Abstract
The present study investigated changes in behaviour associated with oral monosodium glutamate (a flavouring agent), using the open field, elevated plus maze and conditioned place preference (CPP) paradigms, respectively. Mice were assigned to two groups for CPP [monosodium glutamate (MSG)-naïve (n = 40) and MSG-pretreated (n = 40)] and two groups for open field (OF) and elevated plus maze (EPM) tests [n = 40 each], respectively. Animals in respective groups were then divided into four subgroups (n = 10) (vehicle or MSG (80, 160 and 320 mg/kg)). MSG-naïve mice were observed in the CPP box in three phases (pre-conditioning, conditioning and post-conditioning). Mice were conditioned to MSG or an equivalent volume of saline. The MSG pretreatment group received vehicle or respective doses of MSG daily for 21 days, prior to conditioning. Mice in the OF or EPM groups received vehicle or doses of MSG (orally) for 21 days, at 10 ml/kg. Open field or EPM behaviours were assessed on days 1 and 21. At the end of the experiments, mice in the OF groups were sacrificed and brain homogenates used to assay glutamate and glutamine. Results showed that administration of MSG was associated with a decrease in rearing, dose-related mixed horizontal locomotor, grooming and anxiety-related response and an increase in brain glutamate/glutamine levels. Following exposure to the CPP paradigm, MSG-naïve and MSG-pretreated mice both showed ‘drug-paired’ chamber preference. The study concluded that MSG (at the administered doses) was associated with changes in open field activities, anxiety-related behaviours and brain glutamate/glutamine levels; its ingestion also probably leads to a stimulation of the brain reward system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Compulsive consumption of palatable foods has been associated with activation of the brain reward system in humans and animals (Olsen 2011; Hebebrand et al. 2014). Such foods or some of their components are believed to possess an inherent ability to induce dependence (Hebebrand et al. 2014). Studies have shown that different components of food may trigger compulsive eating, and in both humans and animals, differential effects of high-fat, high-sugar or high-protein diet on eating behaviour (Avena et al. 2008; Avena et al. 2012; Tulloch et al. 2015; Schulte et al. 2016) and neurotransmitter chemistry (Bocarsly et al. 2010) have been reported. Recently, debates on the relationship that might exist between food additives (e.g. culinary flavour enhancers) and a repeated urge to consume certain foods have also increased considerably (Morris et al. 2008; Cocores and Gold 2009; Blaylock 2014). There are even suggestions that the consumption of highly processed foods may promote ‘food addiction’ (Johnson and Kenny 2010; Gearhardt et al. 2011). These suggestions are related to the fact that, although many food ingredients lack intoxication capacity, studies have shown that some foods or their additives (e.g. saccharin) appear to induce reinforcement behaviours similar to or possibly exceeding those associated with drugs of abuse (Lenoir et al. 2007; Gearhardt et al. 2011). They could also trigger addiction-like neuroadaptive responses in the brain reward circuits (Johnson and Kenny 2010). Highly processed foods may also have been altered in ways similar to addictive drugs (Gearhardt et al. 2011); indeed, certain formulations of processed foods have been designed to maximise palatability and/or reward (Spence 2012). Despite speculations regarding the factors that are present in food that may stimulate compulsive eating, there is not sufficient scientific evidence to consider any food ingredient, micronutrient or standard food additive as addictive (Hebebrand et al. 2014).
Monosodium glutamate (MSG), a prototype of the umami taste sensation, is a savoury flavour (McCabe and Rolls 2007) which has been used to improve the palatability and acceptability of a variety of foods (Prescott 2004). Despite its wide acceptance and presumed safety, the hint of a possible addictive potential of MSG continues to be the focus of a number of health debates and a central theme of discussion in a number of nutrition and health-related blogs (Moskin 2008; Blaylock 2014). There are suggestions that MSG plays a dual role in nutrition; studies have reported that MSG increases hedonic perception; hence, it has the ability to possibly stimulate appetite and food intake. Secondly, it helps to maintain stable energy levels (Masic and Yeomans 2013). There are also evidences to suggest that MSG reinforces (through conditioning) preferences for its own flavour and flavours related to it (Tsurugizawa and Torii 2010; Ackroff and Sclafani 2013). In previous studies that utilised self-administration protocols (resulting in a total daily consumption of MSG that is higher than the bolus doses given in the present study), MSG at a concentration of 200–300 mM/L (self-administered at approximately 13.5–20.3 g/kg/day) was shown (in mice) to induce conditioned taste preference for itself and MSG-paired flavours (Bachmanov et al. 2000, Ackroff et al. 2012; Ackroff and Sclafani 2013). Tsurugizawa and Torii (2010) also reported that an intragastric load of MSG evoked conditioned flavour preference in rats. However, MSG’s role as a flavour enhancer ordinarily suggests that its presence in foods will enhance consumption, and this does not have to be related to an addictive potential. Ingested MSG undergoes extensive metabolism in the gut (Nakamura et al. 2013); however, the total quantity of glutamate that is directly absorbed increases significantly, as more MSG is ingested (Onaolapo et al. 2016a). Numerous studies evaluating the effects of MSG on the brain (Onaolapo et al. 2015, 2016a, 2016b) at doses within the average range of daily intake in humans (Geha et al. 2000; Beyreuther et al. 2007; Shi et al. 2010) or above it (Gonzalez-Burgos et al. 2001; Hashem et al. 2012) have been published. However, there is still a dearth of information on the potential effects of ingested MSG on the brain reward system.
In the last decade, emphasis has been on the importance of brain glutamate in addiction (Tzschentke and Schmidt 2003); also, imbalances in glutamate homeostasis have been reported to alter brain plasticity, especially as it relates to dopaminergic neurons (in the prefrontal cortex and nucleus accumbens) (Miyamoto et al. 2001, Paz 2005). Neuroplasticity of the cortico-striatal circuitry has been linked to dependence and addictive behaviour (Tzschentke and Schmidt 2003). Glutamate has also been reported to stimulate striatal dopamine release (Nakanishi 1992) and influence the reward system via its effects on dopaminergic neurons in the ventral tegmental area (VTA) (Qi et al. 2014); however, there have been reports that suggest that glutamatergic projections from the VTA to the nucleus accumbens also mediate aversion (Qi et al. 2016). Reports linking glutamate to the reward system (Qi et al. 2014, 2016) raise the question of the possible effect of exogenous glutamate on the development and expression of reward and reward-related behaviours, such as reward-learning and memory consolidation. The rationale for this study was the need to have an insight into this, by assessing the effects of repeated administration of increasing doses of MSG (80, 160 and 320 mg/kg) on open field spontaneous locomotor activity/grooming, anxiety-related behaviours in the elevated plus maze (EPM) and conditioned place preference. However, since there is a dearth of information on the baseline behavioural effects of MSG at these doses (except 80 mg/kg) in the open field, we surmised that we must first assess the general behavioural effects of MSG (to confirm the presence or otherwise of central effects at the doses tested). The EPM model was also used, since it is known that there are neuronal projections between the amygdala bed nucleus of the stria terminalis (BNST) (which modulates anxiety response) and the ventral tegmental area (VTA), a region of the brain that plays an important role in reward or aversion, affirming the possibility of a behavioural link between anxiety and addiction (Jennings et al. 2013). The study also ascertained the place preference response to MSG, in MSG-naïve mice or mice exposed to repeated administration of MSG. We tested the hypotheses that acute or repeated administration of increasing doses of MSG could alter behaviours in the open field, elevated plus maze and/or induce conditioned place preference in a two-chamber paradigm.
