Abstract
Activation of cannabinoid CB1 receptors may offer new therapeutic strategies, but the efficiency of CB1 receptor agonists may be impaired by tolerance development upon prolonged administration. We compared the influence of repeated administration of Δ9-tetrahydrocannabinol (THC) 10 mg/kg on the motility and on basal and CB1 receptor-stimulated 35S-GTPγS binding of adolescent and aged mice. Moreover, we determined the influence of JZL 184 (which inhibits the 2-arachidonoylglycerol, 2-AG, degrading enzyme monoacylglycerol lipase, MAGL) on 35S-GTPγS binding and 2-AG levels of young adult mice. Mouse motility was tested in the open field. 35S-GTPγS binding was studied in hippocampal membranes. THC and CP 55,940 were used as cannabinoid agonists in the behavioural and biochemical studies, respectively. 2-AG levels were quantified by liquid chromatography-multiple reaction monitoring. The THC (10 mg/kg)-induced hypomotility was stronger in untreated than in THC-pretreated adolescent mice but similar in both treatment groups of aged mice. Basal and stimulated 35S-GTPγS binding was decreased in membranes from THC-pretreated adolescent but not affected in membranes from aged mice. Treatment of young adult mice with JZL 184 (4, 10 and 40 mg/kg) for 14 days did not affect basal binding. Stimulated binding tended to be decreased by 25 % only in mice treated with JZL 184 (40 mg/kg). Hippocampal 2-AG level was increased by JZL 184 at 40 and 10 but not affected at 4 mg/kg. In conclusion, CB1 receptor tolerance does not occur in aged mice pretreated with THC and in young adult mice treated with a low dose of the MAGL inhibitor JZL 184.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cannabis preparations have been used for centuries, but their major endogenous target, the endocannabinoid system, has been elucidated during the last 25 years only (Mechoulam and Parker 2013). This signalling system encompasses various endogenous cannabinoids (endocannabinoids), including anandamide (AEA) and 2-arachidonoylglycerol (2-AG) (Piomelli 2014), mechanisms involved in their production and inactivation (Blankman and Cravatt 2013) and receptor-mediated effects of the endocannabinoids (Howlett et al. 2002). The neurotropic and psychotropic effects of the cannabinoids are mainly related to the activation of CB1 receptors, which are Gi/o protein-coupled (Alexander et al. 2013) and typically located presynaptically on the nerve endings of a variety of central neurons (Szabo and Schlicker 2005; Ohno-Shosaku et al. 2012). CB1 receptors are involved in the effects of cannabinoids on functions including learning and memory, anxiety, stress coping, reward, motor coordination, nociception and appetite (Puighermanal et al. 2012; Ruehle et al. 2012; Zogopoulos et al. 2013; Scherma et al. 2014; Vlachou and Panagis 2014; Irie et al. 2015; Morena et al. 2015).
Endogenous and exogenous cannabinoids have CB1 receptor-mediated negative effects on cognition; specifically, they impair working memory and long-term memory without affecting memory acquisition and consolidation. These effects can be related to the inhibition of long-term potentiation, the facilitation of long-term depression, the inhibition of hippocampal acetylcholine release and/or other mechanisms; the major anatomical site is the hippocampus. Opposite effects were found when the animals were treated with an inverse CB1 receptor agonist such as rimonabant or if CB1 −/− mice were examined (for review, see Puighermanal et al. 2012). On the other hand, activation of CB1 receptors could be beneficial in the ageing brain by improving cognitive functions and by alleviating symptoms of neurodegenerative disorders. Several lines of evidence suggest that the cannabinoid system is part of an anti-ageing homeostatic defence system (for review, see Bilkei-Gorzo 2012; Di Marzo et al. 2015), the activity of which declines in ageing (Piyanova et al. 2015). The age-related decrease in CB1 signalling may contribute to the development of brain ageing and increasing susceptibility to neurodegenerative disorders because genetic deletion of CB1 receptors leads to early onset of cognitive deficits (Bilkei-Gorzo et al. 2005; 2012; Albayram et al. 2012), enhanced neuroinflammation, reduced neurogenesis (Albayram et al. 2011) and lipofuscin accumulation (Piyanova et al. 2013), i.e. typical symptoms of brain ageing.
Although CB1 agonists like Δ9-tetrahydrocannabinol (THC) could have a therapeutic value against age-related cognitive disorders, a long-standing therapy might be necessary for this purpose, and therefore, the development of tolerance has to be considered as a potential problem of such a therapeutic strategy. Previous studies on THC tolerance were carried out on adolescent and young adult animals (Bass and Martin 2000; McKinney et al. 2008; Puighermanal et al. 2013); thus, it is not known whether age affects the development of tolerance against chronic THC. Thus, the first aim of the present study was to examine whether CB1 receptor-mediated behavioural (quantified in the open-field test) and biochemical reactivity (determined with the guanosine 5′-[γ-35S]thiotriphosphate (35S-GTPγS) binding assay) to repeated administration of THC differs between adolescent and aged mice. These groups were chosen since a detailed analysis of CB1 receptor activity on cognitive functions and brain ageing is available in these age groups (Bilkei-Gorzo et al. 2005; 2012). Moreover, the activity of the cannabinoid system peaks in adolescent age and decreases later on in ageing (Lee et al. 2013; Long et al. 2012); therefore, we also expect the maximal age-related effect on tolerance development (if any) between adolescent and aged mice.
Provided that CB1 receptor activation becomes a new strategy for treatment of age-related cognitive disorders, THC itself will not be the ideal drug due to its abuse potential. Inhibitors of the degradation of endogenous cannabinoids (e.g. JZL 184, which inhibits 2-AG degradation) might be used instead. In the second series of experiments, we studied whether JZL 184, which both increases brain 2-AG levels and desensitizes CB1 receptors at a high dose (40 mg/kg; Schlosburg et al. 2010), still increases 2-AG levels without inducing desensitization at lower doses (10 or 4 mg/kg).
