Abstract
Nucleoside diphosphate kinase (NDPK) proteins comprise a family of ten human isoforms that participate in the regulation of multiple cellular processes via enzymatic and nonenzymatic functions. The major enzymatic function of NDPKs is the generation of nucleoside triphosphates, such as guanosine triphosphate (GTP). Mechanisms behind the nonenzymatic NDPK functions are not clear but likely involve context-dependent signaling roles of NDPK within multi-protein complexes. This is most evident for NDPK-A, which is encoded by the human NME1 gene, the first tumor metastasis suppressor gene to be identified. Understanding which protein interactions are most relevant for the biological and metastasis-related functions of NDPK will be important in the potential utilization of NDPK as a disease target. Accumulating evidence suggests that NDPK interacts with and affects various components and regulators of the cytoskeleton, including actin-binding proteins, intermediate filaments, and cytoskeletal attachment structures (adherens junctions, desmosomes, and focal adhesions). We review the existing literature on this topic and highlight outstanding questions and potential future directions that should clarify the impact of NDPK on the different cytoskeletal systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nucleoside diphosphate kinase (NDPK) proteins are multifunctional proteins expressed from bacteria to humans and are encoded by ten human genes, NME1–9 and RP2 (Boissan et al. 2009; Desvignes et al. 2009). Mammalian NDPKs (also known as Nm23) are classified into two groups based on their sequence identities. The NME1–4 protein products, NDPK-A, NDPK-B, NDPK-C, and NDPK-D, belong to group I and share higher sequence identity (58–88 %) compared to the other family members, which belong to group II and share 22–44 % sequence identity (Boissan et al. 2009). The two groups also differ substantially in their intracellular distribution, with group I NDPKs exhibiting cytoplasmic (NDPK-A to NDPK-C) or mitochondrial (NDPK-D) localization and most of group II NDPKs being localized within cilia and flagella.
The major enzymatic function of NDPK-A to NDPK-D is to generate nucleoside triphosphates via transfer of a phosphate group from ATP to nucleoside diphosphates (Lacombe et al. 2000; Lascu and Gonin 2000). NDPKs can also function as mammalian histidine kinases (Attwood 2013) and possess 3′-5′-exonuclease activity (Kaetzel et al. 2006), which is important in DNA repair. These and other molecular functions of NDPKs remain to be clarified further since NDPKs are implicated in numerous cellular processes, including cell growth, differentiation, apoptosis, and migration (Boissan et al. 2009; Marino et al. 2012). Importantly, the human NDPK-A (Nm23-H1)-encoding gene, NME1, was the first metastasis suppressor gene identified (Steeg et al. 1988). Forced overexpression of NDPK in various cancer cell lines reduces their in vitro and in vivo migratory and invasive properties (Marino et al. 2012). Knockout of the mouse NDPK-A homologue (Nm23-M1) leads to augmented lung metastasis of primary hepatocellular carcinoma tumors (Boissan et al. 2005). There are reports for both a positive and negative correlation between NDPK-A expression and metastasis in the clinical setting, pointing out context-specific mechanisms. Numerous clinical studies have reported an inverse correlation between NDPK-A expression and the metastatic potential of epithelial tumors, including breast, liver, colon, ovarian, lung, and skin, while the opposite was found for hematopoietic malignancies, neuroblastoma, and osteosarcoma, where NDPK-A expression most often correlates with poor clinical outcome (Lacombe and Boissan 2013; Marino et al. 2012).
Clarification of these mechanisms in future studies should reveal new directions for the design of metastasis-limiting strategies by selective targeting of NDPKs. To that end, it is now well appreciated that NDPKs exert their wide-ranging effects in part by forming complexes with proteins involved in multiple cellular functions, including nucleotide exchange factors, transcriptional regulators, cell growth regulators, and cytoskeletal components (Marino et al. 2011). The goal of this mini-review is to summarize the functional evidence supporting a role for NDPK in modulating cytoskeletal dynamics, in particular as it pertains to its metastasis suppressor function. We focus primarily on systems where functional connections have been established between NDPK and the various cytoskeletal systems, summarized in Table 1. Our discussion is focused on the actin cytoskeleton, intermediate filament cytoskeleton, and cytoskeletal attachment sites (cell junctions).
NDPK modulates actin filament dynamics by regulating actin-associated proteins
Actin filaments are the major cytoskeletal component that contributes to the directional motility of cells (Pollard and Cooper 2009). Actin filaments are composed of globular subunits that are organized in a head-to-tail manner, resulting in molecular polarity that includes a barbed end and a pointed end (Dominguez and Holmes 2011). During cell movement, actin filaments are added to the barbed end, which faces outward relative to the cell surface. As the actin polymers branch and grow just beneath the plasma membrane, physical force is generated to enable cell movement. The membrane curvature is dramatically altered during cell motility due to the presence of actin-based structures important for migration and invasion into the extracellular matrix (ECM), such as membrane ruffles, lamellipodia, invadopodia, and podosomes (Buccione et al. 2004). Numerous proteins associate with actin and modulate actin filament dynamics, such as branching, membrane anchoring, linking to other cytoskeletal components, stabilizing, severing, and capping (Winder and Ayscough 2005). As supported by several mechanistic studies, the effects of NDPK in cancer metastasis can, at least in part, be attributed to interactions with actin-associated proteins, including the GTPase dynamin (Gu et al. 2010) and the actin-severing/capping protein gelsolin (Sun et al. 1999) as highlighted below.
