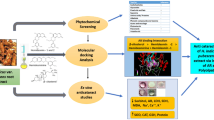
Abstract
Aldose reductase (ALR2) is a rate-limiting component of the polyol pathway, which is essential for the NADPH-mediated conversion from glucose to sorbitol. ALR2 dysregulation has been linked to α-crystallin aggregation, increased oxidative stress, and calcium inflow, all of which contribute to a diabetic cataract. Given its crucial role in occular pathologies, ALR2 has emerged as a promising target to treat oxidative stress and hyperglycaemic condition which form the underlying cause of diabetic cataracts. However, several of them had issues with sensitivity and specificity to ALR2, despite being screened as effective ALR2 inhibitors from a wide range of structurally varied molecules. The current study investigates the inhibitory potential of Nifedipine, an analog of the dihydro nicotinamide class of compounds against ALR2 activity. The enzyme inhibition studies were supported by in vitro biomolecular interactions, molecular modeling approaches, and in vivo validation in diabetic rat models. Nifedipine demonstrated appreciable inhibitory potential with the purified recombinant hAR (human aldose reductase; with an IC50 value of 2.5 µM), which was further supported by Nifedipine-hAR binding affinity (Kd = 2.91 ± 1.87 × 10−4 M) by ITC and fluorescence quenching assays. In the in vivo models of STZ-induced diabetic rats, Nifedipine delayed the onset progression of cataracts by preserving the antioxidant enzyme activity (SOD, CAT, and GPX GSH, TBARS, and protein carbonyls) and was shown to retain the α-crystallin chaperone activity by reducing the calcium levels in the diabetic rat lens. In conclusion, our results demonstrate effective inhibition of ALR2 by Nifedipine, resulting in amelioration of diabetic cataract conditions by lowering oxidative and osmotic stress while retaining the chaperone activity of α-crystallins. The present study could be envisaged to improve the eye condition in older adults upon Nifedipine treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes is a metabolic disease that affects the physiology of a variety of organs, including the eyes, kidneys, and nerves. Uncontrolled or poorly controlled hyperglycemia can result in a range of complications including diabetic cataract. Cataract is the most prevalent cause of blindness in people with diabetes worldwide (Steinmetz et al. 2021). Chronic hyperglycemic condition in cataract lens leads to the activation of the polyol pathway resulting in the conversion of excess glucose to sorbitol by aldose reductase (ALR2). Over accumulation of sorbitol increases osmotic stress, ultimately damaging the lens tissue. The overexpression of ALR2 also reduces reduced glutathione (GSH) levels in the lens, which triggers an increase in reactive oxygen species (ROS) that leads to oxidative stress in the lens (Lee and Chung 1999). Superoxide dismutase, glutathione reductase, catalase, and glutathione peroxidase are antioxidant enzymes that have been evolved by lenses as a protective strategy to control ROS (Demir et al. 2019; Sever et al. 2020). Thus, oxidative stress is one of the main factors contributing to cataract progression. Previous investigations have shown that in diabetic cataracts, several non-specific transporters efflux, excessive calcium (Türkeş et al. 2021a, b), leads to the accumulation of high molecular weight protein aggregates that expedite cataract progression (Yamamoto et al. 2010). In addition, significant accumulation of calcium levels has been shown to discourage chaperone activity of α-crystalline leading to the cataractogenic process (Yamamoto et al. 2010). Thus, cataractogenesis is a multifactorial condition which has been linked to several factors, including elevated oxidative stress, enhanced non-enzymatic glycation, cellular lipid peroxidation events, decreased chaperone activity of α-crystallins, and elevated lens membrane permeability (Türkeş et al. 2022). Thus, a compound with high efficacy to inhibit the ALR2 that can efficiently subside oxidative and osmotic stress can be anticipated to be an anti-cataractogenic drug.
Several ALR2 inhibitors have been reported in recent years, including Quinones, thiazole-based derivatives, 4 H-1,2,4-triazole derivatives, and 2-pyrazolines (Rhodes and Sanderson 2009; Pollreisz and Schmidt-Erfurth 2010; Liu et al. 2015). Furthermore, approved nonsteroidal anti-inflammatory drugs like diclofenac and tenoxicam were also reported to inhibit ALR2 (Ghahramani et al. 2015). Although constant rational design efforts have been pursued to develop aldose reductase inhibitors, non-selective inhibition to ALR homologs (ALR1 and ALR2) has led to their poor toxicity profiles. As a result, we attempted to find a novel entity from existing and approved drugs against ALR2.
Nifedipine has been used in cataractous conditions in independent research conducted by Kametaka et al., Nair et al., and Taalat et al. However, the precise process by which it works is still unknown (Kametaka et al. 2008; Nair et al. 2009; Taalat et al. 2018). Nifedipine is a familiar voltage-gated calcium channel inhibitor that lowers blood pressure by acting as an arterial vasodilator and has its application in a number of cardiovascular disorders. Nifedipine is a member of the 1,4-dihydropyridine group. Chemically, 1,4 dihydropyridine derivatives are dihydronicotinamide analogs (NADPH analogs) in structure and are used as model compounds for reducing oxidative stress (Velena et al. 2016). Because NADPH is a co-factor for ALR2, we presumed that the 1,4 dihydropyridine derivative can suppress ALR2 by competing with it.
