Abstract
Depression is a serious medical illness displaying high lifetime prevalence, early-age onset that adversely affects socio-economic status. The bidirectional association between oxidative stress and calcium-signaling adversely affects the monoaminergic neuron functions that instigate the pathogenesis of depression. The present study investigates the effect of lacidipine (LCD), L-type Ca2+-channel blocker, on reserpine-induced depression in mice. Separate groups of mice (Swiss albino, 18–25 g) were administered lacidipine (0.3, 1 and 3 mg/kg, i.p.) daily for 14 days and reserpine (5 mg/kg, i.p.) was injected on day 14. Rectal temperature, catalepsy, and tail-suspension test (TST) were performed 18 h and ptosis scores at 60, 120, 240, 360 min post-reserpine treatment. Whole-brain TBARS, GSH, nitrite, and superoxide dismutase (SOD) and catalase activities were estimated. Reserpine elevated the catalepsy, ptosis, hypothermia, and immobility period in TST owing to the marked increase in oxidative-nitrosative stress in the brain of mice. LCD attenuated the reserpine triggered the rise in catalepsy, ptosis scores, hypothermia, and immobility period in mice. LCD pretreatment attenuated the increase in TBARS and nitrite levels, and the decline of GSH, SOD, and catalase activities in the brain of reserpine injected mice. Bay-K8644 (0.5 mg/kg, i.p.), Ca2+-channel agonist, attenuated these effects of LCD (3 mg/kg) in reserpine-treated mice. It can be inferred that lacidipine (Ca2+ channel antagonist) attenuates depression-like symptoms in reserpine-treated mice. Furthermore, the abrogation of antidepressant-like effects of LCD by Bay-K8644 revealed that modulation of Ca2+-channels might present a potential strategy in the management of depression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depression is chronic, multi-factorial, and incapacitating psychiatric disorder well characterized by feelings of anhedonia, hopelessness, low self-esteem, and cognitive deficits. The disease carries a high rate of morbidity and mortality throughout the world with a wide population with a preponderance towards women (Nemeroff 2007). At present, ~ 350 million people are suffering from depression (~ 17% lifetime prevalence) which is regarded as the fourth leading cause of disability worldwide and is expected to occupy the second place by 2020. Chronic stress, traumatic events, childhood abuse, and some environmental factors portray chief etiologic culprits in depression (Ahmed et al. 2015; Guan and Liu 2016). Several neurochemicals such as monoamines, glutamate, γ-aminobutyric acid (GABA), and neurotrophic factors (e.g., brain-derived neurotrophic factor) are implicated in the pathology of depression (Hasler 2010; Vavakova et al. 2015). Monoamines (serotonin, noradrenaline, and dopamine) regulate a wide range of functions in the brain, such as mood, cognition, reward processing, and sleep. Deficiency in monoaminergic neurotransmission at the synapse in depression is documented by several previous studies. Drugs promoting monoaminergic transmission in midbrain and brainstem nuclei (e.g., fluoxetine, amitriptyline, phenelzine) have been proven clinically effective in patients of depression. Presently, modulation of serotonin or norepinephrine neurotransmission and monoamine oxidase (MAO) activity is the backbone of depression drug therapy (Hasler 2010). However, a low compliance rate and the emergence of adverse effects of conventional antidepressants have limited the current practice of long-term drug therapy regimen in patients of depression, which permits to seek new alternatives that can modify the progression of the disease.
Recent studies have revealed the implications of oxidative stress in many psychiatric disorders such as depression, anxiety, and psychosis (Vavakova et al. 2015). Several lines of evidence indicate enhanced, free radicals and lipid peroxidation products (e.g., malondialdehyde, isoprostanes) and diminished antioxidant defense in brain autopsies of patients of depression. Neurodegenerative changes in the hippocampus and frontal cortex, including cortico-limbic region, have been observed through modern neuroimaging techniques in depression patients (Hasler 2010). In a meta-analysis study on depression comprising of 3779 human subjects, a significant increase of oxidative markers in urine and plasma samples was reported (Liu et al. 2015). Oxidative stress-mediated immune response shifts tryptophan metabolism towards kynurenine from the serotonin pathway leading to depletion of brain serotonin levels. Oxidative breakdown products of monoamines lead to glutamate excitotoxicity in the brain via increased calcium influx and loss of monoaminergic neurons in the cortex, limbic region, basal ganglia, and brain stem (Spiers et al. 2015). The other Ca2+ triggered excitotoxic cell death pathways include activation of phospholipases, endonucleases, and proteases (Halliwell 2006). Pathogenic activation nitric oxide-guanylyl cyclase signaling is regarded as the primary aspect of Ca2+-induced depressive symptoms by several studies (Dhir and Kulkarni 2011). In clinical settings and animal studies, significant improvement in symptoms of depression through downregulation of the Ca2+-nitric oxide synthase-guanylyl cyclase pathway has been observed (Paul 2001). Two different studies indicated that nitric oxide synthase (NOS) inhibition by 7-nitroindazole (7-NI) and knockdown of NOS-1 gene reversed the depressive hallmarks in rodents (Joca and Guimaraes 2006; Wultsch et al., 2007). Several calcium-regulating proteins such as voltage- and ligand-gated calcium channels, ryanodine-receptors, and glutamate transporters are also implicated in psychiatric abnormalities. Furthermore, in vivo studies with L-type calcium blockers (e.g., nifedipine, verapamil, flunarizine) demonstrated significant benefits in animal models of depression (Aburawi et al. 2007). These studies suggested that calcium channel modulators may be explored for the management of depression and other psychiatric disorders.
