Abstract
Sepsis is a systemic inflammatory response associating severe infection leading to multi-organ failure, such as hepatic dysfunction. This study investigates the possible hepatoprotective effect of the lipoxin A4 agonist (BML-111) in cecal ligation and puncture (CLP) model in rats. Pretreatment with BML-111 (1 mg/kg, i.p., 1 h before CLP) protected against CLP-induced mortality after 24 h. BML-111 prevented marked inflammatory cells in liver tissues and decreased elevation in serum hepatic biomarkers [alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TB), gamma-glutamyl transferase (γ-GT)] induced by CLP. Additionally, BML-111 attenuated elevated serum level of interleukin-6 (IL-6) and downregulated hepatic IL-6 mRNA expression. Meanwhile, BML-111 further increased serum IL-10 and upregulated hepatic IL-10 mRNA expression, while it downregulated hepatic mRNA expression of nuclear factor inhibitory protein kappa-B alpha (NFκBia), toll-like receptor-4 (TLR-4), and 5-lipooxygenase (5-LOX). Moreover, BML-111 prevented NF-κB/p65 nuclear translocation and activation. In conclusion, BML-111 attenuated CLP-induced acute hepatic dysfunction through its anti-inflammatory effect by decreasing NF-κB activity, TLR-4, and 5-LOX expression with subsequent decrease in pro-inflammatory IL-6 and elevation in anti-inflammatory IL-10.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis is a fatal condition that causes multi-organ failure and shock. It activates host immune, inflammatory, and coagulation responses, leading to tissue injury, hypoxia, and organ dysfunction (Takahashi et al. 2013).
Incidence of sepsis-associated hepatic dysfunction among sepsis patients ranges from 34 to 46% (Tsai et al. 2015) and their mortality rates range from 54 to 68% (Yan et al. 2014). Traditionally, sepsis-associated hepatic dysfunction is viewed as a late event. However, recent studies have revealed hepatic dysfunction as an early event in sepsis (Yan et al. 2014).
To study sepsis and associated multi-organ dysfunction, the surgical model, cecal ligation and puncture (CLP), is considered to be the gold standard for the experimental induction of polymicrobial sepsis. This is a realistic model resembling the human condition and is clinically relevant (Brooks et al. 2007).
Since inflammation plays a vital role in sepsis, thus, sepsis research focused mainly on the development of anti-inflammatory regimens (Nullens et al. 2016). However, in clinical trials, several therapies that possibly affect systemic inflammation have widely failed to reduce incidence of mortality in cases with severe sepsis (Gavins et al. 2012). Therefore, new understanding of the pathophysiological mechanisms is needed to develop novel therapeutic strategies.
Lipoxins, particularly lipoxin A4 (LXA4), exhibit anti-inflammatory and proresolution effects, including its capability to reduce vascular permeability, to impair migration of new neutrophils into sites of injury and to promote infiltration of monocytes into the site of inflammation. LXA4, the arachidonic acid metabolite, is biosynthesized by two main routes, and both are influenced by lipoxygenases (LOX) (Sordi et al. 2013).
Since LXA4 is rapidly inactivated, potent analogs have been created, including the LXA4 agonist BML-111. BML-111 exhibits protective effects in many experimental inflammatory conditions, such as ischemic stroke and acute lung injury in rat models and acute pancreatitis-associated lung injury in mice (Wang et al. 2014a). However, its role in sepsis-induced inflammation and hepatic dysfunction is not clear.
Therefore, in this study, we aimed to investigate the possible protective role of BML-111 in acute hepatic dysfunction induced by CLP surgical model in rats.
Materials and methods
Drugs and chemicals
5(S), 6(R), 7-Trihydroxyheptanoic acid methyl ester (BML-111), bovine serum albumin (BSA), and sodium azide were purchased from Sigma Aldrich Chemical Co. (St. Louis, MO, USA) and were dissolved in distilled water. Thiopental sodium (Anapental) was purchased from Sigmatec Pharmaceutical Industries (6 October City, Egypt). Absolute ethanol, chloroform, isopropyl alcohol (20%), disodium hydrogen phosphate (Na2HPO4), sodium dihydrogen phosphate (NaH2PO4), and formalin were obtained from El-Nasr Chemicals Company (Abou-Zaabal, Cairo, Egypt).