NPDK-dynamin interactions regulate endocytosis, cell adhesion, and cytokinesis
Evidence from multiple studies suggests that a major function of NDPKs is to promote the activity of the GTPase dynamin, which is an important regulator of the actin cytoskeleton (Menon and Schafer 2013). There are three dynamin isoforms in mammals. Dynamins 1 and 3 are found predominantly in the brain, whereas dynamin 2 is ubiquitously expressed (Ferguson and De Camilli 2012). The major known function of dynamin is in the regulation of endocytosis, the process by which cellular components from the plasma membrane together with extracellular material are internalized (Ferguson and De Camilli 2012). With respect to its involvement in tumor cell biology, dynamin presence has been noted in focal adhesions, invadopodia, podosomes, and lamellipodia, and its activity has been attributed to the acquisition of migratory and invasive cell phenotypes (Baldassarre et al. 2003; Kruchten and McNiven 2006).
Evidence pointing to physical and functional associations between NDPK and dynamin was presented over a decade ago (Baillat et al. 2002; Krishnan et al. 2001). The functional relevance of this interaction to endocytosis was initially highlighted for dynamin-dependent synaptic vesicle recycling (Krishnan et al. 2001) and loss of cell surface-localized fibroblast growth factor receptor in Drosophila melanogaster (Dammai et al. 2003). Additional work provided further support that NDPK promotes dynamin function in endocytosis as well as in cell division. For example, NDPK was found to promote dynamin-mediated endocytosis of E-cadherin to induce loss of adherens junctions (Palacios et al. 2002). This process involves recruitment of NDPK to adherens junctions by guanosine triphosphate (GTP)-bound ARF6, which is a GTPase that functions in endocytic recycling and actin cytoskeleton remodeling. Upon recruitment, NDPK promotes endocytic vesicle fission and downstream inactivation of Rac1, which is another important regulator of cell migration and invasion (Lawson and Burridge 2014). Additionally, NPDK-A and dynamin associate during cytokinesis to promote chromosome stability. Loss of NDPK-A is linked to cytokinesis failure and tetraploidy, which is phenocopied by a loss of dynamin (Conery et al. 2010). The enzymatic activity of NDPK was found to be important for its association with dynamin during cytokinesis.
The significance of the GTP-generating function of NDPK and its ability to promote dynamin activity was convincingly demonstrated in elegant work by Boissan et al., which showed that NDPKs directly interact with and support the function of dynamin 2 and the mitochondrial dynamin-like GTPase OPA1 by generating high concentrations of GTP in a localized manner to promote membrane remodeling (Boissan et al. 2014). Using several approaches, the authors showed that NDPK-A and NDPK-B are bound to membrane-associated dynamin 2 to promote membrane fission, while loss of NDPK-A and NDPK-B led to inhibition of endocytosis and accumulation of clathrin-coated pits that was not attributed to a global reduction in GTP levels. These effects extended to the mitochondrial compartment, where NDPK-D was found to control mitochondrial fusion by promoting the function of the dynamin-related GTPase OPA1 (Boissan et al. 2014). Loss of NDPK-D phenocopied the loss of OPA1 with respect to the presence of increased number of cells with fragmented, as opposed to tubular, mitochondria.
Therefore, the recent studies in mammalian systems (Boissan et al. 2014; Conery et al. 2010) combined with the earlier work in D. melanogaster (Dammai et al. 2003; Krishnan et al. 2001) provide evidence for an evolutionarily conserved function of NDPKs in regulating the function of dynamin and dynamin-like GTPases.
NDPK-gelsolin interactions limit cell migration and tumor metastasis
Another link between the actin cytoskeleton and NDPK is its interaction with gelsolin. The activation of gelsolin, an actin-severing and monomer-sequestering protein, results in the generation of shorter and more numerous actin filaments, which translates to alterations in cellular morphology, motility, and signaling pathways (Nag et al. 2013). There is in vitro evidence to support a promoting role for gelsolin in cancer cell invasion, and this is mediated through a number of oncogenic signaling effectors (Chen et al. 1996; De Corte et al. 2002), but the role of gelsolin in metastasis is not clear.
A recent proteomics-based study identified and functionally confirmed gelsolin as an interacting partner of NDPK (Marino et al. 2013), validating and expanding upon a prior study that reported this interaction as a component of the anti-metastatic function of NDPK (Garzia et al. 2006). Association between gelsolin and NDPK-A was demonstrated in several cancer cell lines and primary tumors (Marino et al. 2013). Importantly, NDPK reduced the actin depolymerizing activity of gelsolin, antagonized gelsolin-stimulated tumor cell motility in vitro, and attenuated the pro-metastatic function in an in vivo model of mammary tumor metastasis. Therefore, the anti-metastatic function of NDPK may be partially mediated by its ability to indirectly affect actin filament dynamics by modulating the activity of gelsolin.
Involvement of NDPK in the modulation of vimentin and keratin intermediate filaments
Intermediate filament (IF) proteins make up a major cytoskeletal component that is responsible for mechanoprotection and for regulation of growth- and stress-related signaling events (Eriksson et al. 2009; Kim and Coulombe 2007). Keratins, which represent the largest subfamily of IF proteins, are encoded by 54 human genes and expressed in an epithelial cell-specific manner (Schweizer et al. 2006). The other major cytoplasmic IF proteins include mesenchymal vimentin, myocyte desmin, neurofilaments, and glial fibrillary acidic protein, whereas A- and B-type lamin IFs form the nuclear lamina of all nucleated cells. IFs are also known to be involved in modulating cell migration (Chung et al. 2013) through several mechanisms that involve mechanotransduction signaling, associated protein partners, and IF posttranslational modifications (Snider and Omary 2014), in particular, phosphorylation. In general, vimentin IFs promote cell migration, whereas keratin IFs have context-dependent effects that either promote or reduce cell migration (Chung et al. 2013; Ivaska et al. 2007).