Materials and methods
Drug administration
MSG (99.9% purity, Ajinomoto®, West African Seasoning Company, Lagos, Nigeria) was used for this study. Doses of MSG were selected with reference to previous studies (Onaolapo et al. 2015). Mice in the open- field, EPM and conditioned place preference (CPP) (MSG pretreatment) groups received either vehicle (distilled water) or MSG (80, 160 and 320 mg/kg) by gavage via an oral cannula at a volume of 10 ml/kg of body weight, daily (starting at 9.00 a.m.), for 21 days.
Subjects
Adult male Swiss mice (Empire Breeders, Osogbo, Osun State, Nigeria) were used for this study. Mice were housed in groups of five in plastic cages located in a temperature-controlled quarters (22–25 °C) with 12 h light/dark cycle (lights off at 7.00 a.m.). All animals were fed commercial (Top feeds®, Premier feeds Ltd., Nigeria) standard chow (calories: 29% protein, 13% fat, 58% carbohydrate) from weaning. Mice had access to food and tap water except during tests. All animals were naïve to the conditioned place preference (N = 80) apparatus, open field (N = 40) and elevated plus maze (N = 40) at the commencement of the tests. All procedures were conducted in accordance with the approved institutional protocols and within the provisions for animal care and use prescribed in the scientific procedures on living animals, European Council Directive (EU2010/63).
Open field test
The open field apparatus is a rectangular arena made of white-painted wood, measuring 36 × 36 × 26 cm. The floor is made of hard wood and divided by permanent red markings into 16 equal-sized squares. Mice were placed in the centre of the field and covered by a small dome for (5 s), which was removed at the beginning of the 10-min countdown. Generally, spontaneous locomotor activity was monitored in the open field after administration of treatment. Each mouse was introduced into the field and the total horizontal locomotion (number of floor units entered with all paws), rearing frequency (number of times the animal stood on its hind legs either with its forearms against the walls of the observation cage or free in the air) and frequency of grooming (number of body-cleaning with paws, picking of the body and pubis with the mouth and face-washing actions) were recorded and scored (Onaolapo et al. 2015, 2016a).
Animals used for the open field test (N = 40) were divided into four (n = 10) groups: VEH and MSG at 80, 160 and 320 mg/kg/day. Open field spontaneous locomotor activity (horizontal locomotion and rearing) and grooming behaviours were assessed on days 1 (after first dose) and 21 (after last dose), 30 min after administration of VEH or MSG. Tests were conducted in a quiet room between 9 a.m. and 2 p.m. On each of the test days, mice were transported in their home cages to the behavioural testing laboratory and allowed to acclimatise for 30 min before administration of MSG or vehicle. At the beginning of the open field tests, each animal was placed in the box and its behaviour videotaped (by a ceiling-mounted digital video camera (SMX-F543B), placed 1.5 m above the arena) for subsequent analysis, and all interior surfaces of the arena were cleaned thoroughly with 70% ethanol and then wiped dry. At least 5 min was allowed between the testing of individual animals to ensure that the maze was completely dry and that dispersal of the residual odour of alcohol had occurred. The behavioural parameters were later scored by two independent observers who were blind to the groupings.
Anxiety test
Anxiety-related behaviour was measured in the EPM. The EPM is a plus-shaped apparatus made of white-painted wood, with two open arms measuring 25 × 5 × 0.5 cm lying across from each other and perpendicular to two closed arms measuring 25 × 5 × 16 cm with a central platform (5 × 5 × 0.5 cm). The closed arms are enclosed by two high walls (16 cm), while the open arms have no side walls. Animals used for the EPM test (N = 40) were divided into four (n = 10) groups: VEH and MSG at 80, 160 and 320 mg/kg/day on days 1 (after first dose) and 21 (after last dose), 30 min after administration of VEH or MSG. Tests were also conducted in a quiet room between the hours of 9 a.m. and 2 p.m. On each of these test days, mice were transported in their home cages to the behavioural testing laboratory and allowed to acclimatise for 30 min before administration of MSG or vehicle. At the commencement of elevated plus maze tests, animals were placed in the central platform, facing a closed arm and anxiety related behaviours were assessed for 5 min. The criterion for arm visit was considered only when the animal decisively moved all its four limbs into an arm. The following were measured: percentage time spent in the open or closed arms (time in open or closed arms/total time spent in the maze, multiplied by 100) and number of open or closed arm entries (Onaolapo et al. 2016b).
Conditioned place preference
Conditioned place preference apparatus and protocol
The conditioned place preference paradigm is used for determining both the rewarding and aversive properties of drugs in animals (Schechter and Calcagnetti 1993). The apparatus used for the place-conditioning was made of wood and consisted of two compartments (a white and a black, each 15 × 15 × 15 cm high); the white compartment had a wooden floor, while the dark compartment had a glass floor. Compartments were separated by a removable guillotine door (10 × 15 cm high). The light intensity within the conditioning chambers was approximately 30 ± 5 Lux.
Place preference of MSG-naïve mice (N = 40) and mice previously exposed to repeated administration of increasing doses of MSG (N = 40) were observed in the CPP box. Mice in the MSG pretreatment group received vehicle or MSG (80, 160 and 320 mg/kg) orally for 21 days at a volume of 10 ml/kg, before exposure to the CPP protocol. The last dose of either vehicle or MSG was administered 30 min before first pre-conditioning session commenced. At the beginning of the CPP test, mice to be tested were habituated in the test room for 1 h before experiments. Chambers were cleaned with 70% ethanol, to minimise olfactory cues between sessions. Sessions were videotaped for subsequent analysis and interpretation.
The CPP protocol used consists of three phases: pre-conditioning, conditioning and post-conditioning. In the pre-conditioning period, the purpose was to habituate the animals to the novelty and stress associated with the apparatus, handling prior to conditioning, as well as to identify initial chamber preferences (second pre-conditioning day). For the pre-conditioning, all animals received an oral gavage of saline immediately prior to placement in the apparatus. Mice were exposed to the CPP box with the guillotine door left opened to allow free movement between the two compartments for 20 min daily, for 2 days. On the second day of preconditioning, the time each mouse spent in either compartment was measured and recorded as baseline pre-conditioning value for place preference analysis.