Therefore, the present study aims to explore the inhibitory potential of Nifedipine against ALR2 in diabetic cataractous conditions followed by determining its binding affinities through biomolecular interactions assays, concluding with determining its amelioratory effects in hampering the cataract progression in diabetic rats.
Materials and methods
Expression and purification of hAR Protein
pET15b plasmid with hAR (human aldose reductase) gene insert was generously donated by Alberto Podjarny and Alexandra Cousido-Siah, IGBMC-CNRS-University, Strasbourg. Recombinant hAR was expressed in E. coli BL21 (DE3) strains (Novagen) (Lamour et al. 1999) using standard protein expression protocols. 0.1 mM IPTG was used to induce the protein for 4 h at 37 °C, and 500 mM imidazole was used to elute the protein. The purity of the protein was assessed on 12% SDS-PAGE gels, and the concentration was measured with Bradford assay (Türkeş et al. 2019; Demir et al. 2021).
Aldose reductase (hAR) inhibition assay
The inhibitory potential of Nifedipine and sorbinil against hAR was assessed as a function of cellular NADPH levels. Aldose reductase catalyzes the reduction of cytosolic DL-glyceraldehyde substrate to glycerol via the pylol pathway with stoichiometric utilization of NADPH and production of NADP + . Therefore, the gradual decline of NADPH levels following the concentration-dependent addition of inhibitors in turn suggests the potency of the inhibitors against hAR. Hence, inhibition of hAR by Nifedipine and sorbinil was assessed through dose–response curves as previously described (Balendiran and Rajkumar 2005).
During the oxidation step, the NADPH is reduced to NADP +, and the decrease in concentration of NADPH was recorded at 340 nm spectrophotometrically. The reaction mixture contained purified recombinant human aldose reductase protein, 0.1 M sodium phosphate buffer, pH 6.2, containing freshly prepared β-NADPH, 10 mM DL-glyceraldehyde as substrate, and synthesized molecules with concentration ranging from 0.1 to 100 µM. The reaction was started by the addition of substrate, and the decrease in absorbance was monitored for 30 min at 37 °C.
Fluorescence spectroscopy
The fluorescence quenching experiment was performed with a fluorescence spectrophotometer F-7000 (Hitachi). The emission spectra of hAR along with the increasing concentration of Nifedipine were recorded at an excitation wavelength of 280 nm at a range of 300–400 nm (Sekhon and Singh 2019).
Isothermal titration calorimetry
Isothermal titration calorimetry (ITC) was performed with iTC200 microtitration calorimeter to determine the values of ΔH, ΔS, and Kd for Nefidipine and hAR interactions as described by Cornelia Koch et al. (2011).
Molecular docking
The protein–ligand docking was performed with the MolDock scoring function in Molegro Virtual Docker, 2010.4.0.0. The ligand–protein interactions were visualized with Biovia Discovery Studio 2021. Briefly, the docking procedure involved the preparation of ligands and protein to correct the hybridizations and bond orders and include missing residues in protein. The ligand was docked in reference to the co-crystallized ligand in the ALR2 structure (PDB: 1Z3N).
Animal models and induction of diabetes
Three-month male Sprague Dawley (SD) rat weighing 210 ± 8 g was used for the present study. Rats were maintained at the standard sterile conditions (25 °C, and lighting controlled for 12:12 h on- and off-cycle (AnnakaTokusyu Glass)) and fed with AIN-93 Ad libitum diet with water. To induce diabetic conditions, a single intraperitoneal injection of streptozotocin (STZ) (32 mg/kg in 0.1 M citrate buffer at pH 4.5) was administered after a fasting period of 24 h (Kumar Pasala et al. 2021). The control group was fed with an equal volume of vehicles. Following 78 h of induction, the fasting blood glucose levels were assessed. Animals with glucose levels > 100 mg/dl formed the study group.
The negative control group (Group I, n = 10) included the rats that are not induced with STZ. All the diabetic rats were stratified into four groups; Group II (n = 10) includes diabetic controls (positive controls). Group III was fed on Nifedipine at 10 µM/kg, and Group IV (n = 10) was fed at 100 µM/kg body weight/day in laboratory chow (Kametaka et al. 2008). Group V (n = 10) received sorbinil at 100 µM/kg body weight/day (Kumar Pasala et al. 2021). The drug administration was carried out for 8 weeks with weekly monitoring of blood glucose levels, and the cataract progression was recorded using a slit lamp microscope (VISIL 02 Excel). Cardiac vitals of all the rats including heart rate and systolic blood pressure were measured once a week by a tail-cuff sphygmomanometer. The body weights were monitored, and rats were sacrificed to collect the lens by CO2 asphyxiation. Aldose reductase of the rat lens was purified, and inhibition studies were carried out using following the protocols as previously described by Sankeshi, V. et al. (Sankeshi et al. 2013; Çağlayan et al. 2019; Taslimi et al. 2019). Briefly, the eyeball was removed, and the lenses were homogenized in 10 volumes of a 100 mM phosphate buffer with a pH of 6.2. The homogenate underwent a 30-min, 12,000-g, 4 °C centrifugation process followed by ammonium sulfate precipitation. The animal experiments were carried out adhering to the institute’s animal ethical committee and CPCSEA norms (Registration No. IAEC/NRML/Ph.D./03/2021).
