Abstract
In this study, we determined the therapeutic effect of parthenolide (PTL), the active component of Tanacetum parthenium, on neuropathic pain caused by paclitaxel (PTX), a chemotherapeutic drug frequently used in cancer treatment, at the gene and protein levels. To this end, 6 groups were formed: control, PTX, sham, 1 mg/PTL, 2 mg/kg PTL, and 4 mg/kg PTL. Pain formation was tested by Randall-Selitto analgesiometry and locomotor activity behavioral analysis. Then, PTL treatment was performed for 14 days. After the last dose of PTL was taken, Hcn2, Trpa1, Scn9a, and Kcns1 gene expressions were measured in rat brain (cerebral cortex/CTX) tissues. In addition, changes in the levels of SCN9A and KCNS1 proteins were determined by immunohistochemical analysis. Histopathological hematoxylin-eosin staining was also performed to investigate the effect of PTL in treating tissue damage on neuropathic pain caused by PTX treatment. When the obtained data were analyzed, pain threshold and locomotor activity decreased in PTX and sham groups and increased with PTL treatment. In addition, it was observed that the expression of the Hcn2, Trpa1, and Scn9a genes decreased while the Kcns1 gene expression increased. When protein levels were examined, it was determined that SCN9A protein expression decreased and the KCNS1 protein level increased. It was determined that PTL treatment also improved PTX-induced tissue damage. The results of this study demonstrate that non-opioid PTL is an effective therapeutic agent in the treatment of chemotherapy-induced neuropathic pain, especially when used at a dose of 4 mg/kg acting on sodium and potassium channels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is a difficult disease whose incidence increases every day, threatening people’s lives and reducing their quality of life (Addington and Freimer 2016). Cancer requires long-term intensive care and treatment. Therefore, cancer causes many irreversible psychosocial and economic effects on society. This dramatic situation has caused the fight against cancer to be an area of special interest and preventive interventions to be developed and implemented (WHO 2007). Despite the presence of cancer treatment methods, the use of anticancer drugs is one of the most basic approaches. Although treating with these drugs is highly successful, they adversely affect the healing process of patients and their treatment. Neuropathy is one of the most common adverse effects of drugs used in cancer chemotherapy (Alessandri-Haber et al. 2008).
Neuropathic pain is a type of chronic pain that occurs due to injury to nerves located in pain pathways in the nervous system (Colloca et al. 2017). Nerve damage, injury, diabetes, or the use of chemotherapeutics are among the causes of this pain (Huynh et al. 2020). Patients suffering from pain may show various clinical outcomes. In most cases, the conditions causing the pain will improve over time, and the body can enter a normal healing process. In some cases, the pain becomes chronic, lasting for many years (Yang and Chang 2019). Paclitaxel, an anticancer drug obtained from the bark of the plant Taxus brevifolia, is a chemotherapy drug frequently used to treat ovarian, lung, and breast cancers (Gao et al. 2016; Zhou et al. 2020). However, severe neuropathic pain syndrome and some other side effects prevent the use of this drug for a long time and in high doses, which causes cancer recurrence. Normally, paclitaxel-induced neuropathic symptoms begin with paresthesia and fatigue. Afterward, they continue with pain in the hands and feet, difficulties in daily life movements, drowsiness, and balance disorder, especially at night (Maihöfner et al. 2021; Omran et al. 2021). Despite using various analgesics, unrelieved pain remains a major health concern (Testa 1996; Colloca et al. 2017). Thus, it is very important to develop more effective treatment methods or drug candidates to prevent neuropathy caused by chemotherapeutic agents used in cancer treatment (Fields 2011; Nadipelly et al. 2018).
Pain is a complex process shaped by many biological and psychological systems, involving the action of numerous proteins throughout the peripheral and central nervous systems. Therefore, the interaction of genes among themselves and with various environmental factors affects pain sensitivity and expression (Summers et al. 1988). To develop an effective treatment method for chronic pain, it is important to understand the mechanisms involved in pain chronicization (Woolf 1999, 2004; Costigan 2009). Voltage-gated potassium (Kv) channels are very important ion channels due to their fundamental role in pain regulation and the transmission mechanism of nociceptive signals. KCNS1 is the first Kv gene associated with chronic pain in humans. Therefore, genetic analysis has demonstrated that Kcns1 gene polymorphisms are associated with a tendency toward pain development (Costigan et al. 2010; Hendry et al. 2013; Langford et al. 2014). Another gene associated with pain, the Scn9a gene, is the sodium channel called Nav1.7 and is located in the nociceptors that transmit pain signals. Nociceptors are sensory receptors located at nerve endings responsible for transmitting painful stimuli. It is thought that mutations in SCN9A may be associated with painful neuropathy, as in primary erythmalgia (Cox et al. 2006; Dib-Hajj et al. 2007). Members of transient receptor potential (TRP) channels, including TRP ankyrin 1 (TRPA1), also have an important role in pain (Souza Monteiro de Araujo et al. 2020). The expression of cyclic nucleotide gated (HCN) channels activated by hyperpolarization in dorsal root ganglion (DRG) neurons has a role in peripheral nociception, leading to the use of HCN blockers as antinociceptive drugs (Jansen et al. 2021).
Herbal remedies can be effective methods for treating pain. Tanacetum parthenium, known as feverfew, is a herb with a high parthenolide content in the leaves and flower heads (Pittler 2004; Saranitzky 2009; Pareek 2011). Feverfew has been extensively studied more recently, and its efficacy in the treatment and prophylaxis of migraine has been confirmed (Pittler 2004; Saranitzky 2009). Although studies have shown that PTL effectively reduces allodynia and hyperalgesia after sciatic nerve injury, its effect on neuropathic pain has not been fully explained (Fiebich 2002; Uchi 2002). This study aims to investigate the therapeutic effect of PTL, the active component of Tanacetum parthenium, on neuropathic pain caused by an anticancer drug, PTX, at the gene and protein levels.
Materials and methods
Animals and experimental design
Sprague-Dawley male rats (220–295 g, n = 48) were obtained from the Medical Experimental Research and Application Center of Atatürk University. All rats were housed in cages with constant temperature and light cycles (23 ± 1 °C, 12:12 h dark/light cycles) (Fig. 1). Rodent chow and water were given ad libitum. Decision no 193 of Atatürk University Animal Experiments Local Ethics Committee (AUHADYEK) taken during the session dated 07.11.2019 and numbered 14 approved that all stages of our study complied with the ethical rules. Pain tests were performed in accordance with the guidelines of the International Association for the Study of Pain (IASP) (Zimmermann 1983). Rats were randomly assigned to six groups (n = 8). All injections were made intraperitoneally.
-
Group 1 (Control): Animals in this group did not receive any treatment.
-
Group 2 (PTX): The neuropathic pain group was created by administering 2 mg/kg PTX (Huynh et al. 2019).
-
Group 3 (Sham): 2 mg/kg of PTX and DMSO, the solvent of PTL, were given.
