Abstract
Sleep is involved in maintaining energy, regulating heat, and recovering tissues. Furthermore, proper cognitive functions need sufficient sleep. Many studies have revealed the impairment effect of sleep deprivation (SD) on cognitive functions including learning and memory. Alpha lipoic acid (ALA) is a potent free radical scavenger, biological antioxidant, and neuroprotective agent. Furthermore, ALA improves learning and memory performance, decreases oxidative stress, and enhances antioxidant biomarkers. In this study, we aimed to investigate the effect of ALA on social interaction and passive avoidance memories in sleep-deprived rats. Total sleep deprivation (TSD) apparatus was used to induce SD (for 24 h). Three-chamber paradigm test and shuttle box apparatus were used to evaluate social interaction and passive avoidance memory, respectively. Rats’ locomotor apparatus was used to assess locomotion. ALA was administered intraperitoneally at doses of 17 and 35 mg/kg for 3 consecutive days. The results showed SD impaired both types of memories. ALA at the dose of 35 mg/kg restored social interaction memory in sleep-deprived rats; while, at the dose of 17 mg/kg attenuated impairment effect of SD. Moreover, ALA at the dose of 35 mg/kg impaired passive avoidance memory in sham-SD rats and at both doses did not rescue passive avoidance memory in sleep-deprived rats. In conclusion, ALA showed impairment effect on passive avoidance memory, while improved social interaction memory in sleep-deprived rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sleep is a protective circadian biologic rhythm that maintains homeostasis of autonomic, neuroendocrine, and immune systems (Tononi and Cirelli, 2006). In addition, sleep is involved in maintaining energy, regulating heat, and recovering tissues (Maquet, 2001). Sleep is also critical for proper cognitive functions (Havekes et al., 2012, Abel et al., 2013). Studies have shown that sleep deprivation (SD) induces severe pathological alterations that affect psychomotor, cognitive, and behavioral functions (Aldabal and Bahammam, 2011). Furthermore, SD impairs learning and memory functions in various cognitive tasks (Abel et al., 2013). A recent study has reported that SD-exposed rats exhibit cognitive decline in the Y-maze test, object location test, novel object recognition test, and the Morris water maze, due to excessiveness of autophagy in the hippocampus (Yang et al., 2019). SD-exposed rats have more errors in both short- and long-term memory tests of the radial arm water maze, due to attenuating antioxidant effects in the hippocampus (Alzoubi et al., 2019). It has been revealed that SD disrupts neuronal and memory formation processing in the hippocampus by attenuating long-term potentiation (LTP) (Ishikawa et al., 2006), cAMP/PKA signaling (Vecsey et al., 2009), glutamate receptors’ expression (Ravassard et al., 2009), and transcription of cAMP response element binding (CREB) intermediary gene (Alhaider et al., 2011).
Alpha lipoic acid (ALA), also known as lipoic acid or thioctic acid, is an organosulfur compound derived from octanoic acid (Reljanovic et al., 1999, Petersen Shay et al., 2008). ALA is a potent-free radical scavenger, biological antioxidant, and neuroprotective agent that are involved in mitochondrial production of ATP (Packer et al., 1997, Somani et al., 2000). ALA is synthesized in mitochondria from octanoic acid during the process of fatty acid synthesis. An enzyme called lipoyl (octanoyl) transferase 2 catalyzes the transfer of the octanoyl moiety from octanoyl-ACP to a conserved lysine of the H protein of the glycine cleavage system. The next reaction is the insertion of two sulfur atoms at positions 6 and 8 of the protein H-bound octanoyl moiety, thereby producing a dihydrolipoyl moiety. This step is catalyzed by the lipoic acid synthetase, an enzyme containing iron-sulfur clusters that act as sulfur donors in the reaction (Mayr et al., 2014). ALA increases acetylcholine (Ach) production and glucose uptake, scavenges reactive oxygen species (ROS) and lipid peroxidation products, and induces syntheses of antioxidant protective enzymes (Holmquist et al., 2007). ALA has also neuroprotective effects in animal models of seizure, cerebral ischemia, autoimmune encephalomyelitis, subarachnoid hemorrhage, and traumatic brain injury (Freitas, 2009, Ersahin et al., 2010, Chaudhary et al., 2011, Connell et al., 2011, Rocamonde et al., 2012). In a previous study, ALA restored spatial memory impairment induced by bilateral common carotid arteries occlusion (Zhao et al., 2015). Furthermore, the neuroprotective effect of ALA on memory deficit in a neuroendocrine model of depression in mice has been revealed (de Sousa et al., 2018). ALA via modulating muscarinic receptor activity improves hippocampus- and amygdala-dependent memory (Mahboob et al., 2016). The beneficial effects of ALA on spatial learning and memory and hippocampal neuronal morphology in rats have been also reported (Dixit et al., 2020). ALA reduces hippocampus-dependent memory decline without affecting Aβ levels in the mouse model of Alzheimer’s disease (AD) (Quinn et al., 2007). In addition, ALA improves cognitive performance via restoring oxidative stress in another mouse model of AD (Farr et al., 2012). A recent study has also revealed that treatment with ALA protects the postoperative cognitive function and the structure of hippocampal neurons and synapses, and also, prevents the phosphorylation of tau in the hippocampus of wild-type mice (Zhang et al., 2019).
According to the mentioned findings, and with respect to the role of sleep in learning and memory processes, we aimed to investigate the effect of ALA on social interaction and passive avoidance memories in sleep-deprived rats.
Materials and methods
Animals
Ninety-six male Wistar rats (7–8 weeks old, 220-240 g weight) obtained from Institute for Cognitive Science Studies (ICSS) were used in this study. Rats were placed in Plexiglas cages in groups of five, and each group was consisted of eight rats. The lab’s environment was under standard temperature (22 + 2 °C) and light/dark cycle (12/12 h), and free access to food and water was provided for all rats, except during the experiments. All the tests were performed only during the light phase. Our experimental protocol was approved by the Research and Ethics Committee of the School of Advanced Technologies in Medicine, Tehran University of Medical Sciences, and were done in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publications No. 80–23).