Lacidipine (LCD) is a long-acting, highly lipophilic L-type calcium channel antagonist used in the management of hypertension in once-daily dosing (4–6 mg) (McCormack and Wagstaff 2003). A growing body of evidence supports that therapeutic use of calcium channel blockers (CCBs) (e.g., verapamil) in patients with cardiovascular disorders (e.g., coronary artery disease and hypertension) significantly declines the prevalence of depression (Ried et al. 2005, 2006). In addition to calcium channel modulation, LCD is a potent antioxidant with respect to dihydropyridine calcium antagonists (McCormack and Wagstaff 2003) and also comparable to reference antioxidants like vitamin E (van Amsterdam et al. 1992). Moreover, in several studies, calcium channel blockers are found to potentiate the brain monoamine levels and antidepressant effects of tricyclic, atypical, and monoamine oxidase (MAO) inhibitor antidepressants (Prakhie and Oxenkrug 1998; Aburawi et al. 2007; Bergantin and Caricati-Neto 2016). Reserpine is an alkaloid that blocks vesicular monoamine transport-2 (VMAT2) that increases the monoamines turnover in the brain, manifested by symptoms of depression in rodents. Reserpine also enhances redox-imbalance through auto-oxidative catabolism of monoamines and oxidative degradation by MAO that further provide impetus to oxido-nitrosative burden in the brain (Lohr et al. 2003). As intracellular Ca2+ availability instigates MAO activity (Cao et al. 2007), CCBs can resurrect the depleting monoamine levels in the brain. Therefore, the existing study was conducted to examine the potential of lacidipine (CCB) against reserpine-induced depression in mice.
Methods
Experimental animals
Adult mice (Swiss albino, either sex, 18–25 g) were procured from Disease Free Small Animal House, Lala Lajpat Rai University of Veterinary and Animal Science (LUVAS), Hisar (India). The experimental protocol was approved by the Institutional Animal Ethics Committee (ASCB/IAEC/08/15/108). The animals were nurtured in “Animal House Facility” of the institute under a controlled environment and light-dark cycle (12 h each). The lights of Animal House Facility were turned on from 08:00 to 20:00 h and afterwards kept off till 08:00 h morning next day. Mice were given free access to water and pellet diet (Ashirwad Industries, Mohali). The experimental procedures were conducted between 09:00 and 17:00 h. Mice were taken care of as per the instructions outlined by CPCSEA, Ministry of Environment, Forests and Climate Change, Government of India.
Drugs and chemicals
Lacidipine (TCI Chemicals, Chennai); reserpine (Fluka Analytical, Mumbai); fluoxetine (Zee Laboratories, Ponta Sahib); nitro blue tetrazolium (NBT) chloride, p-nitroso-N,N-dimethylaniline (SRL Pvt. Ltd., Mumbai); riboflavin, monosodium phosphate (NaH2PO4), dipotassium phosphate (K2HPO4), sodium nitrite (NaNO2), pyridine, hydrogen peroxide (H2O2), sodium dodecyl sulfate, thiobarbituric acid, trichloroacetic acid, N-1-napthylethylenediamine dihydrochloride, 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), EDTA disodium dehydrate, sodium cyanide (Loba-Chemie, Mumbai); sulfanilamide (SpectroChem, Mumbai); n-butanol (Merck, Mumbai) were used. The intraperitoneal injections were prepared using 0.2% dimethylsulfoxide (DMSO) vehicle.
Experimental protocol
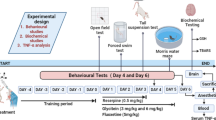
Lacidipine (0.3, 1 and 3 mg/kg, i.p.) was injected to different groups of mice for 14 days once daily (Bellosta et al. 2001). Fluoxetine (standard antidepressant) is a selective serotonin reuptake inhibitor (SSRI) that possesses potent voltage-gated Ca2+ channel (L-type) antagonistic activity (Deak et al. 2000) and was given at dose 20 mg/kg (i.p.) for 14 days. Administration of reserpine in rodents depletes the brain monoamines manifested by characteristic symptoms of depression thereof. In the current protocol, a single dose of reserpine, i.e., 5 mg/kg (i.p.), was given to lacidipine pretreated mice on the 14th day to induce pathogenesis of depression. Bay-K8644 (Ca2+ channel agonist) (0.5 mg/kg, i.p.) was given to mice on the 14th day to delineate the role of Ca2+ in lacidipine (3 mg/kg) and reserpine-treated mice (Jackson and Damaj 2009). Mice were randomly distributed in single-blind fashion into seven groups (n = 6). Vehicle control group was given 0.2% DMSO for 14 days. Reserpine group was administered with reserpine (5 mg/kg, i.p.) on day 14 and vehicle only from day 1 to 13; fluoxetine + reserpine group was administered fluoxetine (20 mg/kg) for 14 days and reserpine; LCD(0.3) + reserpine group was given lacidipine (0.3 mg/kg, i.p.) for 14 days and reserpine; LCD(1) + reserpine group was subjected to lacidipine (1 mg/kg, i.p.) for 14 days and reserpine; LCD(3) + reserpine group was given lacidipine (3 mg/kg, i.p.) for 14 days and reserpine; Bay-K8644 + LCD(3) + reserpine group received Bay-K8644 (0.5 mg/kg, i.p.) on 14th day, lacidipine (3 mg/kg, i.p.) for 14 days and reserpine.
Reserpine-induced catalepsy, ptosis, and fall in rectal temperature are averted through the standard therapy of depression; hence, these parameters were used to evaluate antidepressant effects of lacidipine. The severity of ptosis was observed at 60, 120, 240, and 360 min after the reserpine administration on a scale ranging from 0 to 4 (eyes closed, 4; eyes ¾ closed, 3; eyes ½ closed, 2; eyes ¼ closed, 1; and eyes open, 0). Catalepsy, rectal temperature, and immobility time (TST) were noted 18 h post-reserpine treatment (Fig. 1). Catalepsy was measured using a stair with two cork stoppers with two steps each (height 30 mm). Each animal was placed with hind legs on the top of the cork and head pointing downwards. The mice were placed into a normal position after a cut off period of 60 s, if the cataleptic effect was not reversed. Scoring of catalepsy ranging from 0 to 5 was correlated with duration of catalepsy (> 60 s, 5; between 45 and 60 s, 4; 30–45 s, 3; 10–30 s, 2; 5–10 s, 1; and < 5 s, 0). To measure rectal temperature, the lubricated electronic thermometer was inserted to constant depth (up to 2 cm) into the rectum of animal (Rubin et al. 1957; Askew 1963; Costall and Naylor 1974).
Tail-suspension test
The standard procedure of the tail suspension test was adopted as given by Steru et al. (1985) to evaluate depression-like symptoms in mice. Each animal was suspended freely at a height of 58 cm from the floor by using tape applied at the tip of the tail (~ 1 cm). The passive hanging of the animal completely motionless was defined as immobility. The duration of immobility was noted for 6 min. The decline in the duration of motionless hanging was taken as the measure of antidepressant activity. The result is expressed as the duration of immobility (s) in TST.
