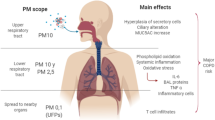
Abstract
Environmental pollution, which contains ambient particulate matter, has been shown to have a significant impact on human health and longevity over the past 30 years. Recent studies clearly showed that exposure to particulate matter directly caused adverse effects on the respiratory system via various mechanisms including the accumulation of free radical peroxidation, the imbalance of intercellular calcium regulation, and inflammation, resulting in respiratory diseases. Recent evidence showed the importance of the role of the respiratory microbiome on lung immunity and lung development. In addition, previous studies have confirmed that several chronic respiratory diseases were associated with an alteration in the respiratory microbiome. However, there is still a lack of knowledge with regard to the changes in the respiratory microbiome with regard to the role of particulate matter exposure in respiratory diseases. Therefore, this review aims to summarize and discuss all the in vivo to clinical evidence which investigated the effect of particulate matter exposure on the respiratory microbiome and respiratory diseases. Any contradictory findings are incorporated and discussed. A summary of all these pieces of evidence may offer an insight into a therapeutic approach for the respiratory diseases related to particulate matter exposure and respiratory microbiome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Particulate matter (PM) is one of the global crisis air pollutants associated with the increase in acute and chronic mortality (Chen and Hoek 2020; Lepeule et al. 2012; Liu et al. 2019). PM can be categorized by aerodynamic diameters including: (1) PM10 or coarse PM, which has an aerodynamic diameter equal to or less than 10 μm; (2) PM2.5 or fine PM, which has an aerodynamic diameter equal to or less than 2.5 μm; and (3) PM0.1 or ultrafine PM, which has an aerodynamic diameter equal to or less than 0.1 μm (Donaldson et al. 2001; U.S. EPA 2021). PM0.1 is the smallest particle, which can penetrate most extensively and deeply, and pass directly into the circulation, also being retained in the body for long periods of time (Ophir et al. 2019). PM also contains metals (such as lead, iron, zinc), solvents [such as volatile organic compounds (VOCs), polycyclic aromatic hydrocarbons (PAHs)], and microbes (such as bacteria, fungus, and viruses), which cause many additional adverse health problems (Anderson et al. 2012). Inhalation is the major route of PM uptake, the PM then primarily affecting the respiratory system as the one initially exposed. Recent studies clearly showed that PM directly caused several effects on the respiratory system via various mechanisms including the accumulation of free radicals and an increase in their peroxidation, the imbalance of intercellular calcium regulation, and inflammation (Leikauf et al. 2020). All of those mechanisms can induce respiratory diseases such as asthma, chronic obstructive pulmonary disease (COPD), and lung cancer (Doiron et al. 2019; Hamra et al. 2014; Orellano et al. 2017; Yang et al. 2020).
Previous evidence showed that the respiratory microbiome plays a role on the immunity and development of the lungs (Dickson and Huffnagle 2015; Marsland et al. 2013). Therefore, the imbalance of the respiratory microbiome may cause the pathogenesis of several respiratory diseases. In accordance with this suggestion, several studies confirmed that many chronic respiratory diseases such as asthma, COPD, cystic fibrosis, and lung cancer were associated with a reduction in the diversity of the respiratory microbiome (Dy and Sethi 2016; Hosgood et al. 2019). Significantly, the Proteobacteria phylum and pathogenic genera such as Haemophilus, Streptococcus, and Moraxella were found to be overrepresented in the lungs of patients with COPD and asthma (Dy and Sethi 2016; Sokolowska et al. 2018).
Although there is much evidence to support the role of the respiratory microbiome in the pathogenesis of respiratory diseases, there is still a lack of knowledge regarding its role in the pathogenesis of respiratory diseases which were related to PM exposure. Therefore, this review aims to summarize and discuss all the in vivo to clinical evidence, which showed the effect of PM exposure on the respiratory microbiome related to respiratory diseases. A summary of all this information may provide insight into a therapeutic approach for the pathogenesis of respiratory diseases related to PM exposure and the respiratory microbiome.