Estimation of biometabolites
Blood glucose levels were estimated by a glucometer (ACCU-CHEK Active). Estimation of sorbitol was performed as described by Vasanth Rao and Seetharam Bhat (1989). To extract sorbitol, the lenses were homogenized in 9 volumes of 0.8 M perchloric acid. The homogenate was centrifuged at 5000 × g for 10 min at 4 °C, followed by an adjustment of the pH of the supernatant to 3.5 with 0.5 M potassium carbonate. The endpoints were recorded with a JASCO FP-750 spectrofluorimeter. Glycated hemoglobin levels were estimated by the ion exchange resin method (MiCARE India Inc.) (Sankeshi et al. 2013). Ellman’s (1959) protocol was followed to estimate the reduced glutathione levels. The lens homogenate was precipitated with 10% tri-chloroacetic acid, and the solution was thoroughly mixed and centrifuged. 2.0 ml of 0.6 mM 5-5ʹdithiobis 2-nitrobenzoic acid was added to 0.5 ml of the supernatant, and the total volume was brought up to 3 ml using 0.2 M phosphate buffer (pH 8.0) and recorded at 412 nm. Protocols described by Ohkawa et al. (1979) and Uchida et al. (1998), respectively, were used to determine TBARS (thiobarbituric acid reactive substances) and protein carbonyls from the lens homogenate (Ellman 1959; Ohkawa et al. 1979).
Assessment of antioxidant enzyme activity
The activity of the antioxidant enzymes—superoxide dismutase (SOD), catalase, and glutathione peroxidase (GPx)—was quantitatively determined by following the protocols described by Marklund and Marklund (1974), Sinha (1972), and Lawrence et al. (1974).
Assessment of chaperone activity
The chaperone activity was analyzed based on the ability of α-crystallins to prevent aggregation of insulin which is induced by DTT. Isolation of α-crystallins from rat lens and its activity was assessed as described by Reddy et al. (2000) and Sankeshi et al. (2013).
Statistical analysis
The comparison between the groups for various analytical endpoints was analyzed with ANOVA and reported as mean ± SD. Statistical significance was defined as a p-value ≤ 0.05. IC50 for Nifedipine and controls standards was calculated by dose–response curves. All the statistical analysis and scientific graphing were carried out by GraphPad Prism 6 for Windows.
Results
Inhibition of human recombinant aldose reductase by Nifedipine
ALR has gained particular importance for its critical involvement in the etiology of diabetic secondary complications. Herein, we evaluated the inhibitory effect of Nifedipine for purified hAR (specific activity is 24.3 EU/mg protein, data provided in supplementary-Fig S1 and S2) under in vitro conditions. IC50 value of Nifedipine was found to be 2.5 ± 0.23 µM (Ki=5.21 ± 0.3 µM) which was approximately equivalent to standard hAR inhibitor sorbinil (IC50 sorbinil = 2.1 ± 0.17 µM). Thus, the observation demonstrates that scaffolds made of 1,4 dihydropyridines present in the Nifedipine can potentially suppress hAR. Nifedipine displayed competitive inhibition against hAR, which was concentration-dependent in kinetic studies using varying concentrations of DL-glyceraldehyde. (Fig. 1). Km and Vmax values of the interaction are provided (Table 1).
Fluorescence inhibition
To elucidate the interaction of Nifedipine with hAR, we used steady-state fluorescence spectroscopic studies. The fluorescence emission signal for protein was recorded in the presence of different Nifedipine concentrations. When hAR was excited at 280 nm, its typical emission peak was observed at 341 nm (Sekhon and Singh 2019). With increasing concentrations of Nifedipine, the hAR fluorescence was gradually quenched (Fig. 2). Quantitative analysis of fluorescence was analyzed with the Stern–Volmer equation as shown below.
where Fo is the fluorescence intensity of hAR, and F is the fluorescence intensity of hAR in the presence of Nifedipine at a given concentration [Q] at pH 8.0. KSV is the Stern–Volmer quenching constant. To obtain the nature of fluorescence quenching, KSV was computed using the standard equation as shown below.
where τo is the lifetime of average integral fluorescence of tryptophan (~ 5.78 × 10−9 s), and his rate of fluorescence quenching was found to be 3 × 1012 M−1 s−1.
It is worthy to note that kq of Nifedipine was nearly 150-fold higher than the standard static quenching value (kq value is usually > 2.0 × 1010 M−1 s−1 in the case of static quenching), suggesting a static mode of fluorescence quenching, meaning bonds form at ground state alone and not at the excited state (Khan et al. 2020). These quenching investigations most likely demonstrated that Nifedipine-hAR interactions are high-affinity interactions that lead to complex formation rather than simple dynamic collisions.
