Abstract
Green tea is an infusion of unfermented leaves of Camellia sinensis (L.) Kuntze (Theaceae), traditionally used for the treatment of obesity, hypercholesterolemia, and gastric complaints. This study evaluated the mechanisms involved in the gastric ulcer healing of the hydroalcoholic extract from green tea (GEt), its ethyl acetate fraction, (GEAc) and epigallocatechin gallate (EGCG) using the model of acetic acid-induced gastric ulcer in rats. The chronic gastric ulcer was induced by application of 80 % acetic acid on serosal mucosa of rats. After 7 days of oral treatment with GEt and GEAc, the ulcer area, mucin content, inflammatory parameters (MPO and NAG), and antioxidant system (GSH and LOOH levels, SOD and GST activities) were evaluated. In vitro, the scavenging activity of GEt and GEAc were also measured. The antisecretory action was studied on the pylorus ligature method in rats. Oral treatment with GEt and GEAc reduced significantly the gastric ulcer area induced by acetic acid. The gastric ulcer healing was accompanied by increasing of mucin content, restoration of GSH levels and SOD activity, and reduction of MPO and LOOH levels. In addition, GEt and GEAc reduced the DPPH free radicals in vitro. Furthermore, the oral treatment of animals with GEt and GEAc did not alter the gastric acid secretion or cause signs of toxicity. Collectively, these results showed that GEt had a pronounced antiulcer effect, possibly through maintenance of mucin content and reduction of inflammation and oxidative stress. In addition, the compounds present in its ethyl acetate fraction could be responsible for the extract activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric ulcers have multifactorial origin and are consequence of an imbalance between the aggressive factors (exogenous and endogenous) and endogenous protective mechanisms (Laine et al. 2008; Tarnawski et al. 2013). The treatment of gastric ulcers aims the inhibition of gastric acid secretion, using H2 receptors antagonists and proton-pump inhibitors (PPIs) (Cryer and Mahaffey 2014). However, long-term acid suppressive therapy is associated to several side effects, and the gastric acid inhibition or Helicobacter pylori eradication during ulcer healing were not sufficient to avoid the gastric ulcer recurrence (Chubineh and Birk 2012; DeVault and Talley 2009, Kangwan et al. 2014). For this reason, the interest in natural products has increased due to efficacy and fewer side effects, becoming a potential source for antiulcer treatment.
Among these natural products with gastroprotective activity are Camellia sinensis (L.) Kuntze (Theaceae) (Hamaishi et al. 2006; Maity et al. 1995; Morikawa et al. 2006; Scoparo et al. 2014). Its leaves are widely used in infusions (tea) and this non-alcoholic beverage is the second most consumed in worldwide, after water (Sharangi 2009). Green tea is prepared from unfermented leaves of C. sinensis, which are processed to inactivate enzymes responsible for the auto-oxidation (Engelhardt 2010; Sharangi 2009). Despite the pleasant flavor and aroma, green tea is also used in traditional medicine for treatment of various disorders such as obesity, cardiovascular problems, and dyspepsia (Borrelli and Izzo 2000; Ogle 2009). Actually, several studies showed different biological activities such as anticarcinogenic (Yang et al. 2011), antiobesity, antidiabetic and hypocholesterolemic (Sae-tan et al. 2011), antioxidant (Cooper et al. 2005a, 2005b), anti-inflammatory (de Mejia et al. 2009), and others. The major chemical constituents in this plant include phenolic compounds (catechin, flavonoids), alkaloids (caffeine, theobromine, theophylline), terpenoids (essential oils, saponins), proteins, carbohydrates, lipids, vitamins, and minerals (Engelhardt 2010). Recently, our group demonstrated that the green tea (GEt) and its fractions presented gastroprotective effects against gastric lesions induced by ethanol (Scoparo et al. 2014). Interestingly, a catechin isolated from green tea, the epigallocatechin gallate (EGCG), seems to be responsible for this activity (Adhikary et al. 2011a; Scoparo et al. 2014).
In this context, this study evaluates for the first time the gastric healing effects of hydroalcoholic extract from green tea, its ethyl acetate fraction, and the major component of this fraction—epigallocatechin gallate, using a model of chronic gastric ulcer induced by acid acetic in rats.
Materials and methods
Preparation and characterization of the hydroalcoholic extract and ethyl acetate fraction
The samples of green tea were purchased in a local market (Curitiba, Paraná, Brazil) as commercially processed leaves (Chá Verde Yamamotoyama, Midori Indústria de Chá, São Miguel Arcanjo, São Paulo, Brazil). The extraction from leaves of C. sinensis (L.) Kuntze and its partition were done as fully described in Scoparo et al. (2014). Briefly, the green tea leaves were extracted with a hydroalcoholic solution (70 % ethanol) giving 21 % of hydroalcoholic extract of GEt. Then, part of the extract was dissolved in water and subjected to liquid/liquid partitioning with chloroform, ethyl acetate, and butanol, giving the respective fractions: 34 % of chloroform fraction (GCl), 18 % of ethyl acetate fraction (GEAc), 30 % of butanolic fraction (GBu), and 12 % of remaining aqueous fraction (GAq). Besides, the hydroalcoholic extract and fractions were analyzed by ultra-high-performance liquid chromatography–mass spectrometry (Scoparo et al. 2014). For the GEt, several compounds were identified, such as catechins and their gallate and oxidation derivatives, glycosylated flavonoids, gallic or hydroxycinnamic acids and also esters of quinic acid, as well as lipids, saponins, and alkaloids. The analysis of GEAc showed the retention of catechins (gallocatechin, epigallocatechin, catechin, gallocatechin-3-O-gallate, gallocatechin dimer, catechin-gallate, and the epigallocatechin-3-O-gallate (EGCG)). Besides, flavonoid glycosides, composed of quercetin or kaempferol attached to different oligosaccharides, and those acylated with a p-coumaroyl group were also found (Scoparo et al. 2014).