Preparation of whole-brain homogenate
The animals were euthanized (n = 6) by cervical dislocation, and whole brains were isolated and immediately positioned on ice followed by washing with ice-cold isotonic saline (0.9% NaCl) to remove the residues and blood. The whole brain wet weight was noted down. Brain tissue homogenate (10% w/v) was prepared using 50 mM phosphate buffer (pH 7.4; 1% v/v Triton X-100; temperature 4 °C) using tissue homogenizer (Remi Motors, Remi Electrotechnik, Vasai, India) (Kumar and Bansal 2018). The homogenate was centrifuged at 12,000×g for 15 min at 4 °C in high-speed refrigerated centrifuge (CPR-30 Remi Compufuge, India), and the resultant supernatant was collected for estimation of biomarkers of oxidative and nitrosative stress.
Estimation of lipid peroxidation in the brain
Quantification of thiobarbituric acid reactive substances (TBARS) by the method of Ohkawa et al. (1979) is a reliable index of lipid peroxidation product malondialdehyde (pink color MDA-TBA2 complex). The 4-ml assay mixture consisted of 0.1 ml homogenate, 1.5 ml glacial acetic acid (20%, pH 3.5), 1.5 ml thiobarbituric acid (TBA) (0.8%), 0.2 ml sodium dodecyl sulfate (8.1%), and 0.7 ml distilled water. The mixture was thoroughly vortexed manually, heated for 1 h on a water bath at 95 °C, and then cooled under tap water. Then, 5 ml mixture of n-butanol and pyridine (15:1) was added and vigorously mixed with the assay and subjected to centrifugation for 10 min at 4000 rpm. The absorbance of the superficial 2 ml organic layer (n-butanol phase) was noted down at λmax = 532 nm using a double-beam spectrophotometer (Shimadzu UV-1700, Pharmaspec). The quantification of malondialdehyde (MDA) formed or TBARS was accomplished by using the molar extinction coefficient of chromophore ε = 1.56 × 105 M−1 cm−1, and the results were reported as nanomole per mg protein (Kumar and Bansal 2018).
Estimation of reduced GSH in the brain of mice
Glutathione (GSH) was analyzed using the procedure described by Ellman (1959). The supernatant (1 ml) was precipitated using 4% sulfosalicylic acid (1 ml), cold digested at 4 °C for 1 h and after 5 min centrifuged at 4 °C for 10 min at 2000 rpm. To 0.1 ml of the supernatant obtained after centrifugation, 0.2 ml DTNB (0.1 mM, pH 8) and 2.7 ml phosphate buffer (0.3 M, pH 8) were added. The absorbance was noted at λmax = 412 nm employing spectrophotometer, and GSH was quantified using a molar extinction coefficient ε = 1.36 × 104 M−1 cm−1 of the chromophore and expressed as micromole GSH per mg protein (Kumar and Bansal 2018).
Determination of SOD activity in the brain of mice
Superoxide dismutase (SOD) activity was evaluated according to the methodology described by Winterbourn et al. (1975). The reaction mixture was prepared using 0.05 ml of the supernatant, 0.1 ml of 1.5 mM nitro blue tetrazolium (NBT), 0.05 ml of riboflavin (0.12 mM), 0.2 ml 0.1 M EDTA (containing 0.0015% or 0.3 mM NaCN), and phosphate buffer (67 mM, pH 7.8) to make up final volume of 3 ml. Uniform lightening was given to all tubes for 15 min under the 60 W Philips® fluorescent tube, and at 560 nm wavelength, change in absorbance of the blue-colored NBT-diformazan was noted for 5 min at 30-s interval. The amount of NBT reduced (micromole NBT reduced/min/mg protein) was calculated from the change in absorbance, based on the molar extinction coefficient for formazan ε = 15,000 M−1 cm−1(Kumar and Bansal 2018).
Determination of CAT activity in the brain of mice
Catalase (CAT) activity was determined as per the procedure described by Claiborne (1985). The assay mixture consisted 0.05 ml supernatant (10%), 1 ml hydrogen peroxide (H2O2) (0.02 M, prepared in 0.05 M phosphate buffer), and 1.95 ml phosphate buffer (0.05 M, pH 7) in a final volume of 3 ml. Change in absorbance was noted at λmax = 240 nm using spectrophotometer for 3 min at 30-s interval. Enzymatic activity (micromole H2O2 decomposed/min/mg protein) was determined using the molar extinction coefficient of ε = 43.6 M−1 cm−1.
Estimation of nitrite in the brain of mice
Total brain nitrites were estimated as per the procedure described by Sastry et al. (2002). The assay mixture consisted of 0.4 ml of carbonate buffer (pH 9), 0.1 ml of supernatant, 150 mg Cu-Cd alloy, incubated for 1 h at room temperature. Then, 0.4 ml zinc sulfate solution (120 mM) and 0.1 ml sodium hydroxide (0.35 M) were added to tubes and allowed to stand for 10 min. Afterward, the mixture was centrifuged at 4000 rpm for 10 min. To 0.1 ml of supernatant, 0.5 ml of Greiss reagent (1:1 solution of 1% sulfanilamide in 3 M HCl, and 0.1% N-1-napthyl ethylene diamine dihydrochloride in water) was added, incubated at room temperature in the dark for 10 min. The absorbance was noted at λmax = 548 nm wavelength using a spectrophotometer. The standard curve of sodium nitrite (10–100 μM) was prepared to quantify the concentration of test sample of nitrite, and the results were reported as micromole per mg of protein (Kumar and Bansal 2018).
Estimation of total proteins in the brain of mice
The total protein was assessed per the procedure developed by Lowry et al. (1951). To supernatant (0.25 ml), 5 ml of Lowry’s reagent (1% w/v copper sulfate solution, 2% w/v sodium-potassium tartrate and 2% w/v sodium carbonate in 0.1 M sodium hydroxide in the ratio of 1:1:98) and phosphate buffer (up to 1 ml) was added, mixed thoroughly, and kept at room temperature in the dark for 15 min. To the mixture, 1 N Folin-Ciocalteu reagent (0.5 ml) was added, vortexed vigorously, and then it was incubated for 30 min at room temperature in dark. The standard curve of bovine serum albumin (0.25–2.50 mg/ml) was plotted, and protein content for the test sample was determined at (λmax) 650 nm using spectrophotometer (Kumar and Bansal 2018).