TSD apparatus
To induce total sleep deprivation (TSD), we used the water box, an automatic TSD apparatus (BorjSanatAzma Co. Tehran, Iran) (Norozpour et al., 2016). The water box was included a water tank made of clear Plexiglas (120 × 30 × 50 cm) with 4 equal-sized boxes (30 × 30 × 50 cm), whereas its water temperature was nearly 30 °C. In each box, one rat was placed (4 rats in general) to observe social stability. Two small platforms with 15 cm diameter and 3 mm deep edge were located next to each other in the middle of the tank. Some holes with 2 mm diameter were at the surface of the platforms to help rats avoid slipping. In fact, these holes facilitated water discharge during upward movements. The motion of platforms was done automatically. At first, the platforms were soaked by going a little deep into the water. Next, each platform regularly moved upward and downward (with the speed of 1 m/s) to force rats move constantly to avoid getting wet. Completion of each platform rotation required 30 s. The peak height of each platform was 10 cm, and the platforms were held at the peak height for 10 s (holding time) to provide food and water for rats. After holding time, the platforms were shifted 60 mm down for 2.5 s and were immediately raised for 2.5 s to the first position. All rats were familiarized with the water box 1 day before the experiments, for 30 min. Our observations during daily SD have shown that rats were awake 100% of the time following using this apparatus (Norozpour et al., 2016). All rats were under TSD condition for 24 h. The effect of new environment’s stress was evaluated by using sham groups in similar situations, when the water box apparatus was off.
Three-chamber paradigm test (social interaction memory test)
The three-chamber paradigm test was used to evaluate social interaction behavior also known as Crawley’s sociability and preference for social novelty (Kaidanovich-Beilin et al., 2011). This apparatus was a rectangular three-chamber box, whereas each chamber was 19 × 45 cm with dividing walls. Rats were individually placed in this apparatus by two equal, wire glass-like compartments with removable tops. The compartments held the familiar/stranger rats. All parameters were recorded by a camera, and the time spent in each compartment was manually determined. The three-chamber paradigm test had three main phases: habituation phase, social affiliation and sociability phase, social novelty/preference, and social memory phase.
Habituation phase
In this phase, the right and left chambers of the rectangular three-chamber box were isolated. Empty containments were located in the center of the right and left chambers. Each rat was individually placed in the center of the middle chamber to adaptation, with the two doorways closed.
Social affiliation and sociability phase
To evaluate social affiliation in rats, one control rat (stranger 1) was placed inside a wire cup that is positioned in one of the side spaces. Then, the walls between the chambers were removed and each rat could discover all the three chambers. Duration of direct interactions between the subject rat and the stranger 1 was recorded. This phase lasted 10 min.
Social novelty/preference and social memory phase
In this phase, a second control rat (stranger 2) was placed inside the other wire cup on the opposite side (that had been empty in previous phase) to distinguish the behavior of the test rat in the presence of stranger 1 (a familiar) when compared with stranger 2 (a novel). This phase lasted 10 min. The procedure used in the three-chamber paradigm test is provided in Fig. 1.
The procedure used in the three-chamber paradigm test. In the habituation phase, the rat was individually placed in the center of the middle chamber for 5 min, to adaptation. The walls between the chambers were closed. In the social affiliation and sociability phase, one rat (stranger 1) was placed inside a wire cup that is positioned in one of the side spaces. Then, the walls between the chambers were removed and each rat could discover all the three chambers. Duration of direct interactions between the subject rat and the stranger 1 was recorded. In the social novelty/preference and social memory phase, a second rat (stranger 2) was placed inside the other wire cup on the opposite side to distinguish the behavior of the first rat in the presence of stranger 1 (a familiar) when compared with stranger 2 (a novel). (Underline = newly added)
Shuttle box apparatus (passive avoidance memory test)
This apparatus consisted of two equal-sized compartments (25 × 25 × 25 cm), including a light and a dark compartment with a grid floor and Plexiglas walls that were separated by a guillotine door. To accustom rats to the apparatus, all rats were placed into the shuttle box 1 day before training, for 5 min. In the training session, each rat was individually put into the light compartment for 60 s. After opening the guillotine door and entrance of the rat into the dark compartment, the door was closed and a 0.5 mA with 50 Hz foot electric shock delivered for 2 s through the grid floor. After 20 s, the rats were transferred to their cage. Twenty-four hours after training, the test session was done. In the test session, rats were placed in the light compartment. The step-through latency to enter the dark compartment was measured as a positive index of memory function. Furthermore, the delay in entering the dark compartment was recorded to a maximum of 300 s.
Rats’ locomotor apparatus (locomotor activity test)
Rats’ locomotion apparatus (BorjSanatAzma Co, Tehran, Iran) consisted of transparent Perspex container (with a height of 40 × 30 × 30 cm). This apparatus had a gray Perspex panel (with a thickness of 2.2 × 30 × 30 cm) with 16 photocells that divided the apparatus into sixteen equal-sized squares. Locomotor activities were evaluated as the number of movements from one square to another during 5 min (Nasehi et al., 2015).
Drug
ALA was purchased from Acros Company (Acros organic, Thermo Fisher Scientific, USA). ALA’s vehicle was 0.1% NaOH. Furthermore, ALA was administered intraperitoneally at the doses of 17 and 35 mg/kg (Thirunavukkarasu et al., 2004, Castro et al., 2014, Andreeva-Gateva et al., 2020).
Experimental groups and procedure
Ninety-six male Wistar rats (8 rats in each group) were used in this study. Furthermore, this study consisted of 3 main groups, where each main group consisted of 4 groups (totally 12 groups):
-
1.
Non-SD groups:
-
Group 1 (Control)—The rats of this group had no intervention
-
Group 2 (Vehicle)—The rats of this group received intraperitoneal injection of NaOH (0.1 μl/g) for 3 days
-
Group 3 (ALA 17 mg/kg)—The rats of this group received intraperitoneal injection of ALA at the dose of 17 mg/kg for 3 days
-
Group 4 (ALA 35 mg/kg)—The rats of this group received intraperitoneal injection of ALA at the dose of 35 mg/kg for 3 days
-
2.
