Abstract
Lung injury is a significant complication associated with cholestasis/cirrhosis. This problem significantly increases the risk of cirrhosis-related morbidity and mortality. Hence, finding effective therapeutic options in this field has significant clinical value. Severe inflammation and oxidative stress are involved in the mechanism of cirrhosis-induced lung injury. Taurine (TAU) is an abundant amino acid with substantial anti-inflammatory and antioxidative properties. The current study was designed to evaluate the role of TAU in cholestasis-related lung injury. For this purpose, bile duct ligated (BDL) rats were treated with TAU (0.5 and 1% w: v in drinking water). Significant increases in the broncho-alveolar lavage fluid (BALF) level of inflammatory cells (lymphocytes, neutrophils, basophils, monocytes, and eosinophils), increased IgG, and TNF-α were detected in the BDL animals (14 and 28 days after the BDL surgery). Alveolar congestion, hemorrhage, and fibrosis were the dominant pulmonary histopathological changes in the BDL group. Significant increases in the pulmonary tissue biomarkers of oxidative stress, including reactive oxygen species formation, lipid peroxidation, increased oxidized glutathione levels, and decreased reduced glutathione, were also detected in the BDL rats. Moreover, significant myeloperoxidase activity and nitric oxide levels were seen in the lung of BDL rats. It was found that TAU significantly blunted inflammation, alleviated oxidative stress, and mitigated lung histopathological changes in BDL animals. These data suggest TAU as a potential protective agent against cholestasis/cirrhosis-related lung injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cholestasis is a serious clinical complication that could progress to severe liver injury, hepatic fibrosis, cirrhosis, and liver failure (Bomzon et al. 1997; Erlinger 2014; Krones et al. 2015; Aniort et al. 2017). Several diseases, as well as xenobiotics, have been identified to be involved in the pathogenesis of cholestasis (Jüngst and Lammert 2013; Levy 2013). Various potentially cytotoxic bile constituents, including bile acids, bilirubin, and manganese, accumulate in the liver during cholestasis (Bomzon et al. 1997; Erlinger 2014; Krones et al. 2015; Aniort et al. 2017; Heidari et al. 2018b). Although the liver is the main organ influenced by cholestasis, toxic bile components enter the systemic circulation and affect other organs (Ommati et al. 2020b, 2021a; Ghanbarinejad et al. 2021). The kidney, lung, skeletal muscle, heart, reproductive system, and brain are organs that are significantly influenced by cholestasis/cirrhosis (Krowka and Cortese 1985; Orellana et al. 2000; Aniort et al. 2017; Ommati et al. 2019, 2020e, 2021a, 2021e, 2021f; Farshad et al. 2020; Heidari et al. 2020).
There is evidence of lung injury in experimental models and human cases of cholestasis/cirrhosis (Krowka and Cortese 1985; Enrico et al. 2007; Zecca et al. 2008; Ding et al. 2014; Herraez et al. 2014; Yu et al. 2014). Cholestasis-induced lung injury could lead to profound hypoxemia in patients (Krowka and Cortese 1985). Severe inflammation or intrapulmonary bleeding is also reported in cholestasis-induced lung injury (Krowka and Cortese 1985; Enrico et al. 2007; Zecca et al. 2008; Ding et al. 2014; Herraez et al. 2014; Yu et al. 2014). Hence, the establishment of effective therapeutic interventions is an urgent need. The accumulation of cytotoxic bile acids and bilirubin is the most suspected factor responsible for cholestasis-induced lung injury (Zecca et al. 2008; Ommati et al. 2021a).
Although the precise mechanism(s) of cholestasis-induced lung injury is far from clear, several studies noted the role of oxidative stress in this complication (Aruoma et al. 1988; Cozzi et al. 1995; Gürer et al. 2001; Pushpakiran et al. 2004; Acharya and Lau-Cam 2013; Hsieh et al. 2014; Abdel-Moneim et al. 2015; Alhumaidha et al. 2015). In this context, severe biomembranes degradation (lipid peroxidation) and decreased cellular antioxidant capacity have been reported in the lung tissue in experimental models of cholestasis/cirrhosis (Salatti Ferrari et al. 2012; Shikata et al. 2014). The accumulation of cytotoxic molecules such as hydrophobic bile acids is the major suspected factor involved in the occurrence of oxidative stress in the lung of cholestatic animals (Zecca et al. 2008; Ommati et al. 2021a). Significant inflammatory cell infiltration is another problem in the lung of cholestatic cases (Schuller-Levis and Park 2003; Marcinkiewicz et al. 2006; Su et al. 2014; Lin et al. 2015). The accumulation of inflammatory cells is also involved in the pathogenesis of lung injury during cholestasis by releasing potentially cytotoxic cytokines and their contribution to the induction of oxidative stress (Shikata et al. 2014; Forrester Steven et al. 2018).
Taurine (TAU) is the most abundant amino acid in the human body that is not corporate in the protein structure (Wright et al. 1986). Many physiological and pharmacological properties have been attributed to TAU (Huxtable et al. 1992; Wójcik et al. 2010; Rashid et al. 2013; Islambulchilar et al. 2015). Most importantly, it has repeatedly been mentioned that TAU could abrogate oxidative stress and its related complications in various experimental models (Cozzi et al. 1995; Pushpakiran et al. 2004; Shimada et al. 2015; Heidari et al. 2019a; Vazin et al. 2020). This amino acid could also robustly blunt inflammation and release cytokines in multiple experiments (Cozzi et al. 1995; Pushpakiran et al. 2004; Shimada et al. 2015; Heidari et al. 2019a; Vazin et al. 2020). Moreover, the positive effects of TAU on pulmonary diseases or xenobiotic-induced lung injury also have been studied (Aruoma et al. 1988; Gürer et al. 2001; Acharya and Lau-Cam 2013; Hsieh et al. 2014; Abdel-Moneim et al. 2015; Alhumaidha et al. 2015; Yang et al. 2016b; Li et al. 2017; Ramos et al. 2018). It has been found that TAU could significantly ameliorate lung pathologies mainly by mitigating oxidative stress and its related complications (Santangelo et al. 2003; Guler et al. 2014; Tu et al. 2018).
The current experimental study aimed to evaluate the protective role of TAU in a rat model of cholestasis-induced lung injury. The effects of this amino acid on several markers, including the population of inflammatory cells in broncho-alveolar lavage fluid (BALF), the level of cytokines and immunoglobulins, biomarkers of oxidative stress, and lung tissue histopathological alterations, were monitored. The data obtained from this study could be translated for human benefit, potentially leading to the establishment of clinical interventions against lung injury in patients with cholestasis/cirrhosis.
Materials and methods
Chemicals and reagents
Iodoacetic acid, 4,2-hydroxyethyl,1-piperazine ethane sulfonic acid (HEPES), hexadecyl-trimethyl-ammonium bromide (HTAB), reduced glutathione (GSH), N-1-naphthyl ethylenediamine dihydrochloride, sodium phosphate dibasic (Na2HPO4), sulphanilamide, dithiothreitol (DTT), fatty acid-free bovine serum albumin fraction V, glacial acetic acid, oxidized glutathione (GSSG), 2′,7′-dichlorofluorescein diacetate (DCF-DA), hydrogen peroxide, methanol HPLC grade, coomassie brilliant blue, acetonitrile HPLC grade, sodium acetate, and ethylenediaminetetraacetic acid (EDTA) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Trichloroacetic acids, meta-phosphoric acid, O-dianisidine hydrochloride, potassium chloride (KCl), sodium chloride (NaCl), hydrochloric acid, and hydroxymethyl aminomethane hydrochloride (Tris–HCl) were purchased from Merck (Merck KGaA, Darmstadt, Germany). All salts for preparing buffer solutions (analytical grade) were prepared from Merck (Merck KGaA®, Darmstadt, Germany). Kits for evaluating serum biochemistry were obtained from ParsAzmoon® (Tehran, Iran). Kits for assessing immunoglobulin and cytokine in BALF were purchased from Shanghai Jianglai Biology® (China). BALF level of bile acids was analyzed by an EnzyFluo™ Bile Acids Assay Kit (BioAssay® Systems, USA).
Animals
Mature male Sprague–Dawley (SD) rats (n = 42, weighing 250 ± 20 g) were obtained from the laboratory animals breeding center of Shiraz University of Medical Sciences, Shiraz, Iran. Animals were maintained in a standard environment (12 h photo-schedule, ≈ 40% relative humidity, and temperature 24 ± 1 °C) with free access to tap water and a regular rat diet (Behparvar®, Tehran, Iran). All procedures using experimental animals were approved by the institutional ethics committee at Shiraz University of Medical Sciences, Shiraz, Iran (IR.SUMS.REC.1399.1353). The ARRIVE guidelines (animal research: reporting of in vivo experiments) were followed.