Association of NDPK with vimentin IFs: potential role in epithelial-to-mesenchymal transition?
Vimentin is normally expressed on cells of mesenchymal origin, such as leukocytes and vascular endothelial cells. This is contrast to epithelial cells, which express keratin IFs. During epithelial-to-mesenchymal transition (EMT), the process by which epithelial cells lose their polarity and intercellular adhesion properties while gaining a pro-migratory function (Kalluri and Weinberg 2009), keratins typically are downregulated while vimentin is upregulated. Therefore, vimentin is considered a marker for EMT but in recent years, it has become apparent that vimentin may also be an active promoter of EMT (Ivaska 2011). Specifically, vimentin can activate oncogenic kinases, such as the tyrosine kinase Axl1 (Vuoriluoto et al. 2011), and may promote the formation of lamellipodia (Helfand et al. 2011). An intact vimentin cytoskeleton is associated with decreased formation of lamellipodia, whereas disassembled vimentin filaments at the cell periphery is associated with increased cell motility (Helfand et al. 2011). This is supported by observations that targeted disassembly of vimentin filaments by microinjection of a vimentin peptide mimetic causes retraction of the filamentous vimentin network from the cell periphery and leads to membrane ruffling and formation of lamellipodia (Helfand et al. 2011).
The existing evidence points to a direct association between NDPK and vimentin (Otero 1997; Roymans et al. 2000), but not with keratin IFs (Snider et al. 2011), and the vimentin-NDPK interaction may be potentially relevant during EMT. The interaction with vimentin was demonstrated biochemically in frog lung, heart, and liver tissues (Otero 1997). Since IFs are highly abundant and may coprecipitate with other proteins nonspecifically, an important control was done with desmin, the major IF in the heart. This showed that, unlike vimentin, the more abundant desmin did not associate with NDPK in heart tissue. A subsequent independent study confirmed the biochemical association and demonstrated co-localization of NDPK with vimentin by indirect immunofluorescence in rat glioma cells (Roymans et al. 2000). Although the in vivo relevance of this interaction is not clear, NPDK promotes the assembly of vimentin filaments in vitro using purified proteins (Otero 2000). One possibility is that association of NDPK with vimentin may promote stabilization of vimentin filaments, thereby, inhibiting the formation of lamellipodia and attenuating cell migration. Since vimentin phosphorylation plays a critical role in filament assembly and disassembly (Hyder et al. 2008), it would also be of interest to investigate the role of NDPK histidine kinase activity in this context.
NDPKs may limit pathologic aggregation of keratin IFs by attenuating the levels of reactive oxygen species
Based on a 2-D difference in gel electrophoresis (2D-DIGE) proteomic analysis, mouse NDPK-A and NDPK-B were identified as candidate proteins that confer resistance to pathologic aggregation of keratin 8/18 IFs in the liver (Snider et al. 2011). Several chronic liver diseases, including alcoholic and nonalcoholic steatohepatitis, are characterized by the presence of keratin 8/18-containing cytoplasmic aggregates in hepatocytes termed Mallory-Denk bodies (MDBs). Elevated oxidative stress and deficient proteasome/autophagy pathways underlie MDB-related liver pathology (Zatloukal et al. 2007). There is a significant genetic and sex component to MDB formation in humans and in mice (Hanada et al. 2008, 2010; Snider et al. 2011). Comparison of C57BL6/J and C3H/HeN, the most and least MDB-susceptible mouse strain, respectively, revealed that hepatocyte and liver NDPK expression and activity were significantly lower in the C57BL6/J mouse livers and hepatocytes (Snider et al. 2011). Furthermore, knockdown of NDPK-A/B in primary hepatocytes led to significant accumulation of reactive oxygen species upon treatment with an MDB-inducing agent. Although NDPK exhibited a filamentous distribution pattern in primary hepatocytes and partially co-localized with keratins 8/18, co-immunoprecipitation analysis under basal conditions did not reveal an association (Snider et al. 2011). The possibility that NDPK associates dynamically with keratins under a specific context (e.g., during stress induction) cannot be excluded. Irrespective of a direct association, NDPK likely functions to limit keratin aggregation by protecting against oxidative stress-induced damage in hepatocytes via mechanisms that are currently not known.