Isothermal calorimetric titration
The binding affinity of the Nifedipine-ALR2 system was investigated with ITC, and the binding constant (Kd = 2.91 ± 1.87 × 10−4 M) of the system suggested a good affinity of Nifedipine in the ALR2 active site, with an “N” value of 1 (~ 1.150). Furthermore, favorable changes in enthalpy (ΔH = − 225 J/mol) and entropy (ΔS = − 35 kJ/mol) suggest stable interactions of Nifedipine with ALR2 (Fig. 3).
Molecular docking analysis
In consensus to the appreciable affinity of Nifedipine for ALR2 as determined by ITC experiments, molecular docking studies, in addition, showed appreciable interaction of Nifedipine at the anion binding pocket of ALR2 (PDB: 1Z3N) with a Moldock score of − 134.77 (Table 2). Nifedipine forms hydrogen bonds with Trp111, Cys298, and Ala299 and stacks with (π-π) with Trp111 and is further stabilized by van der Waals interactions with various active site residues (Fig. 4).
Nifedipine prevents the development of cataracts in STZ-induced diabetic rats
We evaluated Nifedipine for its efficacy to prevent cataract in male Spring Dawley rats under standard monitoring conditions for 8 weeks. Both diabetic control rats and rats treated with Nifedipine or sorbinil showed a negligible difference in systolic blood pressure when compared to the control group (those not induced with STZ). The heart rates of all the diabetic rats did, however, slightly decrease when compared to the healthy control rats (G-I) (data provided in supplementary-Fig S3). We found that bodyweight of the diabetic rats (G-II: 152.26 ± 2.63) was decreased; however, Nifedipine-treated rats (G-III: 195.75 and G-IV: 253.86) weighed more than diabetic control rats but less than the control group (G-III: 195.75 and G-IV: 253.86). Blood glucose levels of STZ-induced diabetic rats (G-II 246.67 ± 2.15) were significantly higher compared to the control group (G-I 83.63 ± 3.54). Upon treatment with Nifedipine (G-III: 145.52 ± 3.17 and G-IV: 113.52 ± 3.139) and sorbinil (G-V: 123.84 ± 4.03), a significant reduction in the glucose was observed (Table 3). In addition, normal HbA1c level (glycated hemoglobin) in Nifedipine-treated groups (G-III: 6.37 ± 0.52, G-IV: 5.51 ± 0.58) was observed than those with diabetic and normal control groups (G-II: 10.34 ± 0.78 and G-I: 4.23 ± 0.91). These observations thus testify to the critical role of Nifedipine in lowering glucose levels and thus preventing the development of cataract condition. Biochemical investigations involving the ALR2 activity and osmotic and oxidative stress were performed in different groups of rat lens. Significant inhibition was observed for ALR2 activity upon Nifedipine administration in a dose-dependent manner (G-III: 41.28 ± 0.67 and G-IV: 35.37 ± 0.54) compared to diabetic control rats (G-II: 46.54 ± 0.65). This observation thus explains why sorbitol levels were normal in Nifedipine-treated rats (G-III and IV) to their untreated counterparts (G-II). Furthermore, the levels of TBARS and protein carbonyls were increased in the treated animals compared to controls. Furthermore, Nifedipine-treated groups showed decreased levels of TBARS and protein carbonyls in group III (TBARS: 17.58 ± 0.54, protein carbonyls: 27.68 ± 0.63) and group IV (TBARS: 14.71 ± 0.67, protein carbonyls: 22.01 ± 0.87) rats compared to diabetic controls (Group II-TBARS: 19.48 ± 0.52, protein carbonyls: 31.02 ± 0.42). In comparison to healthy rats, a significant decrease in GSH levels was noticed in diabetic controls. Conversely, GSH levels were increased in Nifedipine incubated lens (Group III: 8.32 ± 0.28, Group IV: 10.26 ± 0.71 vs. Group II: 6.58 ± 0.61). Next, SOD, catalase, and glutathione peroxidase activities were, in addition, decreased in the diabetic group than in controls supporting the antioxidant-boosting effects of Nifedipine.
In addition to the efficacious effects of Nifedipine, which helps to prevent diabetic cataract conditions as mentioned afore, we found that Nifedipine also mitigates non-specific protein aggregation in the lens by preserving the chaperone activity of α-crystallins. Our findings thus show that the chaperone activity of α-crystallin was improved in Nifedipine-treated rats when compared to the untreated diabetic rats (Figs. 5 and 6).
