Abstract
The present study investigated the role of dopamine D2 receptors (D2Rs) of the dorsal hippocampus (DH) and the nucleus accumbens (NAc) and the effect of their dopaminergic activities on anxiety-like behavior and aversive learning using a test-retest elevated plus-maze (EPM) paradigm in male Wistar rats. Guide cannulae were implanted to allow microinjection of D2R agonist quinpirole or antagonist sulpiride. The pre-test intra-NAc microinjection of quinpirole (0.0625–0.25 μg/rat) or sulpiride (0.125–0.5 μg/rat) increased the percentage of time spent in the open arms (%OAT) of EPM, suggesting an anxiolytic-like effect. However, an increase in open-arm avoidance was observed in the control rats when retested in the EPM, suggesting aversive information storage. Furthermore, a similar result was obtained in the quinpirole-treated rats. In contrast, the sulpiride-treated rats failed to demonstrate further open-arm avoidance, thus proposing an aversive learning deficit. The intra-DH microinjection of drugs alone induced an anxiolytic-like effect and learning deficit. The quinpirole (0.125 μg/rat) injected into each site had no effect on the response induced by sulpiride injected into another site. Finally, a subthreshold dose of quinpirole in both sites did not alter the %OAT; on the contrary, it preserved the aversive memory. The sulpiride induced an anxiolytic-like effect and a learning deficit. Our data suggests that the involvement of D2Rs in the interactions of DH-NAc dopaminergic system helps regulate anxiety-related behavior and EPM-associative memory.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The role of dopamine (DA) in motivated behaviors and cognitive processes is well established; however, its role in regulating anxiety-related behavior and aversive learning has remained a matter of debate (Brandao et al. 1999; Garcia et al. 2005; Alcaro et al. 2007). Depending on the tests applied, various aversive stimuli have been shown to differently affect extracellular DA concentrations and dopaminergic neurotransmission in limbic structures (Tidey and Miczek 1996; Carvalho et al. 2005). The limbic structures, including the hippocampus and nucleus accumbens, receive dopaminergic innervation from the ventral tegmental area (VTA), which is known to be a key dopaminergic center in the brain (Gasbarri et al. 1997; Wahlstrom et al. 2010). Some investigations have revealed that the projections of hippocampal glutamatergic neurons extend to reach the nucleus accumbens, which per se regulate the DA transmission in this structure (Tan 2008). The notion that the nucleus accumbens may indirectly influence the hippocampal plasticity has been supported by some evidence (Kudolo et al. 2010). In addition, DA actions are mediated through two subtypes of DA receptors—namely, D1- and D2-like receptors. All the DA receptors are slow-acting metabotropic receptors coupled with G-proteins. The activation of D1 receptors leads to an increase in the intracellular levels of cAMP, whereas activation of D2 receptors leads to a decrease in the levels of intracellular cAMP (Goto et al. 2007). These receptors may therefore elicit opposite effects on different forms of neuronal plasticity, causing disruptions in consequent learning and memory functions (Floresco and Phillips 2001; Nasehi et al. 2010) as well as anxiety-related behavior (Wall et al. 2003).
The nucleus accumbens, including its core and shell sub-regions, mediates dissociable functions in the processes of acquisition, encoding, and retrieval of aversive learning and memory. It has been suggested that exposure to the elevated plus-maze (EPM) task causes reduced monoaminergic neurotransmission in the limbic structures, including the nucleus accumbens and hippocampus (Carvalho et al. 2005). In addition, the dorsal part of the hippocampus (DH) plays an important role in learning, memory (Nasehi et al. 2009), exploratory behavior (File et al. 2000; Dringenberg et al. 2008), and emotional-cognitive processes (Bertoglio et al. 2006).
The present study aimed to examine the role of dopaminergic D2 agents in DH and NAc core, after considering recent insights including: (1) direct and indirect relations between the hippocampus and nucleus accumbens, and their role in motivation, learning, and memory; (2) the role of D2 dopaminergic system in exploratory behavior, learning, and memory; (3) the expression of D2 dopaminergic receptors at both sites; and (4) the assessment of behavioral expression associated with anxiety (open-arm exploration) and the storage of spatial/emotional information (decreased in exploration, mainly for open arms in a subsequent presentation) in the EPM task. The above has also prompted us to study the interaction of DA activity in specific brain areas with regard to anxiety and/or aversive learning through concurrent microinjection of DA agonist or antagonist into the DH and NAc core prior to the testing session in EPM test-retest protocol.
Materials and methods
Animals
Male Wistar rats (Institute of Cognitive Science Studies, Tehran, Iran) weighing 250–300 g, upon surgery, were housed in groups of five per Plexiglas plastic cage under standard laboratory conditions (temperature 22 ± 2 °C, humidity 60–65%), having free access to food and water, with a 12-h light/dark cycle (lights on at 7:00 am). Each animal was used once only. A total of eight animals were used in each group of experiments. The experiments were carried out during the light phase of the cycle. The animals’ treatment and maintenance were conducted in accordance with the principles of laboratory animal care (NIH publication No. 85-23, revised 1985) in accordance with the animal care and use guidelines laid down by the Tehran University of Medical Sciences (TUMS).
Drugs
Quinpirole (Sigma Chemical Co., St. Louis, CA, USA) and sulpiride (Sigma Chemical Co., St. Louis, CA, USA) were dissolved in saline solution (0.9%) and vehicle, respectively. The vehicle consisted of one drop of glacial acetic acid (using Hamilton microliter syringe), prepared to a volume of 5 ml with sterile 0.9% saline, and subsequently diluted to reach the required volume. The control animals received either saline or vehicle.