-
Group 4 (1 mg/kg PTL): 2 mg/kg paclitaxel was applied, and 1 mg/kg PTL was given for 14 consecutive days.
-
Group 5 (2 mg/kg PTL): 2 mg/kg paclitaxel was applied, and 2 mg/kg PTL was given for 14 consecutive days.
-
Group 6 (4 mg/kg PTL): 2 mg/kg paclitaxel was applied, and 4 mg/kg PTL was given for 14 consecutive days.
Paclitaxel-induced peripheral neuropathy model
The first administration of paclitaxel (2 mg/kg) was performed intraperitoneally in all groups, except for the control group. On days 2, 4, and 6 of the experiment, the 2nd, 3rd, and 4th doses of PTX were administered every other day. Care was taken to administer PTX at the same time every day. The model formation was expected between days 7 and 21 of the experiment (Fig. 2). In this process, the feed and water needs of the subjects were followed, and their weights were measured periodically (Zhou et al. 2020; Son et al. 2021).
Intraperitoneal injection of parthenolide
Between days 22 and 36 of the experiment, PTL, a 14-day treatment drug, was started to be administered intraperitoneally. At the end of day 37, the rats in all groups were decapitated under anesthesia (200 mg/kg sodium thiopental), and their brain tissues were removed (Fig. 2).
Pain behavioral testing
The Randall-Selitto analgesiometry test was used to measure hyperalgesia in the rats in all groups, and the locomotor activity test was used to evaluate their motor activities.
Randall-Selitto analgesiometry test
The Randall-Selitto or paw pressure test is used to evaluate mechanical hyperalgesia by measuring pain response in animals. In this test, a constantly increasing force is applied to the base of the animal's hind paw, and the pressure (grams) with which the animal pulls its paw is recorded as the pain threshold. It is attempted to test the effectiveness of analgesics by observing the response to increased pressure. Pain is said to be present if the animal exhibits an escape response. Pain measurements were made with Randall-Selitto analgesiometry before neuropathic pain was formed and after pain was induced and treated (Neugebauer et al. 2007).
Locomotor activity test
Locomotor activity was measured using a computerized system in which animals were placed individually in a clear plastic cage (30 × 23 × 23 cm). Movement activities were recorded for 10 min after the rats acclimatized to the new environment for a few minutes. The locomotor activity test determines changes in the motor activities of animals exposed to pain and the effect of the applied substances on their motor activities (Jain and Kulkarni 1999; Patil et al. 2003).
Histopathological scoring
Brain tissues were fixed in a 10% neutral formalin solution by necropsies of the rats. After routine alcoh-ol-xylol steps, the tissues were embedded in paraffin blocks. Sections of 5 µm were stained with hematoxylin-eosin, and the numerical reduction in all neurons in five random areas (× 40) in the cerebral cortex was statistically evaluated (Benzer et al. 2018) (Fig. 8c).
Immunohistochemical examinations
After 5-µm tissue sections taken on polylysine slides were passed through xylol and alcohol series, endogenous peroxidase inactivation was achieved with 3% H2O2. They were treated with antigen retrieval solution to reveal the antigen in the tissues. The tissues washed with PBS were then incubated with Nav1.7 antibody (Cell signaling, Cat. no. 14573S) and KCNS1 (Invitrogen, Cat. no. PA568277) primary antibodies at a dilution rate of 1/400 at + 4 ºC overnight. Secondarily, the Large Volume Detection System: anti-Polyvalent, HRP (Thermo Fischer, Cat. no. TP-125-HL) was used in line with the manufacturer's recommendations. DAB (3,3′-Diaminobenzidine) was used as a chromogen. After counterstaining with Mayer’s hematoxylin, it was covered with Entellan and examined under a light microscope. The immunopositive in the motor cortex of the brain tissues were evaluated as none (-), mild ( +), moderate (+ +), and severe (+ + +) (Aksu et al. 2016).
Bioinformatics analysis
Bioinformatics analyses were performed to determine the regions where the genes used in our study were expressed on the brain tissue. For this purpose, the GTEx portal and g: profiles bioinformatics programs were used. According to the data obtained, it was determined that the Scn9a, Kcns1, Trpa1, and Hcn2 genes were predominantly expressed in the cortex region of the brain (Fig. 3). Therefore, analyses were made in the cerebral cortex region. In addition, the visualization of the interaction network of the target proteins examined in the study was performed using the STRING web resource (http://string-db.org) based on different types of evidence.
Real-time PCR
Cerebral cortex samples were taken from the animals in all groups, and RNA was isolated using the RNA isolation kit (Invitrogen 12183025 RNA Mini Kit). cDNA synthesis was performed from the isolated RNA using the cDNA synthesis kit (Biolabs, E6300S). The obtained cDNA was stored at -20 °C (Ceylan et al. 2019). Quantitative changes in the Scn9a, Kcns1, Trpa1, and Hcn2 gene expressions were examined by qPCR using the SYBR Green method (Kocpinar et al. 2020; Budak et al. 2014). Table 1 contains the primers used in this study. Data on the relative gene expression were analyzed using the ΔCT method (Zhang et al. 2003).
Statistical analysis
Three animals were included in each group, and all measurements were made in triplicate for each animal. Experimental results were statistically compared using GraphPad Prism Software version 8.0 for Windows (GraphPad Software, San Diego, CA). The results of the control and experimental groups were analyzed with one-way ANOVA and Tukey’s post hoc test using Prism software. All data were presented as mean ± standard mean error (SEM). P-values < 0.05 were considered significant. Statistically significant changes are indicated with a symbol (*). Symbol expressions are as follows: *P < 0.05 (significant); **P < 0.01 (very significant); ***P < 0.001 and ****P < 0.0001 (highly significant).
Results and discussion
Anticancer treatments with chemotherapeutic agents such as platinum derivatives, taxanes, and vinca alkaloids produce chemotherapy-induced peripheral neuropathy (CIPN), affecting > 60% of patients. This is one of the most common dose-limiting side effects of chemotherapy. CIPN is a non-fatal condition but is considered an unavoidable side effect of chemotherapy since it significantly reduces patients’ quality of life (Canta et al. 2015; Goldlust et al. 2021). Various analgesics, such as antidepressants and opioids, are used to treat neuropathic pain (Bouhassira and Attal 2018; Micheli et al. 2020). Since many analgesic drugs used to fight severe pain cause addiction in patients, studies on developing alternative non-addictive drugs to existing painkillers have been conducted (Majithia et al. 2016; Colvin 2019).