Sham-SD groups:
-
Group 5 (Control)—The rats of this group were placed in the TSD apparatus (when the apparatus was off) without any injection
-
Group 6 (Vehicle)—The rats of this group received intraperitoneal injection of NaOH (0.1 μl/g) for 3 days, and then, they were placed in the TSD apparatus (when the apparatus was off)
-
Group 7 (ALA 17 mg/kg)—The rats of this group received intraperitoneal injection of ALA at the dose of 17 mg/kg for 3 days, and then, they were placed in the TSD apparatus (when the apparatus was off)
-
Group 8 (ALA 35 mg/kg)—The rats of this group received intraperitoneal injection of ALA at the dose of 35 mg/kg for 3 days, and then, they were placed in the TSD apparatus (when the apparatus was off).
-
3.
SD groups:
-
Group 9 (Control)—The rats of this group were placed in the TSD apparatus (under SD condition) without any injection
-
Group 10 (Vehicle)—The rats of this group received intraperitoneal injection of NaOH (0.1 μl/g) for 3 days, and then, they were placed in the TSD apparatus (under SD condition)
-
Group 11 (ALA 17 mg/kg)—The rats of this group received intraperitoneal injection of ALA at the dose of 17 mg/kg for 3 days, and then, they were placed in the TSD apparatus (under SD condition)
-
Group 12 (ALA 35 mg/kg)—The rats of this group received intraperitoneal injection of ALA at the dose of 35 mg/kg for 3 days, and then, they were placed in the TSD apparatus (under SD condition)
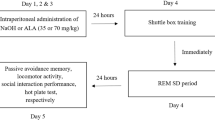
At first, all rats except rats of the control groups (groups 1, 5, and 9) received intraperitoneal injection of NaOH or one of the doses of ALA for 3 consecutive days. At the day 4, all rats were trained in the shuttle box apparatus. Then, all rats except rats of the non-SD groups (groups 1, 2, 3, and 4) were placed in the TSD apparatus for 24 h. At day 5, memory performance and locomotor activity of all rats were assessed (for rats of the non-SD groups, memory performance, and locomotor activity were assessed at the day 4). The order of the cognitive tests was as follows: passive avoidance memory test—locomotor activity test—social interaction memory test. Additionally, a graphical scheme showing the order of performed tests and procedures is provided in Fig. 2.
Statistical analysis
Statistical analyses were done using SPSS software (V. 22.0). Two-way ANOVA, one-way ANOVA, and independent t test analyses were used to assess the significant difference between groups. P < 0.05 was considered as statistically significant.
Results
The effect of ALA on social affiliation and social memory in sleep-deprived rats
Two-way ANOVA analysis for evaluating social affiliation and sociability
The results of two-way ANOVA analysis for non-SD groups showed that the effect of stranger 1 (F1,56 = 12.77, P < 0.001), ALA dose (F3.56 = 9.20, P < 0.001), and the interaction effect (F3.56 = 8.51, P < 0.001) were significant. The results of two-way ANOVA analysis for sham-SD groups showed that the effect of stranger 1 (F1,56 = 74.50, P < 0.001), ALA dose (F3.56 = 5.65, P < 0.01), and the interaction effect (F3.56 = 6.96, P < 0.001) were significant. Furthermore, the results of two-way ANOVA analysis for SD groups showed that the effect of stranger 1 (F1,56 = 44.20, P < 0.001), ALA dose (F3.56 = 4.31, P < 0.01), and the interaction effect (F3.56 = 27.73, P < 0.001) were significant.
Two-way ANOVA analysis for evaluating social memory and novelty
The results of two-way ANOVA analysis for non-SD groups showed that the effect of stranger 2 (F1,56 = 168.94, P < 0.001), ALA dose (F3.56 = 21.04, P < 0.001), and the interaction effect (F3.56 = 39.38, P < 0.001) were significant. The results of two-way ANOVA analysis for sham-SD groups showed that the effect of stranger 2 (F1,56 = 67.97, P < 0.001), ALA dose (F3.56 = 4.08, P < 0.05), and the interaction effect (F3.56 = 10.01, P < 0.001) were significant. Furthermore, the results of two-way ANOVA analysis for SD groups showed that the effect of stranger 2 (F1,56 = 4.52, P < 0.05), ALA dose (F3.56 = 4.26, P < 0.01), and the interaction effect (F3.56 = 35.69, P < 0.001) were significant.
Independent t test analysis
Independent t test analysis also showed that SD impaired social affiliation and sociability (Fig. 3a) and social memory and novelty (Fig. 3b), because sleep-deprived rats did not spend more seconds with stranger 1 in comparison with empty or with stranger 2 in comparison with stranger 1, respectively; while, the rats of the control group spent more times with stranger 1 (in social affiliation) or stranger 2 (in social memory). Furthermore, ALA at both doses improved social affiliation and sociability in sleep-deprived rats (Fig. 3a), and at the dose of 35 mg/kg reversed social memory impairment induced by SD (Fig. 3b), because sleep-deprived rats in these groups spent more seconds with stranger 1 or stranger 2, respectively. It should be noted that ALA at the dose of 17 mg/kg attenuated the impairment effect of SD on social memory, although it was not statistically significant (Fig. 3b).
The effect of ALA on passive avoidance memory and locomotor activity
Passive avoidance memory
The results of one-way ANOVA analysis showed that there were no significant differences between non-SD groups in passive avoidance memory performance (F3.31 = 0.92, P > 0.05). The results of two-way ANOVA analysis for sham-SD groups showed that the effect of sleep (F1,56 = 3.84, P > 0.05) and the interaction effect (F3.56 = 0.81, P > 0.05) were not significant, while the effect of ALA dose (F3.56 = 2.92, P < 0.05) was significant. Furthermore, the results of two-way ANOVA analysis for SD groups showed that the effect of ALA dose (F3.56 = 2.58, P > 0.05) and the interaction effect (F3.56 = 2.45, P > 0.05) were not significant, while the effect of sleep (F1,56 = 149.55, P < 0.001) was significant. Note that, to analyze the results of SD groups, we compared these groups with sham-SD groups, but not with non-SD groups. Sham-SD groups are the most relevant control groups for comparisons, because these groups went through everything the experimental groups experienced, except that the apparatus was not turned on. Independent t test analysis also showed that ALA at the dose of 35 mg/kg impaired passive avoidance memory in sham-SD rats, because rats this group showed a lower latency to enter the dark compartment (Fig. 4). Furthermore, ALA at both doses decreased passive avoidance memory in non-SD rats (although this effect was not significant) (Fig. 4). Moreover, SD impaired passive avoidance memory, and ALA did not restore the impairment effect of SD on passive avoidance memory, because there was no difference between groups in latency to enter the dark compartment (Fig. 4).