Part of the present experiments has been presented at the Annual Meeting of the Deutsche Gesellschaft für experimentelle und klinische Pharmakologie und Toxikologie, Hannover (Feliszek et al. 2014).
Methods
Animals
Mice were kept on a reversed light/dark cycle and housed in standard cages in groups of 3–5 animals receiving food and water ad libitum. For our biochemical studies, we determined the most appropriate cannabinoid receptor agonist using hippocampal membranes from untreated young adult C57BL/6J (wild type, Charles River, Sulzfeld, Germany) and from CB1 −/− or CB1 −/−/CB2 −/− double knockout mice (Zimmer et al. 1999; Karsak et al. 2007).
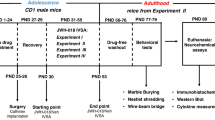
The first major series (treatment of mice with THC) was carried out using 6–8-week-old (adolescent) and 12-month-old (aged) male C57BL/6J mice (Charles River). Biochemical studies (35S-GTPγS binding) were performed on hippocampus homogenates from animals of the behavioural study (open-field test). The second major series (treatment of mice with JZL 184) was carried out on hippocampi from young adult CD-1 IGS mice (Charles River). CD-1 were preferred over C57BL/6J mice due to their bigger size since one hippocampal side was needed for the binding experiments and the other one for the determination of 2-AG and AEA levels.
Pharmacological treatments
THC, JZL 184 or their respective vehicles were administered in a volume of 0.1 mL/10 g of mouse body weight. Adolescent and aged C57BL/6J mice received 8 intraperitoneal (i.p.) injections of 3 or 10 mg/kg THC or its vehicle over a time period of 5 days. One hundred microliters of an ethanolic solution of THC were added to a mixture of 0.5 mL CremophorR and 9.4 mL saline; the vehicle had the same composition except for THC. Injections were administered approximately at 8.00 a.m. and 4.30 p.m. On the fourth and fifth day, animals were treated in the morning only. Thirty minutes after the final injection the motility of the animals was tested in the open-field apparatus. One hour later, the vehicle- and chronic THC-treated animals were killed and their hippocampi were prepared, stored at −80 °C and used for the binding studies. The treatment schedule was modified from Bass and Martin (2000). The CD-1 mice of the second major series received daily i.p. injections of 4, 10 or 40 mg/kg JZL 184 or of its vehicle over a time period of 1, 3 or 14 days. JZL 184 was dissolved in a mixture of 1 mL CremophorR and 9 mL saline; the JZL 184-free mixture served as the vehicle. Animals were sacrificed by cervical dislocation 24 h after the final injection and the hippocampi were rapidly isolated, shock-frozen in dry ice-cooled isopentane within 5 min after killing to avoid post-mortem changes in endocannabinoid levels (Buczynski and Parsons 2010) and stored at −80 °C until further processing. All applicable international, national and institutional guidelines for the care and use of animals were followed. For the experiments, a permit (Az 87–51.04.2011.A038) was obtained from the local ethical committee (Bezirksregierung Köln, Köln, Germany).
Open-field test
Experiments were carried out in a sound and light isolated room in the active phase of the animals between 10 a.m. and 1 p.m. Animals were placed onto an open-field arena (42 cm × 42 cm), and the motility of the animals was followed for 10 min as interruptions of infrared beams using an automatic system (Actimot, TSE-Systems, Bad Homburg vor der Höhe, Germany). The distance travelled was calculated as parameter. Three groups of mice were compared, i.e. mice that received 8 injections of vehicle (“control”), 7 injections of vehicle and 1 injection of THC (“acute THC”) and 8 injections of THC (“chronic THC”).
Binding studies
Hippocampus was thawed and then homogenized (Potter-Elvehjem) in 1.5 mL of ice-cold Tris-EDTA buffer (Tris 50 mM; EDTA 5 mM; pH 7.5) containing 10.27 % sucrose and centrifuged at 1500×g for 10 min (4 °C). The supernatant was centrifuged at 20,000×g for 25 min (4 °C), and the pellet was washed twice with Tris-EDTA buffer. The final pellet, which was used as the membrane fraction, was resuspended in Tris-EGTA buffer (Tris 50 mM, pH 7.4; EGTA 1 mM; MgCl2 3 mM; NaCl 100 mM) and frozen at −80 °C. The protein content was determined according to Bradford (1976).
For the experiments, frozen membranes were thawed and resuspended in reaction buffer (for composition, see below) and preincubated for 10 min at 30 °C with adenosine deaminase (Roche, Mannheim, Germany; final concentration 0.004 U/mL) to remove endogenous adenosine, which increases basal binding by activating G protein-coupled adenosine receptors. Through the inactivation of adenosine, basal binding decreases and the signal-noise ratio is improved (Moore et al. 2000). Binding was performed in Tris-EGTA buffer in a final volume of 0.5 mL containing GDP 30 μM and 6.3 μg protein. 35S-GTPγS was used at a concentration of 0.05 nM. The incubation (30 °C) was terminated after 60 min by filtration through Whatman GF/B filters (Whatman, Maidstone, UK). Non-radioactive GTPγS (10 μM) was used to determine non-specific binding (15–26 % of total binding).
