Abstract
Caffeine is the most consumed psychoactive substance in the world; in general, it is not associated to potentially harmful effects. Nevertheless, few studies were performed attempting to investigate the caffeine addiction. The present review was mainly aimed to answer the following question: is caffeine an abuse drug? To adress this point, the effects of caffeine in preclinical and clinical studies were summarized and critically analyzed taking account the abuse disorders described in the Diagnostic and Statistical Manual of Mental Disorders (DSM-V). We concluded that the diagnostic criteria evidenced on DSM-V to intoxication-continued use and abstinence are not well supported by clinical studies. The fact that diagnostic criteria is not widely supported by preclinical or clinical studies may be due specially to a controversy in its exactly mechanism of action: recent literature point to an indirect, rather than direct modulation of dopamine receptors, and auto-limitant consumption due to adverse sensations in high doses. On the other hand, it reports clear withdrawal-related symptoms. Thus, based on a classical action on reward system, caffeine only partially fits its mechanism of action as an abuse drug, especially because previous research does not report a clear effect of dopaminergic activity enhance on nucleus accumbens; despite this, there are reports concerning dopaminergic modulation by caffeine on the striatum. However, based on human and animal research, caffeine withdrawal evokes signals and symptoms, which are relevant enough to include this substance among the drugs of abuse.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Caffeine is the most famous and consumed psychostimulant substance in the world: it has fans and lovers all over the world, consuming it in a myriad of presentations such as drinks, infusions, nutritional supplements, and medicines. In general, it is consumed in a non-medical context, in fractionated doses during the day. When taken in a moderated use, it can lead to a subtle stimulant effect, useful to relieve the state of mental fatigue and to increase the state of wakefulness and attention (Nehlig et al. 1992). Even though caffeine exerts a mild pharmacological effect when consumed associated to daily common products, it has a complex interaction within the central nervous system (CNS). In addition, its action can be modified or strengthened by other components, for example, antibiotics quinolones (especially ciprofloxacin and ofloxacin) and inhibitors of CYP1A2, such as ketoconazole, and fluvoxamine which increase its toxicity (Fredholm et al. 1999). Unlike other psychoactive drugs, caffeine is legal, affordable, and has no regulatory framework in most of its consuming countries. This study performs a review focusing on the following hypothesis: caffeine can be (H1) or cannot be (Ho) considered a drug of abuse, considering recent studies and DSM-V criteria. Here, we also describe caffeine in summary, considering its molecular structure, its main interactions with central nervous system receptors, since this information provides important framework to the discussion about addiction and abstinence, as well as other symptoms associated to drugs of abuse. In this context, we selected literature information in order to analyze, under different criteria, whether caffeine can be considered a substance of abuse, looking both through the optics of recent research on mechanisms of action and through observed clinical withdrawal symptoms. Finally, we described the main findings of existing literature about the abuse criteria regarding caffeine in the light of the abstinence symptoms enumerated in DSM-V.
Methodology of reviewing process
The Pubmed database was used for selecting studies conducted in humans and in animal models aimed to investigate the potential abuse effects of caffeine; selected manuscripts were published in the period between 1975 and 2018. In the search for original articles, firstly we selected studies conducted in animals and in humans. Exceptions were articles used in the brief description of molecular characteristics and pharmacological profile of caffeine. The dyads of terms used in surveys througout the text were “caffeine and dependence,” “caffeine and withdrawal,” “caffeine and dopamine,” “caffeine and microdialysis,” “caffeine and nucleus accumbens,” “caffeine and ventral tegmental area,” and “caffeine and FMRI.” For human studies, we differentially included “caffeine and abstinence” and for animal studies, we also included “caffeine and microdyalisis,” and “caffeine and effects.” A total of 1.945 manuscritps were found. A total of 1.187 papers were excluded based on the title (further in more detail in the text).
Manuscripts that did not provide data that addressed clinical effects regarding caffeine, its mechanisms of action, abuse, tolerance, withdrawal, as well as dependence or rewarding pathways were excluded from this review. The remaining 758 articles were subjected to abstract reading and then 481 papers excluded after this phase. After full reading, we selected 277 papers, thus, 257 papers were excluded, and, finally, 20 original articles were used in this review (see Fig. 1) to compose the three tables along the manuscript according to classification methods presented in sequence.
Classification methods presented in sequence. We used pairs of words as indicated in PubMed database for clinical trials in humans (at the right side) and animals (at the left side) as indicated. In sequence is presented the following filtering steps in this review. Scores from “A” to “D” were placed according to an order of credibility to guide the concluding positioning about this question
The present review also used some criteria of article credibility considering their utility to address clinical and pharmacological criteria related to the abuse of caffeine. Manuscripts that did not provide data that addressed clinical and preclinical effects regarding caffeine, its mechanisms of action, abuse, tolerance, withdrawal, as well as dependence or rewarding pathways were excluded from this review.
We defined scores from “A” to “D,” related to an order of credibility to guide the concluding positioning about this question. They were here defined as follows:
-
A: Controlled studies in humans or based on questionnaires with 20 or more subjects per group and using consolidated clinical manuals;
-
B: Studies in patients and volunteers with questionnaires and controlled studies with less than 20 subjects per group or with unconsolidated clinical diagnosis or experimental evaluation scales which were not yet consolidated;
-
C: Animal studies with a minimum of three subjects per group for microdialysis, pharmacokinetic, or biochemical studies. In the case of behavioral experiments, with a minimum of six animals per group;
-
D: Studies in animals with less than three animals per group for microdialysis, biochemical, or pharmacokinetic studies. In the case of behavioral experiments, with less than six animals per group
Chemical structure, metabolization, and general action in the organism
Caffeine (1,3,7-trimethylxanthine) is an alkaloid (Brenelli 2003), a group of pharmacologically active, basic pH molecules consisting of bitter crystalline solids. In pure form, it is a bitter white powder.
It is known that by the end of the last century, caffeine had been found in an average of 100 plant species, examples are Camellia sinensis (tea), Coffea arabica (coffee), Paullinia cupana (guaraná), and Ilex paraguariensis (mate). In these plants, caffeine acts as a natural pesticide, inhibiting herbivory by insects. It is usually found in seeds, in the pericarp of fruits and in the leaves of plants (Ashihara 2006). Caffeine is the most popular psychoactive substance in the world, due to the diversity of products that contain it (Nehlig et al. 1992). It is most commonly used ingested as an infusion, orally. Caffeine, however, can be administered, in addition to the oral route, by intramuscular, intraperitoneal, subcutaneous, and rectal routes (suppositories). It rapidly reaches the bloodstream and distributes itself through the tissues (Altimari et al. 2001). About 10 to 35% are bound to plasma proteins (Pardo Lozano et al. 2007). When administered orally, it is absorbed quickly and completely by the gastrointestinal tract, with total bioavailability. It crosses the blood-brain barrier and placenta, and also concentrates on bile, breast milk, semen, and saliva (Pardo Lozano et al. 2007). After ingestion, the caffeine peak in the bloodstream is situated betwen 15 and 120 min, depending on the presence or not of food in the gastrointestinal tract (Altimari et al. 2001). They present plasma half-life of 3 to 5 hours (Pardo Lozano et al. 2007).Caffeine is metabolized in the liver, mainly by cytochrome P450 CYP1A2, which promotes its demethylation. The main metabolites are paraxanthine (1,7-dimethylxanthine) (85%), theobromine (3,7-dimethylxanthine) (10%), and theophylline (1,3-dimethylxanthine) (5%). Caffeine is excreted by the urine, although it appears in a small amount (0.5 to 3%) (Altimari et al. 2001).
Caffeine, as well as its metabolites, is lipophilic substances: they easily cross the blood-brain barrier (Chen et al. 2010) and reach the central nervous system. Upon arriving, caffeine binds to presynaptic A1 adenosine receptor and postsynaptic A2A adenosine receptor (Daly and Fredholm 1998). This action prevents the endogenous adenosine from attaching to these receptors, acting as a classical competitive antagonist of adenosine receptors (Meredith et al. 2013; Altimari et al. 2001). Notably, caffeine displays a variety of functions at the cellular level (Daly and Fredholm 1998), further than the adenosine receptor antagonism, as follows, intracellular calcium mobilization in the endoplasmic reticulum, phosphodiesterase enzyme inhibition, sodium and potassium pump action.
Caffeine is a potent central nervous system stimulant and is often used to reduce physical fatigue and increase alertness. Initially, there is an increase in alertness, a clearer flow of thought, greater focus and better overall bodily coordination (Stoner et al. 1988). The symptoms caused by the consumption of caffeine in humans depend on the ingested dosage. When there is a low amount of caffeine consumption, it is usually observed in an acute condition: increased wakefulness, decreased somnolence, fatigue relief, increased catecholamine release, increased cardiorespiratory frequency, increased metabolism, and diuresis (Braga and Alves 2000). Although these “positive” effects of caffeine can be usually considered safe, if caffeine is chronically consumed without restriction, cnsumers can develop cardiovascular problems, pregnancy risks, anxiety disorders, and insomnia (Striley et al. 2011).
