Abstract
In this study, the effect of diosmin against the adverse effects of lead exposure in rats was investigated. Wistar Albino race 40 male rats weighing 150–200 g 2–3 months were used. A total of 4 groups were assigned, one of which was control and the other 3 were trial groups. The rats in the control group were treated with dimethyl sulfoxide, which was used only as a vehicle in diosmin administration. Groups 2, 3, and 4 from the experimental group were given diosmin at a dose of 50 mg/kg.bw, lead acetate at the dose of 1000 ppm, lead acetate at the dose of 1000 ppm, and diosmin at a dose of 50 mg/kg.bw for 6 weeks, respectively. Application of lead acetate with drinking water and also diosmin was performed by oral catheter. At the end of the experimental period, blood was taken to dry and with heparin by puncture to the heart under light ether anesthesia. Following the blood samples, some organs of the rats (the liver, kidney, brain, heart, and testis) were removed. Some biochemical parameters (glucose, triglyceride, cholesterol, BUN, creatinine, uric acid, LDH, AST, ALT, ALP, total protein, albumin) were measured in serum. Some oxidative stress parameters in tissue samples and blood (MDA, NO, SOD, CAT, GSH-Px, GSH) were evaluated. Body and organ (the liver, kidney, brain, heart, and testis) weights were also evaluated at the end of the study. No significant change was observed in the parameters examined in the diosmin alone-treated group by comparison to control group. On the other hand, significant changes were found in the values of lead acetate-treated group comparing control group. It was observed that the values approached the values of the control group in the combination of lead and diosmin. Exposure to lead acetate at a dose of 1000 ppm for 6 weeks causes organ damage; however the diosmin application at a dose of 50 mg/kg.bw had a positive effect on the regression of tissue damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lead (Pb) is included in the heavy metals whose atomic number is 82. It is a durable metal (Rusyniak et al. 2010; Jomova and Valko 2011) and is used in many industrial applications due to its many physical-chemical properties (Gottesfeld and Pokhrel 2011). The inorganic lead is a cumulative compound mainly absorbed by the lungs and gastrointestinal tract; except for organic lead, its absorption from the skin is very low. Respiratory tract is the most important pathway for workers and 4–50% of inhaled lead enters the bloodstream. Particle size is important in inhalation absorption. Approximately 10% of the dietary intake is absorbed (Sakai 2000; Sanborn et al. 2002; Papanikolaou et al. 2005; Patrick 2006a). Gastrointestinal lead transmission is higher in offspring than in adults. Gastric bowel absorption and retention, structure, and composition of the gastrointestinal cavity vary depending on age and the iron content of the diet (nutritional status of the person) and the chemistry of the environment. Some dietary components (such as ascorbic acid, amino acids, vitamin D, protein, fat, and lactose) increase the absorption of lead. In addition, the exposure reduces the absorption of iron, calcium, and phosphorus or zinc from the intestine (Furst 2002; Ros and Mwanri 2003; Papanikolaou et al. 2005; Alissa and Ferns 2011). Blood lead levels account for 1–5% of the total body weight, especially in the body accumulates in bones and other calcified tissues. Plasma lead concentration is lower than the target organs (i.e., the brain, lungs, spleen, renal cortex, teeth, and bones) (Miller et al. 1983; Papanikolaou et al. 2005). Exposure to lead affects target organ/systems. Exposure to this metal/metalloid triggers a number of common mechanisms underlying toxicity, including oxidative stress activation, reaction with sulfhydryl groups, and interaction with basic metals. In particular, lead binds to enzymes having sulfhydryl groups and causes imbalance of oxidant/antioxidant. It inhibits the activity of delta-aminolevulinic acid dehydratase and glutathione reductase from specific sulfhydryl-containing enzymes. This metal affects the activity of glutathione reductase, glutathione peroxidase, and glutathione-S-transferase (Ercal et al. 2001; Wang and Fowler 2008; Sanders et al. 2009; Hirsch et al. 2010). Lead exposure causes free radical damage in different ways. This can be expressed as the production of reactive oxygen species (ROS), including singlet oxygen and hydrogen peroxide, and depletion of antioxidant natural component reserves. In the biological system where ROS production increased, antioxidant reserves were depleted simultaneously (Patrick 2006a, b; Flora et al. 2012).
Diosmin (C28H32O15, diosmetin 7-O-rutinoside), a part of the flavonoid family, hesperidin derivative, is a natural flavone glycoside and is found in the abundance on the outer shell of various citrus fruits. This flavon is easily obtained by dehydrogenation of glycoside hesperidin and 608.545 g/mol of molecular weight. Diosmin was isolated from Scrophularia nodosa L. in 1925 and was introduced therapeutically in 1969. Diosmin is different from hesperidin by the presence of a double bond between two carbon atoms in the C-ring of the molecule, which contains a sugar molecule bound to the tri-ring flavonoid structure. In addition, diosmin may be produced following the extraction of hesperidin, and this is in the form of the conversion of hesperidin to diosmin (Bogucka-Kocka et al. 2013; Patel et al. 2013; Srinivasan and Pari 2013; Suică-Bunghez et al. 2015; Bertozzi et al. 2017; Buddhan and Manoharan 2017; Mirshekar et al. 2017). Diosmin rapidly converts to diosmetin in the form of aglycone in the intestinal flora. Diosmetin has a plasma half-life of 26 to 43 h. Diosmetin is reduced to phenolic acids or to their glycine-conjugated derivatives and is eliminated by urine (Ramelet 2001; Srinivasan and Pari 2012; Giannini et al. 2015; Russo et al. 2015).