Endocannabinoid extraction and quantification by liquid chromatography-multiple reaction monitoring
Frozen hippocampi were weighed in the cold room. Cold 5-mm steel balls followed by 50 μL acetonitrile containing the internal standards, 300 μL of ice-cold 0.1 M formic acid (homogenization buffer) and 300 μL ethylacetate/hexane (9:1, v/v) (extraction buffer) were added to each tube. Samples were then homogenized using the TissueLyser II (Qiagen, Hilden, Germany) for 1 cycle of 30 s at 30 Hz. Subsequently, samples were centrifuged for 10 min at 10,000×g and 4 °C, and the upper (organic) phase was removed, evaporated to dryness under a gentle stream of nitrogen at 37 °C and re-dissolved in 50 μL acetonitrile to water (1:1, v/v). Quantitative analysis of the endocannabinoids was carried out on a 5500 QTrap triple-quadrupole linear ion trap mass spectrometer equipped with a Turbo V Ion Source (AB SCIEX, Darmstadt, Germany) coupled to an Agilent 1200 series LC system (degasser, pump and thermostated column compartment; Agilent, Waldbronn, Germany) and a CTC HTC PAL autosampler (CTC Analytics AG, Zwingen, Switzerland). Endocannabinoids were separated with a Phenomenex Luna 2.5-μm C18(2)-HST column, 100 mm × 2 mm, combined with a SecurityGuard precolumn (C18, 4 mm × 2 mm; Phenomenex, Aschaffenburg, Germany) with solvents A (0.1 % formic acid in water) and B (0.1 % formic acid in acetonitrile). The liquid chromatography-multiple reaction monitoring (LC-MRM) transitions and other mass spectrometric (MS) parameters were as previously reported (Wenzel et al. 2013). Tissue weights were used for normalization of the endocannabinoid levels.
Drugs and chemicals used
The drugs and chemicals used were 35S-GTPγS (guanosine 5′-[γ-35S]thiotriphosphate, triethylammonium salt; specific activity 1250 Ci/mmol) (PerkinElmer, Boston, MA, USA); CP 55,940 ((−)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol), JZL 184 (4-[bis(1,3-benzodioxol-5-yl)hydroxymethyl]-1-piperidinecarboxylic acid 4-nitrophenyl ester) (Biotrend, Köln, Germany); WIN 55,212-2 ((R)-(+)-[2,3-dihydro-5-methyl-3[(4-morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl)methanone mesylate salt; Sigma-Aldrich, München, Germany). The other chemicals used were of reagent grade. Δ9-Tetrahydrocannabinol was provided as a 100 mg/mL stock in ethanol 96 % by THC-Pharm (Frankfurt am Main, Germany). Stock solutions of CP 55,940 and WIN 55,212-2 were prepared with DMSO and further diluted with reaction buffer containing 0.5 % bovine serum albumin to avoid adsorption of the cannabinoids to surfaces like glass or synthetic materials. The solvents did not affect binding by themselves.
For the endocannabinoid determinations, standard anandamide (AEA), 2-arachidonoylglycerol (2-AG) and their deuterated analogues AEA-d4 and 2-AG-d5 were obtained from Cayman Chemicals (Ann Arbor, MI, USA). Water (H2O), acetonitrile, formic acid, ethylacetate and hexane (all of LC-MS grade) were obtained from Sigma-Aldrich.
Calculations and statistics
Binding data are presented as counts per minute (cpm) per microgram protein. The concentration-response curves of the cannabinoids were fitted as double sigmoidal curves using the GraphPadPrism software (GraphPad Software, San Diego, CA, USA) and EC50 values were determined to characterize their potencies. Due to the restricted availability of knockout mice, the experiments of that series were carried out over a longer time period with different batches of 35S-GTPγS and for this reason stimulated binding was expressed as percent of basal binding.
Results are given as means ± standard error of the mean (SEM) of n experiments; n refers to the number of animals. The analysis of variance (ANOVA) was used for comparison of mean values and the Tukey test (for in vitro experiments) and the LSD (least significant difference) test (for in vivo experiments) were used as post hoc tests. Differences with a P < 0.05 were considered statistically significant.
Results
In initial experiments on hippocampal membranes from young adult C57BL/6J mice, both CP 55,940 and WIN 55,212-2 stimulated 35S-GTPγS binding (concentration-response curves not shown). CP 55,940 was the more potent agonist whereas WIN 55,212-2 elicited the higher maximum effect when compared to CP 55,940 (Table 1). The effect of CP 55,940 was abolished in membranes from CB1 −/− mice. By contrast, WIN 55,212-2 still facilitated binding in membranes from CB1 −/− and CB1 −/− plus CB2 −/− mice although its pEC50 was reduced by almost 1.5 log units (Table 1) and its maximum effect by ~75 % in both strains. Since the effect of CP 55,940 is solely related to the activation of CB1 receptors, this drug was preferred over WIN 55,212-2 for the binding studies described below.
Effect of Δ9-tetrahydrocannabinol
Basal and CP 55,940-induced 35S-GTPγS binding was compared in hippocampal membranes from 6 to 8-week- and 12-month-old C57BL/6J mice pretreated with Δ9-tetrahydrocannabinol (THC) or its vehicle. In hippocampal membranes from vehicle-treated mice, basal binding was ~250 cpm/μg protein for adolescent mice but ~150 cpm/μg protein for aged animals only (Fig. 1). In samples from both age groups, CP 55,940 concentration-dependently increased 35S-GTPγS binding. The maximum effect was higher in 6–8-week- (~350 cpm/μg protein) when compared to 12-month-old mice (~ 150 cpm/μg protein) whereas the pEC50 values did not differ (Fig. 2, Table 1). Next, the effect of 3 mg/kg THC, administered 8 times over a time period of 5 days, on basal and CP 55,940-induced 35S-GTPγS binding was studied. Since no effects occurred (results not shown), the dose of THC was increased to 10 mg/kg. Pretreatment with the higher dose of THC decreased basal 35S-GTPγS binding from adolescent mice by ~25 % without affecting basal binding from aged animals (Fig. 1). In hippocampal samples from adolescent mice, THC decreased the maximum effect of the CP 55,940-induced 35S-GTPγS binding by ~40 % but did not affect the pEC50 (Fig. 2, Table 1). On the other hand, THC did not affect CP 55,940-induced 35S-GTPγS binding in terms of maximum effect and pEC50 in membranes from old animals (Fig. 2, Table 1).