For the conditioning procedure, mice were assigned to one of two conditioning subgroups based on their preference for the black or white compartment (there was a minimum of five mice in each subgroup). Mice in each subgroup received MSG paired with the non-preferred chamber and saline paired with the preferred chamber. Mice were conditioned to MSG or saline on four alternating days of the 8-day conditioning period. Each trial lasts 30 min (one trial per day). On days 3, 5, 7 and 9, animals received MSG before exposure to the drug-paired side, and on days 4, 6, 8 and 10, animals received saline before introduction to the saline-paired side. Mice in the vehicle group were conditioned to only saline. In the post-conditioning test (day 11), the guillotine door was opened and mice allowed to move freely between both compartments for the duration of the 20-min testing trial. Time spent in the MSG-paired chamber in the pre-conditioning (day 2) and post-conditioning (day 11) is recorded, and the development of place preference was defined as a significant difference between pre- and post-conditioning values.
Assessment of brain glutamate and glutamine
All mice in the open field group were sacrificed (within 3 min of the completion of the behavioural test) by cervical dislocation under diethyl-ether anaesthesia and perfused transcardially with ice-cold saline. Whole brains were dissected out, blotted dry and immediately weighed. A 10% brain homogenate was prepared with ice-cold phosphate-buffered saline using Teflon glass homogeniser. The homogenate was centrifuged at 5000 rpm (4 °C) for 15 min and the pellet discarded. The supernatant was used to assay brain glutamate and glutamine levels. Brain glutamate and glutamine levels were assayed using commercially available glutamate and glutamine assay kit (Biovision Inc., Milpitas, CA, USA) as previously described (Onaolapo et al. 2016a, 2016b). The glutamate and glutamine assay kit provides a sensitive detection method for glutamate and glutamine in the sample, respectively.
Statistical analysis
Data was analysed using Chris Rorden’s ezANOVA for Windows, version 0.98. Hypothesis testing was performed using analysis of variance (ANOVA). We tested the hypotheses that acute or repeated administration of increasing doses of MSG could alter behaviours of mice in the open field/elevated plus maze and/or induce a drug-conditioned place preference in a two-chamber CPP paradigm. Two-way ANOVA was used for analyses of (1) open field and elevated plus maze behaviours, with treatment (four levels: VEH, three MSG doses) as between subject and duration of administration (two levels: acute and repeated) as within subject, and (2) MSG-conditioned place preference, with treatment (four levels: VEH, three MSG doses) as between subject and phase (two levels: pre- and post-conditioning) as within subject. One-way ANOVA was used to analyse (1) pre-conditioning test chamber bias and (2) the effect of MSG on brain levels of glutamate and glutamine. Tukey’s honest significant difference (HSD) test was used for post hoc analysis. Results were expressed as mean ± SEM, with p values less than 0.05 considered statistically significant.
Results
Open field novelty-induced behaviours
Effect of monosodium glutamate on horizontal locomotion
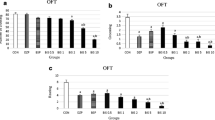
The numbers of lines crossed in 10 min for vehicle or MSG-treated mice are summarised in Fig. 1 (upper panel). Two-factor ANOVA revealed a significant effect of treatment (F(3, 36) = 44.2, p < 0.001), duration of administration (F(1, 36) = 933, p < 0.001) and interactions between treatment and duration of administration (F(3, 36) = 159, p < 0.001). Tukey HSD tests showed a significant increase in locomotor activity following acute administration of MSG at 80 (p < 0.001) and 160 mg/kg (p < 0.001); with repeated administration, locomotor activity decreased significantly at 160 (p < 0.001) and 320 mg/kg (p < 0.001), compared to vehicle. Repeated administration of MSG at 80 (p < 0.001), 160 (p < 0.001) and 320 mg/kg (p < 0.001) significantly decreased locomotor activity compared to acute administration.
Novelty-induced horizontal locomotor activity (upper panel) and rearing (lower panel) following vehicle (VEH) or MSG (80, 160 and 320 mg/kg). Values are means ± SEM (* p < 0.05 significantly different from vehicle, # p < 0.05 repeated administration significantly different from acute administration), VEH vehicle; MSG monosodium glutamate, n = 10
Effect of monosodium glutamate on number of rears
The numbers of rears in 10 min for vehicle or MSG-treated mice are summarised in Fig. 1 (lower panel). Two-factor ANOVA revealed a significant effect of treatment (F(3, 36) = 443, p < 0.001), duration of administration (F(1, 36) = 324, p < 0.001) and interactions between treatment and duration of administration (F(3, 36) = 44.2, p < 0.001). Tukey HSD tests showed a significant decrease in rearing activity following acute and repeated administration of MSG at 80 (p < 0.001, p < 0001) and 160 (p < 0.001, p < 0.001) and 320 mg/kg (p < 0.001, p < 0.001), respectively, compared to vehicle. Repeated administration of MSG at 80 (p < 0.001), 160 (p < 0.001) and 320 mg/kg (p < 0.001) significantly decreased rearing activity compared to acute administration.
Effect of monosodium glutamate on grooming behaviour
The frequency of grooming in 10 min for vehicle or MSG-treated mice is summarised in Fig. 2. Two-factor ANOVA revealed no significant effect of treatment (F(3, 36) = 2.27, p < 0.097) but a significant effect of duration of administration (F(1, 36) = 18.7, p < 0.001) and significant interaction between treatment and duration of administration (F(3, 36) = 21.3, p < 0.001). Tukey HSD tests showed a significant decrease in grooming frequency with MSG at 320 mg/kg (p < 0.002) after acute administration; with repeated administration, grooming decreased at 80 mg/kg (p < 0.041) and increased at 160 (p < 0.012) and 320 mg/kg (p < 0.001), compared to vehicle. Repeated administration of MSG at 80 (p < 0.002) significantly decreased grooming frequency compared to acute administration; at 160 (p < 0.010) and 320 mg/kg (p < 0.002), grooming increased significantly.
Anxiety-related behaviour
Effects of monosodium glutamate on percent time in the EPM open arm
Figure 3 (upper panel) shows the effect of MSG on percent time spent in the open arm of the EPM. Two-factor ANOVA revealed a significant main effect of treatment (F(3, 36) = 12.20, p < 0.001), duration of administration (F(1, 36) = 7.44, p < 0.010) and treatment × duration of administration (F(3, 36) = 4.55, p < 0.002). Tukey HSD tests showed a significant decrease in time spent in the open arms with MSG at 80 (p < 0.002) and a significant increase at 160 (p < 0.001) and 320 mg/kg (p < 0.001), compared to vehicle, following acute administration. With repeated administration, time spent in the open arm decreased with MSG at 80 mg/kg (p < 0.001) and increased at 160(p < 0.002) and 320 mg/kg (p < 0.001). Repeated administration of MSG at 80 (p < 0.001) significantly decreased time spent in the open arm compared to acute administration, while at 160 (p < 0.002) and 320 mg/kg (p < 0.001), percent time spent in the open arm increased significantly.