Although there are studies on the effects of other flavonoids against the potential of lead to oxidative stress of lead/lead acetate (Wang et al. 2012, 2013; Ozkaya et al. 2016, 2018; Aksu et al. 2017), it has not been found that the study of investigation of the possible effect of diosmin in cases of poisoning with this metal in question. Studies on alternative treatment options against lead intoxication include the use of the chelating agent (Llobet et al. 1990; Tandon et al. 1997; Smith and Strupp 2013) which is often preferred to remove the systemic circulating component. However, chelating agents are also known to have individual toxic/adverse effect (Jaffe et al. 1968; Kean et al. 1980; Aposhian 1983, Aposhian et al. 1995; Sachan et al. 1996; Mehta and Flora 2001; Mehta et al. 2002; Blanusa et al. 2005; Flora and Mehta 2003; Flora et al. 2004, 2007a, b; Bradberry and Vale 2009; Cao et al. 2015; Amadi et al. 2019) and cause oxidative stress (Mehta and Flora 2001; Mehta et al. 2002; Flora and Mehta 2003; Flora et al. 2004, 2007a, b). Since lead causes oxidative stress (Wang et al. 2012, 2013; Ozkaya et al. 2016; Aksu et al. 2017) and it is among the poisoning mechanisms of the metal (Jomova and Valko 2011; Rashid et al. 2013; Wani et al. 2015; Valko et al. 2016; Mitra et al. 2017), especially in terms of determining new treatment options against these negative effects of lead, it is very important to investigate the possible effects of diosmin, which has strong antioxidant properties (Silambarasan and Raja 2012; Barreca et al. 2013; Hajimahmoodi et al. 2014; Naso et al. 2016). In this respect, the study is original. In addition, the results will be significant in terms of shedding light on future studies. For this purpose, animals were given alone and together with lead acetate and diosmin for 6 weeks. After the compound application to animals is finished, some biochemical parameters and oxidative stress parameters in blood and tissue samples were evaluated. Body and tissue weights were taken at the end of the study. Thus, the effect of diosmin against the adverse effects of lead acetate exposure with all the parameters expressed was investigated. In particular, in this study, the effect of diosmin antioxidant/radical scavenging activity on lead poisoning process against high levels of free radicals produced by said metal in rats will be examined.
Material and method
Instruments, chemicals, and kits
The instruments and materials used in the study consist of UV-VIS spectrophotometer (Thermo Helios Alpha Double-beam), cooled centrifuge (Sigma 3K30), and mechanical homogenizer (Heidolph Silent Crusher M). In the experiment, diosmin (Sigma D3525) and lead acetate (Sigma 316512) were obtained from Sigma-Aldrich, St. Louis, MD, USA. The chemicals used in the analysis of oxidative stress parameters are of Merck KGaA, Darmstadt, Germany, and Sigma-Aldrich, St. Louis, MD, USA. Serum biochemical parameters were measured by autoanalyzer. The devices and kits used are the same brand (Cobas, Roche Diagnostics, Rotkreuz, Switzerland).
Animal material
Total 40 Wistar Albino race, 150–200 g weight, 2–3 months old male rats were used in the study. The animals were equally divided into 4 groups. The animals were kept in stable conditions (22–24 °C 12 h light/dark). A maximum of 4 rats were placed in each of the cages where the animals were housed (in the polyethylene structure). Therefore, three cages with the same characteristics were used for each group. The feeds (23% crude protein, 7% crude cellulose, and pellet feed containing 3100 kcal/kg metabolic energy) and drinking water were maintained in front of them throughout the trial.
Group 1 was kept as control, and the animals were given only feed, water, and vehicle (dimethyl sulfoxide (DMSO)/deionized water; 4/6; 5 ml/kg.bw volume).
Group 2 was administered to the rats at the oral dose of diosmin 50 mg/kg.bw (in DMSO) once daily for 6 weeks.
Group 3, drinking water containing lead acetate at 1000 ppm was continuously maintained for 6 weeks in front of the rats, and drinking water containing lead acetate was renewed every day.
Group 4, 1000 ppm dose of lead acetate containing drinking water was kept in front of the rats for 6 weeks, and the drinking water was renewed every day. At the same time, the rats were administered once a day on the same day with a catheter at a dose of 50 mg/kg.bw (in DMSO) for 6 weeks. In the determination of the dose of lead acetate (Kang et al. 2009; Asadpour et al. 2013; Samarghandian et al. 2013) and diosmin (Jain et al. 2014; Eraslan et al. 2017) used in the study, the previous studies were based.
Daily water consumption was also detected in all groups during the study, and no significant difference was found between the groups during the trial. The average daily water consumption during trial periods for groups 1, 2, 3, and 4 is 112.4 ml/kg bw/24 h, 111.6 ml/kg bw/24 h, 111.9 ml/kg bw/24 h, and 112.3 ml/kg bw/24 h, respectively.
Collection of samples
At the end of the 6th week of administration of lead acetate and diosmin, the animal was taken to light ether anesthesia and to heart was entered and blood samples were taken to the anticoagulant (lithium heparin)/dry tubes. After cervical dislocation, the liver, kidney, brain, heart, and testes were removed. The body weights were taken before the end of the study, and the weights of the organs were taken after euthanasia.
Washing of erythrocytes and preparation of hemolysate
The blood in the anticoagulant tubes was centrifuged and the plasmas were taken. After removal of the platelet and leukocyte layer of the part containing the blood-shaped elements, the erythrocyte compartment was vortexed by mixing with saline phosphate buffer solution (pH 7.4), centrifuged with 3000 rpm for 10 min, and then washed three times. The remaining erythrocyte layer was mixed gently with the same volume of saline phosphate buffer solution (Winterbourn et al. 1975). The prepared samples were placed in the freezer (-80 °C). Before starting the analyses, samples were allowed to thaw at room temperature and then hemolyzed with 1/5 ice-cold saline buffer. Prepared hemolysate was used to evaluate the amount of hemoglobin and catalase (CAT) and glutathione peroxidase (GSH-Px) activities. At the same time, the remaining hemolysate was vortexed with chloroform/ethanol (6/10 v/v) by rotating for 10 min in a cooled centrifuge (+4 °C) set at 3000 rpm, and the upper phase was used for the measurement of superoxide dismutase (SOD) activity.
Preparation of tissue homogenate
After removing the organs of the animals in all groups, they were cleaned with ice-cold saline phosphate buffer and purified from blood and connective tissue. One gram of the all tissues were homogenized in 4 ml of buffer solution and adjust the pH to 7.2 with 5 N NaOH and 1000 ml for 30 sec at 20000 rpm with the help of mechanical homogenizer. The homogenate was rotated in the Eppendorf tube at 15000 rpm for 45 min, and the supernatant was transferred to separate Eppendorf tubes.