Experimental animals
Male Sprague-Dawley rats, aging 7–8 weeks and weighing 160–200 g, were purchased from Delta University for Science and Technology Breeding Animal House, Gamasa, Egypt. All procedures performed were in accordance with the ethical standards of the Research Ethics Committee, Faculty of Pharmacy, Mansoura University, Egypt.
Experimental protocol
The animals were randomly divided into four groups each consisting of 6–12 rats: group 1, sham group; group 2, BML group—rats receiving BML-111 (1 mg/kg, i.p.) 60 min before sham operation; group 3, CLP group; and group 4, BML + CLP group—rats receiving BML-111 (1 mg/kg, i.p.) 60 min before CLP.
CLP procedure
Rats were anesthetized with Anapental (40 mg/kg, i.p.) (Ferro et al. 2007). A small midline incision was made through the skin and extended till it reached the peritoneal cavity to expose the cecum. The cecum was ligated exactly between the distal pole and base of the cecum with 4/0 surgical silk. Then, the cecum was punctured two times using an 18-gauge needle. A small amount of fecal matter was squeezed out of the perforated cecum. The cecum was relocated back into the abdominal cavity, and the skin was further closed in double layers with 4/0 silk sutures. Pre-warmed 0.9% saline solution (1 mL/100 g; s.c.) was injected to replace fluid loss during surgery (Walker et al. 2011). In sham-operated controls, ceca were exposed without ligation or puncture (Walker et al. 2011).
After 24 h, mortality rate was calculated, then rats were anesthetized and blood was collected to obtain serum for assessment of hepatic and inflammatory biomarkers. In addition, the liver was excised immediately for histopathological examination and measurement of mRNA expression.
Determination of serum alanine aminotransferase (ALT), serum aspartate aminotransferase (AST), total bilirubin (TB), and gamma-glutamyl transferase (γ-GT) activities
Activities of ALT and AST were determined using a commercial kit (ELITech Clinical Systems SAS, Sées, France). Their activities were measured by a kinetic method at a wavelength of 340 nm and expressed as units per liter (U/L) (Bergmeyer et al. 1986a, b).
Level of TB was determined using a commercial kit (BioMed Diagnostics, Badr City, Egypt). The TB level was measured at 578 nm and expressed as milligrams per deciliter (mg/dL) (Garber 1981).
The activity of γ-GT was determined using a commercial kit (Spinreact S.A., Girona, Spain). The γ-GT activity was measured by a kinetic method at a wavelength of 405 nm and expressed as units per liter (Persijn and van der Slik 1976).
Histopathological examination
At the end of the experiment, portions of the right upper hepatic lobes were rapidly dissected out and fixed in 10% buffered formalin for 24 h, embedded in paraffin, sectioned (5 μm thick), stained with hematoxylin and eosin (H&E), and were then assessed microscopically for the presence of inflammation using (Leica Imaging Systems, Cambridge, UK).
Determination of serum IL-6 and IL-10 levels
Levels of IL-6 and IL-10 levels were measured using enzyme-linked immunosorbent assay (ELISA) kits (eBioscience, Vienna, Austria) according to manufacturer’s instructions. Serum IL-6 and IL-10 levels were measured at 450 nm, and the concentration was calculated from a standard curve and expressed as picograms per milliliter.
Quantitative RT-PCR
The right posterior hepatic lobe was isolated, weighed, and preserved in RNA Later (Qiagen, Netherlands, Germany) (50–100 mg tissue/1 mL RNA later). RNA was extracted from the liver by Trizol reagent (Invitrogen, MA, USA). Then, 1 μg from each sample was reverse transcribed into complementary DNA (cDNA) using GoScript Reverse Transcription System (Promega Corporation, Madison, USA) according to the manufacturer’s procedures. Quantitative RT-PCR was performed with a thermocycler Rotor Gene Q (Qiagen), using HOT FIREPol EvaGreen qPCR mix plus kit (Solis BioDyne, Tartu, Estonia).