Pharmacodynamics of caffeine: interactions with dopamine and adenosine systems
Caffeine produces subjective and behavioral effects that are similar, albeit lighter, than the corresponding effects of typical psychomotor stimulators (such as methylphenidate, amphetamines), which that are known to modulate dopaminergic pathways (Kaasinen et al. 2004). However, available findings suggest that the action of caffeine occurs primarily on adenosine rather than in dopaminergic receptors (Ferré 2016). Caffeine induces a psychomotor activation (hyperlocomotion and stereotypes) compatible with the so-called Theory of Stimulating Psychomotor Dependence (Wise and Bozarth 1987), in which reinforcing mechanisms are related to an activity rise in classical dopaminergic pathways. In general, psychostimulants induce behaviors as a consequence of an augmented dopaminergic liberation in nucleus accumbens, as well as in the caudate-putamen (Ferré 2016), but it is not attested for caffeine.
In seminal studies, a singular difference considering an indirect dopamine receptor agonist (such as amphetamine), which facilitates the accumulation of this monoamine within the synaptic cleft, and a direct dopamine receptor agonist (apomorphine; a D1 full agonist), which objectively binds to dopamine receptors, was firstly described.
In this context, caffeine (and theophylline) actions were compatible, when injected in rodents, with a behavioral output which is compatible to direct dopamine receptor agonists (Fuxe and Ungerstedt 1974). In a more recent paper, researchers observed, enhanced locomotor patterns caused by caffeine apparently were extremely related to the upregulation of dopamine receptors, located more specifically on the striatum. (Volkow et al. 2015).
Aditionally, some papers reported rodent behavioral effects of caffeine administration alone as a consequence of an indirect agonistic action of caffeine on dopamine receptors (Fenu and Morelli 1998; Cauli et al. 2003). Adittionally, spaced caffeine treatment predisposed rats to the effect of direct and indirect dopamine receptor agonists (Cauli et al. 2003; Pollack et al. 2010).
In addition, caffeine, when administered chronically in rodents, implies in tolerance-related behaviors (Quarta et al. 2004). It must be stressed, however, that the effects of sensitization and tolerance induced by caffeine are described as distinct features, which implies distinct brain processes (Ferré 2016).
Apparently, unilateral dopamine pathway denervation and previous sensitization with direct dopamine receptor agonists discloses and intensifies the apparent direct dopamine receptor agonistic properties of caffeine (Ferré 2016).
Aiming to better situate the antagonistic action of caffeine on adenosine receptors, it is crucial to state the main physiological function of these receptors, together with adenosine itself. Adenosine is produced by cellular metabolism of the neuron and released from the inside of the cell. It accumulates in the synaptic cleft and has an inhibitory action when it binds to its receptors (Alóe et al. 2005).
Adenosine is a key modulator of dopaminergic and glutamatergic neurotransmission in the striatal local module (Ferré 2010). Your receptors are divided into three classes: A1, A2 (A2B and A2B) and A3. Among them, the A1, A2A and A3 receptors have high affinity for adenosine, whereas the A2B and A3 receptors have low affinity (Ribeiro and Sebastio 2010), having also A3 a weak antagonist action (Daly and Fredholm 1998). The molecular mechanism of action of these receptors, when activated, is not yet well explained (Daly and Fredholm 1998); what is known is that these receptors are coupled to adenylyl cyclase (Paes-De-Carvalho 2002), whether acting through inhibition, as in the case of binding to A1 receptors, or stimulation, as in the case of A2 receptors (Nehlig et al. 1992).
The A3 receptors are poorly expressed in the brain and there are not many studies showing its activation after caffeine administration (Ribeiro and Sebastio 2010). Thus, with the exception of the A3 receptor, the other adenosine receptors are antagonized by methylxanthines, such as caffeine (Paes-De-Carvalho 2002).
In normal brain conditions, adenosine cannot activate all of its receptors. In fact, only the A1 and A2A receptors are normally activated. Other receptors (A3) are only activated in specific pathophysiological conditions, such as ischemia and convulsions (Daly and Fredholm 1998).
Adenosine A1 receptors are co-expressed in dopaminergic and glutamatergic neurons, and are localized in greater amounts in the cerebral cortex, cerebellar cortex, hippocampus, substantia nigra, and some thalamic nuclei (Daly and Fredholm 1998). These receptors appear to be located in synaptic terminals, where activation results in inhibition of neurotransmitter release, but this mechanism remains unknown, as well as the role in controlling the release of neurotransmitters in various central pathways under physiological conditions.
A1 receptor is also related to the arousing effects of caffeine; this brain activating process should be distinghished from the achieved by classical psychostimulants, which may recruit different structures and mechanisms. The arousal effects of caffeine seem not to be dependent on ascending dopamine systems, being instead adenosine a crucial mediator of drowsiness after prolonged wakefulness (Ferré 2016). It is belived that adenosine accumulates at the extracellular space of the basal forebrain, cortex and hypothalamus directly binds to A1 receptor-mediated modulation. Additonally, also an indirect A2A receptor-mediated influence of the hypothalamic histaminergic and orexinergic systems seems to be involved in this process (Ferré 2010).
Studies have pointed that the most probable mechanism underlying caffeine tolerance may involve a general arousal emergence mediated by A1 receptor, but also may require both upregulation in density or response on these receptors and be associated to an increase in plasmatic adenosine ammounts as well (Conlay et al. 1997).
Considering that caffeine has a general effect of arousal that inhibits or causes a delay in sleepiness, we also should look for the relationship between adenosine, caffeine, and sleep. In fact, sleep deprivation in rodents promotes similar alterations as those which are detected in prolonged administration of caffeine: the A1 receptor upregulation and an increment in plasma adenosine levels (Elmenhorst et al. 2009). In addition to A2A effects, Ferre et al. also suggested that, complementarily, rodent studies indicate that A2A receptors also could have a synergic action promoting the global arousal observed within caffeine-induced effects (Ferré 2016).
Adenosine A2 receptors are most commonly expressed in the caudate/putamen, nucleus accumbens and olfactory tubercle, being present in GABAergic and dopaminergic neurons (Daly and Fredholm 1998). The A2 receptor stimulates adenylyl cyclase, whereas the co-localized D2 receptors inhibit adenylyl cyclase. Two types of interactions between A2A and D2 receptors have been described: first type, activation of A2A receptors in the striatal membrane may decrease the potency of D2 receptor agonists; second, the activation of D2 receptors in striatal membranes decreases the accumulation of cyclic AMP induced by the activation of A2A receptors (Daly and Fredholm 1998). Complementarily, heteromers of A2A and D2 were specially described in GABAergic striato-pallidal neurons, anatomically located in striatum (Ferré et al. 1991b).
The caffeine mechanism for maintaining alertness is still not well known. However, rodent studies have shown that, following caffeine administration, there was a significant increase in wakefulness in wild mice and also in those knockouts for A1 adenosine receptors. However, the knockout mice for the adenosine A2A receptor did not show any significant change after consumption of the substance. Thus, it is understood that the increase of caffeine-induced vigilance parameters in rodents depends on adenosine A2A receptors rather than A1 receptors (Stoner et al. 1988; Park et al. 2014). Studies suggest that adenosine A1 receptors and dopamine D1 receptors form an antagonistic heterodimer in GABAergic pathways at the basal nuclei and prefrontal cortex (Stoner et al. 1988). Considering that A1 is implied in general arousal of subjects, according to Ferré (Ferré 2016), an increase in responsiveness to non-rewarding salient stimuli is improved by caffeine, through the disinhibition of adenosine-mediated inhibition of ascending arousal systems.
Adenosine A1 receptors are probably localized at the synaptic terminals of neurons, where the endogenous activation of adenosine is believed to inhibit the release of neurotransmitters (Daly and Fredholm 1998). Adenosine A2A receptors are apparently present in dendritic spines and, probably, the activation of endogenous adenosine would have a stimulatory effect on the activity of these inhibitory GABAergic neurons (Daly and Fredholm 1998).
