Abstract
Non-alcoholic steatohepatitis (NASH) is a more serious condition of non-alcoholic fatty liver disease (NAFLD), a widely spread type of chronic liver disease. Oxidative stress and inflammation induced by lipotoxicity are involved in hepatic injury of NASH. This work aimed to investigate the possible protective effect of adelmidrol (PPAR α and γ agonist) against NASH in mice.Thirty-six mice were divided into six equal groups: sham-operated, NASH, vehicle, adelmidrol 5 mg/kg, adelmidrol 10 mg/kg, and adelmidrol 20 mg /kg. Adelmidrol was injected intraperitoneally once daily for 7 weeks along with high-fat diet (HFD). Mice were euthanized, and livers were removed for light microscopic examination and detection of reduced glutathione (GSH) content while blood samples were collected for assessment of transaminases (ALT& AST), tumor necrosis factor (TNF-α), matrix metalloproteinase-1 (MMP-1), adiponectin, cholesterol, high-density lipoprotein (HDL), and triglyceride. NASH mice had increments in MMP-1, TNF-α, AST, ALT, triglyceride, and cholesterol levels while HDL, adiponectin levels, and GSH content were decreased with vesicular steatosis, lobular inflammation, hepatocyte ballooning, and degeneration. Administration of adelmidrol decreased MMP-1, TNF-α, AST, ALT, triglyceride, and cholesterol levels while increased HDL, adiponectin levels, and GSH content as well as ameliorated the histopathological changes. Adelmidrol protected mice from NASH; an effect could be attributed to its anti-inflammatory and antioxidant actions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction and aim
Non-alcoholic fatty liver disease (NAFLD) is the most common type of chronic liver disease (Uppal et al. 2016). NAFLD may be presented as fatty liver, in which fat accumulation does not induce damage to the liver or non-alcoholic steatohepatitis (NASH) associated with liver inflammation and scarring (Araújo et al. 2018).
The pathogenesis of NASH is complex. Oxidative stress and lipotoxicity are considered the main factors causing hepatic injury in NASH (Lizana et al. 2017). In addition, the immune system shares in NASH by various inflammatory mediators including endotoxins, inflammatory cytokines, and chemokines (Lebensztejn et al. 2016). These molecules are produced by hepatocytes, hepatic stellate cells (HSCs), portal fibroblasts, and immune cells such as neutrophils, macrophages, natural killer (NK) cells, and lymphocytes (Ganz and Szabo 2013). Interactions between liver cells and various immune cell populations in the liver were reported (Magee et al. 2016).
Adelmidrol is a semisynthetic diethanolamide derivative of azelaic acid (Nazzaro-Porro 1987) which belongs to the aliamide family (Aloe et al. 1993) and analogue to palmitoylethanolamide (PEA) which has anti-inflammatory and analgesic proprieties (Genovese et al. 2008). Adelmidrol is used in the treatment of skin inflammation. This compound could exert systemic anti-inflammatory action through activation of peroxisome proliferator-activated receptor (PPAR α and γ) and cannabinoid (CB2) receptor present mainly in immune cells as well as orphan GPR55 receptor (Cordaro et al. 2016).
The anti-inflammatory effect of adelmidrol was documented by reduction of pro-inflammatory mediators such as inducible nitric oxide synthetase (iNOS), tumor necrosis factor-α (TNF-α), and nitric oxide (De Filippis et al. 2011). In addition, adelmidrol inhibited nuclear factor kappa B (NF-kB) pathway in an autoimmune rat model (Impellizzeri et al. 2016).
Thus, the aim of this study was to investigate the possible protective effect of adelmidrol against high-fat diet-induced NASH in mice.
Materials and methods
Drugs and reagents
Adelmidrol was purchased from Sigma-Aldrich (St. Louis, MO). The required doses of adelmidrol used in the studies were dissolved in dimethyl sulfoxide (DMSO) immediately prior to use.