NDPKs are microtubule-binding proteins in vitro
One of the first functions ascribed to NDPKs was their ability to bind microtubules, which was demonstrated 40 years ago with the co-purification of NDPK with a preparation of microtubules from bovine brain (Nickerson and Wells 1984). Since then, links between NDPKs and microtubules have been established in several in vitro situations but evidence that NDPKs can modulate the activity of microtubules is lacking. It was demonstrated that in rat C6 glioma cells, catalytically active NDPK-A was a component of the centrosome and associated with γ-tubulin (Roymans et al. 2001). Additionally, mouse NDPK-A associates with β-tubulin in C2C12 myotubes, and this association was more apparent upon induction of myoblast fusion and the formation of multinucleated fibers (Lombardi et al. 1995). Therefore, the association between NDPK-A and β-tubulin appears to be dynamic. Mouse NDK7, which belongs to the group II NDPKs and is primarily expressed in tissues with motile axonemes (including testis, oviduct, lunch, and trachea), was also identified as a microtubule-binding protein. Purified recombinant mouse NDK7 associated in vitro in a co-sedimentation assay with taxol-stabilized microtubules from porcine brain (Ikeda 2010). Lastly, the thioredoxin-like 2 protein Txl2, which has an N-terminal thioredoxin domain and a C-terminal NDPK domain and is encoded by the NME9 gene, binds microtubule preparations in vitro (Sadek et al. 2003). Therefore associations between NDPK and microtubules may be most relevant to the group II NDPKs, but the in vivo occurrence and significance of these associations remain to be established.
NDPK activity modulates the dynamics of cytoskeletal attachment sites
Some of the mechanisms behind the anti-metastatic effects of NDPK appear to be linked to its ability to modulate cell adhesions, including adherens junctions, desmosomes, and focal adhesions. Adherens junctions and desmosomes are intercellular attachment sites composed of three major protein families: cadherins, armadillo proteins, and cytoskeletal adaptors (Green et al. 2010). In the case of adherens junctions, the cytoplasmic tail of E-cadherin is linked to the actin cytoskeleton via the armadillo protein family member β-catenin. In desmosomes, on the other hand, cadherins communicate with IFs via another armadillo family protein, plakoglobin (also known as γ-catenin). Whereas adherens junctions are considered more dynamic and involved in epithelial reorganization, desmosomes form strong adhesions that enable mechanical integration of cells within tissues (Dusek and Attardi 2011; Green et al. 2010). Focal adhesions are cell-substrate attachment structures that anchor actin filaments and can also serve as signaling platforms (Geiger et al. 2009). They are formed by integrin receptors, which are part of the cellular sensing machinery for environmental chemo- and mechanosensing. Dysfunction of any of these junctional complexes is associated with increased metastasis and cancer progression (Dusek and Attardi 2011; Knights et al. 2012) so this system is highly relevant to the effects of NDPK in cancer metastasis. For example, nuclear translocation of β-catenin orchestrates an oncogenic program by binding to LEF/TCF transcription factors and promoting Wnt signaling, while E-cadherin-mediated cell adhesion is lost during tumor progression (Berx and van Roy 2009). As discussed below, there is evidence for co-regulation as well as direct associations between NDPK and junctional proteins, highlighting several potential mechanisms for NDPK in regulating cytoskeletal attachment sites.
NDPK deficiency is linked to loss of adherens junctions
The acquisition of an invasive phenotype in the human hepatocellular carcinoma (HCC) cell line HepG2 upon silencing of NDPK-A was mechanistically linked to mislocalization of E-cadherin, loss of adherens junctions, and rearrangement of the actin cytoskeleton in the form of filopodia-like structures (Boissan et al. 2010). The loss of adherens junctions upon NDPK-A silencing was not only limited to HepG2 cells but also observed in the human colon carcinoma HCT8/S11 cells, thereby, suggesting a possible general effect. These changes were paralleled by nuclear translocation of β-catenin and subsequent hyperactivation of Wnt signaling and specifically attributed to the loss of NDPK-A, but not NDPK-B expression. The authors also examined a limited set of primary HCC and colorectal cancer tumors and noted a loss of NDPK-A expression along with nuclear β-catenin accumulation in the invasive fronts of some of the tumors, pointing out a potential clinical relevance of these findings. Although the exact mechanisms by which NDPK-A loss leads to loss of adherens junctions remains to be elucidated, it appears that enzymatic activity may not be a critical factor in this case since the authors noted only a 20 % loss of total cellular NDPK activity upon NDPK-A knockdown (Boissan et al. 2010). One possibility is that NDPK may serve as a molecular linker in adherens junctions under specific contexts.
Positive co-regulation and association between NDPK and the desmosomal protein plakoglobin
In recent years, dysfunction of desmosomal components has also been linked to cancer progression via a number of mechanisms (Dusek and Attardi 2011). For example, the desmosomal protein plakoglobin can directly compete with β-catenin for assembly into adherens junctions, which leads to accumulation of β-catenin in the nucleus (Zhurinsky et al. 2000). Furthermore, plakoglobin can translocate to the nucleus and upregulate LEF/TCF target genes and potentially other oncogenic factors independently of its competition with β-catenin in adherens junctions (Conacci-Sorrell et al. 2002). In contrast to these apparent tumor-promoting functions of plakoglobin, loss of plakoglobin expression has also been linked to cell invasiveness (Bailey et al. 2012; Holen et al. 2012), thus, pointing to a context-dependent effect.
