Abstract
We investigated the role of RhoA/Rho-kinase (ROCK) and PKC in the inhibitory effect of L-cysteine/hydrogen sulfide (H2S) pathway on the carbachol-mediated contraction of mouse bladder smooth muscle. Carbachol (10−8–10−4 M) induced a concentration-dependent contraction in bladder tissues. L-cysteine (H2S precursor; 10−2 M) and exogenous H2S (NaHS; 10−3 M) reduced the contractions evoked by carbachol by ~ 49 and ~ 53%, respectively, relative to control. The inhibitory effect of L-cysteine on contractions to carbachol was reversed by 10−2 M PAG (~ 40%) and 10−3 M AOAA (~ 55%), cystathionine-gamma-lyase (CSE) and cystathionine-β-synthase (CBS) inhibitor, respectively. Y-27632 (10−6 M) and GF 109203X (10−6 M), a specific ROCK and PKC inhibitor, respectively, reduced contractions evoked by carbachol (~ 18 and ~ 24% respectively), and the inhibitory effect of Y-27632 and GF 109203X on contractions was reversed by PAG (~ 29 and ~ 19%, respectively) but not by AOAA. Also, Y-27632 and GF 109203X reduced the inhibitory responses of L-cysteine on the carbachol-induced contractions (~ 38 and ~ 52% respectively), and PAG abolished the inhibitory effect of L-cysteine on the contractions in the presence of Y-27632 (~ 38%). Also, the protein expressions of CSE, CBS, and 3-MST enzymes responsible for endogenous H2S synthesis were detected by Western blot method. H2S level was increased by L-cysteine, Y-27632, and GF 109203X (from 0.12 ± 0.02 to 0.47 ± 0.13, 0.26 ± 0.03, and 0.23 ± 0.06 nmol/mg respectively), and this augmentation in H2S level decreased with PAG (0.17 ± 0.02, 0.15 ± 0.03, and 0.07 ± 0.04 nmol/mg respectively). Furthermore, L-cysteine and NaHS reduced carbachol-induced ROCK-1, pMYPT1, and pMLC20 levels. Inhibitory effects of L-cysteine on ROCK-1, pMYPT1, and pMLC20 levels, but not of NaHS, were reversed by PAG. These results suggest that there is an interaction between L-cysteine/H2S and RhoA/ROCK pathway via inhibition of ROCK-1, pMYPT1, and pMLC20, and the inhibition of RhoA/ROCK and/or PKC signal pathway may be mediated by the CSE-generated H2S in mouse bladder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The contraction of detrusor smooth muscle is coordinated by several receptors and signaling pathways (Andersson and Hedlund 2002; Andersson, and Arner 2004). The alterations in intracellular free calcium [Ca2+]I influence the myosin light chain kinase (MLCK) activity, which causes phosphorylation of the myosin light chains (MLC) and regulates the tone of urinary bladder smooth muscle (Fry et al. 2010). Myosin light chain phosphatase (MLCP) reverses the phosphorylation of MLC, and changes of MLCP activity has been defined as essential regulatory mechanism (Stull et al. 1991). The phosphorylation range of MLC is regulated by the rate of MLCK to MLCP activities (Somlyo et al. 1999). It has been shown that intracellular [Ca2+]i-independent pathways contribute to the smooth muscle contraction (Somlyo and Somlyo 1998; Uehata et al. 1997; Kawano et al. 2002). Previous studies reported that several pathways which directly inhibit MLCP may augment the contraction without a corresponding increase in [Ca2+]i. This mechanism is defined as “Ca2+ sensitization” (Chiba and Misawa 2004), and RhoA/Rho-kinase (ROCK) and protein kinase C (PKC) are accepted as two major regulating pathways for Ca2+ sensitivity by MLCP inhibition (Somlyo and Somlyo 2003). Also, Ca2+sensitization has been shown in the urinary bladder smooth muscle by various studies (Durlu-Kandilci and Brading 2006; Takahashi et al. 2004; Wibberley et al. 2003). RhoA/ROCK and PKC pathways exist in bladder smooth muscle and modulate Ca2+sensitivity in the detrusor activated after receptor stimulation (Wibberley et al. 2003; Jezior et al. 2001; Boberg et al. 2012; Anjum 2018; Teixeira et al. 2007). Several studies reported that PKC plays a role in the coordination of ordinary bladder function and that dysfunction of PKC pathway is associated with detrusor over activity, decreased contractility, and attenuated void volume (Hypolite and Malykhina 2015). Besides this, stimulation of G protein-coupled muscarinic M2 and M3 receptors with carbachol produces urinary bladder smooth muscle contraction (Uchiyama and Chess-Williams 2004; Yamaguchi et al. 1996; Mimata et al. 1997; Frazier et al. 2008).
H2S, a gaseous transmitter, has many physiological effects such as mediating smooth muscle relaxation (Dunn et al. 2016), regulating blood pressure (Yang et al. 2008), preventing acute myocardial infarction (Wang et al. 2010), and regulating renin activity and insulin release (Wu et al. 2009). The relaxant effect of H2S has been reported in several smooth muscles including corpus cavernosum, vascular tissues, and gastrointestinal tissues (Cheng et al. 2004; Dhaese and Lefebvre 2009; Aydinoglu and Ogulener 2016). Also, H2S is synthesized in the bladder tissue of various species, including humans, and endogenous H2S is thought to play a role in the regulation of bladder smooth muscle tone and pathological function of the bladder such as overactive bladder (Gai et al. 2013; Fusco et al. 2012). It has been shown that H2S is produced endogenously in mouse, rat, pig and human bladder and plays a role in the regulation of bladder smooth muscle (Fusco et al. 2012; Fernandes et al 2013a, b, 2014; Zou et al. 2018). H2S is endogenously synthesized by cystathionine-gamma-lyase (CSE), cystathionine-beta synthase (CBS), and 3-mercaptopurivate sulfur transferase (3-MST) (Kimura 2011; Abe and Kimura 1996). Expressions of CSE, CBS, and 3-MST were shown in rat (Zou et al. 2018), guinea pig (Fernandes et al. 2014), and human bladder tissues (Fusco et al. 2012). Recently, it has been reported the contribution of Rho-dependent pathway to H2S-induced relaxation in mouse, rabbit, and human colonic smooth muscle (Nalli et al. 2017), rat mesenteric artery (Hedegaard et al. 2016), mice basilar artery (Wen et al. 2019), mouse gastric fundus (Dhaese and Lefebvre 2009), mouse corpus cavernosum (Aydinoglu et al. 2019), bovine retinal arteries (Semiz et al. 2020), and rabbit gastric smooth muscle cells (Nalli et al. 2015). In addition, H2S has been demonstrated to activate PKCα, PKCε, and PKCδ in cardiomyocytes (Pan et al. 2008).
Regardless, the role of RhoA/ROCK/PKC pathway in H2S-induced relaxation in bladder smooth muscle has not been investigated, and to our knowledge, there is no study about the interaction between L-cysteine/H2S and RhoA/ROCK/PKC pathway on agonist-mediated contraction in bladder tissue. Therefore, in the present study, we investigated the role of RhoA/ROCK and PKC in the inhibitory effect of L-cysteine/H2S pathway on the carbachol-induced contraction of mouse bladder smooth muscle. As far as we know, this is the first report of contribution of ROCK and PKC to inhibitory effect of H2S in the urinary bladder. Our main findings suggest that there is an interaction between L-cysteine/H2S and the RhoA/ROCK/PKC pathway, and the interaction mainly occurs through the CSE-generated H2S in the mouse bladder tissues.
Materials and methods
Chemicals
The following drugs were used; amino-oxyacetic acid (o-carboxymethyl), dl-propargylglycine, carbachol chloride, L-cysteine, sodium hydrosulphide hydrate (Sigma Chemical Co., St Louis, MO, USA), trans-4-[(1R)-1-aminoethyl]-N-4-pyridinyl cyclohexane carboxamide dihydrochloride (Y-27632), and 2-[1-(3-dimethylaminopropyl)indol-3-yl]-3-(indol-3-yl) maleimide (GF 109203X) (TOCRIS, Bristol, UK). The stock solutions of GF 109203X were prepared in dimethyl sulfoxide (DMSO). DMSO per se did not affect the tone of the strips. All other drugs were dissolved in distilled water. NaHS was prepared fresh before each experiment and kept on ice.