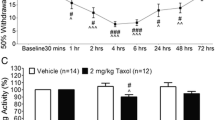
Many putative targets for treating CIPN have been compiled from animal studies, but the available evidence for its effective treatment is scarce. In a study, 75 patients with painful CIPN reported significant improvement in pain when treated with gabapentin up to a dose of 800 mg/day compared to the control group. However, another study involving 115 patients with multiple types of CIPN found that treatment with gabapentin up to 900 mg three times daily caused no improvement in pain compared to the control group (Farquhar-Smith 2011). In a similar study, 82 patients with paclitaxel or docetaxel-induced CIPN reported improvement in pain scores after treatment with pregabalin or duloxetine (Salehifar et al. 2020). Another clinical study examining 231 patients who developed painful CIPN reported a 59% and 38% reduction in pain after duloxetine and placebo treatments, respectively (Smith et al. 2013; Privitera and Anand 2021). This study investigated the therapeutic effect of parthenolide (PTL), the active component of Tanacetum parthenium, on paclitaxel (PTX)-induced neuropathic pain in rats at the gene and protein levels. To this end, 48 rats were first divided into 6 groups, 8 rats in each group. Subsequently, 4 doses of 2 mg/kg PTX were administered to each rat to induce neuropathic pain. Afterward, a 14-day PTL treatment at 1 mg/kg, 2 mg/kg, and 4 mg/kg was administered to the three groups, except for the PTX and sham groups. PTX is one of the chemotherapy drugs causing sensory neuropathy and acute pain in some patients, and pain intensity peaks approximately 3–4 days after the administration of the drug (Polomano and Bennett 2001). In our study, pain threshold measurements were performed with a Randall-Selitto analgesiometer to show the occurrence of pain after PTX administration and determine to what extent the pain was reduced after PTL treatment. When the results were evaluated, it was determined that the pain threshold was significantly reduced in all groups compared to the control group after PTX treatment. As expected, PTX administration induced neuropathic pain in rats. When the measurements were repeated after 14 days of PTL treatment, it was observed that the pain thresholds of the PTX and sham groups continued to decrease compared to the control group but increased depending on the PTL dose in the treatment groups and reached a similar level with the control group, especially in the 4 mg/kg PTL group (Fig. 4).
Patients with CIPN may have motor impairments, balance problems, or problems performing certain manual tasks (such as buttoning up) (Velasco-González and Coffeen 2022). Therefore, in addition to pain threshold measurements, behavioral analysis is performed using automated technologies to study pain generation in rodents. Then, inferences can be made about different “pain” states by comparing the behavior of pain-modeled animals (Deuis et al. 2017). A study conducted on mice with carrageenan-induced inflammatory pain found that the immobility time of the mice increased and locomotor activity decreased in the pain groups. However, locomotor activities improved in the groups receiving indomethacin treatment (Hasriadi et al. 2021). Another study in which the rat acute postoperative pain model was created determined that the escape/avoidance behavior of animals increased and locomotor activity decreased at certain time intervals during the experiment (Bree et al. 2016). In a similar study on rats with traumatic brain injury, ambulatory activity and total distance traveled decreased while resting time increased (Anderson et al. 2021). Likewise, a study investigating the nociceptive mechanisms involved in osteoarthritis pain determined that the distance traveled by mice in the pain group decreased and the resting time increased (Alves et al. 2020). In our study, locomotor activity (distance, resting, and ambulatory movement) was analyzed to evaluate the effects of PTL treatment on locomotor activity in the case of PTX-induced neuropathic pain. The results showed that the distance covered in the initial measurements before the application was similar in all groups, while this level decreased significantly in the other groups compared to the control group after PTX treatment. After the PTL treatment, while this decrease continued in the PTX and sham groups, the total distance traveled in the treatment groups increased and reached similar levels to the control group (Fig. 5c). This shows that PTL reduces the pain level in animals and enables them to move. Considering the resting movement, another behavioral parameter, it was determined that the rats in all groups behaved similarly in the baseline measurements before the applications, while movements decreased significantly in the resting behavior of almost all groups compared to the control group after the PTX treatment. When the measurements taken after the treatment were examined, it was seen that the immobility time in the PTX and sham groups started to increase, but the recognition and entanglement movements of the treatment groups, especially the 2 and 4 mg/kg PTL groups, increased and the resting time decreased and approached the control group (Fig. 5d). Ambulatory movement, another locomotor activity analysis criterion, is all movements performed on the ground. It is all kinds of displacement movements of the experimental animals, such as getting up, sitting, and looking left and right, without standing up. The analysis of the ambulatory movement results in this study showed that the baseline measurements were similar in each group, but there were significant decreases in all groups compared to the control group after PTX administration. After 14 days of PTL treatment, while the movements of the rats in the PTX and sham groups continued to decrease, the 4 mg/kg PTL application, with which animals in the treatment groups became active, further alleviated the pain in the rats and brought their movements to the level of the control group (Fig. 5e). Similar results were obtained upon examining the stereotypical activity of recognizing the environment, head movements, searching movements, sniffing, and grooming movements (Fig. 5f).
Comparative analysis of locomotor activity measurements among groups at baseline, PTX, and after PTL. a The locomotor activity test system used in the study. b The position of the total movements of a selected rat during the test period. c Comparative analysis of total distance traveled among groups. d Comparative analysis of rest time among groups. e Comparative analysis of ambulatory movement activity among groups. f Comparative analysis of stereotypical movement activity among groups
There is a need for effective and safe treatment strategies because CIPN, a common consequence of chemotherapy, significantly reduces the quality of life of cancer patients (Luo et al. 2018). Neuropathy causes changes in the ion channels in nerves affecting spinal and brain sensory signals. The increased expression of sodium channels in sensory nerves results in signal transduction and neurotransmitter release. The role of Nav1.7, one of the subtype-selective sodium channels, has recently been associated with neuropathic pain or trigeminal neuralgia. In particular, it has been determined that gain-of-function mutations in Nav1.7 may cause pain (Chung and Chung 2004; Dabby et al. 2011; Bouhassira and Attal 2018). Studies have shown that changes in sodium channel type and activity may lead to CIPN, as seen in many neuropathic pain conditions. Nav1.7 channel expression has been found to be increased in CIPN-affected segments in human dorsal root ganglia. An acute Na+ channel has been revealed to mediate neuronal excitability in patients with CIPN treated with oxaliplatin (Colvin 2019).
