Abstract
The muscular layer in the GI tract consists of an inner circular muscular layer and an outer longitudinal muscular layer. Acetylcholine (ACh) is the representative neurotransmitter that causes contractions in the gastrointestinal tracts of most animal species. There are many reports of muscarinic receptor-mediated contraction of longitudinal muscles, but few studies discuss circular muscles. The present study detailed the contractile response in the circular smooth muscles of the mouse ileum. We used small muscle strips (0.2 mm × 1 mm) and large muscle strips (4 × 4 mm) isolated from the circular and longitudinal muscle layers of the mouse ileum to compare contraction responses in circular and longitudinal smooth muscles. The time to peak contractile responses to carbamylcholine (CCh) were later in the small muscle strips (0.2 × 1 mm) of circular muscle (5.7 min) than longitudinal muscles (0.4 min). The time to peak contractile responses to CCh in the large muscle strips (4 × 4 mm) were also later in the circular muscle (3.1 min) than the longitudinal muscle (1.4 min). Furthermore, a muscarinic M2 receptor antagonist and gap junction inhibitor significantly delayed the time to peak contraction of the large muscle strips (4 × 4 mm) from the circular muscular layer. Our findings indicate that muscarinic M2 receptors in the circular muscular layer of mouse ileum exert a previously undocumented function in gut motility via the regulation of gap junctions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastrointestinal motility occurs via harmonious movements of longitudinal and circular smooth muscles coordinated by myenteric plexuses that lie between the longitudinal and circular muscle layers. The arrangement of the two types of muscles indicates that contractions of longitudinal muscle shorten the distance that the contents of the digestive tract must travel, and the circular muscles primarily move the contents along the tract. There are major differences in the involvement of the two muscles in gastrointestinal movements.
Investigation of the mechanisms that regulate longitudinal and circular muscular motilities is necessary to understand the coordinated movements of the digestive tract. Comparisons of the regulatory mechanisms of longitudinal and circular muscular motilities was not viewed as important, and most studies on specific areas of the digestive tract were conducted on only longitudinal muscles. There are fewer detailed comparisons of the mechanisms of longitudinal and circular muscular motilities in the same region of the digestive tract, but we previously demonstrated differences in the regulatory mechanisms of muscarinic receptor-mediated contractions of longitudinal (Takeuchi et al. 2004) and circular muscles (Takeuchi et al. 2007a) in distal rat colons.
Gastrointestinal motility occurs through the contraction and relaxation of smooth muscles. Cooperation between contractions on the mouth side of the digestive tract contents and relaxation on the caudal side is physiologically important for peristaltic movement. Contractions of intestinal smooth muscle occur through the actions of neurotransmitters released by intestinal nerves. Acetylcholine (ACh) is the representative neurotransmitter that causes contractions in the digestive tracts of most animal species (McConalouge and Furness 1994). A detailed investigation of ACh-induced contractions of circular smooth muscle is needed to provide insight into the regulatory mechanisms of gastrointestinal motility. This study focused on the ileum to elucidate these mechanisms. The present study investigated the detailed contractile response in circular smooth muscles of the mouse ileum. We used small circular muscle strips (0.2 mm × 1 mm) and large circular muscle strips (4 mm × 4 mm) to specifically assess ACh-induced contractions of circular smooth muscles. These results were compared to respective data from small longitudinal muscle strips (0.2 mm × 1 mm) and large longitudinal muscle strips (4 mm × 4 mm) to identify the mechanisms underlying the altered motility of muscle strips.
Materials and methods
Drugs
Atropine, N-nitro-L-arginine (L-NNA) and tetrodotoxin (TTX) were purchased from Wako Pure Chemical (Osaka, Japan). Carbamylcholine (CCh), carbenoxolone (CBX), methoctramine (MET), and nicardipine were purchased from Sigma (St. Louis, MO, USA). 4-Diphenylacetoxy-N-methylpiperidine methiodide (4-DAMP) was purchased from Tocris Bioscience (Bristol, UK).
Animals
Male C57BL/6 mice (8–10 weeks old) were purchased from CLEA Japan, Inc. WBB6F1 (W/Wv mutant) mice (6–7 weeks old) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). All procedures used in this study complied with the institutional policies of the Osaka Prefecture University Animal Care and Use Committee.