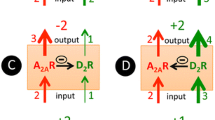
Some preclinical studies have suggested that the pharmacological effects of caffeine are centered on the action of adenosine A1 and A2A receptor antagonists, which are also found in large amounts in the striatum, particularly in striato-palidal GABAergic neurons (Fredholm et al. 1999; Kaasinen et al. 2004; Volkow et al. 2015). Initially, it was postulated that adenosine A1 receptor antagonism could result in increased dopamine efflux in the nucleus accumbens (Solinas et al. 2002), but this finding was observed only with higher doses of caffeine and it was not in agreement with others (Acquas et al. 2002; De Luca et al. 2007). However, it has been shown that the administration of caffeine tends to provide dopamine release or increase of dopaminergic receptor activation in the prefrontal cortex (Acquas et al. 2002). Further explanation suggests that the antagonism of A2A receptor in the ventral striatum (Kaasinen et al. 2004; Volkow et al. 2015) could result in upregulation (Volkow et al. 2015) of dopamine D2/D3 receptors. However, irrespective of the mechanisms responsible for increasing the expression of D2/D3 receptor levels in the striatum, at doses typically consumed by humans, increased dopamine release does not appear to occur (Solinas et al. 2002; Volkow et al. 2015). Although upregulation of dopamine receptors has been proposed in this study as a possible mechanism for withdrawal effects, an alternative hypothesis might be also considered; the acute effects of caffeine could be linked to a modification in the bioavailability or susceptibility to stimulation of receptors that would result from a change in the tertiary structure of the protein, since dopamine and adenosine receptors are found to be associated as heterodimers in the membrane (Ferré et al. 2007; Trifilieff et al. 2011). Comprising an interval of hours, a possible acute consequence of caffeine action could be a specific upregulation of D2 receptors (Volkow et al. 2015), probably linked to membrane externalization of the catalytic receptor domain. However, in observance of the high concentration of adenosine A2A receptors and interaction between A2A-D2 receptors on the striatum, this may also be possible in the thalamus (Kaasinen et al. 2004), which also expresses D2 receptors (De Manzano et al. 2010). The thalamus is described as being involved in global phenomena linked to excitement, to sense of reward and to attention. With a possible blocking effect of adenosine on the thalamus, we would have a thalamic additional effect of increasing the cascade of events mediated by D2 receptors (Kaasinen et al. 2004).
Caffeine is also believed to decrease activation of striatal A2A receptors which are related to an increase of cyclic AMP intracelular levels in GABAergic neurons of the striatum (Daly and Fredholm 1998). Since A2A receptors in these neurons could be co-expressed in heterodymers with D2 dopamine receptors, and considering an antagonic activity of A2A receptor activation on D2 receptor bioavailability at the membrane (Volkow et al. 2015), caffeine would exert an indirect action increasing the coupling of dopamine with the receptor site, and, thus the activation of D2 receptors (Volkow et al. 2015).
According to Ferré (Ferré 2016), a fine regulation of dopamine receptors is achieved by the existence of A2A-D2 receptor heteromers, which promote very detailed reciprocal actions between adenosine and dopamine neurotransmitters. This fine tunning may modulate the firing of GABAergic striato-pallidal cells. More specifically, if the balance tilts to A2A in detriment of D2 receptor signaling, one could observe an increment of neural firing rate mediated by adenylyl cyclase and by allosterically induced modulation of D2; on the contrary, if stimulation favors D2 rather than A2A receptor, the primary effect might result in a diminished cell firing rate and an antagonistic-like effect in A2A receptor activity, due to the counterbalanced action of these two receptors (Ferré et al. 2016). A rational for this regulation is given by previous experiments, in which the magnitude of adenosine receptor agonists potency suggested a specific antagonistic balance considering A2A and D2 receptors (Ferré et al. 1991a). This author also states that “the accumulated knowledge about the function of the striatal A2A-D2 receptor heteromer demonstrates that it is a main target for the psychomotor activating effects of caffeine” (Ferré 2016).
The controversies regarding caffeine action at molecular levels on this scenario of action are still little elucidated. Considering global mechanisms of action of caffeine, while some findings point to an increased effect of dopamine on D2/D3 receptors on the ventral striatum (Volkow et al. 2015) (associated with a general excitatory effect, which includes improvement in attention and decreased fatigue), other findings did not find any correlation between this excitatory and possible plastic modifications in the striatum (Kaasinen et al. 2004).
In summary, here we point out that we find a controversy in caffeine literature about putative mecanisms of action related to addiction and reward. At higher doses, caffeine seems to cause an increase in dopamine at synaptic clefts; in usual doses, it seems to enhance dopamine efects by a mechanism that could indirectly involve both an increase in bioavailability and/or an upregulation of dopamine membrane receptors (Volkow et al. 2015). We hypothesize here that there is less probability of an upregulation process ocurring very acutely (1–2 h) after dosing, since upregulation studies (Peng et al. 1994) are performed at a minimum of 10 to 24 hours after nicotine treatment (Gopalakrishnan et al. 1997). Focusing on D2 receptors, we highlight here a classical effect of haloperidol (a D2 blocker) in D2 upregulation, which in cats are studied in a matter of 1 at 3 weeks (Ginovart et al. 2009).
Another possible mechanism by which caffeine produces its motor and reinforcing effects is by inhibiting the (pre- and postsynaptic) blockade that adenosine imposes on dopaminergic neurotransmission in dendritic spines located on the striatum. By also targeting other type of heterodymers, now the A1-A2A heteromers in glutamatergic terminals and by affecting A1 receptors in dopaminergic terminals (presynaptic brake), and caffeine could induce both glutamate-dependent and glutamate-independent release of dopamine. These presynaptic effects of caffeine are potentiated by the inhibition of the postsynaptic brake imposed by antagonistic adenosine-dopamine receptor-receptor interactions in the A2A-D2 and A1-D1 receptor heteromers (Ferré 2010).
Taking account this view, we may argue that, when chronically ingested at higher doses, caffeine could lead not to an upregulation or to a better membrane exposure of dopamine receptors as proposed for acute action, but to a delayed effect of dopamine receptor downregulation of, which could be related to the observed systemic tolerance in chronic user. This caffeine action, however, is supposed to be indirect, since its direct binding are described not in dopamine, as demonstrated, but in adenosine receptors. Through an indirect stimulation of dopaminergic pathways, downregulation of dopamine receptors linked to drug tolerance could thus emerge as a probable consequence. We emphasize that a possible upregulation in dopamine receptors which can occur in the first hours after intake, as previouslly proposed (Volkow et al. 2015), do not preclude the occurrence of a downregulation mechanism, as considered here, in these receptors, but now comprising a wider chronic use, including weeks or months. This mechanism could be useful to explain much of the tolerance effects described by seminal studies (Ginovart et al. 2009) and by clinical compendiums, such as the DSM-V.
Caffeine effects on mesolimbic system
It has been hypothesized that changes occurring in the nucleus accumbens, due to the presence of drugs of abuse, are related to the general abuse of these drugs, regardless of their specific mechanism of action (Nehlig and Boyet 2000). By contrast, studies have shown that acute caffeine administration does not cause increased levels of dopamine in the shell region of the nucleus accumbens (De Luca et al. 2007). There is only increase at these levels with very large amounts of caffeine, equivalent to five folds normal daily human consumption. In addition, when there is activation of the shell part of the nucleus accumbens with this amount of caffeine, there is also activation of other brain regions, which does not occur in the administration of other drugs of abuse such as amphetamine, cocaine, and nicotine (Nehlig 1999). Other studies, however, report that there is an increase in dopamine concentrations in the nucleus accumbens, but they do not behave in the same way as the increase caused by other drugs of abuse, such as amphetamine and cocaine (Solinas et al. 2002). Because of these differences, caffeine cannot be considered a typical drug of abuse (Solinas et al. 2002).
A relevant study by De Luca et al. (2007) (also detailed in Table 1) showed that, in a paralell manner with some descriptions in humans, microdialysis studies performed in the rat nucleus accumbens, after intraperitoneal administration of different doses of caffeine, show no increase in dopamine concentrations. This study also proposes the investigation of dopamine concentrations in the prefrontal cortex, where, interestingly, they observed a significant increase in dopamine after caffeine administration. That work also brings the plausible hypothesis that this increase of dopamine in the nucleus accumbens showed by some other studies may have occurred due to contamination with the dopamine of the prefrontal cortex itself (De Luca et al. 2007). In addition, in a similar experimental condition, it was reported that at lower doses, which may reflect those normally consumed by humans, caffeine does not cause an increase in dopamine in the nucleus accumbens (Nehlig and Boyet 2000).
QUARTA et al. 2004 (see Table 1) observed that apparently there is an increase in dopamine levels in the shell of the nucleus accumbens after the acute intraperitoneal administration of caffeine at doses ranging from 10 to 30 mg/kg (Quarta et al. 2004). These reported doses of caffeine are much higher than the average normal human consumption, even if we compare it to a rodent whose metabolic rates are usually higher than humans (Caldwell et al. 2004; Atanasov 2007). Following the administration of caffeine at the dose of 3 mg/kg, which would be close to the average human consumption reference values, QUARTA’s report (Quarta et al. 2004) finally reveals that they would not expect such increase of dopamine in nuccleus accumbens.
The work of ACQUAS et al. (Acquas et al. 2002) showed in details the effects of distinct doses of caffeine when administered intravenously (0.25 mg/kg, 0.5 mg/kg, 1 mg/kg, 2.5 mg/kg, and 5 mg/kg), in the rat nucleus accumbens (shell and core) and in the prefrontal cortex, measured by microdialysis. The results go in the same direction of the previously cited ones: there is no increase of dopamine in the nucleus accumbens (shell and core) at any caffeine doses administered; however, there is an increase of dopamine in the prefrontal cortex at all doses used, except to the lower dose. The authors then tested caffeine administration intraperitoneally at doses ranging from 1.5 to 30 mg/kg and they did not find any increase in dopamine levels in the nucleus accumbens (Acquas et al. 2002) (see Table 1).