Like sodium channels, voltage-gated potassium (Kv) channels are a family of ion channels that have been frequently studied in recent times as master regulators of nociceptive excitability. KCNS1 is one of the first potassium channels associated with increased pain intensity and the risk of developing chronic pain (Tsantoulas et al. 2018). It has been determined that decreased potassium channel expression in primary sensory neurons causes an increase in spontaneous neuronal activity in various types of chemotherapy. An increase in Na+ channel activity and a decrease in K+ channel activity predispose patients to hyperexcitability, reducing the properties of CIPN with agents increasing K + channel hyperpolarization (Tsantoulas et al. 2012; Busserolles et al. 2016; Colvin 2019). In the case of cold hyperalgesia induced by the chemotherapy drug oxaliplatin, ambroxol, a Nav channel inhibitor, has been shown to effectively reduce cold allodynia in mice when administered alone or in combination with pregabalin (Furgała et al. 2018). Another study found that lacosamide, a Nav channel antagonist, significantly alleviated the severely painful symptoms of CIPN in a patient treated with cisplatin (Ibrahim et al. 2015). A study examining the effect of loss of gene function of Nav1.7 (Scn9a) on pain showed that in the hot plate experiment, the latency in claw withdrawal decreased with increasing temperature in wild-type (WT) mice, whereas in Scn9a KO, mice were heat-insensitive (Xue et al. 2021). Considering these data, it seems important to focus on these ion channels for a further investigation of Na+ channel activities playing a role in disease states, such as neuropathic pain, and drug development (Hargus and Patel 2007; Wheeler et al. 2014). Upon evaluating the results from the studies as a whole, it is seen that many researchers focus on blocking sodium channels in the treatment of neuropathic pain. However, current treatments seem unable to inhibit Nav1.7 without adverse effects on neuropathic pain (Cai et al. 2018; Xue et al. 2021).
A study in mice found that Kv7 activation was increased and paclitaxel-related symptoms were alleviated in CIPN induced by oxaliplatin and paclitaxel after treatment with minoxidil (Salat 2020). A similar study determined that Kv9.1 expression decreased with the development of hypersensitivity to pain in a rat model. It was observed that siRNA-mediated inhibition of Kv9.1 similarly led to neuropathic pain behaviors (Tsantoulas et al. 2012). Another study investigating the role of potassium channels in chronic pain found that Kv1.2 expression decreased in the spinal cord and dorsal root ganglia of rats with chronic constriction injury (CCI) and mechanical and thermal hypersensitivity occurred (Zhang et al. 2021). Potassium channels are a class of ion channels that have not been adequately studied in the physiology, pharmacology, and pathophysiology of pain. The fact that these channels are effectors of potent analgesic drugs suggests that their direct activation may induce analgesia (Busserolles et al. 2016). Given its role in chronic pain, it is important to further examine the role of KCNS1 and develop pharmacological tools to specifically target KCNS1 (Tsantoulas et al. 2018).
Chronic pain, which affects approximately 30–50% of the population globally, can occur as a result of pathological conditions such as migraine, diabetic neuropathy, nerve damage and treatment with chemotherapeutic agents. Members of transient receptor potential (TRP) channels, including TRP ankyrin 1 (TRPA1), also have an important role in pain (Souza Monteiro de Araujo et al. 2020). Several studies have shown that acute pharmacological inhibition using various TRPA1 inhibitors or TRPA1 gene deletion reduces both mechanical and cold hypersensitivity associated with a persistent inflammation in models of osteoarthritis induced by carrageenan, monosodium iodoacetate, and monosodium (Petrus et al. 2007; Fernandes et al. 2011). Data obtained as a result of spinal nerve ligation in mice also support the effect of TRPA1 in neuropathic pain models (Obata 2005). Downregulation of TRPA1 expression in L5/DRG and upregulation in L4/DRG suggested that a compensatory mechanism may occur after nerve injury. Subsequent studies showed a similar pattern of expression using other nerve injury models, such as sciatic nerve injury through chronic constriction or transection (Katsura et al. 2006; Staaf et al. 2009). All these data revealed a possible analgesic strategy by blocking/inhibiting TRPA1 (Souza Monteiro de Araujo et al. 2020). The expression of cyclic nucleotide gated (HCN) channels activated by hyperpolarization in dorsal root ganglion (DRG) neurons has a role in peripheral nociception, leading to the use of HCN blockers as antinociceptive drugs (Jansen et al. 2021). In a study, the pharmacological effect of MEL55A, which can selectively block HCN1/HCN2 isoforms, on DRG neuron excitability in vitro was investigated. When the results were analyzed, it was determined that MEL55A managed to alleviate the neuropathic pain caused by chemotherapy, and the importance of investigating small molecules with selectivity against HCN channel isoforms involved in nociception was revealed (Dini et al. 2018). In a similar study, the analgesic effect of ivabradine, a non-selective HCN blocker, available by prescription for cardiac indications, was investigated in patients with diabetic neuropathic pain. The results suggested that ivabradine may be an effective analgesic, especially when used in higher doses in patients with diabetic neuropathic pain (Bernard Healey et al. 2021).
Changes in the Scn9a, Kcns1, Trpa1, and Hcn2 gene expression after PTL treatment were also analyzed using real-time PCR. Upon examining the data obtained, it was observed that the Scn9a, Hcn2, and Trpa1 gene expression increased significantly in the PTX and Sham groups compared to the control group. After 14 days of PTL treatment, the amount of expression decreased and reached a similar level to the control group, especially in the 2 and 4 mg PTL groups (Fig. 6a, c, and d). Considering the change in the Kcns1 gene, it was observed that the expression level decreased in the PTX and sham groups, while the expression level increased in the PTL-treated groups, especially in the 2 and 4 mg/kg PTL groups, and approached the control group (Fig. 6b). Hcn2, Trpa1, and Scn9a channels initiate pain transmission by allowing Na+ ions outside the cell to enter the cell. Meanwhile, K+ ions, which are in excess inside the cell, begin to flow out of the cell via the Kcns1 ion channel. In this case, the cell becomes negatively charged and the stimulus to the brain is stopped and pain is relieved. Thus, the nerve cell becomes stable. When the data obtained are evaluated as a whole, it shows that 4 mg/kg PTL treatment stops the transmission of pain by activating the Hcn2, Trpa1, and Scn9a channels that are permeable to Na+ ions and blocking the Kcns1 channels that are permeable to K+ ions, especially in the case of neuropathic pain caused by PTX. This reveals that PTL can be a natural therapeutic agent that can have an analgesic effect.
In this study, SCN9A and KCNS1 proteins were evaluated immunohistochemically to assess the effect of PTL on PTX-induced neuropathic pain. In the analysis of the obtained data, significant differences were found between the groups in immunohistochemical staining with Nav1.7 and KCNS1 in the cerebral cortex (Fig. 7a, b , p <0.05). In the immunohistochemical staining performed for Nav1.7, no significant immunopositivity was observed in the control, 2 mg/kg PTL, and 4 mg/kg PTL groups, while mild immunopositivity was found in the 1 mg/kg PTL group, and moderate immunopositivity was detected in the PTX and sham groups, among the other administration groups. There was an inverse correlation between Nav1.7 and KCNS1 in terms of immunopositivity. KCNS1 immunopositivity was moderate in the control and 4 mg/kg PTL groups and mild in the other administration groups, PTX, sham, 1 mg/kg PTL, and 2 mg/kg PTL. Both Nav1.7 and KCNS1 immunopositivity were intracytoplasmically localized in neurons. A decrease in SCN9A protein level and an increase in KCNS1 protein level are important indicators that PTL reduces mechanical hyperalgesia. When the data were evaluated as a whole, it was determined that 4 mg/kg PTL treatment could be a natural therapy with a possible analgesic effect by acting on the Na+ and K+ channels in the case of PTX-induced neuropathic pain.
