Abstract
Renovascular hypertension is one of the most relevant causes of secondary hypertension, mostly caused by atherosclerotic renovascular stenosis or fibromuscular dysplasia. The increase in angiotensin II production, oxidative stress, and formation of peroxynitrite promotes the decrease in nitric oxide (NO) availability and the development of hypertension, renal and endothelial dysfunction, and cardiac and vascular remodeling. The NO produced by nitric oxide synthases (NOS) acts as a vasodilator; however, endothelial NOS uncoupling (eNOS) also contributes to NO reduced availability in renovascular hypertension. NO donors and NO-derived metabolites have been investigated in experimental renovascular hypertension and have shown promissory effects in attenuating blood pressure and organ damage in this condition. Therefore, understanding the role of decreased NO in the pathophysiology of renovascular hypertension promotes the study and development of NO donors and molecules that can be converted into NO (such as nitrate and nitrite), contributing for the treatment of this condition in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypertension is a chronic condition that damage target organs such as heart, kidneys, vessels, and central nervous system. Primary hypertension is when the cause of high blood pressure is unknown, and when it is possible to determine the etiology, the condition is named secondary hypertension (Charles et al. 2017). Renovascular hypertension is a common cause of secondary hypertension, developed through the reduction of blood flow perfusion to the kidney by renal artery occlusion or stenosis (Herrmann and Textor 2019). This response results in activating the renin–angiotensin–aldosterone-system, with consequently oxidative stress, vascular dysfunction, and thereby increasing blood pressure (Campos et al. 2011; Rossi et al. 2020).
Nitric oxide (NO) is a molecule with important actions on the kidneys, such as natriuresis and diuresis, and a deficiency on its synthesis is related to the development of hypertension (Mount and Power 2006). The activation of the renin-angiotensin system (RAS), which causes the reduction of NO production, leads to an increase in blood pressure (Patzak and Persson 2007). Therefore, understanding the role of decreased NO in the pathophysiology of renovascular hypertension contributes to the development and study of NO donors as possible treatments for renovascular hypertension.
In this review, we discuss the physiopathology of renovascular hypertension and the role of NO in the development and treatment of this dysfunction. A MEDLINE-based search was performed using the following keywords: “hypertension,” “renovascular hypertension,” “kidney,” “nitric oxide,” and “two-kidney, one-clip.” The list of articles was subsequently chosen by those containing abstracts and published in English language. Information analysis started with the title, followed by the abstract and then the complete report.
The role of the kidney in blood pressure control
The role of the kidney in blood pressure control initiated with Richard Bright in the nineteenth century, which suggested that high blood pressure and increased cardiac mass were associated with alterations in the blood and urine caused by the kidney (Bright 1836). Later in the beginning of the twentieth century, Harry Goldblatt was able to induce hypertension in dogs by the stenosis of one of the renal arteries (Goldblatt et al. 1934). Posteriorly, Arthur Guyton suggested that the kidney regulated the blood pressure through a balance of sodium intake and its urinary excretion (Guyton et al. 1972). Nowadays, the concept that the kidneys have the capacity to control the circulation volume through the regulation of sodium and water balance promoting extracellular volume homeostasis is widely accepted (Van Beusecum and Inscho 2015). When there is an increase in sodium and water amounts, the extracellular volume increases, promoting an increase in the cardiac output and blood pressure (Wadei and Textor 2012; Van Beusecum and Inscho 2015). To control these alterations, the nephron increases sodium and water excretion to decrease the extracellular volume and the blood pressure, a process known as pressure natriuresis (Van Beusecum and Inscho 2015). However, a reduction in blood pressure promotes an intense water and sodium absorption by the nephron, increasing the extracellular volume and blood pressure (Coffman 2014; Van Beusecum and Inscho 2015).
Likewise, RAS is another important regulator of sodium balance, body fluid volume, and blood pressure. RAS activation started with increased renin release, which is produced and stored in juxtaglomerular cells and is responsible to convert angiotensinogen to angiotensin I. Thereafter, angiotensin-converting enzyme converts angiotensin I in angiotensin II, which activates angiotensin II type 1 (AT1) receptor causing vasoconstriction, aldosterone synthesis and release, water and sodium retention, pro-inflammatory effects, and tissue growth and remodeling (Danser et al. 1994; Herrmann and Textor 2019). Sodium retention occurs by increasing the expression of the epithelial sodium channel (ENaC) (Mirabito Colafella et al. 2019).
Renovascular hypertension
Epidemiology and causes
Renovascular hypertension is one of the main causes of secondary hypertension, accounting for 1 to 5% of all the cases for hypertension, and being more prevalent in adults over 65 years old (Herrmann and Textor 2019). The major cause of renovascular hypertension is stenosis of the renal artery by atherosclerosis, corresponding to 90% of the cases, followed by fibromuscular dysplasia (9% of the cases) and other diseases, such as renal artery aneurysm and unilateral renal artery disease (1% of the cases) (Samadian et al. 2017; Herrmann and Textor 2019). The individuals who present renovascular hypertension are frequently resistant to medicine treatment, which can contribute to develop other complications, such as left ventricle hypertrophy and reduction in glomerular filtration rate (Textor 2014).
The atherosclerotic renovascular stenosis is a disease with a death rate of 16% per year and mainly developed in men above 50 years old that present previous systemic atherosclerosis and coronary or peripheral vascular alterations (Investigators et al. 2009; Samadian et al. 2017). In this condition, there is a formation of atheroma plaques that can obstruct partially or entirely the origin of the renal artery, as well as the renal vases (Herrmann and Textor 2019). In addition, the condition is characterized by progressive worsening of the stenosis, which may lead to the development of ischemic nephropathy (Bavishi et al. 2016). In general terms, the stenosis of the renal artery caused by atherosclerosis leads to a decrease in the renal perfusion, activating the renin-angiotensin aldosterone system (RAAS), which in turn activates the sympathetic nervous system; increases the production of prostaglandins, nitric oxide, and aldosterone; and decreases the excretion of sodium and water, promoting vasoconstriction (Boutari et al. 2019). The maintained restriction of renal perfusion results in disturbances of the microvascular functionalities and interstitial fibrosis, besides contributing to elevate the blood pressure (Boutari et al. 2019). Symptomatic patients mainly present a progressive decline of the renal function as well as recurrent flash pulmonary edema (Herrmann and Textor 2019).