Animals and experimental design
Male CD1 albino mice (8–10 weeks old, 25–30 g) were obtained from the Faculty of Veterinary Medicine at Zagazig University. Mice were housed at constant environmental conditions (room temperature 23 ± 2 °C with a 12-h light/dark cycle). Mice were provided ad libitum access to standard rodent chow diet and filtered water. The mice were allowed to acclimate for 1 week prior to any use in experiments. All experimental procedures were approved by the local authorities, i.e., the Ethical Committee for Animal Handling at Zagazig University and were according to the guidelines set by the National Institutes of Health (USA). Mice were randomly divided into six groups (6 mice/group): Group 1 (sham-operated) served as control (10% calories from fat); group 2 (NASH) mice received high-fat diet (HFD) (71% fat, 11% carbohydrate, and 18% protein) according to Ching Lau et al. (2017) for 7 weeks; group 3 (vehicle) received DMSO 10 mg/kg by i.p. injection according to Hanslick et al. (2009) daily for 7 weeks; groups 4, 5, and 6 mice received 5, 10, and 20 mg adelmidrol/kg/day, respectively, intraperitoneal for 7 weeks given at the same time with HFD. Mice were weighed daily so that appropriate dosages could be delivered and also for follow-up. The doses of adelmidrol were selected according to Cordaro et al. (2016) who administered 10 mg/kg/day of adelmidrol for the assessment of its protective effect against ulcerative colitis in mice.
Induction of NASH
It was induced using a high-fat diet (40% calories from fat, 40% calories from carbohydrate, and 20% calories from protein) for 7 weeks (Siersbæk et al. 2017). Mice were euthanized by CO2 asphyxiation, and the left and median liver lobes as well as blood from the heart were then collected. The liver tissues in each group were subdivided into two equal sets; one was to be homogenized while the other was designated for histopathological studies. Serum samples were prepared from the collected blood for use in measures of alanine aminotransferase (ALT), aspartate aminotransferase (AST), tumor necrosis factor (TNF-α), matrix metalloproteinase 1 (MMP-1) lipid profile (triglyceride, cholesterol, and HDL), and adiponectin levels. The left and median hepatic lobes were washed with ice-cold saline, blot-dried, and then used for determination of reduced glutathione (GSH) content. In these assays, the liver samples were suspended in a phosphate buffer (50 mmol/L, pH 6) at 5 times the tissue volume and processed in a Potter-Elvehjem homogenizer. The raw homogenate was then aliquoted and frozen at 80 °C until used in the various assays (Helewski et al. 2010).
Determination of liver enzyme activities
Serum ALT and AST activities were assayed enzymatically using commercial kits purchased from Spinreact (Gerona, Spain) according to the method described by Reitman and Frankel (1957).
Determination of serum TNF-α level
Serum TNF-α level was measured by Mouse TNF-α PicoKine™ Elisa kit according to Bonavida (1991).
Determination of hepatic GSH content
Homogenates-reduced GSH content was determined by the colorimetric method according to Beutler et al. (1963).
Determination of MMP-1
Serum MMP-1 levels were measured by mouse matrix MMP-1 Elisa kit according to manufacturer instruction (Lenglet et al. 2014).
Determination of adiponectin
Serum adiponectin levels were measured by Mouse matrix Adipoq (Mouse) Elisa kit (Zhu et al. 2008).
Determination of lipid profile
The total plasma cholesterol, triglyceride, and HDL were measured by quantitative, enzymatic, and colorimetric determination in serum (Yang et al. 2012).
Histopathology
Dedicated specimens were taken from both left and median lobes of the liver, then fixed in 10% buffered formalin (pH 7.2), processed, and embedded in paraffin wax. Sections of 5-mm thickness were then generated and stained with H&E for subsequent light microscope examination (Bancroft and Gamble 2002).