Bile duct ligation surgery and treatments
Animals were randomly allotted into sham-operated and bile duct ligated (BDL) groups. In the BDL group, animals were anesthetized (a mixture of 10 mg/kg of xylazine and 80 mg/kg of ketamine, i.p), and a midline incision (≈ 2 cm) was made through the linea alba. The common bile duct was identified and doubly ligated using a silk suture (no. 04) (Moezi et al. 2013; Heidari et al. 2018d, 2019b; Mousavi et al. 2021). The sham operation involved laparotomy and bile duct manipulation without ligation (Heidari et al. 2019b). Sham-operated and BDL rats (n = 6/group) were monitored at scheduled time intervals (3, 7, 14, and 28 days after surgery). It was found that all markers of lung inflammation and fibrosis were significantly increased at day = 28 after BDL surgery (results) when biomarkers of lung injury were monitored in cholestatic rats. Therefore, in another round of experiments animals were randomly allocated in the following groups: (1) Sham-operated; (2) BDL; (3) BDL + taurine (0.5% w: v in drinking water); (4) BDL + taurine (1% w: v in drinking water). The effects of taurine on BDL-linked lung injury were assessed on day 28 after BDL surgery.
Broncho-alveolar lavage fluid (BALF) preparation
Six animals from each group (sham and BDL) were deeply anesthetized (thiopental, 80 mg/kg, i.p) at scheduled time intervals (3, 7, 14, and 28 days after BDL surgery). Animals were placed in a dorsal position, and the trachea was exposed and cannulated (20 G catheter). The catheter was stabilized with a cotton thread. Then, 1 mL saline–EDTA (2.6 mM EDTA in normal saline; 0.9% w: v NaCl) was injected into the lung, and the chest was gently massaged (10 s) (Daubeuf and Frossard 2014). The solution was re-aspirated and kept on ice. This procedure was repeated five times per animal (1 mL each time). Then, the pooled lavage preparations were centrifuged (5 min, 300 g, 4 °C) to pellet cells. The supernatant was collected to analyze cytokines, IgG, bilirubin, and bile acids (Okada et al. 2013; Daubeuf and Frossard 2014). Then, 500 μL KCl (0.6 M) and 1500 μL of ultrapure water were added to the cell pellet for erythrocyte lysis (10 s). Samples were homogenized by inverting and centrifuged (5 min, 300 g, 4 °C). Finally, the supernatant was discarded, 1 mL of saline–EDTA was added to the cell pellet and homogenized by inverting. The cell suspension was kept at 4 °C for further analysis (Daubeuf and Frossard 2014).
Serum biochemical measurements and BALF cellular analysis
Blood samples (5 mL) were obtained from the abdominal aorta, transported to serum preparation tubes (Improvacuter®; gel and clot activator-coated tubes; Guangzhou, China), and centrifuged (3000 g, 15 min, 4 °C). Commercial kits (Pars-Azmoon®, Tehran, Iran) and a Mindray BS-200® autoanalyzer (Guangzhou, China) were employed to assess serum gamma-glutamyl transpeptidase (γ-GT), total bilirubin, alkaline phosphatase (ALP) alanine aminotransferase (ALT), and aspartate aminotransferase (AST). Kits for assessing IgG and cytokine in BALF were purchased from Shanghai Jianglai Biology® (China). BALF level of bile acids was analyzed by an EnzyFluo™ Bile Acids Assay Kit (BioAssay® Systems, USA). BALF total bilirubin was assessed using a Parsazmoon® kit (Tehran, Iran). A Prokan® automatic blood cell counter analyzed the differential inflammatory cell count of BALF.
Myeloperoxidase (MPO) activity in the lung tissue
The MPO activity in the pulmonary tissue was assessed as an index of inflammatory cell infiltration. Briefly, tissue specimens (100 mg) were homogenized in 1 mL of hexadecyl-trimethyl-ammonium bromide (HTAB) solution (0.5% w: v; dissolved in 50 mM potassium phosphate buffer; pH = 6. 4 °C) and centrifuged (3000 g, 20 min at 4 °C). Then, 100 µL of the supernatant was added to 2.9 mL of potassium phosphate buffer (50 mM; pH = 6) containing 16.7 mg/100 mL of O-dianisidine hydrochloride and 0.0005% v: v of hydrogen peroxide. After incubation (5 min, room temperature), the reaction was stopped by adding 100 µL of hydrochloric acid (1.2 M). Finally, the absorbance of samples was measured at λ = 400 nm (EPOCH® plate reader, USA) (Liu et al. 1999).
Nitric oxide measurement in lung
The Griess assay was used to evaluate nitric oxide (NO) production in the lung tissue of BDL and sham-operated rats (Yang et al. 2016a). The Griess method determines nitrite as the stable end product of NO (Yang et al. 2016a). For this purpose, 300 µL of the lung tissue homogenate (10% in 40 mM Tris–HCl buffer) was added to a solution containing 1 mL of distilled water and 120 μl of NaOH (2% w: v). Then, 1 mL of distilled water and 20 μL of HCl (7.4% v: v) were added, mixed well, and heated (50 °C, 15 min). Afterward, samples were centrifuged (3000 g, 10 min, 4 °C), and 50 μL of supernatant was added to a 96-well plate. Then, 50 μl of sulphanilamide and 50 μl of N-1-naphthyl ethylene diamine dihydrochloride were added. Finally, the absorbance was measured at λ = 540 nm (EPOCH® plate reader, USA). The nitrite concentrations were estimated using a standard curve (sodium nitrite as a standard).
Reactive oxygen species in the lung of BDL rats
Reactive oxygen species (ROS) formation in the lung tissue was assessed using 2′,7′-dichlorofluorescein diacetate (DCF-DA) as a fluorescent probe (Heidari et al. 2018a, 2019b; Heidari and Niknahad 2019; Abdoli et al. 2021; Ahmadi et al. 2021a). For this purpose, 400 mg of the lung tissue was homogenized in 4 mL of ice-cooled Tris–HCl buffer (40 mM, pH = 7.4). Then, 100 µL of the resulted tissue homogenate was added to 1 mL of Tris–HCl buffer (40 mM, pH = 7.4) containing 10 µM of DCF-DA (Heidari et al. 2018c, 2019a) and incubated in the dark (10 min, 37 °C incubator). Finally, the fluorescence intensity was assessed at λ excit = 485 nm and λ emiss = 525 nm (FLUOstar Omega® multifunctional fluorimeter, Germany) (Ommati et al. 2017; Heidari et al. 2018a; Heidari and Niknahad 2019).
Lung tissue lipid peroxidation
Lipid peroxidation in the lung of BDL and sham-operated rats was assessed using the thiobarbituric acid reactive substances (TBARS) test (Heidari et al. 2017; Heidari and Niknahad 2019; Ommati et al. 2020c; Ahmadi et al. 2021b). Briefly, 500 µL of the lung tissue homogenate (10% w: v in 40 mM Tris–HCl buffer, pH = 7.4) was treated with 2 mL of TBARS assay reagent (a mixture of 1 mL of thiobarbituric acid 0.375% w: v, 1 mL of 50% w: v of trichloroacetic acid, pH = 2; adjusted with HCl) (Niknahad et al. 2014; Heidari et al. 2015b, 2016a; Heidari and Niknahad 2019). Samples were vortexed well (1 min) and heated (100 °C water bath, 45 min). Afterward, 2 mL of n-butanol was added. Samples were mixed and centrifuged (10,000 g, 20 min, 4 °C). Finally, the absorbance of the pink-colored supernatant (n-butanol phase) was measured (λ = 532 nm, EPOCH® plate reader, USA) (Niknahad et al. 2017a; Heidari et al. 2018d; Heidari and Niknahad 2019; Ommati et al. 2022).
The total antioxidant capacity of the lung tissue
The pulmonary tissue’s ferric reducing antioxidant power (FRAP) was assessed. For this purpose, a working FRAP mixture was freshly prepared by mixing ten parts of 300 mmol/L acetate buffer (pH = 3.6) with 1 part of 10 mmol/L of 2, 4, 6-tripyridyl-s-triazine in 40 mmol/L HCl, and with 1 part of 20 mmol/L FeCl3. Tissue samples were homogenized in Tris–HCl buffer (40 mM; pH = 7.4; 4 °C), containing 5 mM dithiothreitol and 0.2 M sucrose (Heidari et al. 2017; Mousavi et al. 2020; Ommati et al. 2020f, 2021g). Then, 1.5 mL FRAP reagent and 200 µL deionized water were added to 50 µL tissue homogenate and incubated at 37 °C for 5 min. The intensity of the resultant blue color was assessed (λ = 593 nm, EPOCH plate reader, USA) (Heidari et al. 2016c; Ommati et al. 2020f, 2021c, 2021d).
Reduced and oxidized glutathione in the lung of cholestatic rats
The reduced (GSH) and oxidized (GSSG) glutathione content in the lung of cholestatic rats was assessed based on an HPLC protocol (Meeks and Harrison 1991; Truong et al. 2006; Siavashpour et al. 2020). The HPLC apparatus consisted of an amine column (NH2, 25 cm, Bischoff chromatography, Leonberg, Germany) and a UV detector (wavelength was set at λ = 252) (Meeks and Harrison 1991). Acetate buffer: water; 1:4 v: v as buffer A; methanol: water; 4:1 v: v as buffer B were used as mobile phases. The gradient method steadily increased buffer B to 95% in 30 min (1 mL/min flow rate) (Meeks and Harrison 1991; Niknahad et al. 2017b). For sample preparation, 1 mL of tissue homogenate (10% w: v in 40 mM Tris–HCl buffer, pH = 7.4; 4 °C) was treated with 200 µL of TCA (70% w: v). Samples were mixed well and incubated on ice (10 min, 4 °C) in a shaker incubator (Mohammadi et al. 2020; Ommati et al. 2020a, 2020d). Afterward, samples were centrifuged (17,000 g, 30 min, 4 °C). The supernatant (1000 µL) was collected (in 5 mL tubes), and 400 µL of the NaOH: NaHCO3 (2 M: 2 M) was added. Then, 100 µL of iodoacetic acid (1.5% w: v in HPLC grade water) was added and incubated in the dark (1 h, 4 °C). Afterward, 500 µL of 2, 4-dinitrofluorobenzene (1.5% v: v dissolved in HPLC grade ethanol) was added and mixed well. Samples were incubated in the dark (25 °C, 24 h, in a shaker incubator). After the incubation period, samples were centrifuged (16,000 g, 30 min), filtered, and injected (25 µL) into the mentioned HPLC apparatus (Meeks and Harrison 1991; Truong et al. 2006).