Contraction studies on small muscle strips and large muscle strips
The ileum was removed from mice and washed in Tyrode’s solution as previously described (Takeuchi et al. 2004). The ileum was used approximately 5 cm before the ileocecal junction. The mucosal layers (mucosa and submucosa) were peeled away from the muscular layers (circular and longitudinal muscle layers). Small strips (0.2 mm × 1 mm) of circular and longitudinal muscles were deprived of longitudinal and circular muscles, respectively. Muscle strips (4 mm × 4 mm; large) were prepared via dissection. The large strips were prepared in the orientation of the layer of longitudinal or circular muscle. Isometric tension to CCh (1 μM) was measured using a force displacement transducer (AE801, SensoNor, Horten, Norway) for small strips (0.2 mm × 1 mm) and a force transducer (TD-112A; Nihon Kohden, Tokyo, Japan) for large muscle strips (4 mm × 4 mm). All tested reagents were added at least 10 min prior to the addition of CCh, unless otherwise stated. Contractions relative to baseline tone were analyzed via measurement of the extent of the contraction induced with KCl (60 mM).
Statistical analysis
The results are expressed as the mean ± SE. The statistical significance of parametric data was evaluated using the two-tailed Student’s t test (unpaired) to detect differences between circular and longitudinal strips. Statistical significance was determined using the two-tailed Student’s t test (paired) for comparisons with the control group. A P value less than 0.05 was considered significant.
Results
Responses of small muscle strips
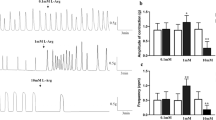
CCh (1 μM) induced spike-like rhythmic contractions and a large contraction after a latent period of approximately 6 min in small circular muscle strips (Fig. 1a, upper). Contractions superimposed with rhythmic contractions were observed soon after CCh (1 μM) treatment in small longitudinal muscle strips (Fig. 1a, lower). In contrast, contractions induced by 60 mM KCl exhibited a similar response pattern of phasic contraction immediately after treatment followed by tonic contraction in both small muscle strips (Fig. 1a). The time from CCh treatment to the peak of the large contraction and the amplitudes of peak contractions were compared in small circular and longitudinal muscle strips. The time to peak was approximately 6 min longer in the circular muscles than longitudinal muscles (Fig. 1b). The amplitudes of CCh-induced contractions were similar in small circular and longitudinal muscle strips (Fig. 1b). Concentration response curves were constructed for small circular and longitudinal muscle strips using various concentrations of CCh (0.1–10 μM) (Fig. 1c). The pD2 values for CCh were 0.86 ± 0.05 μM (n = 4) for circular muscle and 0.82 ± 0.07 μM (n = 4) for longitudinal muscle, which was not significantly different between muscles. These results reveal a marked difference in the time from CCh treatment to the peak of the large contraction between small circular and longitudinal muscle strips.
CCh (1 μM)-induced contractions in small circular and longitudinal muscle strips. Representative recording traces of CCh-induced responses are shown. CCh treatment was performed after the induction of a certain level of contraction tension using a KCl solution (60 mM) (a). Quantitative data of CCh-induced contractions. Time to peak describes the time from CCh treatment to peak contraction. The amplitudes of contractions are expressed as percentages of the contraction induced with a KCl solution (60 mM). C small circular muscle strips (n = 5), L small longitudinal muscle strips (n = 4) (b). Responses of small muscle strips to a range of CCh concentrations. Contractions are expressed as percentages of the contraction induced with a KCl solution (60 mM) (n = 4–6) (c). **P < 0.01 for circular vs. longitudinal
As expected, the nonselective muscarinic receptor (MR) antagonist atropine (1 μM) completely suppressed 1 μM CCh-induced contractions in small circular and longitudinal muscle strips (Fig. 2). This result suggests that MRs mediate CCh-induced contractions.