Statistical analysis
Results are expressed as mean ± S.E.M. The data were analyzed by one-way ANOVA followed by Tukey’s post hoc test and two-way ANOVA followed by Bonferroni’s post hoc test using software GraphPad Prism 5.0 (GraphPad Software Inc., USA). A value of p < 0.05 was considered significant.
Results
Lacidipine attenuates ptosis in reserpine-treated mice
Reserpine significantly (p < 0.001) increased the ptosis scores in comparison to vehicle treatment. Lacidipine (1 and 3 mg/kg) and fluoxetine (20 mg/kg) pretreatments significantly attenuated the reserpine-induced rise in ptosis score (p < 0.01; p < 0.001; p < 0.001) mice in comparison to mice that received reserpine alone [for time F(3, 140) = 190.7, p < 0.001; treatment F(6, 140) = 79.32, p < 0.001; time × treatment F(18, 140) = 9.919, p < 0.001]. However, fluoxetine conspicuously attenuated (p < 0.001) the ptosis score with respect to lacidipine in reserpine-treated mice. Administration of Ca2+ agonist, Bay-K8644, attenuated the effects (p < 0.05) of lacidipine (3 mg/kg) in reserpine-injected mice with respect to mice that received lacidipine (3 mg/kg) and reserpine (Fig. 2a).
Lacidipine attenuates ptosis, catalepsy, and hypothermia in reserpine-treated mice. Statistical analysis of a ptosis score (two-way ANOVA followed by Bonferroni’s test), b catalepsy score, and c rectal temperature (one-way ANOVA followed by Tukey’s post hoc test). Values are expressed as mean ± S.E.M. (n = 6). Significance at *p < 0.05, ***p < 0.001 vs vehicle control; #p < 0.05, ##p < 0.01, ###p < 0.001 vs reserpine group; $p < 0.05, $$p < 0.01 vs LCD(3) + reserpine group, @@p < 0.01, @@@p < 0.001 vs fluoxetine + reserpine group
Lacidipine attenuates catalepsy in reserpine-treated mice
Reserpine significantly (p < 0.001) increased the catalepsy in comparison to vehicle treatment.
Pretreatment of reserpine administered mice with LCD (1 and 3 mg/kg) or fluoxetine significantly lowered the cataleptic score (p < 0.05; p < 0.01; p < 0.001), with respect to mice that received reserpine alone [F(6, 35) = 42.62, p < 0.001]. However, lacidipine-treated groups displayed increased (p < 0.001; p < 0.01) cataleptic scores with respect to the fluoxetine + reserpine group. Bay-K8644 administration abolished the effects (p < 0.05) of lacidipine (3 mg/kg) on the cataleptic score in reserpine-injected mice with respect to mice that received lacidipine (3 mg/kg) and reserpine (Fig. 2b).
Lacidipine decreased hypothermia in reserpine-treated mice
Reserpine significantly (p < 0.001) decreased the rectal temperature of mice in comparison to vehicle treatment. Lacidipine (1 and 3 mg/kg) and fluoxetine significantly attenuated hypothermia (p < 0.05; p < 0.01; p < 0.001) in reserpine-administered mice as compared to mice that received reserpine alone [F(6, 35) = 79.47, p < 0.001]. However, fluoxetine more effectively (p < 0.001) attenuated the hypothermia in comparison to lacidipine in reserpine-administered mice. Bay-K8644 significantly abolished (p < 0.05) the LCD(3)-attenuated hypothermia in reserpine-treated mice in comparison to LCD(3) + reserpine group (Fig. 2c).
Lacidipine decreases reserpine-triggered immobility in mice in TST
Reserpine group showed a profound (p < 0.001) increase in the immobility period in comparison to the vehicle control group. Lacidipine (1 and 3 mg/kg) and fluoxetine (20 mg/kg) pretreatments in separate groups of reserpine-administered mice exhibited a significant decline (p < 0.05; p < 0.01; p < 0.001) in immobility in comparison to mice that received reserpine alone [F(6, 35) = 23, p < 0.001]. Although LCD (0.3 mg/kg)-treated mice showed higher (p < 0.001) immobility period with respect to fluoxetine, LCD (1 and 3 mg/kg) treatments showed modest (p > 0.05) increase in immobility with respect to fluoxetine in reserpine-administered mice. Bay-K8644 significantly abolished (p < 0.05) the LCD(3)-induced fall in immobility in reserpine-treated mice in comparison to LCD(3) + reserpine group (Fig. 3). These results indicated that LCD decreases reserpine-triggered symptoms of depression in mice that were significantly abolished by Bay-K8644 (Ca2+ agonist). It can be inferred that the Ca2+ antagonist activity of LCD may provide relief in symptoms of depression.
Lacidipine attenuates reserpine-induced rise in the duration of immobility in mice. Statistical analysis of the immobility period was achieved using one-way ANOVA followed by Tukey’s post hoc test. Values are expressed as mean ± S.E.M. (n = 6). Significance at *p < 0.05; ***p < 0.001 vs vehicle control; #p < 0.05, ##p < 0.01, ###p < 0.001 vs reserpine group; $p < 0.05 vs LCD(3) + reserpine group, @@@p < 0.001 vs fluoxetine + reserpine group
Lacidipine decreases reserpine-triggered rise in the brain TBARS level in mice
Reserpine group showed profound (p < 0.001) increase in brain TBARS levels in mice when compared to the vehicle control group. Pretreatment of reserpine-administered mice with lacidipine (1 and 3 mg/kg) or fluoxetine (20 mg/kg) exhibited a significant decline (p < 0.001, p < 0.01, p < 0.001) in brain TBARS levels with respect to mice that received reserpine alone [F(6, 35) = 31.69, p < 0.001]. However, fluoxetine markedly suppressed the lipid peroxidation in comparison to lacidipine (0.3 mg/kg, p < 0.001; 1 mg/kg, p < 0.001; 3 mg/kg, p < 0.01) in the brain of reserpine-treated mice. Bay-K8644 significantly abolished (p < 0.05) the LCD(3)-induced decrease in brain TBARS levels in reserpine-treated mice in comparison to mice that were given LCD(3) and reserpine only (Fig. 4a). The present findings corroborated that LCD effectively decreased reserpine-triggered lipid peroxidation that was significantly abolished by Bay-K8644 (Ca2+ agonist), which highlights the neuroprotective role of Ca2+ antagonist activity of LCD in the current model of depression.
