapy for systemic mycosis and the nanobiotechnology era: improving efficacy, biodistribution and toxicity. Front Microbiol 8. https://doi.org/10.3389/FMICB.2017.00336
Spivak ES, Hanson KE (2018) Candida auris: an emerging fungal pathogen. J Clin Microbiol 56. https://doi.org/10.1128/JCM.01588-17
Springer J, Lackner M, Ensinger C et al (2016) Clinical evaluation of a Mucorales-specific real-time PCR assay in tissue and serum samples. J Med Microbiol 65:1414–1421. https://doi.org/10.1099/JMM.0.000375
Srivastava V, Ahmad A (2020) Abrogation of pathogenic attributes in drug resistant Candida auris strains by farnesol. PLoS One 15. https://doi.org/10.1371/JOURNAL.PONE.0233102
Steckiewicz KP, Barcinska E, Malankowska A et al (2019) Impact of gold nanoparticles shape on their cytotoxicity against human osteoblast and osteosarcoma in in vitro model. Evaluation of the safety of use and anti-cancer potential. J Mater Sci Mater Med 30. https://doi.org/10.1007/S10856-019-6221-2
Tan KX, Barhoum A, Pan S, Danquah MK (2018) Risks and toxicity of nanoparticles and nanostructured materials. Emerg Appl Nanoparticles Archit Nanostructures Curr Prospect Futur Trends 121–139. https://doi.org/10.1016/B978-0-323-51254-1.00005-1
Tang D, Lin Y, Zhou Q (2018) Carbon dots prepared from Litchi chinensis and modified with manganese dioxide nanosheets for use in a competitive fluorometric immunoassay for aflatoxin B1. Mikrochim Acta 185. https://doi.org/10.1007/S00604-018-3012-2
Tsai CS, Lee SSJ, Chen WC et al (2022) COVID-19-associated candidiasis and the emerging concern of Candida auris infections. J Microbiol Immunol Infect. https://doi.org/10.1016/J.JMII.2022.12.002
Unser S, Bruzas I, He J, Sagle L (2015) Localized surface plasmon resonance biosensing: current challenges and approaches. Sensors (Basel) 15:15684. https://doi.org/10.3390/S150715684
Vahedi-Shahandashti R, Lass-Flörl C (2020) Novel antifungal agents and their activity against Aspergillus species. J Fungi (Basel, Switzerland) 6:1–21. https://doi.org/10.3390/JOF6040213
Van De Ven H, Paulussen C, Feijens PB et al (2012) PLGA nanoparticles and nanosuspensions with amphotericin B: potent in vitro and in vivo alternatives to Fungizone and Am Bisome. J Control Release 161:795–803. https://doi.org/10.1016/J.JCONREL.2012.05.037
Vazquez-Munoz R, Arellano-Jimenez MJ, Lopez-Ribot JL (2020) Bismuth nanoparticles obtained by a facile synthesis method exhibit antimicrobial activity against Staphylococcus aureus and Candida albicans. BMC Biomed Eng 2. https://doi.org/10.1186/S42490-020-00044-2
Vazquez-Rodriguez A, Vasto-Anzaldo XG, Leon-Buitimea A et al (2020) Antibacterial and antibiofilm activity of biosynthesized silver manoparticles coated with exopolysaccharides obtained from Rhodotorula mucilaginosa. IEEE Trans Nanobiosci 19:498–503. https://doi.org/10.1109/TNB.2020.2985101
Villamizar RA, Maroto A, Rius FX (2009) Improved detection of Candida albicans with carbon nanotube field-effect transistors. Sensors Actuators B Chem 136:451–457. https://doi.org/10.1016/J.SNB.2008.10.013
Wang J, Chen G, Jiang H et al (2013) Advances in nano-scaled biosensors for biomedical applications. Analyst 138:4427–4435. https://doi.org/10.1039/C3AN00438D
White PL (2021) Diagnosis of invasive fungal disease in coronavirus disease 2019: approaches and pitfalls. Curr Opin Infect Dis 34:573–580. https://doi.org/10.1097/QCO.0000000000000791
Wu T, Tang M (2018) Review of the effects of manufactured nanoparticles on mammalian target organs. J Appl Toxicol 38:25–40. https://doi.org/10.1002/JAT.3499
Xing Y, Tang J, Li X et al (2022) Photo-iduced antifungal activity of chitosan composite film solution with nano-titanium dioxide and nano-silver. J Food Prot 85:597–606. https://doi.org/10.4315/JFP-21-290
Xu Y, Gao C, Li X et al (2013) In vitro antifungal activity of silver nanoparticles against ocular pathogenic filamentous fungi. J Ocul Pharmacol Ther 29:270–274. https://doi.org/10.1089/JOP.2012.0155
Xu J, Yang X, Lv Z et al (2021) Risk factors for invasive aspergillosis in patients admitted to the intensive care unit with coronavirus disease 2019: a multicenter retrospective study. Front Med 8. https://doi.org/10.3389/FMED.2021.753659
Yang L, Liu S, Liu J et al (2020a) (2020a) COVID-19: immunopathogenesis and Immunotherapeutics. Signal Transduct Target Ther 51(5):1–8. https://doi.org/10.1038/s41392-020-00243-2
Yang X, Yu Y, Xu J et al (2020b) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 8:475–481. https://doi.org/10.1016/S2213-2600(20)30079-5
Yao Y, Shi L, Zhang C et al (2019) Application of fungal fluorescent staining in oral candidiasis: diagnostic analysis of 228 specimens. BMC Microbiol 19. https://doi.org/10.1186/S12866-019-1467-X
Zafar A, Imam SS, Alruwaili NK et al (2022) Formulation and evaluation of topical nano-lipid-based delivery of butenafine: in vitro characterization and antifungal activity. Gels (Basel, Switzerland) 8. https://doi.org/10.3390/GELS8020133
Zambom CR, da Fonseca FH, Crusca E et al (2019) A novel antifungal system with potential for prolonged delivery histatin 5 to limit growth of Candida albicans. Front Microbiol 10. https://doi.org/10.3389/FMICB.2019.01667
Zhao Z, Song J, Yang C et al (2021) Prevalence of fungal and bacterial co-infection in pulmonary fungal infections: a metagenomic next generation sequencing-based study. Front Cell Infect Microbiol 11. https://doi.org/10.3389/FCIMB.2021.749905
Author information
Authors and Affiliations
Contributions
Amisha Vora, and Vinay Chaudhari visualized the presented idea. Amisha Vora supervised the project. Vinay Chaudhari, Vaishnavi Vairagade, Ami Thakkar, and Himani Shende contributed in doing literature searches and prepared manuscript draft. Amisha Vora revised and approved the manuscript. All authors contributed to the article and approved the submitted version. The authors confirm that no paper mill and artificial intelligence was used.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chaudhari, V., Vairagade, V., Thakkar, A. et al. Nanotechnology-based fungal detection and treatment: current status and future perspective. Naunyn-Schmiedeberg's Arch Pharmacol 397, 77–97 (2024). https://doi.org/10.1007/s00210-023-02662-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02662-8