Effects of atropine (1 μM), MET (100 nM), 4-DAMP (10 nM), and nicardipine (10 nM) on CCh (1 μM)-induced contractions in small circular and longitudinal muscle strips. Representative recording traces of CCh-induced responses are shown (a). Quantitative data of CCh-induced contractions. The amplitudes of contractions are expressed as percentages of the contraction induced with a KCl solution (60 mM) (atropine, n = 4; MET, n = 4; 4-DAMP, n = 4; nicardipine, n = 3) (b). *P < 0.05, **P < 0.01 for control
The neuronal sodium channel blocker TTX (1 μM) had no effect on CCh-induced contractions of small circular and longitudinal muscle strips (Table 1). This result suggests that CCh-induced contractions are not dependent on neuronal excitation and that CCh acts directly on smooth muscles.
M3R activation in the thoracic aorta of rodents induces nitric oxide (NO) production and blood vessel relaxation (Khurana et al. 2005). Therefore, we investigated whether NO exerted an inhibitory effect on CCh-induced contractions of small circular muscle strips. The effect of the NO synthase inhibitor L-NNA on CCh-induced contractions was examined. L-NNA (30 μM) did not affect 1 μM CCh-induced contractions of small circular muscle strips (Table 1), which suggests that NO is not involved in CCh-induced contractions.
ACh produces contractions that are mediated by MRs on smooth muscle. Five MR subtypes were identified (M1–M5) (Caulfield and Birdsall 1998). Gastrointestinal smooth muscles contain mostly (70–80 %) M2Rs and some (20–30 %) M3Rs (Eglen et al. 1996). M3R is the primary MR subtype involved in gastrointestinal smooth muscle contractions in the guinea pig ileum (Honda et al. 1993), mouse ileum (Matsui et al. 2002), and colons (Kondo et al. 2011), with only a small degree of M2R involvement.
Effects of M2R and M3R antagonists were investigated to identify the MR subtypes involved in CCh-induced contractions of small muscle strips of mouse ileum. The selective M2R antagonist MET (100 nM) had no effect on CCh-induced contractions in small circular and longitudinal muscle strips (Fig. 2). In contrast, the preferring M3R antagonist 4-DAMP (10 nM) completely suppressed CCh-induced contractions in small circular and longitudinal muscle strips (Fig. 2). These results suggest that CCh-induced contractions in small circular and longitudinal muscle strips occur via activation of the M3R receptor.
Ca2+ entry signals are crucial to the regulation of agonist-induced smooth muscle contractions (Mayer and Sun 1992; Missiaen et al. 1992; Horowitz et al. 1996). Ca2+ influx triggers Ca2+ release from the sarcoplasmic reticulum. Ca2+ influx from voltage-dependent Ca2+ channels plays a major role in MR-mediated contractions in the circular muscle of dog colon (Sato et al. 1994). We examined the involvement of voltage-dependent Ca2+ channels in CCh-induced contractions of small circular and longitudinal muscle strips. The Ca2+ channel blocker nicardipine (10 nM) markedly suppressed CCh-induced contractions in circular and longitudinal muscles (Fig. 2), which indicates that CCh-induced contractions of circular and longitudinal muscles rely on voltage-dependent Ca2+ channels.
Responses of large muscle strips
The experiments performed in small muscle strips (0.2 mm × 1 mm) revealed marked differences in CCh-induced contractions in circular and longitudinal muscles. These differences were not reported previously, because previous studies were performed using large, but not small, muscle strips. We compared contractile responses to CCh in large circular and longitudinal muscle strips (4 mm × 4 mm) and small muscle strips (0.2 mm × 1 mm). CCh (1 μM)-induced contractions occurred earlier in large longitudinal muscle strips than large circular muscle strips (Fig. 3a). Furthermore, contractions induced by 60 mM KCl consisted of phasic and tonic contractions in large circular and longitudinal muscle strips, and both large muscle strips exhibited similar response patterns (Fig. 3a). The time from CCh treatment to peak contraction and the sizes of contractions were compared between large circular and longitudinal muscle strips. The time to peak in circular muscle was significantly longer, and the size of rhythmic contractions was larger (Fig. 3b). The amplitude of CCh-induced contraction in circular muscle was larger than longitudinal muscle (Fig. 3b). The latent period of the CCh-induced contraction response was shorter in large circular muscle strips compared to small circular muscle strips (Figs. 1b and 3b).