Lacidipine ameliorates reserpine-induced oxidative stress in the brain of mice. Statistical analysis of brain a TBARS content, b GSH level, c SOD, and d catalase activity was achieved using one-way ANOVA followed by Tukey’s post hoc test. Values are expressed as mean ± S.E.M. (n = 6). Significance at *p < 0.05, **p < 0.01 ***p < 0.001 vs vehicle control; #p < 0.05, ##p < 0.01, ###p < 0.001 vs reserpine group; $p < 0.05, $$p < 0.01, $$$p < 0.001 vs LCD(3) + reserpine group, @@p < 0.01, @@@p < 0.001 vs fluoxetine + reserpine group
Lacidipine attenuates reserpine-triggered decline in the brain GSH level in mice
The administration of reserpine in mice markedly decreased (p < 0.001) the GSH levels in the brain in comparison to vehicle treatment. Pretreatment with LCD (1 and 3 mg/kg) and fluoxetine significantly attenuated (p < 0.05; p < 0.01; p < 0.001) the reserpine-induced decline of GSH content in the brain of mice that received reserpine alone [F(6, 35) = 79.47, p < 0.001]. However, the administration of lacidipine (1 and 3 mg/kg) improved the GSH content that was comparable to fluoxetine pretreatment in reserpine-administered mice. Bay-K8644 significantly abolished (p < 0.05) the GSH enhancing effect of LCD (3 mg/kg) in the brain of reserpine-treated mice with respect to mice subjected to LCD(3) and reserpine only treatment (Fig. 4b). The present results exhibited consolidation of GSH levels in the brain by LCD against reserpine-induced oxidative stress, which was abolished by Ca2+ agonist (Bay-K8644). In the presently used model, LCD bestowed antioxidant effect through inhibition of L-type Ca2+ channels.
Lacidipine attenuates reserpine-triggered decline in the brain SOD in mice
A significant decline (p < 0.001) in SOD activity in the brain of mice injected with a single dose of reserpine was observed in comparison to mice subjected to vehicle treatment. Pretreatment with lacidipine (1 and 3 mg/kg) and fluoxetine attenuated (p < 0.01; p < 0.001; p < 0.001) the reserpine-induced decline of brain SOD activity with respect to reserpine alone administration [F (6, 35) = 43.84, p < 0.001]. Although lacidipine (0.3 and 1 mg/kg)-treated mice exhibited a significantly lower (p < 0.001) brain SOD activity in comparison to fluoxetine, lacidipine (3 mg/kg) treatment revived the SOD activity that was comparable to that by standard drug (fluoxetine) in reserpine-treated mice (Fig. 4c). Bay-K8644 + LCD(3) + reserpine group showed a conspicuous decline (p < 0.001) in SOD activity in comparison to LCD(3) + reserpine group. These results indicated that LCD by virtue of Ca2+ channel blockade attenuated the decline of SOD activity in the brain against reserpine in mice, which was highlighted by the abrogation of effects of LCD by Bay-K8644. Furthermore, the effect of LCD (3) in brain of mice was comparable to the standard drug, fluoxetine.
Lacidipine attenuates reserpine-induced decline in the brain catalase activity
Reserpine group showed a significant reduction (p < 0.001) in the brain catalase activity in comparison to the vehicle control group. LCD (1 and 3 mg/kg) or fluoxetine pretreatment in reserpine-administered mice exhibited a significant rise (p < 0.05; p < 0.001; p < 0.001) in CAT activity in comparison to mice that received reserpine alone [F (6, 35) = 30.16, p < 0.001]. However, the administration of lacidipine (1 and 3 mg/kg) improved the brain catalase activity that was comparable to fluoxetine pretreatment in reserpine-administered mice. LCD(0.3) + reserpine group displayed significantly lower (p < 0.01) CAT activity in comparison to the fluoxetine + reserpine group. Bay-K8644 significantly (p < 0.01) attenuated the CAT restorative activity of LCD(3) in reserpine-treated mice in comparison to mice subjected to LCD(3) and reserpine only (Fig. 4d). These findings depicted that LCD (L-type Ca2+ channel antagonist) attenuated the decline of CAT activity in the brain of reserpine-treated mice, which was significantly abolished by Bay-K8644 (Ca2+ agonist), and thus, highlighted the role of Ca2+ channels in the pathology of depression.
Lacidipine attenuates reserpine-induced nitrosative stress in the brain of mice
Reserpine group showed profound (p < 0.001) increase in brain nitrite levels in mice when compared to the vehicle control group. LCD (1 and 3 mg/kg) and fluoxetine significantly declined (p < 0.01; p < 0.001; p < 0.001) the brain nitrite levels in reserpine-administered mice with respect to mice that received reserpine alone [F (6, 35) = 211.7, p < 0.001]. A significantly higher (p < 0.001) brain nitrite content was noted in response to lacidipine with respect to fluoxetine in reserpine-administered mice. Bay-K8644 significantly decreased (p < 0.001) the LCD(3)-induced decrease in brain nitrite levels in reserpine-treated mice in comparison to LCD(3) + reserpine group (Fig. 5). The present findings corroborated that LCD attenuated the reserpine-triggered nitrosative stress that was significantly abolished by Bay-K8644 (Ca2+ agonist), which highlights the role of Ca2+ antagonist activity of the LCD in neuroprotection in the currently employed model of depression.
