Abstract
Lung cancer is the most common type of cancer, with over 2.1 million cases diagnosed annually worldwide. It has a high incidence and mortality rate, leading to extensive research into various treatment options, including the use of nanomaterial-based carriers for drug delivery. With regard to cancer treatment, the distinct biological and physico-chemical features of nano-structures have acquired considerable impetus as drug delivery system (DDS) for delivering medication combinations or combining diagnostics and targeted therapy. This review focuses on the use of nanomedicine-based drug delivery systems in the treatment of lung cancer, including the use of lipid, polymer, and carbon-based nanomaterials for traditional therapies such as chemotherapy, radiotherapy, and phototherapy. The review also discusses the potential of stimuli-responsive nanomaterials for drug delivery in lung cancer, and the limitations and opportunities for improving the design of nano-based materials for the treatment of non-small cell lung cancer (NSCLC).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-small cell lung cancer (NSCLC) remains the leading cause of cancer-related deaths. The complexity and heterogeneity of NSCLC make it difficult to diagnose and treat, leading to a need for ongoing research to better understand the disease. The use of nanotechnology-based tools has resulted in significant improvements in clinical outcomes for NSCLC through retrospective studies of traditional therapies (García-Fernández et al. 2020; Ferlay et al. 2017). Lung cancer (LC) will surpass breast cancer as the next highly detected cancer worldwide in 2020 with two million new cases. However, lung cancer is the primary reason of death worldwide due to its 1,796,144 fatalities, which accounts for 18% of the total 9,958,133 deaths caused by cancer. Even though smoking is the chief causing factor for lung cancer, geography and racial differences have a big impact on how often the disease occurs (Stabile et al. 2017; McIntyre and Ganti 2017). There are primarily two types of lung cancer—non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). Basically 15% of lung cancers are categorized under SCLC, a fatal tumor, while approximately 80–85% of lung cancers are NSCLC in the form of squamous cell carcinomas, large cell carcinomas, and adenocarcinomas (Roca et al. 2017). This particular kind of lung cancer may develop in non-smokers as well, despite smoking being the primary risk factor (Sun et al. 2007). The middle of the lung is where squamous cell lung cancer often develops from the proximal bronchi. It frequently contributes to 25 to 30% of NSCLC and can manifest in smokers (Socinski et al. 2016). Adenomas and squamous cell lung carcinomas progress more rapidly than large cell lung carcinomas, which may develop anywhere in the lung tissue. Large cell carcinoma makes up around 3% of all lung malignancies, although it has a much lower overall survival rate than the other subtypes (Seong et al. 2020). Chemotherapy, targeted therapy, immunotherapy, surgery, and radiation therapy are currently available treatment options for NSCLC. A huge number of patients under clinical stages Ib-IV require systemic therapies (chemotherapy, targeted therapy, or immunotherapy) (Rochigneux et al. 2020). In general, cisplatin- or carboplatin-based doublet platinum-derived chemotherapy is the first-line treatment for NSCLC (Pai-Scherf et al. 2017). Small-sized EGFR tyrosine kinase inhibitors (EGFR-TKIs) such as erlotinib and gefitinib have recently been introduced as the 2nd-generation therapy for the management of NSCLC (Lynch et al. 2004). EGFR-TKIs have minimal toxicity and considerably enhance quality of life when compared to the conventional chemotherapy regimen. The most current EGFR-TKI, osimertinib, has been routinely employed as first-line treatment for individuals having progressed EGFR-mutant NSCLC since it was approved in April 2018 (Zhou and Zhou 2015). Nanomedicine is a subfield of nanotechnology that uses substances with a size range of 5 to 200 nm for medical and health applications (Chen et al. 2017a). By increasing anticancer drugs’ stability and bioavailability, facilitating cancer targeting across biological membranes, expanding circulation and plasma concentration, minimizing enzyme deterioration, and minimizing their toxic effects and antigenicity, a nanoparticle drug delivery system (NDDS) used in nanomedicine has the capabilities to resolve the disadvantages of anticancer drugs (Choi and Han 2018). Through an improved permeability besides enhanced permeation and retention (EPR) effect, nanoparticles are also useful for accelerating the accumulation of therapeutic drugs in cancer tissues. (Børresen et al. 2020). Only a few of the numerous kinds of nanoparticles created for the administration of anticancer drugs have advanced to the clinical stage (Garbuzenko et al. 2019). To our surprise, nanomedicine—which combines treatment and diagnostics—has developed into a workable paradigm for cancer treatments. It provides the best targeting potential and extremely efficient nanocomposites to carry cargo when coating them with targeted moiety (i.e., ligand, peptide, antibodies) for imaging and therapeutics. The concept led to the creation of several nanoparticles that are ideal for both diagnosis and drug administration, accelerating the development of customized medicine (Fernandez-Fernandez et al. 2011). Nanomedicine has made it possible to create multifunctional systems that can both help with diagnosis and deliver treatments with greater precision to the target spot or tissue. Here, we examine how nanotechnology is being applied to the management of NSCLC. Furthermore, combining drug delivery strategies depending upon the extrinsic stimulation and tumor microenvironment (TME) through other chemotherapeutic and/or immune-modulatory therapies may have synergistic benefits in lung cancer treatment. In the near future, numerous innovations will be required to design the most efficient, intelligent, and smart DDS techniques for the management of lung cancer.
Lung cancer: an overview
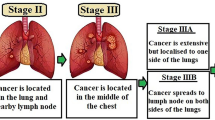
“Small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC)” are the two primary subtypes of lung cancer (Fig. 1). Even though SCLC is less frequent than NSCLC, it is more aggressive. Big cell lung cancer, squamous cell carcinoma, and adenocarcinoma are the three primary histological subtypes of NSCLC. Again, each subtype is distinct and responds to available treatments in a different way. The interplay of ecological factors, including cigarette smoke, and genetic predisposition affects lung carcinogenesis. Infrequent germ line transformations such as “epidermal growth factor receptor (EGFR), retinoblastoma, and p53” prominently enhance the risk of developing cancer. Decreased DNA healing effectiveness can correspondingly be a substantial aspect in the development of lung cancer. According to reports, chemicals in tobacco smoke are highly involved in lung cancer development. Tyrosine kinases are clearly implicated in lung cancer pathogenesis, according to new research. Cancer may develop as a result of constitutive kinase activation, downstream signaling, overexpression, and autocrine paracrine stimulation. Tyrosine kinase oncogenic activation, such as that of MET, PIK3CA, and EGFR is often seen in NSCLC and presents a therapeutic target (Burstein and Schwartz 2008; Paul and Mukhopadhyay 2004). Notwithstanding advances in “non-small cell lung cancer (NSCLC) treatment,” the last 5-year survival rate for carcinogenic progression has increased by fivefold in the past few months. Surgical procedure is a crucial component of treating diseases in their early stages. However, lung cancer surgery is complicated and can have negative effects. Another obstacle to patients receiving good treatment for lung cancer is early diagnosis of lung malignant development. Most lung disease patients will have advanced tumor stages when they are detected, and about 75% of these individuals will have symptoms. Radiation therapy and cancer chemotherapy are relatively successful in the early stages of NSCLC treatment. With the advent of large-scale genome-wide association studies and the availability of precise sequencing tools, it is now obvious that molecular heterogeneity occurs especially inside tumor subgroups. Heterogeneity between the initiating malignancy and its metastatic counterpart may occur, even among the tissues of particular cancer, or depending on the tissue that initially gave rise to the tumor. The variety of tumor cells’ genetic composition, as well as their propensity to gain adaptive tolerance, makes developing an efficacious treatment difficult (Jamal-Hanjani et al. 2017). A new technique that addressed the biochemical particularities of each participant is required.
Nanomedicine as an alternative theranostics
Nanotechnology, which has been utilized to treat and diagnose a number of biological disorders, is one of the rapidly increasing subjects in biomedical research. Nanotechnology has witnessed a tremendous surge in its usage in treating a variety of ailments in recent years for instance, cancer, diabetes, bacterial infections, cardiovascular disease, and others (DiSanto et al. 2015). Because traditional therapy techniques for lung cancer have significant drawbacks, researchers and scientists have concentrated on creating nanoscale chemotherapeutic drugs using delivery systems that include polymeric nanoparticles, liposomal nanoparticles, metal nanoparticles, inorganic nanoparticles, and biointegrated nanoparticles. The number of nanostructures is depicted in Fig. 2, which are commonly employed in tumor targeting.
Because of their small dimension, the nanoparticles have been shown to be efficient theranostic agents for treating lung cancer. This allows them to preferentially concentrate in tumor cells owing to an “EPR effect.” Furthermore, nanoparticles have a significant drug loading capacity owing to their large surface area to volume ratio and accessibility of functionalization (Mukherjee and Patra 2016; Ashique et al. 2022a). Because of their increased biocompatibility and ability to evade renal clearance, nanoparticles outperform traditional medicinal procedures. Because they have exhibited diverse characteristics including diagnostics imaging, sensing, and treatments, researchers may employ a range of nanoparticles for several applications in lung cancer theranostics. The diversified applications of various nanocarrier systems are explained in Fig. 3. This study concentrated on theranostic uses of a range of nanocomposite types that have demonstrated immense ability to be used in the therapeutic based as well as detection of lung cancer that included (i) polymeric nanoparticles, (ii) liposomal nanoparticles, (iii) metal nanoparticles; (iv) bio-nanoparticles (Ashique et al. 2022b).
Routes of administration of NDDS for NSCLC
It has been difficult to deliver a novel drug delivery system (NDDS) for lung-related disorders, but it has a lot of potential. The literature contains several reports of attempts to identify the most effective strategy, path, resource, and method for achieving the intended treatment objectives. In general, regional (inhalation) or systemic (intravenous) administration can be used to deliver medication specifically to the lungs. The preferred delivery strategy, systemic administration, has a number of limitations, including toxicities on healthy cells and an effective drug concentration at the site of tumor (Lee et al. 2015. Localized delivery is still preferable since it reduces the possibility of adverse effects brought on by systemic dispersion. The drug administration via inhalation is beneficial and promising for both its local and potential systemic effects, when drug molecules may get gather in the lymphatic circulatory system after administration (Mangal et al. 2017). A NDDS may decrease the toxicities associated with pharmaceuticals by encapsulating them suggesting that a novel drug delivery approach may also have the potential to minimize the widespread toxicity of medications like doxorubicin (Roa et al. 2011) and cisplatin (Devarajan et al. 2004), when they are delivered through the pulmonary route. It is probable that the pulmonary administration of NDDS and the EPR effect are incompatible. Further research has been done on an active targeted approach for NDDS lung delivery. According to research accomplished by Tseng and his coworkers, the “bEGF-decorated” nano-system has proficiently absorbed by/interacted with tumors overexpressed with EGFR found in animal studies, and large dosages of cisplatin were effectively administered to the malignant lung cells (Tseng et al. 2009.) There are particular issues that must be taken into consideration in order to ensure that NDDS is adequately administered to the lungs. The design of the pulmonary mechanism as well as the lung clearing process may make it more difficult to accumulate a substantial measure of drug-encapsulated NDDS at the site of tumor (Razak et al. 2021). The size of NDDS is concerning because nanometer-sized particles are generally expelled during normal breathing. The appropriate construction of nanostructures is also necessary to enable the collection as well as distribution of the drugs they carry on cancerous cells while preventing damage to normal cells. This minimizes the risk of complications while increasing the efficacy of the treatment. Numerous methods have reportedly been researched, including pH-triggered medication discharge from NDDS (Joshi et al. 2014). Understanding the microenvironment is essential for developing a successful NDDS for tumor targeting, in lung cancer.
Nanomedicine-based treatment strategy for NSCLC
Their function and consequent therapeutic effects are largely determined by the functionalization of nanoparticles as well as their geometry and materials (Shi et al. 2017a). Additionally, by altering these characteristics, it is conceivable to employ them as contrast agents for PET or CT imaging procedures, effectively creating dual theranostic platforms (Li et al. 2016a). For a variety of NSCLC treatments, numerous nanocarrier systems are really being evaluated as prospective medication delivery strategies. Lipidic, polymeric, and metal-based nanoparticles are the competent classes of nanocarriers that are extensively researched for lung cancer.