Ectopic expression of plakoglobin in the human tongue squamous cell carcinoma SCC9 cell line (which lack endogenous plakoglobin) facilitates desmosome formation and an epidermoid phenotype characterized by contact-dependent growth inhibition and limited in vitro migration and invasion (Parker et al. 1998). Therefore, in this context, plakoglobin expression and desmosome formation exert antitumor properties. This phenotypic change in the SCC9 cells was accompanied by elevated expression of several proteins, including NDPK-A and NDPK-B (Aktary et al. 2010). Regulation of NDPK-A was at the transcriptional level and was mediated by a plakoglobin-induced decrease in the expression of the oncogenic chromatin remodeling SATB1, which appears to function as a negative regulator of NME1 expression (Aktary and Pasdar 2013). Immunofluorescence staining analysis revealed partial plasma membrane junctional localization of NDPK-A/B, whereas co-immunoprecipitation of desmosomal component proteins suggested an association between NDPK-A/B and plakoglobin, as well as α-catenin and E-cadherin (Aktary et al. 2010). The biochemical findings extended to other epithelial cell lines, including MCF-7, MCF-10-2A, MDCK, and SW620. Previously reported findings that NDPK promotes adhesion of the oral squamous cell carcinoma of the tongue (CAL 27) cells may be rooted in the same mechanisms (Bago et al. 2009). The outstanding questions from this work pertain to the specific roles of NDPK in the formation and/or stabilization of desmosomes and in mediating the differential effects of plakoglobin in cancer.
NDPK may regulate integrin signaling via its association with integrin cytoplasmic domain-associated protein 1
Integrin activation is required for focal adhesion formation. Conformational changes of the integrin heterodimer lead to increased affinity of integrins for extracellular matrix components (Geiger et al. 2009). Several proteins are known to modulate integrin activation in a positive and negative manner. Two classes of proteins, the talins and the kindlins, link the cytoplasmic domains of β-integrins to the actin cytoskeleton and are critical for integrin activation (Geiger et al. 2009). On the other hand, the integrin cytoplasmic domain-associated protein 1 (ICAP-1) slows focal adhesion assembly by antagonizing talin and kindlin functions (Millon-Fremillon et al. 2008).
Although the functional importance and mechanisms of ICAP-1-mediated inhibition of integrin activation have only recently become appreciated, the association between ICAP-1 and NDPK-B was documented over a decade ago (Fournier et al. 2002). A yeast two-hybrid analysis identified NDPK-B as an interacting partner of ICAP-1. The association between NDPK-B and ICAP-1 was confirmed biochemically by using recombinant proteins in vitro and in cell culture using co-immunoprecipitation and indirect immunofluorescence analysis. Although NDPK-A was also found to co-localize with ICAP-1 within lamellipodia at the initiation of cell spreading, there is no biochemical evidence for a direct interaction between them (Fournier et al. 2003). The biochemical association studies revealed that the C terminus of ICAP-1, which contains a protein tyrosine phosphatase (PTP) domain, is required for binding to NDPK-B. Additionally, crystallization of ICAP-1 bound to β1-integrin revealed that the PTP domain is also involved in this interaction (Liu et al. 2013). This indicates that the interaction of ICAP-1 with NDPK-B may preclude association between ICAP-1 and β1-integrin, and in that sense, NDPK-B could function as a negative regulator of ICAP-1 to allow β1-integrin activation. In support of that, ICAP-1 and NDPK-B co-localize in lamellipodia during early stages of cell spreading, and this process precedes formation of focal adhesions that lack both proteins (Fournier et al. 2002).
One possibility for the functional significance of this interaction could be that, upon NDPK-B binding, ICAP-1 is sequestered intracellularly to allow for integrin activation and focal adhesion formation to proceed. These mechanisms would be analogous to that of another protein, Krev/Rap1 interaction trapped 1 (KRIP1), an antagonist of ICAP-1 that is important for integrin activation (Liu et al. 2013). Therefore, the interaction of NDPK with specific components of the environmental sensing machinery will need to be reevaluated in functional and in vivo studies to determine if it is a significant contributor to NDPK-mediated metastasis suppression.
Conclusions
The most well-established function of NDPK proteins is the tumor metastasis suppressor role of NDPK-A. However, the mechanisms behind this process are still unclear, mostly because the enzymatic activities of NDPKs are not fully understood and because NDPKs appear to mainly work in a complex with other cellular proteins. NDPKs interact directly or indirectly with all of the major cytoskeletal elements and are likely to impact their cellular functions. The interactions of NDPKs with components of the cytoskeletal machinery are highly relevant, given the well-established roles of the cytoskeleton in cell motility. Although few mechanistic studies have examined the ability of NDPK to regulate cytoskeletal dynamics (summarized in Fig. 1), there are important directions that may be considered in future studies. These include a clarification for the isoform-specific roles of NDPK in mediating actin-based cell motility in vivo, effects of NDPK on vimentin and potentially other IF protein assembly and disassembly properties in vivo and the potential consequences of this process to EMT and other cellular responses, and the functional role of NDPK in modulating the formation and/or stability of cell-cell and cell-substrate adhesions. Discriminating between enzymatic and nonenzymatic NDPK functions in all of these processes will also be essential. The use of available genetic mouse models, such as NDPK−/− mice, and generating conditional knockouts to probe NDPK function in a cell-specific manner should significantly aid progress in this field. Ultimately, novel pharmacological approaches targeting specific interactions between NDPK and proteins involved in cytoskeleton remodeling may offer treatment strategies for metastatic cancer in the appropriate patient populations.