Animals
Male Swiss albino mice were obtained from Cukurova University Health Sciences Application and Research Center. Male Swiss albino mice weighing 20–25 g were used in these experiments. Mice were kept under environmental conditions (12 h light/darkness cycles) and allowed free access to food and water. Protocols were conducted in accordance with national and international guidelines for the care and use of laboratory animals and approved by the Institutional Animal Care and Use Committee of Cukurova University and given the approval number 4/3/08.07.2019.
Experimental protocol
The experimental procedures detailed below are summarized in flow chart (Fig. 1).
Functional studies
Mice were killed by stunning and cervical dislocation. The bladder tissue was carefully removed. Strips (0.5 mm wide and 4–5 mm long) from the midportion of the urinary bladder were mounted under 9.80 mN tension in an (5 ml) organ bath filled with Krebs solution (in mM: NaCI 118.1, KCI 4.7, CaCI2 2.5, MgCI26H2O 1.2, KH2PO4 1.2, NaHCO3 25, and glucose 11.5). The bath medium was maintained at 37 °C and gassed with a mixture of 95% O2 and 5% CO2 at pH 7.4. Muscle strips were allowed to equilibrate for 60 min, during which the medium was changed every 15 min. Changes in muscle length were recorded isometrically via an isometric transducer (MP35).
After the equilibration period of 60 min, isolated mouse bladder strips were pre-contracted with isotonic 60 mM KCl to determine contractile ability of the strips. The tissues were then washed out with Krebs solution, and tissues were left in re-equilibration for 30 min. After this period, the cumulative carbachol (10−8–10−4 M) concentration–response curve was obtained. After the first series of cumulative contractile responses was obtained with carbachol, the tissues were left in equilibration for 30 min and the second series of cumulative response curve was obtained with carbachol. Also, a third series of carbachol cumulative response curve was similarly obtained from the same tissue. Thus, the tissues were standardized by repeated contractions with carbachol.
To investigate the effect of L-cysteine/H2S pathway on contractile response induced by carbachol in mouse bladder strips, concentration–response curve to carbachol was studied in the presence of L-cysteine (precursor of H2S; 10−2 M). In this set of experiments, after the second series of contractile responses to cumulative carbachol (10−8–10−4 M) was obtained, the tissues were washed and incubated for 30 min with L-cysteine (10− 2 M), and the third series of cumulative response curve was obtained with carbachol. In addition, the effect of H2S donor NaHS (exogenous H2S; 10−3 M) on carbachol-induced conractile responses were studied in the same manner. In some experiments, to clarify the role of endogenous H2S production in the inhibitory effect of L-cysteine on the carbachol-induced contractions, the effects of propargylglycine (PAG, 10−2 M; a non-competitive cystathionine-gamma-lyase inhibitor) and amino-oxyacetic acid (AOAA, 10−3 M; a cystathionine-β-synthetase inhibitor) were investigated on the carbachol-induced contractile responses in the presence of L-cysteine. After the second series of contractile response to carbachol was obtained, the tissue was incubated in a medium containing PAG (10−2 M) or AOAA (10−3 M) for 30 min with L-cysteine, and the response to carbachol was repeated. Furthermore, we studied the effects of H2S enzyme inhibitors PAG (10−2 M) and AOAA (10−3 M) on the carbachol-induced contractions alone in the same manner.
To assess the involvement of RhoA/ROCK pathway in the contractile responses to carbachol, the effect of specific ROCK enzyme inhibitor (R)-( +)-trans-N-(4-pyridyl)-4-(1-aminoethyl)-cyclohexane carboxamide (Y-27632; 10−6 M) on cumulative carbachol (10−8–10−4 M)-induced contractions was investigated. In this group of experiments, after the second series of cumulative contraction responses to carbachol was obtained, tissues were washed and incubated with 10−6 M Y-27632 for 30 min, and the third series of carbachol cumulative response curves was obtained. The contribution of H2S/L-cysteine to the inhibitory effect of Y-27632 on carbachol-induced contractions was studied in the presence of PAG and AOAA in bladder strips. With this purpose, after the second series of contractile responses to carbachol was obtained cumulatively (10−8–10−4 M), tissues were washed and incubated with PAG (10−2 M) plus Y-27632 (10−6 M) or AOAA (10−3 M) plus Y-27632 (10−6 M) for 30 min, and then contractile responses to carbachol were obtained in the same manner. Also, to investigate the interaction between H2S/L-cysteine and RhoA/ROCK pathway in mouse bladder smooth muscle, L-cysteine (10−2 M) was added to bath medium and then contractile responses to carbachol were obtained in the same manner in the presence of Y-27632 (10−6 M). In some experiments, the effect of Y-27632 on the inhibitory effect of L-cysteine on the carbachol-induced contractions was investigated in the presence of H2S enzyme inhibitors PAG and AOAA in mice bladder strips. Furthermore, the role of RhoA/ROCK in the inhibitory effect of NaHS was studied in the same manner.
In the other sets of experiments, to investigate the contribution of protein kinase C (PKC) to both carbachol-induced contractions and the inhibitory effect of L-cysteine on contractions, we studied the effects of GF 109203X (10−6 M) on carbachol-induced contractions in the presence of L-cysteine or PAG in bladder strips in the same way.
Measurement of endogenous H2S release in mouse bladder strips
H2S production in bladder tissue samples was determined with a commercially available H2S colorimetric assay kit (Elabscience Biotechnology Co., Ltd., Wuhan, China) through the reaction between H2S and zinc acetate, N, N-dimethyl-p-phenylenediamine, and ammonium ferric sulfate. Protein concentration was determined by using a bicinchoninic acid assay kit (Sigma Chemical Co., St. Luis, MO, USA). Bladder tissues were homogenized in extraction solution and centrifuged for 10 min at 4 °C at 10.000 × g, and the supernatant was collected. The supernatant solution was mixed with an equal volume of reagents 1 and 2. After centrifugation, the sediment was dissolved in reagents 1, 3, and 4. The supernatant obtained after centrifugation was mixed with reagent 5. The absorbance of solutions was measured after 20 min at a wavelength of 665 nm and H2S concentrations in bladder tissues, expressed as nmol/mg protein.
Expression of CSE, CBS, and 3-MST in mouse bladder
CSE, CBS, and 3-MST protein expressions of mice bladder were determined by Western blot analysis. Tissues were immediately frozen by liquid nitrogen and stored at − 20 °C. For the homogenization, tissues were weighed (almost 15 mg) and diluted in ice cold RIPA buffer system containing Halt protease inhibitor. Tissues were homogenized by Bandelin Sonopuls HD Ultrasonic Homogeniser (D-12207, Germany). Homogenates were centrifuged 14,000 rpm for 15 min and at 4 °C. Following centrifugation, protein concentrations of supernatants were measured by the Bradford method and supernatants were stored at − 20 °C.
Proteins from bladder tissues were obtained and boiled in the presence of Laemmli gel loading buffer containing SDS and β-mercaptoethanol as reducing agents at pH 6.8 and kept at − 20 °C until use. Proteins were separated in a 10% SDS-PAGE gel containing a 4% stacking gel, under denaturing conditions at 100 V for 1 h and 40 min at room temperature. Proteins in the gel were then transferred to a PVDF membrane (Millipore), which was previously rehydrated in methanol and equilibrated with transfer buffer. Then a sandwich cassette was prepared according to the manufacturer’s (Bio-Rad) instructions, and proteins were electro blotted on to the PVDF membrane for 1 h and 30 min at 4 °C. After transfer, the membrane was briefly washed with phosphate-buffered saline (PBS) solution containing 1% Tween-20. The blots were then blocked for 1 h with 5% nonfat dry milk in PBS and constantly agitated and incubated with the following primary antibodies: CSE (dilution 1:1000, ab151769, RRID: AB_2861405), CBS (dilution 1:500, ab135626, RRID: AB_2814659), 3-MST (dilution 1:5000, sc374326, RRID: AB_10986129), and beta-actin (dilution1:1000, CST-4967S, RRID: AB_330288) overnight at + 4 °C. The membranes were washed three times for 10 min each with PBS-T and incubated with a horseradish peroxidase-conjugated second antibody (dilution 1:5000; Santa Cruz Biotechnology) at room temperature for 1 h with constant agitation. After briefly drying, the membrane was incubated with 3 ml of HRP ECL substrate mixture (1.5 ml hydrogen peroxide and 1.5 ml enhancer) (Biorad) and incubated for 1 min at room temperature. The membranes were wrapped with stretch film and placed in Chemi Doc MP (Bio-rad) for 1–10 min. The bands were quantified using the Image J program. The protein expression was normalized to the β-actin content.