Percentage time spent in the open arm (upper panel) and number of open arm entries (lower panel) in the elevated plus maze following vehicle (VEH) or MSG (80, 160 and 320 mg/kg).Values are means ± SEM (* p < 0.05 significantly different from vehicle, # p < 0.05 repeated administration significantly different from acute administration), VEH vehicle, MSG monosodium glutamate, n = 10
Effects of monosodium glutamate on number of EPM open arm entries
Figure 3 (lower panel) shows the effect of MSG on number of open arm entries in the EPM. Two-factor ANOVA revealed a significant effect of treatment (F(3, 36) = 10.10, p < 0.001), duration of administration (F(1, 36) = 8.10, p < 0.001) and no interaction between treatment and duration of administration (F(3, 36) = 1.12, p < 0.173). Tukey HSD tests revealed a significant decrease in number of open arm entries with MSG at 80 (p < 0001) and a significant increase at 160 (p < 0.001) and 320 mg/kg (p < 0.001) compared to vehicle, after acute administration; with repeated administration, number of open arm entries decreased with MSG at 80 mg/kg (p < 0002) and increased with MSG at 160 (p < 0.002) and 320 mg/kg (p < 0.001). Repeated administration of MSG did not significantly alter number of open arm entries compared to acute administration.
Effects of monosodium glutamate on percent time in the EPM closed arm
Figure 4 (upper panel) shows the effect of MSG on percent time spent in the closed arm of the EPM. Two-factor ANOVA revealed a significant main effect of treatment (F(3, 36) = 13.45, p < 0.001), no significant effect of duration of administration (F(1, 36) = 1.31, p < 0.476) or interactions between treatment × duration of administration (F(3, 36) = 0.21, p < 0.628). Tukey HSD tests showed a significant decrease in time spent in the closed arms with MSG at 320 mg/kg (p < 0.001, p < 0001) compared to vehicle, following acute and repeated administration. Repeated administration of MSG also did not differ significantly compared to acute administration.
Percentage time spent in the closed arm (upper panel) and number of closed arm entries (lower panel) in the elevated plus maze following vehicle (VEH) or MSG (80, 160 and 320 mg/kg). Values are means ± SEM (* p < 0.05 significantly different from vehicle), VEH: vehicle, MSG: monosodium glutamate, n = 10
Effects of monosodium glutamate on number of EPM closed arm entries
Figure 4 (lower panel) shows the effect of MSG on number of closed arm entries in the EPM. Two-factor ANOVA revealed a significant effect of treatment (F(3, 36) = 9.34, p < 0.004), duration of administration (F(1, 36) = 10.21, p < 0.001) and no interactions between treatment and duration of administration (F(3, 36) = 12.80, p < 0.001). Tukey HSD tests revealed a significant increase in the number of closed arms entries with MSG at 80 (p < 0001) and a significant decrease at 160 (p < 0.001) and 320 mg/kg (p < 0.001) compared to vehicle, after acute administration; with repeated administration, number of closed arm entries increased with MSG at 80 mg/kg (p < 0001) and decreased with MSG at 160 (p < 0.001) and 320 mg/kg (p < 0.001). Repeated administration of MSG did not significantly alter number of closed arm entries compared to acute administration. Two-factor ANOVA analysis of the effect of MSG on total arm entry revealed no significant effect of treatment (F(3, 36) = 3.02, p < 0.241) or duration of administration (F(1, 36) = 1.69, p < 0.120) and no significant interactions between treatment and duration of administration (F(3, 36) = 0.50, p < 0.293). Tukey HSD tests revealed no significant difference in total arm entry at any of the doses of MSG tested compared to vehicle following acute or repeated administration. Repeated administration of MSG also showed no significant difference compared to acute administration.
Conditioned place preference
Pre-conditioning chamber bias in MSG-naïve mice
Bias in the two-chamber paradigm was measured as time (seconds) spent in each of the two chambers. There was no significant (F(1, 78) = 0.025, p < 0.873) difference in the time mice spent in the black or white chamber. Although chamber preference was not statistically significant, mice were however assigned to the conditioning chamber based on the time spent in their preferred compartment and place preference data analysis was conducted on the groups, with MSG-paired side being the non-preferred side.
Pre-conditioning chamber bias in MSG-pretreated mice
Bias in the two-chamber paradigm was measured as time (seconds) spent in each of the two chambers by mice pretreated with MSG or vehicle. There was no significant main effect of pretreatment with MSG (F(3, 72) = 0.078, p < 0.972), chamber (F(1, 72 = 0.049, p < 0.825) or interactions between pretreatment and chamber (F(3, 72) = 0.020, p < 0.996). Tukey HSD analysis revealed no significant difference in the time mice spent in the black or white chambers at any of the doses of MSG compared to vehicle. Although chamber preference was not statistically significant, mice were however assigned to the conditioning chamber based on the time spent in their preferred compartment and place preference data analysis was conducted on the groups, with MSG-paired side being the non-preferred side.
Place preference in MSG-naïve mice
We tested the effects of MSG on conditioned place preference in mice. The time (in seconds) spent on the drug-paired side in MSG-naive mice is summarised in Fig. 5 (upper panel). Two-factor ANOVA revealed a significant effect of treatment (F(3, 36) = 5.21, p < 0.001), phase (F(1, 36) = 14.50, p < 0.001) and interactions between treatment and phase (F(3, 36) = 6.03, p < 0.002). Tukey HSD tests showed that MSG-naïve mice conditioned to MSG at 160 (p < 0.046) and 320 mg/kg (p < 0.002) spent significantly more time on the MSG-paired side after four pairing sessions compared to vehicle (saline-conditioned). MSG conditioning at 160 (p < 0.001) and 320 mg/kg (p < 0.001) also resulted in significant increase in time spent on the drug-paired side compared to baseline. This result shows that MSG-conditioned place preference was induced in MSG-naïve mice.
Conditioned place preference with vehicle (VEH) or MSG (80, 160 and 320 mg/kg) in MSG-naïve mice (upper panel) and MSG-pretreated mice (lower panel). Values are means ± SEM (* p < 0.05 significantly different from vehicle, # p < 0.05 significantly different from baseline), VEH vehicle, MSG monosodium glutamate, n = 10
Place preference in MSG-pretreated mice
In this second CPP experiment, we tested for MSG-conditioned place preference in MSG-pretreated mice. The time (seconds) spent on the drug-paired side for vehicle or MSG-pretreated mice is summarised in Fig. 5 (lower panel). Two-factor ANOVA revealed a significant effect of treatment (F(3, 36) = 27.3, p < 0.001), phase (F(1, 36) = 193, p < 0.001) and interactions between treatment and phase (F(3, 36) = 43.5, p < 0.001). Tukey HSD tests showed that MSG-pretreated mice conditioned to MSG at 80 (p < 0.001), 160 (p < 0.001) and 320 mg/kg (p < 0.001) spent significantly more time on the MSG-paired side after four pairing sessions, compared to vehicle (saline-conditioned). MSG conditioning at 80 (p < 0.001), 160 (p < 0.001) and 320 mg/kg (p < 0.001) also resulted in significant increase in time spent on the drug-paired side compared to baseline. This result shows that pretreatment with MSG facilitated MSG-conditioned place preference.