Fibromuscular dysplasia occurs mainly in children and women between 30 and 50 years old (Samadian et al. 2017; Rossi et al. 2020). This condition is idiopathic and compromises the proximal, medium, or distal segments of the kidney arteries (Samadian et al. 2017). Most times the lesion is limited to two-thirds of the renal artery, being multifocal with the appearance of “string-of-beads,” or focal, with the characteristic of tubular stenosis (Samadian et al. 2017; Herrmann and Textor 2019). The disease is characterized by a non-atherosclerotic and non-inflammatory angiopathy, affecting mainly the arteries in the kidneys (Ralapanawa et al. 2016). The reduction of the arterial renal perfusion due to the unifocally or multifocally stenosed arteries activates the RAAS, promoting volume increase and hypertension (Gottsäter and Lindblad 2014).
Renovascular hypertension pathophysiology
The main cause of renovascular hypertension is the decrease in blood flow perfusion to the kidneys, in which the hemodynamic effects are only detected when the occlusion of the vascular lumen is between 75 and 85% (Herrmann and Textor 2019). Studies show that partial or total vascular occlusion in the kidneys leads to a raise in the systemic blood pressure, mainly through the activation of RAS (Textor 2009; Tafur-Soto and White 2015).
The decrease in kidney perfusion increases renin release by the juxtaglomerular cells, converting its substrate angiotensinogen to angiotensin I, which is converted to angiotensin II by the angiotensin converting enzyme. Angiotensin II promotes vasoconstriction and leads to the release of adrenal cortex aldosterone and retention of water and sodium, increasing the cardiac output and blood pressure (Herrmann and Textor 2019).
The animal two-kidney one clip (2K1C) was the first created with the strategy of decreasing renal perfusion through a clip in one of the renal arteries, while the other kidney remains intact (Goldblatt et al. 1934). This clamping leads to the activation of the RAS and the development of renovascular hypertension (Goldblatt et al. 1934). The clip in one of the renal arteries increases plasma renin activity in the perfusion pressure of the other kidney, which promotes sodium excretion to decrease the blood pressure (Garovic and Textor 2005). Hence, blood pressure drops, reducing the pressure in the perfusion of the kidney with stenosis and further increasing renin release, angiotensin II levels, aldosterone secretion, and distal sodium reabsorption, leading to renal vasoconstriction and increased sodium reabsorption in proximal and distal tubules (Garovic and Textor 2005). The increased levels of angiotensin II also contribute to increase the synthesis of collagen type I and III in fibroblasts, promoting the thickening of the vascular walls, the myocardium, and contributing to develop fibrosis (Nair and Vaqar 2021).
Moreover, angiotensin II increases the sympathetic nervous system activity (increases peripheral resistance and cardiac output), activates inflammatory and fibrotic pathways and oxidative stress, and reduces glomerular filtration rate, compromising the renal function (Investigators et al. 2009).
The NAD(P)H oxidase is an enzymatic complex formed by catalytic and regulators subunits which works in the reactive oxygen species (ROS) generation, being activated by angiotensin II. This group of enzymes is named as “Nox family” and each one is characterized by its respectively catalytic and regulators subunits. There are seven members of this family: Nox1, Nox2 (gp91phox), Nox3, Nox4, Nox5, and Duox1 and Duox2 (Rodino-Janeiro et al. 2013). These enzymes use NADPH or NADH as electron donor to promote the O2− production based on the following reaction: 2O2 + NADPH → 2O2•− + NADP+ + H+ (Konior et al. 2014). The catalytic subunits are activated by different regulators mechanisms which involves the calcium action or cytosolic proteins as p47phox and NOXO-1 (Rodino-Janeiro et al. 2013). These enzymes are expressed in different tissues and develop a variety of biological functions. In the kidneys, Nox4 is the most important NAD(P)H oxidase expressed and is originally named as renox (renal oxidase) (Holterman et al. 2015). Besides Nox4, the kidney also expresses Nox1 and Nox2, which regulate physiological actions and play an important role in the renal dysfunction development (Munoz et al. 2020).
The 2K1C Goldblatt model of renovascular hypertension results in an Ang II-induced hypertension with increased oxidative stress by NAD(P)H oxidase activation. Chronic administration of superoxide dismutase mimetic tempol decreases ROS and blood pressure in renovascular hypertension and increases the renal function in 2K1C hypertension more effectively than the AT1 antagonist (Campos et al. 2011). These results suggest that oxidative stress has a role in the development of renal dysfunction in renovascular hypertension. In a rat model of renal unilateral arterial stenosis, there was a progressive increase in ROS production and in the blood pressure, even when the angiotensin II levels decreased, suggesting a systemic action from the oxidative stress in maintaining renovascular hypertension and renal damage caused by hypertension (Lerman et al. 2001).
Besides that, the increase in the oxidative stress is also related to the development of inflammation, which in turn contributes to endothelial dysfunction and hypertension (Dinh et al. 2014). The increased levels of ROS can promote inflammatory processes through activation of transcription factors such as NF-κB (Dinh et al. 2014). In addition, experiments using the model of 2K1C hypertension demonstrated that the angiotensin activation and the decrease in NO production could lead to an increased production of TNF-α and IL-6, promoting tubular degeneration and interstitial mononuclear cell infiltrations (Kalaivani et al. 2013).
Nitric oxide (NO)
NO is a gaseous molecule that is found free in low quantities in the atmosphere, and since it contains an unpaired electron, it tends to be very reactive (Ahmad et al. 2018). NO is synthetized from the amino acid L-arginine, which is produced in low quantities, and is neo-synthesized from citrulline in the renal proximal tubules (Ahmad et al. 2018). In the presence of NAD(P)H and calcium, L-arginine is converted to N-hydroxy-L-arginine, which is transformed into NO and L-citrulline in the presence of oxygen and NAD(P)H (Ahmad et al. 2018). NO synthesis occurs by the enzyme nitric oxide synthase (NOS), which has three isoforms: endothelial (eNOS), neuronal (nNOS) and inducible (iNOS) (Ahmad et al. 2018).
The nNOS is more expressed in the macula densa, but is also located in specialized neurons, as the non-adrenergic, non-cholinergic nerves; within the renal arteries of the hilus, arcuate and interlobular arteries; occasionally in the pre-glomerular afferent arterioles; and also in the outer medullary collecting duct, the cortical collecting duct and the inner medullary thin limb (Mount and Power 2006). The iNOS is induced by renal damage, as during renovascular hypertension or ischemia–reperfusion (Mount and Power 2006). The eNOS is more expressed in renal vascular endothelium of arteries and arterioles, glomerular capillaries, and medullary descending vasa recta. In contrast, eNOS expression is not seen in cortical capillaries or venous endothelium (Bachmann and Mundel 1994). Tubular expression of eNOS has been detected in the inner medullary collecting duct, the thick ascending limb of the loop of Henle, and the proximal convoluted tubule (Mount and Power 2006). RAS activation promotes an equivalent expression of eNOS in cortex and medulla (Higashi et al. 2002).