CCh (1 μM)-induced contractions in large circular and longitudinal muscle strips. Representative recording traces of CCh-induced responses are shown. CCh treatment was performed after the induction of a certain level of contraction tension using a KCl solution (60 mM) (a). Quantitative data of CCh-induced contractions. Time to peak describes the time from CCh treatment to peak contraction. The amplitudes of contractions are expressed as percentages of the contraction induced with a KCl solution (60 mM). C large circular muscle strips (n = 4), L large longitudinal muscle strips (n = 4) (b). Responses of large muscle strips to a range of CCh concentrations. Contractions are expressed as percentages of the contraction induced with a KCl solution (60 mM) (n = 4–6) (c). **P < 0.01 for circular vs. longitudinal
CCh (0.1–10 μM) was applied to large circular and longitudinal muscle strips to construct concentration response curves. Both strips exhibited concentration-dependent increases in contractions (Fig. 3c). No difference was observed between the pD2 values for CCh of circular (0.86 ± 0.11; n = 4) and longitudinal (0.87 ± 0.04; n = 4) muscles. These findings demonstrate clear differences in the time from CCh treatment to peak contraction and amplitude in CCh-induced contractions between large circular and longitudinal muscle strips.
The addition of atropine (1 μM) completely suppressed CCh (1 μM)-induced contractions in large circular and longitudinal muscle strips (Fig. 4). CCh-induced contractions in small muscle strips were due to the activation of M3Rs. We also examined the possible involvement of M3Rs in large muscle strips. In large circular and longitudinal muscle strips, 4-DAMP (10 nM) almost completely suppressed CCh-induced contractions (Fig. 4).
Effects of atropine (1 μM) and 4-DAMP (10 nM) on CCh (1 μM)-induced contractions in large circular (a) and longitudinal (b) muscle strips. Representative recording traces of CCh-induced responses are shown (left panels). Quantitative data of CCh-induced contractions (n = 4). The amplitudes of contraction are expressed as percentages of the contraction induced with a KCl solution (60 mM) (right panels). **P < 0.01 for control
TTX (1 μM) did not affect CCh-induced contractions in either muscle (Table 2). The myenteric plexuses were not removed from large muscle strips. Therefore, we predicted that the enteric nervous system would be more affected in CCh-induced contractions than small muscle strips. The enteric nervous system is more directly involved in motility in large muscle strips than small muscle strips. Therefore, the participation of NO in CCh-induced contractions is more likely observed in large muscle strips than small muscle strips. However, L-NNA (30 μM) had no effect on CCh-induced contractions in large circular muscle strips (Table 2).
Interstitial cells of Cajal (ICCs), which are also called pacemaker cells, create the rhythm of gastrointestinal motility (Sanders and Ward, 2007; Iino et al., 2011). We investigated CCh-induced contractions of large ileal circular muscle strips from W/WV mice, which lack ICCs, and compared the responses to wild-type mice. Treatment of the W/WV mouse strips with CCh (1 μM) resulted in similar patterns to large strips from wild-type mice (Fig. 5). These results suggest that ICCs do not participate in CCh-induced contractions of large circular muscle strips.
CCh (1 μM)-induced contractions in large circular muscle strips of W/WV mouse ileum. Representative recording traces of CCh-induced responses are shown (a). Quantitative data of CCh-induced contractions. Time to peak describes the time from CCh treatment to peak contraction. The amplitudes of contractions are expressed as percentages of the contraction induced with a KCl solution (60 mM) (b)
Gap junctions in circular muscles of the mouse ileum connect circular smooth muscle cells to each other and ICCs. Gap junctions mediate ion exchanges and signal transmission between these cells (Daniel and Wang 1999) and synchronize the activity of large numbers of cells in vascular smooth muscle (Jacobsen et al. 2007). We investigated the possible involvement of gap junctions in CCh-induced contractions of large circular muscle strips. The gap junction inhibitor CBX (30 μM) significantly lengthened the time to peak contraction (Fig. 6), which was similar to the time to peak of 1 μM CCh-induced contractions in small circular muscle strips (Fig. 1b). These results indicate an important role for gap junctions in the time to peak contractions induced by CCh in large circular muscle strips.