The use of ACE-2 and nanotechnology in the treatment of NSCLC was discussed in a prominent publication by Sivalingam and Singh (2023). The most common, deadly, and severe subgroup of NSCLC is “lung adenocarcinoma and squamous cell carcinoma (SCC).” It often develops in scar tissues with regions of persistent irritation toward the lung’s periphery. It has a low mortality rate of fewer than 5% and a greater amount of genetic alteration. (Dong et al. 2020) ACE-2 levels are higher in SCC than in healthy tissue regardless of the stage of the malignancy. It has been suggested that “DNA methylation,” which functions as a cancer regulator, is the cause of elevated ACE-2 expression (Zhang et al. 2020). Samad et al. demonstrated that ACE-2 is upregulated in lung adenocarcinoma and squamous cell carcinoma, with ACE-2 suppressing mortality in “human rat alveolar epithelial cells,” lowering cell migration, and mitigating NSCLC (Samad et al. 2020). In another study, Kim et al. (2022), investigated the antitumor and immunological modulatory properties of the “scL-RB94 nanocomplex” in experimental models of “human non-small cell lung cancer (NSCLC).” Mice with human NSCLC tumors were given systemic therapy with “scL-RB94,” which dramatically reduced tumor development by boosting death and reducing cancer cell development in vivo. Treatment with scL-RB94 also improved antitumor immune function by increasing immunological identification markers and attracting innate immune cells like natural killer (NK) cells (Kim et al. 2022). The “NSCLC cell line H460” was used to investigate the efficacy of “anti-Bcl-xL siRNA” included in an “EGFR-targeted nanomedicine with scFv ligands (NM-scFv)” created by Nguyen and his colleague. “Anti-EGFR scFv ligand”–modified nanostructure was demonstrated to enable active gene transport into “H460 cells” and resulted in roughly 63% of genes being silenced at both the mRNA and protein levels and concluded that “anti-Bcl-xL NM-scFv” variant enhanced the apoptotic activity of cisplatin (Nguyen et al. 2022). Polymeric micelle loaded with paclitaxel for treatment for NSCLC was created by Lu and his group in 2022. They discovered the first indication that “Pm-Pac (polymeric micelle-paclitaxel)” may have extended overall survival in NSCLC patients without pleural metastases while maintaining a favorable safety profile. Overall, this research presents a fresh viewpoint on how nanomedicine is being developed to examine chemotherapy effectiveness (Lu et al. 2022). For the purpose of delivering therapeutic “p53-mRNA” into “p53-null hepatocellular carcinoma (HCC) and non-small cell lung cancer (NSCLC) cells,” Cerami et al. (2012) constructed self-assembling lipid-polymer hybrid nanoparticles. Thirty-six percent of individuals with HCC and 68% of those with NSCLC had defects in the p53 tumor suppressor gene (Cerami et al. 2012). The nanostructures were suggested as effective methods for enhancing macrophage-mediated phagocytosis by Moradinasab et al. 2022. In the interest of achieving an improved antitumor activity in the management of NSCLC, the current study proposes CAR-macrophage as the cutting-edge method and it acts as a bridge between the innate and adaptive immune systems (Moradinasab et al. 2022). Kim et al. investigated whether p53 genetic therapy delivered by a cancer-targeting nanostructure (called SGT-53) can supplement anti-programmed cell death-1 (PD-1) immunotherapy, allowing it to be used in non-responding patients. Utilizing syngeneic mouse models of lung malignancies that are resistant to anti-PD-1, they establish that reinstatement of natural p53 expression stimulates anti-PD-1 to reduce cancer development and extend the lifespan of malignant cells bearing animals. The findings showed that SGT-53 can improve efficient immune function toward NSCLC by lowering immunosuppression chemicals and immunosuppressive cells (M2 macrophages and regulatory T cells) (Kim et al. 2022). In a similar study by Lu and his researcher group, polymeric micellar paclitaxel-based nanoparticle’s antitumor activity was investigated in both “A549/H226 cells and A549/H226-derived xenograft tumor models.” (Lu et al. 2023) For the treatment of NSCLC, inhibiting “YAP expression” may be a promising therapeutic strategy. Huang et al. (2022) developed a “nano-cocktail” treatment method described by using “amphiphilic and block-dendritic-polymer-based nanoparticles (NPs)” for tailored co-delivery of “EGFR-TKI gefitinib (Gef) and YAP-siRNA” to produce a controlled treatment against NSCLC (Huang et al. 2022). According to Das and his researcher group, polyphenol-based tailored nanostructures have shown to bind with a wide range of protein domains and cellular signal-transduction pathways, having a significant impact on key cellular functions. Nano-constructed dietary polyphenols have a number of molecular processes and potential therapeutic targets for the treatment of lung cancer (Das et al. 2022). Shukla et al. (2022), planned to create and evaluate “nintedanib-loaded niosomes” as inhalable carriers for increasing their therapeutic effectiveness by site-specific drug deposition against NSCLC. In vitro tests showed that nintedanib-loaded niosomes had much greater cytotoxicity, which was further supported by 3D spheroids (Shukla et al. 2022). In a similar way, “Indomethacin-loaded liposomes” were created by Sarvepalli et al. (2022) for the successful management of NSCLC. The 3D spheroid experiment results showed that “IND-Lip” nano-conjugate performed noticeably better in ex vivo tumor reduction (Sarvepalli et al. 2022). For the dual delivery of “pemetrexed disodium (PMX) and siRNA” for the treatment of NSCLC, Xiaoyu et al. (2023), created “poly-glutamic acid-modified cationic liposomes (γ-PGA-CL).” The recent research demonstrated the viability of combining “PMX and siRNA via γ-PGA-CL” as a feasible therapy method for NSCLC after in vivo antitumor trials from the complex group significantly inhibited tumor development (Xiaoyu et al. 2023). In order to combat NSCLC, Dominguez-Martinez and his colleague created “folate-decorated cross-linked cytochrome-C nanoparticles.” The findings show that both in vitro and in vivo, the NPs significantly inhibited the development of tumors in cancerous cells that exhibited FR overexpression (Dominguez-Martinez et al. 2022). Thangavelu and his group created liposomes that were loaded with “6-gingerol” for the evaluation of its anti-NSCLC therapeutic effectiveness in both in vitro and in vivo. Whenever compared to “standard Gn, lipo-pharmacological Gn’s” characteristics were considerably enhanced in “BALB/c mice” that had NSCLCs generated. “Lipo-Gn” can therefore be taken into consideration for its expanding uses against lung cancer (Thangavelu et al. 2022). In a similar manner, “amentoflavone-loaded nanoparticles” were created by Zhao et al. (2022) to target NSCLC. The remarkable drug carrying efficiency, excellent water tolerance and dispersion, extended bioavailability, and pH-dependent release of the “AMF@DOX-Fe3 + -PEG nanoparticles (ADPF NPs)” which is the “coordination of ferric ions (Fe3 +), amentoflavone (AMF), PEG-polyphenol, and doxorubicin (DOX).” ADPF NPs resulted in targeted drug delivery and improved drug deposition in the cancer site. Furthermore, by lowering the “aldo–keto reductase family-1B10 (AKR1B10)” activity, the ADPF NPs might prevent the development of the A549 tumor and improve the cardiotoxicity caused by free DOX. This nanoplatform that is combined with AMF and DOX offers a wide range of potential applications in the treatment of clinical tumors (Zhao et al. 2022). For the treatment of NSCLC, Patil and his colleague created “inhalable bedaquiline (BQ)–loaded cubosome (BQLC) nanocarriers.” Upon nebulization, the “BQLC nanocomposites” displayed good aerodynamic qualities. After 48 h of administration, the “BQLC” demonstrated increased cytotoxicity toward NSCLC (A549) cells compared to plain BQ. Moreover, research using 3D tumor simulations shows that cubosomal nanocarriers are more effective in fighting cancer than free BQ (Patil et al. 2021). Another study for the treatment of NSCLC was achieved by the development of quercetin-loaded micelles by Li et al. (2022). The confluence of “enhanced permeability and retention (EPR) and active targeting impact” may be responsible for the good tumor targeting capabilities and anticancer effectiveness shown by quercetin-loaded PEGMA–PDMAEA–PCL/DSPE–PEG–biotin mixed micelles (Que-MMICs). Together, Que-MMICs showed improved anticancer effect in the NSCLC-harboring mouse model and indicated substantial deposition at the tumor site (Li et al. 2022). The “bevacizumab-coated gefitinib-loaded nanoparticles (BCGN)” with dual-responsive drug release were created by Zhao et al. (2023). These nanoparticles were designed to suppress tumor growth and phosphorylation of “epidermal growth factor receptor (EGFR).” Both the “A549 and the HCC827 human NSCLC models” show considerable tumor growth inhibition when treated with a dual-responsive release of the drugs “bevacizumab and gefitinib.”(Zhao et al. 2023) In another work, Asadollahi et al. (2022) created nanostructured lipid carriers (NLCs) in order to assess the synergistic anticancer effects of co-delivering “erlotinib (ELT) and resveratrol (RES),” a naturally occurring phenol derived from herbs, on NSCLC (Asadollahi et al. 2022). In the subsections, they are briefly discussed. The targeting strategy of nanoparticles toward NSCLC is explained in Fig. 4.
Liposomes
When natural or synthesized amphiphilic lipids are spread in water, they spontaneously form vesicles with a bilayer shape known as liposomes. Because of their biocompatibility and advantageous safety profile, they have been extensively researched as drug delivery vehicles ever since they were created (Ashique et al. 2021). Phosphatidylcholine, cholesterol, and other substances make up the bilayer structure of liposomes, which are known to transport a variety of hydrophobic and hydrophilic big and small molecules for therapeutic purposes. Polyethylene glycol (PEG) can be grafted onto their surface to change it, lengthening their half-life in circulation (Moghimi and Szebeni 2003). In 1995 and 1999, respectively, the FDA approved Doxil and Myocet, two of the most popular doxorubicin-based liposomes (Barenholz 2012). Even though there are already sixteen liposomal medications on the market, relatively few formulations are approved as a type of treatment for NSCLC. Here, our group enlisted current instances of liposomal formulations used for therapeutic drug transport to the lungs. In 2014, Cheng et al. used the new peptide GE11’s EGFR binding affinity to study the liposomes’ size distribution (Cheng et al. 2014), and it was discovered that 10% GE11 density in A549 cytotoxicity was ideal. Cellular uptake tests also confirmed the clathrin-mediated endocytosis pathway’s significant role. A dual-ligand-modified triptolide-loaded liposome (dl-TPL-lip) was created, for efficient triptolide (TPL) delivery to NSCLC via pulmonary injection. An apoptosis assay was used to gauge the ability to destroy cells (Lin et al. 2018). Importantly, employing 3D tumor spheroids, the liposomes’ better tumor penetration along with tumor growth suppression efficacy was confirmed. For the treatment of NSCLC, Song et al. (2017) developed a multifunctional targeting liposome in 2017 and got superior in vivo results. Octreotide (OCT) was used to decorate the liposome surface (Song et al. 2017). Honokiol, which reduces tumor metastasis and inhibits the creation of vasculogenic mimicking channels, and epirubicin, an anticancer medication, were both co-encapsulated in the liposome. These liposomes were found to be able to downregulate PI3K, MMP-2, and MMP-9, while activating caspase 3. Researchers from all across the world are paying close attention to the pulmonary medication delivery technique, or local distribution via inhalation, as a viable option. This necessitates the use of treatments with low doses of low toxicity. In order to determine the effectiveness and side effects of giving patients with lung metastases aerosolized interleukin (IL)-2 liposomes. The liposome-aerosol was inhaled three times daily for around 20 min. On the basis of earlier research, the dose was selected. Three cohorts of three patients each received different doses of IL-2 three times per day for a total of nine individuals. According to researchers, inhaling IL-2 liposomes is safe and causes less systemic damage (Skubitz and Anderson 2000). Aerosolized liposomal cisplatin in patients with lung cancer showed that cisplatin was well tolerated (Wittgen et al. 2007). Lowery et al. designed doxorubicin encapsulated liposomal complex engrafted with peptide molecules for targeted delivery of drug into the malignancies site (Lowery et al. 2011). After being labeled with Alexa Fluor 750, tagged liposomes were used to follow the biodistribution in a murine Lewis lung cancer model. Liposomes are often employed in lung cancer theranostics because of their exceptional biocompatibility and biodegradability (Sercombe et al. 2015). Additionally, liposomes have an advantage over other nanoparticles since they can easily be managed for sustained drug administration and are effective for loading a large number of therapeutic compounds (Bolhassani et al. 2014). Nanoliposomes often experienced certain drawbacks, such as reduced stability and high construction charges (Maja et al. 2020). IV drug delivery of liposome, having positively charged surface, has also been shown to elevate liver enzymes and pro-inflammatory cytokines in healthy C57BL/6 mice with efficient therapeutic activity (Kedmi et al. 2010).
Polymeric nanoparticles
For the therapy of NSCLC, extensive research has been done on polymeric nanoparticles using polymers for instance, polycaprolactone (PCL), polylactic acid (PLA), and chitosan (Esim et al. 2020). In addition to being widely available, the properties of polymeric nanoparticles can be controlled for steady as well as prolonged release, facile surface functionalization, simple nano-sizing, freely available cellular internalization, and the ability to enclose a wide range of active compounds (including drugs, peptides, and oligonucleotides) (Senapati et al. 2018). They are also more stable in storage than lipid-based formulations. Despite this fact, the complicated processing approach may make large-scale manufacturing difficult. Nevertheless, numerous in vitro (lab-based experimental setup) and in vivo (experiment based on small animals) investigations have demonstrated the non-toxicity of the PLGA nanoparticulate system, making it excellent polymeric material for the therapy of NSCLC (Semete et al. 2010). The main drawbacks of the drug delivery procedure, such as the harmful consequences of anti-neoplastic therapy, have been addressed using polymeric NPs (Singh and Nalwa 2007). They demonstrate an increase in the effectiveness of chemotherapeutic and targeted treatments by using polymeric NP to encapsulate hydrophobic pharmaceuticals at large concentrations, extending circulation duration, and improving transportation to the targeted region (Allouche 2013). The FDA has authorized “Abraxane®, an albumin-based nanocarrier” filled with paclitaxel for the management of NSCLC and advanced breast cancer (Ma and Mumper 2013). The use of “PEG-modified NPs” that contain taxanes has been shown to increase the effectiveness of combination chem-radiation treatment for non-small cell lung cancer (Jung et al. 2012). Hu et al. presented the effectiveness of “polycaprolactone nanoparticles” loaded with paclitaxel in conjunction with “chronomodulated chemotherapy” in the year 2017, (Hu et al. 2017) and they also suggested a possible role for “circadian rhythms” in tumor progression. Most significantly, Wang et al. revealed intercellular translocation of NPs to cancer cell with in vivo suppressed primary cancer progression using mesenchymal stem cells as a carrier to increase delivery of drug (paclitaxel) from NPs (Wang et al. 2019). “Crizotinib (for EML4-ALK fusion–positive lung cancer) and polylactic tocopheryl PEG 1000 succinate (PLA-TPGS)” were combined in a formulation by Jiang et al. in 2015 that demonstrated sustained release, caused cytotoxic effect in “NCIH3122 lung cancer cells,” and detectable both early and late apoptosis (Jiang et al. 2015). In the interest of overcoming resistance mechanism, erlotinib was loaded onto PLGA. This method has shown improved loading capacity, increased entrapment, and prolonged release (Vaidya et al. 2019). Afatinib and paclitaxel were placed into PLGA inhaled microspheres in another trial. These NPs shown great biological compatibility, prolonged release, and maintained higher concentration toward lung cells than other cell/tissue. These targeted NPs are a suitable therapy for resistant lung cancer because of all these benefits (Yang et al. 2019a). Lipid-based polymeric micelles are a different class of nanoparticles; they are distinguished by an architecture that consists of a hydrophobic core with the ability to transport medicines and a hydrophilic PEG-shell. In South Korea and various European nations, “Genexol-PM,” a micelle formulation of “PLGA-b-methoxy-PEG nanocarrier” carrying paclitaxel, has been authorized for use in the treatment of cancer (Kim et al. 2007). Positive anti-neoplastic outcomes were shown in a phase II study with “Genexol-PM combined with gemcitabine” in those suffering from advanced NSCLC; however, frequent level III–IV toxicities were seen (Ahn et al. 2014). When added to “PEG polymeric nanoparticles,” cisplatin has improved anticancer activity toward cancer cells in vitro (Li 2018). In the treatment of NSCLC, co-encapsulated micelles encapsulating “itraconazole and paclitaxel” have shown reduced cytotoxicity (Zhang et al. 2018). Using modified micelles coated with “a-conotoxin,” it is also able to deliver additional medications, such as “docetaxel,” to the A549 NSCLC cell line (Mei et al. 2018). A new “polyurethane micelle” with promise for MRI diagnostic and chemotherapeutic treatment was created by Ding and his group (Ding et al. 2014). As a potential remedy for drug resistance, the utilization of NPs in the distribution of anticancer drugs has also been investigated. In order to create biocompatible NPs that were extremely efficient in resistant A549 cells and might suppress the production of certain multidrug-resistance proteins, galactoxyloglucan and paclitaxel were utilized (Reshma et al. 2019). Drugs containing polymeric NPs in the form of aerosols may lessen cytotoxic effects (Abdelaziz et al. 2018). Significant anticancer efficacy of cisplatin was reported in “A549 lung adenocarcinoma cells” after lung injection of “gelatin-based NPs.” (Elzoghby 2013) NPs of doxorubicin released from polyisobutyl cyanoacrylate used for the treatment of macrophages resulted in secondary cytotoxicity to lung cancer cells after 8 and 24 h (Al-Hallak et al. 2010). In a different investigation, pulmonary administration of the hyaluronan-cisplatin compound demonstrated enhanced lung drug concentration compared to intravenous cisplatin after 24 h, with reduced tissue/plasma ratio both in kidneys and the central nervous system, lowering dose-limiting toxicities (Xie et al. 2010). Amreddy et al. created and assessed an NP approach based on a “folic acid (FA)–coupled PAMAM-dendrimer-polyethyleneimine” platform for the simultaneous delivery of “cisplatin, siRNA, and human receptor-R” for the treatment of lung cancer and they revealed that composite treatment utilizing “FA receptor (FAR)”–targeted dendrimer nanoparticles demonstrated precision and specificity toward FAR–expressing cancer cells, improving the therapeutic effectiveness and also reducing the cytotoxic effects against healthy cells (Amreddy et al. 2018).