Cataract progression was retarded in Nifedipine-treated diabetic rats. A represents rat eye lens morphology of G-IV (Nifedipine 100 µM /kg body weight) at 0, 2, 4, 6, and 8 weeks of treatment. The data are the mean ± SD (n = 10). Statistically significant from G-I (analyzed by ANOVA; p ≤ 0.05). B Graphical representation of stages of cataract formation scored every week G-I healthy controls (black), G-II diabetic controls (red), G-III and IV were treated with 10 and 100 µM /kg body weight Nifedipine (light green and blue), G-V were treated with100 µM/kg body weight of sorbinil (cyan). C Histogram representation of percentage incidence of cataract development in the 5th and 8th weeks
Nifedipine preserves the chaperone activity of α-crystallins. The chaperone activity was assessed in terms of its ability to subside DTT-induced aggregation of insulin. Insulin was incubated in the absence (trace 1) or presence of α-crystallins from control rats (trace 2), diabetic rats (trace 3), and diabetic rats treated with 10 µM (trace 4) and 100 µM (trace 5) Nifedipine. The single and double asterisk denotes that data is statistically significant from trace 3, against trace 4 and 5 (analyzed by one-way ANOVA; p ≤ 0.05)
Overall, the multi-faceted activity of Nifedipine brings about a decrease of TBARS and protein carbonyl levels, enhancing antioxidants like catalase, SOD, GPx, and GSH and eventually preserving the α-crystallin activity apparently suggests an amelioratory role of Nifedipine to delay cataract progression.
Discussion
Dysregulation of ALR2 has been well indicated in developing secondary complications of diabetic cataracts, which is the primary cause of visual impairment (Pollreisz and Schmidt-Erfurth 2010). Recent studies in diabetic mice show that aldose reductase inhibition augments lens regeneration post-cataract surgery. For years, a variety of structurally diverse compounds demonstrated ALR2 inhibition, but none of them ever received regulatory approval because of either lack of sensitivity, specificity, or toxicity (Gopinath et al. 2016). Epalrestat was the only approved drug used in the treatment of diabetic nephropathy. Tolrestat, a potent ARI, was taken off the market due to fatal liver damage. Similarly, typical carboxylic acid ARIs, zenarestat, zopolrestat, and ponalrestat were recalled due to their adverse drug reactions (Zhu 2013). For example, in a tiny percentage of patients, zenarestat has been associated with renal damage, while ponarestat was taken out of clinical trials due to its ineffectiveness; 10% of patients using sorbinil experienced hypersensitivity events, which included fever, skin rash, and myalgia, due to the accumulation of hazardous oxidative intermediate resulting from the sorbinil metabolism (Zhu 2013).
In view of this, we endeavored to screen Nifedipine a, 1,4-dihydropyridine derivative, an analog of dihydro nicotinamide against hAR. Our present study explores the in vitro inhibition of hAR by Nifedipine, a widely approved anti-hypertensive drug using in vitro and in vivo studies (Snider et al. 2008).
The IC50 of Nifedipine tested against hAR and was determined to be 2.5 ± 0.23 µM, indicating similar inhibitory concentrations as for sorbinil—a potent ALR inhibitor. Nifedipine demonstrated concentration-dependent suppression of hAR activity, according to kinetic tests carried out with various concentrations of DL-glyceraldehyde. Given that Nifedipine’s IC50 value for suppressing AR activity was lower, it might form a candidate for repurposing as an aldose reductase inhibitor (ARI) in the treatment of diabetes. In consensus with the present findings, Türkeş et al. (2019) also reported competitive inhibition of ALR2 by Nifedipine from sheep kidneys (Türkeş et al. 2019).
Because of the significant inhibitory action of Nifedipine for hAR, we next pursued quenching experiments to study stable complex formation between Nifedipine and hAR. In hAR, there are six Trp residues, of which four of them constitute the hydrophobic pocket of the active site in the core of the β-sheet, and two are obscured in the α-helices that surround it. The fluorescence of these Trp residues served as an indicator of tertiary structure alteration (Khan et al. 2020). Fluorescence quenching upon the addition of Nifedipine was observed in a concentration-dependent manner, which explains why Nifedipine-hAR interactions are not merely dynamic collisions but instead are high-affinity contacts that result in complex formation.
To validate stable Nifedipine-hAR interactions, those obtained from quenching experiments, the binding affinity of the Nifedipine-ALR2 system was investigated through ITC. The binding constant, Kd (2.91 ± 1.87 × 10−4 M) found for the system suggested a high affinity of Nifedipine at the active site of hAR. The ITC analysis suggests a single-site binding model at the active site. As a convention, both ΔH and ΔS are positive for hydrophobic interactions, whereas negative ΔH and ΔS changes result from van der Waals force and hydrogen bonding formation in low dielectric mediums (Khan et al. 2020). Negative ΔH, on the other hand, could play a function in electrostatic interactions. A single intermolecular force model however cannot account for the thermodynamic properties of the Nifedipine–ALR2 coordination complex. Thus, the binding of Nifedipine to ALR2 at the active site indicates hydrogen bonds, electrostatic interactions, and van der Waals forces, as confirmed by negative enthalpy and entropy. In concurrence with the ITC experiments, molecular docking, in addition, revealed hydrogen bond formation between the ester group of Nifedipine and Trp111 of anion binding pocket of ALR2 (Fig. 4). Furthermore, the nitrophenyl group of Nifedipine stacks by π-π interactions with Trp111 of ALR2. Overall, our in vitro and molecular modeling approaches elucidated the molecular basis of Nifedipine-ALR2 interactions which in turn explains why Nifedipine forms a potential inhibitor of ALR2 in diabetic cataract conditions.