Literature review methodology
In this review, the effect of particulate matter on the respiratory microbiome was reported in eight in vivo and ten clinical studies. The search carried out on the PubMed database included the search terms “particulate matter” and “respiratory microbiome”. Only original research articles related to the search criteria were included and published in English in the period 2016 to 2022.
Changes in the respiratory microbiome in relation to physiological and pathological conditions
The respiratory microbiome is characterized by the presence of bacteria, fungi, and other microorganisms in the respiratory tract. Although the respiratory microbiome is colonized during infancy, several factors from the host and environment including birth mode, feeding type, medication, smoking, or infection could cause changes in the composition of the respiratory microbiome (Bogaert et al. 2011, 2004b; Bosch et al. 2016, 2017). The respiratory microbiome can be examined from the nasal cavity to the lungs and is frequently divided into two parts: the upper respiratory tract (URT) and lower respiratory tract (LRT). The respiratory microbiome of the URT comprising the nasal cavity, the nasopharynx and oropharynx, and the portion of the larynx above the vocal cords, while the respiratory microbiome in the LRT comprises the trachea, bronchi, bronchioles, and alveoli. The URT microbiome varies as a consequence of maternal origin and environmental exposure. The LRT microbiome varies as a result of micro-aspiration and translocation of microbes from the URT, and the upper gastrointestinal tract (Bassis et al. 2015; Dickson and Huffnagle 2015). The major phyla of respiratory microbiome in both URT and LRT were Firmicutes, Bacteroidetes, and Proteobacteria (Morris et al. 2013). However, the density, diversity, and characteristics of respiratory microbiome were found to differ between the URT and LRT (Man et al. 2017). The nasal cavity is lined with skin and is dominated by a lipophilic microbiome including Staphylococcus spp., Propionibacterium spp., and Corynebacterium spp. The microbiome of nasopharynx was found to be more diverse than the nasal cavity containing overlap microbiomes from the nasal cavity such as Moraxella spp., Staphylococcus spp., and Corynebacterium spp. Meanwhile the different microbiome between nasopharynx and nasal cavity was Haemophilus spp. and Streptococcus spp. (Bosch et al. 2016; Man et al. 2017) The oropharynx showed the highest density and diverse microbiome with the dominant colonizers being streptococcal species, Neisseria spp., Rothia spp. and anaerobes, including Veillonella spp. and Prevotella spp. (Man et al. 2017). While the LRT microbiome was directly transferred from URTs, especially oropharynx, the composition of microbiome was commonly found in oropharynx including Haemophilus spp., Staphylococcus spp., and Streptococcus spp. (Man et al. 2017).
The respiratory microbiome was found to play a role in protecting the respiratory tract from pathogen colonization and in the development of the respiratory tract and immunity (Bäumler and Sperandio 2016; Bogaert et al. 2004a; Olszak et al. 2012). In normal situations, host–microorganism interaction was the most important mechanism in keeping the balance of respiratory health. The resident respiratory microbiome activated immune cells and released a signal to regulatory cells including alveolar macrophages and regulatory T cells (Sommariva et al. 2020; Zheng et al. 2020). To balance the microbes in the respiratory microbiome, the host responds by releasing antimicrobial peptides and secretory immunoglobulin A (sIgA) (Hapfelmeier et al. 2010; Sommariva et al. 2020). These interactions regulate inflammation and the induction of microbial tolerance and contribute to the normal development and the maintenance of respiratory microbiome communities. Furthermore, there was a microbial inter-compartment cross talk between the respiratory and gastrointestinal tracts, known as the “gut–lung axis (GLA)” (Marsland et al. 2015). In this GLA, short-chain fatty acids, produced by the gut microbiome, could notify antigen-presenting cells in the lungs and cause the activation of lung inflammatory responses (McAleer and Kolls 2018). The GLA was found to be complex and unclear, and the mesenteric lymphatic system might be an important system for the translocation of bacteria, biomarkers, and metabolites into the GLA (Bingula et al. 2017; Marsland et al. 2015; McAleer and Kolls 2018).