Nanostructured lipid carriers
Nanostructured lipid carriers are composed of emulsifier dispersion in aqueous medium and partially liquid and solidified solid lipids; nanoscale lipid particles are distributed (Makled et al. 2017). This loosely packed crystalline structure permits drug molecule entrapment, lowers drug leakage during storage, and permits regulated drug release (Iqbal et al. 2012). Additionally, it has been discovered that NLCs are distributed favorably in the organs, including the lungs, which may enhance cancer therapy other than lung cancer. The NLC system’s primary drawbacks are its limited drug loading capacity (Poonia et al. 2016).
NLCs are considered as potential vehicles for the fabrication of efficient individualized cancer chemotherapy therapies against NLC. These lipid-based nanoparticles that are biocompatible and/or biodegradable have a core matrix made of both solid and liquid lipids that is disseminated in a surfactant solution. Most hydrophobic cancer treatments are more soluble in water after NLC. Their surface modification can be employed to produce site-specific tailoring for enhanced efficiency as well as for decreased toxicities related to doses administered, which would help cancer treatment overcome drug resistance. The necessity of an early diagnosis in order to offer an appropriate prognosis and treatment options justifies the significance of a thorough lung cancer assessment. The first lung cancer categorization was offered at the beginning of the twentieth century, and it has been regularly updated to reflect both the rise in cases and the discovery of new subgroups of cell lung cancer (Travis et al. 2015). Many anticancer moieties have so far been effectively created as NLCs. The enhancement in the anticancer effectiveness of cytotoxic medications with fewer side effects has been demonstrated in preclinical research employing cell lines or animal models. Since they lack tumor selectivity characteristics, the majority of cytotoxic medicines have a limited therapeutic window that was previously indicated. The chosen dose is extremely close to the maximum tolerated dose, which makes it difficult to distribute the medication effectively. NLCs have recently demonstrated tremendous potential to target cancerous cells. Drug targeting that is passive or active can target tumor cells (Torchilin 2011). NLCs were recently designed to incorporate or connect multipurpose compounds including anticancer drugs, antibodies as well as ligands addressing MDR cancer cells, nucleic acids, or P-gp inhibitors to block different MDR contributors. The surface of MCF-7 cell line has overexpressed “breast cancer resistance protein (BCRP),” which promotes higher uptake of mitoxantrone hydrochloride (MTO) encapsulated dextran-conjugated NLCs than basic drug solution (Zhang et al. 2008, 2012). The exceptional drug uptake suggests that drug efflux, which is mediated by the breast cancer resistance protein (BCRP), has been inhibited. Docosahexaenoic acid (DHA)– and doxorubicin (DOX)–based NLCs with the resistant “MCF-7/ADR” cell line also demonstrated cytotoxicity at relatively low concentrations (16 m of doxorubicin and 112 m of DHA) (Mussi et al. 2014). Lipid nanoparticle carriers for bioimaging as well as for anticancer treatments that are loaded with camptothecin and quantum dots were developed and tested by Hsu et al (Hsu et al. 2013). Excellent fluorescence labelling of cancer cells was seen in in vivo real-time tumor monitoring using fluorescent imaging. In comparison to the free drug solution, the produced theranostic NLC showed increased cytotoxicity to the B16-F0 melanoma cell line and camptothecin accumulations that were 6.4 times higher. For the therapy of lung cancer, Patel et al. created DIM-CpPhC6H5 (DIM-P) and a tumor-homing PEGylated VEGF peptide (CREKA peptide) encapsulated theranostic NLC system (Patel et al. 2014). When compared to unconjugated NLCs, the developed system showed a threefold increase in attachment with protein molecules present in the tumor plasma site. Studies revealed that CREKA peptide-conjugated designed systems moved through tumor vasculature 40 times more than peptide-unconjugated NLCs. The NLC-based theranostic preparation of MgO NPs (manganese oxide nanoparticles) loaded with MMP2 cleavable peptide and cancer-targeting compound exhibited superior deposition inside the tumor and improved the MRI signaling (Patel et al. 2014; Savla et al. 2014). Remarkable antitumor effectiveness was seen in vitro with the BRAF (gene) inhibitor vemurafenib, which has been loaded into NLCs using Chinese hamster ovary cells (CHOK1), human lung (A549), ovarian (A2780), and melanoma (COLO 829) cancer cells, as well as in vivo using various cancer models. The development of well-organized means for delivering foreign genes, including siRNA or DNA, to tumor cells is a key component of gene therapy techniques. Recently, there has been a lot of interest in the potential of NLCs as a means of delivering genes (Xue and Wong 2011; Han et al. 2014a). The human lung cell line (A549 cells) in vitro cell viability study demonstrated more than 80% of cell viability against control. Shao and his colleague constructed paclitaxel (PTX)–loaded transferrin (Tf) NLCs (Shao et al. 2015). In vitro cytotoxicity study illustrated more than fourfold decline in the IC50 value especially in comparison with PTX solution in limiting the viability of carcinoma cells, taking into account the maximum antitumor activity. Furthermore, the system demonstrated high in vivo and in vitro gene transfection efficacy. Similar to this, Chen et al. developed multifunctional NLCs for simultaneous DNA and temozolomide (TMZ) delivery (Chen et al. 2016). In an in vitro cytotoxicity research using U87MG cells, the formulation showed over fourfold decrement in IC50 value compared to plain temozolomide, indicating that malignant glioma cells have the strongest anticancer effects. NLCs have several benefits as a potential delivery system for genetic material (i.e., DNA) with their increased transfection proficiency, such as lower cytotoxic nature along with higher gene loading efficiency (Han et al. 2016).
Solid lipid nanoparticles
Colloidal nanocarriers called solid lipid nanoparticles (SLNs) circumvent the problems associated with liposomes, emulsions, etc. (Naseri et al. 2015). The medication is either embedded within the solid core of SLNs or is positioned on the exterior portion of the solid lipid that makes up SLNs. They are less susceptible to enzyme breakdown than other nanostructured systems, can resist mild pressure (such as nebulization), and are biocompatible (Duan et al. 2010). Additionally, the technology does not need organic solvents, which makes it easier to pilot scale-up production and gives the enclosed chemotherapeutic drug improved protection (Mishra et al. 2018). Unfortunately, the SLNs share the problem of inadequate drug entrapment with other colloidal systems. SLNs are recently developed substitutes for conventional colloidal delivery systems. SLNs demonstrate substantial potential for drug localization and retention at the actionable site via both passive and active targeting owing to their unique abilities to encapsulate hydrophilic as well as hydrophobic payloads in conjunction with nucleic acids and proteins. For sustained and precise medication and gene delivery, SLNs promise new vistas. Polymeric nanoparticles and liposomes are combined in SLNs, which avoids both acute and long-term toxicity (Scioli Montoto et al. 2020; Chen et al. 2017b). The biocompatible lipids in SLNs, which the body and lungs find bearable, were thought to contribute to their superior safety profile (Dolatabadi et al. 2015). They are therefore highly advised for the delivery of pulmonary drugs, whether in the form of suspension or dry powder, without causing inflammation (Liu et al. 2018). Physiological solid lipids, which are solid at body and room temperatures, have a major contribution to the formation of SLNs. These lipids are stable in surfactant-incorporated water, which aid in blending the aqueous phase (external) with internal lipid phase and preventing particle accumulation (Weber et al. 2014). Scalable nanocarriers with a large surface area and tiny particle size are known as SLNs. By optimizing absorption as well as retention (EPR) based on aberrations in tumor cell behavior including extreme vascularization and inadequate lymph drainage, small particle size of SLNs enables passive targeting, which is critical for improving drug localization at the site of action (Shen et al. 2015). Additionally, the surface of SLNs might be easily customized and embellished by A.O. Elzoghby et al. (2017) (Elzoghby et al. 2017). Additionally, utilizing the proper targeting moiety, which encourages the receptor-mediated endocytosis of nanocarrier toward tumor cells via active targeting based on fusion with receptors overexpressed in the cell surface, the surface of SLNs might be easily tweaked and embellished (Toloza et al. 2006). In addition to proteins and nucleic acids, the solid-lipid nanostructure has the capacity to incorporate both lipophilic and hydrophilic drugs. The entrapment efficiency depends on a number of factors, including the drug’s solubility in lipid, polymorphous state of lipid, lipid-matrix physico-chemical structure, and its miscibility with lipid (Gaber et al. 2017). SLNs are considered a good option for lipophilic drugs for improving the aqueous solubility and bioavailability and ultimately maximize the therapeutic efficacy like chemotherapeutic agents as etoposide, doxorubicin, and paclitaxel. Decoration of targeting ligands on the surface of SLNs by using PEG is also a facile approach preferentially accumulation at the site of action (Yu et al. 2010; Sahu et al. 2015). PEGylation, on the other hand, consequently increases the ability of SLNs to penetrate and accumulate at the tumor site. Cationic SLNs are one of the types that may be considered for enhancing tumor permeability and enhancing therapeutic effectiveness (Elzoghby et al. 2016). In comparison to bigger particles, SLNs offer superior internalization and higher transfection because of their smaller particle size (below 100 nm). Additionally, negatively charged DNA interacts electrostatically with positively charged SLNs to form lipoplex, which enhances cellular uptake. In several lung cancer cell lines, the wildtype p53 gene plays a critical role in tumor suppression (Choi et al. 2008). Unfortunately, p53 mutations are linked to more than 50% of NSCLCs. In order to defeat lung cancer, it is essential to reintroduce or overexpress the p53 gene in mutant cells. Adenovirus vectors that express p53 were successfully transduced into NSCLC patients by Roth et al. Phase I/II of a clinical trial saw the introduction of this tactic with success. These results corroborated other studies that demonstrated the efficacy and safety of transfecting p53 genes to inhibit the spread of cancer (Choi et al. 2008). Utilizing the melt homogenization approach, a brand-new, stable cationic SLN formulation was created to effectively transport p53 to NSCLC mutant cells. Tricaprin (TC) was the core forming lipid bilayer in this study, with cationic lipid “3b [N-(N0, N0-dimethylaminoethane)carbamoyl] cholesterol (DC-chol),” helper lipid “dioleoylphosphatidylethanolamine (DOPE),” and surfactant “Tween-80” being used. Overexpression of typical oncogenes such as “signal transducer and activator of transcription-3” which operate as apoptosis inhibitors caused multidrug-resistance (MDR) STAT3. Overexpression of STAT3 in NSCLC was a sign of poor prognosis. To inhibit the STAT3 and enhance the effect of anticancer drugs, numerous strategies are used, such as use of chemotherapeutic moiety, STAT3 inhibitors, and siRNA (small interfering RNAs). MDR can be defeated by STAT3 suppression at the mRNA level (RNA interference, RNAi) (Kotmakçı et al. 2017). However, since nucleic acids are susceptible to nuclease enzyme breakdown, direct administration of nucleic acids is not practical. A cationic SLN loaded with “plasmid encoding antiSTAT3-short hairpin RNA (STAT3-shRNA)” was synthesized. MicroRNAs (miRNAs) are small (18e24 nucleotide) endogenous noncoding RNAs that operate post-transcriptionally. As an oncogene, microRNA21 (miR-21) is a well-known miRNA that has been linked to tumor metastasis in lung cancer. When overexpressed, miR-21 inhibits apoptosis and promotes proliferation. The “anti-miRNA oligonucleotide (AMO)” has enormous potential for preventing cancer (Shi et al. 2012). One promising method for overcoming MDR and improving therapeutic outcomes in lung cancer patients is to combine chemotherapy and gene (nucleic acid) delivery. Targeting drugs directly to the lungs, whether for localized or systemic treatment, is now possible thanks to pulmonary drug delivery (Elzoghby et al. 2015). Effective pharmacokinetic features, capability of solute exchange, high bioavailability, thin alveolar epithelium, large surface area, and a strong inclination to avoid the first-pass effect are all the facts of this strategy based on the special characteristics of lungs (Uchenna Agu et al. 2001). The biocompatible lipids that are extremely well tolerated by the body and lungs form the foundation of the SLNs safety profile. Proteins and nucleic acids can be included into SLNs together with hydrophilic and lipophilic medicines, opening up new possibilities for medication and gene delivery.
Dendrimers
Dendrimers are polymeric nanoparticles that were initially discovered at the end of the 1970s (Tomalia and Fréchet 2002). Dendrimers are synthesized nano-constructions having three-dimensional architectures that are repeatedly branched and radially symmetrical. The surface of the dendrimers is made up of terminal chemical structures that are covalently bonded to a core of highly repetitive units (Sandoval-Yañez and Castro Rodriguez 2020). Dendrimers are adaptable polymers because of their known molecular weight, nanoscale, and monodisperse nature, as well as their aptitude for encapsulating both hydrophilic and hydrophobic chemotherapeutic drugs (Parajapati et al. 2016). Additionally, their multipurpose surface makes surface modification for targeted distribution simple. Such a smart drug delivery system has the potential to release the drug at a specified site after coming into contact with specific enzymes with the outer functional groups, the medication can also be delivered in a regulated manner in which mAbs and RGD peptide like targeting molecules are employed, and including Doxorubicin (DOX) and paclitaxel (PTX) like hydrophobic medications is commonly used (Hu and Zhang 2012; Mattheolabakis et al. 2012; Lim and Simanek 2012). Drug delivery can also be accomplished using dendrimers that self-assembled into micelles, such as poly(glutamic acid)-b-poly(phenylalanine) copolymers (Webster et al. 2013). Amphiphilic diblock copolymers forming micelles are being used in numerous clinical trials to deliver paclitaxel for the treatment of advanced pancreatic cancer, non-small cell lung cancer, and breast cancer. Human breast adenocarcinoma (MCF-7), colorectal adenocarcinoma (HT-29), non-small-cell lung carcinoma (NCI-H460), and glioblastoma (SF-268) were utilized to investigate the antitumor proficiency of the dendrimer loaded with camptothecin (Morgan et al. 2006). A study demonstrated the usage of dendrimer-targeting peptide conjugates for NSCLC (Liu et al. 2011). These dendrimer-peptide conjugates were successfully absorbed by the tumor cells when administered to a mouse model with a lung tumor, suggesting their promise as a therapeutic vehicle for the targeting and management of tumor. In a separate investigation, a newly developed “PEGylated dendrimer nanoparticle” showed potential as DDS based on aerosol mechanism (Ryan et al. 2013). While larger dendrimer particles are said to be trapped in the lung for a long time, smaller dendrimer particles are said to enter the bloodstream through inhalation. In the future, injectable medication delivery methods could be replaced by this approach of controlled release drug delivery system (CRDDS) to the lungs.
Metal-based nanoparticle
As drug delivery techniques in the treatment of NSCLC, various kinds of metal-based nanoparticles, including quantum dot, carbon nanoparticles, gold, and silver nanostructures, have been studied. The acceptable biocompatibility of metal-based nanoparticles and their simplicity in size and surface modification are the main causes of the exponential expansion in this field of research. They are useful for intracellular tracking due to their visible light extinction capabilities (Oerlemans et al. 2012). Due to its greater ability to load drugs due to pi-pi stacking between graphene sheets, in graphene, a monolayer made up of carbon atoms organized in the form of a honeycomb-like hexagonal lattice structure is now receiving a lot of attention (SreeHarsha et al. 2019). To make the most of graphene’s use in drug delivery systems, a thorough understanding of its physico-chemical properties is still lacking (Yang et al. 2019b). A thorough analysis of the use of graphene as a versatile platform for the delivery of drug molecules was presented by Hoseini-Ghahfarokhi et al (Hoseini-Ghahfarokhi et al. 2020).