Lung tissue histopathology
Lung tissue samples were fixed in 10% v: v buffered formalin solution. Then, samples were embedded in paraffin blocks, and a 5-μm-thick slice of each sample was prepared by a microtome and stained with hematoxylin and eosin (H&E). Trichrome-Masson staining was also used for detecting lung tissue fibrotic changes. Liver tissue was also histopathologically evaluated (H&E and Trichrome stain) to confirm proper BDL induction in the current study. A pathologist blindly analyzed tissue slides.
Statistical analysis
Data are represented as mean ± SD. Data comparison was performed by the one-way analysis of variance (ANOVA) with Tukey’s multiple comparison test as the post hoc. Data of lung tissue histopathological alterations are represented as median and quartiles for five random pictures in each group. The analysis of pulmonary histopathological changes (non-parametric) was performed by the Kruskal–Wallis followed by the Dunn’s multiple comparison test. Values of P < 0.05 were considered statistically significant.
Results
Serum biochemistry assessment revealed a significant increase in the levels of ALT, AST, LDH, ALP, γ-GT, bilirubin, and bile acids (Fig. 1). Moreover, significant liver histopathological changes and fibrosis were detected in the BDL animals (Fig. 1). These data indicate proper induction of cholestasis in the current BDL model.
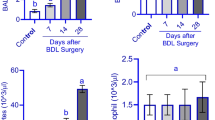
Serum biochemistry and broncho-alveolar fluid (BALF) level of bilirubin and bile acids in bile duct ligated (BDL) rats. In the current study, liver tissue histopathological alterations also confirmed the proper induction of cholestasis (bile duct proliferation: yellow arrow, inflammatory cell infiltration: blue arrow; gibrotic lesions: green arrow). Scale bar = 100 µm. Data are represented as mean ± SD (n = 6/group). Data sets with different alphabetical superscripts are statistically different (P < 0.05)
BALF levels of total bilirubin and bile acids were also evaluated (Fig. 1). It was found that total bilirubin and bile acids levels were significantly increased in the BALF of BDL animals at all time intervals assessed in the current study (Fig. 1). The maximum BALF level of bilirubin and bile acids was detected 14 and 28 days after the BDL operation (Fig. 1).
A significant increase in the BALF level of inflammatory cells was detected at different time points (3, 7, 14, and 28 days) after BDL operation (Fig. 2). Neutrophils were the most abundant inflammatory cells in the BALF of BDL rats (> 10 times than other cells; Fig. 2). The maximum increase in BALF level of neutrophils and lymphocytes was detected 28 days after the BDL surgery. BALF eosinophil levels were also increased at all time points after the BDL operation (Fig. 2). No significant increase in the BALF level of basophil and monocytes was detected in the current study (Fig. 2).
A significant increase in the BALF IgG level was detected in the BDL group (Fig. 2). Besides, the BALF level of TNF-α was also significantly increased in cholestatic animals (Fig. 2). The maximum levels of TNF-α and IgG were detected after day 7 of the BDL induction (Fig. 2).
The effects of TAU on cholestasis-induced lung injury were evaluated after 28 days of the BDL induction because all biomarkers assessed in the current model were maximumly increased at this time point. It was found that TAU (0.5 and 1% in drinking water) significantly decreased inflammatory cell infiltration (neutrophils, lymphocytes, and eosinophils) in the lung tissue of BDL rats (Fig. 3). On the other hand, BALF levels of IgG and TNF-α were declined considerably in the high dose of TAU-treated cholestatic animals (1% w: v in drinking water) (Fig. 3).
Effect of taurine (0.5 and 1% w: v in drinking water) on the level of inflammatory cells, TNF-α, and IgG in the broncho-alveolar lavage fluid (BALF) of bile duct ligated (BDL) rats (28 days after the DBL surgery; BDL 28). Data are shown as mean ± SD (n = 6). Data sets with different alphabetical superscripts are significantly different (P < 0.05)
Markers of oxidative stress were also evaluated in the lung tissue of control and BDL rats (Fig. 4). Significant elevations in ROS levels, in addition to lipid peroxidation, and increased level of oxidized glutathione (GSSG), were detected in cholestatic animals (Fig. 4). Moreover, a significant decrease in the lung tissue level of GSH and total antioxidant capacity was evident in BDL rats (Fig. 4). It was found that both doses of TAU (0.5 and 1%) significantly abrogated cholestasis-induced oxidative stress in the pulmonary tissue of BDL animals (Fig. 4). On the other hand, as an index of nitric acid formation, lung tissue nitrate level was increased in cholestatic rats (Fig. 4). Moreover, a significant increase in lung myeloperoxidase (MPO) activity was detected in the lung tissue of BDL rats in comparison with the control group (Fig. 4). Moreover, as an index of nitric acid formation, lung tissue nitrate level was increased in cholestatic rats (Fig. 4). It was found that TAU (0.5 and 1%) significantly decreased lung tissue nitrate levels and MPO activity in the BDL groups (Fig. 4).
Effects of taurine (TAU) on biomarkers of oxidative stress, myeloperoxidase activity, and nitric oxide levels in the lung tissue of bile duct ligated (BDL) rats (28 days after BDL surgery; BDL 28). Data are represented as mean ± SD (n = 6/group). Data sets with different alphabetical superscripts are statistically different (P < 0.05)
Pulmonary histopathological changes in BDL animals included significant inflammatory cell infiltration, alveolar congestion, and hemorrhage (Fig. 5 and Table 1). On the other hand, significant pulmonary fibrosis was evident in the cholestatic rats, as revealed by the Trichrome stain (Fig. 6 and Table 1). It was found that TAU significantly alleviated cholestasis-related lung histopathological alterations and fibrosis in the current study (Figs. 5 and 6 and Table 1).
Lung histopathological alterations in cholestatic animals (H&E stain; 28 days after BDL surgery). Significant inflammatory cell infiltration (yellow arrow) and hemorrhage (green arrow) were evident in the pulmonary tissue of BDL rats (28 days after the BDL operation). It was found that taurine (TAU) provided significant protective properties against lung inflammation in BDL rats. Scores of pulmonary tissue histopathological alterations and their statistical analysis are represented in Table 1. Scale bar = 100 µm
Taurine (TAU) mitigated pulmonary fibrosis (blue arrow in the Trichrome-Masson stain) in bile duct ligated (BDL) animals (28 days after BDL surgery). Scores of lung tissue fibrosis and its statistical analysis are given in Table 1. Scale bar = 100 µm
Discussion
Pulmonary injury is a serious complication associated with cholestasis/cirrhosis (Krowka and Cortese 1985; Al‐Hussaini et al. 2010; Horvatits et al. 2017). Unfortunately, there is no specific and compelling therapeutic option against this complication. The accumulation of inflammatory cells, secretion of cytokines, and occurrence of oxidative stress seem to play an essential role in cholestasis/cirrhosis-induced lung injury (Merli et al. 2010). In the current study, significant infiltrations of inflammatory cells and MPO activity were detected in the lung tissue of BDL animals. Moreover, a substantial increase in BALF levels of TNF-α, IgG, bilirubin, and bile acids was detected in BDL rats. BDL operation also caused significant histopathological alterations and increased oxidative stress and NO levels in the lung tissue. It was found that TAU (0.5 and 1% w: v in drinking water) significantly blunted BDL-related pulmonary damage. The anti-inflammatory and antioxidative stress properties of TAU seem to play a crucial role in its effects in the current investigation.
Previous studies on cholestasis have reported increased neutrophils and macrophages in the lung tissue in experimental models and human cases (Shikata et al. 2014; Hu et al. 2020). In the current study, we found that neutrophils and lymphocytes populations dramatically increased at all times, with the maximum level at day 28, after the BDL operation (Fig. 2). Moreover, we found that monocytes and eosinophils were significantly increased in the lung 28 days after the BDL surgery (Fig. 2). Our data are in line with previous studies indicating the elevation in the pulmonary tissue level of inflammatory cells and confirm that the inflammation process plays a vital role in the pathogenesis of lung injury during cholestasis.