Animals
Experiments were conducted using adult female Wistar rats weighing 180–200 g, provided by Universidade Federal do Paraná, housed at 22 ± 2 °C under a 12-h light/dark cycle and with free access to food and water. The animals were deprived of food (15–18 h) prior to all experiments. The study was conducted in agreement with the “Principles of Laboratory Animal Care” (NIH Publication 85-23, revised 1985) and approved by the Institutional Animal Ethics Committee of Federal University of Parana (CEUA/BIO-UFPR; approval number 689).
Induction of chronic gastric ulcers by acetic acid
Chronic gastric ulcers were induced with acetic acid according to Okabe et al. (1971) with modifications. The animals were anesthetized with xylazine/ketamine (10 and 5 mg/kg, respectively, i.p.), the abdomen was opened and the stomach exposed. A solution of 80 % acetic acid (v/v, 0.5 ml) was instilled into a cylinder (6 mm of diameter) that was applied to the serosal surface of the stomach and, after 1 min, was removed by aspiration, and the area of contact was washed with sterile saline. Forty-eight hours after the ulcer induction, the rats were orally treated with vehicle (C: water, 1 ml/kg), omeprazole (20 mg/kg), GEt (1, 3, 10, and 30 mg/kg), GEAc (1.8 mg/kg), or EGCG (0.612 mg/kg) twice a day for 7 days. On the day following the last treatment, the animals were sacrificed and the stomachs were removed and opened for the measurement of ulcer area (mm2) as length (mm) × width (mm).
For histological evaluation, the gastric ulcers were fixed in Alfac solution (85 % alcohol 80 °GL, 10 % of formaldehyde at 40 % and 5 % glacial acetic acid) for 16 h. After that, the ulcers were dehydrated with alcohol and xylene, embedded in paraffin wax, sectioned at 5 μm, and stained with hematoxylin/eosin (HE). The gastric sections were observed and photographed with a slide scanner (Meta Viewer Version 2.0 20X, MetaSystems, North Royalton, OH, USA).
Determination of mucin content
The histochemical assay for mucin was performed as described by Mowry and Winkler (1956). Slides containing sections were deparaffinized, rehydrated, oxidized (0.5 % periodic acid for 5 min) and washed in distilled water. Following the staining with Schiff’s reagent for 20 min, the sections were washed with sulfurous water (three times for 2 min) and in tap water for 10 min. After that, the sections were counterstained with hematoxylin for 20 s and dehydrated. Periodic acid-Schiff (PAS)-stained mucin-like glycoproteins positive pixels were evaluated with ImageJ® software (Pereira et al. 2013).
Preparation of subcellular fractions of stomachs
Samples of gastric ulcers were homogenized with 200 mM potassium phosphate buffer (pH 6.5) and the homogenates were used to quantify the reduced glutathione (GSH) and lipid hydroperoxides (LOOH) levels. After that, homogenates aliquots were centrifuged at 9000×g for 20 min and the supernatants were used for the determination of superoxide dismutase (SOD) and glutathione S-transferase (GST) activities. The pellets were used to determine the myeloperoxidase (MPO) and N-acetylglucosaminidase (NAG) levels.
The protein concentrations of the supernatants were determined by the Bradford method (Bio-Rad, Hercules, CA, USA), using bovine serum albumin as standard.
Evaluation of inflammatory parameters: MPO and NAG levels
Neutrophil infiltration in the gastric ulcers was assessed by determination of MPO activity according to the method described by Bradley et al. (1982), with modifications. The pellet was resuspended in 1 ml of potassium phosphate buffer (80 mM, pH 5.4) containing 0.5 % hexadecyltrimethylammonium bromide (HTAB). After that, the mix was centrifuged at 11,000×g for 20 min at 4 °C and 0.017 % H2O2 and 18.4 mM 3,3′, 5,5′-tetramethylbenzidine (TMB) were added to the supernatants. Absorbance of the samples was determined at 620 nm, and the results expressed as units of optic density (O.D.)/mg of protein.
NAG activity is based on the hydrolysis of p-nitrophenyl-N-acetyl-β-d-glucosamine (substrate) by N-acetyl-β-d-glucosaminidase, releasing p-nitrophenol (Bailey 1988). Samples of the supernatant obtained by the centrifugation process described above were incubated with citrate buffer (5 mM, pH 4.5) in the presence of substrate (2.24 mM). The plate was incubated at 37 °C for 60 min and the reaction was interrupted with glycine buffer (200 mM, pH 10.4). Absorbance was measured by spectrophotometer at 405 nm, and the results were expressed as units of optic density (O.D.)/mg of protein.