Statistical analysis
One-way analysis of variance (ANOVA) was used for comparison of all groups. Least significant difference (LSD) was used for comparison of groups. All data are expressed as mean ± SD. Significance was accepted at p values < 0.05. The collected data were analyzed by computer using Statistical Package of Social Services version 22 (SPSS).
Results
Effect of adelmidrol on serum levels of ALT and AST
NASH significantly increased serum levels of ALT and AST compared with the sham group. ALT and AST levels in DMSO treated group were insignificant in relation to the NASH group. Adelmidrol (5, 10, and 20 mg/kg/day) produced dose-dependent significant reductions in ALT and AST levels as compared with NASH and DMSO groups (Table 1).
Effect of adelmidrol on TNF-α and MPP-1
NASH produced significant increments in serum levels of TNF-α and MPP-1 compared with the sham group. In DMSO treated group, TNF-α and MPP-1 levels were insignificantly changed in relation to NASH group. Adelmidrol (5, 10, and 20 mg/kg/day) produced dose-dependent significant reductions in TNF-α and MPP-1 levels in relation to NASH and DMSO groups (Table 2).
Effect of adelmidrol on adiponectin and GSH
Adiponectin serum level and hepatic GSH content were significantly reduced in the NASH group. In the DMSO-treated group, adiponectin level and GSH content were insignificantly different from the sham group. Adelmidrol (5, 10, and 20 mg/kg/day) significantly increased adiponectin level and GSH content as compared with the NASH and DMSO groups in a dose-dependent manner (Table 3).
Effect of adelmidrol on lipid profile
NASH resulted in significant increments in serum levels of triglyceride and cholesterol with significant decrease in serum HDL level compared with the sham group. DMSO treatment produced insignificant changes in the studied parameters in relation to the NASH group. Adelmidrol (5, 10, and 20 mg/kg/day) produced significant decrements in triglyceride and cholesterol levels with significant increase in HDL level as compared with the NASH and DMSO groups in a dose-dependent manner. (Table 4).
Effect of adelmidrol on total body weight
NASH resulted in significant increments in total body weight of mice compared with the sham group. DMSO treatment produced insignificant changes in relation to NASH group. Adelmidrol (5, 10, and 20 mg/kg/day) produced significant decrements in total body weight as compared with the NASH and DMSO groups in a dose-dependent manner (Table 5).
Histopathological results
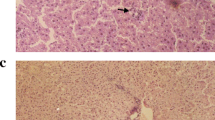
Histopathological findings showed normal hepatic architecture, normal hepatocytes with central nucleus and eosinophilic cytoplasm, normal central veins, no steatosis, no ballooning, and no hepatocyte degeneration (Fig. 1a). In the NASH group, the liver showed macro and microvesicular steatosis, lobular inflammation, hepatocyte ballooning, and degeneration of many hepatocytes (b). The DMSO group showed similar histopathological findings to the NASH group (c). Adelmidrol (5, 10, and 20 mg/kg) treated groups showed decrements in the number of inflammatory cells, degree of steatosis with less prominent hepatocyte ballooning, and degeneration. These effects were dose-dependent (5 mg/kg (d), 10 mg/kg (e), 20 mg/kg (f).
a Photomicrograph of normal liver tissue showing three central veins surrounded by rows of hepatocytes with no hepatocyte ballooning or degeneration (sham group) (H&E × 200). b Photomicrograph of liver tissue showing dilated congested central vein surrounded by heavy aggregates of inflammatory cells (NASH group) (H & E × 400). c Photomicrograph of liver tissue showing severe fatty changes of the hepatocytes with vacuolated cytoplasm and signet-ring nuclei (DMSO group) (H & E × 400). d Photomicrograph of the liver tissue showing fatty changes of hepatocytes and scattered inflammatory cells (adelmidrol 5 mg) (H & E × 400). e Photomicrograph of liver tissue showing mild steatosis (grade 0), moderate lobular inflammation (grade 2), ballooning degeneration of many hepatocytes (adelmidrol 10 mg) (H & E × 400). f Photomicrograph of liver tissue showing minimal steatosis, mild lobular inflammation, ballooning degeneration of few hepatocytes (adelmidrol 20 mg) (H & E × 400)
Discussion
Adelmidrol is an analogue to palmitoylethanolamide (PEA) which has anti-inflammatory and antinociceptive proprieties (Genovese et al. 2008). PEA exerts systemic anti-inflammatory action through activation of PPAR α& γ receptors as well as CB2 and orphan GPR55 receptors (Cordaro et al. 2016).