The connection between oxidative stress and the inflammatory response is the subject of many investigations (MacNee 2001; Stamp et al. 2012; Carrera-Quintanar et al. 2020). It has been well-established that inflammatory cells are the primary sources of ROS (Forrester Steven et al. 2018). Hence, a key source of ROS and oxidative stress could be mediated through the action of these cells. Inflammation-induced ROS formation and oxidative stress could be mediated through several mechanisms. In this context, several enzymes in the inflammatory cells play a pivotal role in ROS formation and oxidative stress. It is well-known that MPO is a mediator for the induction of oxidative stress during the inflammation process (Ndrepepa 2019). MPO belongs to the superfamily of peroxidase enzymes abundantly expressed in inflammatory cells, including neutrophils and monocytes (Ndrepepa 2019). It has long been known that inflammatory cells’ MPO activity plays a critical role in ROS formation. Naturally, MPO-mediated ROS formation is essential for defense against pathogens (Aratani 2018). On the other hand, pathological elevation in the MPO levels, for example, due to severe infiltration of neutrophils into tissues, could entail tremendous ROS production and massive tissue injury (Kolli et al. 2009; Aratani 2018; Chen et al. 2020). In the current study, we found that the MPO activity in the pulmonary samples of BDL animals was significantly elevated (Fig. 4). Hence, a fundamental mechanism linking oxidative stress with inflammation in the pulmonary tissue of cholestatic animals could be mediated through the enhanced MPO activity. Interestingly, the connection between MPO activity and the amino acid TAU is the subject of several investigations (Redmond et al. 1998; Kim and Cha 2014; Marcinkiewicz and Kontny 2014; Kato et al. 2015). One of the most exciting investigations in this field has been carried out by Kim et al. (Kim and Cha 2014). This study described a putative mechanism for the anti-inflammatory properties of TAU (Kim and Cha 2014). Briefly, Kim et al. found that TAU is converted to TAU-chloramine (TauCl) by the inflammatory cells' MPO enzyme (Kim and Cha 2014). The formation of TauCL by inflammatory cells seems to have an immense effect on the protection of TAU. Kim et al. found that TauCl released from neutrophils significantly suppresses the activity of reactive species such as superoxide anion (O2• ‾) and nitric oxide (NO) (Kim and Cha 2014). TauCl can also suppress the release and activity of cytokines such as TNF-α and IL-β (Kim and Cha 2014). More interestingly, it has been found that TauCl could enhance the activity of enzymes such as glutathione reductase, peroxidases, thioredoxin, and peroxiredoxins in macrophages as well as neighbor tissues (Kim and Cha 2014). These events could protect against cytotoxic oxygen metabolites.
Inflammatory cells also contain an enzyme named NADPH oxidase. NADPH oxidase could produce considerable ROS (Bedard and Krause 2007). Therefore, it could play a vital role in the mechanism of ROS formation and oxidative stress observed in the current study. Interestingly, it is known that TAU robustly inhibits NADPH oxidase enzyme (Ekremoğlu et al. 2007; Bhavsar et al. 2009; Li et al. 2009; Miao et al. 2013). Although not investigated in the present study, an essential mechanism for the positive effects of TAU on oxidative stress biomarkers in the current model might be mediated through such a mechanism. Cytokines are cytotoxic agents in organs such as the lung (Muroya et al. 2012). In the current study, a high level of TNF-α and IgG was detected in the BALF 28 days after the BDL surgery (Fig. 2). The inhibitory effect of TAU on the secretion of cytokines by the inflammatory cells is an interesting feature of this compound and has been repeatedly mentioned in various experimental models (Zaki et al. 2011; Liu et al. 2017; Maleki et al. 2020). In this research, we found that TAU significantly decreased cytokines in the lung of cholestatic animals. This could be a crucial mechanism for the protective properties of TAU in the current model.
Oxidative stress is a significant mechanism tightly connected with tissue fibrosis (Saad et al. 2017). It is well-known that oxidative stress could stimulate tissue fibrosis in many organs (Lv et al. 2018; Filippa and Mohamed 2019). As lung tissue contains a considerable oxygen concentration, forming free oxygen radicals is more feasible in this organ (Kinnula et al. 2005; Todd et al. 2012; Cheresh et al. 2013). On the other hand, the presence of several enzymes such as eosinophil peroxidases, MPO, and possibly xanthine oxidase could ease this process (Kinnula et al. 2005; Todd et al. 2012; Cheresh et al. 2013). Although lung tissue developed robust antioxidant systems to encompass this problem (Kinnula et al. 2005; Todd et al. 2012; Cheresh et al. 2013), several investigations revealed that antioxidant defense systems are impaired in the lung during cholestasis/cirrhosis (Ommati et al. 2021a). Collectively, lung tissue can develop significant tissue fibrosis during cholestasis/cirrhosis. Therefore, finding therapeutic strategies against this complication has great clinical value. The current study found that TAU significantly abated cholestasis pulmonary fibrosis. The antifibrotic properties of TAU in the lung of cholestasis animals could be associated with, at least in part, its role in abating oxidative stress biomarkers in this organ. On the other hand, assessing the role of signaling molecules and growth factors (e.g., ET-1, PDGF-BB, and TGF-β) involved in the pathogenesis of tissue fibrosis and organ injury could give a better insight into the role of antifibrotic properties of pharmacological interventions in future studies. Moreover, evaluating some other markers (e.g., arterial blood gas) could clear the degree of lung injury in cholestasis/cirrhosis and estimate the impact of therapeutic interferences in experimental models.
The inhibitory role of TAU on NO synthesis is an essential feature of this amino acid (Schaffer and Kim 2018; Guizoni et al. 2020). In the current study, we found that NO levels were significantly decreased in the lung of TAU-treated BDL rats. NO plays a major role in nitrosative stress (Heinrich et al. 2013). NO could react with ROS such as superoxide anion (O2• −) to produce toxic peroxynitrite anion (ONOO•−) (Heinrich et al. 2013). Hence, preventing NO production in the lung of BDL rats could be an essential mechanism of the protective effects of TAU in the current model.
Cytotoxic bile acids are the most likely chemical suspects responsible for lung complications during cholestasis (Zecca et al. 2008; Yu et al. 2014). These compounds are strong surfactants that could severely damage and denature alveolar structures, leading to harmful events such as lipid peroxidation (Chen et al. 2017; Ommati et al. 2021b). In the current model, supraphysiological concentrations of bile acids were detected in the lung tissue of cholestatic rats. Fortunately, several studies have explored the positive effects of TAU on biomembranes (Shi et al. 1997; You and Chang 1998; Pushpakiran et al. 2004; Das et al. 2009; Heidari et al. 2019a). It has been found that TAU significantly prevents lipid peroxidation through an unknown mechanism. However, it seems that this amino acid stabilizes lipid membranes and prevents phospholipid bilayers oxidation by free radicals (You and Chang 1998; Pushpakiran et al. 2004; Das et al. 2009; Heidari et al. 2019a). In the current study, this mechanism of TAU seems to ideally prevent alveolar damage in cholestasis as the level of lipid peroxidation was significantly lower in the lung of TAU-treated animals. Interestingly, a plethora of investigations revealed that TAU could protect the lung against xenobiotics-induced injury or several pulmonary disorders in many experimental models (Gordon et al. 1986; Gurujeyalakshmi et al. 1998; Schuller-Levis et al. 2003; Li et al. 2017). Interestingly, the positive effects of TAU on markers such as pulmonary hypertension have been reported in previous studies, including experimental animals and human subjects (Ruiz-Feria and Wideman 2001; Militante and Lombardini 2002). All these data mention TAU as a potent protective agent against pulmonary disorders.
The hepatoprotective properties of TAU have been repeatedly mentioned in the previous studies (Heidari et al. 2016b, 2018e; Jamshidzadeh et al. 2017). It has been found that this amino acid significantly protected hepatocytes against xenobiotics and liver diseases (Heidari et al. 2012, 2013, 2014, 2015a, 2015c, 2016d; Miyazaki and Matsuzaki 2014; Karamikhah et al. 2016; Nikkhah et al. 2021). Our research team also mentioned the hepatoprotective effects of TAU in BDL animals (Heidari et al. 2016b). TAU significantly decreased serum biomarkers such as ALT, AST, and LDH in BDL rats (Heidari et al. 2016b). TAU also mitigated liver histopathological changes in the cholestatic animals (Heidari et al. 2016b). Hence, the hepatoprotective effects of TAU might also partly contribute to the benefical role of this amino acid against cholestasis-induced lung injury in the BDL model of cholestasis/cirrhosis.
Fortunately, TAU is a very safe amino acid and could be readily used in humans (e.g., > 6 g/day) (Militante and Lombardini 2002; Schwarzer et al. 2018). Obviously, further investigations are needed to delineate the mechanisms of action of TAU against cholestasis/cirrhosis-induced pulmonary complications and, finally, its application in clinical settings.
Data availability
All data generated or analyzed during this study are included in this published article. Any supplementary data could be available from the corresponding author at reasonable request.