However, at higher doses, caffeine seems to increase the release of dopamine and glutamate in the nucleus acumbens and the dorsal striatum (caudate-putamen), respectively, which in turn could generate psychostimulatory effects (Solinas et al. 2002; Ferré 2016); in parallel, at lower doses, caffeine seems to modulate the activity of VTA (Ventral Tegumentar Area) (Stoner et al. 1988). On the other hand, another study (Acquas et al. 2002) points out that caffeine was described to modulate the prefrontal cortex by modulation of dopamine/acetylcholine concentration increase, but it does not have any direct relationship with the increased dopamine release found in Nacc (Nucleo Accumbens). Also, those authors suggested that tolerance to the effects of caffeine is linked to A1 antagonism effects (Quarta et al. 2004).
Evaluating caffeine as a potential drug of abuse
In order to address the main question, if caffeine can be or not considered a drug of abuse, we consider here two main sets of criteria for characterization of what is a drug of abuse. The first is a preclinical and pharmacological-based set of criteria. A given drug of abuse can potentially activate directly or indirectly the mesolimbic reward pathway, and influence activity of dopamine receptors in related structures. The second way is the symptom-based set of criteria for classifying a drug of abuse, described in clinical studies and on DSM-V.
We proposed here to highlight the definition of some terms, in DSM-V related to drugs of abuse, in order to better illustrate this topic so-called dependence and abstinence. These definitions were based on the Diagnostic and Statistical Manual of Mental Disorders-V (DSM-V) from American Psychiatric Association. The DSM is a manual that describes all mental disorders of adults and children. In DSM-V (American Psychiatric Association 2013), there is a detailing of the major topic “Substance-Related and Addictive Disorder.” Despite including caffeine-related disorders in the topic of substance-related disorders, this manual clearly defines that diagnosis of addiction can be applied to a range of substances other than caffeine and the generic criteria require that only two of the 11 criteria are required for a diagnosis (Budney et al. 2015). For the first time, in DSM-V, the potential clinical significance of a troublesome syndrome caused by habitual heavy use of caffeine was included in the text (see section III: conditions for further study) (American Psychiatric Association 2013) (Budney et al. 2015). Taking a closer look at caffeine abuse, we realized that the description of this disorder differs from the methodological schemas of other substance use disorders. All other substance disorders, except caffeine abuse, include the same generic criteria, but also require the observation of the three most clinically relevant points for diagnosis: (1) repeated unsuccessful attempts to stop or reduce, (2) abstinence, and (3) continued use despite the damage (Budney et al. 2015).
DSM-V manual does not differ between diagnoses between substance abuse and dependence. Instead, it highlights criteria for classifiying substance use disorders, and this classification is accompanied by criteria for intoxication, withdrawal, substance-induced disorders, and non-specified-related disorders, when relevant.
Returning to DSM-V definitions, here we highlight the terms “abstinence” and “tolerance,” closely related to drug abuse and dependence. As found in DSM-V, “tolerance is signaled when a markedly greater dose of the substance is required to achieve the desired effect or when a markedly reduced effect is obtained after consumption of the usual dose.” Tolerance can be difficult to determine from history alone, and laboratory tests may be helpful (for example, elevated levels of the substance in the blood with little evidence of intoxication suggest a good chance of tolerance). Tolerance should also be recognized from individual variation in initial sensitivity to the effects of specific substances (American Psychiatric Association 2013).
Abstinence is a syndrome that occurs when concentrations of a substance in the blood or tissues decrease in an individual who has maintained prolonged intense use. After developing withdrawal symptoms, the individual shows a trend towards to consume the substance to alleviate them. Abstinence symptoms vary greatly from one class of substance to another, and distinct sets of abstinence criteria are provided for drug classes. Marked physiological signals, and usually easy to gauge, are common with alcohol, opioids and with sedatives, hypnotics, and anxiolytics. Signs and symptoms of withdrawal from stimulants (amphetamines and cocaine), as well as tobacco and Cannabis, are often present, but are less visible. No tolerance or withdrawal is required for a substance use disorder diagnosis. However, in most substance classes, prior withdrawal history is associated with a more severe clinical course (earlier onset of substance use disorder, higher levels of substance use, and a greater amount of substance-related problems) (American Psychiatric Association 2013).
Substance-related disorders, in general, according to DSM-V, are based on a pathological behavioral cluster, grouped under social deterioration, risk under use, dependence, pharmacological actions and abstinence but can be reversed with targeted interventions. The diagnosis is performed according to patterns related to its specificities. Symptomatic behaviors were clustered in some criteria, thus assessing the severity of the condition, as follows.
Criteria 1–4 are marked by low control of drug use. The user may consume the substance in larger quantities or in larger time durations (criterion 1). The individual may demonstrate persistent desire to reduce or regulate substance use and may report a number of unsuccessful efforts to decrease or discontinue use (criterion 2). The individual can spend a lot of time getting the substance, using it or recovering from its effects (criterion 3). Specifically, in some cases of more serious disorders due to substance use, practically all the daily activities of the user revolve around the substance. The individual may manifest craving, through an intense desire or need to use the drug that may occur at any time, but more likely within an environment where the drug was previously obtained or used (criterion 4). It has also been shown that craving involves classical conditioning and is associated with the activation of specific reward brain structures.
Criteria 5–7 refer to social prejudice. Intermittent use may result in failure or disruption of daily activities (criterion 5). The individual, even with apparent interpersonal troubles caused or potentiated by the substance, continues its use (criterion 6). Activities, whether recreational, social, academic, or professional, may be discontinued or restricted with use (criterion 7).
The risk under use is related to the next criteria 8–9. Recurrent use can influence scenarios that pose a risk to individual’s physical integrity (criterion 8). The individual, even recognizing the physical or psychological damage caused or potentiated by the substance, persists on its consumption (criterion 9).
Finally, the the criteria 10–11 focus on pharmacological bases of tolerance and abstinence; tolerance is observed when the drug effects are markedly reduced with the usual dose or, when the dose is highly elevated to achieve the desired effect (criterion 10). Abstinence is affected by the decrease or lack of substance in the tissues of individuals who have maintained their prolonged use and, at the first signs of discomfort, the user will tend to consumption for their relief (criterion 11).
In particular, the concurrent syndrome associated with substance use has specific diagnostic criteria according to its nature. In general, behavioral, physiological and/or evident cognitive changes characterize the first criterion (criterion A). Manifestation of clinical signs followed after 1 to 3 days after cessation of consumption defines second criterion (criterion B). The syndrome also may cause clinical suffering and/or impairment in its usual activities (criterion C). The symptoms are not substantiated by intoxication/withdrawal by another substance (criterion D).
The intoxication caused by drug abuse at a time sets the boundary between desired and adverse effects. Intoxication criteria are defined in following lines. The main characteristic of drug intoxication is the manifestation of a reversive syndrome after its consumption (criterion A), being this specific for each substance. The manifestations of clinical signs which are associated to central nervous system (CNS) usually arise between consumption and the end of use (criterion B). The syndrome causes clinical suffering and/or impairment in the individual usual activities (criterion C). The symptoms are not substantiated by intoxication/withdrawal associated to another substance (criterion D).
In summary, these are criteria related to general drug abuse defined by DSM-V. Since DSM-V is a huge psychiatric reference for diagnoses all over the world, here we based the appointments of DSM-V related to caffeine abuse in order to compare with existing data of experimental research in the field.
Following those steps, recent research has pointed to the potential for caffeine dependence and abstinence. It is known that dependence on caffeine is not only observed in people who are high caffeine consumers, but also in those who have below-average consumption (Juliano et al. 2012). Caffeine intoxication occurs during or after its chronic consumption, usually in a daily perspective. According to DSM-V, symptoms include restlessness, nervousness, excitement, insomnia, facial flushing, diuresis, and gastrointestinal complaints, which may occur with low doses (200 mg) in sensitive individuals such as in children, in the elderly, or in individuals who have never been exposed before. The drug symptoms that usually appear at doses greater than 1 g/day of caffeine ingestion include thoughts and speech with erratic flow, tachycardia or cardiac arrhythmia, increased energy, and psychomotor agitation (American Psychiatric Association 2013). It has been observed that regular consumption of higher doses of caffeine leads to high levels of dependence (Juliano et al. 2012). According to Koppelstaetter et al. (see Table 2), the acute intake of caffeine in humans is related to the activation in the medial frontopolar cortex and right anterior cingulate gyrus (Koppelstaetter et al. 2008); its neuroexcitatory action generally involves attentional actions, modulating also a deactivation of regions considered nodes of the default mode network (DMN), related to introspective thinking (not implied in specific tasks) (Park et al. 2014). Although not yet elucidated, apparently there is an increase in the bioavailability of dopamine D2/D3R receptors, caused by the antagonistic action of caffeine on adenosine A2A receptors (in this case, adenosine and dopamine heterodimeric receptors) (Volkow et al. 2015). This antagonic effect in A2A, according to the authors, may occur without necessarily involving an increase in dopamine release, but making possible a greater activation of dopaminergic receptors within the thalamus, linked to the increase of its bioavailability in the cellular membrane (possibly related to a greater exposure to the catalytic site to the binding molecule) (Kaasinen et al. 2004).