Immune negativity in the control group, moderate immunopositivity in the PTX and sham groups, mild immunopositivity at 1 mg/kg PTL (arrowheads), and immune negativity in the 2 mg/kg PTL and 4 mg/kg PTL groups. Nav1.7- IHC. a Moderate immunopositivity in the control group, mild immunopositivity in the PTX, sham, 1 mg/kg PTL, and 2 mg/kg PTL groups, and moderate immunopositivity in the 4 mg/kg PTL group (arrowheads). KCNS1-IHC (b). (Analyses were performed on cerebral cortex tissue)
Histopathological hematoxylin-eosin staining was performed to investigate the role of PTL in treating tissue damage caused by PTX therapy-induced neuropathic pain, and the results showed significant differences between the groups (Fig. 8, p < 0.05). Considering the results, it was determined that the neurons in the cerebral cortex of the control group rats had a normal histological appearance. It was observed that a numerical decrease in healthy neurons was the most significant in the PTX and sham groups among the treatment groups. While the number of healthy neurons in the 1 mg/kg PTL group among the drug administration groups was similar to the PTX and sham groups, the number of healthy neurons was moderate in the 2 mg/kg and 4 mg/kg PTL groups (Fig. 8a, b). These data support the idea that PTL may have a protective effect by preventing new neuron damage and possible death in neurons. In this study, the curative and analgesic effects of PTL on paclitaxel-induced neuropathic pain were investigated. The results showed that PTL reduced PTX-induced tissue damage and neuron death. We think that these data will help to investigate the protective effect of PTL in future studies.
Histopathological staining results. a The presence of normal neurons in the control group, and a slight number of neurons in the PTX and sham groups (□, arrow). H-E. Mild neuron presence in the 1 mg/kg PTL group and moderate neuron presence in the 2 mg/kg PTL and 4 mg/kg PTL groups (□, arrow). H-E. b Numerical scoring of healthy neurons (p < 0.05). c Histopathological scoring. (Microscope images were taken with 40 × magnification)
Protein-protein interaction (PPI) networks play a crucial role in cellular functions and biological processes in all organisms. Identification of protein interactions could lead to a better understanding of infection mechanisms and the development of a variety of pharmaceutical drugs and treatment optimization (Kuzmanov and Emili 2013). The proteomics approach provides an enormous amount of raw data that can be processed with the help of bioinformatics tools such as STRING (Interacting Gens/Proteins Search Tool) available on the World Wide Web (Szklarczyk et al. 2019). The output of proteomic studies is usually a panel of multiple proteins rather than single proteins. Most of the identified proteins do not function independently because they regulate activity and induce/decrease expression levels of other proteins. To better understand the physiology and biological processes that proteins affect, it is important to study protein-protein interactions (Gomez-Varela et al. 2019).
Proteins, which are the main actors in biological functions occurring in cell metabolism, mostly interact with each other and with other molecules in the cell (Barabási and Oltvai 2004). Therefore, relevant proteins must be evaluated together to understand complex processes such as cell functioning, disease mechanisms, and drug action mechanisms (Gerdle and Ghafouri 2020). Today, thanks to the achievements of -omics technologies, it is possible to create biological networks to describe cellular processes. Protein-protein interaction (PPI) networks are a bioinformatics-based tool that is frequently used for this purpose and helps to make sense of complex mechanisms. Therefore, using the STRING online database (Yesilkent and Ceylan 2022), a PPI network was created for the genes targeted in the current study. The data obtained showed that all the genes examined in the study interacted with each other and functioned. Circles show target proteins and lines show protein-protein interactions (Fig. 9).
Screening of genes for neuropathic pain induced by chemotherapy. The visualization of the interaction network of the proteins examined in the study was performed using the STRING web resource (http://string-db.org)
Conclusion
The lack of effective pharmacological methods to prevent and treat the development of CIPN creates a serious therapeutic gap. Although only the non-opioid duloxetine has been recommended as “moderately safe” for treating CIPN, there are currently no FDA-approved treatments for blocking chemotherapy-induced neuropathic pain (Majithia et al. 2016; Goldlust et al. 2021). While pharmacological interventions have predominated traditionally, there is also a need for a holistic approach considering mechanically guided non-pharmacological interventions. The complex interaction between cancer cells, neurons, and the immune system must be considered when investigating new preventive or modifying treatments for CIPN. Any new therapy used, especially during oncological treatment, should not interfere with the tumoricidal effects of chemotherapy (Colvin 2019). Neuropathic pain is still an unresolved problem since most treatments available have moderate efficacy or safety. Therefore, new treatment methods are welcomed (Bouhassira and Attal 2018). Based on the results from this study, we think that non-opioid PTL, especially when used at a dose of 4 mg/kg, is an effective therapeutic agent in treating chemotherapy-induced neuropathic pain, which acts on sodium and potassium channels. In addition, using the data obtained from this study, ion channels can be specifically targeted with electrophysiological techniques and the effect of PTL can be studied in more detail.
Data availability
The data supporting the findings of the present research are available on request from the corresponding author upon reasonable request.