Effects of CBX (30 μM) and MET (100 nM) on CCh (1 μM)-induced contractions in large circular muscle strips. Representative recording traces of CCh-induced responses are shown (a). Quantitative data of CCh-induced contractions (n = 4). Time to peak describes the time from CCh treatment to peak contraction. The amplitudes of contractions are expressed as percentages of the contraction induced with a KCl solution (60 mM) (b, c). *P < 0.05, **P < 0.01 for control
M2R plays a role in MR-mediated contractile responses in the mouse ileum (Unno et al. 2005; Takeuchi et al. 2007b). We examined the effect of the M2R antagonist MET in large circular muscle strips. MET (100 nM) significantly prolonged the time to peak of CCh-induced contractions in large circular muscle strips (Fig. 6). These results closely resemble the values in small circular muscle strips (Fig. 1b). Contractions were also significantly suppressed (Fig. 6). These observations suggest that M2R participates in CCh-induced contractions of large circular muscle strips.
Discussion
Small muscle strips
This study first detailed the contraction responses in small circular muscle strips of the mouse ileum. This study demonstrated that small circular muscle strips exhibit a spike-like rhythmic contraction, a long latent period, and a large contraction with a peak approximately 6 min after treatment. This contraction response to CCh markedly differs from previous studies on muscle strips from the bladder (Callahan and Creed, 1981), trachea (Janssen 2002; Semenov et al., 2011), and other regions. In contrast, numerous previous studies of CCh-induced contractions of gastrointestinal muscle strips, particularly longitudinal muscles, demonstrated a contraction that occurs immediately after treatment (Takeuchi et al. 2007a, b; Frei et al. 2009). The contraction responses in small longitudinal muscle strips of the mouse ileum in this study are similar to previous studies.
In this study, we used TTX as a neuronal sodium channel blocker. In a singular example, some agents possibly act on the nerve endings to release neurotransmitters without membrane depolarization. In general, the membrane depolarization on the nerve endings is mostly needed to release neurotransmitters. Therefore, we guess that CCh-induced contractions are not dependent on neuronal excitation and that CCh acts directly on smooth muscles.
The findings in small strips indicate marked differences, especially time to peak contraction, in the M3R-mediated contractions of circular and longitudinal muscles. We found the following results in this study. First, the pD2 values for CCh were nearly identical in small circular and longitudinal muscle strips, which indicate no difference in CCh sensitivity. Small circular and longitudinal muscle strips also exhibited similar contraction patterns in response to KCl. Second, the preferring M3R antagonist 4-DAMP completely suppressed atropine-sensitive CCh-induced contractions of small circular and longitudinal muscle strips. Atropine has a dissociation constant of about 10−9 M for all subtypes of the MR when measured in a physiological buffer. Thus, atropine concentration used in this study is 1000-fold greater than KD to block enough and completely the muscarinic contractions. Previous studies regarding on the selectivity of 4-DAMP have demonstrated that 4-DAMP has a KD of about 10−9 M for M1, M3, M4, and M5 subtypes, and a KD of about 10−8 M for the M2 subtype when measured in a physiological buffer (Ehlert 1996; White et al. 2011). Namely, 4-DAMP is selective for M3R over M2R. Because the ileum is thought to express mainly M2R and M3R (Eglen et al., 1996), our data implicated that 4-DAMP mediates CCh-induced contractions through M3R. The voltage-dependent Ca2+ channel inhibitor nicardipine also markedly suppressed CCh-induced contractions of small circular and longitudinal muscle strips.
Previous reports of MR activation and voltage-dependent Ca2+ channel opening describe different mechanisms. In one mechanism, the activated MR opens nonselective cation channels, which depolarizes the membrane and opens voltage-dependent Ca2+ channels (Pacaud and Bolton 1991). Voltage-dependent Ca2+ channels are opened without membrane depolarization in the other mechanism (Kamishima et al. 1992). Therefore, the difference observed in CCh-induced contractions between small circular and longitudinal muscle strips may be due to differences in the contraction mechanism itself, or alternatively, the contraction mechanisms may be similar but the regulatory mechanisms differ.
Large muscle strips
Atropine-sensitive CCh-induced contractions of large circular and longitudinal muscle strips were completely suppressed by 4-DAMP, which is similar to small muscle strips. Different receptors did not underlie differences in contraction responses of large circular and longitudinal muscle strips, which is similar to small muscle strips.