NO functions in the kidneys
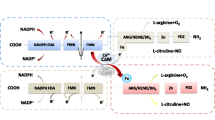
NO has several functions throughout the human body and is known as “endothelial-derived relaxing factor” by the fact that it induces the relaxation of the smooth muscle (Bauer and Sotnikova 2010). In the kidneys, NO controls natriuresis and diuresis, regulates renal hemodynamics, maintains medullar perfusion, controls tubuloglomerular feedback, inhibits sodium reabsorption by renal tubules, and increases sodium excretion and urinary flow, which demonstrates its crucial renal hemodynamic role and action in the sympathetic neural activity present in this organ (Bachmann and Mundel 1994; Mount and Power 2006). Thereby, NO contributes to the maintenance of a low vascular resistance and being responsible for about one third of the renal blood flow (Majid and Navar 2001) (Fig. 1).
NO activates the soluble guanylyl cyclase (sGC), which converts guanosine triphosphate into cyclic guanosine monophosphate (cGMP), leading to vasodilation (Ahmad et al. 2018). Moreover, cGMP may activate protein kinase G (PKG), which phosphorylates different proteins involved in vasodilation, neutrophils activation, modulation of smooth muscle cells tone, and matrix expansion (Ahmad et al. 2018). In the kidneys, eNOS is the main NO producer, which through NO/sGC/cGMP pathway produces vasodilation (Mount and Power 2006).
Renal autoregulation is the kidneys’ capacity to maintain constant blood pressure and the glomerular filtration even with alterations in the systemic blood pressure (Just 1997). The tubuloglomerular feedback is an essential process in this autoregulation, because the increase in glomerular filtration generates an increase in sodium in the loop of Henle detected by the macula densa, which promotes signaling for the vasoconstriction of the afferent arteriole, decreasing the glomerular filtration (Mount and Power 2006). Furthermore, NO derived from nNOS participates in tubuloglomerular feedback regulation through sGC stimuli, producing cGMP and activating the kinase cGMP-dependent protein within the cells of macula densa (Majid and Navar 2001).
The renal medullary flow is related to blood pressure regulation and sodium balance, and NO directly acts in both processes (Cowley et al. 2003; Mount and Power 2006). A decrease in NO production or its inhibition is associated with development of hypertension, since there is a significant decrease on its effects over the renal sodium, fluid excretion, and renal vascular resistance (Martinez et al. 2002). In the renal tubules, NO has the capacity to inhibit sodium reabsorption, which contributes to its natriuretic and diuretic effects (Mount and Power 2006). The use of L-arginine analogue L-NAME decreased 40% renal medullary flow, indicating that NO directly influences the renal vases in the renal medulla (Cowley et al. 2003). Besides, NO in the renal medulla opposes the noradrenaline, vasopressin, and angiotensin II vasoconstriction effects, maintaining the medullary flow normal and preventing the development of hypertension (Mount and Power 2006).
NO role in renovascular hypertension
Alterations in NO bioavailability are observed in renovascular hypertension. Figure 2 shows a schematic representation of physiopathology of renovascular hypertension, with RAS activation, renal inflammation, oxidative stress, and reduction of glomerular filtration rate. All of them promote a decrease of NO and an increase of ONOO− bioavailability, and endothelium dysfunction. Endothelial disfunction leads to a decrease in NO production in 2K1C hypertensive rats, avoiding NO vasodilatory protective action on exaggerated angiotensin II production and increasing even more the blood pressure (Sanchez-Mendoza et al. 1998). Moreover, the NOS inhibitor N omega-nitro-L-arginine increased vasoconstriction and blood pressure in 2K1C model (Nakamoto et al. 1995). Furthermore, genetic therapy with a recombinant adenovirus expressing eNOS prevented the development of hypertension in the animals due to NO vasodilation that compensated angiotensin II vasoconstrictor effects in 2K1C hypertension (Gava et al. 2008).
Schematic representation of physiopathology of renovascular hypertension. Renal stenosis is commonly caused by the presence of renovascular atheroma plaque or fibromuscular dysplasia. Both are able to activate renin angiotensin system which increases angiotensin II-induced activation of AT1 receptor. This response plays an important role on increase of renal inflammation and oxidative stress and also reduction of glomerular filtration rate. All of them promote decrease of NO and increase of ONOO− bioavailability, and also endothelium dysfunction, leading to renovascular hypertension. AT1, Angiotensin II type 1 receptor; NO, nitric oxide; peroxynitrite, ONOO−
Besides hypertension development, NO metabolites present at the renal tissue and eNOS expression were considerably reduced in the clamped kidney of 2K1C hypertension, and when the clip was removed, the blood pressure returned to normal as well as NO metabolites and eNOS expression (Park et al. 2000). The nonclipped kidney did not have NO metabolites or eNOS expression alterations, both during the use of the clip in the lateral kidney and after the clip removal (Park et al. 2000). Chronic inhibition of iNOS present in the macula densa increased blood pressure and renal vascular resistance, besides promoting a sensitization in the juxtaglomerular feedback (Ollerstam et al. 1997). Oxidative stress and AT1 receptor activation are stimulus for an increase in nNOS expression in the macula densa, even though nNOS presents reduced enzymatic activity in 2K1C rats (Pereira et al. 2009).
Renovascular hypertension leads to NAD(P)H oxidase activation in the entire renal tissue and microvasculature, producing ROS, especially superoxide anions (Higashi et al. 2002; Touyz 2004). There is an imbalance between NO production, which is reduced, and ROS (mainly superoxide), which is increased, leading to the development of endothelial dysfunction in renovascular hypertension (Higashi et al. 2002). NO production is reduced in the presence of increased oxidative stress, which decreases endothelium vasodilation once the balance between the normal levels of superoxide and NO are essential to maintain a normal endothelial function (Higashi et al. 2002).
Renal ischemia and reperfusion (I/R) may cause acute renal failure, which can be caused by renal artery stenosis (Guven et al. 2008). The development of this condition is associated to NO production by iNOS, which is induced in the kidneys through cytokines, lipopolysaccharides, and during the I/R development, leading to renal damage (Chatterjee et al. 2002; Aktan 2004). The produced NO by the iNOS interacts with superoxide radicals producing peroxynitrite, which is a toxic molecule capable of nitrating tyrosine residues from proteins and enzymes, promoting damage such as induction of apoptosis, lipidic peroxidation, necrosis, and DNA damage (Guven et al. 2008). Ischemic kidneys present increased nitrotyrosine levels, and the reduction of its levels improves the renal function (Goligorsky et al. 2002). Moreover, the peroxynitrite scavenger ebselen improved renal disfunction of ischemic kidneys, suggesting that peroxynitrite is one of the main responsible compounds to cause damage in the I/R development (Noiri et al. 2001). Treatment with iNOS inhibitor decreased renal dysfunction and renal tissue damage caused by I/R through reduction of peroxynitrite generation in rats (Chatterjee et al. 2002).