The elevated plus-maze apparatus
An EPM made of non-transparent Plexiglas, consisting of two opposite open arms (50 × 10 cm) surrounded by a 1 cm-high ledge, and two enclosed arms (50 × 10 × 40 cm) was used. The maze was placed 50 cm above the floor. The junction area of the four arms (central platform) measured 10 × 10 cm (Eslimi et al. 2011).
Stereotactic surgery and drug infusion
The rats were intraperitoneally anesthetized using ketamine hydrochloride 10% (50 mg/kg; Alfasan, Woerden, Holland) plus xylazine 2% (4 mg/kg; Alfasan, Woerden, Holland), and then positioned in a stereotactic frame. The upper incisor bar was set 3.3 mm below the inter-aural line so that the skull aligned horizontally between bregma and lambda. Then, two unilateral guide cannulae (through which a needle could be inserted for drugs, saline, or vehicle injections 5–7 days later) were stereotaxically implanted over the left NAc core and DH. Based on earlier reports (Nazari-Serenjeh et al. 2011; Nazari-Serenjeh and Rezayof 2013), to prevent further brain damage, the cannula implantation was done unilaterally. Taking bregma as the reference point, the coordinates for the NAc core were AP = +1.8 mm, ML = ±1.5 mm, and DV = 6.5 mm. For DH, these were AP = −2.2 mm, ML = ±1.8 mm, and DV = 3.2 mm, according to the atlas of Paxinos and Watson (2007). The cannulae were fixed to the skull by means of acrylic resin and two stainless steel screws. When surgery was completed, a stylet was inserted into each guide cannula to prevent possible occlusion. Later, the rats were returned to their home cages in groups of five, similar to the pre-surgery condition. Then, 5 to 7 days post-surgery, the awake rats were gently restrained in hand and each given a unilateral infusion either into the DH or NAc core, through a 27-gauge dental needle introduced into the guide cannula. The injection needle was advanced until its tip was at a distance of 1–2 mm below the cannula end. Then, 0.5 and 0.3 μl/side of solutions were injected into DH and NAc core, respectively. This was done over a 60-s period, using a 2.5-μl glass Hamilton syringe. A polyethylene catheter was interposed between the upper end of the dental needles and microliter syringes. The displacement of an air bubble inside the polyethylene catheter was used to monitor the drug flow. To allow proper infusion, the needles were removed 60 s after the completion of the injection.
General experimental conditions and data collection
In the present study, the EPM test-retest protocol was applied to investigate the anxiety and aversive learning processes. Recent studies have shown that using test-retest sessions in the EPM can result in a qualitative shift of the emotional state. As such, an unconditioned fear in the test session could possibly transform into a learned avoidance upon retest (Cruz-Morales et al. 2002; Gianlorenco et al. 2011). In our study, animals were given a pre-test intra-cerebral drug injection, followed by an injection-free (un-drugged) retest, 24 h later. By doing this, the drug’s effect on aversive learning with the subsequent long-term effect on memory was tested 24 h later. It has been reported that prior experience of an un-drugged test session in the EPM alters the behavioral response upon the retest un-drugged session (Carvalho et al. 2005; Stern et al. 2010). It has also been suggested that the EPM retest outcome depends on the length of the test session as well as the baseline anxiety state (Ghizoni et al. 2006). The support for the former test-retest procedure stems from data showing that although pre-test systemic benzodiazepine administration can reliably exert anxiolytic effects, the pre-retest injections fail to affect the response in the second EPM session. In general, regarding the saline- or vehicle-treated groups, the reduced exploratory behavior in the open arms during the retest session indicates aversive learning, associated with the initial exploration of this potentially dangerous environment (Stern et al. 2010; Gianlorenco et al. 2011). All experiments were carried out in a minimally illuminated (40-lx) room during the diurnal phase between 09:00 am and 03:00 pm. A video camera recorded 5-min EPM sessions, while a monitor and DVD-recording system were installed in the next room. After each EPM session, the apparatus was cleaned and towel-dried to avoid urine impregnation. The number of open-arm entries (OAE) and enclosed-arm entries (EAE; with all four paws in an arm), as an EPM index of general exploratory activity, as well as the time spent in the open and enclosed arms were carefully recorded. Raw data were used to calculate the percentage of time spent in open arms (%OAT (time in open arms/300) × 100).
Verification of cannulae placements
Upon the conclusion of each experiment, the rats were deeply anesthetized, after which 1% Methylene Blue solution was injected into the DH (0.5 μl/side) and the NAc core (0.3 μl/side) as described in the drug section. Each animal was then decapitated, its brain removed and placed in a 10% formalin solution. After 7–10 days, the brains were sliced and the injection sites were verified according to the Paxinos and Watson (2007) atlas. Statistical analysis was performed after excluding data from the rats with cannula placements outside the intended sites.
Statistical analysis
The behavioral data was expressed as mean ± SEM and analyzed by SPSS (version 21) software. The two-way analysis variance (ANOVA) with treatment (doses) as one factor and sessions as repeated measures (test × retest sessions) was used to compare the results of experiment 1 (shown in panels 1 and 2 and panels 3 and 4 of respective figures), experiment 2 (shown in panels 1 and 2 and panels 3 and 4 of respective figures), and experiment 4. The results were then evaluated using a two-way ANOVA in experiments 1 or 2 (shown in panels 3 and 5 and panels 4 and 6 of the respective figures). Following a significant F value, post hoc analysis (Tukey’s test) was performed to assess the differences between groups. P values < 0.05 were considered statistically significant.
Experimental design
In all the experiments, microinjection of drugs took place 5 min prior to the EPM testing. After 24 h, the treated groups were retested in the EPM (un-drugged).
Experiment 1: Open-arm exploratory behavior following pre-test intra-NAc core microinjections of quinpirole or sulpiride
In this experiment, all the groups received saline in the DH but different injections in the NAc core. The NAc injections were either saline, vehicle, quinpirole (0.0625, 0.125, and 0.25 μg/rat), or sulpiride (0.125, 0.25, and 0.5 μg/rat).