Measurement of ROCK, phosphorylated MYPT1, and MLC20
To evaluate the contribution of ROCK, MYPT1, and MLC20 in L-cysteine-induced inhibitory effect on contractile responses to carbachol, the expression of the ROCK-1, phosphorylated MYPT1, and MLC20 was studied by the Western blot method. The isolated preparations were incubated in the organ baths under the same conditions as in tension recording experiments (Krebs solution at 37 °C under a stream of 5% CO2 and 95% O2). Some of the tissues were treated with the drugs; some were not to serve as control specimens. Tissues were pre-incubated with L-cysteine (10−2 M; 30 min), NaHS (10−3; 5 min), PAG (10−2 M; 60 min) plus L-cysteine (10−2 M; 30 min), and PAG (10−2 M; 60 min) plus NaHS (10−3 M; 5 min). Afterward, carbachol (10−4 M, 4 min) was added to the bath medium, and the tissues were frozen in liquid nitrogen. Tissues that had been frozen were homogenized in ice-cold RIPA buffer system containing Halt protease inhibitor by Bandelin Sonopuls HD Ultrasonic Homogeniser (D-12207, Germany), and total protein content was measured by the Bradford method as mentioned above. Proteins from bladder tissues were obtained and boiled in the presence of Laemmli gel loading buffer containing SDS and β-mercaptoethanol as reducing agents at pH 6.8 and kept at − 20 °C until ready for use. Proteins were separated in a 10% SDS-PAGE gel containing a 4% stacking gel, under denaturing conditions at 100 V for 1 h and 40 min at room temperature. Proteins in the gel were then transferred to a PVDF membrane (Millipore), which was previously rehydrated in methanol and equilibrated with transfer buffer. Then, a sandwich cassette was prepared according to the manufacturer’s (Bio-Rad) instructions, and proteins were electro-blotted onto the PVDF membrane for 1 h and 30 min at 4 °C. After transfer, the membrane was briefly washed with Tris-buffered saline (TBS) containing 1% Tween-20. Bovine serum albumin (BSA) at concentration 5% was used in the wash buffer as a blocking agent. Membranes were blocked for 1 h at room temperature with gentle and constant agitation and incubated with primary antibodies ROCK-1 (dilution 1:1000, AF7016, RRID: AB_2835321), pMYPT1 (dilution 1:500, CST-5163S, RRID: AB_10691830), pMLC20 (dilution 1:500, 3671S, RRID:AB_330248), and beta-actin (dilution 1:1000, CST-4967S, RRID: AB_330288) overnight. The membranes were washed three times for 10 min each with TBS-T and incubated with a horseradish peroxidase-conjugated second antibody (dilution 1:5000; Santa Cruz Biotechnology) at room temperature for 1 h with constant agitation. After briefly drying, the membrane was incubated with 3 ml of HRP ECL substrate mixture (1.5 ml hydrogen peroxide and 1.5 ml enhancer) (Bio-rad) and incubated for 1 min at room temperature. The membranes were wrapped with stretch film and placed in ChemiDoc MP (Bio-rad) for 1–10 min. The bands were quantified using the Image J program. The protein expression was normalized to the β-actin content.
Statistical analysis
Contractile activity of muscle strips was calculated as maximum force generated in response to carbachol, and the effect of L-cysteine or NaHS was calculated as percent decrease in maximum contraction. The contractile responses to cumulative carbachol were expressed as “mg,” and the maximum contraction of second series carbachol was considered 100 (A). Third series of carbachol contraction (B) was calculated as a percentage of maximal carbachol contraction induced by second series. Emax was expressed as the maximum contraction achieved by the third series of carbachol (C) (C:Bx100/A). The sensitivities of the bladder strip to carbachol were calculated as the effective concentration that elicits 50% of the maximal response by using nonlinear regression curve fit and expressed as pEC50 (− log M) (GraphPAD Software, version 5.00, San Diego, USA). Data are presented as “mean ± SD” while “n” is the number of bladder strips isolated from different animals. The “null” hypothesis was determined as there was no difference between % contractile response of groups. For testing of this hypothesis, statistically analysis was performed by one-way ANOVA, paired and unpaired t-tests. Also, one-way ANOVA was corrected by post hoc Bonferroni test. A P-value less than 0.05 was considered statistically significant. The null hypothesis was rejected at P < 0.05. As statistical analyses included group sizes partly below n = 5 and due to the explorative study design, presented p-values need to be considered preliminary and purely descriptive.
Results
Effect of L-cysteine/H2S pathway on carbachol-induced contractions
Carbachol, muscarinic receptor agonist, caused sustained contraction in concentration-dependent manner (10−8–10−4 M) in isolated mouse bladder strips. There was a difference between first and second contractile responses to carbachol but not between second and third series contractions (Fig. 2 a and b).
Contractile response to muscarinic receptor agonist carbachol in mouse bladder tissues. Representative trace and the cumulative concentration–response curve to carbachol (10−8–10−4 M) (a, b). All values are mean ± SD. (n = 4–6). *P < 0.05 significantly different from Emax of 1st series; one-way ANOVA and paired t-test followed by Bonferroni’s comparison test
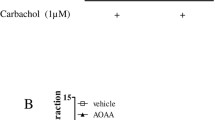
Therefore, the contractile responses to carbachol were expressed as a percentage of the maximal contractile response to second series of cumulative carbachol. To elucidate the inhibitory effect of endogenous and exogenous H2S on carbachol-induced contractions of mouse bladder strips, the effects of L-cysteine (an endogenous H2S precursor) and NaHS (exogenous H2S) were studied. Pre-treatment with L-cysteine (10−2 M) and NaHS (10−3 M) significantly reduced contractile responses to carbachol from 77.63 ± 10.70% to 39.85 ± 5.90 and 36.24 ± 12.40%, respectively (Fig. 3a and b). Maximum contractile responses (Emax) to carbachol but not pEC50 values were reduced by L-cysteine and NaHS (Table 1). The inhibitory effect of L-cysteine on contractile responses induced by carbachol was reversed by 10− 2 M PAG from 39.85 ± 5.90 to 55.78 ± 12.80% (Fig. 3c and d) and 10−3 M AOAA from 61.90 ± 16.60% (Fig. 3e and f), CSE and CBS enzyme inhibitor, respectively, suggesting the endogenous H2S-mediated inhibitory effect of L-cysteine on contractile responses induced by carbachol in mouse bladder strips. Maximum contractile responses (Emax) to carbachol but not pEC50 values were increased by PAG and AOAA in the presence of L-cysteine (Table 1). On the other hand, pre-incubation of bladder strips with PAG (10−2 M) or AOAA (10−3 M) alone did not affect contractile response to carbachol compared to the control group (Fig. 3d and f), suggesting that basal endogenous H2S has no effect on carbachol-induced contractile responses.
The effect of L-cysteine/ H2S pathway on the carbachol-induced contractions in mouse bladder tissues. Representative trace and the cumulative concentration–response curve to carbachol (10−8–10−4 M) in the absence (control) or presence of L-cysteine (10−2 M) or NaHS (10−3 M) (a, b). Representative traces and the cumulative concentration–response curve to carbachol (10−8–10–4 M) in the absence or presence of L-cysteine (10−2 M) or L-cysteine (10−2 M) plus PAG (10−2 M) (c, d) or L-cysteine (10−2 M) plus AOAA (10−3 M) (e, f). Responses are expressed as a percentage of the response evoked by carbachol. All values are mean ± SD (n = 4–6).*P < 0.05 significantly different from Emax of control group; # P < 0.05 significantly different from Emax of L-cysteine group; one-way ANOVA and unpaired t-test followed by Bonferroni’s comparison test
Also, in order to determine L-cysteine/H2S pathway in mouse bladder tissue, the protein expression of CBS, CSE, and MST which enzymes responsible for endogenous H2S synthesis from L-cysteine was identified by using Western blotting methods (Fig. 4), supporting that H2S can be produced endogenously in the mouse bladder tissue.