References
Addington J, Freimer M (2016) Chemotherapy-induced peripheral neuropathy: an update on the current understanding. F1000Res 5. https://doi.org/10.12688/f1000research.8053.1
Aksu EH, Özkaraca MUSTAFA, Kandemir FM, Ömür AD, Eldutar E, Küçükler S, Çomaklı S (2016) Mitigation of paracetamol‐induced reproductive damage by chrysin in male rats via reducing oxidative stress. Andrologia 48(10):1145–1154
Alessandri-Haber N, Dina OA, Joseph EK, Reichling DB, Levine JD (2008) Interaction of transient receptor potential vanilloid 4, integrin, and SRC tyrosine kinase in mechanical hyperalgesia. J Neurosci 28(5):1046–1057. https://doi.org/10.1523/JNEUROSCI.4497-07.2008
Alves CJ, Couto M, Sousa DM, Magalhães A, Neto E, Leitão L, Conceição F, Monteiro AC, Ribeiro-da-Silva M, Lamghari M (2020) Nociceptive mechanisms driving pain in a post-traumatic osteoarthritis mouse model. Sci Rep 10(1):15271. https://doi.org/10.1038/s41598-020-72227-9
Anderson LM, Samineni S, Wilder DM, Lara M, Eken O, Urioste R, Long JB, Arun P (2021) The neurobehavioral effects of buprenorphine and meloxicam on a blast-ınduced traumatic brain ınjury model in the rat. Front Neurol 12:74637012. https://doi.org/10.3389/fneur.2021.746370
Barabási A-L, Oltvai ZN (2004) Network biology: understanding the cell’s functional organization. Nat Rev Genet 5(2):101–113. https://doi.org/10.1038/nrg1272
Benzer F, Kandemir FM, Ozkaraca M, Kucukler S, Caglayan C (2018) Curcumin ameliorates doxorubicin-induced cardiotoxicity by abrogation of inflammation, apoptosis, oxidative DNA damage, and protein oxidation in rats. J Biochem Mol Toxicol 32(2):e22030
Bernard Healey SA, Scholtes I, Abrahams M, McNaughton PA, Menon DK, Lee MC (2021) Role of hyperpolarization-activated cyclic nucleotide-gated ion channels in neuropathic pain: a proof-of-concept study of ivabradine in patients with chronic peripheral neuropathic pain. Paın Rep 6(4):e967. https://doi.org/10.1097/PR9.0000000000000967
Bouhassira D, Attal N (2018) Emerging therapies for neuropathic pain: new molecules or new indications for old treatments? Pain 159(3):576–582. https://doi.org/10.1097/j.pain.0000000000001136
Bree D, Moriarty O, Broom DC, Kelly JP, Roche M, Finn DP (2016) Characterization of the affective component of acute postoperative pain associated with a novel rat model of ınguinal hernia repair pain. CNS Neurosci Ther 22(2):146–153. https://doi.org/10.1111/cns.12483
Budak H, Ceylan H, Kocpinar EF, Gonul N, Erdogan O (2014) Expression of Glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase in oxidative stress ınduced by long- term ıron toxicity in rat liver. J Biochem Mol Toxicol 28(5):217–223. https://doi.org/10.1002/jbt.21556
Busserolles J, Tsantoulas C, Eschalier A, García JAL (2016) Potassium channels in neuropathic pain: advances, challenges, and emerging ideas. Pain 157:S7–S14
Cai W, Zhao Q, Shao J, Zhang J, Li L, Ren X, Zang W (2018) MicroRNA-182 alleviates neuropathic pain by regulating Nav1. 7 following spared nerve injury in rats. Sci Rep 8(1):1–11
Canta A, Pozzi E, Carozzi VA (2015) Mitochondrial dysfunction in chemotherapy-ınduced peripheral neuropathy (CIPN). Toxics 3(2):198–223. https://doi.org/10.3390/toxics3020198
Ceylan H, Budak H, Kocpinar EF, Baltaci NG, Erdogan O (2019) Examining the link between dose-dependent dietary iron intake and Alzheimer’s disease through oxidative stress in the rat cortex. J Trace Elem Med Biol 56:198–206. https://doi.org/10.1016/j.jtemb.2019.09.002
Chung JM, Chung K (2004) Sodium channels and neuropathic pain. Pathological Pain: From Molecular to Clinical Aspects 261:19–27
Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, Raja SN (2017) Neuropathic pain. Nat Rev Dis Primers 3(1):1–19
Colvin LA (2019) Chemotherapy-induced peripheral neuropathy (CIPN): where are we now? Pain 160(Suppl 1):S1–S10. https://doi.org/10.1097/j.pain.0000000000001540
Costigan (2009) Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci 32:1–32
Costigan M, Belfer I, Griffin RS, Dai F, Barrett LB, Coppola G, Wu TX, Kiselycznyk C, Poddar M, Lu Y, Diatchenko L, Smith S, Cobos EJ, Zaykin D, Allchorne A, Shen PH, Nikolajsen L, Karppinen J, Mannikko M, … Woolf CJ (2010) Multiple chronic pain states are associated with a common amino acid-changing allele in KCNS1. Brain 133:2519–2527. https://doi.org/10.1093/brain/awq195
Cox JJ, Reimann F, Nicholas AK, Thornton G, Roberts E, Springell K, Karbani G, Jafri H, Mannan J, Raashid Y, Al-Gazali L, Hamamy H, Valente EM, Gorman S, Williams R, McHale DP, Wood JN, Gribble FM, Woods CG (2006) An SCN9A channelopathy causes congenital inability to experience pain. Nature 444(7121):894–898. https://doi.org/10.1038/nature05413
Dabby R, Sadeh M, Gilad R, Lampl Y, Cohen S, Inbar S, Leshinsky-Silver E (2011) Chronic non-paroxysmal neuropathic pain—novel phenotype of mutation in the sodium channel SCN9A gene. J Neurol Sci 301(1–2):90–92
Deuis JR, Dvorakova LS, Vetter I (2017) Methods used to evaluate pain behaviors in rodents. Front Mol Neurosci 10:28410. https://doi.org/10.3389/fnmol.2017.00284
Dib-Hajj SD, Cummins TR, Black JA, Waxman SG (2007) From genes to pain: Na(v)1.7 and human pain disorders. Trends Neurosci 30(11):555–563. https://doi.org/10.1016/j.tins.2007.08.004
Dini L, Del Lungo M, Resta F, Melchiorre M, Spinelli V, Di Cesare Mannelli L, Ghelardini C, Laurino A, Sartiani L, Coppini R, Mannaioni G, Cerbai E, Romanelli MN (2018) Selective blockade of HCN1/HCN2 channels as a potential pharmacological strategy against pain. Front Pharmacol 9:1252. https://doi.org/10.3389/fphar.2018.01252
Farquhar-Smith P (2011) Chemotherapy-induced neuropathic pain. Curr Opin Support Palliat Care 5(1):1–7
Fernandes ES, Russell FA, Spina D, McDougall JJ, Graepel R, Gentry C, Staniland AA, Mountford DM, Keeble JE, Malcangio M, Bevan S, Brain SD (2011) A distinct role for transient receptor potential ankyrin 1, in addition to transient receptor potential vanilloid 1, in tumor necrosis factor α-induced inflammatory hyperalgesia and Freund’s complete adjuvant-induced monarthritis. Arthritis Rheum 63(3):819–829. https://doi.org/10.1002/art.30150
Fiebich (2002) Inhibition of LPS-induced p42/44 MAP kinase activation and iNOS/NO synthesis by parthenolide in rat primary microglial cells. J Neuroimmunol 132:(1-2):18–24
Fields HL (2011) The doctor’s dilemma: opiate analgesics and chronic pain. Neuron 69(4):591–594. https://doi.org/10.1016/j.neuron.2011.02.001