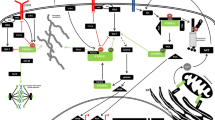
Involvement of NDPK in modulating cytoskeletal dynamics. 1. NDPK associates with and promotes the activity of the GTPase dynamin in endocytosis of fibroblast growth factor receptor and E-cadherin. NDPK also promotes dynamin function by providing GTP during cytokinesis to promote chromosomal stability. 2. NDPK associates with gelsolin and inhibits its actin-severing/capping function. 3. NDPK associates with vimentin intermediate filaments in multiple tissues and promotes vimentin filament assembly in vitro. 4. NDPK supports adherens junction formation through unknown mechanisms. Loss of NDPK leads to loss of adherens junctions and nuclear accumulation of β-catenin. 5. Multiple components of desmosomal junctions, including E-cadherin and plakoglobin, associate with NDPK. Plakoglobin also upregulates NDPK protein levels via induction of NME1 gene expression. 6. NDPK associates with ICAP-1, which is a negative regulator of integrin signaling. A potential relevance of this association is to sequester ICAP-1 to allow integrin activation and formation of focal adhesions
References
Aktary Z, Pasdar M (2013) Plakoglobin represses SATB1 expression and decreases in vitro proliferation, migration and invasion. PLoS One 8:e78388
Aktary Z et al (2010) Plakoglobin interacts with and increases the protein levels of metastasis suppressor Nm23-H2 and regulates the expression of Nm23-H1. Oncogene 29:2118–2129. doi:10.1038/onc.2009.495
Attwood PV (2013) Histidine kinases from bacteria to humans. Biochem Soc Trans 41:1023–1028. doi:10.1042/BST20130019
Bago R, Pavelic J, Maravic Vlahovicek G, Bosnar MH (2009) Nm23-H1 promotes adhesion of CAL 27 cells in vitro. Mol Carcinog 48:779–789
Bailey CK, Mittal MK, Misra S, Chaudhuri G (2012) High motility of triple-negative breast cancer cells is due to repression of plakoglobin gene by metastasis modulator protein SLUG. J Biol Chem 287:19472–19486. doi:10.1074/jbc.M112.345728
Baillat G, Gaillard S, Castets F, Monneron A (2002) Interactions of phocein with nucleoside-diphosphate kinase, Eps15, and Dynamin I. J Biol Chem 277:18961–18966
Baldassarre M et al (2003) Dynamin participates in focal extracellular matrix degradation by invasive cells. Mol Biol Cell 14:1074–1084. doi:10.1091/mbc.E02-05-0308
Berx G, van Roy F (2009) Involvement of members of the cadherin superfamily in cancer. Cold Spring Harb Perspect Biol 1:a003129. doi:10.1101/cshperspect.a003129
Boissan M et al (2005) Increased lung metastasis in transgenic NM23-Null/SV40 mice with hepatocellular carcinoma. J Natl Cancer Inst 97:836–845
Boissan M, Dabernat S, Peuchant E, Schlattner U, Lascu I, Lacombe ML (2009) The mammalian Nm23/NDPK family: from metastasis control to cilia movement. Mol Cell Biochem 329:51–62
Boissan M et al (2010) Implication of metastasis suppressor NM23-H1 in maintaining adherens junctions and limiting the invasive potential of human cancer cells. Cancer Res 70:7710–7722
Boissan M et al (2014) Membrane trafficking. Nucleoside diphosphate kinases fuel dynamin superfamily proteins with GTP for membrane remodeling. Science 344:1510–1515. doi:10.1126/science.1253768
Buccione R, Orth JD, McNiven MA (2004) Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat Rev Mol Cell Biol 5:647–657
Chen P, Murphy-Ullrich JE, Wells A (1996) A role for gelsolin in actuating epidermal growth factor receptor-mediated cell motility. J Cell Biol 134:689–698
Chung BM, Rotty JD, Coulombe PA (2013) Networking galore: intermediate filaments and cell migration. Curr Opin Cell Biol 25:600–612. doi:10.1016/j.ceb.2013.06.008
Conacci-Sorrell ME, Ben-Yedidia T, Shtutman M, Feinstein E, Einat P, Ben-Ze’ev A (2002) Nr-CAM is a target gene of the beta-catenin/LEF-1 pathway in melanoma and colon cancer and its expression enhances motility and confers tumorigenesis. Genes Dev 16:2058–2072. doi:10.1101/gad.227502
Conery AR, Sever S, Harlow E (2010) Nucleoside diphosphate kinase Nm23-H1 regulates chromosomal stability by activating the GTPase dynamin during cytokinesis. Proc Natl Acad Sci U S A 107:15461–15466
Dammai V, Adryan B, Lavenburg KR, Hsu T (2003) Drosophila awd, the homolog of human nm23, regulates FGF receptor levels and functions synergistically with shi/dynamin during tracheal development. Genes Dev 17:2812–2824. doi:10.1101/gad.1096903
De Corte V, Bruyneel E, Boucherie C, Mareel M, Vandekerckhove J, Gettemans J (2002) Gelsolin-induced epithelial cell invasion is dependent on Ras-Rac signaling. EMBO J 21:6781–6790
Desvignes T, Pontarotti P, Fauvel C, Bobe J (2009) Nme protein family evolutionary history, a vertebrate perspective. BMC Evol Biol 9:256