The existence of CBS, CSE, and MST in mouse bladder tissues. Representative image of Western blot analysis showing the expression of CBS, CSE, MST, and β-actin. The graph shows the relative protein expression levels of CBS, CSE, MST, and versus β-actin. Values were normalized by the intensity of each band relative to the intensity of the loading control: values of β-actin (4.7, 8.6, 1, and 7.1). All values are mean ± SD (n = 4)
The role of ROCK in the inhibitory effect of L-cysteine/H2S on carbachol-induced contractions
To clarify the contribution of RhoA/ROCK pathway to the contractile responses to carbachol, we studied the effects of Y-27632 on contractions evoked by carbachol. Pre-treatment with Y-27632 (10−6 M) reduced contractile responses induced by carbachol in mouse bladder strips from 77.63 ± 10.70 to 63.45 ± 11.00% (Fig. 5a and b). Maximum contractile responses to carbachol (Emax) but not pEC50 values were reduced in the presence of Y-27632 (Table 2). To determine the contribution of endogenous H2S to the inhibitory effect of Y-27632 on carbachol-induced contractions, we investigated the effects of PAG and AOAA on the inhibitory responses of Y-27632 on contractions to carbachol. The inhibitory effect of Y-27632 (10−6 M) on contractions to carbachol was reversed by pre-treatment with 10−2 M PAG from 63.45 ± 11.00 to 81.81 ± 8.30% but not with 10−3 M AOAA (69.80 ± 12.20%) (Fig. 5a and b). Maximum contractile responses (Emax) to carbachol were increased by PAG in the presence of Y-27632 (Table 2).
The role of ROCK on the carbachol induced in mouse bladder tissues. Representative trace and the cumulative concentration–response curve to carbachol (10−8–10−4 M) in the absence or presence of Y-27632 (10−6 M) or Y-27632 (10−6 M) plus PAG (10−2 M) or L-cysteine (10−2 M) plus AOAA (10−3 M) (a, b). All values are mean ± SD (n = 4–6).*P < 0.05 significantly different from Emax of control group; + P < 0.05 significantly different from Emax of Y-27632 group; one-way ANOVA and unpaired t-test followed by Bonferroni’s comparison test
To clarify the contribution of ROCK pathway to the inhibitory effect of endogenous and exogenous H2S on carbachol-induced contractions in mouse bladder strips, we studied the effects of Y-27632 on the inhibition of L-cysteine and NaHS on contractions to carbachol. Y-27632 (10−6) partially altered the inhibitory responses of L-cysteine (10−2 M) from 39.85 ± 5.90 to 55.01 ± 13.90% but not NaHS (10−2 M) from 36.24 ± 12.40 to 40.03 ± 10.50% on the carbachol-induced contractions (Fig. 6 a–d). Maximum contractile responses (Emax) to carbachol but not pEC50 values were increased by Y-27632 compared to L-cysteine (Table 2). PAG but not AOAA almost prevented the inhibitory effect of L-cysteine on the contractile responses to carbachol in the presence of Y-27632 on the carbachol-induced contraction from 55.01 ± 13.90 to 76.16 ± 16.30 and 51.49 ± 13.60%, respectively (Fig. 6 e–g). Maximum contractile responses (Emax) and pEC50 values to carbachol were not altered compared to the control group (Table 2). The reversal response induced by PAG in the presence of Y-27623 was greater than that of L-cysteine plus PAG.
The role of ROCK in the inhibitory effect of L-cysteine/H2S on carbachol-induced contractions in mouse bladder tissues. Representative traces and the cumulative concentration–response curve to carbachol (10−8 –10−4 M) in the absence and presence of L-cysteine (10−2 M) and L-cysteine (10−2 M) plus Y-27632 (10−6 M) (a, b) and NaHS (10−3 M), NaHS (10−3 M) plus Y-27632 (10−6 M) (c, d). Representative traces and the cumulative concentration–response curve to carbachol (10−8–10−4 M) in the presence of L-cysteine (10−2 M), L-cysteine (10−2 M) plus Y-27632 (10−6 M), or L-cysteine (10−2 M) plus Y-27632 (10−6 M) plus PAG (10−2 M) (e, f) or L-cysteine (10−2 M) plus Y-27632 (10−6 M) plus AOAA (10−3 M) (g). All values are mean ± SD. (n = 4–6).*P < 0.05 significantly different from Emax of control group; #P < 0.05 significantly different from Emax of L-cysteine group; one-way ANOVA and unpaired t-test followed by Bonferroni’s comparison test.&P < 0.05 significantly different from Emax of L-cysteine plus Y-276322 group; one-way ANOVA followed by Bonferroni’s comparison test
The contribution of PKC to the inhibitory effect of L-cysteine/H2S on carbachol-induced contractions
To investigate the contribution of PKC pathway to the contractile responses to carbachol in mouse bladder strips, we studied the effects of GF 109203X, a specific PKC inhibitor, on contractions to carbachol. Pre-treatment with GF 109203X (10−6 M) reduced contractile responses induced by carbachol in mouse bladder strips from 77.63 ± 10.70 to 58.99 ± 6.20 (Fig. 7a and b). Maximum contractile responses to carbachol (Emax) but not pEC50 values were reduced in the presence of GF 109203X (Table 2). To determine the contribution of endogenous H2S to the inhibitory effect of GF 109203X on carbachol-induced contractions, we investigated the effects of PAG (10−2 M) on the inhibitory responses of GF 109203X on contractions to carbachol. The inhibitory effect of GF 109203X (10−6 M) on contractions to carbachol was reversed by pretreatment with PAG from to 58.99 ± 6.20 to 70.25 ± 7.30% (Fig. 7a and b). Maximum contractile responses (Emax) and pEC50 values to carbachol were not altered compared to GF-109203 (Table 2).
The role of PKC in the inhibitory effect of L-cysteine/H2S on carbachol-induced contractions in mouse bladder tissues. Representative traces and the cumulative concentration–response curve to carbachol (10−8–10−4 M) in the absence and presence of GF-109203x (10−6 M) or GF-109203x (10−6 M) plus PAG (10−2 M) (a, b). Representative traces and the cumulative concentration–response curve to carbachol (10−8–10−4 M) in the absence and presence of L-cysteine (10−2 M) and L-cysteine (10−2 M) plus GF-109203x (10−6 M) (c, d). All values are mean ± SD (n = 4–6).∗P < 0.05 significantly different from Emax of control group;+P < 0.05 significantly different from Emax of GF-109203 × group; #P < 0.05 significantly different from Emax of L-cysteine plus GF-109203 × group; one-way ANOVA and unpaired t-test followed by Bonferroni’s comparison test
To clarify the contribution of PKC pathway to the inhibitory effect of endogenous H2S on carbachol-induced contractions in mouse bladder strips, we studied the effects of GF 109203X (10−6 M) on the inhibition of L-cysteine on contractions to carbachol. The inhibitory effect of L-cysteine on contractile responses induced by carbachol was reversed by GF 109203X from 39.85 ± 5.90 to 60.50 ± 13.60% (Fig. 7c and d). Maximum contractile responses (Emax) but not pEC50 values to carbachol were significantly increased compared to L-cysteine (Table 2).
The effect of ROCK and PKC inhibition on H2S generation
We studied the effects of Y27632 and GF 109203X, specific ROCK and PKC inhibitors, respectively, on the H2S generation. Mouse bladder tissue generated detectable amounts of basal H2S (0.12 ± 0.02 nmol/mg). CSE inhibitor PAG (10−2 M) reduced the increase in H2S production stimulated with L-cysteine from 0.47 ± 0.13 to 0.17 ± 0.02 nmol/mg, suggesting that mouse bladder tissue is capable of synthesizing H2S from L-cysteine. Y-27632 and GF 109203X increased basal H2S generation (0.26 ± 0.03 and 0.23 ± 0.06 nmol/mg, respectively), and PAG (10−2 M) reduced the increase in H2S production in the presence of Y27632 and GF 109203X (0.15 ± 0.03 and 0.07 ± 0.04 nmol/mg, respectively; Fig. 8), suggesting the interaction between H2S and the RhoA/ ROCK and PKC pathway, and the interaction mainly occurs through the CSE enzyme in the mouse bladder tissues.