Strategy and research investigation of metallic nanocarrier, such as silver and gold, aimed toward biomedical and biopharmaceutical characteristics have advanced significantly in recent years. The noble metal gold nanoparticles stand out among these because of their surface-plasmon resonance-enhanced optical capabilities, which have been used recently in biomedical applications with a focus on the detection and treatment of cancer, particularly lung cancer (Huang et al. 2007). Gold nanoparticles have recently been tried with success as sensing agent/diagnostics for identifying and categorizing pathophysiology of various lung cancers. The sensor could differentiate between malignant and healthy cells, SCLC and NSCLC, and two subtypes of LCs (Barash et al. 2012). In a study on Lewis lung cancer animal model, methotrexate (MTX) linked gold nanoparticle, a medication with excellent hydrophilic nature and minimal tumor retention, demonstrated substantial tumor retention and improved therapeutic efficacy (Chen et al. 2007). Numerous studies have been conducted on magnetic nanoparticles, and they have been used in the detection and therapy of numerous malignancies. The delivery of therapeutic drugs and imaging are both made easier by theranostic nanoparticles. A noninvasive treatment method for lung cancer called magnetic hyperthermia involves the heat-induced ablation of desired tumor tissue. Superparamagnetic iron oxide (SPIO) nanoparticles, for example, are magnetic materials that, when exposed to alternating currents, produce sublethal heat that damages local tissue. One study used a mouse model of NSCLC and found that the tumor-targeted SPIO nanoparticles were very effective at inhibiting tumor development and destroying tumors through hyperthermia (Sadhukha et al. 2013a). Because of their variable drug encapsulation potential, controlled release of drugs characteristic, and versatility, “mesoporous silica nanoparticles (MSNs)” have become more frequently utilized as a novel carrier for the delivery of chemotherapeutic agents. Mesoporous silica nanoparticles (MSNs) were the subject of the first study on their viability in vivo, which was released by the Mou group in 2008 (Wu et al. 2008). For animal magnetic resonance imaging studies and intracellular labelling, multifunctional mesoporous silica nanoparticles have been employed. MSNs are largely internalized by human lung cancer cells via endocytosis (Sun et al. 2008). The development of a cancer-targeted mesoporous silica nanoparticle–associated DDS was created for the inhalation-based treatment of lung cancer. The technology was capable of delivering chemotherapeutic medications (cisplatin and doxorubicin) combined with two categories of siRNA tailored to “MRP1 and BCL2 mRNA” in order to successfully reduce pump and non-pump cellular resistance in NSLC (Taratula et al. 2011). Mesoporous silica nanoparticles were capable of targeting tumor cells because of “LHRH peptide” being conjugated to its surface employing a poly(ethylene glycol) (PEG) as linker.
Silver nanoparticles
Fluorescence imaging, biosensors, anticancer applications, and other biological uses of silver nanoparticles have all been around for a while (Mukherjee et al. 2014). He et al. recently showed that biosynthesized silver nanoparticles (AgNPs) have anticancer potential (He et al. 2016). By using the trypan blue and MTT assays, the AgNPs showed a powerful cytotoxic effect. Mechanistic investigations revealed that AgNPs led to apoptosis in lung cancer cells. AgNPs’ cytotoxicity is substantially influenced by their morphology, surface chemistry, size, and shape (Stoehr et al. 2011). Jeong and his group of researchers investigated the fundamental phenomenon of hypoxia on silver nanoparticle–induced apoptosis and found that HIF-1 expression was upregulated in both normoxic and hypoxic circumstances. Additionally, whereas normal cells did not experience programmed cell death from the AgNP treatment, lung cancer cells did. Significantly, HIF-1 prevented the AgNP-induced apoptotic phenomenon by regulating the autophagosome flux via the LC3-II, p62, and ATG5. The results of the research suggest that HIF-1 could be a potential candidate for lung cancer treatment by using hypoxia-mediated autophagy to prevent AgNP-mediated apoptosis (Jeong et al. 2016).
Iron oxide nanoparticles
In addition to their widespread usage as MRI contrast agents, super-magnetic iron oxide nanocarriers may be employed as a delivery mechanism in cancer theranostics applications. Iron oxide nanoparticles have a wide range of medical applications, including long-standing usage in cancer theranostics MRI imaging, magnetic hyperthermia, and medication delivery, in lung cancer (Noh et al. 2009). Wang et al. (2017) have published a study on iron oxide nanoparticle–based targeted ultrasonic ablation therapy for lung cancer (Wang et al. 2017). In a different work, inhalable Fe2O3NPs that are targeted at the EGFR have been shown by Sadhuka et al. to cause hyperthermia associated with magnetic field in lung cancer (Sadhukha et al. 2013b). The researchers demonstrated that EGFR targeting improved iron oxide nanoparticles’ tumor retention. Additionally, EGFR-targeted iron oxide, a nanoparticle, was demonstrated in in vivo magnetic hyperthermia treatment in in vivo orthotopic lung cancer models by resulting in significant suppression of growth in lung cancer.
Other metal nanoparticles
For theranostics applications in lung cancer, silica, nano-diamond, and rare earth were utilized, among other nanoparticles (Sadhukha et al. 2013b). In vitro and in vivo lung cancer imaging using near-infrared tumor targeting and p53 gene therapy was demonstrated by Wu et al. (2015) using a silica-polymer nanocomposite (Wu et al. 2015). When treating lung cancer, paclitaxel was also delivered via a nano-diamond (ND). In a lung cancer cell model, this nanodrug delivery significantly reduced tumor size in immunodeficient animals. According to mechanistic investigations, ND triggered mitotic arrest and apoptosis, which killed lung cancer cells. In a different experiment, Chen et al. showed that Nd2O3 nanoparticles at micromolar concentrations can significantly vacuolate NSCLC cells and trigger a lot of autophagy (Chen et al. 2005). In order to deliver several siRNAs (EGFR and cyclin B1) to lung cancer cells and for bioimaging, Wu et al. (2016) synthesized quantum dot–based carbon nanomaterial with multifunctional moiety attachment on the surface for the establishment of a potential nano-agent (Wu et al. 2016). When activated at 360 nm, this agent creates a visible blue photoluminescence that may be used for bioimaging. Additionally, it was found that this nanodrug preferentially aggregated in cells of lung cancer via endocytosis mediated by the receptor, elevating silencing of gene as well as its anticancer efficacy.
Carbon nanotubes
Despite numerous attempts by professional teams treating lung cancer, a lot of people continue to pass away every year as a result of this. The prevalence of lung cancer is widely acknowledged as being among the most pervasive malignancies on a global level. Nanotherapy is a novel therapeutic approach that is currently under investigation by experts for the management of non-small cell lung cancer (NSCLC). The disease can be effectively treated by carbon nanotubes (CNTs) themselves by activating the apoptotic pathway by concentrating on the organelle of mitochondria in cancer cells. Accordingly, CNTs coupled to polyethylene glycol may be able to target cancer cell repertoires more effectively, thereby enhancing the effectiveness of nanodrug delivery (Kim et al. 2017). In addition to graphene oxide, the anticancer medication paclitaxel also formed bonds with SWCNTs. This nanostructure improved the efficiency and caused the death of cancer cells A549 and NCI-H460 (Arya et al. 2013). SWCNT modified with chitosan was the subject of a different investigation to deliver this medication and increase in vivo compatibility. Hyaluronic acid has also been added to the layer of chitosan to specifically target A549 cells (Yu et al. 2016). Because of the enhanced distribution along with the magnetic localization, doxorubicin delivery by SWCNTs can improve targeting and boost therapeutic effectiveness. The results of boosting the effectiveness of therapy by using MRI technology were in fact validated by this experiment, which was carried out on mice (Al Faraj et al. 2016). In a 2018 study, curcumin was examined in a nano-state using a SWCNT carrier, a substance created with therapeutic promise for A549 cancer cells. The drug’s effectiveness was improved by the carrier, which worked with the polysaccharides in chitosan and alginate (Singh et al. 2018). Gemcitabine is one anticancer drug for non-small cell lung cancer. The medication was evaluated employing a SWCNT carrier in a clinical experiment on B6 mice. The A549 cell line, which displays intriguing suppression results, was the subject of this investigation. Because of the substantial loading propensity of the medicine, the extended time of distribution, and the remarkable permeability of the cell membrane, this study has clearly validated SWCNTs as stimulating carriers for the administration of medications (Razzazan et al. 2016). To reduce drug waste and use it in the intended manner, methotrexate may be conjugated with MWCNTs for the treatment of lung cancer. Animal experiments also showed that this compound had no negative effects on the heart, liver, or kidneys (Das et al. 2013). The anticancer activity of the betulinic acid–loaded MWCNTs with acid functionality can be measured using thermogravimetric and UV light. Actually, certain medication concentrations made lung cancer cells more susceptible (Tan et al. 2014).
Biological system–based nanoparticles for lung cancer theranostics
The incorporation of a bio-mimicking constituent into medicinal nanostructures emerged as the primary topic of recent investigation. This is because of the excellent biodegradability, stability, and biocompatibility of bio-based nanoparticles, which include apoferritin, aptamers, viral-based nanoparticles, SLNs, polymeric nanoparticles, and protein nanoparticles (Sivarajakumar et al. 2018). These kinds of nanoparticles have been successfully created, manufactured, and applied for cancer theranostics purposes in NSCLC (Rizvi et al. 2008).
Viral nanoparticles
Investigators are intrigued by viral nanoparticles (VNPs) produced by viruses or bacteriophages for a variety of biological functions, such as drug delivery, biomedical imaging, biosensing, and vaccine production. This is attributable to the viral nanomaterials’ biocompatibility, adaptability in shapes and sizes, and convenience of surface alteration (Li et al. 2020a). In an effort to combat the challenges posed by drug tolerance, a number of investigators have developed a therapeutic strategy for lung cancer that combines chemotherapy and immunotherapy.
Protein-based nanoparticles
Due to their exceptional biomedical applications, lack of inflammatory response in human pulmonary cell lines, and enhanced cellular uptake, protein nanomaterials having natural polypeptides like legumin, gliadin, albumin, and gelatin have been recently utilized in the delivery of therapeutic agents whether alone or in combination form with biodegradable polymers in the management of lung cancer (Lohcharoenkal et al. 2014). Employing “cationic bovine serum albumin (CBSA),” siRNA has been administered for the management of metastatic lung cancer (Han et al. 2014b).
Apoferritin
Apoferritin is a “ferritin-based multifunctional nanocarrier” utilized to demonstrate the detection of lung cancer in A549 cells by utilizing both fluorescence and MR imaging (Dostalova et al. 2017; Li et al. 2012). This multipurpose apoferritin was employed to image cancer cells with increased αvβ3 integrin. Another study done by Luo and his researcher group showed the utilization of daunomycin encapsulated apoferritin nanocages coupled to hyaluronic acid (HA) employed for intracellular distribution and liberation of anticancer drug daunomycin based on a pH-responsive system (Luo et al. 2015).
Treatment and diagnosis of lung cancer: stimuli-responsive drug delivery systems
Light-responsive nanocarriers
Light has indeed frequently been utilized for remote drug regulation because of its generally high level of safety and non-intrusive nature (Li et al. 2020b). For on-demand medication release, photolabile groups can be directly destroyed by short-wavelength light, like visible light or UV. Their inability to penetrate deeply, however, limits their potential for use in biomedicine. Since near-infrared (NIR) light (780–2500 nm) may permeate the tissues more deeply than short-wavelength light, it is necessary for the release of medication in a controlled manner inside the biological system. When activated by light, some nanoparticles can significantly enhance local temperatures or activate/stimulate “reactive oxygen species (ROS),” which might be used to treat malignancies. In addition, external light sources also cause the therapeutic system’s structural changes and the quick dissolution of photolabile groups (Ko et al. 2019). These two methods of therapy are referred to as photothermal therapy (PTT) and photodynamic therapy (PDT). For particular, employing oxygen-boosted immunogenic PDT, researchers created core–shell gold nanocage@manganese dioxide (AuNC@MnO2) nanoparticles to concurrently eradicate intrinsic triple-negative breast cancer and thereby preventing metastasis of the lung (Liang et al. 2018) and to enhance the antitumor effectiveness while reducing adverse effects by combining various therapeutic modalities (such as PTT, PDT, immunotherapy, and chemotherapy). In the interim, the ROS as well as the heat effects produced by PTT or PDT may possibly cause the drug to be released. Drug release induced by light can potentially be paired with other stimuli, such as pH, enzyme, and glutathione on the inside and other external stimuli (like radio frequency) on the outside, for improved targeted therapy for lung cancer (Li et al. 2021; Gou et al. 2020; Liu et al. 2019; Pan et al. 2019). Light-responsive therapies, as previously mentioned, have a variety of advantages and show tremendous promise for the therapeutic treatment of lung cancer. Furthermore, during the course of the treatment, light-absorbing molecules that produce temperature or increase “ROS” can emit fluorescence or convert the temperature into something like a photoacoustic (PA) signal. These compounds are used in imaging diagnosis.181
pH-responsive nanocarriers
Tumor cells have a significantly more acidic environment (pH 5.7–6.9) due to the presence of lactic acid and perhaps by some end products formed by lung carcinoma cells that is linked to an unusual accelerated metabolism as well as the proliferation in contrast to normal physiological tissues (Kanamala et al. 2016). Besides increasing the accrual of nanocarrier in tumor cells and strengthening the consistency and protection of therapeutic strategies for the treatment of lung cancer, the release of drug/chemotherapeutics can be controlled inside the tumor microenvironment or even can minimize the drug release to the tumor by employing pH-sensitive nanoparticles. Lee and team discovered that an acidic pH could effectively activate cholesteryl hemisuccinate (CHEMS)–based liposomes, indicating that pH-sensitive nanostructures loaded with anticancer drugs have exceptional anticancer activity against NSCLC. The poor outlook of NSCLC patients was attributed to the folate receptor beta (FR) that was commonly highly expressed in M2 tumor-associated macrophages (TAMs) as well as NSCLC cells (Park et al. 2021). To make pH-sensitive liposomes for regulated drug release, CHEMS (which is unstable in acidic conditions) was coupled to PEG-folate. A mixed micellar system including poly-benzyl-glutamate and d-tocopherol polyethylene glycol succinate may be able to modify the secondary poly-benzyl-glutamate complexes by regulating the release of DOX. The DOX encapsulated conjugated/mixed micelles showed exceptional therapeutic effects against lung cancer when used on naked mice injected with human lung cancer A549 cells (Shih et al. 2020). For the management of lung carcinoma, the acidic pH of the tumor microenvironment could also cause charge reversal to encourage intracellular uptake and nuclear translocation. Despite indiscriminate adsorption, nanocomposites having positive exterior potential often have shorter circulation of blood half-lives. This issue was rectified by using TAT peptide by increasing tumor cells’ absorption of the drug. Anhydride (DA) groups can hide TAT’s positive charges. After the aggregation of carriers in the tumor’s acidic medium, the charge was flipped from negative to positive, restoring TAT’s targeting potential. To combat lung tumors, Jing et al. created a “DA-TAT” vehicle for pH-responsive nuclear targeting and cellular uptake (Jing et al. 2018). Shi et al. constructed pH-triggered nanocomposite utilizing a tri-block copolymer consisting of “poly(sulfadimethoxine), methoxy poly (ethylene glycol)-poly(histidine), and poly(histidine) as mPEG-PHis-PSD.” PSD’s charge was immediately changed from negative to neutral by an acidic pH, which caused it to quickly dissociate from the lipid core. This made it possible for NSCLC medication to effectively internalize, accumulate specifically in tumors, and have an anticancer effect (Shi et al. 2018).
