Abstract
Isorhapontigenin (ISO) is one of the main bioactive components of Gnetum cleistostachyum and was shown to possess antioxidant and antitumor functions. Herein, we hope to examine the neuroprotection impacts of ISO in rats subjected to transient middle cerebral artery occlusion/reperfusion (MCAO/R, 2/24 h) injuries. ISO was injected intraperitoneally into the rats immediately after cerebral ischemia. After 24 h of the reperfusion, infarct volume, brain water contents, neurological deficit, and cerebral blood flow were assessed. Hippocampus histopathology change was detected by H&E and TUNEL staining. The expressions of cleaved caspase-3, Bax and Bcl-2, and phospho-Akt (p-Akt) were investigated by real-time RT-PCR or western blot analysis. We found that ISO significantly suppressed the infarct volumes, brain water contents, and neurological deficit, increased CBF, and relieved histopathologic change in a dose-dependent manner. Reduced malondialdehyde (MDA) and elevated activities of superoxide dismutase (SOD) and GSH and glutathione peroxidase (GSH-PX) were observed in ISO group. ISO remarkably decreased caspase-3 and Bax and increased levels of Bcl-2. Additionally, ISO upregulated p-Akt expression. Blocking of PI3K activities by wortmannin can abolish the ISO-caused decrease in infarct volumes and neurologic deficit scores and abrogate the promotion of p-Akt. The data indicated that ISO played neuroprotective impacts against focal I/R injuries, possibly related to the activating of PI3K/Akt signaling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebral I/R injuries are the most common stroke-related deaths and disabilities over the world, with poor prognosis (Jean et al. 1998; Soriano et al. 1999). Due to the obstructions of a brain artery, with inadequate blood supplies flowing back to the brain, reperfusions within the ischemic brain result in complex pathophysiologic procedures leading to further damages of I/R injuries (Aronowski et al. 1997; Huang et al. 2006). In addition, autophagy and inflammation emerge after I/R contribute to further damages to the neuron death and cell death (Jean et al. 1998; Zhang et al. 2013). There are not enough effective treatment exists. Therefore, researchers are paying more and more efforts to protect the neurons after I/R injuries.
ISO, as a novel derivative of stilbene, is originally isolated from Belamcanda chinensis (Li et al. 2005). With a molecular structure of C15H14O4, it has a quite similar structure to resveratrol (Liu and Liu 2004). It has been demonstrated by a variety of researchers that ISO remains the health-beneficial advantage of antioxidative activities (Wang et al. 2001), antitumor (Fang et al. 2012), and cardioprotective impacts (Abbas 2016). For instance, QL. Wang reported that natural ISO had antioxidative activities in liver microsomes, brain mitochondria, and synaptosomes (Wang et al. 2001). In 2012, Y. Fang demonstrated that ISO induced death within cancer cells via inhibiting expressions of anti-apoptosis factor of XIAP (Fang et al. 2012). In particular, Amr M. Abbas discovered the cardioprotective impacts of ISO in myocardial infarction of rats (Abbas 2016). Resveratrol has been widely reported to pose neuroprotection impacts for I/R injuries (Hung et al. 2004; Ray et al. 1999). Considering ISO’s role in previous studies, and its similarity with resveratrol, it is possible that ISO may also have some neuroprotective benefits in I/R injuries. Herein, we are aimed to investigate whether ISO has any positive impacts on cerebral ischemia/reperfusion injuries.
PI3K/Akt signaling was widely investigated due to its activation by the neuroprotection factors in I/R injuries, such as isoflurane (Gray et al. 2005), humanin (Xu et al. 2008), and sevoflurane postconditioning (Wang et al. 2010). According to J. J. Gray in 2005, the activations of the PI3K/Akt pathway may be a critical factor for anesthetic conditioning from isoflurane against the cerebral I/R injuries (Gray et al. 2005). Specifically, GA. Abel-Aleem demonstrated the neuroprotective impacts of resveratrol against brain I/R injuries in rats via PI3K/Akt /GSK3b survival pathway (Wang et al. 2010). ISO, as a new resveratrol analog, may also bring similar impacts on the modulation of the PI3K/Akt pathway during the neuroprotection against cerebral ischemia/reperfusion. Here, we designed a series of experiments to study the roles of ISO in cerebral I/R and its correlations with the PI3K/Akt pathway.