Lacidipine decreases reserpine-induced nitrosative stress in the brain of mice. Statistical analysis of brain total nitrite content in the brain was achieved using one-way ANOVA followed by Tukey’s post hoc test. Values are expressed as mean ± S.E.M. (n = 6). Significance at ***p < 0.001 vs vehicle control; ##p < 0.01, ###p < 0.001 vs reserpine group; $$$p < 0.001 vs LCD(3) + reserpine group, @@@p < 0.001 vs fluoxetine + reserpine group
Discussion
Although CCBs are historically used in a variety of cardiovascular origin diseases (e.g. hypertension), they have also been reported to influence monoaminergic yield, brain redox-balance and, thereby, possessing antidepressant activity (Aburawi et al. 2007; Dhir and Kulkarni 2011). Existing evidences highlight the role of calcium in oxidative stress, excitotoxic pathways, and neurodegeneration, which prompted to test CCBs in different psychiatric disorders (Halliwell 2006). In previous studies, dihydropyridine CCBs have shown considerable antidepressant potential in diverse animal models (Aburawi et al. 2007; Bergantin and Caricati-Neto 2016). Oxidative stress is implicated in the pathogenic rise of intraneuronal calcium load by reducing calcium outflux through targeting L-type calcium channels that lead to upregulation of Ca2+-NOS-guanylyl cyclase pathway and symptoms of depression in rodents (Paul 2001, Salido, 2009). The present study aims to delineate the effects of lacidipine (L-type Ca2+ channel antagonist) on symptoms of depression and underlying etiopathogenic factors such as oxido-nitrosative stress in the brain of mice. Bay-K8644 is a Ca2+ channel agonist employed to enlighten the putative role of L-type Ca2+ channels in depression in LCD and reserpine-treated mice. Reserpine was used at dose of 5 mg/kg to instigate the pathology of depression in LCD pretreated mice. Reserpine is widely used to induce the symptoms of depression in rodents, owing to its ability to deplete the monoamine levels at the synapse (e.g., cortex, striatum, limbic region) and enhancing the oxidative burden (Lohr et al. 2003; Arora et al. 2011).
Ptosis, catalepsy, and hypothermia are indices used worldwide for the characterization of reserpine-induced depression in rodents. Reserpine irreversibly blocks vesicular transportation of monoamines which triggers their oxidative metabolism and thereby generates free radicals. In the present study, reserpine administration enhanced ptosis and cataleptic score of mice and reduced the rectal temperature. LCD pretreatment significantly attenuated the reserpine-induced reduction in rectal temperature as compared to reserpine-only administration. Furthermore, cataleptic and ptosis scores were also reduced significantly by LCD pretreatment for 14 days in reserpine-injected mice. These observations highlight the antidepressant potential of LCD. In the current protocol, protection from reserpine-induced ptosis, catalepsy, and hypothermia by the standard antidepressant drug (fluoxetine) in mice validates these as key parameters in reserpine-induced depression. Bay-K8644 is a structural analog of nifedipine that activates L-type calcium channels and was administered to LCD(3) and reserpine-treated mice. In the present study, Bay-K8644 significantly attenuated the effects of LCD (3) on catalepsy, ptosis, and rectal temperature in reserpine-treated mice.
TST is an inescapable but moderately stressful rodent model, widely used for screening of new antidepressant drugs. This test has a sensitivity and specificity to all major classes of antidepressant drugs. In TST, immobility indicates a state of despair that is decreased by potential antidepressant drugs (Dhingra and Valecha 2007). The results of the current study exhibited a significant increase in the immobility period of mice in TST after 18 h of reserpine administration. However, a marked reduction in the immobility period was observed in reserpine-injected mice that were previously pretreated with LCD for 14 days. Fluoxetine was used as a standard antidepressant drug in the present protocol, which also exhibits inhibition of Ca2+ channels (L-type) and serotonin reuptake at the synapse. Although fluoxetine pretreatment was able to conspicuously decline the immobility period of reserpine-injected mice in TST, this effect was akin to that by lacidipine (1 and 3 mg/kg). Hence, it can be inferred that in the current model of depression, the antidepressant-like effects of lacidipine is comparable to fluoxetine (standard drug) which might be exploited as a substitute to standard antidepressant therapy specifically in patients of cardiovascular diseases. Administration of Bay-K8644 abolished the effect of lacidipine on the immobility period in TST in reserpine-treated mice. These findings demonstrate antidepressant-like effects of LCD (Ca2+ antagonist) against reserpine-induced depression in mice, which were significantly attenuated by Ca2+ channel agonist (Bay-K8644).
Oxidative damage to poly-unsaturated fatty acid (membrane fatty acid) and palmitic and myristic acid deteriorates neurolemma integrity, signal transduction, and cytoskeleton leading to neuron dysfunction, and also influences brain neurotransmitter functions (e.g., glutamate) involved in the pathology of depression (Vavakova et al. 2015). Human studies revealed a profound increase in biomarkers of oxidative stress (e.g., malondialdehyde, 8-iso-prostaglandin F2α), genocide product 8-hydroxy-2′-deoxyguanosine, xanthine oxidase activity, nitrite-adducts, inflammatory (e.g., tumor necrosis factor-α) and immunogenic (e.g., IgG, IgM) cascade in the brain, and serum of patients of major depression (Hasler 2010; Siwek et al. 2013). Some recent evidence indicates that targeting the mitochondrial dysfunction-associated oxidative stress by antioxidants such as vitamin E, coenzyme Q10, N-acetylcysteine, and curcumin may attenuate the symptoms of depression (Maes et al. 2012). TBARS is widely used to assess the lipid peroxidation product, malondialdehyde (MDA), which is highly neurotoxic and immunogenic. MDA forms adducts with a diverse range of biomolecules such as advanced glycation end products (AGEs), acetaldehyde and deoxyribonucleic acid (DNA) that are capable of activating microglial response detrimental to monoaminergic neurons. Pathogenic elevation of nitrite content in the brain leads to the formation of peroxynitrite radical, nitrosylation of proteins and modulation of many neurochemicals (e.g., monoamines). Nitric oxide adducts (e.g. tyrosine, phenylalanine, aspartate, histidine) capable of eliciting IgM immune response are observed in depression (Maes et al. 2012). In comparison to controls, significantly higher content of nitric oxide has been observed in patients with severe depression and suicidal propensity. Antidepressant effect of nitric oxide synthase inhibitors (e.g., L-NAME) is attributed to the elevation of monoaminergic transmission (e.g., serotonin and dopamine) which was also validated by augmentation of antidepressant activity of bupropion (atypical antidepressant) by 7-NI in rodents (Dhir and Kulkarni 2011). In previous studies, it is observed that the bidirectional modulation of association between oxidative stress (e.g., lipid peroxidative products and nitrosative stress) and intracellular Ca2+ burden via L-type channels provide impetus to the pathogenesis of depression (Aburawi et al. 2007; Bergantin and Caricati-Neto 2016). In this study, mice exposed to reserpine exhibited a marked rise in brain TBARS and nitrite content and a decrease in GSH, catalase, and SOD activity, thereby indicating compromised brain antioxidant defense. The present findings support the earlier results that indicated reserpine-triggered oxidative stress in the brain of rodents (Arora et al. 2011). However, pretreatment with LCD attenuated the surge in TBARS and nitrite levels in the brain of mice administered with reserpine. These results are in harmony with earlier literature that reported direct scavenging of numerous radical species in vitro as well as in vivo by LCD (van Amsterdam et al. 1992; McCormack and Wagstaff 2003; Berkels et al. 2005). In earlier studies, a decline in activities of xanthine oxidase, myeloperoxidase, reactive oxygen species output, MDA levels, and rise in GSH content by LCD in animal models of ischemia and diabetes also substantiate the results of the present study (Berkels et al. 2005; Kumbasar et al. 2012). Activities of SOD and catalase and glutathione levels showed considerable improvement in the brain of reserpine-administered mice pretreated with lacidipine for 14 days. The standard drug (fluoxetine) decreased the lipid peroxidation and nitrite content and increased the GSH, SOD, and catalase activity in the brain of reserpine-treated mice. In the reserpine-induced mouse model of depression, the antioxidative potential of fluoxetine and lacidipine were comparable. Bay-K8644 administration antagonized the effects of lacidipine on biomarkers of oxidative stress in brain of reserpine-treated mice. The reversal of antidepressant effects of LCD in reserpine-treated mice by Bay-K8644 indicates that L-type calcium channel antagonism may serve as a possible strategy to curb the pathogenesis of depression and its symptoms.