Effect of pre-treatment of 6–8-week-old (“adolescent”) and 12-month-old (“old”) C57BL/6J mice with Δ9-tetrahydrocannabinol (THC) or its vehicle on basal 35S-GTPγS binding to hippocampal membranes. Mice received 8 i.p. injections of 10 mg/kg THC or its vehicle over a time period of 5 days. Membranes were incubated (30 °C) for 60 min with 0.05 nM 35S-GTPγS. Means ± SEM of 7–9 mice. *P < 0.05, **P < 0.01, ***P < 0.001
Effect of pre-treatment of 6–8-week-old (“adolescent”) and 12-month-old (“old”) C57BL/6J mice with Δ9-tetrahydrocannabinol (THC) or its vehicle on the CP 55,940-induced 35S-GTPγS binding to hippocampal membranes. Mice received 8 i.p. injections of 10 mg/kg THC or its vehicle over a time period of 5 days. Membranes were incubated (30 °C) for 60 min with 0.05 nM 35S-GTPγS. Means ± SEM of 7–9 mice. For statistical evaluation of the Emax values, see Table 1
The question whether chronic administration of THC has a different effect between adolescent and aged mice was also studied in a behavioural model. For this purpose, the motility of the animals was assessed as distance travelled within 10 min in the open field. THC at a dose of 3 mg/kg failed to alter the activity of both adolescent (F2,29 = 1.229; P > 0.05) and aged (F2,28 = 1.810; P > 0.05) mice (not shown). At 10 mg/kg, THC decreased the activity of the animals in both age groups (adolescent: F2,23 = 11.59; P < 0.001; aged: F2,23 = 8.961; P < 0.01) (Fig. 3). Post hoc analysis of the data revealed that acute treatment with THC reduced the motility of animals independent from age. Acute and chronic THC treatment induced similar hypomotility in aged mice, suggesting that no tolerance was developing in this age group (Fig. 3b). By contrast, in adolescent mice, the motor activity of THC-injected animals was significantly higher after chronic than after acute treatment (Fig. 3a).
Effect of acute and chronic Δ9-tetrahydrocannabinol (THC) treatment on the open-field activity of a 6–8-week-old (“adolescent”) and b 12-month-old (“aged”) C57BL/6J mice. Mice received 7 i.p. injections of 10 mg/kg THC (chronic THC) or its vehicle (control and acute THC) before they were challenged with 10 mg/kg THC (acute and chronic THC) or vehicle (control) 30 min before the test. Means ± SEM of 8–10 mice. *P < 0.05, **P < 0.01, ***P < 0.001, compared to the respective control. + P < 0.05, compared to acute THC
Effect of JZL 184
Then, we compared the effect of JZL 184 pretreatment on CP 55,940-induced 35S-GTPγS binding and on endocannabinoid levels in the hippocampus from young adult CD-1 mice. Mice received daily i.p. injections of JZL 184, an inhibitor of monoacylglycerol lipase, or its vehicle. The dose of JZL 184 was 4, 10 or 40 mg/kg and the duration of the treatment was 1, 3 or 14 days. Basal 35S-GTPγS binding, which was 150 ± 36 cpm/μg protein in the group of mice treated with vehicle for 14 days (n = 6), was not affected by JZL 184, irrespective of the dose and the duration of treatment (not shown). CP 55,940-induced 35S-GTPγS binding was not affected by 4, 10 and 40 mg/kg JZL 184 administered for 1 or 3 days (results not shown). When the treatment was extended to 14 days, the two lower doses of JZL 184 again failed to affect this parameter whereas the highest dose, 40 mg/kg, tended to decrease the maximum effect from ~200 to ~150 cpm/μg protein; this effect, however, did not reach statistical significance (P > 0.05) (Fig. 4, Table 1). The pEC50 was not affected by any of the three doses of JZL 184 (Table 1).
Effect of pre-treatment with JZL 184 on the CP 55,940-induced 35S-GTPγS binding to hippocampal membranes from young adult CD-1 mice. Animals were treated for 14 days with 4, 10 or 40 mg/kg JZL 184 or its vehicle. Membranes were incubated (30 °C) for 60 min with 0.05 nM 35S-GTPγS. Means ± SEM of 6 mice
With respect to the hippocampal endocannabinoid levels, 4 mg/kg JZL 184 failed to affect the 2-AG level when given up to 14 days. One injection of the next higher dose, 10 mg/kg, also failed whereas three injections tended to increase the 2-AG level; treatment for 14 days increased the 2-AG level by 260 % (Fig. 5a). JZL 184 at 40 mg/kg given over time periods of 1, 3 and 14 days led to increases by 90, 420 and 820 %, respectively (Fig. 5a). The level of the other endocannabinoid, AEA, was not affected by JZL 184, irrespective of the dose and the duration of the treatment (Fig. 5b and not shown).
Effect of pre-treatment with JZL 184 on endocannabinoid levels in the hippocampus from young adult CD-1 mice. Animals were treated for 1, 3 or 14 days with 4, 10 or 40 mg/kg JZL 184 or its vehicle. Concentrations of 2-arachidonoylglycerol (2-AG; a) and anandamide (AEA; b) were determined by liquid chromatography-multiple reaction monitoring (LC-MRM). Means ± SEM of 7–8 mice. *P < 0.05, **P < 0.01, ***P < 0.001, compared to Vehicle
Discussion
The aim of the present study was to examine whether tolerance to repeated THC is influenced by age and whether a treatment schedule can be found for JZL 184 under which 2-AG is increased but CB1 receptor tolerance does not develop.