Methods and materials
Chemicals and reagents
ISO (≥ 98%) and wortmannin were provided by Sigma, USA. Figure 1A displayed chemical structures. 2,3,5-triphenyl tetrazolium chloride (TTC) was obtained from SiISOa, USA. Activities assay kit for SOD, GSH, GSH-PX, and MDA were supplied by Nanjing Jiancheng, China. Rabbit anti-cleaved caspase-3, anti-Bcl-2, anti-Bax, anti-Akt, and anti-phospho-Akt (anti-p-Akt) (Ser473) were bought from Abcam, USA. Rabbit anti-β-actin and peroxidase-linked goat anti-rabbit IgG were obtained from Santa Cruz Bio, USA.
Rat classification
Male SD rats (200 g to 220 g) were bought from Shandong University, China. Rats were kept at around 23 °C in a 12-h light and 12-h dark. They are free to eat or drink. Animal Care and Use Committee of The Affiliated Hospital of Jining Medical University approved all our experiments, which were performed according to the guidelines for the use of laboratory animals in China.
Focal cerebral I/R models
The rats were anesthetized by 10% (w/v) chloral hydrate (350 mg/kg), intraperitoneally, at 37 °C. After the skins and muscles were incised, the left common carotid arteries (CA) were clipped, and the external CA were ligatured. A nylon monofilament was inserted from the left common CA in internal CA to block the original place of the middle cerebral artery. After 2-h MCAO, reperfusion was carried out by removing the monofilament. The sham was undergoing MCAO, without inserting the nanofilament to common CA.
Classifications and drug administrations
We classified the rats in five groups: (1) sham (n = 42), rat had MCAO, without inserting the nanofilament to common CA; (2) I/R (n = 42), rat had cerebral ischemia by ligation for 2 h and reperfusion for 1 day; (3) ISO (6.25–100 mg/kg) groups (n = 126), rats were injected intraperitoneally with ISO (6.25–100 mg/kg) at 2 h after ischemia; (4) Wortmannin (n = 12), the rat was slowly injected intraventricularly with 0.6 mg/kg wortmannin at half an hour before prior ischemia (Wang et al. 2009); and (5) ISO (100 mg/kg) + wortmannin (0.6 mg/kg) group (n = 12), the processes were similar to the ISO and wortmannin groups. ISO was dissolved in ethanol and diluted with saline to reach an ethanol concentration of 5%. Rats in sham and I/R had the same volume of 5% ethanol in normal saline. After 24-h reperfusion, we anesthetized and decapitated the rats.
Evaluations of infarct volume, neurological deficit, and brain water contents
After reperfusion, six rats were anesthetized and then decapitated. We sliced the brains with 1.5 mm and stained them by 1% TTC for half an hour and fixed them by 4% paraformaldehyde. After taking images of the stained parts, the infarct volume was measured by ImageJ. The infarction percentages were obtained by dividing the infarct volumes by the total volumes. Neurological deficit (n = 6) was determined by zero, rat has normal behaviors; one, a rat cannot fully stretch left front leg; two, rats turned around in a circle; three, rats fall to the left; and four, the rat cannot move and lose consciousness. Brain water contents (n = 6) were measured at 1 day after reperfusions. We got infarct brain hemispheres as wet weight and dried them for a night at 105 °C in as dry weight. Brain water contents were calculated as [(wet weight - dry weight)/wet weight] × 100%.
Cerebral blood flow (CBF) measurements
We used an optical scanner with a low-power laser beam on the exposed cortex. The scanner head was placed parallel to the cerebral cortex at a distance of 20 cm. We took the photos of relative perfusion levels, for 24 h from ischemia to reperfusion.
Histological evaluation
We obtained the brains from six rats, fixed them in 4% formaldehyde and inserted them in paraffin. We sliced the tissue to 5 μm and stained them using TUNEL (Roche, Germany). A light microscope was used to examine the results. The amounts of karyotype-kenosis and dead cells were measured, and the mean values were calculated.