Moreover, excessive superoxide anion production through angiotensin II increase is not counterbalanced by the antioxidant endogenous system, contributing to maintain a high blood pressure in renovascular hypertension (Oliveira-Sales et al. 2008). The antioxidant system is composed mainly by three enzymes: superoxide dismutase, glutathione peroxidase, and catalase, in which the first two enzymes are more responsible for removing ROS (Guven et al. 2008). When an episode of oxidative stress occurs, the excessive production of ROS surpasses the antioxidant system, as it happens during the development of I/R. In this context, in a study using a NOS inhibitor (L-Nil) or a permeable-cell superoxide dismutase in rats with a clamped renal artery and renal ischemia, there was an improvement in the renal function through inhibition of iNOS, with inhibition of peroxynitrite production, therefore inhibiting oxidative stress (Noiri et al. 2001).
Another mechanism that may be present in the worsening of renovascular hypertension is the eNOS uncoupling, which may induce the oxidative stress and endothelial dysfunction through superoxide anion production (Cai and Harrison 2000). This enzyme depends on tetrahydrobiopterin (BH4), which must be connected near its heme group to transfer electrons to L-arginine and produce NO and citrulline (Cai and Harrison 2000). As a result, in the absence of BH4 or L-arginine due to a possible previous oxidation, the electrons are not properly transported and molecular oxygen is reduced to superoxide anion instead of NO, aggravating the damage (Wassmann et al., 2004). Moreover, a possible explanation to cause BH4 oxidation and eNOS uncoupling would be the uncontrolled production of peroxynitrite, which is mainly produced by iNOS activation, though in the eNOS partial uncoupling this enzyme may produce NO and superoxide simultaneously (Cai and Harrison 2000).
Furthermore, humans with renovascular hypertension submitted to angioplasty presented an improvement in vasodilation endothelium-dependent through a decrease in angiotensin II production, decreasing oxidative stress, and an increase in NO production, improving endothelial dysfunction (Higashi et al. 2002). In addition, a decrease in endothelial dysfunction and morphologic alterations and an increased in the glomerular filtration rate and restored the enzymatic activities of the renal antioxidant system in renal I/R in rats by three different compounds (Origanum majorana (OM) methanolic extract, carvacrol, and vitamin E) in the treatment of all the compounds promoted (Gheitasi et al. 2020).
Causes and effects of low renal NO
The NO production can influence many physiologic processes, interacting with the control of short- and long-term renal functions (Lee 2008). In this context, the decrease of NO production or bioactivity in the kidneys may promote the development of several complications, such as acute and chronic kidney injury (Lee 2008).
In the kidneys, NO deficiency may occur through four circumstances: L-arginine deficiency, NOS abundance and activity decrease, increased oxidative stress that inactivates NOS, and increased ADMA (asymmetric dimethylarginine), which is a NOS inhibitor (Hsu and Tain 2019). In humans, L-arginine deficiency is related to the development of kidney disease and hypertension, while a decrease in activity and abundance of nNOS in chronic kidney disease animal models can contribute to decrease NO production and promote hypertension (Hsu and Tain 2019). In addition, a decrease in NO production by eNOS is related to exacerbate the damaging effects of diabetic nephropathy, which is a chronic kidney disease initiated as an excessive increase in glomerular filtration followed by a decline in general renal function, culminating in kidney failure (Hsu and Tain 2019; Hammoud et al. 2021). Considering oxidative stress, it can promote a reduction in NO bioavailability by three mechanisms: causing NOS uncoupling by oxidizing BH4, increasing ADMA, and forming peroxynitrite by the combination of NO and superoxide anion (Hsu and Tain 2019). These reactive species when in elevated levels can decrease NO bioavailability and increase IL-1 in the circulation, promoting vascular smooth muscle cells to become dysfunctional and migrate towards the region intima of the vases, causing hyperplasia and deposition of excessive extracellular matrix and hyaline material, stiffening of arteries by calcification, and high pulse pressure (Ravarotto et al. 2018).
Due to these facts, several functions in the kidneys are impaired when in deficiency of NO. Decreasing its production promotes a deregulation of the glomerular filtration, unbalances the pressure-natriuresis, changes medullary perfusion, decreases tubuloglomerular feedback, promotes tubular sodium reabsorption, and decreases the modulation of the sympathetic nerve activity (Lee 2008). These kidney disturbances may result in serious consequences, such as glomerulosclerosis, interstitial fibrosis, microvascular damage, and proteinuria, which are present in end-stage renal disease and can culminate in kidney failure (Girardi et al. 2011; Baylis 2008).
Treatment of renovascular hypertension
Renovascular hypertension is the most common type of secondary hypertension and has become a challenge in therapy due to its treatment resistance (Textor 2020). The advance in technology and medical treatment through the years has improved the management of this condition, contributing to optimize the drug treatment (Textor 2020). Before starting the medical therapy, it is important to evaluate the severity of the disease, which can be diagnosed by three groups of tests: functional and physiologic tests (evaluation of the stenosis considering the renin-angiotensin system); radiological diagnostic tests (evaluation of the stenosis severity and the blood supply); and diagnostic tests that evaluate the possible interventions of angiography (Samadian et al. 2017).
After testing and diagnosing, the management of renovascular hypertension depends on controlling the blood pressure and promoting renal revascularization to preserve all kidneys functions (Textor 2020). Within this context, the first therapeutic option available to treat this condition is the medical therapy, in which smoking cessation, administration of statins, control of patients’ glycaemia, and anti-hypertensive treatment are the procedures adopted initially, especially in cases of atherosclerotic renovascular stenosis (Boutari et al. 2019). The anti-hypertensive treatment includes ACE inhibitors and angiotensin receptor blockers (ARBs), which can be combined with diuretics and calcium channel blockers to promote an improvement in blood pressure levels (Textor 2020). Nevertheless, it is important to notice that this drug treatment is contraindicated for patients with a single functioning kidney or bilateral injury since these drugs may cause afferent arteriolar vasodilation, interfering in autoregulation and decreasing glomerular filtration, which could compromise the renal function (Nair and Vaqar 2021).