General
To term CB1 receptor plasticity, Martin et al. (2004) used “down-regulation” for decreased CB1 receptor binding, “desensitization” for decreased CB1 receptor-mediated G protein activation, “tolerance” for decreased CB1 receptor function in a general sense (including behavioural effects) and we adhered to this nomenclature in the present paper. In our biochemical studies we used the hippocampus, which shows the greatest magnitude of tolerance in the brain (reviewed in Martin et al. 2004). The development of desensitization on a biochemical level was evaluated as a decrease in the CB1 receptor-related 35S-GTPγS binding after repeated injections, which allows the quantification of receptor function and not only of receptor affinity (for review, see Strange 2010). CP 55,940 was used as CB1 receptor agonist since it totally loses its effect in membranes from the whole mouse brain (Breivogel et al. 2001) or hippocampus (present study) when the CB1 receptor is missing (CB1 −/− mouse). WIN 55,212-2, frequently used as a CB1 receptor agonist (Pertwee et al. 2010) including 35S-GTPγS binding studies (Mato and Pazos 2004; Sim-Selley et al. 2006; Moore et al. 2010), retains some activity in whole brain membranes from CB1 −/− mice (Breivogel et al. 2001) and in hippocampal membranes from CB1 −/− or from CB1 −/− plus CB2 −/− double knockout mice (present study). The remaining low-affinity effect of WIN 55,212-2 may be ascribed to a putative receptor for anandamide and WIN 55,212-2 (discussed in Pertwee et al. 2010).
Tolerance to the behavioural effects of THC was assessed as reduced hypomotility after chronic THC treatment. The degree of tolerance against THC strongly differs between behavioural readouts. Strong tolerance was detected for the hypothermic effect whereas no tolerance occurred for the hypomotor effect after 4 daily injections of 30 mg/kg THC (Tai et al. 2015). Using a lower dose (10 mg/kg) and a different protocol (a total of 7 injections, twice daily), McKinney et al. (2008) reported a similar amplitude of tolerance for hypomotility as we found in our study. A possible reason of the variability is that the striatum and its projection areas show a low level whereas the hippocampus exhibits a high level of CB1 receptor desensitization and down-regulation (Sim-Selley 2003; Martin et al. 2004; McKinney et al. 2008). It was suggested that regional differences in the induction of immediate early genes (Lazenka et al. 2013), the intensity of receptor internalization (Wu et al. 2008) or β2-arrestin levels (Nguyen et al. 2012) due to the different cytoarchitecture of the brain areas could be responsible for this phenomenon.
Age dependence of tolerance/desensitization to Δ9-tetrahydrocannabinol
THC itself was used to induce tolerance since this compound was chosen for this purpose in most of the studies (Sim et al. 1996; Martin et al. 2004; González et al. 2005). Our treatment schedule (8 doses of 10 mg/kg) was almost identical to that used by Bass and Martin (2000). The latter authors could elicit tolerance even at a dose of 3 mg/kg. However, this dose when given to adolescent mice in the present investigation did not induce hypomotility nor did it lead to desensitization in our biochemical studies. THC at 10 mg/kg induced a strong hypomotility after acute treatment and tolerance after chronic administration in adolescent animals, similarly as in other studies (McKinney et al. 2008; Puighermanal et al. 2013). Our biochemical study revealed that THC 10 mg/kg did not only reduce the maximum of the CP 55,940-induced increase in 35S-GTPγS by ~40 % (whereas the pEC50 value was not affected) but also decreased basal binding by ~25 %. The latter phenomenon is not surprising since some receptors and particularly CB1 receptors are spontaneously active in the absence of an exogenous or endogenous agonist (Pertwee 2005). It is very plausible that repeated administration of an agonist at CB1 receptors, which have a particularly high receptor density among the G protein-coupled receptors (Baker et al. 2003), markedly alters basal 35S-GTPγS binding. Comparison of the CP 55,940-induced increase in 35S-GTPγS binding in membranes from vehicle- and THC-treated mice has to be interpreted critically since the additional binding is on top of a different amount of basal binding. This latter phenomenon by itself may influence the size of the CP 55,940-induced binding.
In 12-month-old mice, the level of hypomotility induced by acute or chronic THC treatment was similar suggesting that tolerance to repeated administration of THC develops in aged animals. On the other hand, the degree of hypomotility to acute administration of THC did not differ between adolescent and aged mice. In the biochemical experiments, we observed four age-dependent alterations (identified by Roman numerals). Compared to young vehicle-treated animals, (i) basal and (ii) CP 55,940-induced increase in 35S-GTPγS binding was reduced. A marked age dependence of basal 35S-GTPγS binding in the brain has been also shown for the cerebral cortex of humans without neurological or psychiatric disorders (death mainly by accident; González-Maeso et al. 2002; Mato and Pazos 2004). In the paper by Mato and Pazos (2004), the age dependence of the WIN 55,212-2-stimulated increase in 35S-GTPγS binding was studied as well; the authors expressed stimulated binding as percent of basal binding. The ratio of ~1 did not change with increasing age, suggesting that stimulated 35S-GTPγS binding declines in parallel to basal binding. By contrast, in our hands, ageing led to a much more marked decrease of stimulated (Fig. 2) than of basal binding (Fig. 1) with ratios of 1.6 (adolescent) and 1.0 (old mice). In a study on limbic forebrain membranes from the mouse (Wang et al. 2003), 35S-GTPγS binding stimulated by the cannabinoid receptor agonist HU 210 and expressed as percent of basal binding also showed an age-dependent decrease (whether the decrease also affects basal binding is unclear since absolute values have not been given by Wang et al. 2003). Thus, these studies together suggest that the G protein coupling of CB1 receptors decreases in age.
In our study, treatment of 1-year-old mice with THC did no longer attenuate (iii) the basal and (iv) CP 55,940-induced increase in 35S-GTPγS binding. In order to further prove that ageing interferes with the development of desensitization additional experiments with longer treatment periods, with dose escalation and with mature animals are necessary; a possible influence of pharmacokinetics also has to be considered. Moreover, it would be interesting to study whether CB1 receptor down-regulation (to be quantified with a conventional radioligand like 3H-rimonabant) occurs as well. Although a comparison between adolescent and aged mice has so far not been described for THC, our data are reminiscent of studies in which the effect of opioid treatment on opioid receptor tolerance was compared in (young) adult and aged individuals (for review, see Zhao et al. 2012). Thus, the dose escalation to opioids was higher in younger (≤50 years) than in older patients (≥60 years; Buntin-Mushock et al. 2005) and the tolerance to the analgesic effect of morphine developed faster in young adult than in one year-old rats (Wang et al. 2005). In the latter study, pharmacokinetic differences do not appear to be responsible for the age-dependent difference (Wang et al. 2005). A comparison between opioid and cannabinoid CB1 receptors may be justified since both groups of receptors resemble each other with respect to their transduction machinery (coupling to Gi/o proteins), neuronal location (presynaptic site) and function (e.g. analgesic and addictive effect). On the other hand, such comparisons have to be made with caution since THC, due to its lipophilic character, has a long half-time (reviewed in Grotenhermen 2003).