Locomotor activity
The results of one-way ANOVA analysis showed that there were no significant differences between non-SD groups in locomotor activity (F3.31 = 0.14, P > 0.05). The results of two-way ANOVA analysis for sham-SD groups showed that the effect of sleep (F1,56 = 0.34, P > 0.05), ALA dose (F3.56 = 0.30, P > 0.05), and the interaction effect (F3.56 = 0.14, P > 0.05) were not significant. Furthermore, the results of two-way ANOVA analysis for SD groups showed that the effect of sleep (F1.56 = 0.001, P > 0.05), ALA dose (F3.56 = 0.59, P > 0.05), and the interaction effect (F3.56 = 0.04, P > 0.05) were not significant. Note that, to analyze the results of SD groups, we compared these groups with sham-SD groups, but not with non-SD groups. Sham-SD groups are the most relevant control groups for comparisons, because these groups went through everything the experimental groups experienced, except that the apparatus was not turned on. Independent t test analysis did not show any significant differences between groups in locomotor activity (Fig. 5). Thus, locomotor activity did not change in all groups.
Discussion
Impairment effect of SD on social interaction memory and passive avoidance memory
As the results showed, SD impaired social interaction memory and passive avoidance memory in rats. One of the important elements involved in general health and proper cognitive functions is enough and comfortable sleep (Alzoubi et al., 2019). Sleep maintains homeostasis of autonomic, neuroendocrine, and immune systems (Abel et al., 2013). One of the critical roles of sleep is to set newly acquired memory for long-term durations (Wagner and Born, 2008). Also, sleep plays a critical role in promoting neurogenesis (Sompol et al., 2011). On the other hand, SD induces a deleterious effect on proliferation of newly born neuronal cells in the hippocampus (Meerlo et al., 2009). SD impairs learning and memory functions in various cognitive tasks (Abel et al., 2013, Ahmad et al., 2019). Furthermore, SD impairs memory performance in multiple trial inhibitory avoidance and contextual fear conditioning tasks in male Wistar rats (Oliveira et al., 2019). SD also impairs memory formation in inhibitory passive avoidance apparatus (Javad-Moosavi et al., 2017). There are a lot of hypotheses about the role of SD in memory loss. It has been suggested that SD impairs memory performance by excessiveness of autophagy in the hippocampus (Yang et al., 2019). Furthermore, SD induces cognitive decline in memory tasks via attenuating antioxidant effects in the hippocampus (Alzoubi et al., 2019). It has been also suggested that SD by increasing oxidative stress in the brain, especially in the hippocampus, induces memory decline (Noguti et al., 2013, Alzoubi et al., 2018). SD decreases glutathione levels as an antioxidant marker in the brain (D'Almeida et al., 2000). Some researchers believe that SD-induced memory impairment is related to a stop in memory processing (Jiang et al., 2009, Alhaider et al., 2010). As mentioned, SD disrupts memory formation processing in the hippocampus via attenuating LTP (Ishikawa et al., 2006), cAMP/PKA signaling (Vecsey et al., 2009), glutamate receptors’ expression (Ravassard et al., 2009), and transcription of CREB intermediary gene (Alhaider et al., 2011). Additionally, the negative effect of SD on homeostasis can be an underlying cause of cognitive decline (Goel et al., 2009). Evidence shows that sleep enhances neurogenesis (Sompol et al., 2011), while SD induces a deleterious effect on proliferation and survival of newly born neuronal cells in the hippocampus and leads to memory deficit (Tosini et al., 2012). Furthermore, synaptic homeostasis represents a fundamental mechanism in learning and memory processing (Magistretti, 2011), while SD via increasing oxidative stress and attenuating immune system activity can disrupt synaptic homeostasis, decrease neurogenesis, and induce memory decline (Meerlo et al., 2009, Gibson et al., 2010). Previous reports have also shown SD impairs learning and memory via attenuating hippocampal neurogenesis (Guzman-Marin et al., 2005, Lopez-Virgen et al., 2015). SD also induces body weight loss, probably via complement activation. Complement activation also induces memory impairment, in addition to weight loss (Wadhwa et al., 2019). It has been reported that complement antagonism improves SD-induced body weight loss and memory decline in rats (Wadhwa et al., 2019). Furthermore, activating complement system following SD significantly decreases neurogenesis in the brain (Wadhwa et al., 2019). Increase in the expression of synaptic proteins in the hippocampus following reversing SD-induced complement system activation via complement antagonism has been also reported (Wadhwa et al., 2019). Thus, we suggest that SD impaired memory performance in the present study probably via increasing oxidative stress, attenuating LTP, attenuating antioxidant activity, decreasing neurogenesis, altering synaptic homeostasis, and activating complement system. It should be noted that this study has a limitation. The body weight of the rats was not measured in the present study, and also, we did not assess probable effects of SD on the brain homeostasis or morphology.