Enzyme-responsive nanocarriers
In order to sustain an organism’s regular processes, such as development, growth, disease, metabolism, immunity, and aging, enzymes are crucial biomolecules. Abnormal enzymatic expression was commonly observed in several illnesses’ cellular microenvironments, particularly in lung cancer (Chen et al. 2018; Sharma et al. 2018). Hyaluronidase (HAase), matrix metalloproteinases (MMPs), esterase, quinone oxidoreductase, and NADPH are the most commonly overexpressed enzymes (NQO1). Due to the selectivity, efficacy, and speed of enzymatic responses in treating lung cancer, enzyme-responsive nanoparticles have received a lot of interest (Shahriari et al. 2019; Wang et al. 2018). For instance, the MMP family of proteolytic zinc-dependent secreted endopeptidases may precisely break down a range of extracellular matrix components (ECMs). The introduction of drug delivery based on enzyme-responsive devices may take advantage of abnormally high MMP expression in lung carcinoma tissues (Egeblad and Werb 2002). For targeted lung cancer therapy, MMP-responsive peptides or gelatin is typically adhered to the surfaces of nanoparticles (Fan et al. 2017). This is predicated on the notion that collagen as well as basement membrane can be preferentially broken down by MMP-2 and MMP-9. Gianneschi constructed an MMP-9-associated nanostructure for the purpose of delivering the “immunotherapeutic small molecule (1V209)” for stimulation of the immune system. The previously stated platform improved medication efflux and prevented lung tumor spread in vivo (Li et al. 2019). Additionally, gelatin can act as the carrier skeleton and serve as a substrate for MMPs. As the extensive treatment approach of lung tumors, the complexed gelatin and cisplatin create an intelligent inhalable nanocarrier. When subjected to physiological solutions, cisplatin was swapped with chloride ions after gelatin was broken down, which caused the drug to be quickly released at the tumor location (Vaghasiya et al. 2021). To enhance the efficiency of treatment for lung cancer, HAase is typically coupled with multi-responsive nanoparticles. The hydrophilic shell was built using hyaluronic acid (HA), and hydrophobic chemicals could be added to the HA structure via a conjugation mechanism. In contrast, by employing pH-sensitive hydrazone couplings, Tang and coworkers developed hyaluronic acid (HA) NPs that are both pH and enzymatic responsive (Ren et al. 2019). He and colleagues created HPGBCA nanostructure for the delivery of “afatinib” via carrier-mediated targeting mechanism for the management of NSCLC therapy. Another excellent option is an esterase-sensitive nanocarrier which has been employed to deliver chemotherapeutics toward lung cancer cells based on enzyme-responsive phenomena. For the treatment of lung cancer, Cho and colleagues created the HAPBA nanoparticle, which would release medications in an esterase-rich environment within the tumor tissue. HA was conjugated 4-phenylbutyric acid (PBA) via ester linkage to regulate and control the rapid liberation of PBA-curcumin. PBA served as both the hydrophobic section and an effective histone deacetylase inhibitor in the formation of these nanoparticles (HDAC). When ester bonds were broken, curcumin and PBA were released quickly, effectively slowing down the growth of lung adenocarcinomas (Lee et al. 2019). A curcumin-gold nanorod coupled nanostructure was created by using an “esterase-labile ester link” demonstrated by Ren et al. This compound displayed a curcumin release that was both quick and consistent (Ren et al. 2019). In the absence of esterase, the amount of loaded therapeutic agent was fully constrained within the nanomaterials. Curcumin was released suddenly as esterase concentration increased, indicating that ester hydrolysis was a crucial catalyst for drug release. As a result, the nanorod-associated curcumin-coupled nanocarrier inhibitory potential on human lung cancer cells (A549) was enhanced by the introduction of the ester linkage (Zhu et al. 2018).
Vaccine-based therapy for NSCLC
Historically, the word “vaccine” has been associated with the medical practice of treating infectious disorders by the induction of humoral immunity toward infectious agents. In the process of developing therapeutic vaccines, several forms of vaccinations have acted as a source of motivation (Banchereau and Palucka 2018). The former are intended to cure a condition by enhancing the cellular and humoral responses of the immune system, particularly those of T cells. The discovery of mutant proteins that are undesirably produced by cancer cells gave birth to the idea of developing a vaccination for cancers. These are recognized by the immune function as “tumor-associated antigens (TAA),” and they are able to be separated into two categories: expressed fetal antigens, which are ordinarily lacking in healthy adults, and overexpressed normal proteins (Cuppens and Vansteenkiste 2014; Cortés‐Jofré et al. 2019). The basic operating concept of medicated vaccinations is that they function by instructing the immune system to detect and react appropriately to certain antigens. Numerous vaccination approaches, such as whole-cell (Xia et al. 2016; Ward et al. 2002) peptide/protein-based (Wada et al. 2017) and mRNA-associated (Sebastian et al. 2011, 2014) vaccines have been investigated as potential treatments for non-small cell lung cancer (NSCLC) (Table 1). In this essay, we will concentrate on mRNA, peptide, and protein, peptide since there have been some noteworthy breakthroughs surrounding the encapsulation of these types of vaccines utilizing nanoparticles.
Nanoparticle-associated ongoing clinical trials for management of NSCLC
The therapeutic effects of nanoparticles are mostly determined by their function, which is also highly determined by their shape and materials (Shi et al. 2017b). Additionally, by changing these properties, it is feasible to employ them as contrast agents for CT/PET imaging methods, consequently establishing multifunctional theranostic platforms (Li et al. 2016b). The researchers were capable of identifying possible targets for repurposing depending on the mode of action by employing different repurposing methodologies. Nevertheless, there were only a few medications that made it to the level of clinical trials. Glucocorticoids are the most prevalent class of pharmaceuticals in clinical studies exploring prospective therapies for repurposing in the management of non-small cell lung cancer. The recent/ongoing clinical investigation for the treatment of NSCLC based on nanoparticle system is mentioned in Table 2.
Conclusions and future perspectives
In conclusion, fresh applications for usage in cancer diagnosis, detection, imaging, and treatment are constantly being developed, demonstrating the limitless promise of nanoparticle-based medicine. Nanoparticles are the best delivery systems for the treatment of lung cancer because they may be specifically designed for a personalized medicine approach. Recognizing cancer physiology, tumor’s microenvironment, and the engagement of tumor cells with nanoparticles is essential for the future development of various techniques to specifically deliver medications to lung tumors and lung metastases. Oncologists now have a larger selection of medications at their disposal thanks to polymer conjugates and particulate nanocarriers. These currently rely on passive tissue targeting rather than active cellular targeting, primarily through EPR. Theranostics, or individualized treatment for cancer, is based on a personalized prescription and therefore it is evidence-based to ensure the precise administration of medicines or the effective treatment at the correct time, improving the patient’s condition and lowering the cost of drugs and services. Using a delivery platform based on nanotechnology, theranostics medicine is used to deliver imaging and therapeutic substances to the predetermined specific location via site-specific targeting (i.e., lung cancer cell). Nanomaterials have shown to be extremely useful tools and are now being used in medical settings. Due to their new physical characteristics, nanostructures can frequently resolve problems with solubility as well as stabilization via interface modifications or supplementary preparations. Targeted drug delivery and diagnostics nanostructures are made possible by the assimilated method of integrating biomacromolecules, carriers, medications, and diagnostic material. The nanometric size range molecules have larger surface area which contributes to the higher therapeutic payload as well. Targeting specific cancer cells with nanoparticles and delivering therapeutic payloads to cancer areas with precision through “active and passive targeting” can greatly lower nonspecific cytotoxicity. Despite the benefits, there are still many obstacles that need to be resolved, like cost-effective manufacturing, scale-up concerns, imaging approach, and the pharmacokinetics of drugs. To see lung cancer theranostics reach the clinic, more concerns with nanotoxicity and regulatory rules and barriers need to be tackled. Although nanotechnology has made significant progress, it is still not being utilized to its full potential in lung and other cancers. Stronger sequencing, immunohistochemistry, and proteomic techniques are now available, which will lead to a greater understanding of the mechanisms behind cancer as well as the discovery of novel, conclusive biomarkers. The development of key genomic programs like the “human protein atlas, cancer proteome studies, and The Cancer Genome Atlas (TCGA)” and increased financing for multi-center cohort research all advanced our understanding of cancer. In a single nanoscale package, multifunctional nanocarriers provide earlier detection, diagnostic capabilities, targeted medication distribution via several pathways, and therapy evaluation. These advantages are especially significant for adenocarcinomas that have MDR, such as NSCLC. It is crucial to explore alternatives to traditional chemotherapeutic therapies because NSCLC is one of the most deadly cancers, with one of the highest fatality rates. Nowadays, nanomedicine is a vital option for the treatment of NSCLC, and it may serve a deciding role in the approaching transformation of a tailored comprehensive therapy.
Data availability
This document includes citations for all the data that were analyzed throughout the literature review.
References
Abdelaziz H, Gaber M, Abd-Elwakil MM, Mabrouk MT, Elgohary MM, Kamel NM et al (2018) Inhalable particulate drug delivery systems for lung cancer therapy: nanoparticles, microparticles, nanocomposites and nanoaggregates. J Control Release 269:374–392
Ahn HK, Jung M, Sym SJ, Shin DB, Kang SM, Kyung SY et al (2014) A phase II trial of Cremorphor EL-free paclitaxel (Genexol-PM) and gemcitabine in patients with advanced non-small cell lung cancer. Cancer Chemother Pharmacol 74:277–282
Al Faraj A, Shaik AS, Halwani R, Alfuraih A (2016) Magnetic targeting and delivery of drug-loaded SWCNTs theranostic nanoprobes to lung metastasis in breast cancer animal model: noninvasive monitoring using magnetic resonance imaging. Mol Imag Biol 18:315–324
Al-Hallak KM, Azarmi S, Anwar-Mohamed A, Roa WH, Löbenberg R (2010) Secondary cytotoxicity mediated by alveolar macrophages: a contribution to the total efficacy of nanoparticles in lung cancer therapy? Eur J Pharm Biopharm 76:112–119
Allouche J (2013) Synthesis of organic and bioorganic nanoparticles: an overview of the preparation methods. In: Brayner R, Fiévet F, Coradin T (eds) Nanomaterials: a danger or a promise?. Springer, London. https://doi.org/10.1007/978-1-4471-4213-3_2
Amreddy N, Babu A, Panneerselvam J, Srivastava A, Muralidharan R, Chen A et al (2018) Chemo-biologic combinatorial drug delivery using folate receptor-targeted dendrimer nanoparticles for lung cancer treatment. Nanomedicine 14:373–384
Arya N, Arora A, Vasu KS, Sood AK, Katti DS (2013) Combination of single walled carbon nanotubes/graphene oxide with paclitaxel: a reactive oxygen species mediated synergism for treatment of lung cancer. Nanoscale 5(7):2818–2829
Asadollahi L, Mahoutforoush A, Dorreyatim SS, Soltanfam T, Paiva-Santos AC, Peixoto D, Veiga F, Hamishehkar H, Zeinali M, Abbaspour-Ravasjani S (2022) Co-delivery of erlotinib and resveratrol via nanostructured lipid carriers: a synergistically promising approach for cell proliferation prevention and ROS-Mediated apoptosis activation. Int J Pharm 25(624):122027
Ashique S, Sandhu NK, Chawla V, Chawla PA (2021) Targeted drug delivery: trends and perspectives. Current Drug Deliv 18(10):1435–1455
Ashique S, Almohaywi B, Haider N, Yasmin S, Hussain A, Mishra N, Garg A (2022a) siRNA-based nanocarriers for targeted drug delivery to control breast cancer. Advances in Cancer Biol-Metast 4(2022):100047
Ashique S, Upadhyay A, Kumar N, Chauhan S, Mishra N (2022b) Metabolic syndromes responsible for cervical cancer and advancement of nanocarriers for efficient targeted drug delivery-A review. Adv Cancer Biol-Metast 4(2022):100041
Banchereau J, Palucka K (2018) Cancer vaccines on the move. Nature Rev Clinical Oncol 15(1):9–10
Barash O et al (2012) Classification of lung cancer histology by gold nanoparticle sensors. Nanomed 8(5):580–589
Barenholz YC (2012) Doxil®—the first FDA-approved nano-drug: Lessons learned. J Controlled Rel 160(2):117–134
Bolhassani A, Javanzad S, Saleh T, Hashemi M, Aghasadeghi MR, Sadat SM (2014) Polymeric nanoparticles: potent vectors for vaccine delivery targeting cancer and infectious diseases. Human Vaccine Immunother 10(2):321–332