The mRNA levels of IL-6 (NM_012589), IL-10 (NM_012854.2), inhibitory protein kappa-B alpha (NFκBia) (NM_001105720.2), toll-like receptor-4 (TLR-4) (NM_019178.1), and 5-lipoxygenase (5-LOX) (NM_012822.1) were measured and normalized relative to 18S ribosomal RNA (Rn18S) (NR_046237.1) in the same sample. IL-6, IL-10, NFκBia, TLR-4, 5-LOX, and Rn18S primers were as follows:
-
IL-6 (sense, AGCCAGAGTCATTCAGAGCAA; antisense, TGGAAGTTGGGGTAGGAAGG, amplicon size = 98)
-
IL-10 (sense, CACTTCCCAGTCAGCCAGA; antisense, GTCAGCAGTATGTTGTCCAGC, amplicon size = 107)
-
NFκBia (sense, GTGACTTTGGGTGCTGATGT; antisense, ACACTTCAACAGGAGCGAGA, amplicon size = 111)
-
TLR-4 (sense, TGTTCCTTTCCTGCCTGAGA; antisense, GGTTCTTGGTTGAATAAGGGATG, amplicon size = 129)
-
5-LOX (sense, CAAGATTGTTCCCATCGCCA; antisense, GCCAGTCGTATTTTGAGTCCG, amplicon size = 89)
-
Rn18S (sense, AGTTGGTGGAGCGATTTGTC; antisense, GAACGCCACTTGTCCCTCTA, amplicon size = 121)
The results were expressed as an n-fold change of the relative expression levels of target genes from control group using ∆∆Ct method.
Immunohistochemistry for NF-κB/p65
Paraffin-embedded liver tissue sections were used for the immunohistochemical study of NF-κB/p65 expression. After de-paraffinization, the slides were rehydrated and the antigen was retrieved. Unspecific binding sites were blocked by using 0.2% BSA and 15 mM sodium azide in 10 mM phosphate-buffered saline (PBS), pH 7.6, for 1 h at RT before incubation with the primary antibody. Tissue sections were incubated overnight at 4 °C with rabbit anti-NF-κB/p65 polyclonal antibody (Thermo Fisher Scientific, Fremont, CA, USA) as a primary antibody (Vazquez et al. 2015). After washing with PBS (3–5 min), the sections were incubated with secondary antibody (Genemed Biotechnologies, South San Francisco, USA) for 2 h at RT, then washed with PBS. All antibodies were used in a dilution of 1:100. Immunocompleted slides were visualized according to the manufacturer’s protocol and then counterstained with H&E and washed again with PBS. Negative control sections were performed by omission of the primary antibody from the procedure. Finally, observation and imaging of immunolocalization were carried out using a light microscope (Leica).
Statistical analysis
Data are expressed as mean ± standard deviation (SD). Statistical analysis was carried out using one-way analysis of variance (ANOVA), followed by Tukey-Kramer multiple comparisons post hoc test. Additionally, Kruskal-Wallis test followed by Dunn’s post hoc test were used in non-parametric measurements (mortality rate). The level of significance was set at p <0.05. Statistical analysis and graphing were performed with GraphPad Prism v 5.02 (GraphPad Software Inc., San Diego, CA, USA).
Results
Effect of BML-111 on CLP-induced mortality in rats
Mortality was significantly (p < 0.05, n = 12) increased in CLP group by 50% when compared to sham group (Table 1). Pre-injection of rats with BML-111 produced a significant decrease in CLP-induced mortality rate to 8.3%. Additionally, BML-111 had no significant effect on mortality in sham group (Table 1).
Effect of BML-111 on CLP-induced elevation in hepatic biomarkers in rats
CLP caused a significant (p < 0.05, n = 6) increase in ALT, AST, TB, and γ-GT by 2.3-, 1.8-, 2.7-, and 4.5-fold, respectively, when compared to sham group (Fig. 1). Pre-injection of BML-111 before CLP caused a significant decrease in CLP-induced elevation of ALT, AST, TB, and γ-GT. Moreover, BML-111 had no significant effect on serum hepatic biomarkers in sham group (Fig. 1).
Effect of BML-111 on CLP-induced changes in serum hepatic biomarkers. BML-111 (1 mg/kg, i.p.) was given 1 h before induction of CLP, and after 24 h, serum was collected to measure ALT (a), AST (b), total bilirubin (c), and γ-GT (d). Data are expressed as mean ± SD, n = 6. *, #p < 0.05, significantly different from sham and CLP groups, respectively, using one-way ANOVA followed by Tukey-Kramer multiple comparisons post hoc test. ALT alanine transaminase, AST aspartate transaminase, γ-GT gamma-glutamyl transferase
Effect of BML-111 on CLP-induced changes in histopathological examination of liver tissues of rats
Rats in sham and BML-111 groups revealed normal liver architecture (Fig. 2a, b). CLP caused moderate inflammatory cell infiltration, hepatocellular necrosis, and central vein dilation and congestion (Fig. 2c). The administration of BML-111 prior to CLP attenuated the histopathological changes induced by CLP (Fig. 2d).