Experiment 2: Open-arm exploratory behavior following pre-test intra-DH microinjections of quinpirole or sulpiride
In this experiment, the animals received saline, vehicle, different doses of quinpirole or sulpiride into the DH, but only saline injection into the NAc core. The doses of quinpirole were 0.0625, 0.125, and 0.25 μg/rat and for sulpiride, were 0. 25, 0.5, and 1 μg/rat.
Experiment 3: The effect of quinpirole injection in the NAc core on open-arm exploratory behavior induced by sulpiride injected into the DH and vice versa
A total of four animal groups received concurrent intra-DH microinjection of quinpirole (0.125 μg/rat, intra-NAc core) with vehicle (0.5 μl/rat) or different doses of sulpiride (0.25, 0.5, and 1 μg/rat). Another four groups received concurrent microinjection of quinpirole (0.125 μg/rat, intra-DH) with vehicle (0.3 μl/rat) or different doses of sulpiride (0.125, 0.25, and 0.5 μg/rat) into the NAc core.
Experiment 4: Open-arm exploratory behavior following pre-test concurrent microinjections of quinpirole (0.125 μg/rat, in both sites) or sulpiride (0.125 μg/rat, intra-NAc core, 0.25 μg/rat, intra-DH)
A total of four groups of rats received concurrent microinjection of saline (0.5 μl/rat, intra-DH; 0.3 μl/rat, intra-NAc core), quinpirole (0.125 μg/rat, intra-DH) with saline (0.3 μl/rat, intra-NAc core), quinpirole (0.125 μg/rat, intra-NAc core), along with saline (0.5 μl/rat, intra-DH), and quinpirole (0.125 μg/rat, at both sites). Another four groups of rats received concurrent microinjection of vehicle (0.5 μl/rat, intra-DH, 0.3 μl/rat, intra-NAc core), the subthreshold dose of sulpiride (0.25 μg/rat, intra-DH) with vehicle (0.3 μl/rat, intra-NAc core), the subthreshold dose of sulpiride (0.125 μg/rat, intra-NAc core) with vehicle (0.5 μl/rat, intra-DH), as well as the subthreshold dose of sulpiride (0.125 μg/rat, at both sites).
Results
Histology
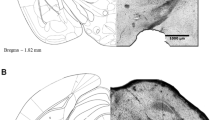
Figure 1a, b (right panels) shows the locations of the injection cannula tips in the NAc core and DH, respectively. The left panels of Fig. 1a, b illustrate the representative sections taken from the rat’s brain atlas of Paxinos and Watson (2007). The shaded and dark areas represent the approximate points at which the cannulae were positioned in each animal. The data from animals with injection sites located outside the NAc core and DH were excluded from the analysis.
A representative photomicrograph of microinjections into the NAc core and DH (a, b right). a, b Left, the locations of injections on cross-sections obtained from the brain atlas of Paxinos and Watson (2007). The shaded and dark areas indicate the sites of injections into the NAc core and DH
Experiment 1
The effect of intra-NAc core quinpirole microinjection on exploratory behavior in naive rats subjected to the EPM is shown in Fig. 2 (panels 1 and 2) and Table 1. The results indicate that the quinpirole (0.25 μg/rat) injected into the NAc core before the test session produced a significant increase in %OAT as compared with the saline-treated rats in the same session. When the animals were retested, the %OAT decreased for different doses of quinpirole and saline, in comparison with the test session. No difference in response was noted between the saline- and quinpirole-treated animals in terms of %OAT and OAE. Similarly, the EAE was similar among the quinpirole-treated groups when compared with the saline-treated group in the same session.
The effects of quinpirole or sulpiride microinjections into the NAc core on exploratory behavior in naive rats subjected to EPM. Naive rats were tested in the EPM, 5 min after concurrent microinjection of saline (0.5 μl/rat) into DH; saline (0.3 μl/rat) or quinpirole (0.0625, 0.125, and 0.25 μg/rat) or vehicle (0.3 μl/rat) or sulpiride (0.125, 0.25, and 0.5 μg/rat) into NAc core. A day later, all groups were retested in EPM, un-drugged. Percentage of open-arm time (a); open-arm entries (b); and enclosed-arm entries (c). Values are expressed as mean ± SEM (n = 8 in each group). *P < 0.05 and **P < 0.01 are different from saline control group in the test session; ++P < 0.01 is different from vehicle control group in the test session; $P < 0.05, $$P < 0.01, and $$$P < 0.001 are different from the vehicle control group in the same session
Furthermore, the effect of intra-NAc core sulpiride microinjection on exploratory behavior in naive rats subjected to the EPM is shown in Fig. 2 (panels 3 and 4) and Table 1. The data revealed that the sulpiride (0.5 μg/rat) injected into the NAc core before the test session produced a significant increase in the %OAT as compared with the vehicle-treated rats in the same session. When the animals were retested, the %OAT and OAE decreased in the vehicle-treated rats but not in the sulpiride-treated group at the doses of 0.25 and 0.5 μg/rat, in comparison with the test session. Therefore, significant differences were observed between the vehicle- and sulpiride-treated animals in terms of %OAT and OAE. Meanwhile, the EAE was not different similar among the sulpiride-treated groups when compared with the vehicle-treated group in the same session.
In conclusion, our findings demonstrate that the intra-NAc core microinjection of quinpirole or sulpiride induced an anxiolytic-like effect. The saline- or vehicle-treated control rats displayed an experience-induced increase in open-arm avoidance when retested in the EPM, suggesting that the aversive information about the maze was preserved. Similar results were obtained in the quinpirole-treated rats too. In contrast, the sulpiride-treated rats failed to show further open-arm avoidance or block the expected normal decrease in open-arm exploration when retested in the EPM, thus indicating an aversive learning deficit.