Based on all the generic criteria for disorders, intoxication and withdrawal presented by the DSM-V, a comparative table presenting the main experimental and clinical findings (categories A, B, C, D), as cited in methods section, was constructed in order to compare human experimental data to the main definitions of symptoms related to regular caffeine consumption.
As presented in the Table 3, with the exception of Juliano et al., the experimental studies conducted in humans do not contain or describe the two main DSM-V criteria, wich are abstinence and tolerance. Possibly, literature in the field is much more focused on the neuropharmacological processes, rather than on symptomatic observation and its consequences for the social field from the point of view of the user, which is the focus of DSM-V. We highlight that in studies with animals, for this propose, we considered only the signs of intoxication or withdrawal. Thus, there is a lack of correspondence when we compare clinical-based description of DSM-V and experimental human research related to caffeine addictive effects.
Caffeine consumption at lower to moderate doses produces several positive subjective effects (Evans and Griffiths 1992; Pardo Lozano et al. 2007), such as an increase in well-being, arousal, and sociability (Striley et al. 2011). Such effects are more related to emphatic attitudes within the context of the first daily dose of caffeine, and a plausible hypothesis would be that, in people with regular consumption, an enhanced empathy may occur because the individual has been on withdrawal since the day before, so that consumption works as a reversal of negative symptoms of caffeine, as headache, tiredness/fatigue/anxiety (Meredith et al. 2013).
The reinforcement criterion is linked to a mechanism that leads to an increase in the probability of administering a previously used drug in the future (Meredith et al. 2013) and maintaining behavior on which the delivery of drug is dependent (Griffiths et al. 1979). This mechanism is mainly responsible for the dependence and consumption pattern of the substance (Meredith et al. 2013). Caffeine is considered a consumption booster drug in both humans and animals, since the consumption of low to moderate doses increases the chances of new use (Pardo Lozano et al. 2007). When compared to the reinforcement produced by caffeine and the reinforcement produced by other psychostimulants, such as cocaine and amphetamines, it is perceived that the reinforcement for the use of caffeine is less intense (Meredith et al. 2013) or it can be also related to self-administration under a more narrow range of parametric conditions (Griffiths and Woodson 1988c).
This reinforcement effect becomes more clear in a study involving baboons (Griffiths et al. 1979) which illustrates the differentiation of the reinforcing effect between caffeine and cocaine. This study revealed that, at some of the tested doses, auto-administrated caffeine maintained steady or erratic daily patterns of self-injection in all experimental animal baboons (Griffiths et al. 1979). However, this work also indicates that caffeine does not influence self-administration behavior with the same predictability seen in classic drugs of abuse, only induced a performance level of this behavior above the range of active drugs (including the amphetamine analog) (Griffiths and Woodson 1988c).
In addition, Evans and Griffiths demonstrated that the subjective responses to caffeine and placebo differed between those subjects who chose caffeine and those who chose placebo (Evans and Griffiths 1992). In caffeine choosers, when compared to placebo, caffeine produced positive subjective effects in choosers. In contrast, caffeine produced “negative” subjective effects in non-choosers, relative to placebo (Evans and Griffiths 1992). On the other hand, placebo produces negative effects in choosers, which is an additional evidence of withdrawal effect caused by this substance (Evans and Griffiths 1992). Thus, we can observe that physical dependence can potentiate the reinforce effects of caffeine (Griffiths and Woodson 1988c; Striley et al. 2011). In addition, both positive and negative effects may contribute to regular and habitual use that can potentially result in excessive consumption, and difficulty in stopping caffeine use (Budney et al. 2015), and caffeine reinforcement can occur as the result of the adverse effects caused by abstinence (Rogers et al. 1995). These subjective effects described this work are consistent with the common reported effects of withdrawal (worn out, headache, and flu-like feelings and decreased ratings of alert and well-being) (Schuh and Griffiths 1997). Some evidence suggests that symptoms may emerge later (less than 24 h) from higher doses (e.g., 900 mg/day) of caffeine (Evans and Griffiths 1992). The fact that subjects preferred forfeitng money and, by consequence, avoiding placebo to ingest caffeine, corroborates the pharmacological criteria in DSM-V defined for the identification of the substance use disorder: subjects exhibit withdrawal symptoms due to discontinuation or reduction of their intense and prolonged use (American Psychiatric Association 2013).
Another important study provided suggestive results (Griffiths and Woodson 1988a) that a component of caffeine withdrawal syndrome can be detected in subjects with relatively low levels of dietary exposure to caffeine (Griffiths and Woodson 1988b). These withdrawal symptoms in susceptible subjects appear to begin within only at 12–24 hours after caffeine intake (Juliano and Griffiths 2004). In this study, individuals were on caffeine abstinence for 7 days, showed no withdrawal effects after a single day of exposure to caffeine (300 mg/day) (Evans and Griffiths 1999). However, significant withdrawal symptoms occurred after three consecutive days of caffeine admistration, with somewhat greater severity demonstrated after 7 and 14 consecutive days of exposure (Juliano and Griffiths 2004). Another interesting finding revealed that rats which had previously received injections of caffeine on each of 12 days avoided a novel solution which had been associated with the aversive properties of absence of caffeine in rats repeatedly exposed to caffeine and suggest that physiological withdrawal (Vitiello and Woods 1977). In humans, an increase in preference showed for caffeinated drinks associated with negative effects of overnight caffeine abstinence (Rogers et al. 1995).
Although caffeine tolerance mechanism has not been well characterized, Griffiths and colleagues (Evans and Griffiths 1992) demonstrated the tolerance effects, comprising subjective effects, of caffeine in moderate consumers (mean = 343 mg/daily). Griffths and colleagues (Evans and Griffiths 1992) evidenced clear tolerance effects, in a very innovative work comprising continuous caffeine administration at three different phases to moderate users. They observed that moderate caffeine consumers that chose caffeine consumption (for 3 days) over placebo and continued to receive caffeine for 18 days disclosed an additional preference for caffeine over placebo after this last period, and a reduction of tension, anxiety, headache, confusion and bewilderment, and fatigue when compared to non-choosers (Evans and Griffiths 1992).
After regular consumption of caffeine, a phenomenon known as tolerance may occur (Pardo Lozano et al. 2007). Tolerance to a drug occurs when the normally used dose no longer causes the same subjective, psychological, and behavioral effects as previously caused in the individual, so a higher dose is required to restore equivalent symptoms (Mandel 2002). It is very relevant to observe that tolerance occurs only in higher doses of daily caffeine consumption, that is, from 400 to 1200 mg/day (Meredith et al. 2013). Tolerance, although it lasts while there is constant consumption of the drug, usually dissipates after about 3 days of interruption of consumption, when there is resensitization (Striley et al. 2011).
A clarifying experimental study (Koppelstaetter et al. 2010) used 15 individuals, moderate caffeine users, abstinent for at least 12 hours to observe verbal work memory. Subjects underwent magnetic resonance imaging for 2 days after consumption of 100 mg of caffeine. Activation was observed in the frontopolar medial cortex in both cerebral hemispheres, which extended to the anterior cingulate cortex. The frontopolar medial cortex integrates the prefrontal cortex, since the cingulate cortex is strictly connected to the prefrontal cortex, so that they modulate one another, so the caffeine present in one of these areas can influence the other. Frontopolar prefrontal cortex plays a role in planning, monitoring, and problem-solving functions. And the anterior cingulate cortex has a role in motivated attention, attention allocation, and error detection (Koppelstaetter et al. 2010).
Another research study examines physiological cues of the effects of caffeine during an attention task where patients needed to be alert. Fourteen volunteers were recruited who had abstinence for at least 12 h (Koppelstaetter et al. 2008). Thus, two imaging tests were performed, one before and the other after controlled consumption of caffeine. Increased activity was observed in the following brain regions: left cerebellum, basal nuclei (including the putamen, thalamus and insula) and right precentral gyrus. In contrast, there was a reduction of magnetic activity in the response of the ventro-medial prefrontal cortex, the posterior medial cortex, and the left posterior lateral cortex. This evidence shows that there is an induction of cognitive control, especially related to attention, and in motor and visual functions (Park et al. 2014).
Other study addressed the same basic technique and was based on the observation of nine caffeine-abstinent individuals, in which researchers observed brain activity 30 min after oral consumption of 200 mg of caffeine. The PET scan was used for 40 min after injecting 5 mCi of F-FDG into the subjects while in the scanner. They observed that caffeine intake leads to a decrease of activity in the motor cortex (Park et al. 2014).