Results of comparison between the time spent on the drug-paired side during the post-conditioning test in MSG-naive and MSG-pretreated mice revealed a significant effect of treatment (F(3, 36) = 4.94, p < 0.006), phase (F(1, 36) = 15, p < 0.001) and interactions between treatment and phase (F(3, 36) = 7.54, p < 0.001). Tukey HSD analysis revealed a significant increase in time spent on the drug-paired side in MSG-pretreated mice at 160 [860 ± 5.63 versus 715 ± 4.03 (p < 0.021)] and 320 mg/kg [968 ± 6.84 versus 820 ± 2.73 (p < 0.006)] compared to MSG-naïve mice.
Effect of MSG administration on brain glutamate and glutamine levels
Table 1 shows the effects of daily administration of MSG on brain levels of glutamate and glutamine. Brain glutamate (F(3, 36) = 35.30, p < 0.001) and glutamine levels (F(3, 36) = 18.20, p < 0.001) increased significantly with MSG at 160 and 320 mg/kg, respectively, compared to vehicle. This result shows that daily administration of MSG at increasing doses may alter brain levels of glutamate and glutamine.
Discussion
In the present study, we examined the effects of increasing doses of MSG on behaviours in the open field and elevated plus maze, place preference (in both MSG-naïve and MSG-pretreated mice) and brain glutamate/glutamine levels. We deduced that MSG was associated with (1) suppression of open field horizontal locomotion and rearing and an increase in frequency of grooming; following repeated administration (2) dose-related anxiolytic response with repeated administration; (3) drug-paired chamber preference in both MSG-naïve and MSG-pretreated mice; and (4) increase in brain levels of glutamate/glutamine levels following repeated administration.
Administration of MSG by gavage was associated with dose- and time-related alterations in open field spontaneous locomotor activity, rearing and self-grooming. These results corroborate previous studies (Onaolapo and Onaolapo 2011; Onaolapo et al. 2015), by demonstrating that acute and repeated oral administration of MSG induces changes in behaviour. In the study, horizontal locomotion and rearing behaviours varied with dose (increasing doses of MSG was associated with locomotor retardation) and duration of administration (repeated administration was also associated with locomotor retardation). Studies have reported differences in MSG-induced alterations in open field behaviours, with dose (Kiss et al. 2007; Onaolapo and Onaolapo 2011; Onaolapo et al. 2016b), age of animals (Kiss et al. 2007) and/or duration of administration (Onaolapo et al. 2016b). Self-grooming, however, showed a dose-related decrease with acute administration and an increase after repeated administration. Grooming is an important aspect of the rodent’s behavioural repertoire, and it consists of a complex hierarchy of patterns that are sensitive to novelty, stress and drugs (Onaolapo et al. 2016c). Results of MSG’s effects on grooming (from previous studies) have also varied with respect to age of animals (Dunn et al. 1985) and dose of MSG (Onaolapo and Onaolapo 2011; Onaolapo et al. 2015). Dunn et al. (1985) reported no effect of neonatal MSG administration on grooming behaviour in adult rats. However, results of previous studies from our laboratory revealed that acute intraperitoneal injection of MSG at doses between 0.5 and 1.5 mg/kg (Onaolapo and Onaolapo 2011) increased grooming (however, not significantly), and following oral administration of MSG at 10–80 mg/kg/day (Onaolapo et al. 2015), a significant dose-related increase in grooming behaviour was observed after acute administration and a decrease after repeated administration. These results suggest that effect of MSG on grooming behaviour is dependent on dose and/or duration of administration.
In rodents, the EPM is a model for studying anxiety-related behaviours. Behaviour in the EPM is based on the natural aversion of rodents for elevated and open spaces. In this study, repeated administration of increasing doses of MSG led to a dose-related decrease in arm entry (locomotor response) and an anxiolytic response. The result of closed arm exploration was similar to the effects observed with locomotor response (horizontal locomotion and rearing) in the open field. A dose-related decrease in anxiety-related behaviours was also observed (number of open arm entries and time spent in the open arm). In a number of studies, increases in anxiety-related behaviours have been demonstrated with MSG administration (Narayanan et al. 2010). In a previous study (Onaolapo et al. 2016b), we reported a dose-related mixed response (with decreased anxiety observed when MSG was administered at 10 and 20 mg/kg and increased anxiety response with MSG at 40 and 80 mg/kg). In this study, at 80 mg/kg, we observed increased anxiety which was exacerbated by repeated administration; this corroborates the findings of our previous study. However, at the two higher doses examined, a decrease in anxiety response was observed.
Glutamate (a key constituent of MSG) is the most abundant free amino acid in the brain, functioning both as a neurotransmitter and a fuel reserve (Hawkins 2009). The interactions between glutamate and other brain neurotransmitters (like GABA, dopamine and serotonin) and their receptors, neuromediators or hormones have been reported to alter horizontal locomotion, rearing (Al-Khatib et al. 1995; Cortese and Phan 2005), grooming (Dunn et al. 1985; Barros et al. 1994) and anxiety-related behaviour (Cortese and Phan 2005; Amiel and Mathew 2007). In a few studies, there have been suggestions that a decrease in spontaneous locomotion (as observed in this study, after repeated administration of increasing doses of MSG) may be a result of down-regulation of dopamine receptors (Xu et al. 1994; Svensson et al. 1994; Rubinstein et al. 1997) possibly from stimulation of GABA receptors (Phillis et al. 2001). The GABA system has also been reported to play an important role in the expression of grooming via its GABA-A and GABA-B receptors (Barros et al. 1994); the stimulation of these receptors decreases novelty-induced grooming behaviour (Barros et al. 1994), which may have been responsible for the decrease in grooming response observed with acute administration of MSG (Silverman et al. 2015). Following repeated administration of MSG however, grooming activity increased, suggesting a possible involvement of interactions between glutamate and other neurotransmitters or brain regions. Hong et al. (2014) reported that stimulation of glutamatergic receptors in the medial amygdala induced self-grooming behaviours in rodents, which would suggest that glutamatergic effects in the amygdala may be responsible for the increased grooming response observed with repeated administration of glutamate in this study. Increased glutamatergic receptor activation and shifts in glutamate-GABA influences have also been associated with anxiety-related disorders (Cortese and Phan 2005; Amiel and Mathew 2007). Lopes et al. (2007) observed that the stimulation of both GABA-A and GABA-B receptors in the nucleus accumbens shell was anxiolytic.