Effect of BML-111 on CLP-induced changes in histopathological examination of liver. BML-111 (1 mg/kg, i.p.) was given 1 h before induction of CLP, and after 24 h, liver tissues were collected for histopathological examination using H&E stain (×200). Rats in sham (a) and BML-111 + sham (b) groups revealed normal liver architecture. Portal liver tissue of CLP group (c) showed moderate infiltration of mononuclear inflammatory cells (arrows), hepatocellular necrosis, and central vein dilation and congestion. BML-111 + CLP group (d) showed mild mononuclear cells infiltrate both portal (long arrow) and parenchymal (short arrow)
Effect of BML-111 on CLP-induced changes in serum level and hepatic mRNA expression of IL-6 and IL-10 in rats
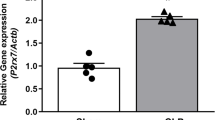
CLP caused a significant (p < 0.05, n = 6) increase in serum IL-6 and IL-10 by 9.4- and 4.5-fold, respectively, when compared to sham group (Fig. 3a, b).
Effect of BML-111 on CLP-induced changes in serum level and hepatic mRNA expression of IL-6 and IL-10. BML-111 (1 mg/kg, i.p.) was given 1 h before induction of CLP and after 24 h serum was collected to measure IL-6 (a) and IL-10 (b). Additionally, liver samples were collected to measure mRNA expression of IL-6 (c) and IL-10 (d). Data are expressed as mean ± SD, n = 6. *, #p < 0.05, significantly different from sham and CLP groups, respectively, using one-way ANOVA followed by Tukey-Kramer multiple comparisons post hoc test
Pre-injection of BML-111 before CLP caused a significant decrease in CLP-induced elevation in serum IL-6 compared to CLP group (Fig. 3a). Conversely, BML-111 produced a significant increase in CLP-induced elevation in serum IL-10 compared to sham and CLP groups (Fig. 3b).
Similarly, the hepatic mRNA expressions of IL-6 and IL-10 were increased in CLP group by 6.7- and 4.7-fold, respectively, compared to sham group (Fig. 3c, d). Pretreatment with BML-111 before CLP significantly downregulated hepatic mRNA expression of IL-6 compared to CLP group, while it upregulated hepatic mRNA expression of IL-10 significantly compared to sham and CLP groups (Fig. 3c, d). The BML-111 had no significant effect on serum IL-6, IL-10, and their hepatic mRNA expression in sham group.
Effect of BML-111 on CLP-induced changes in hepatic mRNA expression of NFκBia, TL-R4, and 5-LOX in rats
CLP upregulated hepatic mRNA expression of NFκBia, TLR-4, and 5-LOX significantly by 2.7-, 5.8-, and 3.3-fold, respectively, compared to sham group (Fig. 4). Pretreatment with BML-111 before CLP downregulated hepatic mRNA expression of NFκBia, TLR-4, and 5-LOX significantly compared to CLP group. Additionally, BML-111 had no significant effect on hepatic mRNA expression of NFκBia, TLR-4, and 5-LOX in sham group (Fig. 4).
Effect of BML-111 on CLP-induced changes in hepatic mRNA expression of NFκBia, TLR-4, and 5-LOX. BML-111 (1 mg/kg, i.p.) was given 1 h before induction of CLP, and after 24 h, liver samples were collected to measure mRNA expression of NFκBia (a), TLR-4 (b), and 5-LOX (c). Data are expressed as mean ± SD, n = 6. *, #p < 0.05, significantly different from sham and CLP groups, respectively, using one-way ANOVA followed by Tukey-Kramer multiple comparisons post hoc test. 5-LOX 5-lipoxygenase, NFκBia nuclear factor inhibitory protein kappa-B alpha, TLR-4 toll-like receptor-4
Effect of BML-111 on CLP-induced changes in immunohistochemical expression of NF-κB/p65 in liver tissues of rats
Hepatic cells obtained from the sham and BML-111 groups revealed marked cytoplasmic staining (Fig. 5a, b) showing that p65 was limited to the cytoplasm, indicating the presence of NF-κB in its inactive form. In liver tissue collected from CLP rats, there was a substantial increase in immunostaining for p65 in the nuclei of hepatic cells (Fig. 5c), indicating translocation of NF-κB to the nucleus. In rats injected with BML-111 prior to CLP, p65 was limited to the cytoplasm in all hepatic cells (Fig. 5d), indicating that BML-111 suppressed translocation of NF-κB into the nucleus.