References
Abdel-Moneim AM, Al-Kahtani MA, El-Kersh MA, Al-Omair MA (2015) Free radical-scavenging, anti-inflammatory/anti-fibrotic and hepatoprotective actions of taurine and silymarin against CCl4 induced rat liver damage. PLoS ONE 10(12):e0144509
Abdoli N, Sadeghian I, Azarpira N, Ommati MM, Heidari R (2021) Taurine mitigates bile duct obstruction-associated cholemic nephropathy: effect on oxidative stress and mitochondrial parameters. Clin Exp Hepatol 7(1):30–40
Acharya M, Lau-Cam CA (2013) Comparative evaluation of the effects of taurine and thiotaurine on activities of antioxidant and glutathione-related enzymes by acetaminophen in the rat. In: Idrissi AE, L’Amoreaux WJ (eds) Taurine 8. Springer, New York, pp 199–215
Ahmadi N, Rezaee Z, Azarpira N, Zahedi S, Saeedi A, Jamshidzadeh A, Heidari R (2021) A histopathological evaluation on the effect of captopril in cyclophosphamide-induced hemorrhagic cystitis. Trend Pharm Sci 7(1):35–48
Ahmadi A, Niknahad H, Li H, Mobasheri A, Manthari RK, Azarpira N, Mousavi K, Khalvati B, Zhao Y, Sun J, Zong Y, Ommati MM, Heidari R (2021a) The inhibition of NFкB signaling and inflammatory response as a strategy for blunting bile acid-induced hepatic and renal toxicity. Toxicol Lett 34912–29
Alhumaidha KA, Saleh DO, Abd El Fattah MA, El-Eraky WI, Moawad H (2015) Cardiorenal protective effect of taurine against cyclophosphamide-induced toxicity in albino rats. Canadian Journal of Physiology and Pharmacology 1–9
Al-Hussaini A, Taylor RM, Samyn M, Bansal S, Heaton N, Rela M, Mieli-Vergani G, Dhawan A (2010) Long-term outcome and management of hepatopulmonary syndrome in children. Pediatr Transplant 14(2):276–282
Aniort J, Poyet A, Kemeny J-L, Philipponnet C, Heng A-E (2017) Bile cast nephropathy caused by obstructive cholestasis. Am J Kidney Dis 69(1):143–146
Aratani Y (2018) Myeloperoxidase: its role for host defense, inflammation, and neutrophil function. Archives of Biochemistry and Biophysics 64047–52
Aruoma OI, Halliwell B, Hoey BM, Butler J (1988) The antioxidant action of taurine, hypotaurine and their metabolic precursors. Biochemical Journal 256(1):251–255
Bedard K, Krause K-H (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87(1):245–313
Bhavsar TM, Cantor JO, Patel SN, Lau-Cam CA (2009) Attenuating effect of taurine on lipopolysaccharide-induced acute lung injury in hamsters. Pharmacol Res 60(5):418–428
Bomzon A, Holt S, Moore K (1997) Bile acids, oxidative stress, and renal function in biliary obstruction. Semin Nephrol 17(6):549–562
Carrera-Quintanar L, Funes L, Herranz-López M, Martínez-Peinado P, Pascual-García S, Sempere JM, Boix-Castejón M, Córdova A, Pons A, Micol V, Roche E (2020) Antioxidant supplementation modulates neutrophil inflammatory response to exercise-induced stress. Antioxidants 9(12):E1242
Chen B, You Wen J, Liu Xue Q, Xue S, Qin H, Jiang Han D (2017) Chronic microaspiration of bile acids induces lung fibrosis through multiple mechanisms in rats. Clin Sci 131(10):951–963
Chen S, Chen H, Du Q, Shen J (2020) Targeting myeloperoxidase (MPO) mediated oxidative stress and inflammation for reducing brain ischemia injury: potential application of natural compounds. Front Physiol 0
Cheresh P, Kim S-J, Tulasiram S, Kamp DW (2013) Oxidative stress and pulmonary fibrosis. Biochim Biophys Acta 1832(7):1028–1040
Cozzi R, Ricordy R, Bartolini F, Ramadori L, Perticone P, De Salvia R (1995) Taurine and ellagic acid: two differently-acting natural antioxidants. Environ Mol Mutagen 26(3):248–254
Das J, Ghosh J, Manna P, Sinha M, Sil PC (2009) Taurine protects rat testes against NaAsO2-induced oxidative stress and apoptosis via mitochondrial dependent and independent pathways. Toxicol Lett 187(3):201–210
Daubeuf F, Frossard N (2014) Eosinophils and the ovalbumin mouse model of asthma. Methods Mol Biol 1178283–293
Ding Y-L, Zhang L-J, Wang X, Zhou Q-C, Li N, Wang C-X, Zhang X-Q (2014) Fetal lung surfactant and development alterations in intrahepatic cholestasis of pregnancy. World J Obstet Gynecol 3(2):78–84
Ekremoğlu M, Türközkan N, Erdamar H, Kurt Y, Yaman H (2007) Protective effect of taurine on respiratory burst activity of polymorphonuclear leukocytes in endotoxemia. Amino Acids 32(3):413–417
Enrico Z, Daniele DL, Marco M, Giada B, Costantino R (2007) Intrahepatic cholestasis of pregnancy and bile acids induced lung injury in newborn infants. Curr Pediatr Rev 3(2):167–176
Erlinger S (2014) Bile acids in cholestasis: bad for the liver, not so good for the kidney. Clin Res Hepatol Gastroenterol 38(4):392–394
Farshad O, Ommati MM, Yüzügülen J, Jamshidzadeh A, Mousavi K, Ahmadi Z, Azarpira N, Ghaffari H, Najibi A, Shafaghat M, Niknahad H, Heidari R (2020) Carnosine mitigates biomarkers of oxidative stress, improves mitochondrial function, and alleviates histopathological alterations in the renal tissue of cholestatic rats. Pharm Sci 27(1):32–45
Filippa VP, Mohamed FH (2019) Lithium therapy effects on the reproductive system. Psychiatry and Neuroscience Update. Springer 187–200
Forrester Steven J, Kikuchi Daniel S, Hernandes Marina S, Xu Q, Griendling Kathy K (2018) Reactive oxygen species in metabolic and inflammatory signaling. Circ Res 122(6):877–902
Ghanbarinejad V, Jamshidzadeh A, Khalvati B, Farshad O, Li H, Shi X, Chen Y, Ommati MM, Heidari R (2021) Apoptosis-inducing factor plays a role in the pathogenesis of hepatic and renal injury during cholestasis. Naunyn-Schmiedeberg’s Arch Pharmacol 394(6):1191–1203
Gordon RE, Shaked AA, Solano DF (1986) Taurine protects hamster bronchioles from acute NO2-induced alterations. A histologic, ultrastructural, and freeze-fracture study. Am J Pathol 125(3):585–600
Guizoni DM, Vettorazzi JF, Carneiro EM, Davel AP (2020) Modulation of endothelium-derived nitric oxide production and activity by taurine and taurine-conjugated bile acids. Nitric Oxide 9448–53
Guler L, Tavlasoglu M, Yucel O, Guler A, Sahin MA, Kurkluoglu M, Sirin Y, Eken A, Gamsizkan M, Dakak M, Gurkok S, Genc O (2014) Taurine attenuates lung ischemia–reperfusion injury after lung transplantation in rats. J Anesth 28(3):347–353
Gürer H, Ozgünes H, Saygin E, Ercal N (2001) Antioxidant effect of taurine against lead-induced oxidative stress. Arch Environ Contam Toxicol 41(4):397–402
Gurujeyalakshmi G, Hollinger MA, Giri SN (1998) Regulation of transforming growth factor- β1 mRNA expression by taurine and niacin in the bleomycin hamster hodel of lung fibrosis. Am J Respir Cell Mol Biol 18(3):334–342
Hamza RZ, El-Shenawy NS (2017) Anti-inflammatory and antioxidant role of resveratrol on nicotine-induced lung changes in male rats. Toxicology Reports 4399–407
Heidari R, Niknahad H (2019) The role and study of mitochondrial impairment and oxidative stress in cholestasis. In: Vinken M (ed) Experimental Cholestasis Research. Springer, New York, NY, pp 117–132
Heidari R, Babaei H, Eghbal MA (2012) Ameliorative effects of taurine against methimazole-induced cytotoxicity in isolated rat hepatocytes. Sci Pharm 80(4):987–1000
Heidari R, Babaei H, Eghbal MA (2013) Cytoprotective effects of taurine against toxicity induced by isoniazid and hydrazine in isolated rat hepatocytes. Arh Hig Rada Toksikol 64(2):201–210
Heidari R, Babaei H, Eghbal MA (2014) Amodiaquine-induced toxicity in isolated rat hepatocytes and the cytoprotective effects of taurine and/or N-acetyl cysteine. Res Pharm Sci 9(2):97–105
Heidari R, Jamshidzadeh A, Keshavarz N, Azarpira N (2015) Mitigation of methimazole-induced hepatic injury by taurine in mice. Sci Pharm 83(1):143–158
Heidari R, Niknahad H, Jamshidzadeh A, Azarpira N, Bazyari M, Najibi A (2015) Carbonyl traps as potential protective agents against methimazole-induced liver injury. J Biochem Mol Toxicol 29(4):173–181
Heidari R, Sadeghi N, Azarpira N, Niknahad H (2015) Sulfasalazine-induced hepatic injury in an ex vivo model of isolated perfused rat liver and the protective role of taurine. Pharm Sci 21(4):211–219