Evaluation of antioxidant system
Determination of GSH levels
GSH levels were quantified according to Sedlak and Lindsay (1968). Aliquots of gastric ulcer homogenate were added to 12.5 % trichloroacetic acid, vortexed for 10 min and centrifuged for 15 min at 900×g. Then, the supernatant were mixed with Tris-HCl buffer (0.4 M, pH 8.9) and 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB, 0.01 M) at 4 °C. After that, absorbance of the samples were read at 415 nm in a microplate reader and the individual values were interpolated into a standard curve of GSH (0.375–3 μg). The results were expressed as μg/g of tissue.
Determination of LOOH content
LOOH content in gastric tissue was quantified by the method of ferrous oxidation-xylenol orange (FOX2) according to Jiang et al. (1991). A solution of 90 % methanol was added to a homogenate aliquot, sonicated and centrifuged at 9000×g for 20 min at 4 °C. The supernatant was added to FOX2 reagent (4 mM butylated hydroxytoluene (BHT), 250 mM FeSO4, 25 mM H2SO4, and 100 mM xylenol orange) and incubated for 30 min at room temperature. The absorbance was determined at 560 nm by spectrophotometry and the concentration of LOOH was calibrated in a base of 1 mg of tissue from the homogenized sample. The results were expressed as mmol/mg of tissue.
Determination of GST activity
To measure the GST activity, the method described by Habig et al. (1974) was used. Supernatant aliquots were added to 1-chloro-2,4-dinitrobenzene (CDNB, 1 mM), 1 mM GSH, and 100 mM potassium phosphate buffer (pH 6.5) at room temperature. Conjugation of CDNB with GSH was monitored at 340 nm for 90 s. Specific activity was calculated using an extinction coefficient of 9.6/mM/cm for GSH, and the results were expressed as μmoles/min/mg of protein.
Determination of SOD activity
SOD activity was determined according to Marklund and Marklund (1974) and Gao et al. (1998). Pyrogallol (1 mM) was mixed with Tris-HCl–EDTA buffer (200 mM, pH 8.5) and aliquots of the supernatant and vortexed for 1 min. After incubation for 20 min at room temperature, the reaction was stopped with the addition of HCl (1 N) and centrifuged for 4 min at 18,700×g. After that, the absorbance supernatant was measured at 405 nm using a microplate reader. The amount of SOD that inhibited the oxidation of pyrogallol by 50 %, relative to the control, was defined as one unit of SOD activity and the results were expressed as U/mg of protein.
2,2-Diphenyl-1-picrylhydrazyl (DPPH) free-radical scavenging assay
The free-radical scavenging activity of GEt and GEAc on the DPPH assay was determined using the method described by Blois (1958), with modifications. GEt (1, 10, 100, and 1000 μg/ml) and its fraction, GEAc (0.18, 1.8, 18, and 180 μg/ml), were mixed with DPPH methanolic solution (10 μg/ml). The vehicle (water) was used as negative control and ascorbic acid (50 μg/ml) was used as positive control. The absorbance was measured at 517 nm after 5 min, and the individual values were interpolated into a standard curve of DPPH (0–60 μM) and expressed as μM.
Determination of gastric acid secretion and peptic activity
Gastric acid secretion was assessed after pylorus ligature in rats under anesthesia (Shay et al. 1945). The animals were treated with vehicle (C: water, 1 ml/kg, intraduodenal (i.d.) or oral (p.o.) or saline, 1 ml/kg, intraperitoneal (i.p.)), omeprazole (40 mg/kg, p.o.), or GEt (1, 3, and 10 mg/kg, (i.d.) or 10 mg/kg, (p.o.) or 1 mg/kg, (i.p.)) immediately after (when treated intraduodenally or intraperitoneally) or 1 h before (when treated orally) pylorus ligature. After 4 h of pyloric ligature, the animals were sacrificed, the stomach was carefully removed after clamping the lower esophagic sphincter, and the gastric secretion was collected for centrifugation at 1077×g for 30 min. The volume of gastric juice was determined and total acidity was quantified by simple titration with 0.1 N NaOH using 2 % phenolphthalein as acid-base indicator as previously described (Baggio et al. 2003).
The peptic activity was determined through incubation of 100 μl of gastric acid secretion with 500 μl of bovine albumin solution (5 mg/ml in 0.06 N HCl) at 37 °C for 10 min. The reaction was stopped by adding 500 μl of 10 % trichloroacetic acid and centrifuged at 1500×g for 20 min. Then, 1 ml of the supernatant was basified with 5 ml of 0.55 M sodium carbonate. Thereafter, 500 μl of 1 N Folin reagent was added to the mixture and incubated for 30 min at room temperature. After the incubation period, the absorbance was determined by spectrophotometric reading at 660 nm. Individual values were interpolated at a tyrosine standard curve (30–1000 μmol/ml), and results expressed as μmol of tyrosine/ml (Anson 1938).
Evaluation of toxicity
Toxicity of GEt and GEAc was evaluated after treatment during 7 days in the chronic gastric ulcer model. The body weight of all groups was recorded daily, and the animals were observed for detection of possible signs of acute toxicity such as diarrhea, piloerection, salivation, or death. At the end of the treatment (twice a day for 7 days), the animals were sacrificed and the selected organs (adrenal, heart, kidney, liver, lung, spleen, ovaries, and uterus) were removed and weighted. Organ weights were reported as relative weight ((organ weight/body weight) × 100). Serum samples were used for evaluation of alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine, and urea using a commercial kit (Bioclin/Quibasa, Belo Horizonte, MG, Brazil).