SOD, GSH, GSH-PX, and MDA
SOD level was evaluated in the hippocampus through the calculation of the rate of inhibitions of nucleotide oxidations. The activity of GSH-PX and GSH was quantified by calculating the rates of oxidations of the decreased glutathione to oxidized glutathione. The concentrations of MDA were detected by the measurement of thiobarbituric acid at 532 nm. Protein contents were measured through Coomassie brilliant blue method by BSA as reference.
qRT-PCR
Total RNAs were extracted by Trizol. Reverse transcription was conducted (Invitrogen, CA). The primer sequences were below:
Caspase-3: forward, 5-CTACCGCACCCGGTTACTAT-3; reverse, 5-TTCCGGTTAACACGAGTGAG-3. For Bcl-2, forward, 5-CCTGTGGATGACTGAGTACCT-3; reverse, 5- GAGCAGGGTCTTCAGAGACA-3. Bax: forward, 5- CTGAGCTGACCTTGGAGC-3; reverse, 5- GACTCCAGCCACAAAGATG-3. Beta-actin, forward, 5-CCAGCCGAGCCACATCGCTC-3; reverse, 5-ATGAGCCCCAGCCTTCTCCAT-3.
Western blot
After 1-day reperfusion, brain tissue (n = 6) were homogenized, lysed, and centrifuged at 13,200 × g for 20 min at 4 °C. Protein from the supernatant was detected by a BCA protein assay kit (KeyGEN, China). About 50 μg sample was separated by 10% (w/v) SDS-polyacrylamide gel and transferred to nitrocellulose membranes (Amersham, USA). The membrane was blocked by 5% (w/v) skim milk for 2 h. Then, the membranes were treated with rabbit anti-cleaved caspase-3 (1: 1000), anti-Bcl-2 (1: 1000 dilution), anti-Bax (1: 1000), anti-Akt (1: 1000), anti-p-Akt (1: 1000), and anti-β-actin (1:5000) for a night at 4 °C. After washing, the membrane was treated for 2 h with peroxidase-labeled goat anti-rabbit IgG (1: 5000). ECL was employed visualizing the signal intensity, analyzed by Quantity One (Bio-Rad, USA).
Data analysis
We displayed the data as the mean ± SD. Differences were compared by one-way ANOVA and post hoc LSD tests. P < 0.05 was treated as statistically significant.
Results
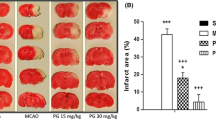
ISO reduced infarct volume, behavioral score, water contents, and increased CBF in I/R rats
Figure 1B, C, and D displayed that neurological score, and infarct volume was substantially elevated in I/R than sham (##P < 0.01). However, ISO markedly decreased the neurological score and infarct volume induced from I/R at all doses (#P < 0.05, ##P < 0.01). Figure 1E displayed that cerebral I/R considerably lifted the brain water contents (##P < 0.01) but were significantly relieved by ISO (#P < 0.05, ##P < 0.01). Figure 1F displayed that CBF was reduced to ~ 17% of basic CBF after ischemia (##P < 0.01) and was rescued to ~ 80% of baseline after reperfusion in I/R rats. About 100 mg/kg ISO induced a considerable elevation in CBF at 1 day after reperfusion (#P < 0.05). There was no noticeable difference that existed in the 25 or 50 mg/kg ISO groups. Our data displayed that ISO reduced infarct volume, behavioral score, and water contents and increased CBF in I/R rats.
ISO on histological change in the hippocampus
According to Fig. 2, H&E staining results revealed that the neuron in sham was better aligned and structured, but I/R had shrunken neuron nuclear and disordered neurons. In the ISO group, neuron karyopyknosis was decreased than the I/R group (**P < 0.01) (Fig. 2). In Fig. 3, the neuron in the hippocampus in I/R rats had more cell death, and cell death occurrences were attenuated by ISO (*P < 0.05, **P < 0.01). The results revealed that ISO can reduce I/R-related neuron cell death.