Børresen B, Hansen AE, Fliedner FP, Henriksen JR, Elema DR, Brandt-Larsen M, Kristensen LK, Kristensen AT, Andresen TL, Kjær A (2020) Noninvasive molecular imaging of the enhanced permeability and retention effect by 64Cu-liposomes: in vivo correlations with 68Ga-RGD, fluid pressure, diffusivity and 18F-FDG. Int J Nanomed 15:8571
Burstein HJ, Schwartz RS (2008) Molecular origins of cancer New England J Med 358(5):527
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N (2012) Cancer Discov 2:401–404
Chen Y, Yang L, Feng C, Wen LP (2005) Nano neodymium oxide induces massive vacuolization and autophagic cell death in non-small cell lung cancer NCI-H460 cells. Biochem Biophysical Res Commun 337(1):52–60
Chen YH et al (2007) Methotrexate conjugated to gold nanoparticles inhibits tumor growth in a syngeneic lung tumor model. Mol Pharm 4(5):713–722
Chen Z, Lai X, Song S, Zhu X, Zhu J (2016) Nanostructured lipid carriers based temozolomide and gene co-encapsulated nanomedicine for gliomatosis cerebri combination therapy. Drug Deliv 23(4):1369–1373
Chen CH, Huang TH, Elzoghby AO, Wang PW, Chang CW, Fang JY (2017b) Squarticles as the nano antidotes to sequester the overdosed antidepressant for detoxification. Int J Nanomed 12:8071
Chen H, Jin Y, Wang J, Wang Y, Jiang W, Dai H, Pang S, Lei L, Ji J, Wang B (2018) Design of smart targeted and responsive drug delivery systems with enhanced antibacterial properties. Nanoscale 10(45):20946–20962
Chen H, Zhang W, Zhu G, Xie J, Chen X (2017a) Rethinking cancer nanotheranostics. Nature Revi Mat 9 2 (7):1–8
Cheng L, Huang FZ, Cheng LF, Zhu YQ, Hu Q, Li L, Wei L, Chen DW (2014) GE11-modified liposomes for non-small cell lung cancer targeting: preparation, ex vitro and in vivo evaluation. Int J Nanomed 9:921
Choi YH, Han HK (2018) Nanomedicines: current status and future perspectives in aspect of drug delivery and pharmacokinetics. J Pharm Invest 48(1):43–60
Choi SH, Jin SE, Lee MK, Lim SJ, Park JS, Kim BG, Ahn WS, Kim CK (2008) Novel cationic solid lipid nanoparticles enhanced p53 gene transfer to lung cancer cells. European J Pharm Biopharm 68(3):545–554
ClinicalTrials.gov Identifier: NCT00020124 (2003)
ClinicalTrials.gov Identifier: NCT00006036 (2004)
ClinicalTrials.gov Identifier: NCT00199849 (2005)
ClinicalTrials.gov Identifier: NCT00277082 (2006)
ClinicalTrials.gov Identifier: NCT00442754 (2007)
ClinicalTrials.gov Identifier: NCT00503568 (2007)
ClinicalTrials.gov Identifier: NCT00654030 (2008)
ClinicalTrials.gov Identifier: NCT00676507 (2008)
ClinicalTrials.gov Identifier: NCT00729612 (2008)
ClinicalTrials.gov Identifier: NCT00960115 (2009)
ClinicalTrials.gov Identifier: NCT01023347 (2009)
ClinicalTrials.gov Identifier: NCT01051362 (2010)
ClinicalTrials.gov Identifier: NCT01159288 (2010)
ClinicalTrials.gov Identifier: NCT01219348 (2010)
ClinicalTrials.gov Identifier: NCT01620190 (2012)
ClinicalTrials.gov Identifier: NCT01720836 (2012)
ClinicalTrials.gov Identifier: NCT01789099 (2013)
ClinicalTrials.gov Identifier: NCT01792479 (2013)
ClinicalTrials.gov Identifier: NCT01853878 (2013)
ClinicalTrials.gov Identifier: NCT02283320 (2014)
ClinicalTrials.gov Identifier: NCT02470468 (2015)
ClinicalTrials.gov Identifier: NCT02654587 (2016)
ClinicalTrials.gov Identifier: NCT02667743 (2016)
ClinicalTrials.gov Identifier: NCT02818426 (2016)
ClinicalTrials.gov Identifier: NCT03164772 (2017)
ClinicalTrials.gov Identifier: NCT03289962 (2017)
ClinicalTrials.gov Identifier: NCT03548467 (2018)
ClinicalTrials.gov Identifier: NCT03948763 (2019)
ClinicalTrials.gov Identifier: NCT04078269 (2019)
Cortés‐Jofré M, Uranga R, Pombert AT, Prado MD, Aguirrechu IC, Pacheco C, Reyes RM, Chuecas F, Bermejo PI (2019) Therapeutic vaccines for advanced non‐small cell lung cancer. The Cochrane Database Systematic Rev 2019(8):CD013377
Cuppens K, Vansteenkiste J (2014) Vaccination therapy for non-small-cell lung cancer. Current Opin Oncol 26(2):165–170
Das M, Datir SR, Singh RP, Jain S (2013) Augmented anticancer activity of a targeted, intracellularly activatable, theranostic nanomedicine based on fluorescent and radiolabeled, methotrexate-folic acid-multiwalled carbon nanotube conjugate. Mol Pharma 10(7):2543–2557
Das SS, Tambe S, Prasad Verma PR, Amin P, Singh N, Singh SK, Gupta PK (2022) Molecular insights and therapeutic implications of nanoengineered dietary polyphenols for targeting lung carcinoma: part I. Nanomedicine 17(23):1799–1816
Devarajan P, Tarabishi R, Mishra J, Ma Q, Kourvetaris A, Vougiouka M, Boulikas T (2004) Low renal toxicity of lipoplatin compared to cisplatin in animals. Anticancer Res 24(4):2193–2200
Ding M, Zeng X, He X, Li J, Tan H, Fu Q et al (2014) Cell internalizable and intracellularly degradable cationic polyurethane micelles as a potential platform for efficient imaging and drug delivery. Biomacromol 15:2896–2906
DiSanto RM, Subramanian V, Gu Z (2015) Recent advances in nanotechnology for diabetes treatment. Wiley Interdisciplinary Reviews: Nanomed Nanobiotechnol 7(4):548–564
Dolatabadi JE, Valizadeh H, Hamishehkar H (2015) Solid lipid nanoparticles as efficient drug and gene delivery systems: recent breakthroughs. Adv Pharmaceuti Bull 5(2):151
Dominguez-Martinez I, Joaquin-Ovalle F, Ferrer-Acosta Y, Griebenow KH (2022) Folate-decorated cross-linked cytochrome c nanoparticles for active targeting of non-small cell lung carcinoma (NSCLC). Pharmaceutics 14(3):490
Dong S, Men W, Yang S, Xu S (2020) Identification of lung adenocarcinoma biomarkers based on bioinformatic analysis and human samples. Oncol Rep 43:1437–1450. https://doi.org/10.3892/or.2020.7526
Dostalova S, Vasickova K, Hynek D, Krizkova S, Richtera L, Vaculovicova M, Eckschlager T, Stiborova M, Heger Z, Adam V (2017) Apoferritin as an ubiquitous nanocarrier with excellent shelf life. Int J Nanomed 12:2265
Doumat G, Daher D, Zerdan MB, Nasra N, Bahmad HF, Recine M, Poppiti R (2023) Drug repurposing in non-small cell lung carcinoma: old solutions for new problems. Current Oncol 30(1):704–719
Duan Y, Dhar A, Patel C, Khimani M, Neogi S, Sharma P, Kumar NS, Vekariya RL (2010) A brief review on solid lipid nanoparticles: part and parcel of contemporary drug delivery systems. RSC Adv 10(45):26777–26791
Egeblad M, Werb Z (2002) New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2(3):161–174
Elzoghby AO (2013) Gelatin-based nanoparticles as drug and gene delivery systems: reviewing three decades of research. J Control Release 172:1075–1091
Elzoghby O, A, M Abd-Elwakil M, Abd-Elsalam K, T Elsayed M, Hashem Y, Mohamed O, (2016) Natural polymeric nanoparticles for brain-targeting: implications on drug and gene delivery. Current Pharma Design 22(22):3305–3323
Elzoghby AO, Vranic BZ, Samy WM, Elgindy NA (2015) Swellable floating tablet based on spray-dried casein nanoparticles: near-infrared spectral characterization and floating matrix evaluation. Int J Pharm 491(1–2):113–122
Elzoghby AO, El-Lakany SA, Helmy MW, Abu-Serie MM, Elgindy NA (2017) Shell-crosslinked zein nanocapsules for oral codelivery of exemestane and resveratrol in breast cancer therapy. Nanomed 12(24):2785–2805
Esim O, Bakirhan NK, Yildirim N, Sarper M, Savaser A, Ozkan SA, Ozkan Y (2020) Development, optimization and in vitro evaluation of oxaliplatin loaded nanoparticles in non-small cell lung cancer. DARU J Pharm Sci 28(2):673–684
Fan Y, Yuan S, Huo M, Chaudhuri AS, Zhao M, Wu Z, Qi X (2017) Spatial controlled multistage nanocarriers through hybridization of dendrimers and gelatin nanoparticles for deep penetration and therapy into tumor tissue. Nanomed Nanotech, Biol Med 13(4):1399–410
Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A (2017) Bray F (2019) Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 144(8):1941–1953
Fernandez-Fernandez A, Manchanda R, McGoron AJ (2011) Theranostic applications of nanomaterials in cancer: drug delivery, image-guided therapy, and multifunctional platforms. Applied Biochem Biotechn 165(7):1628–1651
Gaber M, Medhat W, Hany M, Saher N, Fang JY, Elzoghby A (2017) Protein-lipid nanohybrids as emerging platforms for drug and gene delivery: challenges and outcomes. J Controlled Rel 254:75–91
Garbuzenko OB, Kuzmov A, Taratula O, Pine SR, Minko T (2019) Strategy to enhance lung cancer treatment by five essential elements: inhalation delivery, nanotechnology, tumor-receptor targeting, chemo-and gene therapy. Theranostics 9(26):8362
García-Fernández C, Fornaguera C, Borrós S (2020) Nanomedicine in non-small cell lung cancer: from conventional treatments to immunotherapy. Cancers 12(6):1609
Gou S, Yang J, Ma Y, Zhang X, Zu M, Kang T, Liu S, Ke B, Xiao B (2020) Multi-responsive nanococktails with programmable targeting capacity for imaging-guided mitochondrial phototherapy combined with chemotherapy. J Controlled Rel 327:371–383
Han Y, Zhang Y, Li D, Chen Y, Sun J, Kong F (2014a) Transferrin-modified nanostructured lipid carriers as multifunctional nanomedicine for codelivery of DNA and doxorubicin. Int J Nanomed 9:4107
Han J, Wang Q, Zhang Z, Gong T, Sun X (2014b) Cationic bovine serum albumin based self-assembled nanoparticles as siRNA delivery vector for treating lung metastatic cancer. Small 10(3):524–535
Han Y, Li Y, Zhang P, Sun J, Li X, Sun X, Kong F (2016) Nanostructured lipid carriers as novel drug delivery system for lung cancer gene therapy. Pharm Develop Technol 21(3):277–281
He Y, Du Z, Ma S, Liu Y, Li D, Huang H, Jiang S, Cheng S, Wu W, Zhang K, Zheng X (2016) Effects of green-synthesized silver nanoparticles on lung cancer cells in vitro and grown as xenograft tumors in vivo. Int J Nanomed 11:1879
Hoseini-Ghahfarokhi M, Mirkiani S, Mozaffari N, Abdolahi Sadatlu MA, Ghasemi A, Abbaspour S, Akbarian M, Farjadian F, Karimi M (2020) Applications of graphene and graphene oxide in smart drug/gene delivery: is the world still flat?. Int J Nanomed 15:9469–9496
Hsu SH, Wen CJ, Al-Suwayeh SA, Huang YJ, Fang JY (2013) Formulation design and evaluation of quantum dot-loaded nanostructured lipid carriers for integrating bioimaging and anticancer therapy. Nanomed 8(8):1253–1269
Hu CM, Zhang L (2012) Nanoparticle-based combination therapy toward overcoming drug resistance in cancer. Biochem Pharmacol 83(8):1104–1111
Hu J, Fu S, Peng Q, Han YW, Xie J, Zan N et al (2017) Paclitaxel-loaded polymeric nanoparticles combined with chronomodulated chemotherapy on lung cancer: in vitro and in vivo evaluation. Int J Pharm 516:313–322
Huang X, Jain PK, El-Sayed IH, El-Sayed MA (2007) Gold nanoparticles:interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomedicine (lond) 2(5):681–693
Huang J, Zhuang C, Chen J, Chen X, Li X, Zhang T, Wang B, Feng Q, Zheng X, Gong M, Gong Q (2022) Targeted drug/gene/photodynamic therapy via a stimuli-responsive dendritic-polymer-based nanococktail for treatment of EGFR-TKI-resistant non-small-cell lung cancer. Adv Mater 34(27):2201516
Iqbal MA, Md S, Sahni JK, Baboota S, Dang S, Ali J (2012) Nanostructured lipid carriers system: recent advances in drug delivery. J Drug Targeting 20(10):813–830
Jamal-Hanjani M, Wilson GA, McGranahan N, Birkbak NJ, Watkins TB, Veeriah S, Shafi S, Johnson DH, Mitter R, Rosenthal R, Salm M (2017) Tracking the evolution of non–small-cell lung cancer. New Eng J Med 376(22):2109–2121
Jeong JK, Gurunathan S, Kang MH, Han JW, Das J, Choi YJ, Kwon DN, Cho SG, Park C, Seo HG, Song H (2016) Hypoxia-mediated autophagic flux inhibits silver nanoparticle-triggered apoptosis in human lung cancer cells. Scientific Rep 6(1):21688
Jiang ZM, Dai SP, Xu YQ, Li T, Xie J, Li C et al (2015) Crizotinib-loaded polymeric nanoparticles in lung cancer chemotherapy. Med Oncol 32:193
Jing Y, Xiong X, Ming Y, Zhao J, Guo X, Yang G, Zhou S (2018) A multifunctional micellar nanoplatform with pH-triggered cell penetration and nuclear targeting for effective cancer therapy and inhibition to lung metastasis. Adv Healthcare Mat 7(7):1700974
Joshi N, Shirsath N, Singh A, Joshi KS, Banerjee R (2014) Endogenous lung surfactant inspired pH responsive nanovesicle aerosols: pulmonary compatible and site-specific drug delivery in lung metastases. Sci Rep 4(1):1–1
Jung J, Park SJ, Chung HK, Kang HW, Lee SW, Seo MH et al (2012) Polymeric nanoparticles containing taxanes enhance chemoradiotherapeutic efficacy in non-small cell lung cancer. Int J Radiat Oncol Biol Phys 84:e77-83
Kanamala M, Wilson WR, Yang M, Palmer BD, Wu Z (2016) Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: a review. Biomat 85:152–167
Kedmi R, Ben-Arie N, Peer D (2010) The systemic toxicity of positively charged lipid nanoparticles and the role of Toll-like receptor 4 in immune activation. Biomat 31(26):6867–6875
Kim DW, Kim SY, Kim HK, Kim SW, Shin SW, Kim JS et al (2007) Multicenter phase II trial of Genexol-PM, a novel cremophor-free, polymeric micelle formulation of paclitaxel, with cisplatin in patients with advanced non-small-cell lung cancer. Ann Oncol 18:2009–2014
Kim SW, Lee YK, Lee JY, Hong JH, Khang D (2017) PEGylated anticancer-carbon nanotubes complex targeting mitochondria of lung cancer cells. Nanotechnol 28(46):465102
Kim SS, Doherty C, Moghe M, Rait A, Pirollo KF, Harford JB, Chang EH (2022) Nanomedicine-based gene delivery for a truncated tumor suppressor RB94 promotes lung cancer immunity. Cancers 14(20):5092
Kim SS, Harford JB, Moghe M, Doherty C, Chang EH (2022) A novel P53 nanomedicine reduces immunosuppression and augments anti-PD-1 therapy for non-small cell lung cancer in syngeneic mouse models. Cells 11(21):3434
Ko S, Park JY, Oh YK (2019) A microbial siderophore-inspired self-gelling hydrogel for noninvasive anticancer phototherapy. Cancer Res 79(24):6178–6189