Statistical analysis
The data were subjected to analysis of normality using the Kolmogorov–Smirnov test and expressed as the mean ± standard error of the mean (SEM) of 6–10 animals per group. Statistical significance was determined using one-way analysis of variance (ANOVA) followed by Bonferroni’s test or the Kruskal–Wallis’ test followed by Dunns’ test using GraphPad software (GraphPad software, San Diego, CA, USA). Differences were considered to be significant when P < 0.05.
Results
Effect of GEt, GEAc, and EGCG on chronic gastric ulcer induced by acid acetic
Oral treatment with GEt (3, 10, and 30 mg/kg, twice a day for 7 days) reduced significantly the gastric ulcer induced by 80 % acetic acid by 32, 63, and 71 %, respectively, when compared to the control group (C: 146.3 ± 9.0 mm2). The positive control of the test, omeprazole (20 mg/kg, p.o.), also inhibited the gastric ulcer area by 65 % (Fig. 1a).
Effect of GEt (a), GEAc (b), and EGCG (c) on chronic gastric ulcer induced by 80 % acetic acid in rats. The animals were orally treated with vehicle (C: water, 1 ml/kg), omeprazole (Ome: 20 mg/kg), GEt (1, 3, 10, and 30 mg/kg), GEAc (1.8 mg/kg), or EGCG (0.612 mg/kg) twice a day for 7 days after the gastric ulcer induction. The results are expressed as mean ± S.E.M. (n = 8). ANOVA followed by Bonferroni’s test. *P < 0.05 when compared to the control group
In other set of experiments, the administration of GEAc (1.8 mg/kg) and omeprazole (20 mg/kg) by oral route decreased the ulcer area by 75 and 70 %, respectively, compared to the control group (C: 138.8 ± 16.2 mm2) (Fig. 1b). However, the oral treatment with EGCG (0.612 mg/kg) did not alter the ulcer area when compared with the control group (C: 90.6 ± 9.7 mm2) but omeprazole (20 mg/kg, p.o.) also reduced the area of acetic acid-induced ulcer by 49 % (Fig. 1c).
The observation of histological slices of gastric ulcers also demonstrates a deep gastric mucosal injury caused by acetic acid (Fig. 2a, b) and a regression of the ulcer size when orally treated with omeprazole (20 mg/kg) (Fig. 2c, d), GEt (10 mg/kg) (Fig. 2e, f), or GEAc (1.8 mg/kg) (Fig. 2g, h).
Representative macroscopic photograph of stomachs and histological hematoxylin/eosin (HE) sections (20×) of chronic gastric ulcer induced by 80 % acetic acid in rats. Animals were orally treated with vehicle (C: water, 1 ml/kg; a, b), omeprazole (20 mg/kg; c, d), GEt (10 mg/kg; e, f), or GEAc (1.8 mg/kg; g, h) twice a day for 7 days after the gastric ulcer induction. Bars = 1 cm (a, c, e, and g) and 1 mm (b, d, f, and g), where M indicates margin of ulcer and B indicates base of ulcer
Effect of GEt and GEAc on gastric mucin content
In Fig. 3a, it was observed that the gastric ulcer induced by acetic acid application possessed a smaller amount of mucin-like glycoproteins. However, when the animals were treated with omeprazole (20 mg/kg, p.o.), GEt (10 mg/kg, p.o.), or GEAc (1.8 mg/kg, p.o.), the PAS-staining for mucin was increased by 86, 80, and 66 %, respectively (Fig. 3b–d, respectively), compared to the control group (C: 11.0 ± 1.0 pixels/field × 104) (Fig. 3e).
Effect of GEt and GEAc on histochemical staining for mucin-like glycoproteins (PAS) in chronic gastric ulcer induced by 80 % acetic acid in rats. a–d Representative images of ulcer margin of groups orally treated with vehicle (C: water, 1 ml/kg; a), omeprazole (20 mg/kg; b), GEt (10 mg/kg; c), or GEAc (1.8 mg/kg; d) twice a day for 7 days after the gastric ulcer induction. Magnification = 100×, bars = 50 μm. e The results are expressed as mean ± S.E.M. (n = 8). ANOVA followed by Bonferroni’s test. *P < 0.05 when compared to the control group
Effect of GEt and GEAc on MPO and NAG levels
Application of acetic acid to the gastric mucosa increased the MPO levels to 15.3 ± 1.0 mO.D./mg of protein, when compared to non-ulcerated stomachs (N: 1.5 ± 0.7 mO.D./mg of protein). Oral treatment of rats with GEt (10 mg/kg), GEAc (1.8 mg/kg), and omeprazole (20 mg/kg) significantly reduced the MPO levels in 76, 50, and 79 %, respectively, when compared to the control group (Fig. 4a).