Dominguez R, Holmes KC (2011) Actin structure and function. Annu Rev Biophys 40:169–186. doi:10.1146/annurev-biophys-042910-155359
Dusek RL, Attardi LD (2011) Desmosomes: new perpetrators in tumour suppression. Nat Rev Cancer 11:317–323. doi:10.1038/nrc3051
Eriksson JE, Dechat T, Grin B, Helfand B, Mendez M, Pallari HM, Goldman RD (2009) Introducing intermediate filaments: from discovery to disease. J Clin Invest 119:1763–1771. doi:10.1172/JCI38339
Ferguson SM, De Camilli P (2012) Dynamin, a membrane-remodelling GTPase. Nat Rev Mol Cell Biol 13:75–88
Fournier HN, Albigès-Rizo C, Block MR (2003) New insights into Nm23 control of cell adhesion and migration.J Bioenerg Biomembr. 35(1):81–7
Fournier HN, Dupe-Manet S, Bouvard D, Lacombe ML, Marie C, Block MR, Albiges-Rizo C (2002) Integrin cytoplasmic domain-associated protein 1alpha (ICAP-1alpha) interacts directly with the metastasis suppressor nm23-H2, and both proteins are targeted to newly formed cell adhesion sites upon integrin engagement. J Biol Chem 277:20895–20902
Garzia L, Roma C, Tata N, Pagnozzi D, Pucci P, Zollo M (2006) H-prune-nm23-H1 protein complex and correlation to pathways in cancer metastasis. J Bioenerg Biomembr 38:205–213
Geiger B, Spatz JP, Bershadsky AD (2009) Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol 10:21–33. doi:10.1038/nrm2593
Green KJ, Getsios S, Troyanovsky S, Godsel LM (2010) Intercellular junction assembly, dynamics, and homeostasis. Cold Spring Harb Perspect Biol 2:a000125. doi:10.1101/cshperspect.a000125
Gu C et al (2010) Direct dynamin-actin interactions regulate the actin cytoskeleton. EMBO J 29:3593–3606. doi:10.1038/emboj.2010.249
Hanada S, Strnad P, Brunt EM, Omary MB (2008) The genetic background modulates susceptibility to mouse liver Mallory-Denk body formation and liver injury. Hepatology 48:943–952. doi:10.1002/hep.22436
Hanada S, Snider NT, Brunt EM, Hollenberg PF, Omary MB (2010) Gender dimorphic formation of mouse Mallory-Denk bodies and the role of xenobiotic metabolism and oxidative stress. Gastroenterology 138:1607–1617. doi:10.1053/j.gastro.2009.12.055
Helfand BT et al (2011) Vimentin organization modulates the formation of lamellipodia. Mol Biol Cell 22:1274–1289. doi:10.1091/mbc.E10-08-0699
Holen I et al (2012) Loss of plakoglobin promotes decreased cell-cell contact, increased invasion, and breast cancer cell dissemination in vivo. Breast Cancer Res BCR 14:R86
Hyder CL, Pallari HM, Kochin V, Eriksson JE (2008) Providing cellular signposts—post-translational modifications of intermediate filaments. FEBS Lett 582:2140–2148
Ikeda T (2010) NDP kinase 7 is a conserved microtubule-binding protein preferentially expressed in ciliated cells. Cell Struct Funct 35:23–30
Ivaska J (2011) Vimentin: central hub in EMT induction? Small GTPases 2:51–53. doi:10.4161/sgtp.2.1.15114
Ivaska J, Pallari HM, Nevo J, Eriksson JE (2007) Novel functions of vimentin in cell adhesion, migration, and signaling. Exp Cell Res 313:2050–2062
Kaetzel DM, Zhang Q, Yang M, McCorkle JR, Ma D, Craven RJ (2006) Potential roles of 3′-5′ exonuclease activity of NM23-H1 in DNA repair and malignant progression. J Bioenerg Biomembr 38:163–167
Kalluri R, Weinberg RA (2009) The basics of epithelial-mesenchymal transition. J Clin Invest 119:1420–1428. doi:10.1172/JCI39104
Kim S, Coulombe PA (2007) Intermediate filament scaffolds fulfill mechanical, organizational, and signaling functions in the cytoplasm. Genes Dev 21:1581–1597. doi:10.1101/gad.1552107
Knights AJ, Funnell AP, Crossley M, Pearson RC (2012) Holding tight: cell junctions and cancer spread. Trends Cancer Research 8:61–69
Krishnan KS et al (2001) Nucleoside diphosphate kinase, a source of GTP, is required for dynamin-dependent synaptic vesicle recycling. Neuron 30:197–210
Kruchten AE, McNiven MA (2006) Dynamin as a mover and pincher during cell migration and invasion. J Cell Sci 119:1683–1690. doi:10.1242/jcs.02963
Lacombe M, Boissan M (2013) NME1 (NME/NM23 nucleoside diphosphate kinase 1). Atlas Genet Cytogenet Oncol Haematol 17:526–538
Lacombe ML, Milon L, Munier A, Mehus JG, Lambeth DO (2000) The human Nm23/nucleoside diphosphate kinases. J Bioenerg Biomembr 32:247–258
Lascu I, Gonin P (2000) The catalytic mechanism of nucleoside diphosphate kinases. J Bioenerg Biomembr 32:237–246
Lawson CD, Burridge K (2014) The on-off relationship of Rho and Rac during integrin-mediated adhesion and cell migration.Small GTPases. 5:e27958. doi:10.4161/sgtp.27958
Liu W, Draheim KM, Zhang R, Calderwood DA, Boggon TJ (2013) Mechanism for KRIT1 release of ICAP1-mediated suppression of integrin activation. Mol Cell 49:719–729