In cases where patients are young and present fibromuscular dysplasia, several clinicians are in favor of endovascular or surgical revascularization to treat this condition (Textor 2020). Besides that, revascularization is indicated for patients with atherosclerotic renal stenosis that are resistant to the medical treatment as well as fibromuscular dysplasia patients (Nair and Vaqar 2021). The interventional treatment for renal revascularization includes a conventional percutaneous transluminal renal angioplasty (with or without stenting) that is preferred to a surgical renal vascularization once the surgeries have been related to high rates of morbidity and mortality (Samadian et al. 2017; Boutari et al. 2019). Therefore, it is important to search for the best treatment for renovascular hypertension according to each patient, taking into consideration achieving satisfactory blood pressure levels and preserving/improving the renal functions.
NO donors in renovascular hypertension
As NO availability is impaired in renovascular hypertension, drugs that increase NO as NO donors have been investigated to find alternatives to the treatment of this condition. NO donors are molecules that can develop NO-related activity when used in biological pathways, acting as a substitute for a NO deficiency or with the capacity to mimic an endogenous NO response (Lunardi et al. 2009). Sodium nitroprusside (SNP) is a compound that can act as a NO donor or can decrease superoxide anion release, promoting effects in the arteries and veins (Buzinari et al. 2017). SNP is a vasodilator that acts rapidly and is used clinically in hypertensive emergencies, being used during surgery due to its controlled hypotensive effect (Araujo et al. 2019). The incubation of the aorta from 2K1C rats with SNP at low doses decreased superoxide anion concentration and peroxynitrite formation and increased NO availability in this model of renovascular hypertension (Buzinari et al. 2017). However, SNP presented some undesired effects in vivo, such as reflex tachycardia and toxicity (Araujo et al. 2019).
Another NO donor used in the clinic is nitroglycerin, which is a molecule that can be used in the treatment of many cardiovascular diseases, such as hypertension and angina pectoris (Paulo et al. 2013). This molecule is a potent venodilator, which can be used in three different forms: sublingual, topical, or IV infusion (Prasanna et al. 2018). In a study with 37 patients with a hypertension emergency and evidence of end organ dysfunction, a sublingual nitroglycerin spray was administered once to the patients, and after around 10 min, the patients presented a significant reduction in blood pressure (Prasanna et al. 2018).
Therefore, several other compounds have been developed to increase NO concentration. The compound called cis-[Ru(bpy)2(py)(NO2)](PF6) (RuBPY) is a non-classic NO donor that releases NO in a controlled manner, inducing a hypotensive effect and promoting vasodilation in several blood vessels. A comparison between SNP and RuBPY using mesenteric resistance arteries from 2K1C protocol showed that both compounds can induce vascular relaxation in hypertension (Araujo et al. 2019). In another study using 2K1C and normotensive rats treated with RuBPY or SNP, RuBPY was shown to be more advantageous, because it does not induce a hypotensive effect in normotensive rats, while hypotensive effect in hypertensive rats was more long lasting, without causing reflex tachycardia (Pereira et al. 2017).
In addition, a ruthenium complex called trans-[RuCl([15]aneN4)NO]2+ promoted a reduction in the mean arterial pressure in moderate hypertensive 2K1C rats, when the dose was 10 mmol/L/Kg, and in the severe hypertensive animals when the dose was 0.1 mmol/L/kg (de Gaitani et al. 2009). A second ruthenium complex called cis-[Ru(H-dcbpy-)2(Cl)(NO)] (DCBPY) inactivated superoxide anions and improved endothelial function in the aorta of 2K1C rats (Oishi et al. 2015). A third ruthenium complex called [Ru(terpy)(bdq)NO +]3 + (Terpy) was compared with SNP in 2K1C rats. The results demonstrated that the hypotensive effect obtained from Terpy was longer than the obtained from SNP, even though SNP was more potent in inducing the decrease in the arterial pressure than Terpy (Rodrigues et al. 2012).
Furthermore, the incubation of the aorta from 2K1C rats with 100 μmol/L of NCX2121, a vasodilator compound that presents NO in its structure, led to a direct vasodilatation associated to NO release inside the smooth muscle and sGC activation (de Paula et al. 2017). However, NCX 2121-induced relaxation was associated with eNOS activation and inhibition of prostanoids production in aortas presenting endothelium (de Paula et al. 2017).
Considering all these possibilities, these known compounds by the literature and new molecules being created to donate NO can become important interventions in the treatment of many cardiovascular diseases, such as hypertension.
NO-derived metabolites in renovascular hypertension
Nitrite is a molecule known as a biochemical marker for NO production in general since it is mainly produced through NO oxidative breakdown (Ling et al. 2020). However, it has been proven that nitrite under pathological conditions can be recycled back to NO, which can promote a hypotensive effect and prevent endothelial dysfunction (Ling et al. 2020). In this context, under conditions where there is a physiological hypoxia, the inorganic anions nitrite and nitrate are reduced to form NO in tissues and blood, while NO production by NOS is decreased due to a fall in oxygen levels (the L-arginine-NOS pathway is oxygen dependent) (Lundberg et al. 2008). By the parallel of L-arginine-NOS and nitrite-nitrate pathways, the generation of NO is ensured in both physiological and pathological conditions, contributing to maintain NO functions (Lundberg et al. 2008).
Considering renovascular hypertension and kidney damage, there are many studies in the literature with the usage of nitrite to increase NO availability. 2K1C hypertensive rats treated orally with sodium nitrite presented a decrease in blood pressure attributed to the formation of gastric S-nitrosothiols, which are dependent on gastric pH (Pinheiro et al. 2015). When 2K1C rats were orally treated with both nitrite and nitrate while using an antiseptic mouthwash, even though the antiseptic mouthwash caused the disruption of the enterosalivary circulation of nitrate, oral nitrite promoted hypotensive effects (Pinheiro et al. 2016).
Moreover, in 2K1C rats treated with oral sodium nitrite as a therapy for both hypertensive and non-hypertensive groups, the vascular remodeling caused by hypertension was reverted by the use of nitrite, which can be related to the decrease of NADPH oxidase activity and formation of NO from nitrite through xanthine oxidase mediation (Rizzi et al. 2019). In an experiment with angiotensin II infused mouse model of hypertension, chronic administration of sodium nitrite for 2 weeks promoted a decrease in the systolic blood pressure as well as an improvement in the endothelial dysfunction presented by the resistance vessels due to restoring NO bioavailability, which also demonstrates that the increase in NO levels promotes relevant changes regarding blood pressure and endothelial injuries (Ling et al. 2018). In another study, 2K1C rats were treated with atorvastatin, sildenafil, or both drugs for 8 weeks. Atorvastatin but not sildenafil treatment increased the bioavailability of NO, even though both promoted an improvement in endothelial function and in antioxidant effects as well as a decrease in blood pressure (Guimaraes et al. 2013).