ISO on MDA and antioxidant enzymes in the hippocampus
In order to corroborate the effects of ISO on oxidative stress during I/R injury of rat, the levels of MDA and the activities of antioxidant enzymes (SOD, GSH, and GSH-PX) in hippocampus were evaluated. From Fig. 4A, MDA (lipid peroxidation indicator) was substantially elevated in I/R rats (**P < 0.01), in contrast with sham. After injections of ISO (25, 50, 100 mg/kg), MDA was obviously reduced (*P < 0.05, **P < 0.01), compared with the I/R group. Activities of SOD in ischemic rats were lowered significantly (##P < 0.01) than the sham (Fig. 4B). However, ISO (50 and 100 mg/kg) promoted SOD activities (**P < 0.01) than I/R rats. Moreover, in contrast with the sham group, I/R rats reduced a great decreasing GSH (##P < 0.01) and GSH-PX (##P < 0.01) in the hippocampus (Fig. 4C and D). ISO can dramatically elevate GSH (**P < 0.01) and GSH-PX (*P < 0.05, **P < 0.01), comparing to the I/R group (Fig. 4C and D).
ISO on cell apoptosis factor in hippocampus
We examined the expressions of caspase-3, Bcl-2, and Bax by real-time RT-PCR and western blot analysis. Apoptotic cells were detected by measuring the levels of cleaved caspase-3, Bcl-2, and Bax in the brain. Figure 5A and B revealed that cleaved caspase-3 and Bax were greatly elevated; Bcl-2 was markedly reduced in I/R rats, than the sham group. However, ISO can increase the expressions of Bcl-2 and inhibit cleaved caspase-3 and Bax (*P < 0.05, **P < 0.01). It was evident that ISO had neuroprotection for I/R-related brain injuries via anti-apoptosis.
Effect of ISO on the cell death factor in the hippocampus. (A) Effect of ISO on mRNA expression of caspase-3, Bcl-2, and Bax in rat hippocampus. (B) Western blot and quantitative densitometry of the expressions of cleaved caspase-3, Bcl-2, and Bax proteins. n = 6. ##P < 0.01 versus sham group; *P < 0.05, **P < 0.01 versus I/R group
Effects of ISO on Akt activation
From Fig. 6A and B, ISO lowered the infarct volume and neurologic deficit score of I/R rats, but wortmannin attenuated these impacts. Figure 6C, D, and E evaluated the impacts of ISO on the expressions of Akt and p-Akt (Ser473) via western blot. Akt’s expressions were similar in all groups. I/R injuries increased p-Akt than the sham group (P < 0.01), and ISO promoted p-Akt activities (P < 0.05). ISO + wortmannin significantly suppressed the phosphorylations of Akt in contrast to ISO (&P < 0.05, &&P < 0.01). Our data indicated that ISO induced the activation of Akt.
Wortmannin can suppress the neuroprotective impacts of ISO treatment. (A) Wortmannin suppressed the decreasing of infarct volume induced by ISO treatment. (B) Wortmannin attenuated the reducing impacts on neurologic deficit scores caused by ISO treatment. (C) The representative band displayed that wortmannin suppressed p-Akt on Ser473. (D) The relative densities of total-Akt to β-actin. (E) The density of p-Akt to total-Akt. n = 6. #P < 0.05, ##P < 0.01 vs sham group; *P < 0.05, **P < 0.01 versus I/R group; &P < 0.05, &&P < 0.01 versus ISO group
Discussions
It was well established that I/R injuries in rats would cause the increasing of infarct volume and the neurological scores, as well as the inhibition of normal behavior outcomes (Ray et al. 1999). The evaluations of those parameters could effectively indicate the protective effects from ISO. Therefore, we carried a series of experiments to examine the outcomes from ISO on infarct volume, behavioral score, water contents, and CBF in I/R rats. In our experiments on ISO, we first found that infarct volume was substantially elevated in the I/R group, and ISO markedly decreased infarct volume resulted from I/R. Cerebral I/R considerably lifted the brain water contents but were significantly relieved by ISO. CBF was reduced to ~ 17% of basic CBF after ischemia, and this level was increased to ~ 80% of baseline after reperfusion in I/R rats. About 100 mg/kg ISO induced a considerable elevation in CBF at 1 day after reperfusion. In consistence with the impacts of resveratrol on I/R injured rats (Lin et al. 2013), we also established the fact that ISO reduced infarct volume, behavioral outcome, water contents, and increased CBF in I/R rats.