JZL 184 10 mg/kg increases 2-AG levels without inducing desensitization
For activation of CB1 receptors, exogenously administered agonists may be used although, due to their side effects and abuse potential (particularly true for THC), they may be not ideal. For a long-term treatment, e.g. of individuals suffering from age-dependent cognitive decline (see “Introduction”), drugs that increase the level of endogenously formed cannabinoids by blocking their degradation may be preferable. The MAGL inhibitor JZL 184 (Long et al. 2009) led to a marked increase of brain 2-AG levels of mice when administered at 40 mg/kg over 6 days; the compound, however, also led to CB1 receptor down-regulation, desensitization and tolerance (determined by behavioural experiments) (Schlosburg et al. 2010).
We were interested whether lower doses of the compound increase 2-AG without leading to tolerance; this part of the study was restricted to young adult mice. First, we could confirm that 40 mg/kg JZL 184 markedly increases 2-AG (determined in the hippocampus and not in the whole brain as in the study by Schlosburg et al. 2010); the increase was already significant after one injection and more than 9-fold after 14 days. Unlike in the study of Schlosburg et al. (2010), the level of AEA, another endocannabinoid, was not affected; the reason for the discrepancy may be that this short-lived effect (Schlosburg et al. 2010) could not be detected since we determined the endocannabinoids 24 h (and not 2 h) after the final administration of JZL 184. In our study, basal 35S-GTPγS binding was not affected whereas the CP 55,940-induced increase in 35S-GTPγS binding showed a clear tendency towards a desensitization although this effect, unlike in the study by Schlosburg et al. (2010), did not reach a significant level. Next, 4 mg/kg JZL 184 was studied but proved unsuited for our studies since it failed to affect 2-AG even after 14 days. Increasing the dose of JZL 184 to 10 mg/kg led to a time-dependent rise in 2-AG (almost four-fold after 14 days) without affecting the CP 55,940-induced increase in 35S-GTPγS binding, suggesting that a treatment schedule with JZL 184 can be titrated leading to continuous activation of CB1 receptors without desensitizing them.
When the experiments of the present study were in progress, two papers appeared in which the effects of lower doses of JZL 184 on 2-AG and CB1 receptors of the mouse brain were studied as well (Ghosh et al. 2013; Kinsey et al. 2013); like in the study by Schlosburg et al. (2010), JZL 184 was administered over a time period of 6 days and experiments were done or brain samples were prepared already 2 h after the final JZL 184 injection. JZL 184 at a dose of 8 mg/kg did not desensitize CB1 receptors in brain homogenates nor did it lead to tolerance of the CB1 receptor-mediated antinociceptive and gastroprotective effects (Kinsey et al. 2013). Moreover, 4 mg/kg JZL 184 did not down-regulate CB1 receptors in brain homogenates (Kinsey et al. 2013) nor did it cause tolerance of the CB1 (plus CB2) receptor-mediated anti-allodynic effect in the carrageenan test (Ghosh et al. 2013). However, all parameters underwent tolerance when JZL was increased to 16 mg/kg (Ghosh et al. 2013; Kinsey et al. 2013). Although most of the data in the latter two papers and in the present one fit together, the level of 2-AG was increased by 4 mg/kg JZL 184 in the study by Kinsey et al. (2013) but not affected in the present one. The discrepancy may be related to the fact that Kinsey et al. (2013) used the whole brain (as opposed to the hippocampus) and/or determined 2-AG already 2 h (as opposed to 24 h) after the final administration of JZL 184.
Conclusion
Our study shows that the age of the animals influences the development of desensitization/tolerance against THC. Repeated administration of THC decreases hypomotility, basal 35S-GTPγS binding and (as expected) desensitizes CB1 receptors in adolescent mice whereas no tolerance was detected in aged animals. Moreover, our data confirm that the MAGL inhibitor JZL 184 at 10 mg/kg still increases the hippocampal level of the endocannabinoid 2-AG without inducing CB1 receptor desensitization. The above findings are encouraging, but much work has still to be done to prove that a CB1 receptor-based treatment is a useful treatment strategy for patients suffering from age-related cognitive decline.