Protective role of ALA in social interaction memory of sleep-deprived rats
As the results showed, ALA at the dose of 17 mg/kg slightly attenuated the impairment effect of SD on social memory and novelty; while, at the dose of 35 mg/kg significantly reversed the impairment effect of SD on social memory and novelty. ALA is potently involved in combating free radicals and promoting the reduction of lipid peroxidation (Chng et al., 2009). ALA has been also identified as an ideal neuroprotective antioxidant based on its ability to cross the blood-brain barrier and its uniform uptake by different parts of the nervous system (Packer et al., 1995). Many studies have reported the protective effects of ALA against neurodegeneration in models of AD, Parkinson’s disease, diabetes, seizure, cerebral ischemia, autoimmune encephalomyelitis, subarachnoid hemorrhage, and traumatic brain injury (Freitas, 2009, Siedlak et al., 2009, Ersahin et al., 2010, Connell et al., 2011, Rocamonde et al., 2012, Zaitone et al., 2012, Nebbioso et al., 2013). Furthermore, the protective effect of ALA on memory performance has been reported. For example, ALA improves spatial learning and memory after bilateral common carotid arteries occlusion (BCCAO) surgery in rats (Zhao et al., 2015). ALA restores the attenuating effect of BCCAO on Ach and choline acetyltransferase levels, and also, the stimulating effect of BCCAO on acetycholinesterase (AchE) level in the hippocampus of rats (Zhao et al., 2015). Furthermore, ALA restores social interaction memory impairment induced by early postnatal administration of thimerosal in male rats (Namvarpour et al., 2018). It seems that the potent modulatory role of ALA in mitochondrial dehydrogenase reactions plays a critical role for its pro-cognitive and antioxidant properties (Packer et al., 1995). Additionally, ALA via increasing the expression of muscarinic receptors in both the hippocampus and the amygdala improves memory performance (Soares et al., 2006). Note that, the amygdala plays a crucial role in mediating emotional behavior in social novelty preference (Daenen et al., 2002, Antunes and Biala, 2012). Therefore, ALA-induced higher expression of muscarinic receptors in both the hippocampus and the amygdala has been suggested as an underlying cause of improving social behavior (Mahboob et al., 2016). On the other hand, SD attenuates antioxidant activity in the hippocampus (Alzoubi et al., 2019), increases oxidative stress in the hippocampus (Noguti et al., 2013, Alzoubi et al., 2018), and decreases glutathione levels as an antioxidant marker in the brain (D'Almeida et al., 2000). Also, the attenuating effect of ALA on oxidative stress and ROS accumulation and its stimulating effect on the induction of antioxidant enzymes synthesis may decrease the impairment effect of SD on memory processing (Holmquist et al., 2007). In addition, ALA improves the structure of hippocampal neurons and synapses, spine density, and the number of intersections both in pyramidal and granule cells of the hippocampus (Dixit et al., 2020). Interestingly, the crucial role of the hippocampus in mediating social memory and social behavior has been also reported (Montagrin et al., 2018). Furthermore, previous studies have reported the impairment effect of SD on hippocampal structure and neurons. For example, it has been shown that SD attenuates hippocampal cell proliferation and neurogenesis in both the dorsal and ventral dentate gyrus (Murata et al., 2018). Even a brief period of SD impacts a wealth of signaling events in the hippocampus including transcriptional and translational processes and synaptic structure (Havekes et al., 2016, Tudor et al., 2016). The results of the present study also showed that ALA restored SD-induced impaired social memory and novelty. Thus, we suggest that ALA via increasing muscarinic transmission in both the amygdala and the hippocampus increase in anti-oxidative stress system activity, increase in free radicals’ clearance, and improve the function and the structure of the hippocampus, improved social memor, and novelty.
ALA impaired passive avoidance memory and did not restore the impairment effect of SD on passive avoidance memory
As the results showed, ALA at the dose of 35 mg/kg impaired passive avoidance memory and at both doses did not improve passive avoidance memory in sleep-deprived rats. We did not find any published paper about the effect of ALA on passive avoidance memory; however, based on the findings which show that the stimulation or inhibition of the basolateral amygdala improve or impair passive avoidance memory, respectively, it seems that amygdala has a key role in passive avoidance memory formation (Parent et al., 1992, McGaugh et al., 1996). Furthermore, there is evidence that emotionally arousing experiences activate the amygdala. In the basolateral amygdala, this activation leads to the modulation of memory-related processes in other brain regions (McIntyre et al., 2003). Drugs which affect neuromodulatory systems within the basolateral amygdala can modulate the memory of many emotionally arousing training tasks, including the inhibitory avoidance (Roozendaal et al., 2008). Previous research has shown that lesion of the basolateral amygdala impairs passive avoidance memory consolidation or retrieval in rats (Tomaz et al., 1992). It is important to note that blockade of the N-methyl-d-aspartate (NMDA) subtype of glutamate receptors in the basolateral amygdala inhibits passive avoidance memory formation. These receptors play a critical role in the acquisition and storage of fear-motivated learning in rats (LaLumiere et al., 2004, Delaney et al., 2013). It seems that activation of the basolateral amygdala possibly through the NMDA receptors modulates hippocampal LTP (Li and Richter-Levin, 2013). Interestingly, previous study has reported that ALA oxidizes NMDA receptors’ redox site and inhibits NMDA-induced responses (Tang and Aizenman, 1993). Any modification of this site affects other NMDA-mediated phenomena, including LTP and neurotransmitter releasing that are involved in learning and memory (Tauck and Ashbeck, 1990, Woodward and Blair, 1991). Therefore, we suggest that for the effect of ALA on NMDA receptors’ function in the amygdala, we did not observe any protective effect of ALA on impaired passive avoidance memory in sleep-deprived rats. On the other hand, we observed the impairment effect of ALA at its high dose on passive avoidance memory. It should be noted that there is no published data about the effect of ALA on passive avoidance memory. Therefore, to better understand the effect of ALA on passive avoidance memory and NMDA function in the amygdala, more detailed molecular and behavioral researches are needed. We hope that the results of this study will help researchers in future studies.
Conclusion
The results of this study showed that 24-h SD impaired social affiliation and memory and passive avoidance memory. Furthermore, ALA at the dose of 35 mg/kg reversed the impairment effect of SD on social interaction memory, while at the dose of 17 mg/kg attenuated this effect. ALA at the dose of 35 mg/kg impaired passive avoidance memory and at both doses did not rescue passive avoidance memory in sleep-deprived rats.