Effect of BML-111 on CLP-induced hepatic changes in immunohistochemical expression of NF-κB p65. BML-111 (1 mg/kg, i.p.) was given 1 h before induction of CLP. After 24 h, liver tissues were collected for immunohistochemistry (IHC) staining using H&E stain (×200). Liver tissue of sham (a) and BML-111 + sham (b) groups showed diffuse cytoplasmic staining for NF-κB/p65 in hepatocytes mostly within the centrolobular areas. Liver tissue of CLP group (c) showed marked intranuclear expression and translocation for NF-κB/p65 within hepatocytes (arrows). Liver tissue of BML-111 + CLP group (d) showing cytoplasmic staining for NF-κB/p65 and marked intracytoplasmic expression of NF-κB within hepatocytes
Discussion
In this study, the lipoxin A4 analog BML-111, was able to protect against CLP-induced acute hepatic dysfunction. Sepsis is commonly associated with hepatic dysfunction (Wang et al. 2015). Various human (Groger et al. 2016; Tsai et al. 2015) and animal models (Bu and Yu 2015; Dangi et al. 2016) confirmed the association between sepsis and hepatic dysfunction.
A previous study showed that CLP-induced hepatic dysfunction is associated with elevation in serum ALT, AST, TB, and γ-GT activities (Bu and Yu 2015). Our work showed that pretreatment with BML-111 protected against CLP-induced hepatic dysfunction through decreasing hepatic biomarkers (ALT, AST, TB, and γ-GT).
Moreover, a marked liver injury is characterized by the deterioration of histopathology (Wang et al. 2015). Our results showed that CLP caused a marked inflammation in the liver tissue of rats with significant granulocyte infiltration and disruption of cellular morphology which confirms the presence of liver dysfunction. In our study, BML-111 was able to improve survival and restore the histologic architecture of liver of CLP rats. These results confirm that BML-111 resulted in a marked protection against CLP-induced hepatic dysfunction.
In CLP-induced hepatic dysfunction, inflammation plays a major role (Li et al. 2015). A previous study showed that CLP-induced hepatic dysfunction is associated with elevation in TLR-4, NF-κB, IL-6, and IL-10 (Deng et al. 2013).
TLR-4 is expressed on most cell types in the liver, including Kupffer cells, endothelial cells, and hepatocytes (Rivera et al. 2010). NF-κB is a transcription factor that is triggered by TLR-4 activation during inflammation (Kim et al. 2016). NF-κB is responsible for activation of an inflammatory cascade leading to stimulation of the expression of pro-inflammatory cytokines involved in sepsis, such as IL-6, in addition to anti-inflammatory cytokines, such as IL-10 (Deng et al. 2013; Murakami et al. 2011).
The p65 subunit of NF-κB is a key transcriptional factor responsible for regulating most of NF-κB’s transcriptional activity (Fan et al. 2011). NF-κB/p65 heterodimer is present in the cytoplasm in inactive form. Activation of NF-κB/p65 allows nuclear translocation of NF-κB transcription factor and increases the expression of many pro-inflammatory cytokines (Shafik et al. 2015).
Previous studies showed an increased TLR-4 and NF-κB expression after CLP induction in rat and mice models (Petronilho et al. 2012; Wu et al. 2015b). Another study reported that NF-κB transcriptional activity was increased secondary to overexpression of NF-κB/p65 (Wang et al. 2013). Therefore, we examined the effect of CLP on mRNA expression of TLR-4 and NFκBia as a main regulator of NF-κB activation (Harvey et al. 2013) in addition to the immunohistochemical expression of NF-κB/p65 and mRNA expression of the inflammatory cytokines IL-6 and IL-10.
Our results showed that CLP increased hepatic mRNA expression of TLR-4, NFκBia, and nuclear translocation of NF-κB/p65. In addition, CLP increased serum level and hepatic mRNA expression of IL-6 and IL-10. Meanwhile, pretreatment with BML-111 decreased CLP-induced increase in mRNA expression of TLR-4, NFκBia, and NF-κB/p65 nuclear translocation and activation. Moreover, BML-111 prevented CLP-induced elevation in serum level and mRNA expression of IL-6. Conversely, BML-111 further increased CLP-induced elevation in serum and mRNA expression of the anti-inflammatory cytokine IL-10.