Heidari R, Esmailie N, Azarpira N, Najibi A, Niknahad H (2016) Effect of thiol-reducing agents and antioxidants on sulfasalazine-induced hepatic injury in normotermic recirculating isolated perfused rat liver. Toxicol Res 32(2):133–140
Heidari R, Jamshidzadeh A, Niknahad H, Safari F, Azizi H, Abdoli N, Ommati MM, Khodaei F, Saeedi A, Najibi A (2016) The hepatoprotection provided by taurine and glycine against antineoplastic drugs induced liver injury in an ex vivo model of normothermic recirculating isolated perfused rat liver. Trend Pharm Sci 2(1):59–76
Heidari R, Rasti M, ShiraziYeganeh B, Niknahad H, Saeedi A, Najibi A (2016) Sulfasalazine-induced renal and hepatic injury in rats and the protective role of taurine. BioImpacts 6(1):3–8
Heidari R, Moezi L, Asadi B, Ommati MM, Azarpira N (2017) Hepatoprotective effect of boldine in a bile duct ligated rat model of cholestasis/cirrhosis. PharmaNutrition 5(3):109–117
Heidari R, Jafari F, Khodaei F, ShiraziYeganeh B, Niknahad H (2018) Mechanism of valproic acid-induced Fanconi syndrome involves mitochondrial dysfunction and oxidative stress in rat kidney. Nephrology 23(4):351–361
Heidari R, Jamshidzadeh A, Ghanbarinejad V, Ommati MM, Niknahad H (2018) Taurine supplementation abates cirrhosis-associated locomotor dysfunction. Clin Exp Hepatol 4(2):72–82
Heidari R, Ommati MM, Alahyari S, Azarpira N, Niknahad H (2018) Amino acid-containing Krebs-Henseleit buffer protects rat liver in a long-term organ perfusion model. Pharm Sci 24(3):168–179
Heidari R, Ahmadi A, Ommati MM, Niknahad H (2020) Methylene blue improves mitochondrial function in the liver of cholestatic rats. Trend Pharm Sci 6(2):73–86
Heidari R, Jamshidzadeh A, Niknahad H, Mardani E, Ommati MM, Azarpira N, Khodaei F, Zarei A, Ayarzadeh M, Mousavi S, Abdoli N, Yeganeh BS, Saeedi A, Najibi A (2016b) Effect of taurine on chronic and acute liver injury: focus on blood and brain ammonia. Toxicology Reports 3870–879
Heidari R, Ghanbarinejad V, Mohammadi H, Ahmadi A, Esfandiari A, Azarpira N, Niknahad H (2018a) Dithiothreitol supplementation mitigates hepatic and renal injury in bile duct ligated mice: potential application in the treatment of cholestasis-associated complications. Biomed Pharmacother 991022–1032
Heidari R, Ghanbarinejad V, Mohammadi H, Ahmadi A, Ommati MM, Abdoli N, Aghaei F, Esfandiari A, Azarpira N, Niknahad H (2018b) Mitochondria protection as a mechanism underlying the hepatoprotective effects of glycine in cholestatic mice. Biomed Pharmacother 971086–1095
Heidari R, Behnamrad S, Khodami Z, Ommati MM, Azarpira N, Vazin A (2019a) The nephroprotective properties of taurine in colistin-treated mice is mediated through the regulation of mitochondrial function and mitigation of oxidative stress. Biomed Pharmacother 109103–111
Heidari R, Mandegani L, Ghanbarinejad V, Siavashpour A, Ommati MM, Azarpira N, Najibi A, Niknahad H (2019b) Mitochondrial dysfunction as a mechanism involved in the pathogenesis of cirrhosis-associated cholemic nephropathy. Biomed Pharmacother 109271–280
Heinrich TA, Silva RSd, Miranda KM, Switzer CH, Wink DA, Fukuto JM (2013) Biological nitric oxide signalling: chemistry and terminology. Br J Pharmacol 169(7):1417–1429
Herraez E, Lozano E, Poli E, Keitel V, De Luca D, Williamson C, Marin JJG, Macias RIR (2014) Role of macrophages in bile acid-induced inflammatory response of fetal lung during maternal cholestasis. J Mol Med 92(4):359–372
Horvatits T, Drolz A, Rutter K, Roedl K, Fauler G, Müller C, Kluge S, Trauner M, Schenk P, Fuhrmann V (2017) Serum bile acids in patients with hepatopulmonary syndrome. Z Gastroenterol 55(4):361–367
Hsieh Y-L, Yeh Y-H, Lee Y-T, Huang C-Y (2014) Effect of taurine in chronic alcoholic patients. Food Funct 5(7):1529–1535
Hu Z-H, Kong Y-Y, Ren J-J, Huang T-J, Wang Y-Q, Liu L-X (2020) Kidney and lung tissue modifications after BDL-induced liver injury in mice are associated with increased expression of IGFBPrP1 and activation of the NF-κB inflammation pathway. Int J Clin Exp Pathol 13(2):192–202
Huxtable RJ et al (1992) Physiological actions of taurine. Physiol Rev 72(1):101–163
Islambulchilar M, Asvadi I, Sanaat Z, Esfahani A, Sattari M (2015) Taurine attenuates chemotherapy-induced nausea and vomiting in acute lymphoblastic leukemia. Amino Acids 47(1):101–109
Jamshidzadeh A, Heidari R, Abasvali M, Zarei M, Ommati MM, Abdoli N, Khodaei F, Yeganeh Y, Jafari F, Zarei A, Latifpour Z, Mardani E, Azarpira N, Asadi B, Najibi A (2017) Taurine treatment preserves brain and liver mitochondrial function in a rat model of fulminant hepatic failure and hyperammonemia. Biomed Pharmacother 86514–520
Jüngst C, Lammert F (2013) Cholestatic liver disease. Dig Dis 31(1):152–154
Karamikhah R, Jamshidzadeh A, Azarpira N, Saeidi A, Heidari R (2016) Propylthiouracil-induced liver injury in mice and the protective role of taurine. Pharm Sci 21(2):94–101
Kato T, Okita S, Wang S, Tsunekawa M, Ma N (2015) The effects of taurine administration against inflammation in heavily exercised skeletal muscle of rats. Advances in Experimental Medicine and Biology 803773–784
Kim C, Cha Y-N (2014) Taurine chloramine produced from taurine under inflammation provides anti-inflammatory and cytoprotective effects. Amino Acids 46(1):89–100
Kinnula VL, Fattman CL, Tan RJ, Oury TD (2005) Oxidative stress in pulmonary fibrosis. Am J Respir Crit Care Med 172(4):417–422
Kolli VK, Abraham P, Isaac B, Selvakumar D (2009) Neutrophil infiltration and oxidative stress may play a critical role in methotrexate-induced renal damage. Chemotherapy 55(2):83–90
Krones E, Wagner M, Eller K, Rosenkranz AR, Trauner M, Fickert P (2015) Bile acid-induced cholemic nephropathy. Dig Dis 33(3):367–375
Krowka MJ, Cortese DA (1985) Pulmonary aspects of chronic liver disease and liver transplantation. Mayo Clin Proc 60(6):407–418
Levy C (2013) Cholestatic liver diseases, an issue of clinics in liver disease. Elsevier Health Sciences
Li Y, Arnold JMO, Pampillo M, Babwah AV, Peng T (2009) Taurine prevents cardiomyocyte death by inhibiting NADPH oxidase-mediated calpain activation. Free Radic Biol Med 46(1):51–61
Li X, Yang H, Sun H, Lu R, Zhang C, Gao N, Meng Q, Wu S, Wang S, Aschner M, Wu J, Tang B, Gu A, Kay SA, Chen R (2017) Taurine ameliorates particulate matter-induced emphysema by switching on mitochondrial NADH dehydrogenase genes. Proc Natl Acad Sci U S A 114(45):E9655–E9664
Lin C-J, Chiu C-C, Chen Y-C, Chen M-L, Hsu T-C, Tzang B-S (2015) Taurine attenuates hepatic inflammation in chronic alcohol-fed rats through inhibition of TLR4/MyD88 signaling. J Med Food 18(12):1291–1298
Liu SF, Ye X, Malik AB (1999) Pyrrolidine dithiocarbamate prevents I-κB degradation and reduces microvascular injury induced by lipopolysaccharide in multiple organs. Mol Pharmacol 55(4):658–667
Liu Y, Li F, Zhang L, Wu J, Wang Y, Yu H (2017) Taurine alleviates lipopolysaccharide-induced liver injury by anti-inflammation and antioxidants in rats. Mol Med Report 16(5):6512–6517
Lv W, Booz GW, Fan F, Wang Y, Roman RJ (2018) Oxidative stress and renal fibrosis: recent insights for the development of novel therapeutic strategies. Front Physiol 9
MacNee W (2001) Oxidative stress and lung inflammation in airways disease. Eur J Pharmacol 429(1):195–207
Maleki V, Mahdavi R, Hajizadeh-Sharafabad F, Alizadeh M (2020) The effects of taurine supplementation on oxidative stress indices and inflammation biomarkers in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Diabetol Metab Syndr 12(1):9
Marcinkiewicz J, Kurnyta M, Biedroń R, Bobek M, Kontny E, Maśliński W (2006) Anti-inflammatory effects of taurine derivatives (taurine chloramine, taurine bromamine, and taurolidine) are mediated by different mechanisms. Advances in Experimental Medicine and Biology 583481–492
Marcinkiewicz J, Kontny E (2014) Taurine and inflammatory diseases. Amino Acids 46(1):7–20