The role of CSE, Rho- kinase, and PKC inhibition on endogenous H2S formation in mouse bladder tissue. The graph showing to endogenous H2S production in the absence or presence of L-cysteine (10−2 M), L-cysteine (10−2 M) plus PAG (10−2 M), Y-27632 (10−6 M), Y-27632 (10−6 M) plus PAG (10−2 M), GF-109203x (10−6 M), and GF-109203x (10−6 M) plus PAG (10−2 M) in mouse bladder. All values are mean ± SD (n = 3). ∗P < 0.05 significantly different from control; +P < 0.05 significantly different from L-cysteine; #P < 0.05 significantly different from Y-27632; &P < 0.05 significantly different from GF-109203x; one-way ANOVA and unpaired t-test followed by Bonferroni’s comparison test
The effect of L-cysteine/H2S pathway on ROCK1
To determine whether the inhibitory effect of CSE-produced H2S on carbachol-induced contractions is associated with RhoA/ROCK pathway, ROCK1 protein was studied with Western blot method. Treatment of bladder tissues for 4 min with carbachol (10−4 M) caused an increase in ROCK1 level compared to control. Addition of L-cysteine (10−2 M) or NaHS (10−3 M) reduced the carbachol-increased ROCK1 level (Fig. 8), and CSE inhibitor PAG (10−2 M) increased the ROCK1 level in the presence of L-cysteine but not NaHS (Fig. 9).
The effect of L-cysteine/H2S pathway on ROCK-1 in mouse bladder tissue. Representative image of Western blot analysis showing the effect of carbachol (10−4 M) on ROCK-1 protein in the absence and presence of L-cysteine (10−2 M), exogenous H2S (NaHS; 10−3 M), L-cysteine (10−2 M) plus PAG (10−2 M), and NaHS (10−3 M) plus PAG (10−2 M). The graph showing the relative protein levels of ROCK-1 versus β-actin in mouse bladder. Values were normalized by the intensity of each band relative to the intensity of the loading control: values of β-actin (1.3, 1.0, 1.0, 1.5, 1.1, 1.0, 1.2, 1.8, 1.5, 2.3, 1.5, 1.7, 2.2, 2.5, 1.5, 1.0, and 2.0). All values are mean ± SD (n = 2–3). ∗P < 0.05 significantly different from control; +P < 0.05 significantly different from carbachol; &P < L-cysteine (unpaired t-test)
The effect of L-cysteine/H2S pathway on pMYPT1 and pMLC20 levels
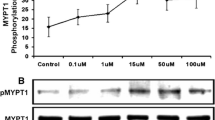
It has been shown that activation of ROCK and PKC leads to inhibition of MLC20 phosphatase activity via phosphorylation of MYPT1 at Thr696. The pMLC20 and pMYTP1 levels increased in carbachol-treatment tissues compared to the control (Fig. 10 a–c). To investigate that the inhibitory effect of H2S on muscle contraction is mediated via inhibition of pMLC20 and pMYTP-1, we next measured levels of MLC20 and MYPT1 phosphorylation in the presence of L-cysteine and NaHS. Consistent with inhibition of ROCK1, endogenous (L-cysteine; 10−2 M) and exogenous H2S (NaHS; 10−3 M) caused decrease of carbachol-induced phosphorylation of MLC20 and MYPT1 (Fig. 10 a–c), and L-cysteine-induced inhibition of pMLC20 and pMYPT1 levels was reversed in the presence of PAG (10−2 M) in bladder strips (Fig. 10 a–c). PAG increased the carbachol-induced phosphorylation of MLC20 but not of pMYPT1 (Fig. 10a–c).
The effect of L-cysteine/H2S pathway on pMYPT1 and pMLC20 levels in mouse bladder tissue. Representative image of Western blot analysis (a) showing the effect of carbachol (10−4 M) on pMYPT1 and pMLC20 protein levels in the absence and presence of L-cysteine (10−2 M), exogenous H2S (NaHS; 10−3 M), L-cysteine (10−2 M) plus PAG (10−2 M), and NaHS (10−3 M) plus PAG (10−2 M). The graphs showing the relative protein levels of pMYPT1 (b) and pMLC20 (c) versus β-actin in mouse bladder. Values were normalized by the intensity of each band relative to the intensity of the loading control: values of β-actin (1.3, 1.0, 1.0, 1.5, 1.1, 1.0, 1.2, 1.8, 1.5, 2.3, 1.5, 1.7, 2.2, 2.5, 1.5, 1.0, 2.0, 2.0, 1.0, and 1.4) All values are mean ± SD (n = 2–3). ∗P < 0.05 significantly different from control; +P < 0.05 significantly different from carbachol; &P < 0.05 significantly different from L-cysteine (unpaired t-test)
Discussion
In the present study, we investigated the role of RhoA/ROCK and PKC in the inhibitory effect of L-cysteine/H2S pathway on the carbachol-mediated contraction of mouse bladder smooth muscle. Our main findings suggest that there is an interaction between L-cysteine/H2S and RhoA/ROCK pathway via inhibition of ROCK-1, pMYPT1, and pMLC20, and the inhibition of RhoA/ROCK and/or PKC signal pathway may be mediated by the CSE-generated H2S in mouse bladder.
Expressions of CSE, CBS, and 3-MST enzymes and L-cysteine-mediated H2S production were shown in rat, guinea pig, and human bladder tissues (Gai et al. 2013; Fernandes et al. 2013a, b; Zou et al. 2018). Matsunami et al. (2012) demonstrated that CSE is the responsible enzyme for endogenous H2S synthesis in mouse bladder, but Wang et al. (2018) showed that CBS and CSE enzymes are present in bladder tissue. In the present study, we determined protein expressions of CSE, CBS, and 3-MST enzymes by Western blot technique in mouse bladder tissue. Our molecular results are consistent with studies reporting the presence of endogenous H2S-generating enzymes in mouse bladder tissue (Matsunami et al. 2012; Wang et al. 2018). In our study, another piece of evidence supporting these data was our functional experiment findings on mouse bladder tissue. We studied the effect of L-cysteine (as precursor for H2S) and CSE/CBS enzyme inhibitors on carbachol-mediated contractions of mouse bladder strips. L-cysteine inhibited contractile responses to carbachol, and this inhibition was reversed by the PAG (inhibitor of CSE enzyme) and AOAA, (inhibitor of CBS enzyme), suggesting that these enzymes are responsible for H2S synthesis in mouse bladder tissue and that the L-cysteine/H2S pathway is partially responsible for the inhibition of agonist-mediated bladder smooth muscle contractions. The present results are consistent with the human bladder studies that L-cysteine-elicited relaxation was diminished by PAG and AOAA (Gai et al. 2013; Fusco et al. 2012). Another finding of ours that corroborates this supposition is that NaHS (an exogenous H2S donor) inhibited carbachol-induced contractions in a similar way. Also, we measured H2S levels in bladder tissues to confirm the production of endogenous H2S by L-cysteine and to evaluate the possible interaction at the synthesis level. We observed that bladder tissue released basal H2S, which amounted to tissue concentration of approximately 0.12 nm/mg, and L-cysteine increased tissue H2S level around 0.47 nm/mg, which decreased in the presence of PAG (0.17 nm/mg). In line with this, H2S enzyme inhibitors also inhibited L-cysteine-induced inhibition on carbachol-induced contractions but did not affect carbachol contractions alone, suggesting that the basal H2S level may be insufficient for inhibitory effectiveness on contractions. Our results are in agreement with studies showing that bladder smooth muscle can generate H2S (Fusco et al. 2012; Zou et al. 2018). The present molecular and functional findings suggest that the existence of the L-cysteine/H2S pathway and H2S plays a role in the inhibition of carbachol-mediated contractions in mouse bladder tissues.