These results are in accordance with previous findings that alterations in the CLP-induced elevated expression of TLR-4 may be one of the molecular mechanisms underlying the protective effect of BML-111 in rat model (Liu et al. 2015). Furthermore, BML-111 was shown to possess potent anti-inflammatory activities as it can effectively suppress NF-κB activation (Kong et al. 2015). This protective role was strongly associated with reduced expression and production of pro-inflammatory cytokine IL-6 and increased production and expression of anti-inflammatory IL-10 protein level (Wang et al. 2014b). However, Walker et al. (2011) showed that LXA4 pretreatment before CLP in rats reduced IL-10 expression compared to CLP-operated rats 48 h after surgery (Walker et al. 2011). This variation from our results may be due to time variability of examination after CLP surgery. It was previously reported that examination of the anti-inflammatory effect of LXA4 and its analog BML-111 24 h after surgery is mediated by the release of IL-10 via increasing its production and mRNA expression (Souza et al. 2007; Wang et al. 2014a).
In addition, 5-LOX has been shown to increase in sepsis/CLP. 5-LOX products derived from arachidonic acid are pathogenic mediators in endotoxemia and are responsible for inflammatory reactions (Chen 2011).
Therefore, we examined the effect of CLP on hepatic mRNA expression of 5-LOX. In the present study, CLP increased hepatic mRNA expression of 5-LOX. Administration of BML-111 decreased CLP-induced elevation in mRNA expression of 5-LOX. This is in accordance with previous findings that BML-111 effectively inhibited 5-LOX enzyme produced after sepsis induction by bacterial inoculation in mice model (Sordi et al. 2013). Measurement of protein levels of 5-LOX and TLR-4 would further confirm our results and requires further investigation.
In addition, previous studies reported that LXA4 and its analog BML-111 increased neutrophil bacterial clearing function and reduced blood bacteria load in CLP and bacterial inoculation sepsis models, indicating an antibacterial effect of BML-111 (Sordi et al. 2013; Wu et al. 2015a). Although we did not measure this antibacterial effect of BML-111 in our study, however, this antibacterial effect cannot be excluded as a mechanism of the hepatoprotective effect of BML-111 in this study.
In conclusion, this study showed that BML-111 has a protective effect against CLP-induced acute hepatic dysfunction in rats through its anti-inflammatory effect. This effect may be mediated by decreasing the hepatic mRNA expression of TLR-4, 5-LOX, and NFκBia in addition to suppressing NF-κB/p65 nuclear translocation and activation with subsequent increase in the hepatic mRNA expression and serum level of IL-10 and the decrease in the hepatic mRNA expression and serum level of IL-6. Therefore, these findings provide a potential beneficial effect of BML-111 in the prevention of acute sepsis-associated hepatic dysfunction.
Abbreviations
- CLP:
-
Cecal ligation and puncture
- NFκBia:
-
Nuclear factor inhibitory protein kappa-B alpha
References
Bergmeyer HU, Horder M, Rej R (1986a) International Federation of Clinical Chemistry (IFCC) Scientific Committee, Analytical Section: approved recommendation (1985) on IFCC methods for the measurement of catalytic concentration of enzymes. Part 2. IFCC method for aspartate aminotransferase (L-aspartate: 2-oxoglutarate aminotransferase, EC 2.6.1.1). Journal of clinical chemistry and clinical biochemistry Zeitschrift fur klinische Chemie und klinische Biochemie 24:497–510
Bergmeyer HU, Horder M, Rej R (1986b) International Federation of Clinical Chemistry (IFCC) Scientific Committee, Analytical Section: approved recommendation (1985) on IFCC methods for the measurement of catalytic concentration of enzymes. Part 3. IFCC method for alanine aminotransferase (L-alanine: 2-oxoglutarate aminotransferase, EC 2.6.1.2). Journal of clinical chemistry and clinical biochemistry Zeitschrift fur klinische Chemie und klinische Biochemie 24:481–495