Moreover, the NO donor cyclohexane nitrate (HEX) promoted vasodilatation via NO/cGMP/PKG pathway and activation of the ATP-sensitive K+ channels in 2K1C rats treated orally (10 mg/Kg/day) for 7 days. As a consequence, the present NO donor induced a reduction of blood pressure and heart rate, leading to an antihypertensive effect in renovascular hypertensive rats (Mendes-Junior et al. 2015). Moreover, 2K1C rats treated orally with nitrite (15 mg/kg) presented a reduction in blood pressure and reduction of vascular dysfunction. These responses occurred in parallel to reduced concentration of ROS and increased expression of antioxidant enzymes in arterial mesenteric bed via the activation of nuclear factor erythroid-2 transcription factor (Amaral et al. 2019).
Conclusions
Renovascular hypertension is characterized by RAS activation, promoting vasoconstriction, oxidative stress, and increasing the blood pressure. NO is a vasodilator, but its bioavailability is reduced in injured kidney in this type of hypertension. Increased oxidative stress in renovascular hypertension directly contributes to endothelial damage and kidney dysfunction. The produced NO combines with superoxide anion, producing peroxynitrite that is very reactive, and promotes nitrotyrosine formation. The reduction of NO bioavailability and peroxynitrite formation have a fundamental role in renovascular hypertension physiopathology, which may result in renal failure. Recent experimental studies investigating the use NO donors and NO-derived metabolites have shown promissory effects in attenuating blood pressure and organ damage in this condition. These observations suggest that the development of NO donors for clinical use may be an interesting alternative for the treatment of renovascular hypertension.
Data availability
Not applicable
References
Ahmad A, Dempsey SK, Daneva Z, Azam M, Li N, Li PL, Ritter JK (2018) Role of nitric oxide in the cardiovascular and renal systems. Int J Mol Sci 19: 2605
Aktan F (2004) iNOS-mediated nitric oxide production and its regulation. Life Sci 75:639–653
Amaral JH, Rizzi ES, Alves-Lopes R, Pinheiro LC, Tostes RC, Tanus-Santos JE (2019) Antioxidant and antihypertensive responses to oral nitrite involves activation of the Nrf2 pathway. Free Radical Biol Med 141:261–268
Araujo AV, Andrade FA, Paulo M, de Paula TD, Potje SR, Pereira AC, Bendhack LM (2019) NO donors induce vascular relaxation by different cellular mechanisms in hypertensive and normotensive rats. Nitric Oxide Biol Chem 86:12–20
Bachmann S, Mundel P (1994) Nitric oxide in the kidney: synthesis, localization, and function. Am J Kidney Dis 24:112–129
Bauer V, Sotnikova R (2010) Nitric oxide-the endothelium-derived relaxing factor and its role in endothelial functions. Gen Physiol Biophys 29:319–340
Bright R (1836) R. Observations on the treatment of fever. Case of simple fever, protracted by irritation of the bowels, and attended by relapse. Guys Hosp Rep 1:1–8
Buzinari TC, Oishi JC, De Moraes TF, Vatanabe IP, Selistre-de-Araujo HS, Pestana CR, Rodrigues GJ (2017) Treatment with sodium nitroprusside improves the endothelial function in aortic rings with endothelial dysfunction. Eur J Pharm Sci 105:144–149
Bavishi C, De Leeuw PW, Messerli FH (2016) Atherosclerotic renal artery stenosis and hypertension: pragmatism, pitfalls, and perspectives. Am J Med 129:635.e5-635.e14. https://doi.org/10.1016/j.amjmed.2015.10.010
Baylis C (2008) Nitric oxide deficiency in chronic kidney disease. Am J Physiol - Ren Physiol 294:F1–F9. https://doi.org/10.1152/ajprenal.00424.2007
Boutari C, Georgianou E, Sachinidis A et al (2019) Renovascular hypertension: novel insights. Curr Hypertens Rev 16:24–29. https://doi.org/10.2174/1573402115666190416153321
Cai H, Harrison DG (2000) Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87:840–844
Campos RR, Oliveira-Sales EB, Nishi EE, Boim MA, Dolnikoff MS, Bergamaschi CT (2011) The role of oxidative stress in renovascular hypertension. Clin Exp Pharmacol Physiol 38:144–152
Charles L, Triscott J, Dobbs B (2017) Secondary hypertension: discovering the underlying cause. Am Fam Physician 96:453–461
Chatterjee PK, Patel NS, Kvale EO, Cuzzocrea S, Brown PA, Stewart KN, Mota-Filipe H, Thiemermann C (2002) Inhibition of inducible nitric oxide synthase reduces renal ischemia/reperfusion injury. Kidney Int 61:862–871
Coffman TM (2014) The inextricable role of the kidney in hypertension. J Clin Investig 124:2341–2347
Cowley AW Jr, Mori T, Mattson D, Zou AP (2003) Role of renal NO production in the regulation of medullary blood flow. Am J Physiol Regul Integr Comp Physiol 284:R1355-1369
Danser AH, van Kats JP, Admiraal PJ, Derkx FH, Lamers JM, Verdouw PD, Saxena PR, Schalekamp MA (1994) Cardiac renin and angiotensins. Uptake from plasma versus in situ synthesis. Hypertension 24:37–48
de Gaitani CM, de Melo MC, Lunardi CN, de S Oliveira F, da Silva RS, Bendhack LM (2009) Hypotensive effect of the nitrosyl ruthenium complex nitric oxide donor in renal hypertensive rats. Nitric Oxide Biol Chem 20:195–199
de Paula TD, Silva BR, Grando MD, Pernomiando Prado LAF, Bendhack LM (2017) Relaxation induced by the nitric oxide donor and cyclooxygenase inhibitor NCX2121 in renal hypertensive rat aortas. Eur J Pharm Sci: Official Journal of the European Federation for Pharmaceutical Sciences 107:45–53
Dinh QN, Drummond GR, Sobey CG, Chrissobolis S (2014) Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed Res Int 2014:1–11. https://doi.org/10.1155/2014/406960
Garovic VD, Textor SC (2005) Renovascular hypertension and ischemic nephropathy. Circulation 112:1362–1374
Gava AL, Peotta VA, Cabral AM, Vasquez EC, Meyrelles SS (2008) Overexpression of eNOS prevents the development of renovascular hypertension in mice. Can J Physiol Pharmacol 86:458–464
Gheitasi I, Azizi A, Omidifar N, Doustimotlagh AH (2020) Renoprotective effects of origanum majorana methanolic L and carvacrol on ischemia/reperfusion-induced kidney injury in male rats. Evidence-Based Complement Altern Med 2020:1–9