References
Albayram O, Alferink J, Pitsch J, Piyanova A, Neitzert K, Poppensieker K, Mauer D, Michel K, Legler A, Becker A, Monory K, Lutz B, Zimmer A, Bilkei-Gorzo A (2011) Role of CB1 cannabinoid receptors on GABAergic neurons in brain aging. Proc Natl Acad Sci 108:11256–11261
Albayram O, Bilkei-Gorzo A, Zimmer A (2012) Loss of CB1 receptors leads to differential age-related changes in reward-driven learning and memory. Front Aging Neurosci 4:34
Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ, Collaborators CGTP (2013) The concise guide to pharmacology 2013/14: G protein-coupled receptors. Br J Pharmacol 170:1459–1581
Baker D, Pryce G, Giovannoni G, Thompson AJ (2003) The therapeutic potential of cannabis. Lancet Neurol 2:291–298
Bass CE, Martin BR (2000) Time course for the induction and maintenance of tolerance to Δ9-tetrahydrocannabinol in mice. Drug Alcohol Depend 60:113–119
Bilkei-Gorzo A (2012) The endocannabinoid system in normal and pathological brain ageing. Phil Trans R Soc B 367:3326–3341
Bilkei-Gorzo A, Racz I, Valverde O, Otto M, Michel K, Sarstre M, Zimmer A (2005) Early age-related cognitive impairment in mice lacking cannabinoid CB1 receptors. Proc Natl Acad Sci 102:15670–15675
Bilkei-Gorzo A, Drews E, Albayram O, Piyanova A, Gaffal E, Tueting T, Michel K, Mauer D, Maier W, Zimmer A (2012) Early onset of aging-like changes is restricted to cognitive abilities and skin structure in CNR1−/− mice. Neurobiol Aging 33:200.e11–22
Blankman JL, Cravatt BF (2013) Chemical probes of endocannabinoid metabolism. Pharmacol Rev 65:849–871
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantitites of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Breivogel CS, Griffin G, Di Marzo V, Martin BR (2001) Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol Pharmacol 60:155–163
Buczynski MW, Parsons LH (2010) Quantification of brain endocannabinoid levels: methods, interpretations and pitfalls. Brit J Pharmacol 160:423–442
Buntin-Mushock C, Phillip L, Moriyama K, Palmer PP (2005) Age-dependent opioid escalation in chronic pain patients. Anesth Analg 100:1740–1745
Di Marzo V, Stella N, Zimmer A (2015) Endocannabinoid signalling and the deteriorating brain. Nat Rev Neurosci 16:30–42
Feliszek M, Bilkei-Gorzo A, Schlicker E (2014) Does aging influence the tolerance development after chronic Δ9-tetrahydrocannabinol treatment in mice? Naunyn-Schmiedeberg's Arch Pharmacol 387(Suppl 1):S39
Ghosh S, Wise L, Chen Y, Gujjar R, Mahadevan A, Cravatt BF, Lichtman AH (2013) The monoacylglycerol lipase inhibitor JZL 184 suppresses inflammatory pain in the mouse carrageenan model. Life Sci 92:498–505
González S, Cebeira M, Fernández-Ruiz J (2005) Cannabinoid tolerance and dependence: a review of studies in laboratory animals. Pharmacol Biochem Behav 81:300–318
González-Maeso J, Torre I, Rodríguez-Puertas R, García-Sevilla JA, Guimón J, Meana JJ (2002) Effects of age, postmortem delay and storage time on receptor-mediated activation of G-proteins in human brain. Neuropsychopharmacology 26:468–478
Grotenhermen F (2003) Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet 42:327–360
Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG (2002) International Union of Pharmacology. XXVII. classification of cannabinoid receptors. Pharmacol Rev 54:161–202
Irie T, Kikura-Hanajiri R, Usami M, Uchiyama N, Goda Y, Sekino Y (2015) MAM-2201, a synthetic cannabinoid drug of abuse, suppresses the synaptic input to cerebellar Purkinje cells via activation of presynaptic CB1 receptors. Neuropharmacology 95:479–491
Karsak M, Gaffal E, Date R, Wang-Eckhardt L, Rehnelt J, Petrosino S, Starowicz K, Steuder R, Schlicker E, Cravatt B, Mechoulam R, Buettner R, Werner S, Di Marzo V, Tüting T, Zimmer A (2007) Attenuation of allergic contact dermatitis through the endocannabinoid system. Science 316:1494–1497
Kinsey SG, Wise LE, Ramesh D, Abdullah R, Selley DE, Cravatt BF, Lichtman AH (2013) Repeated low-dose administration of the monoacylglycerol lipase inhibitor JZL184 retains cannabinoid receptor type 1-mediated antinociceptive and gastroprotective effects. J Pharmacol Exp Ther 345:492–501
Lazenka MF, Selley DE, Sim-Selley LJ (2013) Brain regional differences in CB1 receptor adaptation and regulation of transcription. Life Sci 92:446–452
Lee TT, Hill MN, Hillard CJ, Gorzalka BB (2013) Temporal changes in N-acylethanolamine content and metabolism throughout the periadolescent period. Synapse 67:4–10
Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavón FJ, Serrano AM, Selley DE, Parsons LH, Lichtman AH, Cravatt BF (2009) Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol 5:37–44
Long LE, Lind J, Webster M, Weickert CS (2012) Developmental trajectory of the endocannabinoid system in human dorsolateral prefrontal cortex. BMC Neurosci 13:87
Martin BR, Sim-Selley L, Selley DE (2004) Signaling pathways involved in the development of cannabinoid tolerance. Trends Pharmacol Sci 25:325–330
Mato S, Pazos A (2004) Influence of age, postmortem delay and freezing storage period on cannabinoid receptor density and functionality in human brain. Neuropharmacology 46:716–726
McKinney DL, Cassidy MP, Collier LM, Martin BR, Wiley JL, Selley DE, Sim-Selley LJ (2008) Dose-related differences in the regional pattern of cannabinoid receptor adaptation and in vitro tolerance development to Δ9-tetrahydrocannabinol. J Pharmacol Exp Ther 324:664–673
Mechoulam R, Parker LA (2013) The endocannabinoid system and the brain. Annu Rev Psychol 64:21–47
Moore RJ, Xiao R, Sim-Selley LJ, Childers SR (2000) Agonist-stimulated [35S]GTPγS binding in brain: modulation by endogenous adenosine. Neuropharmacology 39:282–289
Moore NLT, Greenleaf ALR, Acheson SK, Wilson WA, Swartzwelder HS, Kuhn CM (2010) Role of cannabinoid receptor type 1 desensitization in greater tetrahydrocannabinol impairment of memory in adolescent rats. J Pharmacol Exp Ther 335:294–301