Brooks HF, Osabutey CK, Moss RF, Andrews PL, Davies DC (2007) Caecal ligation and puncture in the rat mimics the pathophysiological changes in human sepsis and causes multi-organ dysfunction. Metab Brain Dis 22:353–373. doi:10.1007/s11011-007-9058-1
Bu Y, Yu J (2015) Rosiglitazone protects against endotoxin-induced acute liver injury in rats. Zhonghua Yi Xue Za Zhi 95:3180–3183
Chen S (2011) Natural products triggering biological targets—a review of the anti-inflammatory phytochemicals targeting the arachidonic acid pathway in allergy asthma and rheumatoid arthritis. Curr Drug Targets 12:288–301
Dangi A, Huang C, Tandon A, Stolz D, Wu T, Gandhi CR (2016) Endotoxin-stimulated rat hepatic stellate cells induce autophagy in hepatocytes as a survival mechanism. J Cell Physiol 231:94–105. doi:10.1002/jcp.25055
Deng Y et al (2013) Toll-like receptor 4 mediates acute lung injury induced by high mobility group box-1. PLoS One 8:e64375. doi:10.1371/journal.pone.0064375
Fan L, Wang K, Shi Z, Die J, Wang C, Dang X (2011) Tetramethylpyrazine protects spinal cord and reduces inflammation in a rat model of spinal cord ischemia-reperfusion injury. J Vasc Surg 54:192–200. doi:10.1016/j.jvs.2010.12.030
Ferro MM, Angelucci ME, Anselmo-Franci JA, Canteras NS, Da Cunha C (2007) Neuroprotective effect of ketamine/xylazine on two rat models of Parkinson’s disease. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica [et al.] 40:89–96
Garber CC (1981) Jendrassik–Grof analysis for total and direct bilirubin in serum with a centrifugal analyzer. Clin Chem 27:1410–1416
Gavins FN, Hughes EL, Buss NA, Holloway PM, Getting SJ, Buckingham JC (2012) Leukocyte recruitment in the brain in sepsis: involvement of the annexin 1-FPR2/ALX anti-inflammatory system. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 26:4977–4989. doi:10.1096/fj.12-205971
Groger M et al (2016) Monocyte-induced recovery of inflammation-associated hepatocellular dysfunction in a biochip-based human liver model. Scientific reports 6:21868. doi:10.1038/srep21868
Harvey SA, Dangi A, Tandon A, Gandhi CR (2013) The transcriptomic response of rat hepatic stellate cells to endotoxin: implications for hepatic inflammation and immune regulation. PLoS One 8:e82159. doi:10.1371/journal.pone.0082159
Kim SJ, Park JS, Lee DW, Lee SM (2016) Trichostatin A protects liver against septic injury through inhibiting toll-like receptor signaling. Biomol Ther. doi:10.4062/biomolther.2015.176
Kong X, Wu SH, Zhang L, Chen XQ (2015) Roles of lipoxin A4 receptor activation and anti-interleukin-1beta antibody on the toll-like receptor 2/mycloid differentiation factor 88/nuclear factor-kappaB pathway in airway inflammation induced by ovalbumin. Mol Med Rep 12:895–904. doi:10.3892/mmr.2015.3443
Li M et al (2015) Oral administration of escin inhibits acute inflammation and reduces intestinal mucosal injury in animal models. Evidence-based complementary and alternative medicine : eCAM 2015:503617. doi:10.1155/2015/503617
Liu H, Liu Z, Zhao S, Sun C, Yang M (2015) Effect of BML-111 on the intestinal mucosal barrier in sepsis and its mechanism of action. Mol Med Rep 12:3101–3106. doi:10.3892/mmr.2015.3746
Murakami T, Suzuki K, Tamura H, Nagaoka I (2011) Suppressive action of resolvin D1 on the production and release of septic mediators in D-galactosamine-sensitized endotoxin shock mice. Experimental and therapeutic medicine 2:57–61. doi:10.3892/etm.2010.170
Nullens S et al (2016) Beneficial effects of anti-interleukin-6 antibodies on impaired gastrointestinal motility, inflammation and increased colonic permeability in a murine model of sepsis are most pronounced when administered in a preventive setup. PloS One 11:e0152914. doi:10.1371/journal.pone.0152914
Persijn JP, van der Slik W (1976) A new method for the determination of gamma-glutamyltransferase in serum. Journal of clinical chemistry and clinical biochemistry Zeitschrift fur klinische Chemie und klinische Biochemie 14:421–427