Experiment 2
The effect of intra-DH quinpirole microinjection on the exploratory behavior of naive rats subjected to the EPM is shown in Fig. 3 (panels 1 and 2) and Table 1. The results show that quinpirole (0.25 μg/rat) injected into the DH before the test session produced a significant increase in %OAT as compared with saline-treated rats in the same session. When the animals were retested, decreased %OAT was found by saline, in comparison with the test session. In addition, upon retest, a significant increase in %OAT was observed in the animals which received quinpirole (0.25 μg/rat), in comparison with the saline control group. The EAE was not different between the quinpirole-treated and saline-treated groups in the same session.
The effects of intra-DH quinpirole or sulpiride microinjection on exploratory behavior of naive rats subjected to EPM. Naive rats were tested in the EPM, 5 min after concurrent microinjection of saline (0.3 μl/rat) into the NAc core; saline (0.5 μl/rat) or quinpirole (0.0625, 0.125 and 0.25 μg/rat) or vehicle (0.5 μl/rat) or sulpiride (0.25, 0.5, and 1 μg/rat) into DH. A day later, all the groups were retested in the EPM, un-drugged. Percentage of open-arm time (a); open-arm entries (b); and enclosed-arm entries (c). Values are expressed as mean ± SEM (n = 8 in each group); ###P < 0.001 is different from respective group in Fig. 3 (panel 4); ^P < 0.05 and ^^P < 0.01 are different from respective group in Fig. 2 (panel 4)
Additionally, the effect of intra-DH sulpiride microinjection on exploratory behavior in naive rats subjected to the EPM is shown in Fig. 3 (panels 3 and 4) and Table 1. The data reveals that sulpiride (0.5 and 1 μg/rat) injected into the DH before the test session, produced a significant increase in %OAT as compared with vehicle-treated rats in the same session. When the animals were retested, the %OAT showed a decrease for vehicle but not by sulpiride at the dose of 1 μg/rat, in comparison with the test session. Therefore, significant differences in %OAT were observed between the vehicle- and sulpiride-treated (1 μg/rat) animals. The EAE was significantly different between the animals which received sulpiride at the doses 0.25 or 1 μg/rat when compared with the vehicle-treated group in the retest session.
In conclusion, the results demonstrate that microinjection of quinpirole or sulpiride into the DH induced an anxiolytic-like effect as well as an aversive learning deficit.
Experiment 3
The effects of subthreshold dose injection of quinpirole into the NAc on the anxiolytic-like response and learning deficit induced by sulpiride into the DH is shown in Fig. 4 (panels 1 and 2) and Table 1. The two-way ANOVA and post hoc analysis were performed and the comparative results are illustrated in Figs. 4 (panel 1) and 3 (panels 3 and 4), and 4 (panel 2). The analyses reveal that injecting quinpirole (0.125 μg/rat) into the NAc does not alter the effect of sulpiride on the DH in terms of %OAT, OAE, or EAE in the test session; however, upon retest, it significantly decreased the %OAT in response to sulpiride.
The effects of concurrent microinjections of a subthreshold dose of quinpirole in one site and different doses of sulpiride in another site on exploratory behavior of naive rats subjected to EPM. The animals were divided into two sets of four groups. The first four groups received concurrent microinjections of quinpirole (0.125 μg/rat, intra-NAc core), vehicle (0.5 μl/rat), or different doses of sulpiride (0.25, 0.5, and 1 μg/rat) into the DH. The other four groups received concurrent microinjections of quinpirole (0.125 μg/rat, intra-DH), vehicle (0.3 μl/rat), or different doses of sulpiride (0.125, 0.25, and 0.5 μg/rat) into the NAc core. The rats were tested in the EPM, 5 min after infusion. The treated groups were then retested in the EPM 24 h later, un-drugged. Percentage of open-arm time (a); open-arm entries (b); and enclosed-arm entries (c). Values are expressed as mean ± SEM (n = 8 in each group). ##P < 0.01 and ###P < 0.001 are different from respective group in Fig. 3 (panel 2); ^P < 0.05 and ^^P < 0.01 are different from respective group in Fig. 2 (panel 4)
The effect of subthreshold dose injection of quinpirole into the DH on the anxiolytic-like effect and learning deficit induced by sulpiride injection into the NAc is shown in Fig. 4 (panels 3 and 4) and Table 1. The two-way ANOVA and post-hoc analysis were performed and the results are illustrated in Figs. 2 (panels 3 and 4) and 4 (panels 3 and 4). The analyses revealed that the quinpirole injection (0.125 μg/rat) in the DH did not alter the response of intra-NAc sulpiride injection in regard to %OAT, OAE, or EAE in the test session. However, upon retest, it significantly decreased the response of sulpiride in terms of %OAT.
In conclusion, our findings demonstrate that microinjection of a subthreshold dose of quinpirole into a site does not alter the anxiolytic-like effects induced by sulpiride injected into the other site. Interestingly, the quinpirole reversed the aversive learning deficit induced by sulpiride, after injection into the other site.
Experiment 4
The effect of concurrent microinjections of similar doses (0.125 μg/rat) of quinpirole into the NAc core and DH on the exploratory behavior of naive rats subjected to EPM are shown in Fig. 5 (panels 1 and 2) and Table 1. The results show that the concurrent microinjection of quinpirole (0.125 μg/rat) into the DH and NAc core before the test session produced insignificant changes in %OAT and OAE as compared with the saline/saline control group. When the animals were retested, the saline/saline group produced a normal decrease in %OAT, in comparison with its respective levels in the test session. No significant difference was noted in the %OAT or OAE of animals which received quinpirole/quinpirole as compared with the saline/saline group upon retest. The EAE was similar between groups which received the drug in either of the sites when compared with the saline/saline-treated group in the same session.