Oxidative stress parameters
In the determination of plasma malondialdehyde (MDA) concentration, the method defined by Yoshioka et al. (1979) was preferred. The measurement of tissue MDA levels was based on the method reported by Ohkawa et al. (1978). Absorbances read was calculated using the 1.1.3.3 tetraethoxypropane prepared curve as the nmol/ml and nmol/mg-protein. In the measurement of erythrocyte hemoglobin amount, the method presented by Fairbanks and Klee (1987) was used. The data obtained were evaluated in terms of mgHb/ml hemolysate. In the measurement of tissue protein level, the method modified by Miller (1959) was used based on the method of Lowry et al. (1951). Quantification of absorbance readings was calculated as mg-protein/ml supernatant. In the measurement of erythrocyte and tissue CAT activity, Luck’s (1965) method was used. The data were calculated in k/gHb and k/g-protein. The analysis of SOD activity in the samples was performed according to the method described by Sun et al. (1988). The absorbances obtained were evaluated as U/mgHb and U/mg-protein. GSH-Px activity in test samples was performed by method of Paglia and Valentine (1967). The absorbance values obtained were calculated as μmol NADPH+/min/gHb and μmol NADPH+/min/g-protein. Nitric oxide (NO) levels in the samples were determined by the method described by Tracey et al. (1995). The results were calculated as nmol/ml or nmol/mg-protein. Beutler (1975) method was used in the analysis of tissue glutathione (GSH) level. The results obtained were calculated in nmol/ml or nmol/mg-protein.
Analysis of some biochemical parameters
The determination of serum glucose, triglyceride, cholesterol, blood urea nitrogen (BUN), creatinine, uric acid, lactate dehydrogenase (LDH), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total protein, and albumin levels/activities, Roche Cobas brand kit, and the same brand autoanalyzer were used for detection.
Statistical calculations
SPSS 13.0 was used as the statistical program for the statistical calculation. Evaluations were given as arithmetic mean and standard deviation. One-way analysis of variance (ANOVA) and Tukey test (based on p < 0.05) were used to determine the differences between the groups.
Results
Diosmin alone-applied group
Biochemical and oxidative stress parameters examined, body weight, organ weight, and organ weight/body weight ratio of diosmin-treated group did not change significantly compared to the control group (Table 1, 2, 3, 4, 5, 6, 7 and 8).
Lead acetate alone-applied group
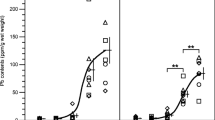
There was a significant increase in MDA and NO levels of all the plasma/tissues examined, while a decrease of the plasma GSH levels and erythrocyte SOD, CAT, and GSH-Px activities was observed in the animals treated with lead acetate compared to the control group. The activity of liver SOD and GSH-Px and the levels of GSH were decreased, but CAT activity was increased. Elevation of SOD and CAT activity and decrease of GSH-Px and GSH level/activity were observed in renal tissue. There was an increase in brain tissue CAT activity and a decrease in SOD and GSH-Px activity and GSH levels. SOD, CAT, GSH-Px activity, and GSH levels were decreased in cardiac tissue. There was a decrease in SOD, GSH-Px, and GSH activity/level of testis tissue, while there was an increase in CAT activity (Tables 1, 2, 3, 4, 5, 6). Reduction of glucose, total protein, and albumin levels in biochemical parameters in the lead acetate-applied group according to the control group and an increase in triglyceride, cholesterol, BUN, creatinine, uric acid, LDH, AST, ALT, and ALP activity/level were observed. While there was a rising in the weight of liver, kidney, and heart tissue, the decrease in the weight of brain and testicular tissue was determined (Table 7). There is an increase in the ratio of organ/body weight of the liver, kidney, heart, and testis. A meaningful decrease in body weight was also showed (Table 8).
Group applied the combination of diosmin and lead acetate
The obtained values converge towards the control group. According to the control, important changes are observed in the activity of erythrocyte CAT, the activity of liver SOD, renal NO level and GSH-Px activity, brain GSH level, heart SOD, CAT, GSH-Px activities and GSH level, testis SOD, and CAT activity (Tables 1, 2, 3, 4, 5, 6). There are significant changes in cholesterol level and LDH activity from biochemical parameters (Table 7). There are also significant differences in the organ weight, organ weight/body weight ratio of the kidney tissue and organ weight/body weight ratio of testis, and the body weight (Table 8).
When the values of lead acetate-treated group were compared with lead acetate plus diosmin-treated group, plasma MDA, NO and GSH levels, and erythrocyte SOD and CAT activities; liver MDA, NO, CAT, and GSH-Px activities/levels; kidney MDA, NO, SOD, CAT, and GSH-Px activities/levels; brain MDA, SOD, CAT, GSH-Px, and GSH levels/activities; heart MDA, NO, SOD, and GSH-Px activities/levels; and testis SOD, CAT, GSH-Px, and NO levels/activities have significant differences (Tables 1, 2, 3, 4, 5, 6). Evident changes were observed in uric acid, LDH, AST, ALT, and total protein levels/activities (Table 7). A meaningful difference was found in the weight of the liver and testis; the liver, kidney, and heart weight/body weight ratio; and body weight (Table 8).