Morena M, Patel S, Bains JS, Hill MN (2016) Neurobiological interactions between stress and the endocannabinoid system. Neuropsychopharmacology 41:80–102
Nguyen PT, Schmid CL, Raehal KM, Selley DE, Bohn LM, Sim-Selley LJ (2012) ß-Arrestin2 regulates cannabinoid CB1 receptor signalling and adaptation in a central nervous system region-dependent manner. Biol Psychiat 71:714–724
Ohno-Shosaku T, Tanimura A, Hashimotodani Y, Kano M (2012) Endocannabinoids and retrograde modulation of synaptic transmission. Neuroscientist 18:119–132
Pertwee RG (2005) Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci 76:1307–1324
Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos G, Mackie K, Mechoulam R, Ross RA (2010) International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev 62:588–631
Piomelli D (2014) More surprises lying ahead. The endocannabinoids keep us guessing. Neuropharmacology 76:228–234
Piyanova A, Albayram O, Rossi CA, Farwanah H, Michel K, Nicotera P, Sandhoff K, Bilkei-Gorzo A (2013) Loss of CB1 receptors leads to decreased cathepsin D levels and accelerated lipofuscin accumulation in the hippocampus. Mech Ageing Dev 134:391–399
Piyanova A, Lomazzo E, Bindila L, Lerner R, Albayram O, Ruhl T, Lutz B, Zimmer A, Bilkei-Gorzo A (2015) Age-related changes in the endocannabinoid system in the mouse hippocampus. Mech Ageing Dev 150:55–64
Puighermanal E, Busquets-Garcia A, Maldonado R, Ozaita A (2012) Cellular and intracellular mechanisms involved in the cognitive impairment of cannabinoids. Phil Trans R Soc B 367:3254–3263
Puighermanal E, Busquets-Garcia A, Gomis-González M, Marsicano G, Maldonado R, Ozaita A (2013) Dissociation of the pharmacological effects of THC by mTOR blockade. Neuropsychopharmacology 38:1334–1343
Ruehle S, Rey AA, Remmers F, Lutz B (2012) The endocannabinoid system in anxiety, fear memory and habituation. J Psychopharmacol 26:23–39
Scherma M, Fattore L, Castelli MP, Fratta W, Fadda P (2014) The role of the endocannabinoid system in eating disorders: neurochemical and behavioural preclinical evidence. Curr Pharm Des 20:2089–2099
Schlosburg JE, Blankman JL, Long JZ, Nomura DK, Pan B, Kinsey SG, Nguyen PT, Ramesh D, Booker L, Burston JJ, Thomas EA, Selley DE, Sim-Selley LJ, Liu QS, Lichtman AH, Cravatt BF (2010) Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci 13:1113–1119
Sim LJ, Hampson RE, Deadwyler SA, Childers SR (1996) Effects of chronic treatment with Δ9-tetrahydrocannabinol on cannabinoid-stimulated [35S]GTPγS autoradiography in rat brain. J Neurosci 16:8057–8066
Sim-Selley LJ (2003) Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Crit Rev Neurobiol 15:91–119
Sim-Selley LJ, Schechter NS, Rorrer WK, Dalton GD, Hernandez J, Martin BR, Selley DE (2006) Prolonged recovery rate of CB1 receptor adaptation after cessation of long-term cannabinoid administration. Mol Pharmacol 70:986–996
Strange PG (2010) Use of the GTPγS ([35S]GTPγS and Eu-GTPγS) binding assay for analysis of ligand potency and efficacy at G protein-coupled receptors. Br J Pharmacol 161:1238–1249
Szabo B, Schlicker E (2005) Effects of cannabinoids on neurotransmission. Handb Exp Pharmacol 168:327–365
Tai S, Hyatt WS, Gu C, Franks LN, Vasiljevik T, Brents LK, Prather PL, Fantegrossi WE (2015) Repeated administration of phytocannabinoid Δ9-THC or synthetic cannabinoids JWH-018 and JWH-073 induces tolerance to hypothermia but not locomotor suppression in mice, and reduces CB1 receptor expression and function in a brain region-specific manner. Pharmacol Res 102:22–32
Vlachou S, Panagis G (2014) Regulation of brain reward by the endocannabinoid system: a critical review of behavioral studies in animals. Curr Pharm Des 20:2072–2088
Wang L, Liu J, Harvey-White J, Zimmer A, Kunos A (2003) Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc Natl Acad Sci 100:1393–1398
Wang Y, Mitchell J, Moriyama K, Kim KJ, Sharma M, Xie GX, Palmer PP (2005) Age-dependent morphine tolerance development in the rat. Anesth Analg 100:1733–1739
Wenzel D, Matthey M, Bindila L, Lerner R, Lutz B, Zimmer A, BK F (2013) Endocannabinoid anandamide mediates hypoxic pulmonary vasoconstriction. Proc Natl Acad Sci 110:18710–18715
Wu DF, Yang LQ, Goschke A, Stumm R, Brandenburg LO, Liang YJ, Höllt V, Koch T (2008) Role of receptor internalization in the agonist-induced desensitization of cannabinoid type 1 receptors. J Neurochem 104:1132–1143
Zhao J, Xin X, Xie GX, Palmer PP, Huang YG (2012) Molecular and cellular mechanisms of the age-dependency of opioid analgesia and tolerance. Mol Pain 10:38
Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI (1999) Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci 96:5780–5785
Zogopoulos P, Vasileiou I, Patsouris E, Theocharis SE (2013) The role of endocannabinoids in pain modulation. Fundam Clin Pharmacol 27:64–80
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft to Andras Bilkei-Gorzo, Beat Lutz, Eberhard Schlicker and Andreas Zimmer within the Forschergruppe 926. We are grateful to Kerstin Michel, Doris Petri, Ildiko Racz and Claudia Schwitter for their assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable international, national and institutional guidelines for the care and use of animals were followed. For the experiments, a permit (Az 87–51.04.2011.A038) was obtained from the local ethical committee (Bezirksregierung Köln, Köln, Germany).
Additional information
A. Bilkei-Gorzo and E. Schlicker share senior authorship
Rights and permissions
About this article
Cite this article
Feliszek, M., Bindila, L., Lutz, B. et al. Lack of hippocampal CB1 receptor desensitization by Δ9-tetrahydrocannabinol in aged mice and by low doses of JZL 184. Naunyn-Schmiedeberg's Arch Pharmacol 389, 603–612 (2016). https://doi.org/10.1007/s00210-016-1226-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-016-1226-6