Evidence suggests that high levels of glucose in lens epithelial cells overexpress ALR2, which in turn converts glucose into sorbitol. Sorbitol, being impermeable to cell membranes, builds up in the lens, leading to an increase in calcium levels causing oxidative and osmotic stress, ultimately resulting in cataract formation (Mathebula 2015; Ghahramani et al. 2015). Studies by Shah et al. (1995) have shown that Nifedipine can lower blood glucose levels and prevent sorbitol accumulation in diabetic rats. Our findings support these studies wherein we also observed that the administration of Nifedipine to STZ-induced diabetic rats decreased glucose levels and prevented sorbitol accumulation (Table 3) (Shah et al. 1995). Furthermore, Nifedipine was shown to have an insignificant effect on systolic blood pressure in STZ-induced diabetic rats. This can be attributed to a steady and gradual increase in drug concentration in the blood in the case of oral administration.
Our results were consistent with the studies of Kametaka et al. (2008), which reported that intravenous administration of Nifedipine markedly decreases systolic blood pressure when compared to oral administration. While Nifedipine administered intravenously did not cause any discernible alteration in blood glucose levels. The differences in heart rate and body weight are in line with Kametaka et al. (2008).
Elevated levels of calcium and high oxidative stress in the lens play a crucial role in the development of diabetic cataracts. Our observations in consistent with the findings of Mak and Weglicki (1990), that suggests Nifedipine not only inhibits ALR2 activity, but also controls TBARS protein carbonyl levels, prevents GSH loss, and increases antioxidant enzyme-specific activity (SOD, catalase, and glutathione peroxidase) (Table 3) (Mak and Weglicki 1990).
Our results are further supported by studies of Maddala et al. (2013) which emphasized the significant role of L-type calcium channels in maintaining lens transparency. Any dysregulation in calcium levels causes the cataractous condition, and inhibition of the L-type calcium channel with Nifedipine also has a major role in regulating cataract formation (Maddala et al. 2013; Velena et al. 2016). α-crystallin, a chaperone, is known to impede non-specific interactions of proteins that amount to cloudy protein aggregates in the healthy lens (Rao et al. 1994). Like any chaperone, the activity of α-crystallin is vulnerable to oxidative stress. This explains why the activity of α-crystallin is reduced in cataract lens (Fig. 6) (Yan and Harding 2006), and supplementing diabetic rats with Nifedipine that prevents loss of antioxidant-glutathione, retained its chaperone activity and prevented protein aggregates in the lens (Clark and Huang 1996). The present findings hence suggest that Nifedipine can avert the depletion of cellular glutathione to reduce oxidative stress, in order to preserve α-crystallin for lens transparency and eventually prevent diabetic cataracts.
Conclusion
The present study shows that Nifedipine delays the development of diabetic cataracts through its multi-faceted mechanisms including blocking of ALR2, exerting anti-oxidant effects, and driving the chaperone function of α-crystallin to maintain lens transparency. Based on the findings, Nifedipine can be repurposed to ameliorate cataract conditions in normotensive diabetics and treat presbyopia, a posterior capsular opacification.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. The corresponding author can provide the raw data from this study upon reasonable request.
References
Balendiran GK, Rajkumar B (2005) Fibrates inhibit aldose reductase activity in the forward and reverse reactions. Biochem Pharmacol 70:1653–1663. https://doi.org/10.1016/j.bcp.2005.06.029
Çağlayan C, Taslimi P, Demir Y et al (2019) The effects of zingerone against vancomycin-induced lung, liver, kidney and testis toxicity in rats: the behavior of some metabolic enzymes. J Biochem Mol Toxicol 33:1–8. https://doi.org/10.1002/jbt.22381
Clark JI, Huang QL (1996) Modulation of the chaperone-like activity of bovine α-crystallin. Proc Natl Acad Sci U S A 93:15185–15189. https://doi.org/10.1073/pnas.93.26.15185
Demir Y, Özaslan MS, Duran HE et al (2019) Inhibition effects of quinones on aldose reductase: antidiabetic properties. Environ Toxicol Pharmacol 70. https://doi.org/10.1016/j.etap.2019.103195
Demir Y, Ceylan H, Türkeş C, Beydemir Ş (2021) Molecular docking and inhibition studies of vulpinic, carnosic and usnic acids on polyol pathway enzymes. J Biomol Struct Dyn 1–6. https://doi.org/10.1080/07391102.2021.1967195