Conclusion
In the present study, reserpine instigated characteristic symptoms of depression in mice through elevation of oxido-nitrosative stress in the brain. Pretreatment with lacidipine showed profound improvement in depression-like symptoms by virtue of its Ca2+ channel blocking and antioxidant activities in reserpine-administered mice.
References
Aburawi S, Al-Tubuly R, Alghzewi E, Gorash Z (2007) Effects of calcium channel blockers on antidepressant action of alprazolam and imipramine. Libyan J Med 2(4):169–175. https://doi.org/10.4176/070909
Ahmed HH, Abd El Dayem SM, Aly Foda FM, Mohamed HA (2015) Significance of vitamin D in combination with calcium in modulation of depression in the experimental model. Der Pharma Chemica 7:128–147
Arora V, Kuhad A, Tiwari V, Chopra K (2011) Curcumin ameliorates reserpine-induced pain-depression dyad: behavioural, biochemical, neurochemical and molecular evidences. Psychoneuroendocrinology 36(10):1570–1581. https://doi.org/10.1016/j.psyneuen.2011.04.012
Askew BM (1963) A simple screening procedure for imipramine-like antidepressant agents. Life Sci 2(10):725–730. https://doi.org/10.1016/0024-3205(63)90076-6
Bellosta S, Canavesi M, Favari E, Cominacini L, Gaviraghi G, Fumagalli R, Paoletti R, Bernini F (2001) Lacidipine modulates the secretion of matrix metalloproteinase-9 by human macrophages. J Pharmacol Exp Ther 296(3):736–743
Bergantin LB, Caricati-Neto A (2016) Impact of interaction of Ca2+/cAMP intracellular signalling pathways in clinical pharmacology and translational medicine. Clin Pharmacol Transl Med 1(1):2–5
Berkels R, Breitenbach T, Bartels H, Taubert D, Rosenkranz A, Klaus W, Roesen R (2005) Different antioxidative potencies of dihydropyridine calcium channel modulators in various models. Vasc Pharmacol 42(4):145–152. https://doi.org/10.1016/j.vph.2004.11.003
Cao X, Wei Z, Gabriel GG, Li X, Mousseau DD (2007) Calcium-sensitive regulation of monoamine oxidase-a contributes to the production of peroxyradicals in hippocampal cultures: implications for Alzheimer disease-related pathology. BMC Neurosci 8:73
Claiborne A (1985) Catalase activity. In: Greenwald RA (ed) CRC handbook of methods for oxygen radical research, 3rd edn. CRC, Boca Raton, pp 283–284
Costall B, Naylor RJ (1974) On catalepsy and catatonia and the predictability of the catalepsy test for neuroleptic activity. Psychopharmacologia 34(3):233–241
Deak F, Lasztoczi B, Pacher P, Petheo GL, Kecskemeti V, Spat A (2000) Inhibition of voltage-gated calcium channels by fluoxetine in rat hippocampal pyramidal cells. Neuropharmacology 39(6):1029–1036. https://doi.org/10.1016/S0028-3908(99)00206-3
Dhingra D, Valecha R (2007) Evaluation of the antidepressant-like activity of Convolvulus pluricaulischoisy in the mouse forced swim and tail suspension tests. Med Sci Monit 13(7):BR155–BR161
Dhir A, Kulkarni SK (2011) Nitric oxide and major depression. Nitric Oxide 24(3):125–131. https://doi.org/10.1016/j.niox.2011.02.002
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Guan LP, Liu BY (2016) Antidepressant-like effects and mechanisms of flavonoids and related analogues. Eur J Med Chem 121:47–57. https://doi.org/10.1016/j.ejmech.2016.05.026
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97(6):1634–1658. https://doi.org/10.1111/j.1471-4159.2006.03907.x
Hasler G (2010) Pathophysiology of depression: do we have any solid evidence of interest to clinicians? World Psychiatry 9:155–161. https://doi.org/10.1002/j.2051-5545.2010.tb00298.x
Jackson KJ, Damaj MI (2009) L-type calcium channels and calcium/calmodulin-dependent kinase II differentially mediate behaviors associated with nicotine withdrawal in mice. J Pharmacol Exp Ther 330(1):152–161. https://doi.org/10.1124/jpet.109.151530
Joca SR, Guimaraes FS (2006) Inhibition of neuronal nitric oxide synthase in the rat hippocampus induces antidepressant-like effects. Psychopharmacology 185(3):298–305. https://doi.org/10.1007/s00213-006-0326-2
Kumar M, Bansal N (2018) Ellagic acid prevents dementia through modulation of PI3-kinase-endothelial nitric oxide synthase signalling in streptozotocin-treated rats. Naunyn Schmiedeberg's Arch Pharmacol 391(9):987–1001. https://doi.org/10.1007/s00210-018-1524-2
Kumbasar S, Yapca OE, Bilen H, Suleyman B, Ozgeris FB, Borekci B, Suleyman H (2012) The effect of Lacidipine on ischemia-reperfusion induced oxidative damage in ovaries of female rats. Biomed Res 23(4):495–500
Liu T, Zhong S, Liao X, Chen J, He T, Lai S, Jia Y (2015) A meta-analysis of oxidative stress markers in depression. PLoS One 10(10):e0138904. https://doi.org/10.1371/journal.pone.0138904
Lohr JB, Kuczenski R, Niculescu AB (2003) Oxidative mechanisms and tardive dyskinesia. CNS Drugs 17(1):47–62. https://doi.org/10.2165/00023210-200317010-00004