Effect of GEt and GEAc on MPO (a) and NAG (b) levels in chronic gastric ulcer induced by 80 % acetic acid in rats. The animals were orally treated with vehicle (C: water, 1 ml/kg), omeprazole (Ome: 20 mg/kg), GEt (10 mg/kg), or GEAc (1.8 mg/kg) twice a day for 7 days after the gastric ulcer induction. Naive (N): non-ulcerated group. The results are expressed as mean ± S.E.M. (n = 8). ANOVA followed by Bonferroni’s test. # P < 0.05 when compared to naive group, *P < 0.05 when compared to the control group
Similarly, the NAG levels were increased in gastric ulcer induced by acetic acid to 20.5 ± 2.1 mO.D./mg of protein, when compared to non-ulcerated stomachs (N: 11.5 ± 1.3 mO.D./mg of protein). However, the treatments with GEt (10 mg/kg, p.o.) or GEAc (1.8 mg/kg, p.o.) did not alter the NAG levels. The omeprazole (20 mg/kg) inhibited the NAG levels in 48 % when compared to the control group (Fig. 4b).
Effect of GEt and GEAc on antioxidant system
In chronic ulcer, the acetic acid decreased the GSH levels in 64 % and increased the LOOH content in 57 %, compared to non-ulcerated group (naive: 544.3 ± 37.0 μg/g of tissue and 36.1 ± 2.5 mmol/mg of tissue) (Table 1). The treatment of animals with GEt (10 mg/kg, p.o.) and GEAc (1.8 mg/kg, p.o.) prevented the decrease of GSH levels to 528.7 ± 41.2 and to 415.4 ± 58.9, respectively, when compared to the control group (C: 195.0 ± 21.4 μg/g of tissue) (Table 1). Besides, GEt administration (10 mg/kg, p.o.), but not GEAc (1.8 mg/kg, p.o.), also decreased the LOOH content to 21.6 ± 2.3 mmol/mg of tissue when compared to the control group (C: 56.7 ± 2.9 mmol/mg of tissue) (Table 1). Omeprazole (20 mg/kg, p.o.) also prevented the depletion of GSH content and the increasing of LOOH levels when compared to the control group (Table 1).
Moreover, in the ulcerated gastric mucosa by acetic acid, the SOD and GST activities were decreased in 44 and 51 %, respectively, compared to the non-ulcerated group (N: 15.4 ± 1.4 U/mg of protein and 374.3 ± 28.0 μmoles/min/mg of protein) (Table 1). However, the oral administration of GEt (10 mg/kg) and GEAc (1.8 mg/kg) restored the SOD activity to 13.3 ± 0.4 and 15.1 ± 0.9 U/mg of protein, respectively. Regarding the GST activity, both treatments with GEt or GEAc did not alter the enzymatic activity (Table 1). Already the omeprazole (20 mg/kg, p.o.), the positive control, was able to restore SOD and GST activities (Table 1).
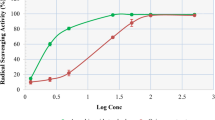
The results in Fig. 5a showed the scavenging effect of GEt on DPPH radicals with inhibition of 25, 62 and, and 80 %, respectively, compared to the control group. In addition, GEAc, at concentrations of 18 and 180 μg/ml, also decreased the DPPH levels in 54 and 62 %, respectively (Fig. 5b). Ascorbic acid, used as a positive control, also reduced the DPPH levels in 69 % when compared to the control group (C: 46.6 ± 1.5 μg/ml) (Fig. 5).
Effect of GEt (a) and GEAc (b) on the ability to scavenge the free-radical DPPH in vitro. The figure shows the scavenging of DPPH radical by GEt (1, 10, 100, and 1000 μg/ml), GEAc (0.18, 1.8, 18, and 180 μg/ml), or ascorbic acid (Aa, 50 μg/ml). The results are expressed as mean ± S.E.M. (n = 8). ANOVA followed by Bonferroni’s test. *P < 0.05 when compared to the control group
Effect of GEt on gastric acid secretion and peptic activity
The administration of GEt (1, 3, and 10 mg/kg (i.d.) or 10 mg/kg (p.o.) or 1 mg/kg (i.p.)) did not alter the volume, total acidity, and peptic activity of gastric secretion produced for 4 h. However, omeprazole (40 mg/kg, p.o.), the positive control of the test, inhibited the gastric volume, total acidity and peptic activity by 52, 83, and 55 %, respectively, when compared with the control group (C: 10.8 ± 0.5 ml; 0.074 ± 0.005 mEq[H+]/ml and 431.8 ± 42.3 μmol of tyrosine/ml) (data not shown).
Effects of GEt and GEAc on body and organ weights
During the 7-day treatment with GEt (10 mg/kg, p.o.) or GEAc (1.8 mg/kg, p.o.), no mortality or signs of toxicity were observed in animals. Furthermore, GEt and GEAc did not change the body and organ weights and the biochemical parameters (AST, ALT, creatinine, or urea), when compared to control animals (data not shown).
Discussion
The present study shows for the first time the activity of C. sinensis on chronic gastric ulcer model. Interestingly, GEt accelerated the healing of chronic ulcer in rats, with maintenance of mucus and antioxidants (SOD and GSH) protection and reduction of the harmful oxidative stress and inflammation. In accordance with our data, previous studies showed the gastroprotective property of green tea in different ulcer models (Adhikary et al. 2011b; Hamaishi et al. 2006; Lee et al. 2005; Rozza et al. 2012).