References
Abel T, Havekes R, Saletin JM, Walker MP (2013) Sleep, plasticity and memory from molecules to whole-brain networks. Curr Biol 23:R774–R788
Ahmad L, Mujahid M, Mishra A, Rahman MA (2019). Protective role of hydroalcoholic extract of Cajanus cajan Linn leaves against memory impairment in sleep deprived experimental rats. J Ayurveda Integr Med
Aldabal L, Bahammam AS (2011) Metabolic, endocrine, and immune consequences of sleep deprivation. Open Respir Med J 5:31–43
Alhaider IA, Aleisa AM, Tran TT, Alkadhi KA (2010) Caffeine prevents sleep loss-induced deficits in long-term potentiation and related signaling molecules in the dentate gyrus. Eur J Neurosci 31:1368–1376
Alhaider IA, Aleisa AM, Tran TT, Alkadhi KA (2011) Sleep deprivation prevents stimulation-induced increases of levels of P-CREB and BDNF: protection by caffeine. Mol Cell Neurosci 46:742–751
Alzoubi KH, Malkawi BS, Khabour OF, El-Elimat T, Alali FQ (2018) Arbutus andrachne L. reverses sleep deprivation-induced memory impairments in rats. Mol Neurobiol 55:1150–1156
Alzoubi KH, Mayyas F, Abu Zamzam HI (2019) Omega-3 fatty acids protects against chronic sleep-deprivation induced memory impairment. Life Sci 227:1–7
Andreeva-Gateva P, Traikov L, Sabit Z, Bakalov D, Tafradjiiska-Hadjiolova R (2020) Antioxidant effect of alpha-lipoic acid in 6-Hydroxydopamine unilateral intrastriatal injected rats. Antioxidants (Basel) 9
Antunes M, Biala G (2012) The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13:93–110
Castro MC, Francini F, Gagliardino JJ, Massa ML (2014) Lipoic acid prevents fructose-induced changes in liver carbohydrate metabolism: role of oxidative stress. Biochim Biophys Acta 1840:1145–1151
Chaudhary P, Marracci G, Yu X, Galipeau D, Morris B, Bourdette D (2011) Lipoic acid decreases inflammation and confers neuroprotection in experimental autoimmune optic neuritis. J Neuroimmunol 233:90–96
Chng HT, New LS, Neo AH, Goh CW, Browne ER, Chan EC (2009) Distribution study of orally administered lipoic acid in rat brain tissues. Brain Res 1251:80–86
Connell BJ, Saleh M, Khan BV, Saleh TM (2011) Lipoic acid protects against reperfusion injury in the early stages of cerebral ischemia. Brain Res 1375:128–136
D'Almeida V, Hipolide DC, Lobo LL, de Oliveira AC, Nobrega JN, Tufik S (2000) Melatonin treatment does not prevent decreases in brain glutathione levels induced by sleep deprivation. Eur J Pharmacol 390:299–302
Daenen EW, Wolterink G, Gerrits MA, Van Ree JM (2002) The effects of neonatal lesions in the amygdala or ventral hippocampus on social behaviour later in life. Behav Brain Res 136:571–582
de Sousa CNS, Meneses LN, Vasconcelos GS, da Silva MI, Silva MCC, Mouaffak F, Kebir O, da Silva Leite CMG, Patrocinio MCA, Macedo D, Vasconcelos SMM (2018) Neuroprotective evidence of alpha-lipoic acid and desvenlafaxine on memory deficit in a neuroendocrine model of depression. Naunyn Schmiedeberg's Arch Pharmacol 391:803–817
Delaney AJ, Sedlak PL, Autuori E, Power JM, Sah P (2013) Synaptic NMDA receptors in basolateral amygdala principal neurons are triheteromeric proteins: physiological role of GluN2B subunits. J Neurophysiol 109:1391–1402
Dixit S, Mehra RD, Dhar P (2020) Effect of alpha-lipoic acid on spatial memory and structural integrity of developing hippocampal neurons in rats subjected to sodium arsenite exposure. Environ Toxicol Pharmacol 75:103323
Ersahin M, Toklu HZ, Cetinel S, Yuksel M, Erzik C, Berkman MZ, Yegen BC, Sener G (2010) Alpha lipoic acid alleviates oxidative stress and preserves blood brain permeability in rats with subarachnoid hemorrhage. Neurochem Res 35:418–428
Farr SA, Price TO, Banks WA, Ercal N, Morley JE (2012) Effect of alpha-lipoic acid on memory, oxidation, and lifespan in SAMP8 mice. J Alzheimers Dis 32:447–455
Freitas RM (2009) The evaluation of effects of lipoic acid on the lipid peroxidation, nitrite formation and antioxidant enzymes in the hippocampus of rats after pilocarpine-induced seizures. Neurosci Lett 455:140–144
Gibson EM, Wang C, Tjho S, Khattar N, Kriegsfeld LJ (2010) Experimental ‘jet lag’ inhibits adult neurogenesis and produces long-term cognitive deficits in female hamsters. PLoS One 5:e15267
Goel N, Rao H, Durmer JS, Dinges DF (2009) Neurocognitive consequences of sleep deprivation. Semin Neurol 29:320–339
Guzman-Marin R, Suntsova N, Methippara M, Greiffenstein R, Szymusiak R, McGinty D (2005) Sleep deprivation suppresses neurogenesis in the adult hippocampus of rats. Eur J Neurosci 22:2111–2116
Havekes R, Park AJ, Tudor JC, Luczak VG, Hansen RT, Ferri SL, Bruinenberg VM, Poplawski SG, Day JP, Aton SJ, Radwanska K, Meerlo P, Houslay MD, Baillie GS, Abel T (2016) Sleep deprivation causes memory deficits by negatively impacting neuronal connectivity in hippocampal area CA1. Elife 5
Havekes R, Vecsey CG, Abel T (2012) The impact of sleep deprivation on neuronal and glial signaling pathways important for memory and synaptic plasticity. Cell Signal 24:1251–1260
Holmquist L, Stuchbury G, Berbaum K, Muscat S, Young S, Hager K, Engel J, Munch G (2007) Lipoic acid as a novel treatment for Alzheimer’s disease and related dementias. Pharmacol Ther 113:154–164