The CPP paradigm has been applied widely to study the reward value of a number of drugs of abuse (Tzschenke 1998). One of the advantages of the CPP paradigm is that it allows the assessment of the reward value of substances over a relatively brief exposure period. The CPP utilises the pairing of a conditioned stimulus (chamber), with an unconditioned stimulus (administered drug) to unmask the behavioural response to stimulation (or otherwise) of the brain reward system (Watanabe 2013). Studies have shown that drugs such as morphine (Tzschenke 1998) and nicotine (Pascual et al. 2009) can induce place preference. In this study, we observed that in MSG-naïve mice, conditioning with MSG at 160 and 320 mg/kg (not at 80 mg/kg) resulted in a significant increase in time spent on the MSG-paired side, when compared to either baseline (preconditioning) or saline-pairing (VEH), while pretreatment with MSG was associated with an increase in time spent on the MSG-paired side at 80, 160 and 320 mg/kg. This result suggests successful induction of MSG-conditioned place preference in MSG-naïve or MSG-pretreated mice and also shows that pretreatment enhanced MSG’s potency in inducing CPP. A few studies have reported that MSG induces conditioned taste preference for itself and MSG-paired flavours (Bachmanov et al. 2000, Ackroff et al. 2012; Ackroff and Sclafani 2013). There are also reports of MSG preference to water across a range of concentrations in a two-bottle choice test in mice (Bachmanov et al. 2000). The rewarding stimulus developed during conditioning is determined by the association between the treatment (MSG) and the chamber, which from the results of this study suggest that the administration of MSG was rewarding and an association was learned. A number of researchers have also suggested the involvement of memory processes in reward, since animals were observed while in a drug-free state (Hsu et al. 2002; Koob et al. 2004). In contrast, these behaviours were absent in vehicle group. There have been associations between learning, memory and reward which involve several brain regions, neurotransmitters influences and neural circuitry (McHugh et al. 2013). Glutamatergic neurons found within the mesolimbic reward system have been reported to overlap with the neuronal circuitry associated with learning, memory processes and goal-directed behaviours (Kelley 2004). There are reports that mechanisms involved in the reward response of a treatment (for example cocaine) are strongly related to the aspect of CPP measured (White and Carr 1985; Hsu et al. 2002). Different phases have been described, which include the initial acquisition and expression of drug-induced CPP (memory consolidation), reinstatement, extinction and reconsolidation (Aguilar et al. 2008). In the present study, the phase that we observed was the initial acquisition and expression of MSG-induced CPP. Several receptors, neuronal pathways and neuroanatomically discrete sites have been implicated in the brain reward mechanisms (McBride et al. 1999, Hyman and Malenka 2001), which involve glutamatergic (Tzschentke and Schmidt, 2003; Kawasaki et al. 2005; He et al. 2014), dopaminergic (Thomas et al. 2008), GABA and peptidergic neurons (Groenewegen et al. 1996). Projections to and from the dorsal raphe nucleus (DRN), the VTA (Qi et al. 2014), corticolimbic and thalamic brain regions (Groenewegen et al. 1996) stimulate preference (Qi et al. 2014) or aversion (Qi et al. 2016).
Ingestion of MSG has been associated with increased stimulation of certain regions of the brain, either directly or indirectly. Kondoh et al. (2009) reported that stimulation of l-glutamate receptors in the gut by luminal glutamate activates vagal afferent nerve fibres, which (via vagal inputs) stimulate brain regions (like the habenular nucleus) that are known to influence other regions of the brain. Tsurugizawa et al. (using functional magnetic resonance imaging studies and blood oxygen level-dependent signals) observed that an intragastric load of MSG significantly activates the insular cortex, amygdala and the hypothalamic regions, including the lateral hypothalamus, dorsomedial hypothalamus and the medial preoptic area (Tsurugizawa et al. 2009; Tsurugizawa and Torii 2010), hence linking luminal presence of glutamate to the ability to influence the brain reward system circuit.
MSG dissociates to yield glutamate, following oral administration (Blachier et al. 2009). Numerous studies have observed that although plasma glutamate levels remain generally stable, it can increase (to varying degrees), following administration of MSG (Caccia et al. 1983; Fernstrom 2000). In a previous study (Onaolapo et al. 2016a), we reported that oral administration of MSG at 80 mg/kg/day was associated with a significant rise in plasma glutamate, while an increase in brain glutamate and glutamine levels observed was not statistically significant. In this study however, we observed a dose-related increase in brain levels of glutamate and glutamine. There are reports that under normal conditions, most of the free brain glutamate is derived from local synthesis (Smith 2000), although glutamate flux from plasma (at physiologic plasma concentrations) into the brain also occurs via a high affinity, saturable and stereoselective transport system at the blood–brain barrier. Also, efflux from brain back into plasma is driven by a sodium-dependent active transport system at the capillary abluminal membrane (Lee et al. 1998). This allows glutamate concentrations in the brain to be maintained fairly independently of small fluctuations in plasma concentration but also suggests that this balance may be undermined by increases in plasma glutamate levels.
Conclusion
The results of this study show that repeated oral administration of MSG alters open field and anxiety-related behaviours, while its effects on place preference suggest the possibility of stimulation of the brain reward system. The behavioural changes observed could be attributed to the effects of the increased brain levels of glutamate and glutamine although gut stimulation of specific regions of the brain following an oral load of MSG is also possible; however, further research is needed to evaluate the strengths of these associations.