Discussion
Some of the known biochemical mechanisms of lead toxicity are the effects of antioxidant defense system components and lead to oxidative damage by causing degradation in the pro-oxidant/antioxidant balance. As a result of lead exposure, changes in hepatic cholesterol metabolism, lipid peroxidation as a result of increased ROS production, suppression of antioxidant enzymes activity, decrease in GSH levels, mitochondrial dysfunction, oxidative DNA damage, and ultimately apoptosis are observed (Gurer and Ercal 2000; Hsu and Guo 2002). Oxidative stress is one of the known pathways of the mechanism of lead exposure/toxicity at the molecular level. This occurs when one or more unpaired electron structures of free radicals exceed the capacity of antioxidant defense mechanisms that provide protection against the harmful effects of free radicals. Along with the depletion of glutathione and other sulfhydryl groups, changes in the activity of various antioxidant enzymes that prevent lipid peroxidation play an important role in lead-induced oxidative tissue damage. In the case of lead exposure, superoxide ions, hydroxyl radicals, and hydrogen peroxide production are accelerated from free radicals. Lead has toxic effects on cell membrane structure and function. The effects on the erythrocyte membrane are highly sensitive to lead. Another mechanism for lead-induced membrane oxidative damage is its effect on changes in membrane fatty acid composition. Since there is a correlation between fatty acid chain length and unsaturation and peroxidation and membrane sensitivity, the increase in arachidonic acid leads to an aggravation of membrane lipid peroxidation (Croft 1998; Ercal et al. 2001; Firuzi and Miri 2011; Jomova and Valko 2011; Flora et al. 2012; Carocci et al. 2016). The most commonly used parameters for the evaluation of lead poisoning are the GSH level and the antioxidant enzymes SOD, CAT, and GSH-Px. GSH-Px, CAT, and SOD known as also metalloproteins show antioxidant effect by detoxifying peroxides (-OOH), hydrogen peroxide (H2O2), and single oxygen (1O2), enzymatically. The CAT converts H2O2 into H2O and O2, respectively. It takes an important role in the degradation of H2O2. GSH-Px requires GSH for the degradation of peroxides and serves to decompose lower and less stable H2O2. These antioxidant enzymes are potential targets for lead toxicity. The other is SOD and there are two subtypes, MnSOD and CuSOD. There is a close relationship between SOD and low copper levels in the blood. Exposure to lead leads to a decline in the level of copper and consequently indirectly decreases SOD activity (Croft 1998; Firuzi and Miri 2011; Carocci et al. 2016). One of the effects of lead exposure is glutathione metabolism. Glutathione is a tripeptide containing a reactive -SH group having a reducing power. Accordingly, GSH plays a vital role to protect living cells or tissues against oxidative stress/lipid peroxidation. The direct interaction of the -SH groups with ROS acts as a nonenzymatic antioxidant or is involved in enzymatic detoxification reactions as a cofactor/coenzyme for ROS. There are a carboxylic acid group, an amino group, a sulfhydryl group, and two peptide bonds to which the metals are attached. The lead binds to the -SH group, which reduces the level of GSH, and a low GSH level directly or indirectly affects antioxidant activity (Gurer and Ercal 2000; Hsu and Guo 2002; Patrick 2006a, b). The inactivation of these antioxidant enzymes by the lead (SOD, CAT, and GSH-Px) is also associated with the replacement of zinc, an important cofactor. Apart from lipid peroxidation, it induces Hb oxidation which causes hemolysis of erythrocytes. This is associated with ALAD inhibition and leads to an increase in the concentration of urine/blood ALA substrate. The high level of ALA results in the formation of hydrogen peroxide and superoxide radicals which interact with hydroxyl radicals and oxy-Hb. The mechanism makes the cell highly sensitive to oxidative stress and causes cell death. Single/divalent cations such as Ca2+, Mg+2, Fe+2, and Na+ are replaced by lead (Assi et al. 2016; Singh et al. 2018).
Diosmin alone-applied group
One of the various potential mechanisms of flavonoids is that they function as antioxidants. Most importantly, it is a radical scavenger by breaking the free radical chain reaction. That compound is capable of significantly delaying or inhibiting the oxidation of the substrate when exposed to oxidizable substrate, even at low concentrations. The special antioxidant effect of flavonoids is the chelation of metal in cellular structures that use essential trace elements such as copper or iron for oxidation. The chelate of catalytic metal ions prevents reactions that can form highly reactive hydroxyl radicals, for example, the Fenton reaction. The chelation of the catalytic metal ions prevents the reactions (Fenton reaction) that can form highly reactive hydroxyl radicals. The ability of the polyphenols to react with metal ions also makes them pro-oxidant (Croft 1998; Erlund 2004; Flora et al. 2012). Within the scope of the study, no significant difference was observed in the parameters examined in the diosmin-treated group, compared to the control group, indicating that this compound does not cause a change in the given dose and duration, which may affect the biological system. They reported that they did not cause significant differences in blood/tissue oxidative stress parameters (Srinivasan and Pari 2012; Germoush 2016; Eraslan et al. 2017; Sharmila Queenthy et al. 2018) and some biochemical values (Germoush 2016; Eraslan et al. 2017; Sharmila Queenthy et al. 2018).
Lead acetate alone-applied group
There was a significant increase in all tissue and plasma MDA and NO levels in animals treated with lead acetate compared with the control group, whereas liver SOD, GSH-Px activity, and GSH level decreased, increased CAT activity, elevated SOD and CAT activity, decline in GSH level and GSH-Px activity in renal tissue; increase in CAT activity and decrease in GSH levels, SOD and GSH-Px activity in brain tissue; decreased SOD, CAT, and GSH-Px activity and decreased GSH levels in cardiac tissue; decreased testicular SOD, GSH-Px, and GSH activity/levels, increased CAT activity; reduction of erythrocyte SOD, CAT, and GSH-Px activities; and a decrease in plasma GSH levels clearly show that lead acetate leads to oxidative stress in animals at the specified dose and duration. As mentioned above in detail, the mechanisms that it relies on are as follows: accelerating the formation of free radicals, disrupting the structure of cellular membrane and thus altering the composition of fatty acids, consuming GSH stores, replacing metals that act as cofactors in many antioxidant enzymes, increasing the level of ALA and indirectly increasing the level of free radicals, and changing the ion transport mechanism it affects and its calcium-dependent processes, and consequently takes place in the cause of mitochondrial dysfunction (Gurer and Ercal 2000; Hsu and Guo 2002; Patrick 2006a, b; Assi et al. 2016; Singh et al. 2018).