It has been hypothesized that the respiratory microbiome might be associated with respiratory disease. Differences between the respiratory microbiome in healthy individuals and chronic pulmonary patients have been found (Dickson et al. 2015). In addition, an increase in potential respiratory pathogens such as S. pneumoniae, H. influenzae, and M. catarrhalis and a decrease in microbial diversity have been observed in association with respiratory tract infection (Laufer et al. 2011; Pettigrew et al. 2012). In asthmatic children, many studies reported the reduction in microbial diversity (Koppen et al. 2015; Kozyrskyj et al. 2007). Furthermore, the pathogenesis of each respiratory disease, such as architectural distortion of the lung in COPD, local immunologic response in the lungs, antibiotic use, biofilm formation, osmotic changes, and thickened mucus in cystic fibrosis, also influenced the respiratory microbiome and led to alterations in the microbial communities (O'Dwyer et al. 2016). Although there is much evidence to support the role of the respiratory microbiome in the pathogenesis of respiratory disease, there is still a lack of knowledge regarding the role of the respiratory microbiome on the pathogenesis of respiratory diseases related to PM exposure. Therefore, the following paragraph summarizes and discusses all the in vivo and clinical evidence concerning the effect of PM exposure on the respiratory microbiome.
Effects of acute exposure to particulate matter on respiratory microbiomes: reports from in vivo studies
Two studies have reported the effects of acute exposure to particulate matter on respiratory microbiomes. The first study investigated the impact of various concentrations of PM2.5 exposure on the lung microbiome in a mice model (Li et al. 2020). Interestingly, only exposure to medium (5.4 mg/kg) and high concentrations of PM2.5 (16.2 mg/kg), but not to a low concentration of PM2.5 (1.8 mg/kg) caused changes in the respiratory microbiome as indicated by increased α-diversity, Bacteroidetes, Cyanobacteria, and Firmicutes, and a decrease in Proteobacteria in the lungs (Li et al. 2020). The second study investigated the acute effect of PM on the lung microbiome via exposure to total suspended particles (TSP) into the broilers (Shen et al. 2022). Both doses of TSP exposure (4 and 8 mg/m3) increased α-diversity, β-diversity, Epsilonbacteraeota, Tenericutes, Firmicutes and decreased Bacteroidetes, Acidobacteria, Planctomycetes, and Gemmatimonadetes in the lung tissue of broilers (Shen et al. 2022). Both findings suggested that acute exposure to PM increased microbial α-diversity and perturbed the bacterial profile, major profile changes being in Firmicutes and Bacteroidetes. Firmicutes increased in both studies, but Bacteroidetes increased in the lungs of mice and decreased in those of broilers. The differences between the changes in the respiratory microbiomes in broilers and mice may depend on the species. The dominant phyla in broiler's lungs in order of prevalence were found to be Firmicutes, Bacteroidetes, and Proteobacteria, respectively (Shen et al. 2022), while the major phylum in the mouse lungs was Proteobacteria, followed by Bacteroidetes, Cyanobacteria, and Firmicutes, respectively (Chen et al. 2020). This could explain that the difference in the dominant respiratory microbiome between species may depend on the source of PM. The source of PM from indoor farms might input microbes as a result of aerosolization of animal feces, feedstuff, skin, and feather fragments (Dai et al. 2020); therefore, the microbial composition from indoor PM could differ from the standardized PM.
Both studies reported the effects of acute exposure to particulate matter on lung inflammation and lung metabolites. In the mice model, all doses of exposure to PM2.5 (1.8, 5.4, 16.2 mg/kg) caused lung inflammation as indicated by increased inflammatory cell infiltration into the lungs and an increase in inflammatory cytokines such as interleukin (IL)-1β, IL-6, IL-8, IL-17, and tumor necrosis factor alpha (TNF-α) in both bronchoalveolar lavage fluid (BALF) and serum (Li et al. 2020). In addition, only exposure to medium (5.4 mg/kg) and high concentrations of PM2.5 (16.2 mg/kg) decreased lung metabolites such as valine, L-isoleucine, acetic acid, caproic acid, fumaric acid, and valeric acid (Li et al. 2020). In broilers, both doses of TSP exposure (4 and 8 mg/m3) also increased inflammatory cytokines (IL-8) in the lungs, indicating lung inflammation (Shen et al. 2022). Both doses of TSP exposure also increased some lung metabolites (mostly in the carnitine category) and decreased some lung metabolites (crenolanib, dibutyl phthalate) in broilers (Shen et al. 2022). All of these findings demonstrated that acute exposure to PM altered the composition of the lung microbiome, affecting pulmonary inflammation and pulmonary metabolism. All of these findings are summarized in Table 1. The possible mechanisms involved in the induction of lung pathology as a result of acute PM exposure are summarized in Fig. 1.