The effects of concurrent microinjections of subthreshold dose of quinpirole or sulpiride in both sites on exploratory behavior of naive rats subjected to EPM. The animals received concurrent microinjections of saline (0.5 or 0.3 μl/rat, intra-DH or intra-NAc core, respectively) or quinpirole (0.125 μg/rat, intra-DH), saline (0.3 μl/rat, intra-NAc core) or quinpirole (0.125 μg/rat, intra-NAc core), saline (0.5 μl/rat, intra-DH) or quinpirole (0.125 μg/rat, at both sites) or vehicle (0.5 or 0.3 μl/rat, intra-DH or intra-NAc core, respectively) or sulpiride (0.125 μg/rat, intra-DH), vehicle (0.3 μl/rat, intra-NAc core) or sulpiride (0.25 μg/rat, intra-NAc core), or vehicle (0.5 μl/rat, intra-DH) or sulpiride (0.25 μg/rat, intra-DH, 0.125 μg/rat, intra-NAc core). The rats were tested in the EPM, 5 min after infusion. The treated groups were then retested in the EPM test session 24 h later, un-drugged. Percentage of open-arm time (a); open-arm entries (b); and enclosed-arm entries (c). Values are expressed as mean ± SEM (n = 8 in each group). **P < 0.01 is different from saline/saline control group in the test session; +P < 0.05 and +++P < 0.001 are different from vehicle/vehicle group in the test session; $$P < 0.01 is different from vehicle/vehicle group in the same session
The effects of concurrent microinjections of sulpiride intra-NAc core and intra-DH at doses of 0.125 μg/rat and 0.25 μg/rat, respectively, on the exploratory behavior of naive rats subjected to EPM are shown in Fig. 4 (panels 3 and 4) and Table 1. The results show that the injection of sulpiride into the DH and the NAc prior to the test session significantly increases the %OAT in vehicle/vehicle control group for the same session. When the animals were retested, the vehicle/vehicle group showed a decrease in %OAT and OAE in comparison with its respective levels of the test session. However, the sulpiride/sulpiride group showed an increased %OAT in comparison with the vehicle/vehicle group upon retest. In addition, the EAE significantly decreased in the sulpiride/sulpiride and vehicle/vehicle groups for the same session.
In conclusion, simultaneous microinjection of quinpirole into both sites did not affect open-arm exploratory behavior as compared with the control group in the same session, while simultaneous microinjection of sulpiride into both sites induced an anxiolytic-like effect and an aversive learning deficit. Notably, the concurrent microinjection of sulpiride resulted in decreased locomotor activity in the same session.
Discussion
The EPM test is primarily used to measure anxiety-like behavior in rodents and is modified to evaluate spatial learning and memory. It is clear that during the EPM test, the rodents acquire information about the spatial environment. They learn to identify the safe and dangerous areas in the maze (Cardenas et al. 2000). Animals retested in the EPM exhibit a statistically significant decrease in open-arm exploration relative to their respective levels on testing (Bertoglio and Carobrez 2000). As evidenced by min-by-min analysis, this response of further open-arm avoidance is gradually acquired throughout the testing, and it is thought to reflect the retrieval of aversive memory related to the initial EPM exploration (Carobrez and Bertoglio 2005). Therefore, EPM-associative memory can be considered an aversive memory (Stern et al. 2010). To investigate the interaction between DA activity in the NAc and DH on anxiety-like behavior and aversive learning, EPM test/retest protocol was used with the following findings observed.
Open-arm exploratory behavior following pre-test intra-NAc core microinjections of quinpirole or sulpiride
The present data shows that injection of intra-NAc quinpirole or sulpiride (as dopamine D2-like receptor agonist or antagonist, respectively) prior to the test session increases open-arm exploratory behavior, suggesting an anxiolytic-like response. When the animals were retested, the saline- or vehicle-treated control rats displayed experience-induced increases in open-arm avoidance, suggesting that the aversive information about the maze was preserved. A similar result was observed in intra-NAc quinpirole-treated rats. In contrast, the sulpiride-treated rats failed to display further open-arm avoidance or block the normally expected decrease in open-arm exploration, indicating an aversive learning deficit. Moreover, the two higher doses of sulpiride increased the open-arm entries upon retest. Some investigations report that dopaminergic agonists and antagonists can induce anxiolytic-like, anxiogenic-like, and null effects in animal models of anxiety (Rodgers et al. 1994; de Oliveira et al. 2006). Besides, memory retention is shown to be enhanced by post-training injections of the D2 agonist, quinpirole (Packard and White 1989). We suggest that quinpirole, via activation of post-synaptic D2 receptors, inhibits the post-synaptic D2 receptors-mediated efferent projections. Thus, quinpirole may plausibly induce an anxiolytic-like effect while the aversive learning process is still maintained. On the other hand, the anxiolytic-like effects of DA receptor antagonists have been reported in a variety of animal models. In agreement with our results, sulpiride is shown to render anxiolytic-like profiles in punished drinking and light/dark exploration tests in rats (Rodgers et al. 1994). Our previous study showed that the unilateral microinjection of sulpiride at doses of 0.4 and 0.6 μg/μl into the left nucleus accumbens shell produces an anxiolytic-like effect. Another key finding is that SKF96365 (0.125 μg/μl), as a Ca2+ blocker, reverses the sulpiride-induced anxiolytic-like response (Ahmadi et al. 2013). Therefore, the present study concludes that sulpiride at applied doses blocks the pre-synaptic D2 receptors which in turn increases the release of dopamine. As a result, the increased dopamine in the synapse induces an anxiolytic-like effect and an aversive learning deficit. Furthermore, the systemic administration of sulpiride has been shown to reduce the avoidance response in a two-way avoidance test (Carvalho et al. 2009). There exists some evidence supporting that the dopaminergic responses of NAc core decrease upon aversive stimuli (Louilot and Besson 2000). In addition to the above, it has been suggested that the DA mechanisms in the NAc play an important role in the expression of learned-fear responses. Therefore, there is a clear enhancement of DA release in extracellular NAc nuclei in response to the aversive contextual conditioning cues (Martinez et al. 2008). It seems that sulpiride may produce anxiolytic-like effects by inhibiting an already-enhanced DA transmission. Based on the literature, DA D2-class receptors have indeed been implicated both in learning and memory storage of spatial tasks. These findings are in line with data showing that the pre-training intra-NAc infusions of D2-acting antagonists impair memory acquisition both in radial arm and spatial water maze tasks (Cools et al. 1993; Ploeger et al. 1994). Further to the above evidence, earlier reports have documented the improved memory consolidation of the passive-avoidance task after injection of DA as well as the impaired memory consolidation by the intra-NAc core or shell injection of the D2 receptor antagonist in the Morris water maze task (Setlow and McGaugh 1998; LaLumiere et al. 2005). Taken together, these results suggest that accumbal D2-class receptors possibly play a role in the process of memory storage.