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Ghahramani M, Yousefi R, Khoshaman K, Alavianmehr M-M (2015) The impact of calcium ion on structure and aggregation propensity of peroxynitrite-modified lens crystallins: new insights into the pathogenesis of cataract disorders. Colloids Surf B Biointerfaces 125:170–180. https://doi.org/10.1016/j.colsurfb.2014.11.002
Gopinath G, Sankeshi V, Perugu S et al (2016) Design and synthesis of chiral 2H-chromene-N-imidazolo-amino acid conjugates as aldose reductase inhibitors. Eur J Med Chem 124:750–762. https://doi.org/10.1016/j.ejmech.2016.08.070
Kametaka S, Kasahara T, Ueo M et al (2008) Effect of nifedipine on severe experimental cataract in diabetic rats. J Pharmacol Sci 106:651–658. https://doi.org/10.1254/jphs.FP0072294
Khan MS, Qais FA, Rehman MT et al (2020) Mechanistic inhibition of non-enzymatic glycation and aldose reductase activity by naringenin: binding, enzyme kinetics and molecular docking analysis. Int J Biol Macromol 159:87–97. https://doi.org/10.1016/j.ijbiomac.2020.04.226
Koch C, Heine A, Klebe G (2011) Tracing the detail: How mutations affect binding modes and thermodynamic signatures of closely related aldose reductase inhibitors. J Mol Biol 406:700–712. https://doi.org/10.1016/j.jmb.2010.11.058
Kumar Pasala V, Gudipudi G, Sankeshi V et al (2021) Design, synthesis and biological evaluation of selective hybrid coumarin-thiazolidinedione aldose reductase-II inhibitors as potential antidiabetics. Bioorg Chem 114:104970. https://doi.org/10.1016/j.bioorg.2021.104970
Lamour V, Barth P, Rogniaux H et al (1999) Production of crystals of human aldose reductase with very high resolution diffraction. Acta Crystallogr Sect D Biol Crystallogr 55:721–723. https://doi.org/10.1107/S0907444998013365
Lawrence RA, Sunde RA, Schwartz GL, Hoekstra WG (1974) Glutathione peroxidase activity in rat lens and other tissues in relation to dietary selenium intake. Exp Eye Res 18:563–569. https://doi.org/10.1016/0014-4835(74)90062-1
Lee AYW, Chung SSM (1999) Contributions of polyol pathway to oxidative stress in diabetic cataract. FASEB J 13:23–30
Liu K, Lyu L, Chin D et al (2015) Altered ubiquitin causes perturbed calcium homeostasis, hyperactivation of calpain, dysregulated differentiation, and cataract. Proc Natl Acad Sci U S A 112:1071–1076. https://doi.org/10.1073/pnas.1404059112
Maddala R, Nagendran T, de Ridder GG et al (2013) L-type calcium channels play a critical role in maintaining lens transparency by regulating phosphorylation of aquaporin-0 and myosin light chain and expression of connexins. PLoS One 8:e64676. https://doi.org/10.1371/journal.pone.0064676
Mak IT, Weglicki WB (1990) Comparative antioxidant activities of propranolol, nifedipine, verapamil, and diltiazem against sarcolemmal membrane lipid peroxidation. Circ Res 66:1449–1452. https://doi.org/10.1161/01.RES.66.5.1449
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474. https://doi.org/10.1111/j.1432-1033.1974.tb03714.x
Mathebula SD (2015) Polyol pathway: A possible mechanism of diabetes complications in the eye. African Vis Eye Heal 74:1–5. https://doi.org/10.4102/aveh.v74i1.13
Nair J, Howlin S, Porter J, Rimmer T (2009) Using low dose oral nifedipine to prevent cancellation of cataract surgery for patients with preoperative hypertension. Eye 23:989–990. https://doi.org/10.1038/eye.2008.72
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Pollreisz A, Schmidt-Erfurth U (2010) Diabetic cataract—pathogenesis, epidemiology and treatment. J Ophthalmol 2010:1–8. https://doi.org/10.1155/2010/608751
Rao PV, Horwitz J, Zigler JS (1994) Chaperone-like activity of α-crystallin. The effect of NADPH on its interaction with ζ-crystallin. J Biol Chem 269:13266–13272. https://doi.org/10.1016/s0021-9258(17)36828-x
Reddy GB, Das KP, Petrash JM, Surewicz WK (2000) Temperature-dependent chaperone activity and structural properties of human αA- and αB-crystallins. J Biol Chem 275:4565–4570. https://doi.org/10.1074/jbc.275.7.4565
Rhodes JD, Sanderson J (2009) The mechanisms of calcium homeostasis and signalling in the lens. Exp Eye Res 88:226–234
Sankeshi V, Anil Kumar P, RavindarNaik R et al (2013) Inhibition of aldose reductase by Aegle marmelos and its protective role in diabetic cataract. J Ethnopharmacol 149:215–221. https://doi.org/10.1016/j.jep.2013.06.025
Sekhon G, Singh R (2019) Human aldose reductase unfolds through an intermediate. F1000Research 8:564. https://doi.org/10.12688/f1000research.18963.1
Sever B, Altıntop MD, Demir Y et al (2020) Design, synthesis, in vitro and in silico investigation of aldose reductase inhibitory effects of new thiazole-based compounds. Bioorg Chem 102:104110. https://doi.org/10.1016/j.bioorg.2020.104110
Shah TS, Satia MC, Gandhi TP et al (1995) Effects of chronic nifedipine treatment on streptozotocin-induced diabetic rats. J Cardiovasc Pharmacol 26:6–12. https://doi.org/10.1097/00005344-199507000-00002
Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47:389–394. https://doi.org/10.1016/0003-2697(72)90132-7
Snider ME, Nuzum DS, Veverka A (2008) Long-acting nifedipine in the management of the hypertensive patient. Vasc Health Risk Manag 4:1249–1257. https://doi.org/10.2147/vhrm.s3661
Steinmetz JD, Bourne RRA, Briant PS et al (2021) Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Heal 9:e144–e160. https://doi.org/10.1016/S2214-109X(20)30489-7
Taalat M, Aly E, Mohamed E et al (2018) Caffeine and nifedipine effect on cataract induced by selenite in rats. J Arab Soc Med Res 13:32. https://doi.org/10.4103/jasmr.jasmr_6_18
Taslimi P, Kandemir FM, Demir Y et al (2019) The antidiabetic and anticholinergic effects of chrysin on cyclophosphamide-induced multiple organ toxicity in rats: pharmacological evaluation of some metabolic enzyme activities. J Biochem Mol Toxicol 33:1–7. https://doi.org/10.1002/jbt.22313
Türkeş C, Demir Y, Beydemir Ş (2019) Anti-diabetic properties of calcium channel blockers: inhibition effects on aldose reductase enzyme activity. Appl Biochem Biotechnol 189:318–329. https://doi.org/10.1007/s12010-019-03009-x
Türkeş C, Demir Y, Beydemir Ş (2021a) Calcium channel blockers: molecular docking and inhibition studies on carbonic anhydrase I and II isoenzymes. J Biomol Struct Dyn 39:1672–1680. https://doi.org/10.1080/07391102.2020.1736631
Türkeş C, Kesebir AÖ, Demir Y et al (2021b) Calcium channel blockers: the effect of glutathione S-transferase enzyme activity and molecular docking studies. ChemistrySelect 6:11137–11143. https://doi.org/10.1002/slct.202103100
Türkeş C, Demir Y, Beydemir Ş (2022) Some calcium-channel blockers: kinetic and in silico studies on paraoxonase-I. J Biomol Struct Dyn 40:77–85. https://doi.org/10.1080/07391102.2020.1806927
Uchida K, Kanematsu M, Sakai K, Matsuda T, Hattori N, Mizuno Y, Osawa T (1998) Protein-bound acrolein: potential markers for oxidative stress. Proc Natl Acad Sci 95(9), 4882–4887
VasanthRao P, SeetharamBhat K (1989) Effect of riboflavin deficiency on sorbitol pathway in rat lens. Nutr Res 9:1143–1149. https://doi.org/10.1016/S0271-5317(89)80049-1
Velena A, Zarkovic N, Gall Troselj K, et al (2016) 1,4-Dihydropyridine derivatives: dihydronicotinamide analogues - model compounds targeting oxidative stress. Oxid Med Cell Longev 2016. https://doi.org/10.1155/2016/1892412
Yamamoto E, Nakamura T, Kataoka K et al (2010) Nifedipine prevents vascular endothelial dysfunction in a mouse model of obesity and type 2 diabetes, by improving eNOS dysfunction and dephosphorylation. Biochem Biophys Res Commun 403:258–263. https://doi.org/10.1016/j.bbrc.2010.11.008
Yan H, Harding JJ (2006) Carnosine inhibits modifications and decreased molecular chaperone activity of lens α-crystallin induced by ribose and fructose 6-phosphate. Mol vis 12:205–214
Zhu C (2013) Aldose reductase inhibitors as potential therapeutic drugs of diabetic complications. In: Diabetes mellitus - insights and perspectives. InTech, p 13 https://doi.org/10.5772/54642
Acknowledgements
The authors are grateful to the University Grants Commission, Government of India, for sponsoring the research (JRF: 2121330730, 340931). We would also like to express our gratitude to the DST-PURSE II grant (C-DST-PURSE-II/26/2017), Osmania University for financial support in carrying out this research. CFRD-OU and CCMB, Hyderabad are duly acknowledged for providing the necessary facilities and infrastructure.
Author information
Authors and Affiliations
Contributions
AMD and SSR conceived the idea and provided critical inputs to the concept. AMD, VS, RA, and SSR planned and executed the experiment. AMD, RA, and VKT contributed to analyze, interpret data, and wrote the manuscript. All the authors (AMD, VS, RA, SB, VKT, and SSR) contributed to revise and documentation of the final manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethical approval
The Institute’s animal ethics committee’s guidelines and CPCSEA (Control and Supervision of Experiments on Animals) standards were followed when conducting the animal research. (Registration no. IAEC/NRML/Ph.D./03/2021).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Findings of the study
• Nifedipine, a calcium channel blocker, delays cataract progression in diabetic rat models.
• Nifedipine treatment reduced the osmotic and oxidative stress in STZ-induced diabetic rats.
• Nifedipine preserves the chaperone activity of α-crystallins in cataract conditions in diabetic rats.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Devi, A.M., Sankeshi, V., Ravali, A. et al. Inhibitory effect of Nifedipine on aldose reductase delays cataract progression. Naunyn-Schmiedeberg's Arch Pharmacol 397, 161–171 (2024). https://doi.org/10.1007/s00210-023-02588-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02588-1