Adelmidrol anti-inflammatory effect through different receptor systems, namely PPAR α& γ and CB2 receptors (Impellizzeri et al. 2016). Cordaro et al. (2016) showed that adelmidrol’s protective effect against ulcerative colitis was mainly through PPAR γ as its effect was abolished with the PPAR γ blocker GW 9662 but not abolished either with CB2 blocker, SR144528, or in PPAR α knockout mice. This result cope also with Impellizzeri et al. (2016) who showed that adelmidrol’s protective effect in collagen-induced arthritis was mainly through PPAR γ pathway confirmed as the PPAR γ blocker GW 9662 abolished its effect but not the PPAR α-blocker GW 6471.
The results of the present study showed that administration of adelmidrol with three different doses (5, 10, 20 mg/kg) together with high-fat diet caused significant reduction of the level of hepatic transaminases, AST and ALT, as well as improvement in the histopathological picture of the liver. Adelmidrol reduced the levels of MMP-1 and TNF-α while increased the hepatic GSH content and adiponectin level in serum with the best results obtained with the largest dose.
TNF-α is one of the proinflammatory cytokines released from Kupffer cells to enhance neutrophil recruitment with subsequent hepatocytes damage (Lentsch et al. 2000). Indeed, TNF-α activates proteases like caspase-3 and caspase-8 with subsequent DNA destruction and apoptosis (Arumugam et al. 2004).
TNF-α binds to TNF receptor forming TNFR1 complex, which mediates the activation of the nuclear factor kappa beta (NF-ĸβ) pathway which induce as a result expression of ROS generating enzymes and thus there is a strong relationship between oxidative stress, lipid peroxidation, and the inflammatory cytokine TNF-α (Fischer and Maier 2015).
The present histopathological results showed that NASH was associated with disturbed histopathological picture of the liver in the form of hepatocyte inflammation, fatty infiltration, degeneration, and ballooning. These results are in accordance with Takahashi and Fukusato (2014) who reported the presence of steatosis, lobular inflammation, and hepatocellular ballooning in NAFLD. Adelmidrol administration improved the histopathological picture in a dose-dependent manner. These results cope with Jain et al. (2018) who showed that dual PPARα/γ agonist, saroglitazar, improved liver histopathology in experimental NASH models.
In the present results, NASH was associated with decrease in HDL level. These results are consistent with Jensen et al. (2018) who showed decrease in HDL level in a rat model of NAFLD. The present results showed decrements in adiponectin serum level and hepatic GSH content. These findings cope with Sandhya et al. (2010) who reported that NAFLD was associated with reduced level of GSH content in the liver and adiponectin level in serum. NASH group in the present work showed increased MPP-1 level. Similar results obtained by Okazaki et al. (2014) who found increased level of matrix metalloproteinase in NASH.
The present findings showed that adelmidrol decreased the AST, ALT, triglyceride, and cholesterol levels while increased HDL and adiponectin level. Mattace et al. (2014) stated that PEA, adelmidrol one of its analogues, showed significant decrease in the level of ALT in mice model of diet-induced obesity. Nakaya et al. (2011) confirmed our result as GW7647, the potent PPAR-α agonist, the main receptor of adelmidrol, increased the level of HDL and promoted macrophage reverse cholesterol transport to inhibit atherosclerosis.