Petronilho F et al (2012) Gastrin-releasing peptide receptor antagonism induces protection from lethal sepsis: involvement of toll-like receptor 4 signaling. Molecular medicine (Cambridge, Mass) 18:1209–1219. doi:10.2119/molmed.2012.00083
Rivera CA, Gaskin L, Singer G, Houghton J, Allman M (2010) Western diet enhances hepatic inflammation in mice exposed to cecal ligation and puncture. BMC Physiol 10:20. doi:10.1186/1472-6793-10-20
Shafik NM, Mohamed DA, Bedder AE, El-Gendy AM (2015) Significance of tissue expression and serum levels of angiopoietin-like protein 4 in breast cancer progression: link to NF-kappaB /P65 activity and pro-inflammatory cytokines. Asian Pacific journal of cancer prevention : APJCP 16:8579–8587
Sordi R, Menezes-de-Lima O Jr, Horewicz V, Scheschowitsch K, Santos LF, Assreuy J (2013) Dual role of lipoxin A4 in pneumosepsis pathogenesis. Int Immunopharmacol 17:283–292. doi:10.1016/j.intimp.2013.06.010
Souza DG et al (2007) The required role of endogenously produced lipoxin A4 and annexin-1 for the production of IL-10 and inflammatory hyporesponsiveness in mice. Journal of Immunology (Baltimore, Md : 1950) 179:8533–8543
Takahashi W et al (2013) Kinetics and protective role of autophagy in a mouse cecal ligation and puncture-induced sepsis. Critical care (London, England) 17:R160. doi:10.1186/cc12839
Tsai TN et al (2015) Role of exogenous Hsp72 on liver dysfunction during sepsis. Biomed Res Int 2015:508101. doi:10.1155/2015/508101
Vazquez E et al (2015) Systemic changes following carrageenan-induced paw inflammation in rats. Inflammation research : official journal of the European Histamine Research Society [et al.] 64:333–342. doi:10.1007/s00011-015-0814-0
Walker J, Dichter E, Lacorte G, Kerner D, Spur B, Rodriguez A, Yin K (2011) Lipoxin a4 increases survival by decreasing systemic inflammation and bacterial load in sepsis. Shock (Augusta, Ga) 36:410–416. doi:10.1097/SHK.0b013e31822798c1
Wang D, Yin Y, Yao Y (2015) Advances in sepsis-associated liver dysfunction. Burns & Trauma 2:97–105. doi:10.4103/2321-3868.132689
Wang L, Kang F, Li J, Zhang J, Shan B (2013) Overexpression of p65 attenuates celecoxib-induced cell death in MDA-MB-231 human breast cancer cell line. Cancer Cell Int 13:14. doi:10.1186/1475-2867-13-14
Wang YZ et al (2014a) BML-111, a lipoxin receptor agonist, ameliorates ‘two-hit’-induced acute pancreatitis-associated lung injury in mice by the upregulation of heme oxygenase-1. Artificial cells, nanomedicine, and biotechnology 42:110–120. doi:10.3109/21691401.2013.794355
Wang YZ, Zhang YC, Cheng JS, Ni Q, Li PW, Han W, Zhang YL (2014b) Protective effects of BML-111 on cerulein-induced acute pancreatitis-associated lung injury via activation of Nrf2/ARE signaling pathway. Inflammation 37:1120–1133. doi:10.1007/s10753-014-9836-y
Wu B, Walker J, Spur B, Rodriguez A, Yin K (2015a) Effects of lipoxin A4 on antimicrobial actions of neutrophils in sepsis. Prostaglandins Leukot Essent Fat Acids 94:55–64. doi:10.1016/j.plefa.2014.11.005
Wu KH et al (2015b) Time-series expression of toll-like receptor 4 signaling in septic mice treated with mesenchymal stem cells. Shock (Augusta, Ga). doi:10.1097/shk.00000s00000000546
Yan J, Li S, Li S (2014) “The role of the liver in sepsis”. Int Rev Immunol 33:498–510. doi:10.3109/08830185.2014.889129
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed were in accordance with the ethical standards of the Research Ethics Committee, Faculty of Pharmacy, Mansoura University, Egypt.
Rights and permissions
About this article
Cite this article
El-Tanbouly, G.S., El-Awady, M.S., Megahed, N.A. et al. The lipoxin A4 agonist BML-111 attenuates acute hepatic dysfunction induced by cecal ligation and puncture in rats. Naunyn-Schmiedeberg's Arch Pharmacol 390, 361–368 (2017). https://doi.org/10.1007/s00210-016-1335-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-016-1335-2