Furgała A, Fijałkowski Ł, Nowaczyk A, Sałat R, Sałat K (2018) Time-shifted co-administration of sub-analgesic doses of ambroxol and pregabalin attenuates oxaliplatin-induced cold allodynia in mice. Biomed Pharmacother 106:930–940
Gao W, Zan Y, Wang ZJJ, Hu XY, Huang F (2016) Quercetin ameliorates paclitaxel-induced neuropathic pain by stabilizing mast cells, and subsequently blocking PKCε-dependent activation of TRPV1. Acta Pharmacol Sin 37(9):1166–1177
Gerdle B, Ghafouri B (2020) Proteomic studies of common chronic pain conditions - a systematic review and associated network analyses. Expert Rev Proteomics 17(6):483–505. https://doi.org/10.1080/14789450.2020.1797499
Goldlust SA, Kavoosi M, Nezzer J, Kavoosi M, Korz W, Deck K (2021) Tetrodotoxin for chemotherapy-ınduced neuropathic pain: a randomized, double-blind, placebo-controlled, parallel-dose finding trial. Toxins 13(4). ARTN 235. https://doi.org/10.3390/toxins13040235
Gomez-Varela D, Barry AM, Schmidt M (2019) Proteome-based systems biology in chronic pain. J Proteomics 190:1–11. https://doi.org/10.1016/j.jprot.2018.04.004
Hargus NJ, Patel MK (2007) Voltage-gated Na+ channels in neuropathic pain. Expert Opin Investig Drugs 16(5):635–646
Hasriadi, Wasana PWD, Vajragupta O, Rojsitthisak P, Towiwat P (2021) Automated home-cage for the evaluation of innate non-reflexive pain behaviors in a mouse model of inflammatory pain. Sci Rep 11(1):12240. https://doi.org/10.1038/s41598-021-91444-4
Hendry L, Lombard Z, Wadley A, Kamerman P (2013) KCNS1, but not GCH1, ıs associated with pain ıntensity in a black southern african population with HIV-Associated sensory neuropathy: a genetic association study. Jaids-J Acquir Immune Defic Syndr 63(1):27–30. https://doi.org/10.1097/QAI.0b013e318285cf36
Huynh PN, Giuvelis D, Christensen S, Tucker KL, McIntosh JM (2019) RgIA4 accelerates recovery from paclitaxel-ınduced neuropathic pain in rats. Mar Drugs 18(1):12. https://doi.org/10.3390/md18010012
Huynh PN, Giuvelis D, Christensen S, Tucker KL, McIntosh JM (2020) RgIA4 accelerates recovery from paclitaxel-ınduced neuropathic pain in rats. Mar Drugs 18(1). ARTN 12. https://doi.org/10.3390/md18010012
Ibrahim SA, Albany Z, Albany C (2015) Significant response to lacosamide in a patient with severe chemotherapy-induced peripheral neuropathy. J Community Support Oncol 13(5):202–204
Jain NK, Kulkarni SK (1999) Antinociceptive and anti-inflammatory effects of Tanacetum parthenium L. extract in mice and rats. J Ethnopharmacol 68(1–3):251–259. https://doi.org/10.1016/s0378-8741(99)00115-4
Jansen L-AR, Forster LA, Smith XL, Rubaharan M, Murphy AZ, Baro DJ (2021) Changes in peripheral HCN2 channels during persistent inflammation. Channels 15(1):164–178. https://doi.org/10.1080/19336950.2020.1870086
Katsura H, Obata K, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Sakagami M, Noguchi K (2006) Antisense knock down of TRPA1, but not TRPM8, alleviates cold hyperalgesia after spinal nerve ligation in rats. Exp Neurol 200(1):112–123. https://doi.org/10.1016/j.expneurol.2006.01.031
Kocpinar EF, Baltaci NG, Ceylan H, Kalin SN, Erdogan O, Budak H (2020) Effect of a prolonged dietary ıron ıntake on the gene expression and activity of the testicular antioxidant defense system in rats. Biol Trace Elem Res 195(1):135–141. https://doi.org/10.1007/s12011-019-01817-0
Kuzmanov U, Emili A (2013) Protein-protein interaction networks: probing disease mechanisms using model systems. Genome Med 5(4):37. https://doi.org/10.1186/gm441
Langford DJ, West C, Elboim C, Cooper BA, Abrams G, Paul SM, Schmidt BL, Levine JD, Merriman JD, Dhruva A, Neuhaus J, Leutwyler H, Baggott C, Sullivan CW, Aouizerat BE, Miaskowski C (2014) Variations in potassium channel genes are associated with breast pain in women prior to breast cancer surgery. J Neurogenet 28(1–2):122–135. https://doi.org/10.3109/01677063.2013.856430
Luo J, Bavencoffe A, Yang P, Feng J, Yin S, Qian A, Hu H (2018) Zinc inhibits TRPV1 to alleviate chemotherapy-induced neuropathic pain. J Neurosci 38(2):474–483
Maihöfner C, Diel I, Tesch H, Quandel T, Baron R (2021) Chemotherapy-induced peripheral neuropathy (CIPN): current therapies and topical treatment option with high-concentration capsaicin. Support Care Cancer 29(8):4223–4238. https://doi.org/10.1007/s00520-021-06042-x
Majithia N, Loprinzi CL, Smith TJ (2016) New practical approaches to chemotherapy-ınduced neuropathic pain: prevention, assessment, and treatment. Oncology-New York 30(11):1020–1029
Micheli L, Di Cesare Mannelli L, Del Bello F, Giannella M, Piergentili A, Quaglia W, Ghelardini C (2020) The use of the selective imidazoline I1 receptor agonist carbophenyline as a strategy for neuropathic pain relief: preclinical evaluation in a mouse model of oxaliplatin-induced neurotoxicity. Neurotherapeutics 17(3):1005–1015
Nadipelly J, Sayeli V, Kadhirvelu P, Shanmugasundaram J, Cheriyan BV, Subramanian V (2018) Effect of certain trimethoxy flavones on paclitaxel-induced peripheral neuropathy in mice. Integr Med Res 7(2):159–167
Neugebauer V, Han JS, Adwanikar H, Fu Y, Ji G (2007) Techniques for assessing knee joint pain in arthritis. Mol Pain 3:1744–8069
Obata K (2005) TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Investig 115(9):2393–2401. https://doi.org/10.1172/JCI25437
Omran M, Belcher EK, Mohile NA, Kesler SR, Janelsins MC, Hohmann AG, Kleckner IR (2021) Review of the role of the brain in chemotherapy-ınduced peripheral neuropathy. Front Mol Biosci 8:693133. https://doi.org/10.3389/fmolb.2021.693133
Pareek (2011) Feverfew (Tanacetum parthenium 535 L.): a systematic review. Pharmacogenet Rev 5:103–110
Patil CS, Singh VP, Satyanarayan PSV, Jain NK, Singh A, Kulkarni SK (2003) Protective effect of flavonoids against aging-and lipopolysaccharide-induced cognitive impairment in mice. Pharmacology 69(2):59–67
Petrus M, Peier AM, Bandell M, Hwang SW, Huynh T, Olney N, Jegla T, Patapoutian A (2007) A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological ınhibition. Mol Pain 3:1744-8069-3-40. https://doi.org/10.1186/1744-8069-3-40
Pittler (2004) Feverfew or preventing migraine. Cochrane Database Syst 537 Rev 1 CD002286
Polomano RC, Bennett GJ (2001) Chemotherapy-evoked painful peripheral neuropathy. Pain Med 2(1):8–14. https://doi.org/10.1046/j.1526-4637.2001.002001008.x