Adiponectin is a protein hormone which is involved in regulating glucose levels as well as fatty acid breakdown (Maeda et al. 1996). It associates negatively with obesity and its comorbidities (Arita et al. 1999); therefore, hypoadiponectinemia serves as a marker of adipose tissue dysfunction and the metabolic syndrome (Trujillo and Scherer 2005). Adiponectin exerts insulin-sensitizing and anti-inflammatory actions in liver and muscle cells and has vasoprotective effects. It binds at the cellular level to two specific receptors, adiponectin receptor 1 (AdipoR1) and adiponectin receptor 2 (AdipoR2), which provokes an intracellular activation of PPAR-α receptor (Peter et al. 2013).
The present study showed that adelmidrol increased GSH content in the liver and decreased TNF-α. Indeed, GSH is a free radical scavenger to protect the liver against oxidative damage (Koken and Inal 1999). Previous work done by Impellizzeri et al. (2016) showed that adelmidrol decreased the level of TNF-α in mice model of autoimmune arthritis through PPAR α and γ activation.
Adelmidrol treatment resulted in decreased MPP-1 level, and this is in accordance with Di Paola et al. (2016) who studied the effect of adelmidrol against osteoarthritis and reported a decrease in MMP-1 level. Indeed, metalloproteinase is an enzyme produced by fibroblasts, chondrocytes, macrophages, keratinocytes, endothelial cells, and osteoblasts and is implicated in a wide variety of inflammatory reactions where collagen degradation occurs (Smith 2006).
Adelmidrol treatment resulted in decreased total body weight of mice, and this is in accordance with Mattace et al. (2014) showing that treatment with PEA (30 mg/kg) given subcutaneously for 5 weeks decreased body weight and fat mass in a rat model of ovariectomized rats mainly through activation of PPAR γ and α and also with Barbosa-da-Silva et al. (2015) who reported that treatment with bezafibrate (100 mg/kg), pan PPAR agonist, and WY-14,643 (3 mg/kg), PPAR-α agonist combined into the diet for 4 weeks caused reduction of both body and liver mass in a mice model of NASH.
Conclusion
Adelmidrol protected against high-fat diet induced NASH. This effect might be attributed partially to its anti-inflammatory and antioxidant actions.
References
Aloe L, Leon A, Levi-Montalcini R (1993) A proposed autacoid mechanism controlling mastocyte behaviour. Agents Actions 39:145–147
Araújo AR, Rosso N, Bedogni G, Tiribelli C, Bellentani S (2018) Global epidemiology of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: what we need in the future. Liver Int 38(1):47–51
Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K (1999) Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257(1):79–83
Arumugam TV, Shiels IA, Woodruff TM, Granger DN, Taylor SM (2004) The role of the complement system in ischemia-reperfusion injury. Shock 21(5):401–409
Bancroft JD, Gamble M (2002) Theory and practice of histological techniques, 5th edn. Churchill Livingstone pub.: 172-5, Edinburgh, pp 593–620
Barbosa-da-Silva S, Souza-Mello V, Magliano DC, Marinho T, Aguila MB, Mandarim-de-Lacerda CA (2015) Singular effects of PPAR agonists on nonalcoholic fatty liver disease of diet-induced obese mice. Life Sci 127:73–81
Beutler E, Duron O, Kelly MB (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Bonavida B (1991) Immunomodulatory effect of tumor necrosis factor. Biotherapy 3:127–133
Ching Lau JK, Zhang X, Yu J (2017) Animal models of non-alcoholic fatty liver disease: current perspectives and recent advances. J Pathol 241:36–44