GEt protected the gastric mucosa against lesions induced by ethanol avoiding the depletion of mucus barrier (Scoparo et al. 2014), considered the first line of mucosal protection (Phillipson et al. 2008), contributing to the healing process and to the reepithelialization (Wallace 2008). Here, the treatment of animals with GEt on acetic acid-induced gastric ulcer model was able to prevent the reduction of mucin-like glycoproteins stained with PAS, one of the mucus components. Corroborating our observations, it was observed that an increase of mucus levels by phenolic compounds from green tea (Adhikary et al. 2011a; Rozza et al. 2012), and Alanko et al. (1999) suggested that the phenols stimulate the production of prostaglandin E2 (PGE2), which in turn are related to various protection mechanisms of the gastrointestinal tract, such as mucus production.
In addition, there is a close relationship between inflammation and gastric ulcer formation. Activation of leukocytes during the inflammatory process triggered in the ulcer is followed by enzyme activation that promotes increased oxygen consumption for the production of superoxide anion, the precursor to several reactive oxygen species (ROS) (Mathy-Hartert et al. 1998). Furthermore, infiltration of neutrophils induces abnormalities in the microcirculation, which delay the gastric healing (Bou-Abboud et al. 1988). In the present study, the administration of GEt decreased MPO levels, an enzyme considered a good index of neutrophil infiltration (Potrich et al. 2010). However, no significant difference was observed in the NAG levels, an indicator of the presence of mononuclear cells, which are secondary in the inflammatory process. These results suggest that a longer treatment with extract is necessary to observe an effect on NAG activity, but we cannot exclude a reduction of inflammatory process in its mechanism of ulcer healing. Additionally, GEt also reduced LOOH levels, prevented the depletion of GSH, and increased the enzymatic activity of SOD, demonstrating a modulation of the antioxidant system. Moreover, GEt had direct ability to scavenge free radicals, as shown by the in vitro DPPH assay. These results were expected because of the high percentage of phenolic compounds present in green tea (Scoparo et al. 2012), whose antioxidant effect has been known for almost 30 years (Scalbert et al. 2005).
Continuing with the evaluation of the mechanisms of action of the extract, we verified the ability of GEt in decreasing aggressive parameters of gastric mucosa. The acid secretion is the main endogenous aggressive factor that combined with the action of pepsin, a proteolytic enzyme, may contribute to the onset and aggravation of gastric lesions (Raufman 1996). Our results revealed that GEt did not inhibit gastric acid secretion nor peptic activity. However, a study showed that the black tea from C. sinensis was able to significantly reduce the acidity of gastric juice (Banerjee et al. 2010), and Rozza et al. (2012) observed an antisecretory action of epicatechin, a compound present in green tea.
According to Tarnawski et al. (2013), the protection mechanisms can be distinct and separated from gastric inhibition. Thereby, GEt accelerated the ulcer healing through the strengthening of gastric mucosal protective factors, without inhibition of acid secretion and peptic activity. It is important to note that this activity is a great opportunity for the development of improved antiulcer drugs, with very low probability to produce hypergastrinemia caused by intense achlorhydria as adverse effect. In addition, the inhibition of acid secretion could decrease the protection of the stomach against excessive bacterial growth and affect the absorption of other drugs (Schubert and Peura 2008).
To investigate the possible chemical groups of compounds responsible for the antiulcer activity of GEt, we studied the effect of the ethyl acetate fraction (GEAc). In a previous study of bio-guided purification of the GEt on gastroprotection by chemical partition with solvents of decreasing polarity, GEAc proved to be six times more potent than the initial extract (Scoparo et al. 2014). Interestingly, GEAc also reduced the ulcer area, revealing mechanisms of protection very similar to promoted by the extract and suggesting that the compounds present in the GEAc could be responsible for the antiulcer activity of GEt. Chemical analysis showed that catechins, such as epicatechin, epicatechin gallate, and epigallocatechin gallate (EGCG) are the major substances present in the GEAc (Scoparo et al. 2012). Indeed, it was already demonstrated that catechins possess antioxidant, anti-inflammatory, and gastroprotective actions (Adhikary et al. 2011a; Borrelli and Izzo 2000; Hamaishi et al. 2006; Thawonsuwan et al. 2010).
Recently, Scoparo et al. (2014) showed that EGCG protected the gastric mucosa against ethanol-induced lesions and Adhikary et al. (2011a, 2011b) demonstrated the antiulcer activity of EGCG in model of lesions induced by indomethacin. However, in this study, EGCG at a dose based on its yield from the fraction (GEAc) did not reduce the ulcer area induced by acetic acid. As previously reported, the bioavailability of the catechins contained in green tea is low, and specifically of EGCG is even lower when provided alone (Chen et al. 1997; Lee et al. 2002). Moreover, EGCG could synergistically act with other catechins as reported by Horie et al. (2005), which observed this action on the induction of apoptosis in gastric carcinoma cell lines.
Noteworthy, the subchronic treatment of animals with GEt and GEAc did not present any sign of toxicity, but further studies with a longer administration must be carried out to identify possible adverse effects.
Collectively, GEt presented an important ulcer-healing property in the model of chronic ulcer induced by acetic acid in rats. This effect is associated with the maintenance of gastric mucus and reduction of oxidative stress and inflammatory process. Furthermore, GEAc seems to concentrate the main compounds responsible for the GEt activity. However, additional studies are needed to determine whether other mechanisms could be involved in the antiulcer effect of GEt and GEAc.