Ishikawa A, Kanayama Y, Matsumura H, Tsuchimochi H, Ishida Y, Nakamura S (2006) Selective rapid eye movement sleep deprivation impairs the maintenance of long-term potentiation in the rat hippocampus. Eur J Neurosci 24:243–248
Javad-Moosavi BZ, Vaezi G, Nasehi M, Haeri-Rouhani SA, Zarrindast MR (2017) Critical role of CA1 muscarinic receptors on memory acquisition deficit induced by total (TSD) and REM sleep deprivation (RSD). Prog Neuro-Psychopharmacol Biol Psychiatry 79:128–135
Jiang F, Shen XM, Li SH, Cui ML, Zhang Y, Wang C, Yu XG, Yan CH (2009) Effects of chronic partial sleep deprivation on growth and learning/memory in young rats. Zhongguo Dang Dai Er Ke Za Zhi 11:128–132
Kaidanovich-Beilin O, Lipina T, Vukobradovic I, Roder J, Woodgett JR (2011) Assessment of social interaction behaviors. JoVE (Journal of Visualized Experiments):e2473
LaLumiere RT, Pizano E, McGaugh JL (2004) Intra-basolateral amygdala infusions of AP-5 impair or enhance retention of inhibitory avoidance depending on training conditions. Neurobiol Learn Mem 81:60–66
Li Z, Richter-Levin G (2013) Priming stimulation of basal but not lateral amygdala affects long-term potentiation in the rat dentate gyrus in vivo. Neuroscience 246:13–21
Lopez-Virgen V, Zarate-Lopez D, Adirsch FL, Collas-Aguilar J, Gonzalez-Perez O (2015) Effects of sleep deprivation in hippocampal neurogenesis. Gac Med Mex 151:99–104
Magistretti PJ (2011) Neuron-glia metabolic coupling and plasticity. Exp Physiol 96:407–410
Mahboob A, Farhat SM, Iqbal G, Babar MM, Zaidi NU, Nabavi SM, Ahmed T (2016) Alpha-lipoic acid-mediated activation of muscarinic receptors improves hippocampus- and amygdala-dependent memory. Brain Res Bull 122:19–28
Maquet P (2001) The role of sleep in learning and memory. Science 294:1048–1052
Mayr JA, Feichtinger RG, Tort F, Ribes A, Sperl W (2014) Lipoic acid biosynthesis defects. J Inherit Metab Dis 37:553–563
McGaugh JL, Cahill L, Roozendaal B (1996) Involvement of the amygdala in memory storage: interaction with other brain systems. Proc Natl Acad Sci U S A 93:13508–13514
McIntyre CK, Power AE, Roozendaal B, McGaugh JL (2003) Role of the basolateral amygdala in memory consolidation. Ann N Y Acad Sci 985:273–293
Meerlo P, Mistlberger RE, Jacobs BL, Heller HC, McGinty D (2009) New neurons in the adult brain: the role of sleep and consequences of sleep loss. Sleep Med Rev 13:187–194
Montagrin A, Saiote C, Schiller D (2018) The social hippocampus. Hippocampus 28:672–679
Murata Y, Oka A, Iseki A, Mori M, Ohe K, Mine K, Enjoji M (2018) Prolonged sleep deprivation decreases cell proliferation and immature newborn neurons in both dorsal and ventral hippocampus of male rats. Neurosci Res 131:45–51
Namvarpour Z, Nasehi M, Amini A, Zarrindast MR (2018) Protective role of alpha-lipoic acid in impairments of social and stereotyped behaviors induced by early postnatal administration of thimerosal in male rat. Neurotoxicol Teratol 67:1–9
Nasehi M, Tabatabaie M, Khakpai F, Zarrindast MR (2015) The effects of CA1 5HT4 receptors in MK801-induced amnesia and hyperlocomotion. Neurosci Lett 587:73–78
Nebbioso M, Pranno F, Pescosolido N (2013) Lipoic acid in animal models and clinical use in diabetic retinopathy. Expert Opin Pharmacother 14:1829–1838
Noguti J, Andersen ML, Cirelli C, Ribeiro DA (2013) Oxidative stress, cancer, and sleep deprivation: is there a logical link in this association? Sleep Breath 17:905–910
Norozpour Y, Nasehi M, Sabouri-Khanghah V, Torabi-Nami M, Zarrindast MR (2016) The effect of CA1 alpha2 adrenergic receptors on memory retention deficit induced by total sleep deprivation and the reversal of circadian rhythm in a rat model. Neurobiol Learn Mem 133:53–60
Oliveira SLB, Oliveira MGM, Hipolide DC (2019) A1 adenosine receptors in the striatum play a role in the memory impairment caused by sleep deprivation through downregulation of the PKA pathway. Neurobiol Learn Mem 160:91–97
Packer L, Tritschler HJ, Wessel K (1997) Neuroprotection by the metabolic antioxidant alpha-lipoic acid. Free Radic Biol Med 22:359–378
Packer L, Witt EH, Tritschler HJ (1995) alpha-Lipoic acid as a biological antioxidant. Free Radic Biol Med 19:227–250
Parent MB, Tomaz C, McGaugh JL (1992) Increased training in an aversively motivated task attenuates the memory-impairing effects of posttraining N-methyl-D-aspartate-induced amygdala lesions. Behav Neurosci 106:789–797
Petersen Shay K, Moreau RF, Smith EJ, Hagen TM (2008) Is alpha-lipoic acid a scavenger of reactive oxygen species in vivo? Evidence for its initiation of stress signaling pathways that promote endogenous antioxidant capacity. IUBMB Life 60:362–367
Quinn JF, Bussiere JR, Hammond RS, Montine TJ, Henson E, Jones RE, Stackman RW Jr (2007) Chronic dietary alpha-lipoic acid reduces deficits in hippocampal memory of aged Tg2576 mice. Neurobiol Aging 28:213–225
Ravassard P, Pachoud B, Comte JC, Mejia-Perez C, Scote-Blachon C, Gay N, Claustrat B, Touret M, Luppi PH, Salin PA (2009) Paradoxical (REM) sleep deprivation causes a large and rapidly reversible decrease in long-term potentiation, synaptic transmission, glutamate receptor protein levels, and ERK/MAPK activation in the dorsal hippocampus. Sleep 32:227–240