Girardi JM, Farias RE, Ferreira AP, Raposo NRB (2011) Rosuvastatin prevents proteinuria and renal inflammation in nitric oxide-deficient rats. Clinics 66:1457–1462. https://doi.org/10.1590/S1807-59322011000800025
Goldblatt H, Lynch J, Hanzal RF, Summerville WW (1934) Studies on experimental hypertension: I. The production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med 59:347–379
Goligorsky MS, Brodsky SV, Noiri E (2002) Nitric oxide in acute renal failure: NOS versus NOS. Kidney Int 61:855–861
Gottsäter A, Lindblad B (2014) Optimal management of renal artery fibromuscular dysplasia. Ther Clin Risk Manag 10:583–595. https://doi.org/10.2147/TCRM.S48746
Guimaraes DA, Rizzi E, Ceron CS, Pinheiro LC, Gerlach RF, Tanus-Santos JE (2013) Atorvastatin and sildenafil lower blood pressure and improve endothelial dysfunction, but only atorvastatin increases vascular stores of nitric oxide in hypertension. Redox Biol 1:578–585
Guven A, Uysal B, Akgul O, Cermik H, Gundogdu G, Surer I, Ozturk H, Korkmaz A (2008) Scavenging of peroxynitrite reduces renal ischemia/reperfusion injury. Ren Fail 30:747–754
Guyton AC, Coleman TG, Cowley AV Jr, Scheel KW, Manning RD Jr, Norman RA Jr (1972) Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. Am J Med 52:584–594
Hammoud SH, AlZaim I, Mougharbil N et al (2021) Peri-renal adipose inflammation contributes to renal dysfunction in a non-obese prediabetic rat model: Role of anti-diabetic drugs. Biochem Pharmacol 186:114491. https://doi.org/10.1016/j.bcp.2021.114491
Herrmann SM, Textor SC (2019) Renovascular hypertension. Endocrinol Metab Clin North Am 48:765–778
Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Oshima T, Chayama K (2002) Endothelial function and oxidative stress in renovascular hypertension. N Engl J Med 346:1954–1962
Holterman CE, Read NC, Kennedy CR (2015) Nox and renal disease. Clin Sci 128:465–481
Hsu CN, Tain YL (2019) Regulation of nitric oxide production in the developmental programming of hypertension and kidney disease. Int J Mol Sci 20:681. https://doi.org/10.3390/ijms20030681
Investigators A, Wheatley K, Ives N, Gray R, Kalra PA, Moss JG, Baigent C, Carr S, Chalmers N, Eadington D, Hamilton G, Lipkin G, Nicholson A, Scoble J (2009) Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 361:1953–1962
Just A (1997) Nitric oxide and renal autoregulation. Kidney Blood Press Res 20:201–204
Kalaivani P, Saranya RB, Ramakrishnan G et al (2013) Cuminum cyminum, a dietary spice, attenuates hypertension via endothelial nitric oxide synthase and no pathway in renovascular hypertensive rats. Clin Exp Hypertens 35:534–542. https://doi.org/10.3109/10641963.2013.764887
Konior A, Schramm A, Czesnikiewicz-Guzik M, Guzik TJ (2014) NADPH oxidases in vascular pathology. Antioxid Redox Signal 20:2794–2814
Lee J (2008) Nitric oxide in the kidney: its physiological role and pathophysiological implications. Electrolyte Blood Press 6:27–34
Lerman LO, Nath KA, Rodriguez-Porcel M, Krier JD, Schwartz RS, Napoli C, Romero JC (2001) Increased oxidative stress in experimental renovascular hypertension. Hypertension 37:541–546
Ling WC, Mustafa MR, Murugan DD (2020) Therapeutic implications of nitrite in hypertension. J Cardiovasc Pharmacol 75:123–134
Ling WC, Mustafa MR, Vanhoutte PM, Murugan DD (2018) Chronic administration of sodium nitrite prevents hypertension and protects arterial endothelial function by reducing oxidative stress in angiotensin II-infused mice. Vascul Pharmacol 102:11–20
Lunardi CN, da Silva RS, Bendhack LM (2009) New nitric oxide donors based on ruthenium complexes. Braz J Med Biol Res 42:87–93
Lundberg JO, Weitzberg E, Gladwin MT (2008) The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov 7:156–167. https://doi.org/10.1038/nrd2466
Majid DS, Navar LG (2001) Nitric oxide in the control of renal hemodynamics and excretory function. Am J Hypertens 14:74S-82S
Martinez Y, Martinez S, Meaney A, Meaney E, Escalante B (2002) Angiotensin II type 1 receptor blockade restores nitric oxide-dependent renal vascular responses in renovascular hypertension. J Cardiovasc Pharmacol 40:381–387
Mendes-Junior L, Guimaraes DD, Gadelha DD, Diniz TF, Brandao MC, Athayde-Filho PF, Lemos VS, Franca-Silva Mdo S, Braga VA (2015) The new nitric oxide donor cyclohexane nitrate induces vasorelaxation, hypotension, and antihypertensive effects via NO/cGMP/PKG pathway. Front Physiol 6:243
Mirabito Colafella KM, Bovee DM, Danser AHJ (2019) The renin-angiotensin-aldosterone system and its therapeutic targets. Exp Eye Res 186:107680
Mount PF, Power DA (2006) Nitric oxide in the kidney: functions and regulation of synthesis. Acta Physiol 187:433–446
Munoz M, Lopez-Oliva ME, Rodriguez C, Martinez MP, Saenz-Medina J, Sanchez A, Climent B, Benedito S, Garcia-Sacristan A, Rivera L, Hernandez M, Prieto D (2020) Differential contribution of Nox1, Nox2 and Nox4 to kidney vascular oxidative stress and endothelial dysfunction in obesity. Redox Biol 28:101330
Nair R, Vaqar S (2021) Renovascular hypertension. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing
Nakamoto H, Ferrario CM, Fuller SB, Robaczewski DL, Winicov E, Dean RH (1995) Angiotensin-(1–7) and nitric oxide interaction in renovascular hypertension. Hypertension 25:796–802
Noiri E, Nakao A, Uchida K, Tsukahara H, Ohno M, Fujita T, Brodsky S, Goligorsky MS (2001) Oxidative and nitrosative stress in acute renal ischemia. Am J Physiol Renal Physiol 281:F948-957
Oishi JC, Buzinnari TC, Pestana CR, De Moraes TF, Vatanabe IP, Wink DA Jr, da Silva RS, Bendhack LM, Rodrigues GJ (2015) In vitro Treatment with cis-[Ru(H-dcbpy-)2(Cl)(NO)] improves the endothelial function in aortic rings with endothelial dysfunction. J Pharm Pharm Sci 18:696–704