References
Ackroff K, Sclafani A (2013) Flavour preferences conditioned by oral monosodium glutamate in mice. Chem Senses 38:745–758. doi:10.1093/chemse/bjt049
Ackroff K, Weintraub R, Sclafani A (2012) MSG intake and preference in mice are influenced by prior testing experience. Physiol Behav 107:207–217
Aguilar MA, Rodriguez-Arias M, Minarro J (2008) Neurobiological mechanisms of the reinstatement of drug-conditioned place preference. Brain Res Rev 59:253–277
Al-Khatib IMH, Doè Kmeci I, Fujiwara M (1995) Differential role of nucleus accumbens and caudate-putamen in mediating the effect of nomifensine and methamphetamine on ambulation and rearing of rats in the open-field test. The Japanese J Pharmacol 67:69–77
Amiel JM, Mathew SJ (2007) Glutamate and anxiety disorders. Curr Psychiatry Rep 9:278–283
Avena NM, Bocarsly ME, Rada P, Kim A, Hoebel BG (2008) After daily bingeing on a sucrose solution, food deprivation induces anxiety and accumbens dopamine/acetylcholine imbalance. Physiol Behav 94:309–315
Avena NM, Gold JA, Kroll C, Gold MS (2012) Further developments in the neurobiology of food and addiction: update on the state of the science. Nutrition 28:341–343
Bachmanov AA, Tordoff MG, Beauchamp GK (2000) Intake of umami-tasting solutions by mice: a genetic analysis. J Nutr 130:935S–941S
Barros HM, Tannhauser SL, Tannhauser MA, Tannhauser M (1994) The effects of GABAergic drugs on grooming behaviour in the open field. Pharmacol Toxicol 74:339–344
Beyreuther K, Biesalski HK, Fernstrom JD, Grimm P, Hammes WP, Heinemann U, Kempski O, Stehle P, Steinhart H, Walker R (2007) Consensus meeting: monosodium glutamate: an update. Eur J Clin Nutr 61:304–313
Blachier F, Boutry C, Bos C, Tomé D (2009) Metabolism and functions of L-glutamate in the epithelial cells of the small and large intestines. Am J Clin Nutr 90:814S–821S
Blaylock MD (2014) MSG: the hidden addiction. Newsmax health, Newsmax.com. Accessed November 2016
Bocarsly ME, Powell ES, Avena NM, Hoebel BG (2010) High fructose corn syrup causes characteristics of obesity in rats: increased body weight, body fat, and triglyceride levels. Pharmacol Biochem Behav 97:101–106. doi:10.1016/j.pbb.2010.02.012
Caccia S, Ghezzi P, Garattini S, Salmona M, Takasaki Y, Torii K (1983) Pyroglutamate kinetics and neurotoxicity studies in mice. Toxicol Lett 16:225–229
Cocores JA, Gold MS (2009) The salted food addiction hypothesis may explain overeating and the obesity epidemic. Med Hypotheses 73:892–899
Cortese BM, Phan KL (2005) The role of glutamate in anxiety and related disorders. CNS spectrum 10:820
Dunn AJ, Webster EL, Nemeroff CB (1985) Neonatal treatment with monosodium glutamate does not alter grooming behavior induced by novelty or adrenocorticotropic hormone. Behav Neural Biol 44:80–89
Fernstrom JD (2000) Pituitary hormone secretion in normal male humans: acute responses to a large, oral dose of monosodium glutamate. J Nutr 130:1053S–1057S
Gearhardt AN, Davis C, Kuschner R, Brownell KD (2011) The addiction potential of hyperpalatable foods. Curr Drug Abuse Rev 140-145:1874–4737/11
Geha RS, Beiser A, Ren C, Patterson R, Greenberger PA, Grammer LC, Ditto AM, Harris KE, Shaughnessy MA, Yarnold PR, Corren J, Saxon A (2000) Review of alleged reaction to monosodium glutamate and outcome of a multicenter double-blind placebo-controlled study. The J Nutr 130:1058S–1062S
Gonzalez-Burgos I, Perez-Vega MI, Beas-Zarate C (2001) Neonatal exposure to monosodium glutamate induces cell death and dendritic hypotrophy in rat prefrontocortical pyramidal neurons. Neurosci Lett 297:69–72
Groenewegen HJ, Wright CI, Beijer AV (1996) The nucleus accumbens: gateway for limbic structures to reach the motor system? Progressive Brain Res 107:485–511
Hashem H, Safwat EM, Algaidi S (2012) The effect of monosodium glutamate on the cerebellar cortex of male albino rats and the protective role of vitamin C: histological and immunohistochemical study. J Mol Histol 43:179–186
Hawkins RA (2009) The blood-brain barrier and glutamate. Am J Clin Nutr 90:867S–874S. doi:10.3945/ajcn.2009.27462BB
He Z, Chen Y, Dong H, Su R, Gong Z, Yan L (2014) Inhibition of vesicular glutamate transporters contributes to attenuate methamphetamine-induced conditioned place preference in rats. Behav Brain Res 267:1–5. doi:10.1016/j.bbr.2014.02.047
Hebebrand J, Albayrak Ö, Adan R, Antel J, Dieguez C, de Jong J, Leng G, Menzies J, Mercer JG, Murphy M, van der Plasse G, Dickson SL (2014) Eating addiction, rather than food addiction, better captures addictive-like eating behavior. Neurosci Biobehav Rev 47:295–306
Hong W, Kim D, Anderson DJ (2014) Antagonistic control of social versus repetitive self-grooming behaviors by separable amygdala neuronal subsets. Cell 158:1348–1361
Hsu EH, Schroeder JP, Packard MG (2002) The amygdala mediates memory consolidation for an amphetamine conditioned place preference. Behav Brain Res 129:93–100
Hyman SE, Malenka RC (2001) Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci 2:695–703
Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD (2013) Distinct extended amygdala circuits for divergent motivational states. Nature 496:224–228
Johnson PM, Kenny PJ (2010) Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci 13:635–641
Kawasaki Y, Jin C, Suemaru K, Kawasaki H, Shibata K, Choshi T, Hibino S, Gomita Y, Araki H (2005) Effect of glutamate receptor antagonists on place aversion induced by naloxone in single-dose morphine-treated rats. Br J Pharmacol 145:751–757. doi:10.1038/sj.bjp.0706228
Kelley AE (2004) Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron 44:161–79
Kiss P, Hauser D, Tamás A, Lubics A, Rácz B, Horvath ZS, Farkas J, Zimmermann F, Stepien A, Lengvari I, Reglódi D (2007) Changes in open-field activity and novelty-seeking behavior in periadolescent rats neonatally treated with monosodium glutamate. Neurotox Res 12:85–93
Kondoh T, Mallick HN, Torii K (2009) Activation of the gut-brain axis by dietary glutamate and physiologic significance in energy homeostasis. American J Clin Nutr 90:832S–837S
Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, O'Dell LE, Parsons LH, Sanna PP (2004) Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev 27:739–749
Lee WJ, Hawkins RA, Viña JR, Peterson DR (1998) Glutamine transport by the blood-brain barrier: a possible mechanism for nitrogen removal. American J Physiol 274:C1101–C1107
Lenoir M, Serre F, Cantin L, Ahmed SH (2007) Intense sweetness surpasses cocaine reward. PLoS One 2:698–708
Lopes APF, Da Cunha IC, Steffens SM, Ferraz A, Vargas JC, De Lima TCM et al (2007) GABAA and GABAB agonist microinjections into medial accumbens shell increase feeding and induce anxiolysis in an animal model of anxiety. Behav Brain Res 184:142–149
Masic U, Yeomans MR (2013) Does monosodium glutamate interact with macronutrient composition to influence subsequent appetite? Physiol Behav 116–117:23–29
McBride WJ, Murphy JM, Ikemoto S (1999) Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res 101:129–152