It has been reported that RhoA, ROCK1, ROCK2, and CPI-17 are expressed in human bladder smooth muscle, and Ca2+sensitization has been demonstrated in carbachol-mediated contractions (Wibberley et al. 2003). In addition, ROCK1 and ROCK2 expressions have been demonstrated in mouse (Boberg et al. 2012), rat (Wibberley et al.. 2003), and rabbit (Bing et al. 2003) bladder tissues. Also, Braverman et al. (2006) showed that carbachol-mediated contraction by muscarinic M3 receptors occurs via the ROCK and PKC pathways in the rat bladder. In the present study, we confirmed the role of the ROCK pathway in carbachol-induced contractions in mouse bladder muscle. Carbachol-mediated contractile responses were reduced by Y-27632, the specific inhibitor of ROCK1 and ROCK2 (Uehata et al. 1997; Davies et al. 2000). Our results are consistent with that of mouse and rabbit bladders where Y-27632 inhibited contractions to muscarinic agonists (Boberg et al. 2012). Also, in rat bladder, Y-27632 diminished carbachol-mediated Ca2+ sensitization, indicating a possible role of ROCK (Durlu-Kandilci and Brading 2006). Also, we obtained that the ROCK-1, pMLC20, and pMYTP1 increased in carbachol-treatment tissues compared to the control, confirming the contribution of RhoA/ROCK pathway to carbachol-mediated Ca2+ sensitization and contraction of bladder. This finding is in agreement with prior reports on human (Takahashiet al. 2004), mouse (Boberg et al. 2012), and rat (Wibberley et al. 2003) bladder tissues and indicate the contribution of the ROCK pathway in carbachol contractions in mouse bladder tissue. Our data support the hypothesis that muscarinic receptor-stimulated contractions of bladder smooth muscle are regulated by RhoA/ROCK-induced Ca2+ sensitization. On the otherhand, our findings suggest an upregulation of ROCK-1 protein expression by carbachol within 4 min. Certainly, this needs to be regarded as preliminary, as the time frame is, in fact, short for a change in protein expression and the conclusiveness may be limited to small group sizes or technical reasons at this stage. Similar limitations may apply to our other Western blot experiments where group sizes were small.
To determine the contribution of ROCK pathway to the inhibitory effect of L-cysteine/H2S pathway on carbachol-mediated contractions of mouse bladder tissue, we first evaluated the effect of Y-27623 on agonist-mediated contractions in the presence of PAG or AOAA. The inhibitory effect of Y-27632 on carbachol contractions was abolished by PAG but not by AOAA, suggesting that the CSE-produced H2S may be partially involved in inhibition of the RhoA/ROCK pathway. Also, we observed that Y27632 reduced the inhibitory responses of L-cysteine on the carbachol-mediated contractions and PAG abolished the inhibitory effect of L-cysteine on the contractile responses to carbachol in the presence of Y-27632, supporting the idea that CSE-produced H2S inhibits RhoA/ROCK pathway in mouse bladder tissues. To our knowledge, this is the first study of functional finding related to the effect of Y-27632 on endogenous L-cysteine/H2S pathway in bladder tissue. Our previous study on mouse corpus cavernosum demonstrated that Y-27632 almost extinguished the contractile response to phenylephrine in the presence of L-cysteine (Aydinoglu et al. 2019). This difference may be a tissue and contractile agonist discrepancy. On the other hand, we obtained that the inhibitory effect of NaHS (exogenous H2S) on contractions did not change in the presence of Y-27632, showing that specific ROCK inhibitor Y-27632 interacts with CSE at the synthesis level of H2S. Similarly, it has been reported that Y-27632 did not affect NaHS responses in mouse gastric fundus (Dhaese, and Lefebvre 2009)] and bovine retinal artery (Semiz et al. 2020). Furthermore, we investigated the ROCK1, pMLC20, and pMYTP1 levels in the presence of L-cysteine and NaHS. Endogenous and exogenous H2S decreased carbachol-mediated phosphorylation of ROCK1, pMLC20, and pMYPT1. Inhibition mediated via L-cysteine but not via NaHS was reversed in the presence of PAG, suggesting that H2S is produced through CSE activation from L-cysteine and has led to decrease of sustained contraction by inhibition of ROCK -induced phosphorylation of MLC20 and MYPT1. Similarly, L-cysteine and NaHS inhibited the carbachol-mediated ROCK activity and MYPT1 phosphorylation at Thr696, and the inhibitory effect of L-cysteine was prevented by PAG in the rabbit gastric smooth muscle cells, suggesting that inhibitory effect of L-cysteine on ROCK /PKC activities and muscle contraction was mediated via CSE activation (Nalli et al. 2015). Also, it has been identified that the CSE knockout mice exhibited increased ROCK1, ROCK2, and membrane protein MLC1 levels (Jiao et al. 2019). Furthermore, the RhoA activity and expression of ROCK1/ROCK2 were significantly increased in the CSE knockout mice, and this enhancement of the RhoA activity and ROCK1/ROCK2 expression was decreased by NaHS and Y-27632, suggesting that CSE-generated H2S may inhibit the RhoA/ROCK pathway cerebral artery of mice (Wen et al. 2019). Also, Nalli et al. (2015) reported that L-cysteine or NaHS inhibited carbachol-mediated stimulation of ROCK activity and muscle contraction in colonic muscle cells from mouse and human, suggesting that H2S causes inhibition of RhoA/ ROCK activities and muscle contraction via sulfhydration of RhoA. On the other hand, opposing findings were found in the retinal artery that the mechanism of NaHS-induced relaxation occurred by decrease of MYPT1-subunit of MLCP phosphorylation but not inhibition of RhoA/ ROCK (Semiz et al. 2020). This difference may be arising from different experimental conditions including the type of tissue or contractile agonist. Another piece of evidence supporting the interaction between CSE-produced H2S and ROCKpathway was that ROCK inhibition with Y-27632 enhanced basal H2S formation compared to the control group, and PAG markedly reduced the augmentation in H2S production in the presence of Y-27632, suggesting that the interaction between L-cysteine/H2S and RhoA/ ROCK pathway mainly occurs through the CSE enzyme in the mouse bladder tissues. There may be a two-way interaction between ROCK and H2S, such as ROCK inhibiting H2S production through CSE enzyme inhibition and H2S causing inhibition on contractions via upregulation of ROCK. This is the first time the interaction between L-cysteine/H2S and RhoA/ ROCK pathway has been demonstrated in bladder tissue.
Furthermore, we studied the role of PKC, another pathway that contributes to contractions through Ca sensitization, in the inhibitory effect of L-cysteine/H2S pathway on the carbachol-mediated contraction of mouse bladder smooth muscle. In the present study, we showed that pretreatment with GF 109203X, a specific PKC inhibitor, significantly reduced contractile responses to carbachol in mouse bladder strips, confirming that PKC participates in carbachol-induced contractions. Consistent with our finding, it has been reported that PKC inhibitor GF- 109203X inhibited contractions to muscarinic agonist in mouse, rat, guinea-pig, rabbit, and human bladder tissues (Durlu-Kandilci and Brading 2006; Takahashi et al. 2004; Boberg et al. 2012; Ratz and Miner 2003). In contrast, Fleichman et al. (2004) and Schneider et al. (2004) demonstrated that bisindolylmaleimide, calphostin C, and chelerythrine, the PKC inhibitors, did not affect contractions to carbachol of rat and human bladder, suggesting that carbachol-induced contraction does not involve PKC. These differences may be due to use of different PKC inhibitors and/or experimental conditions such as concentration and species. We next investigated whether the PKC pathway is related to CSE-produced H2S. We first evaluated the effect of GF 109203X, a specific PKC inhibitor, on carbachol-mediated contractions in the presence of PAG, CSE enzyme inhibitor. The inhibitory effect of GF 109203X on carbachol contractions was abolished by PAG, suggesting that the CSE-produced H2S may be partially involved in inhibition of PKC pathway. Also, we observed that GF 109203X reduced the inhibitory responses of L-cysteine on the carbachol-mediated contractions, supporting that CSE-produced H2S inhibits PKC pathway in mouse bladder. Furthermore, PKC inhibition with GF 109203X increased basal H2S formation compared to the control group, and PAG markedly attenuated the increase in H2S generation in the presence of GF 109203X, suggesting that CSE-produced H2S-induced inhibition of bladder contractions to carbachol was partially involved in inhibition of the PKC in the mouse bladder tissues. Consistent with present findings, it has been reported that L-cysteine and NaHS caused inhibition of carbachol-mediated PKC activity and phosphorylation of CPI-17 at Thr38, and PAG prevented the inhibitory effect of L-cysteine on carbachol-mediated PKC activity, suggesting that NaHS and CSE-induced H2S inhibited the sustained contraction through inhibition of PKC-mediated phosphorylation of CPI-17 in rabbit gastric muscle cells (Nalli et al. 2015). In contrast, Semiz et al. (2020) recently demonstrated that calphostin C, a PKC inhibitor, did not change NaHS-induced relaxations and pCPI-17 protein levels in bovine retinal artery, suggesting that PKC and CPI-17 dephosphorylation are not related to NaHS-mediated relaxations. There is no available data in the literature to directly correlate H2S and PKC in bladder smooth muscle.