Considering the scientific findings of humans and animals, we suggest that the habitual use of caffeine could lead to a sense of reward for a preponderant route, which would be more directly linked to the activation of the mesocortical pathway, acting in the prefrontal cortex, than to a possible increase of dopaminergic activity in the mesolimbic pathway (linked to activity in the nucleus accumbens). The main action of caffeine would involve an activation of areas related to motor planning and control, having observable effects of increased wakefulness, decreased drowsiness and fatigue relief (Striley et al. 2011). This set of caffeine actions, especially for its cortical activation, would lead to a sharply increased focus, more productivity and perception of the reality of the present moment, consequently promoting improvement in working memory (Koppelstaetter et al. 2008, 2010). Taking this into account, mainly in situations of stress or sleep, where a faster performance improvement is needed, individuals could feel more comfortable and productive. The sense of improvement in task execution and information processing could then provide a sense of reward and pleasure indirectly; individuals, rewarded for increased productivity from caffeine activity in the prefrontal cortex, would have a tardive and discrete increase in the release of dopamine in mesolimbic brain centers such as nucleus accumbens, and related to this, a light sense of pleasure as consequence.
The mechanism of action of caffeine, therefore, would be slightly different from that known for classical drugs of abuse. Experimental human research indicates that caffeine has a more prominent action in the prefrontal cortex and may probably be more directly linked to the mesocortical dopaminergic route than the classical, addiction-related, mesolimbic dopaminergic pathway.
Concluding remarks
Considering the analysis of relevant experimental research in caffeine abuse, and its comparison with DSM-V, we concluded that caffeine can be considered a drug of abuse, with the special characteristic of possible auto-regulatory effects occurring when higher doses are ingested. In particular, the view of caffeine as an abuse drug is better supported when we consider the symptoms observed by chronic users who report an ingestion of caffeinated beverages per day, starting from 100 to 200 mg of caffeine when compared to placebo ingestion, 100 mg produced significant increases in subjective positive effects (i.e., content/at ease/relaxed/satisfied and active/stimulated/energetic) and decrease in negative effects (i.e., tired/drowsy/half-awake) (Griffiths and Woodson 1988a; Griffiths et al. 1990; Juliano and Griffiths 2004).
Studies in humans and animals step to a confluence: they both report an action of the drug directed to species-related encephalic structures. These structures are linked to increased alertness, attention, and better control of motor activity. In both humans and rats, there is no robust evidence for a main action of direct general stimulation of the main structure attached to the sensation of pleasure, the nucleus acumbens, or structures specifically linked to emotional processing such as the amygdala, but there are some important reports for an action of this molecule in the striatum. Therefore, we propose here that, in parallel to an indirect stimulator of dopamine at rewarding brain pathways, at least one of the reinforcement mechanisms is probably due to the increase in performance, and the improvement of the attention and motor processes of individuals. Downregulation processes of receptors and other mechanisms of adaptation in a situation of chronic consumption of caffeine need further studies to be better elucidated.
Findings among studies illustrated that individuals reported multiple caffeine use disorder symptoms, including persistent desire for consumption or unsuccessful efforts to cut down or control caffeine use (Juliano et al. 2012). These studies also confirm the specific criterion for “low control,” that is continued use despite harm, and points, to another guiding criterion, that is “risky use of substance,” in which the individual fails to refrain from using the substance. Experimental works also base pharmacological (American Psychiatric Association 2013) and withdrawal criteria (Evans and Griffiths 1992; Schuh and Griffiths 1997; Daly and Fredholm 1998; Nehlig 1999; Dews et al. 2002; Juliano et al. 2012) although excessive and problematic caffeine use has not been associated with serious medical consequences such those observed with alcohol or tobacco use (Budney et al. 2015); on its singularity, excessive caffeine use and abrupt withdrawal are in general associated with increased anxiety, insomnia, headache, drowsiness, and fatigue (Stringer and Watson 1987; Griffiths and Woodson 1988b, c; Griffiths et al. 1990; Evans and Griffiths 1992; Strain and Griffiths 1995; Dews et al. 2002; Juliano and Griffiths 2004; Juliano et al. 2012). Therefore, we point to a need for more clinical studies in the field, to possibly identify specific susceptibility factors for caffeine abuse within the population.
In general, this review evaluates relevant criteria that may give pharmacological significance to caffeine, one of the most consumed stimulants in the world. At doses typically consumed, caffeine acts as an adenosine A2A receptor antagonist (Daly and Fredholm 1998; Solinas et al. 2002; Quarta et al. 2004; Ferré 2010; Volkow et al. 2015) and, in sequence, an increase in dopamine D2 receptors bioavailability (Kaasinen et al. 2004). But in relevant studies, there is no increase in dopamine in the nucleus accumbens (Acquas et al. 2002; De Luca et al. 2007) an important pathway of reward and recognized by the neuroadaptations associated with the phenotype of dependence (Volkow et al. 2015). This aspect possibly explains the poor characterization of caffeine addiction by classical action on the reward system, and, as a consequence, if we consider nucleus accumbens activation as a critical parameter for an abuse drug, we have some difficulty of considering caffeine into this group, in spite of important studies showing a striatal mechanism of indirectly enhancing dopamine D2 receptor-based neurotransmission (Ferré 2016; Dews et al. 2002; Volkow et al. 2015). Nevertheless, withdrawal-related effects are described both in previous literature and in DSM-V. In this sense, if we look at caffeine for its side effects, related to tolerance and withdrawal, considering symptoms criteria, there is less doubt in considering caffeine a drug of abuse. Even though, this conclusion might be taken carefully, since the DSM-V criteria for dependence and withdrawal effects are still not widely accepted or verified in traditional literature (see Table 3).
Based on these prior studies, here we propose that usual daily caffeine doses could produce an acute, transient upregulation of D2-dopamine receptor by antagonism of A2A-adenosine receptors. We did not find specific studies showing the time course of D2 receptor up/downregulation within the cellular membrane. Nevertheless, there are studies in which nicotine exposure in a regular basis, which is an abuse drug, is used to produce locomotor sensitization, and leads to a transient upregulation of ach-nicotinic receptors in VTA (2 hours after a nicotine injection, but not 3 days) (Baker et al. 2013). Based on the time of an up-receptor regulation of in reward system, we can estimate that a possible upregulation of dopamine receptors induced by caffeine could work in an analog time interval.
One study described the high concentration of adenosine A2A receptors and interaction between A2A and D2 as heterodimeric receptors on the striatum, thus opening the possibility of a modulation exerted by adenosine ligands on dopamine receptors; these authors believe that this may also be possible in the thalamus (Kaasinen et al. 2004), which also expresses D2 receptors (De Manzano et al. 2010). The thalamus is described as being involved in global phenomena linked to excitement, sense of reward and attention (Kaasinen et al. 2004). With a possible blocking effect of adenosine on the thalamus by caffeine, we would have a thalamic complementary effect of increasing the cascade of events mediated by D2 receptors (Kaasinen et al. 2004).
Based on these experimental works, and on the limited intensity of abuse symptoms described in DSM-V, we propose here that adenosine receptors may work as fine tunning regulators of dopaminergic receptor activity, especially in the reward pathways. This regulation could be within a strict, buffered band of activity; thus, adenosine receptors could work as “partial dopamine receptor modulators” with an auto-limiting characteristic of modulation. This possibility should be adressed in future studies.
Using classical pharmacological definitions, abuse drugs lead to increased brain activity and the specific release of dopamine in the shell of the nucleus accumbens, the key structure for reward, motivation and dependence (Nehlig 1999). However, usual caffeine doses that reflect daily human consumption do not seem to induce the release of dopamine in the shell of the nucleus accumbens, but probably are related to a release of dopamine in the prefrontal cortex and striatum,which is consistent with the enhancing properties of caffeine in focus and performance (Nehlig 1999; Kaasinen et al. 2004; De Luca et al. 2007; Volkow et al. 2015; Ferré 2016). Thus, caffeine, despite its ability to induce tolerance and physical dependence, does not consistently induce all typical behavioral symptoms of classic abuse drugs. According to DSM-V, only a part of symptoms are observed with caffeine consumption: those related only to abstinence intoxication and not to other neurocognitive disorders (American Psychiatric Association 2013).
In a synthesis: considering pharmacological criterium, some difficulty in classifying caffeine as an abuse drug relies on a slightly different mode of action in the reward system from that of classic abuse drugs, because it does not seem to cause direct dopamine release in reward pathways, but can indirectly influence dopaminergic receptor activity in the striatum. In addition, dopamine pathways could be transiently stimulated through an increase in bioavailability/upregulation of dopamine D2 receptors heterodimerically associated with adenosine A2A receptors in reward system; also, it could induce a subtle, indirect reward sense throughtout the stimulation of mesocortical pathway behaviorally associated to performance/focus enhancement (supported by animal end human experimental studies). On the other hand, considering clinical, symptomatological criteria, caffeine can fit in the drug of abuse group if we consider that its consumption induces some of the most important behavioral abnormalities which characterizes a drug of abuse, according to DSM-V (such as erratic thinking flux or discourse, psichomotor agitation, disphoric humor, irritability).
Recent studies have shown that caffeine has unique psychostimulant characteristics that can enhance the effects of other classical psychostimulants and all other abuse drugs (Ferré 2016). This rational implicates in a need for a serious reflexion in our society, especially concerning the use of caffeine and related adenosine psychostimulants, since they may interact with other more pontentially hazardous drugs. The mechanism underlying these effects goes back to the A2A-D2 receptor heteromer model in striatum. Thus, through the blockade of the adenosine A2A receptor, caffeine leads to a diminished influence of these receptors in D2 receptor activity, resulting in an outcome of full D2-mediated activity. In terms of behavior, this activity is associated with motor psychostimulant effects. The effect centered in D2 activation than can be synergically activated by other indirect dopamine agonists, such as cocaine or amphetamine.