Lowry OH, Rosebrough NJ, Farr A, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Maes M, Fišar Z, Medina M, Scapagnini G, Nowak G, Berk M (2012) New drug targets in depression: inflammatory, cell-mediated immune, oxidative and nitrosative stress, mitochondrial, antioxidant, and neuroprogressive pathways. And new drug candidates--Nrf2 activators and GSK-3 inhibitors. Inflammopharmacology Jun 20(3):127–150. https://doi.org/10.1007/s10787-011-0111-7
McCormack PL, Wagstaff AJ (2003) Lacidipine: a review of its use in the management of hypertension. Drugs 63(21):2327–2356. https://doi.org/10.2165/00003495-200363210-00008
Nemeroff CB (2007) The burden of severe depression: a review of diagnostic challenges and treatment alternatives. J Psychiatr Res 41(3–4):189–206. https://doi.org/10.1016/j.jpsychires.2006.05.008
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Paul IA (2001) Antidepressant activity and calcium signaling cascades. Hum Psychopharmacol 16(1):71–80. https://doi.org/10.1002/hup.186
Prakhie IV, Oxenkrug GF (1998) The effect of nifedipine, Ca(2+) antagonist, on activity of MAO inhibitors, N-acetylserotonin and melatonin in the mouse tail suspension test. Int J Neuropsychopharmacol 1(1):35–40. https://doi.org/10.1017/S1461145798001096
Ried LD, Tueth MJ, Handberg E, Kupfer S, Pepine CJ, INVEST Study Group (2005) A study of antihypertensive drugs and depressive symptoms (SADD-Sx) in patients treated with a calcium antagonist versus an atenolol hypertension treatment strategy in the International Verapamil SR-Trandolapril study (INVEST). Psychosom Med 67(3):398–406. https://doi.org/10.1097/01.psy.0000160468.69451.7f
Ried LD, Tueth MJ, Taylor MD, Sauer BC, Lopez LM, Pepine CJ (2006) Depressive symptoms in coronary artery disease patients after hypertension treatment. Ann Pharmacother Apr 40(4):597–604. Epub 2006 Mar 28. https://doi.org/10.1345/aph.1G438
Rubin B, Malone MH, Waugh MH, Burke JC (1957) Bioassay of Rauwolfia roots and alkaloids. J Pharmacol Exp Ther 120(2):125–136
Salido GM (2009) Oxidative stress, intracellular calcium signals and apoptotic processes. In: Salido GM, Rosado JA (eds) Apoptosis: involvement of oxidative stress and intracellular Ca2+ homeostasis, 1st edn. Springer, Dordrecht, pp 1–16
Sastry KV, Moudgal RP, Mohan J, Tyagi JS, Rao GS (2002) Spectrophotometric determination of serum nitrite and nitrate by copper-cadmium alloy. Anal Biochem 306(1):79–82. https://doi.org/10.1006/abio.2002.5676
Siwek M, Sowa-Kućma M, Dudek D, Styczeń K, Szewczyk B, Kotarska K, Misztakk P, Pilc A, Wolak M, Nowak G (2013) Oxidative stress markers in affective disorders. Pharmacol Rep 65(6):1558–1571. https://doi.org/10.1016/S1734-1140(13)71517-2
Spiers JG, Chen HJ, Sernia C, Lavidis NA (2015) Activation of the hypothalamic-pituitary-adrenal stress axis induces cellular oxidative stress. Front Neurosci 8:456. https://doi.org/10.3389/fnins.2014.00456
Steru L, Chermat R, Thierry B, Simon P (1985) The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology 85(3):367–370. https://doi.org/10.1007/BF00428203
van Amsterdam FT, Roveri A, Maiorino M, Ratti E, Ursini F (1992) Lacidipine: a dihydropyridine calcium antagonist with antioxidant activity. Free Radic Biol Med 12(3):183–187. https://doi.org/10.1016/0891-5849(92)90025-C
Vavakova M, Durackova Z, Trebaticka J (2015) Markers of oxidative stress and neuroprogression in depression disorder. Oxidative Med Cell Longev 2015(898393):1–12. https://doi.org/10.1155/2015/898393
Winterbourn CC, Hawkins RE, Brian M, Carrell RW (1975) The estimation of red cell superoxide dismutase activity. J Lab Clin Med 85(2):337–341
Wultsch T, Chourbaji S, Fritzen S, Kittel S, Grünblatt E, Gerlach M, Gutknecht L, Chizat F, Golfier G, Schmitt A, Gass P, Lesch KP, Reif A (2007) Behavioural and expressional phenotyping of nitric oxide synthase-I knockdown animals. J Neural Transm Suppl 72:69–85. https://doi.org/10.1007/978-3-211-73574-9_10
Acknowledgements
The authors are thankful to I. K. Gujral Punjab Technical University, Kapurthala (India) and ASBASJSM College of Pharmacy, Bela (Ropar), Punjab, India for providing the necessary facilities for carrying out research work.
Author information
Authors and Affiliations
Contributions
Prof. (Dr.) Nitin Bansal designed this study. Kunal Khurana (Ph.D. Research scholar) conducted the research and analyzed and interpreted the data. Both authors wrote the initial and final drafts of the article.
Corresponding author
Ethics declarations
The experimental protocol was approved by the Institutional Animal Ethics Committee (ASCB/IAEC/08/15/108).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khurana, K., Bansal, N. Lacidipine attenuates reserpine-induced depression-like behavior and oxido-nitrosative stress in mice. Naunyn-Schmiedeberg's Arch Pharmacol 392, 1265–1275 (2019). https://doi.org/10.1007/s00210-019-01667-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-019-01667-6