Oliveira-Sales EB, Dugaich AP, Carillo BA, Abreu NP, Boim MA, Martins PJ, D’Almeida V, Dolnikoff MS, Bergamaschi CT, Campos RR (2008) Oxidative stress contributes to renovascular hypertension. Am J Hypertens 21:98–104
Ollerstam A, Pittner J, Persson AE, Thorup C (1997) Increased blood pressure in rats after long-term inhibition of the neuronal isoform of nitric oxide synthase. J Clin Investig 99:2212–2218
Park Y-W, Park Y-H, Kim S-W, Lee J-U (2000) Increased expression of nitric oxide synthase coincides with reversal of renovascular hypertension. Korean J Physiol Pharmacol 4:143–147
Patzak A, Persson AE (2007) Angiotensin II-nitric oxide interaction in the kidney. Curr Opin Nephrol Hypertens 16:46–51
Paulo M, Araujo AV, Bendhack LM (2013) Sodium nitroprusside activates potassium channels in the vena cava in normotensive but not in hypertensive rats. Hypertens Res 36:765–769. https://doi.org/10.1038/hr.2013.49
Pereira AC, Araujo AV, Paulo M, Andrade FA, Silva BR, Vercesi JA, da Silva RS, Bendhack LM (2017) Hypotensive effect and vascular relaxation in different arteries induced by the nitric oxide donor RuBPY. Nitric Oxide Biol Chem 62:11–16
Pereira TM, Balarini CM, Silva IV, Cabral AM, Vasquez EC, Meyrelles SS (2009) Endogenous angiotensin II modulates nNOS expression in renovascular hypertension. Braz J Med Biol Res 42:685–691
Pinheiro LC, Amaral JH, Ferreira GC, Portella RL, Ceron CS, Montenegro MF, Toledo JC Jr, Tanus-Santos JE (2015) Gastric S-nitrosothiol formation drives the antihypertensive effects of oral sodium nitrite and nitrate in a rat model of renovascular hypertension. Free Radical Biol Med 87:252–262
Pinheiro LC, Ferreira GC, Amaral JH, Portella RL, Tella SOC, Passos MA, Tanus-Santos JE (2016) Oral nitrite circumvents antiseptic mouthwash-induced disruption of enterosalivary circuit of nitrate and promotes nitrosation and blood pressure lowering effect. Free Radical Biol Med 101:226–235
Prasanna N, Dissanayake HA, Constantine GR (2018) Sublingual nitroglycerin for early blood pressure control in hypertensive emergencies: observations from an emergency department clinical audit in Sri Lanka. BMC Res Notes 11:10–12. https://doi.org/10.1186/s13104-018-3460-0
Ralapanawa DMPUK, Jayawickreme KP, Ekanayake EMM (2016) A case of treatable hypertension: fibromuscular dysplasia of renal arteries Case Reports. BMC Res Notes 9:10–13. https://doi.org/10.1186/s13104-015-1835-z
Ravarotto V, Simioni F, Pagnin E et al (2018) Oxidative stress – chronic kidney disease – cardiovascular disease: A vicious circle. Life Sci 210:125–131. https://doi.org/10.1016/j.lfs.2018.08.067
Rizzi E, Amaral JH, Guimaraes DA, Conde-Tella SO, Pinheiro LC, Gerlach RF, Castro MM, Tanus-Santos JE (2019) Nitrite treatment downregulates vascular MMP-2 activity and inhibits vascular remodeling in hypertension independently of its antihypertensive effects. Free Radical Biol Med 130:234–243
Rodino-Janeiro BK, Paradela-Dobarro B, Castineiras-Landeira MI, Raposeiras-Roubin S, Gonzalez-Juanatey JR, Alvarez E (2013) Current status of NADPH oxidase research in cardiovascular pharmacology. Vasc Health Risk Manag 9:401–428
Rodrigues GJ, Pereira AC, Vercesi JA, Lima RG, Silva RS, Bendhack LM (2012) Long-lasting hypotensive effect in renal hypertensive rats induced by nitric oxide released from a ruthenium complex. J Cardiovasc Pharmacol 60:193–198
Rossi GP, Bisogni V, Rossitto G, Maiolino G, Cesari M, Zhu R, Seccia TM (2020) Practice Recommendations for Diagnosis and Treatment of the Most Common Forms of Secondary Hypertension. High Blood Press Cardiovas Prev 27:547–560
Samadian F, Dalili N, Jamalian A (2017) New insights into pathophysiology, diagnosis, and treatment of renovascular hypertension. Iran J Kidney Dis 11:79–89
Sanchez-Mendoza A, Hong E, Escalante B (1998) The role of nitric oxide in angiotensin II-induced renal vasoconstriction in renovascular hypertension. J Hypertens 16:697–703
Tafur-Soto JD, White CJ (2015) Renal artery stenosis. Cardiol Clin 33:59–73
Textor SC (2009) Current approaches to renovascular hypertension. The Medical clinics of North America 93: 717–732, Table of Contents
Textor SC (2014) Secondary hypertension: renovascular hypertension. J Am Soc Hypertens 8:943–945
Textor SC (2020) Management of renovascular hypertension. Curr Opin Cardiol 35:627–635. https://doi.org/10.1097/HCO.0000000000000790
Touyz RM (2004) Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance? Hypertension 44:248–252
Van Beusecum J, Inscho EW (2015) Regulation of renal function and blood pressure control by P2 purinoceptors in the kidney. Curr Opin Pharmacol 21:82–88
Wadei HM, Textor SC (2012) The role of the kidney in regulating arterial blood pressure. Nat Rev Nephrol 8:602–609
Wassmann S, Wassmann K, Nickenig G (2004) Modulation of oxidant and antioxidant enzyme expression and function in vascular cells. Hypertension 44:381–386
Acknowledgements
Figures were created with BioRender.com.
Funding
This review was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil CNPq [grant number 406177/2016–3]; Fundação de Amparo à Pesquisa do Estado de Minas Gerais, Brazil [grant numbers APQ-01239–16, PPM-00383–18]; and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil.
Author information
Authors and Affiliations
Contributions
Pinheiro, B.P.: conceptualization and original draft preparation
do Valle, G.T: conceptualization and original draft preparation
Ceron, C.S.: conceptualization, funding acquisition, original draft preparation, reviewing, and editing
Corresponding author
Ethics declarations
Ethical approval
Not applicable
Consent to participate
Not applicable
Consent for publication
Not applicable
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pereira, B.P., do Vale, G.T. & Ceron, C.S. The role of nitric oxide in renovascular hypertension: from the pathophysiology to the treatment. Naunyn-Schmiedeberg's Arch Pharmacol 395, 121–131 (2022). https://doi.org/10.1007/s00210-021-02186-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-021-02186-z