Cordaro M, Impellizzeri D, Gugliandolo E, Siracusa R, Crupi R, Esposito E, Cuzzocrea S (2016) Adelmidrol, a palmitoylethanolamide analogue, as a new pharmacological treatment for the management of inflammatory bowel disease. Mol Pharmacol 90(5):549–561
De Filippis D, Luongo L, Cipriano M, Palazzo E, Cinelli MP, de Novellis V, Maione S, Iuvone T (2011) Palmitoylethanolamide reduces granuloma-induced hyperalgesia by modulation of mast cell activation in rats. Mol Pain 7:3
Di Paola R, Fusco R, Impellizzeri D, Cordaro M, Britti D, Morittu VM, Evangelista M, Cuzzocrea S (2016) Adelmidrol, in combination with hyaluronic acid, displays increased anti-inflammatory and analgesic effects against monosodium iodoacetate-induced osteoarthritis in rats. Arthritis Res Ther 18(1):291
Fischer R, Maier O (2015) Interrelation of oxidative stress and inflammation in neurodegenerative disease: role of TNF. Oxidative Med Cell Longev 2015(610813)
Ganz M, Szabo G (2013) Immune and inflammatory pathways in NASH. Hepatol Int 7(2):771–781
Genovese T, Esposito E, Mazzon E, Di Paola R, Meli R, Bramanti P, Piomeli D, Calignano A, Cuzzocrea S (2008) Effects of palmitoylethanolamide on signaling pathways implicated in the development of spinal cord injury. J Pharmacol Exp Ther 326:12–23
Hanslick JL, Lau K, Noguchi KK, Olney JW, Zorumski CF, Mennerick S, Farber NB (2009) Dimethyl sulfoxide (DMSO) produces widespread apoptosis in the developing central nervous system. Neurobiol Dis 34(1):1–10
Helewski KJ, Kowalczyk-Ziomek GI, Czecior E, Swietochowska E, Wielkoszynski T, Czuba ZP, Szliszka E, Krol W (2010) Administration of low doses of TNFa protects rat liver from ischemic damage and re-perfusion injury. J Physiol Pharmacol 61:273–278
Impellizzeri D, Di Paola R, Cordaro M, Gugliandolo E, Casili G, Morittu VM, Britti D, Esposito E, Cuzzocrea S (2016) Adelmidrol, a palmitoylethanolamide analogue, as a new pharmacological treatment for the management of acute and chronic inflammation. Biochem Pharmacol 119:27–41
Jain MR, Giri SR, Bhoi B, Trivedi C, Rath A, Rathod R, Ranvir R, Kadam S, Patel H, Swain P, Roy SS, Das N, Karmakar E, Wahli W, Patel PR (2018) Dual PPARα/γ agonist saroglitazar improves liver histopathology and biochemistry in experimental NASH models. Liver Int 38(6):1084–1094
Jensen S, Hvid H, Damgaard J, Nygaard H, Ingvorsen C, Wulff EM, Lykkesfeldt J, Fledelius C (2018) Dietary fat stimulates development of NAFLD more potently than dietary fructose in Sprague–Dawley rats. Diabetol Metab Syndr 10:4
Koken T, Inal M (1999) The effect of nitric oxide on I/R injury in rat liver. Clin Chim Acta 288:55–62
Lebensztejn DM, Flisiak-Jackiewicz M, Białokoz-Kalinowska I, Bobrus-Chociej A, Kowalska I (2016) Hepatokines and non-alcoholic fatty liver disease. Acta Biochim Pol 63(3):459–467
Lenglet S, Montecucco F, Mach F, Schaller K, Gasche Y, Copin JC (2014) Analysis of the expression of nine secreted matrix metalloproteinases and their endogenous inhibitors in the brain of mice subjected to ischaemic stroke. Thromb Haemost 112(2):363–378
Lentsch A, Kato A, Yoshidome H, McMasters K, Edwards M (2000) Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology 32(2):169–173
Lizana P, Galdames M, Rodrigo R (2017) Oxidative stress and endoplasmic reticulum stress as potential therapeutic targets in non-alcoholic fatty liver disease. React Oxygen Species 4(10):266–274
Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K (1996) cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose most abundant gene transcript 1). Biochem Biophys Res Commun 221(2):286–289
Magee N, Zou A, Zhang Y (2016) Pathogenesis of nonalcoholic steatohepatitis: interactions between liver parenchymal and nonparenchymal cells. Biomed Res Int 2016:5170402