References
Adhikary B, Yadav SK, Bandyopadhyay SK, Chattopadhyay S (2011a) Epigallocatechin gallate accelerates healing of indomethacin-induced stomach ulcers in mice. Pharmacol Rep 63:527–536
Adhikary B, Yadav SK, Bandyopadhyay SK, Chattopadhyay S (2011b) Role of the COX-independent pathways in the ulcer-healing action of epigallocatechin gallate. Food Funct 2:338–347
Alanko J, Riutta A, Holm P, Mucha I, Vapaatalo H, Metsa-Ketela T (1999) Modulation of arachidonic acid metabolism by phenols: relation to their structure and antioxidant/prooxidant properties. Free Radic Biol Med 26:193–201
Anson ML (1938) The estimation of pepsin, trypsin, papain, and cathepsin with hemoglobin. J Gen Physiol 22:79–89
Baggio CH, Freitas CS, Rieck L, Marques MC (2003) Gastroprotective effects of a crude extract of Baccharis illinita DC in rats. Pharmacol Res 47:93–98
Bailey PJ (1988) Sponge implants as models. Methods Enzymol 162:327–334
Banerjee D, Hassarajani SA, Maity B, Narayan G, Bandyopadhyay SK, Chattopadhyay S (2010) Comparative healing property of kombucha tea and black tea against indomethacin-induced gastric ulceration in mice: possible mechanism of action. Food Funct 1:284–293
Blois MS (1958) Antioxidant determinations by use of a stable free radical. Nature 181:1199–1200
Borrelli F, Izzo AA (2000) The plant kingdom as a source of anti-ulcer remedies. Phytother Res 14:581–591
Bou-Abboud CF, Wayland H, Paulsen G, Guth PH (1988) Microcirculatory stasis precedes tissue necrosis in ethanol-induced gastric mucosal injury in the rat. Dig Dis Sci 33:872–877
Bradley PP, Priebat DA, Christensen RD, Rothstein G (1982) Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol 78:206–209
Chen L, Lee MJ, Li H, Yang CS (1997) Absorption, distribution, elimination of tea polyphenols in rats. Drug Metab Dispos 25:1045–1050
Chubineh S, Birk J (2012) Proton pump inhibitors: the good, the bad, and the unwanted. South Med J 105:613–618
Cooper R, Morre DJ, Morre DM (2005a) Medicinal benefits of green tea: part I. Review of noncancer health benefits. J Altern Complement Med 11:521–528
Cooper R, Morre DJ, Morre DM (2005b) Medicinal benefits of green tea: part II. Review of anticancer properties. J Altern Complement Med 11:639–652
Cryer B, Mahaffey KW (2014) Gastrointestinal ulcers, role of aspirin, and clinical outcomes: pathobiology, diagnosis, and treatment. J Multidiscip Healthc 7:137–146
de Mejia EG, Ramirez-Mares MV, Puangpraphant S (2009) Bioactive components of tea: cancer, inflammation and behavior. Brain Behav Immun 23:721–731
DeVault KR, Talley NJ (2009) Insights into the future of gastric acid suppression. Nat Rev Gastroenterol Hepatol 6:524–532
Engelhardt UH (2010) Chemistry of tea. In: Mander L, Liu H-WB (eds) Comprehensive natural products II, chemistry and biology. Elsevier, UK, pp. 999–1032
Gao RM, Yuan ZB, Zhao ZQ, Gao XR (1998) Mechanism of pyrogallol autoxidation and determination of superoxide dismutase enzyme activity. Bioelectroch Bioener 45:41–45
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hamaishi K, Kojima R, Ito M (2006) Anti-ulcer effect of tea catechin in rats. Biol Pharm Bull 29:2206–2213
Horie N, Hirabayashi N, Takahashi Y, Miyauchi Y, Taguchi H, Takeishi K (2005) Synergistic effect of green tea catechins on cell growth and apoptosis induction in gastric carcinoma cells. Biol Pharm Bull 28:574–579
Jiang ZY, Woollard AC, Wolff SP (1991) Lipid hydroperoxide measurement by oxidation of Fe2+ in the presence of xylenol orange. Comparison with the TBA assay and an iodometric method. Lipids 26:853–856
Kangwan N, Park JM, Kim EH, Hahm KB (2014) Quality of healing of gastric ulcers: natural products beyond acid suppression. World J Gastrointest Pathophysiol 5:40–47
Laine L, Takeuchi K, Tarnawski A (2008) Gastric mucosal defense and cytoprotection: bench to bedside. Gastroenterology 135:41–60
Lee JS, Oh TY, Kim YK, Baik JH, So S, Hahm KB, Surh YJ (2005) Protective effects of green tea polyphenol extracts against ethanol-induced gastric mucosal damages in rats: stress-responsive transcription factors and MAP kinases as potential targets. Mutat Res 579:214–224
Lee MJ, Maliakal P, Chen L, Meng X, Bondoc FY, Prabhu S, Lambert G, Mohr S, Yang CS (2002) Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol Biomark Prev 11:1025–1032
Maity S, Vedasiromoni JR, Ganguly DK (1995) Anti-ulcer effect of the hot water extract of black tea (Camellia sinensis). J Ethnopharmacol 46:167–174