Notably, our results indicate that contractions in large circular muscle strips occurred later than large longitudinal muscle strips. The difference between large muscle strips was not as large as the small muscle strips. However, no reports have compared the time to peak contraction in smaller circular and large muscle strips.
The ICC network contributes to the regulation of smooth muscle motility in the gastrointestinal tract (Fujita et al. 2004), and gap junctions connect ICCs with gastrointestinal smooth muscle cells. Mutual transmission likely exists among ICCs, circular smooth muscle, and gap junctions (Daniel and Wang 1999). However, a marked difference in CCh-induced contractions of circular large muscle strips was not observed between W/WV mice lacking ICCs and wild-type mice in our experiments.
The most notable observation in this study was that the time to peak of CCh-induced contractions in large circular muscle strips treated with the gap junction inhibitor CBX was significantly longer and resembled the value in small circular muscle strips. We used CBX as a gap junction inhibitor. CBX has other pharmacological actions that CBX inhibits the rise of intracellular Ca2+ possibly by blocking voltage-gated Ca2+ channels and affects an ionic channel conductance in certain cell types (Benfenati et al. 2009; Bramley et al. 2011). Our results showed that the effect of CBX is markedly different from the effect of Ca2+ channel blocker nicardipine. It seems that CBX through the inhibition of gap junction was longer time to peak, although we do not completely exclude the possibility of other ability of CBX. These results indicate that gap junctions participate in CCh-induced contractions of large circular muscle strips. Therefore, we hypothesized that the faster appearance of CCh-induced contractions in large circular muscle strips compared to small circular muscle strips was due to contractions of individual circular smooth muscles facilitated by gap junctions.
M2R did not participate in CCh-induced contractions in small muscle strips, but a previous report demonstrated that M2R plays a role in MR-mediated contraction responses of mouse ileum longitudinal muscle strips (Unno et al. 2005; Takeuchi et al. 2007b). Therefore, the effect of MET was examined in large muscle strips and small muscle strips. Previous studies regarding on the selectivity of MET have demonstrated that MET has a KD of about 10−7 M for M1 and M2 subtypes (Esqueda et al. 1996; White et al. 2011). Because the ileum is thought to express mainly M2R and M3R (Eglen et al., 1996), our data implicated that MET mediates CCh-induced contractions through M2R. Notably, MET significantly prolonged the time to peak of CCh-induced contractions in large circular muscle strips. These values were similar to small circular muscle strips and large circular muscle strips under CBX treatment. These results suggest that M2R is involved in the synchronization of contractions in circular smooth muscle. A role for M2R in the synchronization of circular smooth muscles is further suggested by the significant suppression of contractions. These results represent new findings that M2R is involved in the synchronization of gastrointestinal circular muscle motility.
There is another possibility for later time to peak as observed in the circular muscle compared to the longitudinal muscle. Neuronal inhibitory innervation in circular muscles is generally predominant than that in longitudinal muscle tissues. Other inhibitory neurotransmitters, such as ATP, adenosine, and vasoactive intestinal polypeptide (VIP) in addition to NO are significantly involved in the inhibitory innervation (El-Yazbi et al. 2007; Wang et al. 2007; Zizzo et al. 2007). Thus, it is possible that MR stimulation releases inhibitory transmitters, and thereby induces slower contractions. MR, which is involved in the release of inhibitory transmitters, is M2R. To answer this possibility, we already demonstrated the addition of M2R antagonist MET. If ATP, adenosine, or VIP are involved in later time to peak (and slow contraction), the treatment MET may results in faster time to peak. However, our results showed that MET had no effect on time to peak of CCh-induced contractions in small circular muscle strips (Fig. 2), and that MET prolonged, but not shorten, the time to peak of CCh-induced contractions in large circular muscle strips. Observation indicated that we can exclude the possible relations of ATP, adenosine, or VIP on the mechanism of later time to peak.
This study found marked differences between the M3R-mediated contraction responses of circular and longitudinal smooth muscles in mouse ileum. Our results also indicate that the time to peak contraction, i.e., the time from M3R activation to contraction, was faster in large circular muscle strips than small circular muscle strips. This faster responsiveness occurred through M2Rs and gap junctions via a mechanism that synchronizes contractions among circular smooth muscle cells.