Kotmakçı M, Çetintaş VB, Kantarcı AG (2017) Preparation and characterization of lipid nanoparticle/pDNA complexes for STAT3 downregulation and overcoming chemotherapy resistance in lung cancer cells. Int J Pharmaceutics 525(1):101–111
Lee WH, Loo CY, Traini D, Young PM (2015) Inhalation of nanoparticle-based drug for lung cancer treatment: advantages and challenges. Asian J Pharm Sci 10(6):481–489
Lee SY, Hong EH, Jeong JY, Cho J, Seo JH, Ko HJ, Cho HJ (2019) Esterase-sensitive cleavable histone deacetylase inhibitor-coupled hyaluronic acid nanoparticles for boosting anticancer activities against lung adenocarcinoma. Biomat Sci 7(11):4624–4635
Li LP (2018) Cisplatin-loaded polymeric micelles with aggregation-induced emission feature for cellular imaging and chemotherapy. Chem Eur 3:13541–13781
Li K, Zhang ZP, Luo M, Yu X, Han Y, Wei HP, Cui ZQ, Zhang XE (2012) Multifunctional ferritin cage nanostructures for fluorescence and MR imaging of tumor cells. Nanoscale 4(1):188–193
Li X, Zhang XN, Li XD, Chang J (2016a) Multimodality imaging in nanomedicine and nanotheranostics. Cancer Bio Med 13(3):339
Li X, Zhang XN, Li XD, Chang J (2016b) Multimodality imaging in nanomedicine and nanotheranostics. Cancer Biology Med 13(3):339
Li S, Chen L, Huang K, Chen N, Zhan Q, Yi K, Qi H, Liu C, Tan Y, Hou X, Lu Y (2019) Extracellular delivery: tumor microenvironment-tailored weakly cell-interacted extracellular delivery platform enables precise antibody release and function (Adv. Funct. Mater. 43/2019). Adv Funct Mat 29(43):1970301
Li K, Nguyen HG, Lu X, Wang Q (2020a) Viruses and their potential in bioimaging and biosensing applications. Analyst 135(1):21–27
Li F, Qin Y, Lee J, Liao H, Wang N, Davis TP, Qiao R, Ling D (2020b) Stimuli-responsive nano-assemblies for remotely controlled drug delivery. J Control Release 322:566–592
Li J, Zhang Z, Deng H, Zheng Z (2021) Cinobufagin-loaded and folic acid-modified polydopamine nanomedicine combined with photothermal therapy for the treatment of lung cancer. Front Chem 9:637754
Li K, Zang X, Meng X, Li Y, Xie Y, Chen X (2022) Targeted delivery of quercetin by biotinylated mixed micelles for non-small cell lung cancer treatment. Drug Delivery 29(1):970–985
Liang R, Liu L, He H, Chen Z, Han Z, Luo Z, Wu Z, Zheng M, Ma Y, Cai L (2018) Oxygen-boosted immunogenic photodynamic therapy with gold nanocages@ manganese dioxide to inhibit tumor growth and metastases. Biomat 177:149–160
Lim J, Simanek EE (2012) Triazine dendrimers as drug delivery systems: from synthesis to therapy. Adv Drug Deliv Rev 64(9):826–835
Lin C, Zhang X, Chen H, Bian Z, Zhang G, Riaz MK, Tyagi D, Lin G, Zhang Y, Wang J, Lu A (2018) Dual-ligand modified liposomes provide effective local targeted delivery of lung-cancer drug by antibody and tumor lineage-homing cell-penetrating peptide. Drug Deliv 25(1):256–266
Liu J, Liu J, Chu L, Wang Y, Duan Y, Feng L, Yang C, Wang L, Kong D (2011) Novel peptide–dendrimer conjugates as drug carriers for targeting nonsmall cell lung cancer. Int J Nanomed 6:59
Liu FC, Yu HP, Lin CY, Elzoghby AO, Hwang TL, Fang JY (2018) Use of cilomilast-loaded phosphatiosomes to suppress neutrophilic inflammation for attenuating acute lung injury: the effect of nanovesicular surface charge. J Nanobiotechnol 16(1):1–4
Liu J, Li F, Zheng J, Li B, Zhang D, Jia L (2019) Redox/NIR dual-responsive MoS2 for synergetic chemo-photothermal therapy of cancer. J Nanobiotech 17(1):1–6
Lohcharoenkal W, Wang L, Chen YC, Rojanasakul Y (2014) Protein nanoparticles as drug delivery carriers for cancer therapy. BioMed Res Int 2014:180549
Lowery A, Onishko H, Hallahan DE, Han Z (2011) Tumor-targeted delivery of liposome-encapsulated doxorubicin by use of a peptide that selectively binds to irradiated tumors. J Cont Rel 150(1):117–124
Lu J, Gu A, Wang W, Huang A, Han B, Zhong H (2022) Polymeric micellar paclitaxel (pm-Pac) prolonged overall survival for NSCLC patients without pleural metastasis. Int J Pharm 25(623):121961
Lu J, Lou Y, Zhang Y, Zhong R, Zhang W, Zhang X, Wang H, Chu T, Han B, Zhong H (2023) Paclitaxel has a reduced toxicity profile in healthy rats after polymeric micellar nanoparticle delivery. Int J Nanomed 31:263–276
Luo Y, Wang X, Du D, Lin Y (2015) Hyaluronic acid-conjugated apoferritin nanocages for lung cancer targeted drug delivery. Biomat Sci 3(10):1386–1394
Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non–small-cell lung cancer to gefitinib. New Eng J Med 350(21):2129–2139
Ma P, Mumper RJ (2013) Paclitaxel nano-delivery systems: a comprehensive review. J Nanomed Nanotechnol 4:100164
Maja L, Željko K, Mateja P (2020) Sustainable technologies for liposome preparation. J Supercrit Fluids 165:104984
Makled S, Nafee N, Boraie N (2017) Nebulized solid lipid nanoparticles for the potential treatment of pulmonary hypertension via targeted delivery of phosphodiesterase-5-inhibitor. Int J Pharm 517(1–2):312–321
Mangal S, Gao W, Li T, Zhou QT (2017) Pulmonary delivery of nanoparticle chemotherapy for the treatment of lung cancers: challenges and opportunities. Acta Pharmacol Sin 38(6):782–797
Mattheolabakis G, Rigas B, Constantinides PP (2012) Nanodelivery strategies in cancer chemotherapy: biological rationale and pharmaceutical perspectives. Nanomed 7(10):1577–1590
McIntyre A, Ganti AK (2017) Lung cancer-a global perspective. J Surg Oncol 115(5):550–554
Mei D, Zhao L, Chen B, Zhang X, Wang X, Yu Z et al (2018) a-Conotoxin Imi-modified polymeric micelles as potential nanocarriers for targeted docetaxel delivery to a7-nAChR overexpressed non-small cell lung cancer. Drug Deliv 25:493–503
Mishra V, Bansal KK, Verma A, Yadav N, Thakur S, Sudhakar K, Rosenholm JM (2018) Solid lipid nanoparticles: Emerging colloidal nano drug delivery systems. Pharmaceutics 10(4):191
Moghimi SM, Szebeni J (2003) Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Progress in Lipid Res 42(6):463–478
Moradinasab S, Pourbagheri-Sigaroodi A, Ghaffari SH, Bashash D (2022) Targeting macrophage-mediated tumor cell phagocytosis: an overview of phagocytosis checkpoints blockade, nanomedicine intervention, and engineered CAR-macrophage therapy. Int Immunopharmacol 1(103):108499
Morgan MT, Nakanishi Y, Kroll DJ, Griset AP, Carnahan MA, Wathier M, Oberlies NH, Manikumar G, Wani MC, Grinstaff MW (2006) Dendrimer-encapsulated camptothecins: increased solubility, cellular uptake, and cellular retention affords enhanced anticancer activity in vitro. Cancer Res 66(24):11913–11921
Mukherjee S, Patra CR (2016) Therapeutic application of anti-angiogenic nanomaterials in cancers. Nanoscale 8(25):12444–12470
Mukherjee S, Chowdhury D, Kotcherlakota R, Patra S, Vinothkumar B, Bhadra MP, Sreedhar B, Patra CR (2014) Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system). Theranostics 4(3):316
Mussi SV, Sawant R, Perche F, Oliveira MC, Azevedo RB, Ferreira LA, Torchilin VP (2014) Novel nanostructured lipid carrier co-loaded with doxorubicin and docosahexaenoic acid demonstrates enhanced in vitro activity and overcomes drug resistance in MCF-7/Adr cells. Pharm Res 31(8):1882–1892
Naseri N, Valizadeh H, Zakeri-Milani P (2015) Solid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and application. Adv Pharm Bull 5(3):305
Nguyen PV, Hervé-Aubert K, Lajoie L, Misericordia Y, Chourpa I, David S, Allard-Vannier E (2022) In vitro synergistic activity of cisplatin and EGFR-targeted nanomedicine of anti-Bcl-xL siRNA in a non-small lung cancer cell line model. International Journal of Pharmaceutics: x 1(4):100139
Noh MS, Jun BH, Kim S, Kang H, Woo MA, Minai-Tehrani A, Kim JE, Kim J, Park J, Lim HT, Park SC (2009) Magnetic surface-enhanced Raman spectroscopic (M-SERS) dots for the identification of bronchioalveolar stem cells in normal and lung cancer mice. Biomat 30(23–24):3915–3925
Oerlemans C, Bult W, Bos M, Storm G, Nijsen JF, Hennink WE (2012) Polymeric micelles in anticancer therapy: targeting, imaging and triggered release. Pharmaceutical Res 27(12):2569–2589
Pai-Scherf L, Blumenthal GM, Li H, Subramaniam S, Mishra-Kalyani PS, He K, Zhao H, Yu J, Paciga M, Goldberg KB, McKee AE (2017) FDA approval summary: pembrolizumab for treatment of metastatic non-small cell lung cancer: first-line therapy and beyond. Oncologist 22(11):1392–1399
Pan A, Jakaria MG, Meenach SA, Bothun GD (2019) Radiofrequency and near-infrared responsive core–shell nanostructures using layersome templates for cancer treatment. ACS Appl Bio Mater 3(1):273–281
Parajapati SK, Maurya SD, Das MK, Tilak VK, Verma KK, Dhakar RC (2016) Potential application of dendrimers in drug delivery: a concise review and update. J Drug Deliv Therapeutics 6(2):71–88
Park YI, Kwon SH, Lee G, Motoyama K, Kim MW, Lin M, Niidome T, Choi JH, Lee R (2021) pH-sensitive multi-drug liposomes targeting folate receptor β for efficient treatment of non-small cell lung cancer. J Controlled Rel 330:1–4
Patel AR, Chougule MB, Lim E, Francis KP, Safe S, Singh M (2014) Theranostic tumor homing nanocarriers for the treatment of lung cancer. Nanomed Nanotechnol, Biol Med 10(5):e1053-63
Patil SM, Sawant SS, Kunda NK (2021) Pulmonary delivery of bedaquiline-loaded cubosomes for non-small cell lung cancer (NSCLC) treatment. Drug Delivery to the Lungs, vol 32. https://ddl-conference.com/ddl2021/conference-papers/pulmonary-delivery-of-bedaquiline-loaded-cubosomes-for-non-small-cell-lung-cancer-nsclc-treatment/
Paul MK, Mukhopadhyay AK (2004) Tyrosine kinase–role and significance in cancer. Internat J Med Sci 1(2):101
Poonia N, Kharb R, Lather V, Pandita D (2016) Nanostructured lipid carriers: versatile oral delivery vehicle. Future Sc OA 2(3):FSO135. https://doi.org/10.4155/fsoa-2016-0030
Razak A, Mohd SA, Gazzali A, Fisol FA, Abdulbaqi M, Parumasivam I, Mohtar T, N A Wahab H (2021) Advances in nanocarriers for effective delivery of docetaxel in the treatment of lung cancer: an overview Cancers 13(3):400
Razzazan A, Atyabi F, Kazemi B, Dinarvand R (2016) In vivo drug delivery of gemcitabine with PEGylated single-walled carbon nanotubes. Mat Sci Engineering: C 62:614–625
Ren Q, Liang Z, Jiang X, Gong P, Zhou L, Sun Z, Xiang J, Xu Z, Peng X, Li S, Li W (2019) Enzyme and pH dual-responsive hyaluronic acid nanoparticles mediated combination of photodynamic therapy and chemotherapy. Int J Biol Macromol 130:845–852
Reshma P, Unnikrishnan B, Preethi GU, Syama HP, Archana MG, Remya K et al (2019) Overcoming drug-resistance in lung cancer cell by paclitaxel loaded galactoxyloglucan nanoparticles. Int J Biol Macromol 136:266–274
Rizvi NA, Riely GJ, Azzoli CG, Miller VA, Ng KK, Fiore J, Chia G, Brower M, Heelan R, Hawkins MJ, Kris MG (2008) Phase I/II trial of weekly intravenous 130-nm albumin-bound paclitaxel as initial chemotherapy in patients with stage IV non–small-cell lung cancer. J Clin Oncol 26(4):639–643
Roa WH, Azarmi S, Al-Hallak MK, Finlay WH, Magliocco AM, Löbenberg R (2011) Inhalable nanoparticles, a non-invasive approach to treat lung cancer in a mouse model. J Controlled Rel 150(1):49–55
Roca E, Gurizzan C, Amoroso V, Vermi W, Ferrari V, Berruti A (2017) Outcome of patients with lung adenocarcinoma with transformation to small-cell lung cancer following tyrosine kinase inhibitors treatment: a systematic review and pooled analysis. Cancer Treatment Rev 59:117–122
Rochigneux P, Garcia AJ, Chanez B, Madroszyk A, Olive D, Garon EB (2020) Medical treatment of lung cancer: can immune cells predict the response? A Systematic Review Front Immunol 11:1036
Ryan GM, Kaminskas LM, Kelly BD, Owen DJ, McIntosh MP, Porter CJ (2013) Pulmonary administration of PEGylated polylysine dendrimers: absorption from the lung versus retention within the lung is highly size-dependent. Mole Pharm 10(8):2986–2995
Sadhukha T, Wiedmann TS, Panyam J (2013a) Inhalable magnetic nanoparticles for targeted hyperthermia in lung cancer therapy. Biomat 34(21):5163–5171
Sadhukha T, Wiedmann TS, Panyam J (2013b) Inhalable magnetic nanoparticles for targeted hyperthermia in lung cancer therapy. Biomaterials 34(21):5163–5171. https://doi.org/10.1016/j.biomaterials.2013.03.061
Sahu PK, Mishra DK, Jain N, Rajoriya V, Jain AK (2015) Mannosylated solid lipid nanoparticles for lung-targeted delivery of paclitaxel. Drug Devel Industrial Pharmacy 41(4):640–649
Samad A, Jafar T, Rafi JH (2020) Identification of angiotensin-converting enzyme 2 (ACE2) protein as the potential biomarker in SARS-CoV-2 infection-related lung cancer using computational analyses. Genomics 112:4912–4923. https://doi.org/10.1016/j.ygeno.2020.09.002
Sandoval-Yañez C, Castro Rodriguez C (2020) Dendrimers: amazing platforms for bioactive molecule delivery systems. Materials 13(3):570
Sarvepalli S, Parvathaneni V, Chauhan G, Shukla SK, Gupta V (2022) Inhaled indomethacin-loaded liposomes as potential therapeutics against non-small cell lung cancer (NSCLC). Pharm Res 39(11):2801–2815
Savla R, Garbuzenko OB, Chen S, Rodriguez-Rodriguez L, Minko T (2014) Tumor-targeted responsive nanoparticle-based systems for magnetic resonance imaging and therapy. Pharmaceutical Res 31(12):3487–3502
Scioli Montoto S, Muraca G, Ruiz ME (2020) Solid lipid nanoparticles for drug delivery: pharmacological and biopharmaceutical aspects. Front Mol Biosci 7:587997
Sebastian M, Papachristofilou A, Weiss C, Früh M, Cathomas R, Hilbe W, Wehler T, Rippin G, Koch SD, Scheel B, Fotin-Mleczek M (2014) Phase Ib study evaluating a self-adjuvanted mRNA cancer vaccine (RNActive®) combined with local radiation as consolidation and maintenance treatment for patients with stage IV non-small cell lung cancer. BMC Cancer 14:1