McCabe C, Rolls ET (2007) Umami: a delicious flavour formed by convergence of taste and olfactory pathways in the human brain. Eur J Neurosci 25:1855–1864
McHugh MJ, Demers CH, Braud J, Briggs R, Adinoff B, Stein EA (2013) Striatal insula circuits in cocaine addiction: implications for impulsivity and relapse risk. American J Drug Alcohol Abuse 39:424–432. doi:10.3109/00952990.2013.847446
Miyamoto Y, Yamada K, Noda Y (2001) Hyperfunction of dopaminergic and serotonergic neuronal systems in mice lacking the NMDA receptor E1 subunit. J Neurosci 21(2):750–757
Morris MJ, Na ES, Johnson AK (2008) Salt craving: the psychobiology of pathogenic sodium intake. Physiol Behav 94:70
Moskin J (2008) Yes, MSG, the secret behind the savour. The New York Times nytimes.com. Accessed November 2016
Nakamura H, Kawamata Y, Kuwahara T, Torii K, Sakai R (2013) Nitrogen in dietary glutamate is exclusively utilized for the synthesis of amino acids in the rat intestine. American J Physiol Endocrinol Metab 304:E100–E108
Nakanishi S (1992) Molecular diversity of glutamate receptors and implications for brain function. Science 258:597–603
Narayanan SN, Paval KJ, Nayak S (2010) Effect of ascorbic acid on monosodium glutamate induced neurobehavioral changes in periadolescent rats. Bratisel Lek Listy 111:247–252
Olsen CM (2011) Natural rewards, neuroplasticity, and non-drug addictions. Neuropharmacology 61:1109–1122
Onaolapo OJ, Onaolapo AY (2011) Acute low dose monosodium glutamate retards novelty induced behaviours in male Swiss albino mice. J Neuro Brain Health 3:51–56
Onaolapo OJ, Onaolapo AY, Akanmu MA, Olayiwola G (2015) Foraging enrichment modulates open field response to monosodium glutamate in mice. Ann Neurosci 22:162–170
Onaolapo OJ, Onaolapo AY, Akanmu MA, Olayiwola G (2016a) Evidence of alterations in brain structure and antioxidant status following ‘low-dose’ monosodium glutamate ingestion. Pathophysiology 23:147–156
Onaolapo OJ, Onaolapo AY, Akanmu MA, Olayiwola G (2016b) Changes in spontaneous working-memory, memory-recall and approach-avoidance following “low dose” monosodium glutamate in mice. AIMS Neurosci 3:317–337
Onaolapo OJ, Onaolapo AY, Akanmu MA, Olayiwola G (2016c) Caffeine and sleep-deprivation mediated changes in open-field behaviours, stress response and antioxidant status in mice. Sleep Science (in press). doi:10.1016/j.slsci.2016.10.008
Pascual MM, Pastor V, Bernabeu RO (2009) Nicotine-conditioned place preference induced CREB phosphorylation and Fos expression in the adult rat brain. Psychopharmacol (Berl) 207:57–71
Paz HR (2005) Pathophysiological models of schizophrenia; glutamate dopamine, glutamate to GABA. Rev Chil Neuro-Psiquiat 43:314–328
Phillis BD, Ong J, White JM, Bonnielle C (2001) Modification of d-amphetamine-induced responses by baclofen in rats. Psychopharmacol 153:277–284
Prescott J (2004) Effects of added glutamate on liking for novel food flavours. Appetite 42:143–150
Qi J, Zhang S, Wang H-l, Wang H, Buendia FA, Hoffman AF, Lupica CR, Seal RP, Morales M (2014) A glutamatergic reward input from the dorsal raphe to ventral tegmental area dopamine neurons. Nature Comm 5:5390. doi:10.1038/ncomms6390
Qi J, Zhang S, Wang H-l, Barker DJ, Miranda-Barrientos J, Morales M (2016) VTA glutamatergic inputs to nucleus accumbens drive aversion by acting on GABAergic interneurons. Nature Neurosci 19:725–733. doi:10.1038/nn.4281
Rubinstein M, Phillips TJ, Bunzow JR, Falzone TL, Dziewczapolski G, Zhang G, Fang Y, Larson JL, McDougal JA, Chester JA, Saez C, Pugsley TA, Gershanik O, Low MJ, Grandy DK (1997) Mice lacking dopamine D4 receptors are supersensitive to ethanol, cocaine, and methamphetamine. Cell 90:991–1001
Schechter MD, Calcagnetti DJ (1993) Trends in place preference conditioning with a cross-indexed bibliography: 1957–1991. Neurosci Biobehav Rev 17:21–41
Schulte EM, Avena NM, Gearhardt AN (2016) Which foods may be addictive? The roles of processing, fat content, and glycemic load. PLoS One 10:e0117959. doi:10.1371/journal.pone.0117959
Shi Z, Luscombe-Marsh ND, Wittert GA, Yuan B, Dai Y, Pan X, Taylor AW (2010) Monosodium glutamate is not associated with obesity or a greater prevalence of weight gain over 5 years: findings from the Jiangsu Nutrition Study. Br J Nutr 104:457–463
Silverman JL, Pride MC, Hayes JE, Puhger KR, Butler-Struben HM, Baker S (2015) GABAB receptor agonist r-baclofen reverses social deficits and reduces repetitive behaviour in two mouse models of autism. Neuropsychopharmacology 40:2228–2239
Smith QR (2000) Transport of glutamate and other amino acids at the blood-brain barrier. J Nutr 130:1016S–1022S
Spence C (2012) Auditory contributions to flavour perception and feeding behaviour. Physiol Behav 107:505–515
Svensson J, Carlsson A, Huff RM, Kling-Petersen T, Waters N (1994) Behavioural and neurochemical data suggest functional differences between dopamine D2 and D3 receptors. Eur J Pharmacol 263:235–243
Thomas MJ, Kalivas PW, Shaham Y (2008) Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. British J Pharmacol 154:327–342
Tsurugizawa T, Torii K (2010) Physiological roles of glutamate signaling in gut and brain function. Biol Pharm Bull 33:1796–1799
Tsurugizawa T, Uematsu A, Nakamura E, Hasumura M, Hirota M, Kondoh T, Uneyama H, Torii K (2009) Mechanisms of neural response to gastrointestinal nutritive stimuli: the gut-brain axis. Gastroenterol. 137:262–273
Tulloch AJ, Murray S, Vaicekonyte R, Avena NM (2015) Neural responses to macronutrients: hedonic and homeostatic mechanisms. Gastroenterol 148:1205–1218
Tzschenke TM (1998) Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects. Recent progress and new issues Prog Neurobiol 56:613–672
Tzschentke TM, Schmidt WJ (2003) Glutamatergic mechanisms in addiction. Mol Psychiatry 8:373–382
Watanabe S (2013) Possible false negative results in conditioned place preference induced by low-dose morphine in mice. J Addiction Res Therapy S4:013. doi:10.4172/2155-6105.S4-013
White NM, Carr GD (1985) The conditioned place preference is affected by two independent reinforcement processes. Pharmacol Biochem Behav 23:37–42
Xu M, Moratalla R, Gold LH, Hiroi N, Koob GF, Graybiel AM, Tonegawa S (1994) Dopamine D1 receptor mutant mice are deficient in striatal expression of dynorphin and in dopamine-mediated behavioral responses. Cell 79:729–742
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors of this paper declare that there is no conflict of interest related to the content of this manuscript.
Source of funding
This research did not receive any specific grant from agencies in the public, commercial or not-for-profit sectors.
Rights and permissions
About this article
Cite this article
Onaolapo, O.J., Aremu, O.S. & Onaolapo, A.Y. Monosodium glutamate-associated alterations in open field, anxiety-related and conditioned place preference behaviours in mice. Naunyn-Schmiedeberg's Arch Pharmacol 390, 677–689 (2017). https://doi.org/10.1007/s00210-017-1371-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-017-1371-6