The decrease in antioxidant enzyme activities revealed that free radicals are consumed during conversion to less harmful and harmless products. On the other hand, as mentioned above, lead can also lead to enzyme inhibition by replacing other metals that act as cofactors. The presence of some antioxidant enzyme activities in some of the studied samples shows that the metal directly or indirectly (with the free radicals produced) leads to the induction of the enzyme. A decrease in glucose, total protein, and albumin levels was observed in lead exposure group for biochemical parameters compared to control group; however, an increase in triglyceride, cholesterol, BUN, creatinine, uric acid, LDH, AST, ALT, and ALP activity shows that changes in the biological system triggers tissue damage. At the same time, differences in organ and body weight support this situation. Other earlier studies on lead exposure have shown similar changes in blood/tissue oxidative stress parameters (Sandhir and Gill 1995; Abdel-Moneim et al. 2011; Abdou and Hassan 2014; Agrawal et al. 2015; Hasanein et al. 2016; Mabrouk 2017; Ozkaya et al. 2018) and biochemical parameters (Suradkar et al. 2010; Abdou and Hassan 2014; Abdel-Moneim et al. 2015; Agrawal et al. 2015; Hasanein et al. 2016; Mohammed et al. 2017). Alterations in the body weight and changes in the organs have been observed in previous studies (Sidhu and Nehru 2004; Ibrahim et al. 2012; Wang et al. 2012; Ekeh et al. 2015; Alwaleedi 2016; Hasanein et al. 2016; Mohammed et al. 2017). In the study, it was determined that there was an agreement between the parameters examined and the parameters obtained from previous studies.
Group applied the combination of diosmin and lead acetate
In the group where both compounds were administered, all the parameters evaluated were closer to the control group. Compared with the control group; erythrocyte CAT activity, liver SOD activity, kidney GSH-Px activity and NO level, brain GSH level, heart SOD, CAT, GSH-Px activities and GSH level, and activity of testis SOD and CAT, as well as the presence of significant differences in biochemical parameters cholesterol level and LDH activity in addition to the presence of significant changes in kidney weight, ratio of kidney weight/body weight, testis weight/body weight, and body weight show that diosmin administration does not completely eliminate the negative effects of lead acetate exposure. Similarly, when the values of lead acetate group are compared with lead acetate and diosmin co-administration group, the levels of plasma MDA, NO, and GSH as well as erythrocytes SOD and CAT activities; MDA, NO, CAT, and GSH-Px activity/level of the liver; also MDA, NO, SOD, CAT and GSH-Px activity/level of the kidney; in addition brain MDA, SOD, CAT, GSH-Px, and GSH levels/activity; on the other hand heart MDA, NO, SOD, and GSH-Px activity/level; and finally testis NO, SOD, CAT, and GSH-Px activity/level of the presence of significant differences in the same thesis are supported. Flavonoids are generally known to scavenge free radicals (Pietta 2000; Bubols et al. 2013). It also results in similar effects in that it prevents lipid oxidation by binding certain cofactor essential trace elements (such as iron and copper) involved in free radical formation reactions (Fernandez et al. 2002; Nkhili et al. 2014). The mechanism of action of diosmin can be said to be similar (Cypriani et al. 1993; Firuzi et al. 2011; Bogucka-Kocka et al. 2013; Hajimahmoodi et al. 2014). Considering previous studies on diosmin, when the data obtained from previous studies in terms of oxidative stress parameters (Srinivasan and Pari 2012; Senthamizhselvan et al. 2014; Eraslan et al. 2017; Sharmila Queenthy et al. 2018) and biochemical parameters (Senthamizhselvan et al. 2014; Eraslan et al. 2017; Sharmila Queenthy et al. 2018) were analyzed, it was noted that similar results were obtained.
Conclusion
In conclusion, the lead acetate administered at the specified concentration with drinking water and duration caused severe organ damage. The changes to oxidative stress parameters, biochemical parameters, and body weight/organ weights indicated this situation. One of the ways to make this effect is the potential to generate free radicals. Diosmin in the same time and at the dose given by the oral dose was found to be effective in alleviating the damage caused by lead acetate. With the radical scavenging and antioxidant effect of diosmin, the toxic effect of lead acetate inducing through this pathway was alleviated. The reversing of the examined parameters to the control group values also supports this evaluation. Therefore, diosmin may be used in case of possible intoxication due to lead acetate exposure, both as a protective agent and as a supportive agent for the purpose of supporting the basic treatment methods. In this way, the use as an additive agent will reduce the negative effects of not only lead but also probably chelating agent.
References
Abdel-Moneim AE, Dkhil MA, Al-Quraishy S (2011) The redox status in rats treated with flaxseed oil and lead-induced hepatotoxicity. Biol Trace Elem Res 143:457–467
Abdel-Moneim AM, El-Toweissy MY, Ali AM, Awad Allah AA, Darwish HS, Sadek IA (2015) Curcumin ameliorates lead (Pb(2+))-induced hemato-biochemical alterations and renal oxidative damage in a rat model. Biol Trace Elem Res 168:206–220
Abdou HM, Hassan MA (2014) Protective role of omega-3 polyunsaturated fatty acid against lead acetate-induced toxicity in liver and kidney of female rats. Biomed Res Int 435857
Agrawal S, Bhatnagar P, Flora SJ (2015) Changes in tissue oxidative stress, brain biogenic amines and acetylcholinesterase following co-exposure to lead, arsenic and mercury in rats. Food Chem Toxicol 86:208–216