Sebastian M, Von Boehmer L, Zippelius A, Mayer F, Reck M, Atanackovic D, Thomas M, Schneller F, Stoehlmacher J, Goekkurt E, Bernhard H, Groeschel A, Bals R, Schmidt S, Scheel B, Koch SD, Lander T. Kallen K, Knuth A (2011) Messenger RNA vaccination in NSCLC: findings from a phase I/IIa clinical trial. J Clinical Oncol 29(15_suppl):2584
Semete B, Booysen L, Lemmer Y, Kalombo L, Katata L, Verschoor J, Swai HS (2010) In vivo evaluation of the biodistribution and safety of PLGA nanoparticles as drug delivery systems. Nanomed Nanotechnol, Biol Med 6(5):662–71
Senapati S, Mahanta AK, Kumar S, Maiti P (2018) Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transd Targeted Therapy 3(1):1–9
Seong GM, Hyun CL, Lee J, Kim C (2020) Large cell carcinoma of the lung presenting as diffuse pulmonary infiltrates with haemoptysis. Respirol Case Rep 8(7):e00632
Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S (2015) Advances and challenges of liposome assisted drug delivery. Front Pharmacol 6(286):1–13
Shahriari M, Zahiri M, Abnous K, Taghdisi SM, Ramezani M, Alibolandi M (2019) Enzyme responsive drug delivery systems in cancer treatment. J Controlled Rel 308:172–189
Shao Z, Shao J, Tan B, Guan S, Liu Z, Zhao Z, He F, Zhao J (2015) Targeted lung cancer therapy: preparation and optimization of transferrin-decorated nanostructured lipid carriers as novel nanomedicine for co-delivery of anticancer drugs and DNA. Int J Nanomed 10:1223
Sharma A, Kim EJ, Shi H, Lee JY, Chung BG, Kim JS (2018) Development of a theranostic prodrug for colon cancer therapy by combining ligand-targeted delivery and enzyme-stimulated activation. Biomat 155:145–151
Shen H, Shi S, Zhang Z, Gong T, Sun X (2015) Coating solid lipid nanoparticles with hyaluronic acid enhances antitumor activity against melanoma stem-like cells. Theranostics 5(7):755
Shi SJ, Zhong ZR, Liu J, Zhang ZR, Sun X, Gong T (2012) Solid lipid nanoparticles loaded with anti-microRNA oligonucleotides (AMOs) for suppression of microRNA-21 functions in human lung cancer cells. Pharm Res 29(1):97–109
Shi J, Kantoff PW, Wooster R, Farokhzad OC (2017a) Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer 17(1):20–37
Shi J, Kantoff PW, Wooster R, Farokhzad OC (2017b) Cancer nanomedicine: progress, challenges and opportunities. Nature Rev Cancer 17(1):20–37
Shi M, Zhao X, Zhang J, Pan S, Yang C, Wei Y, Hu H, Qiao M, Chen D, Zhao X (2018) pH-responsive hybrid nanoparticle with enhanced dissociation characteristic for siRNA delivery. Internat J Nanomed 13:6885
Shih FY, Jiang WP, Lin X, Kuo SC, Huang GJ, Hou YC, Chang CS, Liu Y, Chiang YT (2020) A novel pH-tunable secondary conformation containing mixed micellar system in anticancer treatment. Cancers 12(2):503
Shukla KS, Nguyen V, Goyal M, Gupta V (2022) Cationically modified inhalable nintedanib niosomes: enhancing therapeutic activity against non-small-cell lung cancer. Nanomedicine 17(13):935–958
Singh S, Nalwa HS (2007) Nanotechnology and health safety-toxicity and risk assessment of nanostructured material son human health. J Nanosci Nanotechnol 7:3048–3070
Singh N, Sachdev A, Gopinath P (2018) Polysaccharide functionalized single walled carbon nanotubes as nanocarriers for delivery of curcumin in lung cancer cells. J Nanosci Nanotech 18(3):1534–1541
Sivalingam D, Singh M (2023) Targeting the ACE2 receptor using nanomedicine: novel approach to lung cancer therapy. Trends in Immunotherapy 7(1):1–1
Sivarajakumar R, Mallukaraj D, Kadavakollu M, Neelakandan N, Chandran S, Bhojaraj S, Karri VV (2018) Nanoparticles for the treatment of lung cancers. J Young Pharmacists 10(3):276
Skubitz KM, Anderson PM (2000) Inhalational interleukin-2 liposomes for pulmonary metastases: a phase I clinical trial. Anticancer Drugs 11(7):555–563
Socinski MA, Obasaju C, Gandara D, Hirsch FR, Bonomi P, Bunn P, Kim ES, Langer CJ, Natale RB, Novello S, Paz-Ares L (2016) Clinicopathologic features of advanced squamous NSCLC. J Thoracic Oncol 11(9):1411–1422
Song XL, Ju RJ, Xiao Y, Wang X, Liu S, Fu M, Liu JJ, Gu LY, Li XT, Cheng L (2017) Application of multifunctional targeting epirubicin liposomes in the treatment of non-small-cell lung cancer. Int J Nanomed 12:7433
SreeHarsha N, Maheshwari R, Al-Dhubiab BE, Tekade M, Sharma MC, Venugopala KN, Tekade RK, Alzahrani AM (2019) Graphene-based hybrid nanoparticle of doxorubicin for cancer chemotherapy. Int J Nanomed 14:7419
Stabile L, Buonanno G, Ficco G, Scungio M (2017) Smokers’ lung cancer risk related to the cigarette-generated mainstream particles. J Aerosol Sci 107:41–54
Stoehr LC, Gonzalez E, Stampfl A, Casals E, Duschl A, Puntes V, Oostingh GJ (2011) Shape matters: effects of silver nanospheres and wires on human alveolar epithelial cells. Particle Fiber Toxicol 8:1–5
Sun S, Schiller JH, Gazdar AF (2007) Lung cancer in never smokers—a different disease. Nature Rev Cancer 7(10):778–790
Sun W et al (2008) Endocytosis of a single mesoporous silica nanoparticle into a human lung cancer cell observed by differential interference contrast microscopy. Anal Bioanal Chem 391(6):2119–2125
Tan JM, Karthivashan G, Arulselvan P, Fakurazi S, Hussein MZ (2014) Characterization and in vitro studies of the anticancer effect of oxidized carbon nanotubes functionalized with betulinic acid. Drug Des Devel Ther 8:2333–2343
Taratula O, Garbuzenko OB, Chen AM, Minko T (2011) Innovative strategy for treatment of lung cancer: targeted nanotechnology-based inhalation co-delivery of anticancer drugs and siRNA. J Drug Target 19(10):900–914
Thangavelu P, Sundaram V, Gunasekaran K, Mujyambere B, Raju S, Kannan A, Arasu A, Krishna K, Ramamoorthi J, Ramasamy S, Velusamy T (2022) Development of optimized novel liposome loaded with 6-gingerol and assessment of its therapeutic activity against NSCLC In vitro and In vivo experimental models. Chem Phys Lipid 1(245):105206
Toloza EM, Morse MA, Lyerly HK (2006) Gene therapy for lung cancer. J Cellular Biochem 99(1):1–22
Tomalia DA, Fréchet JM (2002) Discovery of dendrimers and dendritic polymers: a brief historical perspective. J Polymer Sci Part a: Polymer Chem 40(16):2719–2728
Torchilin V (2011) Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev 63(3):131–135
Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JH, Beasley MB, Chirieac LR, Dacic S, Duhig E, Flieder DB, Geisinger K (2015) The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thoracic Oncol 10(9):1243–1260
Tseng CL, Su WY, Yen KC, Yang KC, Lin FH (2009) The use of biotinylated-EGF-modified gelatin nanoparticle carrier to enhance cisplatin accumulation in cancerous lungs via inhalation. Biomat 30(20):3476–3485
Uchenna Agu R, Ikechukwu Ugwoke M, Armand M, Kinget R, Verbeke N (2001) The lung as a route for systemic delivery of therapeutic proteins and peptides. Resp Res 2(4):1–2
Vaghasiya K, Ray E, Singh R, Jadhav K, Sharma A, Khan R, Katare OP, Verma RK (2021) Efficient, enzyme responsive and tumor receptor targeting gelatin nanoparticles decorated with concanavalin-A for site-specific and controlled drug delivery for cancer therapy. Mat Sci Eng: C 123:112027
Vaidya B, Parvathaneni V, Kulkarni NS, Shukla SK, Damon JK, Sarode A et al (2019) Cyclodextrin modified erlotinib loaded PLGA nanoparticles for improved therapeutic efficacy against non-small cell lung cancer. Int J Biol Macromol 122:338–347
Wada S, Yada E, Ohtake J, Sasada T (2017) Personalized peptide vaccines for cancer therapy: current progress and state of the art. Expert Rev Precis Med Drug Dev 2:371–381
Wang Z, Qiao R, Tang N, Lu Z, Wang H, Zhang Z, Xue X, Huang Z, Zhang S, Zhang G, Li Y (2017) Active targeting theranostic iron oxide nanoparticles for MRI and magnetic resonance-guided focused ultrasound ablation of lung cancer. Biomat 127:25–35
Wang X, Gu M, Toh TB, Abdullah NL, Chow EK (2018) Stimuli-responsive nanodiamond-based biosensor for enhanced metastatic tumor site detection. SLAS TECHNOLOGY: Translating Life Sci Innovat 23(1):44–56
Wang X, Chen H, Zeng X, Guo W, Jin Y, Wang S et al (2019) Efficient lung cancer-targeted drug delivery via a nanoparticle/MSC system. Acta Pharm Sin b 9:167–176
Ward S, Casey D, Labarthe MC, Whelan M, Dalgleish A, Pandha H, Todryk S (2002) Immunotherapeutic potential of whole tumour cells. Cancer Immunol, Immunotherapy 51:351–357
Weber S, Zimmer A, Pardeike J (2014) Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for pulmonary application: a review of the state of the art. Europ J Pharm Biopharm 86(1):7–22
Webster DM, Sundaram P, Byrne ME (2013) Injectable nanomaterials for drug delivery: carriers, targeting moieties, and therapeutics. Europ J Pharm Biopharmaceutics 84(1):1–20
Wittgen BP, Kunst PW, Van Der Born K, Van Wijk AW, Perkins W, Pilkiewicz FG, Perez-Soler R, Nicholson S, Peters GJ, Postmus PE (2007) Phase I study of aerosolized SLIT cisplatin in the treatment of patients with carcinoma of the lung. Clin Cancer Res 13(8):2414–2421
Wu SH et al (2008) Multifunctional mesoporous silica nanoparticles for intracellular labeling and animal magnetic resonance imaging studies. ChemBioChem 9(1):53–57
Wu H, Zhao Y, Mu X, Wu H, Chen L, Liu W, Mu Y, Liu J, Wei X (2015) A silica–polymer composite nano system for tumor-targeted imaging and p53 gene therapy of lung cancer. J Biomater Sci Polym Ed 26(6):384–400
Wu YF, Wu HC, Kuan CH, Lin CJ, Wang LW, Chang CW, Wang TW (2016) Multi-functionalized carbon dots as theranostic nanoagent for gene delivery in lung cancer therapy. Scientific Rep 6(1):1–2
Xia L, Schrump DS, Gildersleeve JC (2016) Whole-cell cancer vaccines induce large antibody responses to carbohydrates and glycoproteins. Cell Chem Biol 23(12):1515–1525
Xiaoyu H, Ruonan S, Xiao W, He K, Shan R, Fei X, Huang G (2023) Study on co-delivery of pemetrexed disodium and Bcl-2 siRNA by poly-γ-glutamic acid-modified cationic liposomes for the inhibition of NSCLC. Drug Dev Ind Pharm 49(1):62–74
Xie Y, Aillon KL, Cai S, Christian JM, Davies NM, Berkland CJ et al (2010) Pulmonary delivery of cisplatin-hyaluronan conjugates via endotracheal instillation for the treatment of lung cancer. Int J Pharm 392:156–163
Xue HY, Wong HL (2011) Tailoring nanostructured solid-lipid carriers for time-controlled intracellular siRNA kinetics to sustain RNAi-mediated chemosensitization. Biomat 32(10):2662–2672
Yang Y, Huang Z, Li J, Mo Z, Huang Y, Ma C et al (2019a) PLGA porous microspheres dry powders for codelivery of afatinib-loaded solid lipid nanoparticles and paclitaxel: novel therapy for EGFR tyrosine kinase inhibitors resistant nonsmall cell lung cancer. Adv Healthc Mater 8:1900965
Yang W, Deng X, Huang W, Qing X, Shao Z (2019b) The physicochemical properties of graphene nanocomposites influence the anticancer effect. J Oncol 2019:7254534
Yu W, Liu C, Liu Y, Zhang N, Xu W (2010) Mannan-modified solid lipid nanoparticles for targeted gene delivery to alveolar macrophages. Pharmaceutical Res 27(8):1584–1596
Yu B, Tan L, Zheng R, Tan H, Zheng L (2016) Targeted delivery and controlled release of paclitaxel for the treatment of lung cancer using single-walled carbon nanotubes. Mat Sci Eng: C 68:579–584
Zhang XG, Miao J, Dai YQ, Du YZ, Yuan H, Hu FQ (2008) Reversal activity of nanostructured lipid carriers loading cytotoxic drug in multi-drug resistant cancer cells. Int J Pharma 361(1–2):239–244
Zhang P, Ling G, Pan X, Sun J, Zhang T, Pu X, Yin S, He Z (2012) Novel nanostructured lipid-dextran sulfate hybrid carriers overcome tumor multidrug resistance of mitoxantrone hydrochloride. Nanomedicine Nanotech, Biol Med 8(2):185–93
Zhang L, Liu Z, Kong C, Liu C, Yang K, Chen H et al (2018) Improving drug delivery of micellar paclitaxel against non-small cell lung cancer by co-loading itraconazole as a micelle stabilizer and a tumor vascular manipulator. Small 14:e1802112
Zhang H, Penninger J, Li Y et al (2020) Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 46:586–590
Zhao F, Qian Y, Li H, Yang Y, Wang J, Yu W, Li M, Cheng W, Shan L (2022) Amentoflavone-loaded nanoparticles enhanced chemotherapy efficacy by inhibition of AKR1B10. Nanotechnology 33(38):385101
Zhao ZT, Wang J, Fang L, Qian XD, Cai Y, Cao HQ, Wang GR, He ML, Jiang YY, Wang DG, Li YP (2023) Dual-responsive nanoparticles loading bevacizumab and gefitinib for molecular targeted therapy against non-small cell lung cancer. Acta Pharmacol Sin 44(1):244–254
Zhou F, Zhou CC (2015) Targeted therapies for patients with advanced NSCLC harboring wild-type EGFR: what’s new and what’s enough. Chinese J Cancer 34(3):1
Zhu F, Tan G, Jiang Y, Yu Z, Ren F (2018) Rational design of multi-stimuli-responsive gold nanorod–curcumin conjugates for chemo-photothermal synergistic cancer therapy. Biomat Sci 6(11):2905–2917
Author information
Authors and Affiliations
Contributions
Conceptualization: Sumel Ashique, Ashish Garg, and Neeraj Mishra; methodology: Sumel Ashique, Neha Raina, Radha Rani, and Long Chiau Ming; formal analysis and investigation: Sumel Ashique, Neha Raina, Madhu Gupta; writing—original draft preparation: Sumel Ashique, Ashish Garg, and Neeraj Mishra; writing—review and editing: Ashish Garg, Neeraj Mishra, and Madhu Gupta. The authors confirm that no paper mill and artificial intelligence was used.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All the authors have read the manuscript and have approved this submission.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ashique, S., Garg, A., Mishra, N. et al. Nano-mediated strategy for targeting and treatment of non-small cell lung cancer (NSCLC). Naunyn-Schmiedeberg's Arch Pharmacol 396, 2769–2792 (2023). https://doi.org/10.1007/s00210-023-02522-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02522-5