Reljanovic M, Reichel G, Rett K, Lobisch M, Schuette K, Moller W, Tritschler HJ, Mehnert H (1999) Treatment of diabetic polyneuropathy with the antioxidant thioctic acid (alpha-lipoic acid): a two year multicenter randomized double-blind placebo-controlled trial (ALADIN II). Alpha lipoic acid in diabetic neuropathy. Free Radic Res 31:171–179
Rocamonde B, Paradells S, Barcia JM, Barcia C, Garcia Verdugo JM, Miranda M, Romero Gomez FJ, Soria JM (2012) Neuroprotection of lipoic acid treatment promotes angiogenesis and reduces the glial scar formation after brain injury. Neuroscience 224:102–115
Roozendaal B, Castello NA, Vedana G, Barsegyan A, McGaugh JL (2008) Noradrenergic activation of the basolateral amygdala modulates consolidation of object recognition memory. Neurobiol Learn Mem 90:576–579
Siedlak SL, Casadesus G, Webber KM, Pappolla MA, Atwood CS, Smith MA, Perry G (2009) Chronic antioxidant therapy reduces oxidative stress in a mouse model of Alzheimer’s disease. Free Radic Res 43:156–164
Soares JC, Fornari RV, Oliveira MG (2006) Role of muscarinic M1 receptors in inhibitory avoidance and contextual fear conditioning. Neurobiol Learn Mem 86:188–196
Somani SM, Husain K, Whitworth C, Trammell GL, Malafa M, Rybak LP (2000) Dose-dependent protection by lipoic acid against cisplatin-induced nephrotoxicity in rats: antioxidant defense system. Pharmacol Toxicol 86:234–241
Sompol P, Liu X, Baba K, Paul KN, Tosini G, Iuvone PM, Ye K (2011) N-acetylserotonin promotes hippocampal neuroprogenitor cell proliferation in sleep-deprived mice. Proc Natl Acad Sci U S A 108:8844–8849
Tang LH, Aizenman E (1993) Allosteric modulation of the NMDA receptor by dihydrolipoic and lipoic acid in rat cortical neurons in vitro. Neuron 11:857–863
Tauck DL, Ashbeck GA (1990) Glycine synergistically potentiates the enhancement of LTP induced by a sulfhydryl reducing agent. Brain Res 519:129–132
Thirunavukkarasu V, Anitha Nandhini AT, Anuradha CV (2004) Cardiac lipids and antioxidant status in high fructose rats and the effect of alpha-lipoic acid. Nutr Metab Cardiovasc Dis 14:351–357
Tomaz C, Dickinson-Anson H, McGaugh JL (1992) Basolateral amygdala lesions block diazepam-induced anterograde amnesia in an inhibitory avoidance task. Proc Natl Acad Sci U S A 89:3615–3619
Tononi G, Cirelli C (2006) Sleep function and synaptic homeostasis. Sleep Med Rev 10:49–62
Tosini G, Ye K, Iuvone PM (2012) N-acetylserotonin: neuroprotection, neurogenesis, and the sleepy brain. Neuroscientist 18:645–653
Tudor JC, Davis EJ, Peixoto L, Wimmer ME, van Tilborg E, Park AJ, Poplawski SG, Chung CW, Havekes R, Huang J, Gatti E, Pierre P, Abel T (2016) Sleep deprivation impairs memory by attenuating mTORC1-dependent protein synthesis. Sci Signal 9:ra41
Vecsey CG, Baillie GS, Jaganath D, Havekes R, Daniels A, Wimmer M, Huang T, Brown KM, Li XY, Descalzi G, Kim SS, Chen T, Shang YZ, Zhuo M, Houslay MD, Abel T (2009) Sleep deprivation impairs cAMP signalling in the hippocampus. Nature 461:1122–1125
Wadhwa M, Prabhakar A, Anand JP, Ray K, Prasad D, Kumar B, Panjwani U (2019) Complement activation sustains neuroinflammation and deteriorates adult neurogenesis and spatial memory impairment in rat hippocampus following sleep deprivation. Brain Behav Immun 82:129–144
Wagner U, Born J (2008) Memory consolidation during sleep: interactive effects of sleep stages and HPA regulation. Stress 11:28–41
Woodward JJ, Blair R (1991) Redox modulation of N-methyl-D-aspartate-stimulated neurotransmitter release from rat brain slices. J Neurochem 57:2059–2064
Yang SQ, Jiang L, Lan F, Wei HJ, Xie M, Zou W, Zhang P, Wang CY, Xie YR, Tang XQ (2019) Inhibited endogenous H2S generation and excessive autophagy in hippocampus contribute to sleep deprivation-induced cognitive impairment. Front Psychol 10:53
Zaitone SA, Abo-Elmatty DM, Shaalan AA (2012) Acetyl-L-carnitine and alpha-lipoic acid affect rotenone-induced damage in nigral dopaminergic neurons of rat brain, implication for Parkinson’s disease therapy. Pharmacol Biochem Behav 100:347–360
Zhang Y, Lv YL, Si YN, Zhou J, Qian Y, Bao HG (2019) Alpha-lipoic acid attenuates spatial learning and memory impairment induced by hepatectomy. Exp Ther Med 17:2329–2333
Zhao RR, Xu F, Xu XC, Tan GJ, Liu LM, Wu N, Zhang WZ, Liu JX (2015) Effects of alpha-lipoic acid on spatial learning and memory, oxidative stress, and central cholinergic system in a rat model of vascular dementia. Neurosci Lett 587:113–119
Author information
Authors and Affiliations
Contributions
M. Rezaie and MH. Mohammadi-Mahdiabadi-Hasani collected animal data. S. Vaseghi and M. Nasehi wrote and edited the manuscript and analyzed data. MR. Zarrindast and MA. Nasiri-Khalili designed the study. All the authors have approved the final manuscript. All data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rezaie, M., Nasehi, M., Vaseghi, S. et al. The protective effect of alpha lipoic acid (ALA) on social interaction memory, but not passive avoidance in sleep-deprived rats. Naunyn-Schmiedeberg's Arch Pharmacol 393, 2081–2091 (2020). https://doi.org/10.1007/s00210-020-01916-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-020-01916-z