Aksu DS, Sağlam YS, Yildirim S, Aksu T (2017) Effect of pomegranate (Punica granatum L.) juice on kidney, liver, heart and testis histopathological changes, and the tissues lipid peroxidation and antioxidant status in lead acetate-treated rats. Cell Mol Biol 63:33–42
Alissa EM, Ferns GA (2011) Heavy metal poisoning and cardiovascular disease. J Toxicol 870125
Alwaleedi SA (2016) Hemato-biochemical changes induced by lead intoxication in male and female albino mice. Int J Recent Sci Res 6:46–51
Amadi CN, Offor SJ, Frazzoli C, Orisakwe OE (2019) Natural antidotes and management of metal toxicity. Environ Sci Pollut Res Int 26:18032–18052
Aposhian HV (1983) DMSA and DMPS-water soluble antidotes for heavy metal poisoning. Annu Rev Pharmacol Toxicol 23:193–215
Aposhian HV, Maiorino RM, Gonzalez-Ramirez D, Zuniga-Charles M, Xu Z, Hurlbut KM, Junco-Munoz P, Dart RC, Aposhian MM (1995) Mobilization of heavy metals by newer, therapeutically useful chelating agents. Toxicology 97:23–38
Asadpour R, Azari M, Hejazi M, Tayefi H, Zaboli N (2013) Protective effects of garlic aqueous extract (Allium sativum), vitamin E, and N-acetylcysteine on reproductive quality of male rats exposed to lead. Vet Res Forum 4:251–257
Assi MA, Hezmee MN, Haron AW, Sabri MY, Rajion MA (2016) The detrimental effects of lead on human and animal health. Vet World 9:660–671
Barreca D, Laganà G, Bruno G, Magazù S, Bellocco E (2013) Diosmin binding to human serum albumin and its preventive action against degradation due to oxidative injuries. Biochimie 95:2042–2049
Bertozzi MM, Rossaneis AC, Fattori V, Longhi-Balbinot DT, Freitas A, Cunha FQ, Alves-Filho JC, Cunha TM, Casagrande R, Verri WA Jr (2017) Diosmin reduces chronic constriction injury-induced neuropathic pain in mice. Chem Biol Interact 273:180–189
Beutler E (1975) Reduced glutathione (GSH). In Bergmeyer HU (ed). Red cell metabolism: A manual of biochemical methods. New York, Grune and Stratton, pp 112–114
Blanusa M, Varnai VM, Piasek M, Kostial K (2005) Chelators as antidotes of metal toxicity: therapeutic and experimental aspects. Curr Med Chem 12:2771–2794
Bogucka-Kocka A, Woźniak M, Feldo M, Kockic J, Szewczyk K (2013) Diosmin-isolation techniques, determination in plant material and pharmaceutical formulations, and clinical use. Nat Prod Commun 8:545–550
Bradberry S, Vale A (2009) A comparison of sodium calcium edetate (edetate calcium disodium) and succimer (DMSA) in the treatment of inorganic lead poisoning. Clin Toxicol 47:841–858
Bubols GB, Vianna Dda R, Medina-Remon A, von Poser G, Lamuela-Raventos RM, Eifler-Lima VL, Garcia SC (2013) The antioxidant activity of coumarins and flavonoids. Mini-Rev Med Chem 13:318–334
Buddhan R, Manoharan S (2017) Diosmin reduces cell viability of A431 skin cancer cells through apoptotic induction. J Cancer Res Ther 13:471–476
Cao Y, Skaug MA, Andersen O, Aaseth J (2015) Chelation therapy in intoxications with mercury, lead and copper. J Trace Elem Med Biol 31:188–192
Carocci A, Catalano A, Lauria G, Sinicropi MS, Genchi G (2016) Lead toxicity, antioxidant defense and environment. Rev Environ Contam Toxicol 238:45–67
Croft KD (1998) The chemistry and biological effects of flavonoids and phenolic acids. Ann N Y Acad Sci 854:435–442
Cypriani B, Limasset B, Carrié ML, Le Doucen C, Roussie M, de Paulet AC, Damon M (1993) Antioxidant activity of micronized diosmin on oxygen species from stimulated human neutrophils. Biochem Pharmacol 45:1531–1535
Ekeh FN, Ikele CB, Obiezue R (2015) The effect of lead acetate on the testes of male albino rats. Adv in Life Sci Tech 38:70–74
Eraslan G, Sarıca ZS, Bayram LÇ, Tekeli MY, Kanbur M, Karabacak M (2017) The effects of diosmin on aflatoxin-induced liver and kidney damage. Environ Sci Pollut Res Int 24:27931–27941
Ercal N, Gürer-Orhan H, Aykin-Burns N (2001) Toxic metals and oxidative stress part I: mechanism involved in metal induced oxidative damage. Curr Top Med Chem 1:529–539
Erlund I (2004) Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutr Res 24:851–874
Fairbanks VF, Klee GG (1987) Biochemical aspect of hematology. In: Tietz NW (ed) Fundamentals of clinical chemistry, 3rd Edn. WB Saunders, Philadelphia, pp 803–806
Fernandez MT, Mira ML, Florêncio MH, Jennings KR (2002) Iron and copper chelation by flavonoids: an electrospray mass spectrometry study. J Inorg Biochem 92:105–111
Firuzi O, Miri R, Tavakkoli M, Saso L (2011) Antioxidant therapy: current status and future prospects. Curr Med Chem 18:3871–3888
Flora SJ, Mehta A (2003) Haematological, hepatic and renal alterations after repeated oral and intraperitoneal administration of monoisoamyl DMSA. II. Changes in female rats. J Appl Toxicol 23:97–102
Flora SJ, Pande M, Kannan GM, Mehta A (2004) Lead induced oxidative stress and its recovery following co-administration of melatonin or N-acetylcysteine during chelation with succimer in male rats. Cell Mol Biol (Noisy-le-grand) 50, Online Pub: OL543-51
Flora SJ, Bhadauria S, Kannan GM, Singh N (2007a) Arsenic induced oxidative stress and the role of antioxidant supplementation during chelation: a review. J Environ Biol 28:333–347
Flora SJ, Saxena G, Gautam P, Kaur P, Gill KD (2007b) Response of lead-induced oxidative stress and alterations in biogenic amines in different rat brain regions to combined administration of DMSA and MiADMSA. Chem Biol Interact 170:209–220
Flora G, Gupta D, Tiwari A (2012) Toxicity of lead: a review with recent updates. Interdiscip Toxicol 5:47–58
Furst A (2002) Can nutrition affect chemical toxicity? Int J Toxicol 21:419–424