Abbreviations
- ACh:
-
Acetylcholine
- 4-DAMP:
-
4-Diphenylacetoxy-N-methylpiperidine methiodide
- L-NNA:
-
N-nitro-L-arginine
- TTX:
-
Tetrodotoxin
- CCh:
-
Carbamylcholine
- CBX:
-
Carbenoxolone
- MET:
-
Methoctramine
- MR:
-
Muscarinic receptors
- NO:
-
Nitric oxide
- ICCs:
-
Interstitial cells of Cajal
References
Benfenati V, Caprini M, Nicchia GP, Rossi A, Dovizio M, Cervetto C, Nobile M, Ferroni S (2009) Carbenoxolone inhibits volume-regulated anion conductance in cultured rat cortical astroglia. Channels (Austin) 3:323–336
Bramley JR, Wiles EM, Sollars PJ, Pickard GE (2011) Carbenoxolone blocks the light-evoked rise in intracellular calcium in isolated melanopsin ganglion cell photoreceptors. PLoS One 6:e22721
Callahan SM, Creed KE (1981) Electrical and mechanical activity of the isolated lower urinary tract of the guinea-pig. Br J Pharmacol 74:353–358
Caulfield MP, Birdsall NJM (1998) Classification of muscarinic acetylcholine receptors. Pharmacol Rev 50:279–290
Daniel EE, Wang YF (1999) Gap junctions in intestinal smooth muscle and interstitial cells of Cajal. Microsc Res Tech 47:309–320
Eglen RM, Hegde SS, Watson N (1996) Muscarinic receptor subtypes and smooth muscle function. Pharmacol Rev 48:531–565
Ehlert FJ (1996) The interaction of 4-DAMP mustard with subtypes of the muscarinic receptor. Life Sci 58:1971–1978
El-Yazbi AF, Schulz R, Daniel EE (2007) Differential inhibitory control of circular and longitudinal smooth muscle layers of Balb/C mouse small intestine. Auton Neurosci 131:36–44
Esqueda EE, Gerstin EH Jr, Griffin MT, Ehlert FJ (1996) Stimulation of cyclic AMP accumulation and phosphoinositide hydrolysis by M3 muscarinic receptors in the rat peripheral lung. Biochem Pharmacol 52:643–658
Frei E, Huster M, Smital P, Schlossmann J, Hofmann F, Wegener JW (2009) Calcium-dependent and calcium-independent inhibition of contraction by cGMP/cGKI in intestinal smooth muscle. Am J Physiol Gastrointest Liver Physiol 297:G834–G839
Fujita A, Okishio Y, Takeuchi T, Hata F (2004) Roles of interstitial cells of Cajal in regulation of motility of the mouse intestine. Folia Pharmacol Jpn 123:170–178
Honda K, Takano Y, Kamiya H (1993) Pharmacological profiles of muscarinic receptors in the longitudinal smooth muscle of guinea pig ileum. Jpn J Pharmacol 62:43–47
Horowitz A, Menice CB, Laporte R, Morgan KG (1996) Mechanisms of smooth muscle contraction. Physiol Rev 76:967–992
Iino S, Horiguchi S, Horiguchi K (2011) Interstitial cells of Cajal in the gastrointestinal musculature of Wjic c-kit mutant mice. J Smooth Muscle Res 47:111–121
Jacobsen JC, Aalkjaer C, Nilsson H, Matchkov VV, Freiberg J, Holstein-Rathlou NH (2007) A model of smooth muscle cell synchronization in the arterial wall. Am J Physiol Heart Circ Physiol 293:H229–H237
Janssen LJ (2002) Ionic mechanisms and Ca2+ regulation in airway smooth muscle contraction:do the data contradict dogma? Am J Physiol Lung Cell Mol Physiol 282:L1161–L1178
Kamishima T, Nelson MT, Patlak JB (1992) Carbachol modulates voltage sensitivity of calcium channels in bronchial smooth muscle of rats. Am J Physiol 263:C69–C77
Khurana S, Yamada M, Wess J, Kennedy RH, Raufman JP (2005) Deoxycholyltaurine-induced vasodilation of rodent aorta is nitric oxide- and muscarinic M3 receptor-dependent. Eur J Pharmacol 517:103–110