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Mathy-Hartert M, Bourgeois E, Grulke S, Deby-Dupont G, Caudron I, Deby C, Lamy M, Serteyn D (1998) Purification of myeloperoxidase from equine polymorphonuclear leucocytes. Can J Vet Res 62:127–132
Morikawa T, Li N, Nagatomo A, Matsuda H, Li X, Yoshikawa M (2006) Triterpene saponins with gastroprotective effects from tea seed (the seeds of Camellia sinensis). J Nat Prod 69:185–190
Mowry R, Winkler CH (1956) The coloration of acidic carbohydrates of bacteria and fungi in tissue sections with special reference to capsules of Cryptococcus neoformans, pneumococci and staphilococci. Am J Pathol 32:628–629
Ogle N (2009) Green tea Camellia sinensis. Austr J Med Herb 21:44–48
Okabe S, Roth JL, Pfeiffer CJ (1971) A method for experimental, penetrating gastric and duodenal ulcers in rats. Observations on normal healing. Am J Dig Dis 16:277–284
Pereira IT, Burci LM, da Silva LM, Baggio CH, Heller M, Micke GA, Pizzolatti MG, Marques MC, Werner MF (2013) Antiulcer effect of bark extract of Tabebuia avellanedae: activation of cell proliferation in gastric mucosa during the healing process. Phytother Res 27:1067–1073
Phillipson M, Johansson ME, Henriksnas J, Petersson J, Gendler SJ, Sandler S, Persson AE, Hansson GC, Holm L (2008) The gastric mucus layers: constituents and regulation of accumulation. Am J Physiol Gastrointest Liver Physiol 295:G806–G812
Potrich FB, Allemand A, da Silva LM, Dos Santos AC, Baggio CH, Freitas CS, Mendes DA, Andre E, Werner MF, Marques MC (2010) Antiulcerogenic activity of hydroalcoholic extract of Achillea millefolium L.: involvement of the antioxidant system. J Ethnopharmacol 130:85–92
Raufman JP (1996) Peptic activity and gastroduodenal mucosal damage. Yale J Biol Med 69:85–90
Rozza AL, Hiruma-Lima CA, Tanimoto A, Pellizzon CH (2012) Morphologic and pharmacological investigations in the epicatechin gastroprotective effect. Evid Based Complement Alternat Med:708156
Sae-tan S, Grove KA, Lambert JD (2011) Weight control and prevention of metabolic syndrome by green tea. Pharmacol Res 64:146–154
Scalbert A, Johnson IT, Saltmarsh M (2005) Polyphenols: antioxidants and beyond. Am J Clin Nutr 81:215S–217S
Schubert ML, Peura DA (2008) Control of gastric acid secretion in health and disease. Gastroenterology 134:1842–1860
Scoparo CT, Borato DG, de Souza LM, Dartora N, da Silva LM, Ferreira-Maria D, Sassaki GL, Gorin PAJ, Baggio CH, Iacomini M (2014) Gastroprotective bio-guiding fractionation of hydroalcoholic extracts from green- and black-teas (Camellia sinensis). Food Res Int 64:577–586
Scoparo CT, de Souza LM, Dartora N, Sassaki GL, Gorin PA, Iacomini M (2012) Analysis of Camellia sinensis green and black teas via Ultra high performance liquid chromatography assisted by liquid-liquid partition and two-dimensional liquid chromatography (size exclusion x reversed phase). J Chromatogr A 1222:29–37
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205
Sharangi AB (2009) Medicinal and therapeutic potentialities of tea (Camellia sinensis L.)—a review. Food Res Int 42:529–535
Shay H, Komarov SA, Fels SS, Meranze D, Gruenstein M, Siplet H (1945) A simple method for the uniform production of gastric ulceration in the rat. Gastroenterology 5:43–61
Tarnawski A, Ahluwalia A, Jones MK (2013) Gastric cytoprotection beyond prostaglandins: cellular and molecular mechanisms of gastroprotective and ulcer healing actions of antacids. Curr Pharm Des 19:126–132
Thawonsuwan J, Kiron V, Satoh S, Panigrahi A, Verlhac V (2010) Epigallocatechin-3-gallate (EGCG) affects the antioxidant and immune defense of the rainbow trout, Oncorhynchus mykiss. Fish Physiol Biochem 36:687–697
Wallace JL (2008) Prostaglandins, NSAIDs, and gastric mucosal protection: why doesn’t the stomach digest itself? Physiol Rev 88:1547–1565
Yang CS, Wang H, Li GX, Yang Z, Guan F, Jin H (2011) Cancer prevention by tea: evidence from laboratory studies. Pharmacol Res 64:113–122
Acknowledgments
This study was supported by the Fundação Araucária (protocol 38.512) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Borato, D.G., Scoparo, C.T., Maria-Ferreira, D. et al. Healing mechanisms of the hydroalcoholic extract and ethyl acetate fraction of green tea (Camellia sinensis (L.) Kuntze) on chronic gastric ulcers. Naunyn-Schmiedeberg's Arch Pharmacol 389, 259–268 (2016). https://doi.org/10.1007/s00210-015-1200-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-015-1200-8