Germoush MO (2016) Diosmin protects against cyclophosphamide-induced liver injury through attenuation of oxidative stress, inflammation and apoptosis. Int J Pharmacol 12:644–654
Giannini I, Amato A, Basso L, Tricomi N, Marranci M, Pecorella G, Tafuri S, Pennisi D, Altomare DF (2015) Flavonoids mixture (diosmin, troxerutin, hesperidin) in the treatment of acute hemorrhoidal disease: a prospective, randomized, triple-blind, controlled trial. Tech Coloproctol 19:339–345
Gottesfeld P, Pokhrel AK (2011) Review: Lead exposure in battery manufacturing and recycling in developing countries and among children in nearby communities. J Occup Environ Hyg 8:520–532
Gurer H, Ercal N (2000) Can antioxidants be beneficial in the treatment of lead poisoning? Free Radic Biol Med 29:927–945
Hajimahmoodi M, Moghaddam G, Mousavi SM, Sadeghi N, Oveisi MR, Jannat B (2014) Total antioxidant activity, and hesperidin, diosmin, eriocitrin and quercetin contents of various lemon juices. Trop J Pharm Res 13:951–956
Hasanein P, Kazemian-Mahtaj A, Khodadadi I (2016) Bioactive peptide carnosin protects against lead acetate-induced hepatotoxicity by abrogation of oxidative stress in rats. Pharm Biol 54:1458–1464
Hirsch HV, Possidente D, Possidente B (2010) Pb2+: an endocrine disruptor in Drosophila? Physiol Behav 99:254–259
Hsu PC, Guo YL (2002) Antioxidant nutrients and lead toxicity. Toxicology 180:33–44
Ibrahim NM, Eweis EA, El-Beltagi HS, Abdel-Mobdy YE (2012) Effect of lead acetate toxicity on experimental male albino rat. Asian Pac J Trop Biomed 2:41–46
Jaffe AI, Treser G, Suzuki Y, Ehrenreich T (1968) Nephropathy induced by d-penicillamine. Ann Intern Med 69:549–556
Jain D, Bansal MK, Dalvi R, Upganlawar A, Somani R (2014) Protective effect of diosmin against diabetic neuropathy in experimental rats. J Integ Med 12:35–41
Jomova K, Valko M (2011) Advances in metal-induced oxidative stress and human disease. Toxicology 283:65–87
Kang HG, Jeong SH, Cho MR, Cho JH, Bischoff K (2009) Time-dependent changes in lead and delta-aminolevulinic acid after subchronic lead exposure in rats. Hum Exp Toxicol 28:647–654
Kean WF, Dwosh IL, Anastassiades TP, Ford PM, Kelly HG (1980) The toxicity pattern of d-penicillamine therapy. Arthritis Rheum 23:158–164
Llobet JM, Domingo JL, Paternain JL, Corbella J (1990) Treatment of acute lead intoxication. A quantitative comparison of a number of chelating agents. Arch Environ Contam Toxicol 19:185–189
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Luck H (1965) Catalase. In: Bergmeyer H (ed) Methods of enzymatic analysis. Academic Press, New York, pp 885–894
Mabrouk A (2017) Protective effect of thymoquinone against lead-induced antioxidant defense system alteration in rat liver. Acta Biol Hung 68:248–254
Mehta A, Flora SJ (2001) Possible role of metal redistribution, hepatotoxicity and oxidative stress in chelating agents induced hepatic and renal metallothionein in rats. Food Chem Toxicol 39:1029–1038
Mehta A, Kannan GM, Dube SN, Pant BP, Pant SC, Flora SJ (2002) Haematological, hepatic and renal alterations after repeated oral or intraperitoneal administration of monoisoamyl DMSA. I Changes in male rats. J Appl Toxicol 22:359–369
Miller GL (1959) Protein determination for large numbers of samples. Anal Chem 31:964–964
Miller GD, Massaro TF, Granlund RW, Massaro EJ (1983) Tissue distribution of lead in the neonatal rat exposed to multiple doses of lead acetate. J Toxicol Environ Health 11:121–128
Mirshekar MA, Fanaei H, Keikhaei F, Javan FS (2017) Diosmin improved cognitive deficit and amplified brain electrical activity in the rat model of traumatic brain injury. Biomed Pharmacother 93:1220–1229
Mitra P, Sharma S, Purohit P, Sharma P (2017) Clinical and molecular aspects of lead toxicity: an update. Crit Rev Clin Lab Sci 54:506–528
Mohammed GM, Sedky A, Elsawy H (2017) A study of the modulating action of quercetin on biochemical and histological alterations induced by lead exposure in the liver and kidney of rats. Chin J Phys 60:183–190
Naso L, Martínez VR, Lezama L, Salado C, Valcarcel M, Ferrer EG, Williams PAM (2016) Antioxidant, anticancer activities and mechanistic studies of the flavone glycoside diosmin and its oxidovanadium (IV) complex. Interactions with bovine serum albumin. Bioorg Med Chem 24:4108–4119
Nkhili E, Loonis M, Mihai S, El Hajji H, Dangles O (2014) Reactivity of food phenols with iron and copper ions: binding, dioxygen activation and oxidation mechanisms. Food Funct 5:1186–1202
Ohkawa H, Ohishi N, Yagi K (1978) Reaction of linoleic acid hydroperoxide with thiobarbituric acid. J Lipid Res 19:1053–1057
Ozkaya A, Sahin Z, Dag U, Ozkaraca M (2016) Effects of naringenin on oxidative stress and histopathological changes in the liver of lead acetate administered rats. J Biochem Mol Toxicol 30:243–248
Ozkaya A, Sahin Z, Kuzu M, Saglam YS, Ozkaraca M, Uckun M, Yologlu E, Comakli V, Demirdag R, Yologlu S (2018) Role of geraniol against lead acetate-mediated hepatic damage and their interaction with liver carboxylesterase activity in rats. Arch Physiol Biochem 124:80–87
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Papanikolaou NC, Hatzidaki EG, Belivanis S, Tzanakakis GN, Tsatsakis AM (2005) Lead toxicity update. A brief review. Med Sci Monit 11:RA329–RA336
Patel K, Gadewar M, Tahilyani V, Patel DK (2013) A review on pharmacological and analytical aspects of diosmetin: a concise report. Chin J Integr Med 19:792–800
Patrick L (2006a) Lead Toxicity, A review of the literature. Part I: Exposure, evaluation, and treatment. Altern Med Rev 11:2–22
Patrick L (2006b) Lead toxicity part II: the role of free radical damage and the use of antioxidants in the pathology and treatment of lead toxicity. Altern Med Rev 11:114–127
Pietta PG (2000) Flavonoids as antioxidants. J Nat Prod 63:1035–1042