Privitera R, Anand P (2021) Capsaicin 8% patch Qutenza and other current treatments for neuropathic pain in chemotherapy-induced peripheral neuropathy (CIPN). Curr Opin Support Palliat Care 15(2):125–131. https://doi.org/10.1097/SPC.0000000000000545
Salat K (2020) Chemotherapy-induced peripheral neuropathy: part 1-current state of knowledge and perspectives for pharmacotherapy. Pharmacol Rep 72(3):486–507. https://doi.org/10.1007/s43440-020-00109-y
Salehifar E, Janbabaei G, Hendouei N, Alipour A, Tabrizi N, Avan R (2020) Comparison of the efficacy and safety of pregabalin and duloxetine in taxane-ınduced sensory neuropathy: a randomized controlled trial. Clin Drug Investig 40(3):249–257. https://doi.org/10.1007/s40261-019-00882-6
Saranitzky (2009) Feverfew for 544 migraine prophylaxis: a systematic review. J Diet Suppl 6:91–103
Smith EML, Pang H, Cirrincione C, Fleishman S, Paskett ED, Ahles T, Bressler LR, Fadul CE, Knox C, Le-Lindqwister N, Gilman PB, Shapiro CL, Alliance for Clinical Trials in Oncology (2013) Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA Oncol 309(13):1359–1367. https://doi.org/10.1001/jama.2013.2813
Son DB, Choi W, Kim M, Go EJ, Jeong D, Park CK, Kim YH, Lee H, Suh JW (2021) Decursin alleviates mechanical allodynia in a paclitaxel-ınduced neuropathic pain mouse model. Cells 10(3). ARTN 547. https://doi.org/10.3390/cells10030547
Souza Monteiro de Araujo D, Nassini R, Geppetti P, De Logu F (2020) TRPA1 as a therapeutic target for nociceptive pain. Expert Opin Ther Targets 24(10):997–1008. https://doi.org/10.1080/14728222.2020.1815191
Staaf S, Oerther S, Lucas G, Mattsson JP, Ernfors P (2009) Differential regulation of TRP channels in a rat model of neuropathic pain. Pain 144(1):187–199. https://doi.org/10.1016/j.pain.2009.04.013
Summers MN, Haley WE, Reveille JD, Alarcon GS (1988) Radiographic assessment and psychologic variables as predictors of pain and functional ımpairment in osteo-arthritis of the knee or hip. Arthritis Rheum 31(2):204–209. https://doi.org/10.1002/art.1780310208
Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, Jensen LJ, von Mering C (2019) STRING v11: protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 47(D1):D607–D613. https://doi.org/10.1093/nar/gky1131
Testa (1996) Assessment of qality-of-life outcomes. N Engl J Med 334(13):835–840
Tsantoulas C, Zhu L, Shaifta Y, Grist J, Ward JP, Raouf R, McMahon SB (2012) Sensory neuron downregulation of the Kv9. 1 potassium channel subunit mediates neuropathic pain following nerve injury. J Neurosci 32(48):17502–17513
Tsantoulas C, Denk F, Signore M, Nassar MA, Futai K, McMahon SB (2018) Mice lacking Kcns1 in Peripheral neurons show ıncreased basal and neuropathic pain sensitivity. Pain 159(8):1641–1651. https://doi.org/10.1097/j.pain.0000000000001255
Uchi (2002) The sesquiterpene lactone parthenolide inhibits LPS- but not TNFalpha- induced maturation of human monocyte-derived dendritic cells by inhibition of the p38 mitogen-activated protein kinase pathway. J Allergy Clin Immunol 110(2):269–276
Velasco-González R, Coffeen U (2022) Neurophysiopathological aspects of paclitaxel-induced peripheral neuropathy. Neurotox Res 40(6):1673–1689. https://doi.org/10.1007/s12640-022-00582-8
Wheeler DW, Lee MC, Harrison EK, Menon DK, Woods CG (2014) Case Report: Neuropathic pain in a patient with congenital insensitivity to pain. F1000Research 26;3:135. https://doi.org/10.3389/fmolb.2021.693133
WHO (2007) Normative Guidelines On Pain Management. Report of a Delphi Study to Determine the Need for Guidelines and to Identify the Number and Topics of Guidelines That Should Bedeveloped by WHO. Report Prepared by Prof Neeta Kumar, Consultant. Geneva
Woolf (1999) Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet 353(9168):1959–64
Woolf (2004) American College of Physicians; American Physiological Society. Pain: Moving from Symptom Control toward Mechanism-Specific Pharmacologic Management. Ann Intern Med 140(6):441–51
Xue Y, Chidiac C, Herault Y, Gaveriaux-Ruff C (2021) Pain behavior in SCN9A (Nav1. 7) and SCN10A (Nav1. 8) mutant rodent models. Neurosci Lett 753:135844
Yang S, Chang MC (2019) Chronic pain: structural and functional changes in brain structures and associated negative affective states. Int J Mol Sci 20(13). ARTN 3130. https://doi.org/10.3390/ijms20133130
Yesilkent EN, Ceylan H (2022) Investigation of the multi-targeted protection potential of tannic acid against doxorubicin-induced kidney damage in rats. Chem-Biol Interact 365:110111. https://doi.org/10.1016/j.cbi.2022.110111
Zhang J, Rong L, Shao J, Zhang Y, Liu Y, Zhao S, Cao J (2021) Epigenetic restoration of voltage‐gated potassium channel Kv1. 2 alleviates nerve injury‐induced neuropathic pain. J Neurochem 156(3):367–378
Zhang YL, Zhang DB, Li WQ, Chen JQ, Peng YF, Cao W (2003) A novel real-time quantitative PCR method using attached universal template probe. Nucleic Acids Res 31(20). ARTN e123. https://doi.org/10.1093/nar/gng123
Zhou Y, Liu D, Chen S, Chen N, Sun J, Wang X, Cao F, Tian Y, Ye D (2020) Nrf2 activation ameliorates mechanical allodynia in paclitaxel-induced neuropathic pain. Acta Pharmacol Sin 41(8):1041–1048. https://doi.org/10.1038/s41401-020-0394-6
Zimmermann M (1983) Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16(2):109–110. https://doi.org/10.1016/0304-3959(83)90201-4
Funding
Financial support for the present research was received from Atatürk University Scientific Research Projects Coordination Commission [Grant Number: FDK-2020-8345].
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: HB (group leader), ET and AH. Performed the experiments: ET, CB, SS and MÖ. Analyzed the data: ET, CB and MÖ. Contributing reagents/materials/analysis tools: HB. Wrote the paper: HB, ET and MÖ. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethical approval
With the decision no. 193 of Atatürk University Animal Experiments Local Ethics Committee (AUHADYEK) in its session dated 07.11.2019 and numbered 14, all stages of our study were approved to comply with ethical rules.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Toraman, E., Bayram, C., Sezen, S. et al. Parthenolide as a potential analgesic in the treatment of paclitaxel-induced neuropathic pain: the rat modeling. Naunyn-Schmiedeberg's Arch Pharmacol 396, 3707–3721 (2023). https://doi.org/10.1007/s00210-023-02568-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02568-5