The underlying molecular targets of H2S in the pathways that lead to inhibition of Rho- kinase/PKC signaling activity are not exactly known. Nalli et al. (2017) showed that H2S inhibits muscle contraction via S-sulfhydration of RhoA and inhibition of RhoA/ROCK activity in colonic smooth muscle cells (Nalli et al. 2017). In the S-sulfhydration process, hydropersulfide (-SSH) moiety is produced by the addition of sulfur from H2S to the –SH groups of cysteine residues. This causes chemical and biological reactivity of proteins to change. To clarify molecular mechanism of the interaction between H2S and RhoA/ROCK and/or PKC in bladder tissue, further studies are needed.
In conclusion, these results suggest that there is an interaction between L-cysteine/H2S and RhoA/ROCK through inhibition of ROCK-1, pMYPT1, and pMLC20. Furthermore, the inhibitory mechanism of L-cysteine/H2S on contractions involves, at least in part, the inhibition of RhoA/ROCK and/or PKC pathway by CSE-generated H2S in mouse bladder. There may be a two-way interaction between ROCK/PKC and H2S, such as ROCK/PKC inhibiting H2S production through CSE enzyme inhibition and H2S causing inhibition on contractions via upregulation of ROCK. However, we cannot exclude the possibility that the other kinases such as ZIP-kinase and IL-kinase contribute to inhibitory effect of L-cysteine/H2S pathway on agonist-mediated smooth muscle contraction. Further studies are needed to clarify the role of kinases in H2S-induced inhibition.
Data availability
All the data in this study are transparent and available upon request.
References
Abe K, Kimura H (1996) The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 16:1066–1071. https://doi.org/10.1523/jneurosci.16-03-01066.1996
Andersson KE, Arner A (2004) Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev 84:935–986. https://doi.org/10.1152/physrev.00038.2003
Andersson KE, Hedlund P (2002) Pharmacologic perspective on the physiology of the lower urinary tract. Urology 60:13–21. https://doi.org/10.1016/s0090-4295(2002)01786-7
Anjum I (2018) Calcium sensitization mechanisms in detrusor smooth muscles. J Basic Clin Physiol Pharmacol 29:227–235. https://doi.org/10.1515/jbcpp-2017-0071
Aydinoglu F, Ogulener N (2016) Characterization of relaxant mechanism of H2S in mouse corpus cavernosum. Clin Exp Pharmacol Physiol 43:503–511. https://doi.org/10.1111/1440-1681.12554
Aydinoglu F, Adıbelli EÖ, Yılmaz-Oral D, Ogulener N (2019) Involvement of RhoA/Rho-kinase in L-cysteine/H 2 S pathway-induced inhibition of agonist-mediated corpus cavernosal smooth muscle contraction. Nitric Oxide 85:54–60. https://doi.org/10.1016/j.niox.2019.02.001
Bing W, Chang S, Hypolite JA et al (2003) Obstruction-induced changes in urinary bladder smooth muscle contractility: a role for Rho kinase. Am J Physiol Renal Physiol 285:F990–F997. https://doi.org/10.1152/ajprenal.00378.2002
Boberg L, Poljakovic M, Rahman A, Eccles R, Arner A (2012) Role of Rho-kinase and protein kinase C during contraction of hypertrophic detrusor in mice with partial urinary bladder outlet obstruction. BJU Int 109:132–140. https://doi.org/10.1111/j.1464-410X.2011.10435.x
Braverman AS, Tibb AS, Ruggieri MR (2006) M2 and M3 muscarinic receptor activation of urinary bladder contractile signal transduction. I. Normal rat bladder. J Pharmacol Exp Ther 316:869–874. https://doi.org/10.1124/jpet.105.097303
Cheng Y, Ndisang JF, Tang G et al (2004) Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol 287:H2316–H2323. https://doi.org/10.1152/ajpheart.00331.2004
Chiba Y, Misawa M (2004) The role of RhoA-mediated Ca2+ sensitization of bronchial smooth muscle contraction in airway hyperresponsiveness. J Smooth Muscle Res. 40:155–67. https://doi.org/10.1540/jsmr.40.155
Davies SP, Reddy H, Caivano M, Cohen P (2000) Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351:95–105. https://doi.org/10.1042/0264-6021:3510095
Dhaese I, Lefebvre RA (2009) Myosin light chain phosphatase activation is involved in the hydrogen sulfide-induced relaxation in mouse gastric fundus. Eur J Pharmacol 606:180–186. https://doi.org/10.1016/j.ejphar.2009.01.011
Dunn WR, Alexander SPH, Ralevic V, Roberts RE (2016) Effects of hydrogen sulphide in smooth muscle. Pharmacol Ther 158:101–113. https://doi.org/10.1016/j.pharmthera.2015.12.007
Durlu-Kandilci NT, Brading A (2006) F Involvement of Rho kinase and protein kinase C in carbachol-induced calcium sensitization in beta-escin skinned rat and guinea-pig bladders. Br J Pharmacol 148:376–384. https://doi.org/10.1038/sj.bjp.0706723
Fernandes VS, Ribeiro ASF, Martínez MP et al (2013a) MP Endogenous hydrogen sulfide has a powerful role in inhibitory neurotransmission to the pig bladder neck. J Urol 189:1567–1773. https://doi.org/10.1016/j.juro.2012.10.006
Fernandes VS, Ribeiro ASF, Barahona MV et al (2013b) Hydrogen sulfide mediated inhibitory neurotransmission to the pig bladder neck: role of KATP channels, sensory nerves and calcium signaling. J Urol 190:746–756. https://doi.org/10.1016/j.juro.2013.02.103
Fernandes VS, Ribeiro ASF, Martinez P et al (2014) Hydrogen sulfide plays a key role in the inhibitory neurotransmission to the pig intravesical ureter. PLoS ONE 9:1–19. https://doi.org/10.1371/journal.pone.0113580
Fleichman M, Schneider T, Fetscher C, Michel MC (2004) Signal transduction underlying carbachol-induced contraction of rat urinary bladder. II. Protein kinases. J Pharmacol Exp Ther. 308:54–58. https://doi.org/10.1124/jpet.103.058255
Frazier EP, Peters SLM, Braverman AS, Ruggieri MR, Michel MC (2008) Signal transduction underlying the control of urinary bladder smooth muscle tone by muscarinic receptors and beta-adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol 377:449–462. https://doi.org/10.1007/s00210-007-0208-0
Fry CH, Meng E, Young JS (2010) The physiological function of lower urinary tract smooth muscle. Auton Neurosci 154:3–13. https://doi.org/10.1016/j.autneu.2009.10.006
Fusco F, Di Villa D, Bianca R, Mitidieri E et al (2012) Sildenafil effect on the human bladder involves the L-cysteine/hydrogen sulfide pathway: a novel mechanism of action of phosphodiesterase type 5 inhibitors. Eur Urol 62:1174–1180. https://doi.org/10.1016/j.eururo.2012.07.025
Gai JW, Wahafu W, Guo H et al (2013) Further evidence of endogenous hydrogen sulphide as a mediator of relaxation in human and rat bladder. Asian J Androl 15:692–696. https://doi.org/10.1038/aja.2013.32
Hedegaard ER, Gouliaev A, Winther AK (2016) Involvement of potassium channels and calcium-independent mechanisms in hydrogen sulfide-induced relaxation of rat mesenteric small arteries. J Pharmacol Exp Ther 356:53–63. https://doi.org/10.1124/jpet.115.227017
Hypolite JA, Malykhina AP (2015) Regulation of urinary bladder function by protein kinase C in physiology and pathophysiology. BMC Urol 15(110):1–11. https://doi.org/10.1186/s12894-015-0106-6