Ramelet AA (2001) Clinical benefits of Daflon 500 mg in the most severe stages of chronic venous insufficiency. Angiology 52:49–56
Rashid K, Sinha K, Sil PC (2013) An update on oxidative stress-mediated organ pathophysiology. Food Chem Toxicol 62:584–600
Ros C, Mwanri L (2003) Lead exposure, interactions and toxicity: food for thought. Asia Pac J Clin Nutr 12:388–395
Russo R, Mancinelli A, Ciccone M, Terruzzi F, Pisano C, Severino L (2015) Pharmacokinetic profile of μSMIN Plus™, a new micronized diosmin formulation, after oral administration in rats. Nat Prod Commun 10:1569–1572
Rusyniak DE, Arroyo A, Acciani J, Froberg B, Kao L, Furbee B (2010) Heavy metal poisoning: management of intoxication and antidotes. EXS 100:365–396
Sachan AS, Kannan GM, Kumar P, Flora SJ (1996) Effects of chelation therapy on hepatic glutathione, lipid peroxidation and phospholipid contents in lead-poisoned rats. Indian J Physiol Pharmacol 40:180–182
Sakai T (2000) Biomarkers of lead exposure. Ind Health 38:127–142
Samarghandian S, Borji A, Afshari R, Delkhosh MB, Gholami A (2013) The effect of lead acetate on oxidative stress and antioxidant status in rat bronchoalveolar lavage fluid and lung tissue. Toxicol Mech Methods 23:432–436
Sanborn MD, Abelsohn A, Campbell M, Weir E (2002) Identifying and managing adverse environmental health effects: 3. Lead exposure. CMAJ 166:1287–1292
Sanders T, Liu Y, Buchner V, Tchounwou PB (2009) Neurotoxic effects and biomarkers of lead exposure: a review. Rev Environ Health 24:15–45
Sandhir R, Gill KD (1995) Effect of lead on lipid peroxidation in liver of rats. Biol Trace Elem Res 48:91–97
Senthamizhselvan O, Manivannan J, Silambarasan T, Raja B (2014) Diosmin pretreatment improves cardiac function and suppresses oxidative stress in rat heart after ischemia/reperfusion. Eur J Pharmacol 736:131–137
Sharmila Queenthy S, Stanely Mainzen Prince P, John B (2018) Diosmin prevents isoproterenol-induced heart mitochondrial oxidative stress in rats. Cardiovasc Toxicol 18:120–130
Sidhu P, Nehru B (2004) Lead intoxication: Histological and oxidative damage in rat cerebrum and cerebellum. J Trace Elem Exp Med 17:45–53
Silambarasan T, Raja B (2012) Diosmin, a bioflavonoid reverses alterations in blood pressure, nitric oxide, lipid peroxides and antioxidant status in DOCA-salt induced hypertensive rats. Eur J Pharmacol 679:81–89
Singh N, Kumar A, Gupta VK, Sharma B (2018) Biochemical and molecular bases of lead-induced toxicity in mammalian systems and possible mitigations. Chem Res Toxicol 31:1009–1021
Smith D, Strupp BJ (2013) The scientific basis for chelation: animal studies and lead chelation. J Med Toxicol 9:326–338
Srinivasan S, Pari L (2012) Ameliorative effect of diosmin, a citrus flavonoid against streptozotocin-nicotinamide generated oxidative stress induced diabetic rats. Chem Biol Interact 195:43–51
Srinivasan S, Pari L (2013) Antihyperlipidemic effect of diosmin: a citrus flavonoid on lipid metabolism in experimental diabetic rats. J Funct Foods 5:484–492
Suică-Bunghez IR, Fierăscu RC, Fierăscu I, Ion RM (2015) Flavonoids, polyphenols content and antioxidant activity of citrus fruits extracts. The XIXth International Conference, Inventica. June 24th-26th, IASI, Romania
Sun Y, Oberley LW, Li Y (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem 34:497–500
Suradkar SG, Vihol PD, Patel JH, Ghodasara DJ, Joshi BP, Prajapati KS (2010) Patho-morphological changes in tissues of Wistar rats by exposure of lead acetate. Vet World 3:82–84
Tandon SK, Singh S, Prasad S (1997) Chelation in metal intoxication LI: efficacy of amphipathic dithiocarbamates in mobilization of lead in the rat. Hum Exp Toxicol 16:557–562
Tracey WR, Tse J, Carter G (1995) Lipopolysaccharide-induced changes in plasma nitrite and nitrate concentrations in rats and mice: pharmacological evaluation of nitric oxide synthase inhibitors. J Pharmacol Exp Ther 272:1011–1015
Valko M, Jomova K, Rhodes CJ, Kuča K, Musílek K (2016) Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch Toxicol 90:1–37
Wang G, Fowler BA (2008) Roles of biomarkers in evaluating interactions among mixtures of lead, cadmium and arsenic. Toxicol Appl Pharmacol 233:92–99
Wang J, Yang Z, Lin L, Zhao Z, Liu Z, Liu X (2012) Protective effect of naringenin against lead-induced oxidative stress in rats. Biol Trace Elem Res 146:354–359
Wang J, Zhu H, Yang Z, Liu Z (2013) Antioxidative effects of hesperetin against lead acetate-induced oxidative stress in rats. Indian J Pharm 45:395–398
Wani AL, Ara A, Usmani JA (2015) Lead toxicity: a review. Interdiscip Toxicol 8:55–64
Winterbourn CC, Hawkins RE, Brian M, Carrell RW (1975) The estimation of red cell superoxide dismutase activity. J Lab Clin Med 85:337–341
Yoshioka T, Kawada K, Shimada T, Mori M (1979) Lipid peroxidation in maternal and cord blood and protective mechanism against activated-oxygen toxicity in the blood. Am J Obstet Gynecol 135:372–376
Funding
This research (project code: TYL-2015-5859) was supported by the Research Fund of Erciyes University.
Author information
Authors and Affiliations
Contributions
All authors have equal contributions to the manuscript.
Corresponding author
Ethics declarations
The approval of the Erciyes University Animal Experiments Local Ethics Committee was taken for this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This study is the MSC thesis of Mehmet Bozdağ.
Rights and permissions
About this article
Cite this article
Bozdağ, M., Eraslan, G. The effect of diosmin against lead exposure in rats‡. Naunyn-Schmiedeberg's Arch Pharmacol 393, 639–649 (2020). https://doi.org/10.1007/s00210-019-01758-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-019-01758-4