Meeks RG, Harrison S (1991) Hepatotoxicology. CRC Press
Merli M, Lucidi C, Giannelli V, Giusto M, Riggio O, Falcone M, Ridola L, Attili AF, Venditti M (2010) Cirrhotic patients are at risk for health care–associated bacterial infections. Clin Gastroenterol Hepatol 8(11):979-985.e971
Miao J, Zhang J, Ma Z, Zheng L (2013) The role of NADPH oxidase in taurine attenuation of Streptococcus uberis-induced mastitis in rats. Int Immunopharmacol 16(4):429–435
Militante JD, Lombardini JB (2002) Treatment of hypertension with oral taurine: experimental and clinical studies. Amino Acids 23(4):381–393
Miyazaki T, Matsuzaki Y (2014) Taurine and liver diseases: a focus on the heterogeneous protective properties of taurine. Amino Acids 46(1):101–110
Moezi L, Heidari R, Amirghofran Z, Nekooeian AA, Monabati A, Dehpour AR (2013) Enhanced anti-ulcer effect of pioglitazone on gastric ulcers in cirrhotic rats: the role of nitric oxide and IL-1β. Pharmacol Rep 65(1):134–143
Mohammadi H, Sayad A, Mohammadi M, Niknahad H, Heidari R (2020) N-acetyl cysteine treatment preserves mitochondrial indices of functionality in the brain of hyperammonemic mice. Clin Exp Hepatol 6(2):106–115
Mousavi K, Niknahad H, Ghalamfarsa A, Mohammadi H, Azarpira N, Ommati MM, Heidari R (2020) Taurine mitigates cirrhosis-associated heart injury through mitochondrial-dependent and antioxidative mechanisms. Clin Exp Hepatol 6(3):207–219
Mousavi K, Niknahad H, Li H, Jia Z, Manthari RK, Zhao Y, Shi X, Chen Y, Ahmadi A, Azarpira N, Khalvati B, Ommati MM, Heidari R (2021) The activation of nuclear factor-E2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) signaling blunts cholestasis-induced liver and kidney injury. Toxicol Res 10(4):911–927
Muroya M, Chang K, Uchida K, Bougaki M, Yamada Y (2012) Analysis of cytotoxicity induced by proinflammatory cytokines in the human alveolar epithelial cell line A549. Biosci Trends 6(2):70–80
Ndrepepa G (2019) Myeloperoxidase - a bridge linking inflammation and oxidative stress with cardiovascular disease. Clin Chim Acta 49336–51
Nikkhah E, Shirani K, Rezaee R, Karimi G (2021) Protective effects of taurine against hepatotoxicity induced by pharmaceuticals and environmental chemicals. Toxicol Environ Chem 103(1):56–84
Niknahad H, Heidari R, Alzuhairi AM, Najibi A (2014) Mitochondrial dysfunction as a mechanism for pioglitazone-induced injury toward HepG2 cell line. Pharm Sci 20(4):169–174
Niknahad H, Heidari R, Mohammadzadeh R, Ommati MM, Khodaei F, Azarpira N, Abdoli N, Zarei M, Asadi B, Rasti M, Yeganeh BS, Taheri V, Saeedi A, Najibi A (2017) Sulfasalazine induces mitochondrial dysfunction and renal injury. Ren Fail 39(1):745–753
Niknahad H, Jamshidzadeh A, Heidari R, Zarei M, Ommati MM (2017) Ammonia-induced mitochondrial dysfunction and energy metabolism disturbances in isolated brain and liver mitochondria, and the effect of taurine administration: relevance to hepatic encephalopathy treatment. Clin Exp Hepatol 3(3):141–151
Okada S, Hasegawa S, Hasegawa H, Ainai A, Atsuta R, Ikemoto K, Sasaki K, Toda S, Shirabe K, Takahara M, Harada S, Morishima T, Ichiyama T (2013) Analysis of bronchoalveolar lavage fluid in a mouse model of bronchial asthma and H1N1 2009 infection. Cytokine 63(2):194–200
Ommati MM, Jamshidzadeh A, Niknahad H, Mohammadi H, Sabouri S, Heidari R, Abdoli N (2017) N-acetylcysteine treatment blunts liver failure-associated impairment of locomotor activity. PharmaNutrition 5(4):141–147
Ommati MM, Amjadinia A, Mousavi K, Azarpira N, Jamshidzadeh A, Heidari R (2021) N-acetyl cysteine treatment mitigates biomarkers of oxidative stress in different tissues of bile duct ligated rats. Stress 24(2):213–228
Ommati MM, Farshad O, Azarpira N, Ghazanfari E, Niknahad H, Heidari R (2021) Silymarin mitigates bile duct obstruction-induced cholemic nephropathy. Naunyn-Schmiedeberg’s Arch Pharmacol 394(6):1301–1314
Ommati MM, Farshad O, Azarpira N, Shafaghat M, Niknahad H, Heidari R (2021) Betaine alleviates cholestasis-associated renal injury by mitigating oxidative stress and enhancing mitochondrial function. Biologia 76(1):351–365
Ommati MM, Hojatnezhad S, Abdoli N, Manthari RK, Jia Z, Najibi A, Akbarizadeh AR, Sadeghian I, Farshad O, Azarpira N, Niknahad H, Heidari R (2021) Pentoxifylline mitigates cholestasis-related cholemic nephropathy. Clinical and Experimental Hepatology 7(4):377–389
Ommati MM, Niknahad H, Farshad O, Azarpira N, Heidari R (2021) In vitro and in vivo evidence on the role of mitochondrial impairment as a mechanism of lithium-induced nephrotoxicity. Biol Trace Elem Res 199(5):1908–1918
Ommati MM, Li H, Jamshidzadeh A, Khoshghadam F, Retana-Márquez S, Lu Y, Farshad O, Nategh Ahmadi MH, Gholami A, Heidari R (2022) The crucial role of oxidative stress in non-alcoholic fatty liver disease-induced male reproductive toxicity: the ameliorative effects of Iranian indigenous probiotics. Naunyn-Schmiedeberg’s Arch Pharmacol 395(2):247–265
Ommati MM, Farshad O, Niknahad H, Arabnezhad MR, Azarpira N, Mohammadi HR, Haghnegahdar M, Mousavi K, Akrami S, Jamshidzadeh A, Heidari R (2019) Cholestasis-associated reproductive toxicity in male and female rats: the fundamental role of mitochondrial impairment and oxidative stress. Toxicol Lett 31660–72
Ommati MM, Attari H, Siavashpour A, Shafaghat M, Azarpira N, Ghaffari H, Moezi L, Heidari R (2020a) Mitigation of cholestasis-associated hepatic and renal injury by edaravone treatment: evaluation of its effects on oxidative stress and mitochondrial function. Liver Res In-Press
Ommati MM, Farshad O, Mousavi K, Jamshidzadeh A, Azmoon M, Heidari S, Azarpira N, Niknahad H, Heidari R (2020b) Betaine supplementation mitigates intestinal damage and decreases serum bacterial endotoxin in cirrhotic rats. PharmaNutrition 12100179
Ommati MM, Farshad O, Mousavi K, Taghavi R, Farajvajari S, Azarpira N, Moezi L, Heidari R (2020c) Agmatine alleviates hepatic and renal injury in a rat model of obstructive jaundice. PharmaNutrition 13100212
Ommati MM, Farshad O, Niknahad H, Mousavi K, Moein M, Azarpira N, Mohammadi H, Jamshidzadeh A, Heidari R (2020d) Oral administration of thiol-reducing agents mitigates gut barrier disintegrity and bacterial lipopolysaccharide translocation in a rat model of biliary obstruction. Curr Res Pharmacol Drug Discov 110–18
Ommati MM, Mohammadi H, Mousavi K, Azarpira N, Farshad O, Dehghani R, Najibi A, Kamran S, Niknahad H, Heidari R (2020e) Metformin alleviates cholestasis-associated nephropathy through regulating oxidative stress and mitochondrial function. Liver Res
Ommati MM, Shi X, Li H, Zamiri MJ, Farshad O, Jamshidzadeh A, Heidari R, Ghaffari H, Zaker L, Sabouri S, Chen Y (2020f) The mechanisms of arsenic-induced ovotoxicity, ultrastructural alterations, and autophagic related paths: an enduring developmental study in folliculogenesis of mice. Ecotoxicology and Environmental Safety 204110973
Ommati MM, Arabnezhad MR, Farshad O, Jamshidzadeh A, Niknahad H, Retana-Marquez S, Jia Z, Nateghahmadi MH, Mousavi K, Arazi A, Azmoon MR, Azarpira N, Heidari R (2021b) The role of mitochondrial impairment and oxidative stress in the pathogenesis of lithium-induced reproductive toxicity in male mice. Frontiers in Veterinary Science 8(125):
Ommati MM, Arabnezhad MR, Farshad O, Jamshidzadeh A, Niknahad H, Retana-Marquez S, Jia Z, Nateghahmadi MH, Mousavi K, Arazi A, Azmoon MR, Azarpira N, Heidari R (2021c) The role of mitochondrial impairment and oxidative stress in the pathogenesis of lithium-induced reproductive toxicity in male mice. Front Vet Sci 8603262
Orellana M, Rodrigo R, Thielemann L, Guajardo V (2000) Bile duct ligation and oxidative stress in the rat: effects in liver and kidney. Comp Biochem Physiol 126(2):105–111
Pushpakiran G, Mahalakshmi K, Anuradha CV (2004) Taurine restores ethanol-induced depletion of antioxidants and attenuates oxidative stress in rat tissues. Amino Acids 27(1):91–96