Open-arm exploratory behavior following pre-test intra-DH microinjection of quinpirole or sulpiride
Our results indicate that the pre-test intra-DH microinjection of quinpirole or sulpiride induces an anxiolytic-like response while impairing the process of aversive learning. Moreover, the lowest and highest doses of sulpiride decreased locomotion as compared with the controls upon the retest session. Several distinct pharmacological manipulations in the hippocampus reduce the excitation of neurons in the dorsal part, inducing anxiolytic-like effects (Stefanski et al. 1993; Xu et al. 1997; Menard and Treit 2001). It appears that the dorsal part of the hippocampus partly modulates defensive behavior through its glutamatergic efferent neurons (Walaas and Fonnum 1980) projecting to the lateral septum (Risold and Swanson 1996). In addition, the hippocampal D2 receptor dopaminergic system is suggested to be involved in the mnemonic function and regulation of hippocampal release of acetylcholine (ACh) (Fujishiro et al. 2005). An electrophysiological study showed that a D2R antagonist leads to disturbances of long-term potentiation, a key phenomenon involved in memory consolidation, in the rat hippocampus (Frey et al. 1990; Yanagihashi et al. 1991). To develop the present experiments, we considered two regions of the rat brain (i.e., the DH and the NAc core) as potentially implicated in the phenomenon of aversive memory acquisition and anxiety-like behavior in animal models.
The interaction of NAc and DH dopaminergic systems on open-arm exploratory behavior in test-retest protocol
Data showed that the microinjection of a subthreshold dose of quinpirole into the NAc did not alter the anxiolytic-like response induced by the sulpiride injected into the DH. In addition, the same dose of quinpirole injected into the DH induced the same effects on exploratory behavior as induced by sulpiride after injection into the NAc core. On the other hand, the injection of quinpirole into each site improved the aversive learning deficit induced by the sulpiride, once injected into the other site. Meanwhile, the simultaneous microinjection of quinpirole’s subthreshold dose in both sites did not produce an anxiolytic-like response, while the sulpiride potentiated anxiolytic-like behavior. Furthermore, the quinpirole-treated rats acquired aversive learning, while the sulpiride-treated rats showed a potentiated aversive learning deficit. Fujishiro et al. reports that memory impairment induced by injection of scopolamine could be ameliorated by focal injection of a D2R agonist into the same site. They suggest possible involvement of hippocampal (acetylcholine) ACh-DA interaction in mnemonic processing (Fujishiro et al. 2005). Therefore, we hypothesized that quinpirole activates the cholinergic system in the DH and NAc and in turn, improves the impaired aversive memory induced by sulpiride. Both subregions of NAc (core and shell) receive inputs from different regions of the brain, and are potentially involved in anxiety-like behavior, learning, and memory. Such brain regions include the amygdala, hippocampus, and prefrontal cortex (PFC) which form a convergent and complex network of mediating anxiety-like behavior and memory formation (Fendt and Fanselow 1999). On the other hand, a number of studies have demonstrated that anxiety is not regulated by just one system of neurotransmitters but is controlled by a complex combination of such systems which may further be modulated by hormones (Pandaranandaka et al. 2006).
Conclusion
In summary, the conclusions which emerge from the present investigation include: (1) the activation of dopamine D2 receptor of NAc core or DH tend to improve the aversive memory impairment induced by dopamine D2 receptor antagonists injected into either site; (2) the stimulation of D2 dopamine receptors in both the DH and NAc core seems to induce anxiolytic-like behavior; and (3) the blockade of these receptors in both sites may induce anxiolytic-like behavior and impair acquisition of aversive memory. Consequently, there seems to be a solid connection between dopaminergic systems of NAc and DH. These relations may be manipulated either directly or indirectly through other neurotransmitters and other potentially regulating sites such as VTA.