Jezior JR, Brady JD, Rosenstein DI et al (2001) Dependency of detrusor contractions on calcium sensitization and calcium entry through LOE-908-sensitive channels. Br J Pharmacol 134:78–87. https://doi.org/10.1038/sj.bjp.0704241
Jiao Y. Li Y, Chen Z, Guo Y (2019) Mechanism of H2S-mediated ROCK inhibition of total flavones of Rhododendra against myocardial ischemia injury. Exp Ther Med. 3783-3792. https://doi.org/10.3892/etm.2019.8004
Kawano Y, Yoshimura T, Kaibuchi K (2002) Smooth muscle contraction by small GTPase Rho. Nagoya J Med Sci 65:1–8
Kimura H (2011) Hydrogen sulfide: its production, release and functions. Amino Acids 41:113–121. https://doi.org/10.1007/s00726-010-0510-x
Matsunami M, Miki T, Nishiura K et al (2012) Involvement of the endogenous hydrogen sulfide/Cav3.2 T-type Ca2+ channel pathway in cystitis-related bladder pain in mice. Br J Pharmacol 167:917–928. https://doi.org/10.1111/j.1476-5381.2012.02060.x
Mimata H, Nomura Y, Emoto A et al (1997) Muscarinic receptor subtypes and receptor-coupled phosphatidylinositol hydrolysis in rat bladder smooth muscle. Int J Urol 4:591–596. https://doi.org/10.1111/j.1442-2042.1997.tb00315.x
Nalli AD, Rajagopal S, Mahavadi S et al (2015) Inhibition of RhoA-dependent pathway and contraction by endogenous hydrogen sulfide in rabbit gastric smooth muscle cells. Am J Physiol Cell Physiol 308:C485–C495. https://doi.org/10.1152/ajpcell.00280.2014
Nalli AD, Wang H, Bhattacharya S, Blakeney BA, Murthy KS (2017) Inhibition of RhoA/Rho kinase pathway and smooth muscle contraction by hydrogen sulfide. Pharmacol Res Perspect 5:1–14. https://doi.org/10.1002/prp2.343
Pan TT, Neo KL, Hu LF et al (2008) H2S preconditioning-induced PKC activation regulates intracellular calcium handling in rat cardiomyocytes. Am J Physiol Cell Physiol 294:C169–C177. https://doi.org/10.1152/ajpcell.00282.2007
Ratz PH, Miner AS (2003) Length-dependent regulation of basal myosin phosphorylation and force in detrusor smooth muscle. Am J Physiol Regul Integ. Comp Physiol 284:R1063–R1070. https://doi.org/10.1152/ajpregu.00596.2002
Schneider T, Fetscher C, Krege S, Michel MC (2004) Signal transduction underlying carbachol-induced contraction of human urinary bladder. J Pharmacol Exp Ther 309:1148–1153. https://doi.org/10.1124/jpet.103.063735
Semiz AT, Teker AB, Yapar K (2020) Hydrogen sulfide dilates the isolated retinal artery mainly via the activation of myosin phosphatase. Life Sci 255:117834. https://doi.org/10.1016/j.lfs.2020.117834
Somlyo AP, Somlyo AV (2003) Ca2+ sensitivity of smooth muscle and non-muscle myosin II: Modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83:1325–1358. https://doi.org/10.1152/physrev.00023
Somlyo AP, Wu X, Walker LA, Somlyo AV (1999) Pharmacomechanical coupling: the role of calcium, G-proteins, kinases and phosphatases. Rev Physiol Biochem Pharmacol 134:203–236. https://doi.org/10.1007/3-540-64753-8-5
Somlyo AP, Somlyo AV (1998) From pharmacomechanical coupling to G-proteins and myosin phosphatase. Acta Physiol Scand 437–448. https://doi.org/10.1046/j.1365-201X.1998.00454.x
Stull JT, Tansey MG, Word RA et al (1991) Myosin light chain kinase phosphorylation: regulation of the Ca2+ sensitivity of contractile elements. Adv Exp Med Biol 304:129–138. https://doi.org/10.1007/978-1-4684-6003-2-12
Takahashi R, Nishimura J, Hirano K et al (2004) Ca2+ sensitization in contraction of human bladder smooth muscle. J Urol 172:748–752. https://doi.org/10.1097/01.ju.0000130419.32165.6b
Teixeira CE, Jin L, Priviero FBM, Ying Z, Webb RC (2007) Comparative pharmacological analysis of Rho-kinase inhibitors and identification of molecular components of Ca2+ sensitization in the rat lower urinary tract. Biochem Pharmacol 74:647–658. https://doi.org/10.1016/j.bcp.2007.06.004
Uchiyama T, Chess-Williams R (2004) Muscarinic receptor subtypes of the bladder and gastrointestinal tract. J Smooth Muscle Res 40:237–247. https://doi.org/10.1540/jsmr.40.237
Uehata M, Ishizaki T, Satoh HT (1997) Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 389:990–994. https://doi.org/10.1038/40187
Wang Q, Wang XL, Liu HR et al (2010) Protective effects of cysteine analogues on acute myocardial ischemia: novel modulators of endogenous H(2)S production. Antioxid Redox Signal 12:1155–1165. https://doi.org/10.1089/ars.2009.2947
Wang W, Bo Q, Du J et al (2018) Endogenous H2S sensitizes PAR4-induced bladder pain. Am J Physiol Renal Physiol 314:F1077–F1086. https://doi.org/10.1152/ajprenal.00526.2017
Wen JY, Gao SS, Chen FL (2019) Role of CSE-produced H2S on cerebrovascular relaxation via RhoA-ROCK inhibition and cerebral ischemia-reperfusion injury in mice. ACS Chem Neurosci 10:1565–1574. https://doi.org/10.1021/acschemneuro.8b00533
Wibberley A, Chen Z, Hu EJP et al (2003) Expression and functional role of Rho-kinase in rat urinary bladder smooth muscle. Br J Pharmacol 138:757–766. https://doi.org/10.1038/sj.bjp.0705109
Wu L, Yang W, Jia X et al (2009) Pancreatic islet overproduction of H2S and suppressed insulin release in Zucker diabetic rats. Lab Invest 89:59–67. https://doi.org/10.1038/labinvest.2008.109
Yamaguchi O, Shishido K, Tamura K et al (1996) Evaluation of mRNAs encoding muscarinic receptor subtypes in human detrusor muscle. J Urol 156:1208–1213. https://doi.org/10.1016/S0022-5347(01)65752-5
Yang G, WuL JB, Yang W et al (2008) H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science 322:87–590. https://doi.org/10.1126/science.1162667
Zou S, Shimizu T, Shimizu S et al (2018) Possible role of hydrogen sulfide as an endogenous relaxation factor in the rat bladder and prostate. Neurourol Urodyn 37:2519–2526. https://doi.org/10.1002/nau.23788
Acknowledgements
The authors thank Kenneth J. Cox for English editing.
Funding
This study was granted by the Çukurova University Research Foundation (TDK-2019–12362).
Author information
Authors and Affiliations
Contributions
Fatma Tugçe Dalkir, Fatma Aydinoglu, and Nuran Ogulener conceived and designed research. Fatma Tugçe Dalkir and Fatma Aydinoglu conducted experiments. Fatma Tugçe Dalkir and Fatma Aydinoglu contributed new reagents or analytical tools. Fatma Tugçe Dalkir, Fatma Aydinoglu, and Nuran Ogulener analyzed data. Nuran Ogulener wrote and submitted the manuscript. All authors read and approved submission of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Protocols were conducted in accordance with national and international guidelines for the care and use of laboratory animals and approved by the Institutional Animal Care and Use Committee of Cukurova University and given the approval number 4/3/08.07.2019.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dalkir, F.T., Aydinoglu, F. & Ogulener, N. The role of rhoA/rho-kinase and PKC in the inhibitory effect of L-cysteine/H2S pathway on the carbachol-mediated contraction of mouse bladder smooth muscle. Naunyn-Schmiedeberg's Arch Pharmacol 396, 2023–2038 (2023). https://doi.org/10.1007/s00210-023-02440-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02440-6