In 2015, L. Fang demonstrated that cerebral I/R in mice could cause inflammation and cell death (Fang et al. 2015). From their results, the MCAO group had a severe neuronal loss and neuron death with shrunken cytoplasm and pyknotic nuclei (Fang et al. 2015). In our experiments, the H&E staining results revealed neurons in sham were better aligned, but I/R had shrunken neuron nuclear and disordered neurons. In the ISO group, neuron karyopyknosis was decreased than the I/R group. Neuron in the hippocampus in I/R rats had more cell apoptosis, and cell death occurrences were attenuated by ISO. For the first time, we found that ISO can reduce I/R-related neuron cell death.
According to previous studies, I/R injuries in rats could cause a significant increase in antioxidants of GSH, SOD, and catalase (Abbas 2016). In our experiments, the lipid peroxidation indicator of MDA was substantially elevated in I/R rats, while administrations of 25 mg/kg, 50 mg/kg, or 100 mg/kg ISO obviously reduced MDA. In addition, the level of antioxidants of SOD in ischemic rats was lowered significantly, but ISO promoted SOD activities. In addition, I/R rats substantially decreased the expressions of GSH and GSH-PX. In agreement with previous studies, we found that ISO can significantly elevate the levels of antioxidants of GSH and GSH-PX, and reduce the expressions of lipid peroxidation indicator of MDA, comparing to I/R group.
According to Min Shen, resveratrol attenuated I/R injuries in neonatal cardiomyocytes (Shen et al. 2012). The TUNEL staining displayed that the apoptotic cells increased dramatically in the I/R group and Res lowered apoptotic cells obviously (Shen et al. 2012). In our experiments, we detected cell death by measuring cleaved caspase-3, Bcl-2, and Bax in the brain tissues. It was noticed that caspase-3 and Bax were greatly elevated and Bcl-2 was markedly reduced in I/R rats, than the sham. However, ISO can increase the expressions of Bcl-2 and inhibit cleaved caspase-3 and Bax. It was obvious that ISO had neuroprotection for I/R-related brain injuries via anti-apoptosis.
It was widely reported that many neuroprotective factors, such as isoflurane (Gray et al. 2005), humanin (Xu et al. 2008), and sevoflurane postconditioning (Wang et al. 2010), can activate PI3K/Akt signaling pathway during the alleviation of I/R injuries. For instance, D. He reported that resveratrol can inhibit I/R-induced cardiocyte death, which was involved with the PI3K-Akt signaling pathway. In our experiments, we found that I/R injuries increased p-Akt than the sham group and ISO promoted p-Akt activities. ISO + wortmannin significantly suppressed the phosphorylations of Akt in contrast to ISO. It was obvious that ISO induced the activation of Akt. The neuroprotective impacts of ISO on I/R rats were related to PI3K/Akt signaling pathways.
Conclusions
ISO played neuroprotection impacts against focal I/R injuries, possibly related to the PI3K/Akt signaling activation.