In spite of no specifical effect observed up to date in humans, rodent experiments revealed that, in adolescent rats, stimulant motor and tolerance effects are more pronounced that it is in adults (Marin et al. 2011), indicating more probabilistic dependence of caffeine in an associated to abrain still under development (Rhoads et al. 2011). Considering that adolescents are also more susceptible to classical drugs of abuse than adult population, caffeine consumption in adolescence and its potential relation to other dopaminergic drugs should be a critical issue of future clinical related research.
We suggest that future DSM editions could define more accuratedly criteria for defining caffeine abstinence and intoxication possibly by classifying them according to ponderate weights and not only based on linear sum, as currently suggested. Complementarily, the manual and its criteria do not seem to be so much based on the experimental literature, since drug abuse descriptions are generally focused on clinical symptoms rather than on neural and pharmacological mechanisms. We also recommend that further researches may better elucidate intrinsic pathways which follows caffeine interaction with adenosine receptors, and should focus on a possible upregulation of dopamine D2/D3 receptors, as well as on the interaction between A2A-D2 heterodimers and the relationship with the thalamus.
In this sense, considering current related literature, we conclude that the effects of caffeine can be situated, in a pharmacological perspective, as an auto-limitant rewarding substance, which can be better described as an indirect agonist of dopamine-mediated reward pathways; in a clinical approach, we have support from several studies for both tolerance and withdrawal caffeine-induced effects. Nevertheless, more studies on the field could provide a better understanding of related cellular and molecular mechanisms involved; also, of interest are future studies detailing a possible susceptibility of children and adolescents, as well as the relation of caffeine with other drugs of abuse.
Finally, we suggest, regarding the potential public health damage caused by that substance, which could be of better choice in terms of public policies to provide to the population a better understanding about the potential harmful effects of coffee consumption. In addition, educational campaigns should clarify the recommended doses, and that, in terms of product consumption, campaigns should address the maximum tolerable ammount in daily basis in order to avoid dependence. The population should profit not the harmful, but the optimal effects that caffeine can provide.
References
Acquas E, Tanda G, Di CG (2002) Differential effects of caffeine on dopamine and acetylcholine transmission in brain areas of drug-naive and caffeine-pretreated rats. Neuropsychopharmacology. https://doi.org/10.1016/s0893-133x(02)00290-7
Alóe F, de Azevedo AP, Hasan R (2005) Sleep-wake cycle mechanisms. Rev Bras Psiquiatr 27(Suppl 1):33–39
Altimari LR, Serpeloni E, Zucas SM, Okano H, Burini RC (2001) Cafeína: ergogênico nutricional no esporte Caffeine: nutritional ergogenic in Sports Origem e descrição. R Bras Ciêne Mov 9:57–64
American Psychiatric Association (2013) Diagnostic and statistical manual of mental, 5th edn. American Psychiatric Publishing
Ashihara H (2006) Metabolism of alkaloids in coffee plants. Braz J Plant Physiol 18:1–8. https://doi.org/10.1590/S1677-04202006000100001
Atanasov AT (2007) The linear allometric relationship between total metabolic energy per life span and body mass of mammals. Biosystems 90:224–233. https://doi.org/10.1016/j.biosystems.2006.08.006
Baker LK, Mao D, Chi H, Govind AP, Vallejo YF, Iacoviello M, Herrera S, Cortright JJ, Green WN, McGehee DS, Vezina P (2013) Intermittent nicotine exposure upregulates nAChRs in VTA dopamine neurons and sensitises locomotor responding to the drug. Eur J Neurosci 37:1004–1011. https://doi.org/10.1111/ejn.12114
Braga LC, Alves MP (2000) A cafeína como recurso ergogênico nos exercícios de endurance. Rev Bras Ciênc Mov 8:33–37
Brenelli ECS (2003) A extração de cafeína em bebidas estimulantes: uma nova abordagem para um experimento clássico em química orgânica. Quim Nova 26:136–138. https://doi.org/10.1590/S0100-40422003000100023
Budney AJ, Lee DC, Juliano LM (2015) Evaluating the validity of caffeine use disorder. Curr Psychiatry Rep 17:74. https://doi.org/10.1007/s11920-015-0611-z
Caldwell GW, Masucci JA, Yan Z, Hageman W (2004) Allometric scaling of pharmacokinetic parameters in drug discovery: can human CL, Vss and t1/2 be predicted from in-vivo rat data? Eur J Drug Metab Pharmacokinet 29:133–143
Cauli O, Pinna A, Valentini V, Morelli M (2003) Subchronic caffeine exposure induces sensitization to caffeine and cross-sensitization to amphetamine ipsilateral turning behavior independent from dopamine release. Neuropsychopharmacology 28:1752–1759. https://doi.org/10.1038/sj.npp.1300240
Chen X, Ghribi O, Geiger JD (2010) Caffeine protects against disruptions of the blood-brain barrier in animal models of Alzheimer’s and Parkinson’s diseases. J Alzheimers Dis 20:127–141. https://doi.org/10.3233/JAD-2010-1376
Conlay LA, Conant JA, deBros F, Wurtman R (1997) Caffeine alters plasma adenosine levels. Nature 389:136–136. https://doi.org/10.1038/38160
Daly JW, Fredholm BB (1998) Caffeine—an atypical drug of dependence. Drug Alcohol Depend 51:199–206
De Luca MA, Bassareo V, Bauer A, Di Chiara G (2007) Caffeine and accumbens shell dopamine. J Neurochem 103:157–163. https://doi.org/10.1111/j.1471-4159.2007.04754.x
De Manzano O, Cervenka S, Karabanov A, Farde L, Ullén F (2010) Thinking outside a less intact box: thalamic dopamine D2 receptor densities are negatively related to psychometric creativity in healthy individuals. PLoS One 5:e10670. https://doi.org/10.1371/journal.pone.0010670
Dews PB, O’Brien CP, Bergman J (2002) Caffeine: behavioral effects of withdrawal and related issues. Food Chem Toxicol 40:1257–1261
Elmenhorst D, Basheer R, McCarley RW, Bauer A (2009) Sleep deprivation increases a(1) adenosine receptor density in the rat brain. Brain Res 1258:53–58. https://doi.org/10.1016/j.brainres.2008.12.056
Evans SM, Griffiths RR (1992) Caffeine tolerance and choice in humans. Psychopharmacology 108:51–59
Evans SM, Griffiths RR (1999) Caffeine withdrawal: a parametric analysis of caffeine dosing conditions. J Pharmacol Exp Ther 289:285–294
Fenu S, Morelli M (1998) Motor stimulant effects of caffeine in 6-hydroxydopamine-lesioned rats are dependent on previous stimulation of dopamine receptors: a different role of D1 and D2 receptors. Eur J Neurosci 10:1878–1884
Ferré S (2016) Mechanisms of the psychostimulant effects of caffeine: implications for substance use disorders. Psychopharmacology 233:1963–1979. https://doi.org/10.1007/s00213-016-4212-2
Ferré S (2010) Role of the central ascending neurotransmitter systems in the psychostimulant effects of caffeine. J Alzheimers Dis 20:S35–S49. https://doi.org/10.3233/JAD-2010-1400
Ferré S, Bonaventura J, Tomasi D, Navarro G, Moreno E, Cortés A, Lluís C, Casadó V, Volkow ND (2016) Allosteric mechanisms within the adenosine A2A–dopamine D2 receptor heterotetramer. Neuropharmacology 104:154–160. https://doi.org/10.1016/J.NEUROPHARM.2015.05.028
Ferré S, Ciruela F, Woods AS, Lluis C, Franco R (2007) Functional relevance of neurotransmitter receptor heteromers in the central nervous system. Trends Neurosci 30:440–446. https://doi.org/10.1016/j.tins.2007.07.001
Ferré S, Herrera-Marschitz M, Grabowska-Andén M, Ungerstedt U, Casas M, Andén N-E (1991a) Postsynaptic dopamine/adenosine interaction: I. Adenosine analogues inhibit dopamine D2-mediated behaviour in short-term reserpinized mice. Eur J Pharmacol 192:25–30. https://doi.org/10.1016/0014-2999(91)90064-W
Ferré S, von Euler G, Johansson B, Fredholm BB, Fuxe K (1991b) Stimulation of high-affinity adenosine A2 receptors decreases the affinity of dopamine D2 receptors in rat striatal membranes. Proc Natl Acad Sci U S A 88:7238–7241
Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE (1999) Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev 51:83–133