Acute effects of PM2.5 and TSP on pulmonary parameters and respiratory microbiomes. Once PM2.5 and TSP have built up in the respiratory tract, TSP and PM2.5 have a bi-directional effect on the microbiomes at the phyla level, causing both increases and decreases. TSP and PM2.5 also caused elevation of the inflammatory markers in BALF. There is a correlation between pulmonary inflammatory markers and respiratory microbiomes. Increased inflammatory processes led to morphologic alterations including cell infiltration, septal thickening, and fibrosis. BALF bronchoalveolar lavage fluid, IL interleukin, PM2.5 particulate matter 2.5, TNF tumor necrosis factor, TSP total suspended particulate
Effects of chronic exposure to particulate matter on respiratory microbiomes: reports from in vivo studies
Five studies reported on the effects of chronic exposure to particulate matter on respiratory microbiomes (Table 2). Two studies investigated PM2.5 exposure via intratracheal instillation, and the other three studies investigated PM2.5 exposure via inhalation. In the case of PM2.5 exposure via intratracheal instillation, 200 μg of PM 2.5 exposure for 3 weeks increased β-diversity, but decreased α-diversity in a mouse model (Chen et al. 2020). In addition, the alterations in bacterial profiles were observed in BALF, as indicated by decreasing Streptococcus and Prevotella and an increase in Lachnoanaerobaculum, Peptoniphilus, and Actinomyces. These changes in the respiratory microbiomes also caused changes in the lung morphometry as indicated by increased inflammatory cell infiltration around the bronchi of mice exposed to PM2.5 for 3 weeks (Chen et al. 2020). Interestingly, exposure to PM2.5 for 4 weeks via intratracheal instillation did not affect the changes in the microbial diversity in BALF of SCID mice (Yang and Xiao 2018). However, PM2.5 exposure resulted in increases in the Cyanobacteria phyla, Streptococcaceae family, and Carnobacterium genus and decreased Bifidobacterium, Propionibacterium, and Campyrobacter in BALF of SCID mice (Yang and Xiao 2018). Four weeks of PM exposure also caused changes in the lung morphometry as indicated by an increase in lung cancer tumors, tumor nodules, expression of IL-1β, matrix metalloproteinase-1 (MMP1), and vascular endothelial growth factor (VEGF) (Yang and Xiao 2018).
With regard to PM2.5 exposure via inhalation, the study into ambient exposure to PM2.5 for 8 weeks showed a rise in β-diversity and changes in microbial profiles in BALF of mice as indicated by an increase in some microbiomes profiles (Proteobacteria, Tenericutes, Planctomycetes, and Acidobacteria) and a decrease in others (Firmicutes, Bacteroidetes, Actinobacteria, Chloroflexi, and Euryarchaeota) (Ran et al. 2021). In addition, 8 weeks of exposure to ambient PM2.5 caused severe changes in the lung morphometry in a mouse model as indicated by bronchopneumonia, interstitial pneumonitis, thickening of the alveolae, and pulmonary edema (Ran et al. 2021). These changes were associated with an increment in inflammatory markers in the lung, as indicated by increases in IL-6, IL-1β, TNF-α, mast cell protease-1(Mcpt-1), and immunity-related GTPase (IRGM) (Ran et al. 2021). In addition, the changes in lung morphometry in PM2.5-treated mice were related to the imbalance of pulmonary metabolism which were related to an imbalance in energy consumption, including in the pyruvate pathway, glutamine pathway, lipid metabolism, and choline metabolism (Ran et al. 2021).