Ramos CdO, Campos KKD, Costa GdP, Cangussú SD, Talvani A, Bezerra FS (2018) Taurine treatment decreases inflammation and oxidative stress in lungs of adult mice exposed to cigarette smoke. Regulatory Toxicology and Pharmacology 9850–57
Rashid K, Das J, Sil PC (2013) Taurine ameliorate alloxan induced oxidative stress and intrinsic apoptotic pathway in the hepatic tissue of diabetic rats. Food and Chemical Toxicology 51317–329
Redmond HP, Stapleton PP, Neary P, Bouchier-Hayes D (1998) Immunonutrition: the role of taurine. Nutrition 14(7):599–604
Ruiz-Feria CA, Wideman RF (2001) Taurine, cardiopulmonary hemodynamics, and pulmonary hypertension syndrome in broilers. Poult Sci 80(11):1607–1618
Saad AB, Rjeibi I, Alimi H, Ncib S, Smida A, Zouari N, Zourgui L (2017) Lithium induced, oxidative stress and related damages in testes and heart in male rats: the protective effects of Malva sylvestris extract. Biomed Pharmacother 86127–135
Salatti Ferrari R, da Rosa DP, Forgiarini LF, Bona S, Simões Dias A, Marroni NP (2012) Oxidative stress and pulmonary changes in experimental liver cirrhosis. Oxid Med Cell Longev 2012e486190
Santangelo F, Cortijo J, Morcillo E (2003) Taurine and the lung. In: Lombardini JB, Schaffer SW, Azuma J (eds) Taurine 5: Beginning the 21st Century. Springer, US, Boston, MA, pp 403–410
Schaffer S, Kim HW (2018) Effects and mechanisms of taurine as a therapeutic agent. Biomol Ther (seoul) 26(3):225–241
Schuller-Levis GB, Gordon RE, Wang C, Park E (2003) Taurine reduces lung inflammation and fibrosis caused by bleomycin. Advances in Experimental Medicine and Biology 526395–402
Schuller-Levis GB, Park E (2003) Taurine: new implications for an old amino acid. FEMS Microbiol Lett 226(2):195–202
Schwarzer R, Kivaranovic D, Mandorfer M, Paternostro R, Wolrab D, Heinisch B, Reiberger T, Ferlitsch M, Gerner C, Trauner M, Peck-Radosavljevic M, Ferlitsch A (2018) Randomised clinical study: the effects of oral taurine 6g/day vs placebo on portal hypertension. Aliment Pharmacol Ther 47(1):86–94
Shi X, Flynn DC, Porter DW, Leonard SS, Vallyathan V, Castranova V (1997) Efficacy of taurine based compounds as hydroxyl radical scavengers in silica induced peroxidation. Ann Clin Lab Sci 27(5):365–374
Shikata F, Sakaue T, Nakashiro K-i, Okazaki M, Kurata M, Okamura T, Okura M, Ryugo M, Nakamura Y, Yasugi T, Higashiyama S, Izutani H (2014) Pathophysiology of lung injury induced by common bile duct ligation in mice. PLoS ONE 9(4):e94550
Shimada K, Jong CJ, Takahashi K, Schaffer SW (2015) Role of ROS production and turnover in the antioxidant activity of taurine.
Siavashpour A, Khalvati B, Azarpira N, Mohammadi H, Niknahad H, Heidari R (2020) Poly (ADP-Ribose) polymerase-1 (PARP-1) overactivity plays a pathogenic role in bile acids-induced nephrotoxicity in cholestatic rats. Toxicol Lett 330144–158
Stamp LK, Khalilova I, Tarr JM, Senthilmohan R, Turner R, Haigh RC, Winyard PG, Kettle AJ (2012) Myeloperoxidase and oxidative stress in rheumatoid arthritis. Rheumatology 51(10):1796–1803
Su Y, Fan W, Ma Z, Wen X, Wang W, Wu Q, Huang H (2014) Taurine improves functional and histological outcomes and reduces inflammation in traumatic brain injury. Neuroscience 26656–65
Todd NW, Luzina IG, Atamas SP (2012) Molecular and cellular mechanisms of pulmonary fibrosis. Fibrogenesis & Tissue Repair 5(1):11
Truong DH, Eghbal MA, Hindmarsh W, Roth SH, O’Brien PJ (2006) Molecular mechanisms of hydrogen sulfide toxicity. Drug Metab Rev 38(4):733–744
Tu S, Zhang X-L, Wan H-F, Xia Y-Q, Liu Z-Q, Yang X-H, Wan F-S (2018) Effect of taurine on cell proliferation and apoptosis human lung cancer A549 cells. Oncol Lett 15(4):5473–5480
Vazin A, Heidari R, Khodami Z (2020) Curcumin supplementation alleviates polymyxin E-induced nephrotoxicity. J Exp Pharmacol 12129–136
Wójcik OP, Koenig KL, Zeleniuch-Jacquotte A, Costa M, Chen Y (2010) The potential protective effects of taurine on coronary heart disease. Atherosclerosis 208(1):19–25
Wright CE, Tallan HH, Lin YY (1986) Taurine: biological update. Annu Rev Biochem 55(1):427–453
Yang K, Wu Y, Xie H, Li M, Ming S, Li L, Li M, Wu M, Gong S, Huang X (2016) Macrophage-mediated inflammatory response decreases mycobacterial survival in mouse MSCs by augmenting NO production. Sci Rep 6(1):27326
Yang L, Tang J, Chen H, Ge D, Sui T, Que J, Cao X, Ge Y (2016) Taurine reduced epidural fibrosis in rat models after laminectomy via downregulating EGR1. Cell Physiol Biochem 38(6):2261–2271
You JS, Chang KJ (1998) Taurine protects the liver against lipid peroxidation and membrane disintegration during rat hepatocarcinogenesis. Advances in Experimental Medicine and Biology 442105–112
Yu L, Ding Y, Huang T, Huang X (2014) Effect of bile acid on fetal lung in rat model of intrahepatic cholestasis of pregnancy. Int J Endocrinol 2014e308274
Zaki HF, Salem HA, El-Yamany MF (2011) Taurine: a promising agent of therapeutic potential in experimentally-induced arthritis. Egypt Rheumatol 33(3):131–137
Zecca E, De Luca D, Baroni S, Vento G, Tiberi E, Romagnoli C (2008) Bile acid-induced lung injury in newborn infants: a bronchoalveolar lavage fluid study. Pediatrics 121(1):e146-149
Acknowledgements
The authors acknowledge the Pharmaceutical Sciences Research Center of Shiraz University of Medical Sciences for providing technical facilities for this investigation.
Funding
This study was financially supported by the Vice-Chancellor of Research Affairs of Shiraz University of Medical Sciences, Shiraz, Iran (grants #23701/23031/23040/23028/16428), Shanxi Agricultural University (Youth Fund project of Applied Basic Research in Shanxi Province; K272104065), and Natural Science Foundation of Shanxi Province (grant no. 201901D111232). Ali Mobasheri is supported by the Academy of Finland Profi6 336449 grant awarded to the University of Oulu, the European Commission, and the European Structural and Social Funds (ES Struktūrinės Paramos) awarded through the Research Council of Lithuania (Lietuvos Mokslo Taryba) and the funding program: Attracting Foreign Researchers for Research Implementation (2018–2022), grant no 01.2.2-LMT-K-718–02-0022.
Author information
Authors and Affiliations
Contributions
M.M. Ommati, R. Heidari, H. Niknahad, RK Manthari, N. Azarpira, and A. Mobasheri were involved in subject conceptualization, funding acquisition, methodology, data analysis, validation, project administration, resources, and supervision, writing the original draft, and review and editing the manuscript. Y. Ma, D. Xu, Zh. Tang, Y. Lu, RK. Manthari, N. Abdoli, I. Sadeghian, A. Mousavifaraz, H. Xin, and Y. Mingyu were involved in data visualization, literature review, data analysis, and writing the original manuscript draft. I. Sadeghian, A. Mousavifaraz, A. Nadgaran, A. Nikoozadeh, S. Mazloomi, P. Mehrabani, M. Rezaei, N. Azarpira, and R. Heidari were involved in data collection. All authors read and approved the final version of the manuscript. The authors declare that all data were generated in-house, and no paper mill was used.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All procedures using experimental animals were approved by the institutional ethics committee at Shiraz University of Medical Sciences, Shiraz, Iran (IR.SUMS.REC.1399.1353). This study does not include any human participants.
Consent to participate
Not applicable. This study contains no human data.
Consent for publication
Not applicable. This study contains no human data.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ommati, M.M., Mobasheri, A., Ma, Y. et al. Taurine mitigates the development of pulmonary inflammation, oxidative stress, and histopathological alterations in a rat model of bile duct ligation. Naunyn-Schmiedeberg's Arch Pharmacol 395, 1557–1572 (2022). https://doi.org/10.1007/s00210-022-02291-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-022-02291-7