Fuxe K, Ungerstedt U (1974) Action of caffeine and theophyllamine on supersensitive dopamine receptors: considerable enhancement of receptor response to treatment with DOPA and dopamine receptor agonists. Med Biol 52:48–54
Ginovart N, A a W, Hussey D, Houle S, Kapur S (2009) D2-receptor upregulation is dependent upon temporal course of D2-occupancy: a longitudinal [11C]-raclopride PET study in cats. Neuropsychopharmacology 34:662–671. https://doi.org/10.1038/npp.2008.116
Gopalakrishnan M, Molinari EJ, Sullivan JP (1997) Regulation of human alpha4beta2 neuronal nicotinic acetylcholine receptors by cholinergic channel ligands and second messenger pathways. Mol Pharmacol 52:524–534
Griffiths R, Evans S, Heishman S, Preston K, Sannerud C, Wolf B, Woodson P (1990) Low-dose caffeine physical dependence in humans. J Pharmacol Exp Ther 255
Griffiths RR, Brady JV, Bradford LD (1979) Predicting the abuse liability of drugs with animal drug self-administration procedures: psychomotor stimulants and hallucinogens. Adv Behav Pharmacol 2:163–208. https://doi.org/10.1016/B978-0-12-004702-4.50010-2
Griffiths RR, Woodson PP (1988a) Reinforcing effects of caffeine in humans. J Pharmacol Exp Ther 246:21–29. https://doi.org/10.1016/0091-3057(91)90448-B
Griffiths RR, Woodson PP (1988b) Caffeine physical dependence: a review of human and laboratory animal studies. Psychopharmacology 94:437–451
Griffiths RR, Woodson PP (1988c) Reinforcing properties of caffeine: studies in humans and laboratory animals. Pharmacol Biochem Behav 29:419–427. https://doi.org/10.1016/0091-3057(88)90180-3
Juliano LM, Evatt DP, Richards BD, Griffiths RR (2012) Characterization of individuals seeking treatment for caffeine dependence. Psychol Addict Behav 26:948–954. https://doi.org/10.1037/a0027246
Juliano LM, Griffiths RR (2004) A critical review of caffeine withdrawal: empirical validation of symptoms and signs, incidence, severity, and associated features. Psychopharmacology 176:1–29
Kaasinen V, Aalto S, Någren K, Rinne JO (2004) Dopaminergic effects of caffeine in the human striatum and thalamus. Neuroreport 15:281–285. https://doi.org/10.1097/00001756-200402090-00014
Koppelstaetter F, Poeppel TD, Siedentopf CM, Ischebeck A, Kolbitsch C, Mottaghy FM, Felber SR, Jaschke WR, Krause BJ (2010) Caffeine and cognition in functional magnetic resonance imaging. J Alzheimers Dis 20:S71–S84
Koppelstaetter F, Poeppel TD, Siedentopf CM, Ischebeck A, Verius M, Haala I, Mottaghy FM, Rhomberg P, Golaszewski S, Gotwald T, Lorenz IH, Kolbitsch C, Felber S, Krause BJ (2008) Does caffeine modulate verbal working memory processes? An fMRI study. Neuroimage 39:492–499. https://doi.org/10.1016/j.neuroimage.2007.08.037
Mandel HG (2002) Update on caffeine consumption, disposition and action. Food Chem Toxicol 40:1231–1234
Marin MT, Zancheta R, Paro AH, Possi APM, Cruz FC, Planeta CS (2011) Comparison of caffeine-induced locomotor activity between adolescent and adult rats. Eur J Pharmacol 660:363–367. https://doi.org/10.1016/j.ejphar.2011.03.052
Meredith SE, Juliano LM, Hughes JR, Griffiths RR (2013) Caffeine use disorder: a comprehensive review and research agenda. J Caffeine Res 3:114–130. https://doi.org/10.1089/jcr.2013.0016
Nehlig A (1999) Are we dependent upon coffee and caffeine? A review on human and animal data. Neurosci Biobehav Rev 23:563–576
Nehlig A, Boyet S (2000) Dose-response study of caffeine effects on cerebral functional activity with a specific focus on dependence. Brain Res 858:71–77. https://doi.org/10.1016/S0006-8993(99)02480-4
Nehlig A, Daval JL, Debry G (1992) Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res Brain Res Rev 17:139–170
Paes-De-Carvalho R (2002) Adenosine as a signaling molecule in the retina: biochemical and developmental aspects. An Acad Bras Cienc 74:437–451. https://doi.org/10.1590/S0001-37652002000300007
Pardo Lozano R, Alvarez García Y, Barral Tafalla D, Farré Albaladejo M (2007) Caffeine: a nutrient, a drug or a drug of abuse. Adicciones 19:225–238
Park C-A, Kang C-K, Son Y-D, Choi E-J, Kim S-H, Oh S-T, Kim Y-B, Park C-W, Cho Z-H (2014) The effects of caffeine ingestion on cortical areas: functional imaging study. Magn Reson Imaging 32:366–371. https://doi.org/10.1016/j.mri.2013.12.018
Peng X, Gerzanich V, Anand R, Whiting PJ, Lindstrom J (1994) Nicotine-induced increase in neuronal nicotinic receptors results from a decrease in the rate of receptor turnover. Mol Pharmacol 46:523–530
Pollack AE, Dimitrov KD, Drake JD (2010) Prior treatment (priming) with caffeine sensitizes D2-dopamine-mediated contralateral rotational behavior in 6-hydroxydopamine-lesioned rats. Pharmacology 86:73–78. https://doi.org/10.1159/000315496
Quarta D, Ferré S, Solinas M, You ZB, Hockemeyer J, Popoli P, Goldberg SR (2004) Opposite modulatory roles for adenosine A1 and A2A receptors on glutamate and dopamine release in the shell of the nucleus accumbens. Effects of chronic caffeine exposure. J Neurochem 88:1151–1158. https://doi.org/10.1046/j.1471-4159.2003.02245.x
Rhoads DE, Huggler AL, Rhoads LJ (2011) Acute and adaptive motor responses to caffeine in adolescent and adult rats. Pharmacol Biochem Behav 99:81–86. https://doi.org/10.1016/j.pbb.2011.04.001
Ribeiro JA, Sebastio AM (2010) Caffeine and adenosine. J Alzheimer’s Dis S3–15
Rogers PJ, Elliman NA, Richardson NJ (1995) Overnight caffeine abstinence and negative reinforcement of preference for caffeine-containing drinks. Psychopharmacology 120:457–462
Schuh KJ, Griffiths RR (1997) Caffeine reinforcement: the role of withdrawal. Psychopharmacology 130:320–326
Solinas M, Ferré S, You Z-B, Karcz-Kubicha M, Popoli P, Goldberg SR (2002) Caffeine induces dopamine and glutamate release in the shell of the nucleus accumbens. J Neurosci 22:6321–6324
Stoner GR, Skirboll LR, Werkman S, Hommer DW (1988) Preferential effects of caffeine on limbic and cortical dopamine systems. Biol Psychiatry 23:761–768. https://doi.org/10.1016/0006-3223(88)90064-9
Strain E, Griffiths R (1995) Caffeine dependence: fact or fiction? J R Soc Med 88:437–440
Striley CLW, Griffiths RR, Cottler LB (2011) Evaluating dependence criteria for caffeine. J Caffeine Res 1:219–225. https://doi.org/10.1089/jcr.2011.0029
Stringer KA, Watson WA (1987) Caffeine withdrawal symptoms. Am J Emerg Med 5:469. https://doi.org/10.1016/0735-6757(87)90416-5
Trifilieff P, Rives M-L, Urizar E, Piskorowski R, Vishwasrao H, Castrillon J, Schmauss C, Slättman M, Gullberg M, Javitch J (2011) Detection of antigen interactions ex vivo by proximity ligation assay: endogenous dopamine D2-adenosine A2A receptor complexes in the striatum. Biotechniques 51:111–118. https://doi.org/10.2144/000113719
Vitiello MV, Woods SC (1977) Evidence for withdrawal from caffeine by rats. Pharmacol Biochem Behav 6:553–555. https://doi.org/10.1016/0091-3057(77)90116-2
Volkow ND, Wang G-J, Logan J, Alexoff D, Fowler JS, Thanos PK, Wong C, Casado V, Ferre S, Tomasi D (2015) Caffeine increases striatal dopamine D2/D3 receptor availability in the human brain. Transl Psychiatry 5:e549. https://doi.org/10.1038/tp.2015.46
Wise RA, Bozarth MA (1987) A psychomotor stimulant theory of addiction. Psychol Rev 94:469–492. https://doi.org/10.1037/0033-295X.94.4.469
Acknowledgements
We are grateful to CNPQ, for providing financial support to this work CNPQ-FAPESP–465458/2014-9 and 2014/50891-1-INCT Translacional em Medicina (465458/2014-9).
Author information
Authors and Affiliations
Contributions
VSR and BLS conceived the general structure of the article and selection methods. LSR and MKFS performed literature search and paper selection. MKFS, BLS, and ECG wrote the manuscript. VG, BLS, and ECG revised the manuscript for submission and the responses to reviewers. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
dos Santos, M.K.F., Gavioli, E.C., Rosa, L.S. et al. Craving espresso: the dialetics in classifying caffeine as an abuse drug. Naunyn-Schmiedeberg's Arch Pharmacol 391, 1301–1318 (2018). https://doi.org/10.1007/s00210-018-1570-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-018-1570-9