Lombardi D, Sacchi A, D’Agostino G, Tibursi G (1995) The association of the Nm23-M1 protein and beta-tubulin correlates with cell differentiation. Exp Cell Res 217:267–271
Marino N, Marshall JC, Steeg PS (2011) Protein-protein interactions: a mechanism regulating the anti-metastatic properties of Nm23-H1. Naunyn Schmiedebergs Arch Pharmacol 384:351–362. doi:10.1007/s00210-011-0646-6
Marino N, Nakayama J, Collins JW, Steeg PS (2012) Insights into the biology and prevention of tumor metastasis provided by the Nm23 metastasis suppressor gene. Cancer Metastasis Rev 31:593–603
Marino N, Marshall JC, Collins JW, Zhou M, Qian Y, Veenstra T, Steeg PS (2013) Nm23-h1 binds to gelsolin and inactivates its actin-severing capacity to promote tumor cell motility and metastasis. Cancer Res 73:5949–5962
Menon M, Schafer DA (2013) Dynamin: expanding its scope to the cytoskeleton. Int Rev Cell Mol Biol 302:187–219. doi:10.1016/B978-0-12-407699-0.00003-0
Millon-Fremillon A, Bouvard D, Grichine A, Manet-Dupe S, Block MR, Albiges-Rizo C (2008) Cell adaptive response to extracellular matrix density is controlled by ICAP-1-dependent beta1-integrin affinity. J Cell Biol 180:427–441
Nag S, Larsson M, Robinson RC, Burtnick LD (2013) Gelsolin: the tail of a molecular gymnast. Cytoskeleton 70:360–384. doi:10.1002/cm.21117
Nickerson JA, Wells WW (1984) The microtubule-associated nucleoside diphosphate kinase. J Biol Chem 259:11297–11304
Otero AS (1997) Copurification of vimentin, energy metabolism enzymes, and a MER5 homolog with nucleoside diphosphate kinase. Identification of tissue-specific interactions. J Biol Chem 272:14690–14694
Otero AS (2000) NM23/nucleoside diphosphate kinase and signal transduction. J Bioenerg Biomembr 32:269–275
Palacios F, Schweitzer JK, Boshans RL, D’Souza-Schorey C (2002) ARF6-GTP recruits Nm23-H1 to facilitate dynamin-mediated endocytosis during adherens junctions disassembly. Nat Cell Biol 4:929–936
Parker HR, Li Z, Sheinin H, Lauzon G, Pasdar M (1998) Plakoglobin induces desmosome formation and epidermoid phenotype in N-cadherin-expressing squamous carcinoma cells deficient in plakoglobin and E-cadherin. Cell Motil Cytoskeleton 40:87–100. doi:10.1002/(SICI)1097-0169(1998)40:1<87::AID-CM8>3.0.CO;2-C
Pollard TD, Cooper JA (2009) Actin, a central player in cell shape and movement. Science 326:1208–1212. doi:10.1126/science.1175862
Roymans D et al (2000) Nucleoside diphosphate kinase beta (Nm23-R1/NDPKbeta) is associated with intermediate filaments and becomes upregulated upon cAMP-induced differentiation of rat C6 glioma. Exp Cell Res 261:127–138
Roymans D et al (2001) Identification of the tumor metastasis suppressor Nm23-H1/Nm23-R1 as a constituent of the centrosome. Exp Cell Res 262:145–153
Sadek CM et al (2003) Characterization of human thioredoxin-like 2. A novel microtubule-binding thioredoxin expressed predominantly in the cilia of lung airway epithelium and spermatid manchette and axoneme. J Biol Chem 278:13133–13142. doi:10.1074/jbc.M300369200
Schweizer J et al (2006) New consensus nomenclature for mammalian keratins. J Cell Biol 174:169–174. doi:10.1083/jcb.200603161
Snider NT, Omary MB (2014) Post-translational modifications of intermediate filament proteins: mechanisms and functions. Nat Rev Mol Cell Biol 15:163–177. doi:10.1038/nrm3753
Snider NT et al (2011) Energy determinants GAPDH and NDPK act as genetic modifiers for hepatocyte inclusion formation. J Cell Biol 195:217–229. doi:10.1083/jcb.201102142
Steeg PS, Bevilacqua G, Kopper L, Thorgeirsson UP, Talmadge JE, Liotta LA, Sobel ME (1988) Evidence for a novel gene associated with low tumor metastatic potential. J Natl Cancer Inst 80:200–204
Sun HQ, Yamamoto M, Mejillano M, Yin HL (1999) Gelsolin, a multifunctional actin regulatory protein. J Biol Chem 274:33179–33182
Vuoriluoto K et al (2011) Vimentin regulates EMT induction by Slug and oncogenic H-Ras and migration by governing Axl expression in breast cancer. Oncogene 30:1436–1448. doi:10.1038/onc.2010.509
Winder SJ, Ayscough KR (2005) Actin-binding proteins. J Cell Sci 118:651–654. doi:10.1242/jcs.01670
Zatloukal K et al (2007) From Mallory to Mallory-Denk bodies: what, how and why? Exp Cell Res 313:2033–2049. doi:10.1016/j.yexcr.2007.04.024
Zhurinsky J, Shtutman M, Ben-Ze’ev A (2000) Plakoglobin and beta-catenin: protein interactions, regulation and biological roles. J Cell Sci 113(Pt 18):3127–3139
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Snider, N.T., Altshuler, P.J. & Omary, M.B. Modulation of cytoskeletal dynamics by mammalian nucleoside diphosphate kinase (NDPK) proteins. Naunyn-Schmiedeberg's Arch Pharmacol 388, 189–197 (2015). https://doi.org/10.1007/s00210-014-1046-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-014-1046-5