References
Ahmadi H, Nasehi M, Rostami P, Zarrindast MR (2013) Involvement of the nucleus accumbens shell dopaminergic system in prelimbic NMDA-induced anxiolytic-like behaviors. Neuropharmacology 71:112–123. https://doi.org/10.1016/j.neuropharm.2013.03.017
Alcaro A, Huber R, Panksepp J (2007) Behavioral functions of the mesolimbic dopaminergic system: an affective neuroethological perspective. Brain Res Rev 56:283–321. https://doi.org/10.1016/j.brainresrev.2007.07.014
Bertoglio LJ, Carobrez AP (2000) Previous maze experience required to increase open arms avoidance in rats submitted to the elevated plus-maze model of anxiety. Behav Brain Res 108:197–203
Bertoglio LJ, Joca SR, Guimaraes FS (2006) Further evidence that anxiety and memory are regionally dissociated within the hippocampus. Behav Brain Res 175:183–188. https://doi.org/10.1016/j.bbr.2006.08.021
Brandao ML, Anseloni VZ, Pandossio JE et al (1999) Neurochemical mechanisms of the defensive behavior in the dorsal midbrain. Neurosci Biobehav Rev 23:863–875
Cardenas FP, Lamprea MR, Silveira R et al (2000) Dissociation of memory and anxiety in a repeated elevated plus-maze paradigm: forebrain cholinergic mechanisms. Behav Brain Res 117:97–105
Carobrez AP, Bertoglio LJ (2005) Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev 29:1193–1205. https://doi.org/10.1016/j.neubiorev.2005.04.017
Carvalho JD, de Oliveira AR, da Silva RC, Brandao ML (2009) A comparative study on the effects of the benzodiazepine midazolam and the dopamine agents, apomorphine and sulpiride, on rat behavior in the two-way avoidance test. Pharmacol Biochem Behav 92:351–356
Carvalho LR, Vasconcellos PC, Mantovani W et al (2005) Measurements of biogenic hydrocarbons and carbonyl compounds emitted by trees from temperate warm Atlantic rainforest, Brazil. J Env Monit 7:493–499. https://doi.org/10.1039/b414881a
Cools AR, Ellenbroek B, Heeren D, Lubbers L (1993) Use of high and low responders to novelty in rat studies on the role of the ventral striatum in radial maze performance: effects of intra-accumbens injections of sulpiride. Can J Physiol Pharmacol 71:335–342
Cruz-Morales SE, Santos NR, Brandao ML (2002) One-trial tolerance to midazolam is due to enhancement of fear and reduction of anxiolytic-sensitive behaviors in the elevated plus-maze retest in the rat. Pharmacol Biochem Behav 72:973–978
de Oliveira AR, Reimer AE, Brandao ML (2006) Dopamine D2 receptor mechanisms in the expression of conditioned fear. Pharmacol Biochem Behav 84:102–111. https://doi.org/10.1016/j.pbb.2006.04.012
Dringenberg HC, Levine Y, Menard JL (2008) Electrical stimulation of dorsal, but not ventral hippocampus reduces behavioral defense in the elevated plus maze and shock-probe burying test in rats. Behav Brain Res 186:143–147. https://doi.org/10.1016/j.bbr.2007.07.030
Eslimi D, Oryan S, Nasehi M, Zarrindast MR (2011) Effects of opioidergic systems upon anxiolytic-like behaviors induced in cholestatic rats. Eur J Pharmacol 670:180–185. https://doi.org/10.1016/j.ejphar.2011.08.024
Fendt M, Fanselow MS (1999) The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev 23:743–760
File SE, Kenny PJ, Cheeta S (2000) The role of the dorsal hippocampal serotonergic and cholinergic systems in the modulation of anxiety. Pharmacol Biochem Behav 66:65–72
Floresco SB, Phillips AG (2001) Delay-dependent modulation of memory retrieval by infusion of a dopamine D1 agonist into the rat medial prefrontal cortex. Behav Neurosci 115:934–939
Frey U, Schroeder H, Matthies H (1990) Dopaminergic antagonists prevent long-term maintenance of posttetanic LTP in the CA1 region of rat hippocampal slices. Brain Res 522:69–75
Fujishiro H, Umegaki H, Suzuki Y et al (2005) Dopamine D2 receptor plays a role in memory function: implications of dopamine-acetylcholine interaction in the ventral hippocampus. Psychopharmacol 182:253–261. https://doi.org/10.1007/s00213-005-0072-x
Garcia AM, Martinez R, Brandao ML, Morato S (2005) Effects of apomorphine on rat behavior in the elevated plus-maze. Physiol Behav 85:440–447. https://doi.org/10.1016/j.physbeh.2005.04.027
Gasbarri A, Sulli A, Packard MG (1997) The dopaminergic mesencephalic projections to the hippocampal formation in the rat. Prog Neuro-Psychopharmacol Biol Psychiatry 21:1–22
Ghizoni DM, Joao LM, Moratelli Neto L et al (2006) The effects of metabolic stress and vagotomy on emotional learning in an animal model of anxiety. Neurobiol Learn Mem 86:107–116. https://doi.org/10.1016/j.nlm.2006.01.005
Gianlorenco AC, Canto-de-Souza A, Mattioli R (2011) Microinjection of histamine into the cerebellar vermis impairs emotional memory consolidation in mice. Brain Res Bull 86:134–138. https://doi.org/10.1016/j.brainresbull.2011.05.014
Goto Y, Otani S, Grace AA (2007) The Yin and Yang of dopamine release: a new perspective. Neuropharmacology 53:583–587. https://doi.org/10.1016/j.neuropharm.2007.07.007
Kudolo J, Tabassum H, Frey S et al (2010) Electrical and pharmacological manipulations of the nucleus accumbens core impair synaptic plasticity in the dentate gyrus of the rat. Neuroscience 168:723–731. https://doi.org/10.1016/j.neuroscience.2010.04.015
LaLumiere RT, Nawar EM, McGaugh JL (2005) Modulation of memory consolidation by the basolateral amygdala or nucleus accumbens shell requires concurrent dopamine receptor activation in both brain regions. Learn Mem 12:296–301. https://doi.org/10.1101/lm.93205
Louilot A, Besson C (2000) Specificity of amygdalostriatal interactions in the involvement of mesencephalic dopaminergic neurons in affective perception. Neuroscience 96:73–82