Kondo T, Nakajima M, Teraoka H, Unno T, Komori S, Yamada M, Kitazawa T (2011) Muscarinic receptor subtypes involved in regulation of colonic motility in mice: functional studies using muscarinic receptor-deficient mice. Eur J Pharmacol 670:236–243
Matsui M, Motomura D, Fujikawa T, Jiang J, Takahashi S, Manabe T, Taketo MM (2002) Mice lacking M2 and M3 muscarinic acetylcholine receptors are devoid of cholinergic smooth muscle contractions but still viable. J Neurosci 22:10627–10632
Mayer EA, Sun XP (1992) Contraction-coupling in colonic smooth muscle. Annu Rev Physiol 54:395–414
McConalouge K, Furness JB (1994) Gastrointestinal neurotransmitters. Baillieres Clin Endocrinol Metab 8:51–76
Missiaen L, Smedt HD, Droogmans G, Himpens B, Casteels R (1992) Calcium ion homeostasis in smooth muscle. Pharmaco Ther 56:191–231
Pacaud P, Bolton T (1991) Relation between muscarinic receptor cationic current and internal calcium in guinea-pig jejunal smooth muscle cells. J Physiol 441(477–499)
Sanders KM, Ward SM (2007) Kit mutants and gastrointestinal physiology. J Physiol 578:33–42
Sato K, Sanders KM, Gerthoffer WT, Publicover NG (1994) Sources of calcium utilized in cholinergic responses in canine colonic smooth muscle. Am J Physiol 267:C1666–C1673
Semenov I, Wang B, Herlihy JT, Brenner R (2011) BK channel β1 subunits regulate airway contraction secondary to M2 muscarinic acetylcholine receptor mediated depolarization. J Physiol 589:1803–1817
Takeuchi T, Kushida M, Hirayama N, Kitayama M, Fujita A, Hata F (2004) Mechanisms involved in carbachol-induced Ca2+ sensitization of contractile elements in rat proximal and distal colon. Br J Pharmacol 142:657–666
Takeuchi T, Nakajima H, Hata F, Azuma YT (2007a) A minor role for Ca2+ sensitization in sustained contraction through activation of muscarinic receptor in circular muscle of rat distal colon. Eur J Physiol 454:565–574
Takeuchi T, Tanaka K, Nakajima H, Matsui M, Azuma YT (2007b) M2 and M3 muscarinic receptors are involved in enteric nerve-mediated contraction of the mouse ileum: findings obtained with muscarinic-receptor knockout mouse. Am J Physiol Gastrointest Liver Physiol 292:G154–G164
Unno T, Matsuyama H, Sakamoto T, Uchiyama M, Izumi Y, Okamoto H, Yamada M, Wess J, Komori S (2005) M2 and M3 muscarinic receptor-mediated contractions in longitudinal smooth muscle of the ileum studied with receptor knockout mice. Br J Pharmacol 146:98–108
Wang GD, Wang XY, Hu HZ, Liu S, Gao N, Fang X, Xia Y, Wood JD (2007) Inhibitory neuromuscular transmission mediated by the P2Y1 purinergic receptor in guinea pig small intestine. Am J Physiol Gastrointest Liver Physiol 292:G1483–G1489
White CW, Short JL, Haynes JM, Matsui M, Ventura S (2011) Contractions of the mouse prostate elicited by acetylcholine are mediated by M(3) muscarinic receptors. J Pharmacol Exp Ther 339:870–877
Zizzo MG, Mulè F, Serio R (2007) Inhibitory purinergic transmission in mouse caecum: role for P2Y1 receptors as prejunctional modulators of ATP release. Neuroscience 150:658–664
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Azuma, YT., Samezawa, N., Nishiyama, K. et al. Differences in time to peak carbachol-induced contractions between circular and longitudinal smooth muscles of mouse ileum. Naunyn-Schmiedeberg's Arch Pharmacol 389, 63–72 (2016). https://doi.org/10.1007/s00210-015-1177-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-015-1177-3