All of these findings suggested that the route of administration, dose, and duration of exposure to PM2.5 affected the changes of the respiratory microbiome such as microbiome diversity and microbiome profiles in the respiratory system. Moreover, the differences in the route of administration, dose, and duration of exposure to PM2.5 also caused a dose–response relationship of respiratory parameters. Intratracheal instillation, low dose (200 μg/2 day/week or 20 μl/twice/week), and short term (3–4 weeks) of PM2.5 exposure altered some immune responses and some pathological changes in the lung. However, by inhalation, high dose (226.50 mg/m3, 8 h/day, 7 days/week), and long term (8 weeks) of PM2.5 exposure aggravated these pulmonary immune responses and pathological conditions.
In addition to the study regarding only the exposure to PM2.5, a study into chronic exposure to PM10, PM 2.5, and PM1 from biomass fuel (BMF) for 4 weeks also showed alterations in the respiratory microbiome in rats, specifically increased α-diversity, Clostridiaceae, Ruminococcaceae, Hyphomonadaceae, and Veillonellaceae (Li et al. 2017). Chronic exposure to BMF also stimulated the lung immune responses as indicated by increased levels of IgA, IgG, and macrophages in the BALF of rats (Li et al. 2017). In addition, chronic exposure to motor vehicle exhaust (MVE), which contained PM10, PM 2.5 and PM1, increased α-diversity, Clostridiaceae, Brocadiaceae, Hyphomonadaceae Planococcaceae, Hyphomicrobiaceae, and Veillonellaceae in the BALF of rats (Li et al. 2017). However, decreases in the bacterial profiles of Aerococcaceae, Pseudomonadaceae, Comamonadaceae, Oxalobacteraceae, and Caulobacteraceae were observed in the BALF of rats exposed to MVE (Li et al. 2017). In addition, only IgA was increased in the BALF of rats exposed to MVE (Li et al. 2017). All of these findings suggested that the exposure to BMF and MVE generated different respiratory microbiomes and consequently caused different respiratory immune responses. A possible explanation of the different responses of the respiratory microbiome and respiratory immune response between exposure to BMF and MVE could be due to the differences in sources and concentration of PM between BMF and MVE. In addition, BMF exposure contained higher PM (PM10, PM2.5, and PM1) and gas concentrations (NO1, NOx, and CO) than MVE. Therefore, the respiratory immune responses in the BMF exposed-group were higher than in the MVE exposed-group.
It has been found that the change of microbiome is affected by several factors, such as environmental stimuli and diet consumption (Ahn and Hayes 2021; Leeming et al. 2019). Several studies confirmed that consumption of a high-fat diet altered the gut microbiota and consequently caused inflammation in several organs (Mulders et al. 2018; Silva Figueiredo et al. 2017; Wypych et al. 2017). However, only one study investigated the comparative effect of PM exposure between mice consuming whether a high-fat diet (HFD) or low-fat diet (LFD) (Daniel et al. 2021). Daniel and colleagues found that 30 days of PM exposure from diesel exhaust did not cause any changes in the respiratory microbiomes of LFD-fed mice (Daniel et al. 2021). Interestingly, 30 days of PM exposure from diesel exhaust decreased Firmicutes and Bacteroidetes in BALF of HFD-fed mice (Daniel et al. 2021). Although PM exposure activated lung immune responses and the changes in lung morphology in both LFD- and HFD-fed mice, PM exposure aggravated systemic neutrophils, systemic lymphocytes, lung nitrotyrosine, lung monocytes, and macrophages in HFD-fed mice. The findings of this study suggested that PM exposure altered the respiratory microbiome and consequently aggravated lung immune responses, leading to changes in the lung morphology in HFD-fed mice.
In the gut–lung axis, the gastrointestinal system is connected to the respiratory system by the mesenteric lymphatic system, which leads to the translocation of the microbiome, metabolites, and mediators between both systems (Enaud et al. 2020). Therefore, we speculated that this aggravative effect of the respiratory microbiome and lung immune responses in HFD-fed rats with PM exposure may be caused by the translocation of microbiomes, metabolites, and mediators from the gastrointestinal system.
All of these findings demonstrate that chronic exposure to PM altered the composition of the lung microbiome, which leads to pulmonary inflammation and lung morphometry. Furthermore, chronic PM exposure also aggravates these changes in the HFD-fed condition. The possible mechanisms of chronic PM exposure inducing these pathologies are summarized in Fig. 2.