Martinez A, Pittaluga S, Rudelius M et al (2008) Expression of the interferon regulatory factor 8/ICSBP-1 in human reactive lymphoid tissues and B-cell lymphomas: a novel germinal center marker. Am J Surg Pathol 32:1190–1200. https://doi.org/10.1097/PAS.0b013e318166f46a
Menard J, Treit D (2001) The anxiolytic effects of intra-hippocampal midazolam are antagonized by intra-septal L-glutamate. Brain Res 888:163–166
Nasehi M, Piri M, Nouri M et al (2010) Involvement of dopamine D1/D2 receptors on harmane-induced amnesia in the step-down passive avoidance test. Eur J Pharmacol 634:77–83. https://doi.org/10.1016/j.ejphar.2010.02.027
Nasehi M, Sahebgharani M, Haeri-Rohani A, Zarrindast MR (2009) Effects of cannabinoids infused into the dorsal hippocampus upon memory formation in 3-days apomorphine-treated rats. Neurobiol Learn Mem 92:391–399. https://doi.org/10.1016/j.nlm.2009.05.005
Nazari-Serenjeh F, Rezayof A (2013) Cooperative interaction between the basolateral amygdala and ventral tegmental area modulates the consolidation of inhibitory avoidance memory. Prog Neuro-Psychopharmacology Biol Psychiatry 40:54–61. https://doi.org/10.1016/j.pnpbp.2012.10.003
Nazari-Serenjeh F, Rezayof A, Zarrindast MR (2011) Functional correlation between GABAergic and dopaminergic systems of dorsal hippocampus and ventral tegmental area in passive avoidance learning in rats. Neuroscience 196:104–114. https://doi.org/10.1016/j.neuroscience.2011.08.073
Packard MG, White NM (1989) Memory facilitation produced by dopamine agonists: role of receptor subtype and mnemonic requirements. Pharmacol Biochem Behav 33:511–518
Pandaranandaka J, Poonyachoti S, Kalandakanond-Thongsong S (2006) Anxiolytic property of estrogen related to the changes of the monoamine levels in various brain regions of ovariectomized rats. Physiol Behav 87:828–835. https://doi.org/10.1016/j.physbeh.2006.02.002
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates. Elsevier, New York, NY
Ploeger GE, Spruijt BM, Cools AR (1994) Spatial localization in the Morris water maze in rats: acquisition is affected by intra-accumbens injections of the dopaminergic antagonist haloperidol. Behav Neurosci 108:927–934
Risold PY, Swanson LW (1996) Structural evidence for functional domains in the rat hippocampus. Science (80-) 272:1484–1486
Rodgers RJ, Nikulina EM, Cole JC (1994) Dopamine D1 and D2 receptor ligands modulate the behaviour of mice in the elevated plus-maze. Pharmacol Biochem Behav 49:985–995
Setlow B, McGaugh JL (1998) Sulpiride infused into the nucleus accumbens posttraining impairs memory of spatial water maze training. Behav Neurosci 112:603–610
Stefanski R, Palejko W, Bidzinski A et al (1993) Serotonergic innervation of the hippocampus and nucleus accumbens septi and the anxiolytic-like action of the 5-HT3 receptor antagonists. Neuropharmacology 32:987–993
Stern CA, Do Monte FH, Gazarini L et al (2010) Activity in prelimbic cortex is required for adjusting the anxiety response level during the elevated plus-maze retest. Neuroscience 170:214–222. https://doi.org/10.1016/j.neuroscience.2010.06.080
Tan SE (2008) Roles of hippocampal NMDA receptors and nucleus accumbens D1 receptors in the amphetamine-produced conditioned place preference in rats. Brain Res Bull 77:412–419. https://doi.org/10.1016/j.brainresbull.2008.09.007
Tidey JW, Miczek KA (1996) Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res 721:140–149
Wahlstrom D, White T, Luciana M (2010) Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neurosci Biobehav Rev 34:631–648. https://doi.org/10.1016/j.neubiorev.2009.12.007
Walaas I, Fonnum F (1980) Biochemical evidence for gamma-aminobutyrate containing fibres from the nucleus accumbens to the substantia nigra and ventral tegmental area in the rat. Neuroscience 5:63–72
Wall PM, Blanchard RJ, Yang M, Blanchard DC (2003) Infralimbic D2 receptor influences on anxiety-like behavior and active memory/attention in CD-1 mice. Prog Neuro-Psychopharmacology Biol Psychiatry 27:395–410. https://doi.org/10.1016/S0278-5846(02)00356-1
Xu L, Anwyl R, De Vry J, Rowan MJ (1997) Effect of repeated ipsapirone treatment on hippocampal excitatory synaptic transmission in the freely behaving rat: role of 5-HT1A receptors and relationship to anxiolytic effect. Eur J Pharmacol 323:59–68
Yanagihashi R, Yamanouchi K, Ishikawa T (1991) The effects of apomorphine on the hippocampal field potential in freely moving rats: pharmacological evidence of the involvement of D2 receptors. Neuropharmacology 30:177–182
Funding
The authors wish to thank the Iran National Science Foundation (INSF) for providing financial support to this project.
Author information
Authors and Affiliations
Contributions
ME acquired the animal data and wrote the manuscript. MN and MZ were responsible for the study concept, design, and proteomics analysis, as well as assisted with the data analysis and interpretation of findings. All authors critically reviewed the content and approved the final version for publication.
Corresponding author
Ethics declarations
The study was carried out in accordance with ethical standards in all aspects.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ebrahimi-Ghiri, M., Nasehi, M. & Zarrindast, MR. The modulatory role of accumbens and hippocampus D2 receptors in anxiety and memory. Naunyn-Schmiedeberg's Arch Pharmacol 391, 1107–1118 (2018). https://doi.org/10.1007/s00210-018-1534-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-018-1534-0