References
Abbas AM (2016) Cardioprotective effect of resveratrol analogue isorhapontigenin versus omega-3 fatty acids in isoproterenol-induced myocardial infarction in rats. J Physiol Biochem 72:469–484
Aronowski J, Strong R, Grotta JC (1997) Reperfusion injury: demonstration of brain damage produced by reperfusion after transient focal ischemia in rats. J Cereb Blood Flow Metab 17:1048–1056
Fang Y, Yu Y, Hou Q, Zheng X, Zhang M, Zhang D, Li J, Wu XR, Huang C (2012) The Chinese herb isolate isorhapontigenin induces apoptosis in human cancer cells by down-regulating overexpression of antiapoptotic protein XIAP. J Biol Chem 287:35234–35243
Fang L, Gao H, Zhang W, Zhang W, Wang Y (2015) Resveratrol alleviates nerve injury after cerebral ischemia and reperfusion in mice by inhibiting inflammation and apoptosis. Int J Clin Exp Med 8:3219
Gray JJ, Bickler PE, Fahlman CS, Zhan X, Schuyler JA (2005) Isoflurane neuroprotection in hypoxic hippocampal slice cultures involves increases in intracellular Ca2+ and mitogen-activated protein kinases. Anesthesiology 102:606–615
Huang J, Upadhyay UM, Tamargo RJ (2006) Inflammation in stroke and focal cerebral ischemia. Surg Neurol 66:232–245
Hung L-M, Su M-J, Chen J-K (2004) Resveratrol protects myocardial ischemia–reperfusion injury through both NO-dependent and NO-independent mechanisms. Free Radic Biol Med 36:774–781
Jean WC, Spellman SR, Nussbaum ES, Low WC (1998) Reperfusion injury after focal cerebral ischemia: the role inflammation and the The rapeutic Horizon. Neurosurgery 43:1382–1396
Li H-L, Wang AB, Huang Y, Liu DP, Wei C, Williams GM, Zhang CN, Liu G, Liu YQ, Hao DL, Hui RT, Lin M, Liang CC (2005) Isorhapontigenin, a new resveratrol analog, attenuates cardiac hypertrophy via blocking signaling transduction pathways. Free Radic Biol Med 38:243–257
Lin Y, Chen F, Zhang J, Wang T, Wei X, Wu J, Feng Y, Dai Z, Wu Q (2013) Neuroprotective effect of resveratrol on ischemia/reperfusion injury in rats through TRPC6/CREB pathways. J Mol Neurosci 50:504–513
Liu Y, Liu G (2004) Isorhapontigenin and resveratrol suppress oxLDL-induced proliferation and activation of ERK1/2 mitogen-activated protein kinases of bovine aortic smooth muscle cells. Biochem Pharmacol 67:777–785
Ray PS, Maulik G, Cordis GA, Bertelli AA, Bertelli A, Das DK (1999) The red wine antioxidant resveratrol protects isolated rat hearts from ischemia reperfusion injury. Free Radic Biol Med 27:160–169
Shen M et al (2012) Resveratrol attenuates ischemia/reperfusion injury in neonatal cardiomyocytes and its underlying mechanism. PLoS One 7:e51223
Soriano SG, Coxon A, Wang YF, Frosch MP, Lipton SA, Hickey PR, Mayadas TN (1999) Mice deficient in mac-1 (CD11b/CD18) are less susceptible to cerebral ischemia/reperfusion injury. Stroke 30:134–139
Wang QL, Lin M, Liu GT (2001) Antioxidative activity of natural isorhapontigenin. Jpn J Pharmacol 87:61–66
Wang HY, Wang GL, Yu YH, Wang Y (2009) The role of phosphoinositide-3-kinase/Akt pathway in propofol-induced postconditioning against focal cerebral ischemia-reperfusion injury in rats. Brain Res 1297:177–184. https://doi.org/10.1016/j.brainres.2009.08.054
Wang J-K, Yu L-N, Zhang F-J, Yang M-J, Yu J, Yan M, Chen G (2010) Postconditioning with sevoflurane protects against focal cerebral ischemia and reperfusion injury via PI3K/Akt pathway. Brain Res 1357:142–151
Xu X, Chua CC, Gao J, Chua K-W, Wang H, Hamdy RC, Chua BH (2008) Neuroprotective effect of humanin on cerebral ischemia/reperfusion injury is mediated by a PI3K/Akt pathway. Brain Res 1227:12–18
Zhang X et al (2013) Cerebral ischemia-reperfusion-induced autophagy protects against neuronal injury by mitochondrial clearance. Autophagy 9:1321–1333
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Animal Care and Use Committee of The Affiliated Hospital of Jining Medical University approved all our experiments. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, X., Cui, X. Isorhapontigenin alleviates cerebral ischemia/reperfusion injuries in rats and modulated the PI3K/Akt signaling pathway. Naunyn-Schmiedeberg's Arch Pharmacol 393, 1753–1760 (2020). https://doi.org/10.1007/s00210-019-01794-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-019-01794-0