Chronic effects of PM2.5 exposure on pulmonary parameters and respiratory microbiomes. PM2.5 exposure influences the microbiomes in both an upward and a downward direction. In addition, PM2.5 exposure increased inflammatory markers in both blood and BALF. Consumption of an HFD aggravated the increase of respiratory microbial profiles (Bacteroidetes and Firmicutes), which consequently aggravated white blood cells and nitrotyrosine in BALF. All of this cause an increase in inflammatory processes which led to morphological alterations such as cell infiltration, alveolar thickening, and pneumonitis. BALF bronchoalveolar lavage fluid, IL interleukin, BALF bronchoalveolar lavage fluid, HFD high-fat diet, Ig Immunoglobulin, IL interleukin, MΦ macrophages, ROS–RNS reactive oxygen species and reactive nitrogen species, PM particulate matter, TNF tumor necrosis factor, TSP total suspended particulate
Effects of particulate matter on respiratory microbiomes: reports from clinical studies
Ten studies reported on the effects of PM exposure on the respiratory microbiome. Three studies performed their research on children and infants, which demonstrated a reduction in microbial diversity and changes in the respiratory microbiome following PM exposure (Gisler et al. 2021; Li et al. 2021; Wu et al. 2021). In elementary school children, ambient PM exposure caused changes in the respiratory microbiome, as indicated by a reduction in α-diversity, an increase in some bacterial profiles (Proteobacteria, Streptococcus, Gemella), and a decrease in others (Fusobacteria and Actinomyces) (Li et al. 2021; Wu et al. 2021). In infants, the ambient PM exposure caused the changes in the respiratory microbiome, as indicated by a reduction in β-diversity and Corynebacteriaceae (Gisler et al. 2021). PM exposure not only caused respiratory microbiome changes, but also increased lung inflammation and reduced lung function in elementary school children, as indicated by increased lung inflammatory response including fractional exhaled nitric oxide (FeNO) and increased TNF-α, reduced forced expiratory volume (FEV), and diminished forced vital capacity (FVC) (Li et al. 2021; Wu et al. 2021).
In contrast to the studies in children and infants, the studies into the respiratory microbiome in adults showed divergent findings. A study on vendors in an open-air market reported changes in the respiratory microbiome of an increase in α-diversity, β-diversity, bacterial phyla (Firmicutes, Fusobacteria, Actinobacter), and many types of bacterial genus after acute exposure to ambient PM (Qin et al. 2019). In healthy subjects, Rylance and colleagues reported non-significant diversity changes, but presented differences in lung microbiome profiles with an increase in Neisseria and Streptococcus, and a decrease in Tropheryma in BALF of non-smoking adults (Rylance et al. 2016). However, Mariani and colleagues reported that short-term exposure to PM decreased α- diversity in healthy subjects (Mariani et al. 2021). In addition, these changes in microbial diversity were only associated with PM2.5 exposure, but not with PM10 exposure (Mariani et al. 2021). In contrast to short-term exposure, long-term exposure to both PM2.5 and PM10 increased Lachnospiraceae and Ruminococcaceae, while decreasing Prevotellaceae, Veillonellaceae, Porphyromonadaceae, Fusobacteriaceae, Paraprevollaceae, and Flavobacteriaceae in throat swabs of healthy subjects (Li et al. 2019). Furthermore, these changes in microbial profiles were associated with the reduction of lung function in healthy subjects, as indicated by decreased FVC and peak expiratory flow rate (PEFR) (Li et al. 2019). The findings from healthy subjects suggested that PM exposure caused changes in the respiratory microbiome with consequential stimulation of lung inflammation and impaired lung function.
Several studies demonstrated that PM exposure also affected the respiratory microbiome in patients with respiratory disorders (Hosgood et al. 2019; Mariani et al. 2021; Smit et al. 2017; Wang et al. 2019). In allergic rhinitis, only exposure to PM2.5, but not PM10 increased α-diversity in allergic rhinitis subjects (Mariani et al. 2021). In addition, exposure to PM2.5 also increased α-diversity in participants with chronic obstructive pulmonary disease (COPD) risk (Wang et al. 2019). In non-smoking female lung cancer patients, PM exposure also increased α-diversity without any change in microbial profiles (Hosgood et al. 2019). Furthermore, a study into hospitalized community-acquired pneumonia patients showed an increase in S. pneumoniae and a decrease in Lactobacillus samples taken from oropharyngeal swabs (Smit et al. 2017). All of these clinical findings suggested PM exposure was related to the changes in the respiratory microbiome in both the normal flora and the potential pathogens, both of which may decline pulmonary function in patients.