Mattace RG, Santoro A, Russo R, Simeoli R, Paciello O, Di Carlo C, Diano S, Calignano A, Meli R (2014) Palmitoylethanolamide prevents metabolic alterations and restores leptin sensitivity in ovariectomized rats. Endocrinology. 155(4):1291–1301
Nakaya K, Tohyama J, Naik SU, Tanigawa H, MacPhee C, Billheimer JT, Rader DJ (2011) Peroxisome proliferator-activated receptor activation promotes macrophage reverse cholesterol transport through a liver X receptor–dependent pathway. Arterioscler Thromb Vasc Biol 31(6):1276–1282
Nazzaro-Porro M (1987) Azelaic acid. J Am Acad Dermatol 17:1033–1041
Okazaki I, Noro T, Tsutsui N, Yamanouchi E, Kuroda H, Nakano M, Yokomori H, Inagaki Y (2014) Fibrogenesis and carcinogenesis in nonalcoholic steatohepatitis (NASH): involvement of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinase (TIMPs). Cancers (Basel) 6(3):1220–1255
Peter MS, Markus R, Christian S, Christoph B, Guenter AR, Andreas L, Dierk HE (2013) Antidiabetic treatment restores adiponectin serum levels and APPL1 expression, but does not improve adiponectin-induced vasodilation and endothelial dysfunction in Zucker diabetic fatty rats. Cardiovasc Diabetol 12:46
Reitman S, Frankel S (1957) A colorimetricmethod for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28(1):56–63
Sandhya N, Gokulakrishnan K, Ravikumar R, Mohan V, Balasubramanyam M (2010) Association of hypoadiponectinemia with hypoglutathionemia in NAFLD subjects with and without type 2 diabetes. Dis Markers 29(5):213–221
Siersbæk M, Varticovski L, Yang S, Baek S, Nielsen R, Mandrup S, Hager GL, Chung JH, Grøntved L (2017) High fat diet-induced changes of mouse hepatic transcription and enhancer activity can be reversed by subsequent weight loss. Sci Rep 7:40220
Smith GN (2006) The role of collagenolytic matrix metalloproteinases in the loss of articular cartilage in osteoarthritis. Front Biosci 11:3081–3095
Takahashi Y, Fukusato T (2014) Histopathology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol 20(42):15539–15548
Trujillo ME, Scherer PE (2005) Adiponectin–journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med 257(2):167–175
Uppal V, Mansoor S, Furuya K (2016) Pediatric non-alcoholic fatty liver disease. Curr Gastroenterol Rep 18(5):24
Yang L, Fan B, Yang K, Zhu H (2012) A simple and sensitive method for lipoprotein and lipids profiles analysis of individual micro-liter scale serum samples. Chem Phys Lipids 165(2):133–141
Zhu W, Cheng KK, Vanhoutte PM, Lam KS, Xu A (2008) Vascular effects of adiponectin: molecular mechanisms and potential therapeutic intervention. Clin Sci 114(5):361–374
Author information
Authors and Affiliations
Contributions
M.M.N. and N.A.T. performed the research. M.I.K. and A.A.A. designed the research study. A.A.A. and M.M.N. analyzed the data. M.M.N. wrote the paper. A.A.A. and M.I.K. read and approved the manuscript.
Corresponding author
Ethics declarations
All experimental protocols were approved by the Ethics Committee of Zagazig University. The study was approved by the local Animal Ethical Committee of Zagazig University, Egypt. All experimental procedures were carried out in accordance with the guidelines set forth by the National Institutes of Health (USA).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nageeb, M.M., Khatab, M.I., Abdel-sameea, A.A. et al. Adelmidrol protects against non-alcoholic steatohepatitis in mice. Naunyn-Schmiedeberg's Arch Pharmacol 393, 777–784 (2020). https://doi.org/10.1007/s00210-019-01785-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-019-01785-1