Although clinical findings showed PM exposure was related to the changes in the respiratory microbiome, the microbial diversity and the microbial profiles in each study were different. A possible elucidation of this different response of respiratory microbiome could be due to the differences in the source of the PM (ambient pollutant, indoor pollutant, or fuel combustion), the host (infants, children, adult, healthy, or patient), site of sample collection (saliva, buccal, nasal, or BALF), and the type of study design (case–control, cross-sectional, or cohort).
Different sources of PM were found to affect microbial profiles in different ways. The major sources of outdoor PM were from transportation, biomass burning, construction, and industrial usage, the major source of indoor PM being from household fuel combustion. According to this information, microbes associated with outdoor PM mainly came from the soil, water, and marine sources, while microbes associated with indoor PM were found to be specific microbes related to a specific place or source (Cao et al. 2014). For example, household smoky coal, which represents a major indoor PM source, was associated with Fusobacterium, whereas a biomass fuel, which represents a major outdoor PM source, was associated with Petrobacteria (Hosgood et al. 2019; Rylance et al. 2016).
The host was also the other factors that affected the respiratory microbiome. The determinants of lung bacterial colonization in children were related to the mode of delivery and feeding type (Bosch et al. 2017, 2016). Furthermore, children were more susceptible to respiratory effects than adults, due to their lungs being less well developed in comparison to adults (Burtscher and Schuepp 2012). In addition, the respiratory airways of children are smaller in diameter and shorter in length, and there is a higher respiratory demand, which may cause high exposure to PM. In adults, healthy individuals and respiratory disease patients also had different microbial communities. Subjects with lung cancer and allergic rhinitis had lower microbial diversity compared with healthy matched controls (Hosgood et al. 2019; Mariani et al. 2021). A possible elucidation of these changes may relate to medication. Medication, especially antibiotics or steroids, was one of the factors that had an impact in the case of respiratory disease patients (Bosch et al. 2017): patients taking medication may have a lower microbial diversity in comparison with healthy subjects.
The site of sample collection was also important; the characteristics and quantity of normal flora differed depending on the site of the respiratory tract. The nasal cavity was the first point of contact to PM; therefore, it had a higher chance of being disrupted by all types of PM. Furthermore, the method of specimen collection was also important. The collection of LRTs samples could be performed by BALF and from sputum; however, even though sputum collection was less invasive it had higher chance of contamination by URTs material which may affect the results (Wang et al. 2019).
Although several pieces of evidence confirmed that PM exposure could cause changes in the respiratory microbiome, the mechanism regarding this effect is still unclear. One of the most common mechanisms related to inflammation, PM directly activated immunity, disrupted the balance of normal flora, and finally caused the changes of respiratory microbiome. PM exposure itself activates the inflammatory process and recruits alveolar macrophages to eliminate PM. Then alveolar macrophages have a reduced capacity to engulf bacteria, which results in the rapid growth of Streptococcus pneumoniae and results in an increase in the abundance of Streptococcus (Rylance et al. 2015). Another mechanism associated with PM exposure that affected the respiratory microbiome was via the indirect effect of epithelial cell damage, which lost the permeability properties as a result of an increase in the inflammatory process (Jungnickel et al. 2015). After a change in airway permeability, the airway defense mechanism was found to be disrupted, and it consequently released more antimicrobial peptides and mucus which caused an imbalance in the host–microbiome interaction (Hiemstra et al. 2015). Even though several studies showed an association between PM exposure and the microbial community, respiratory metabolism, and inflammation, findings were inconclusive regarding the determination of the causal pathway of these findings due to limitations of the studies. Therefore, additional studies are needed to investigate more fully the mechanisms of PM induction changes of respiratory microbiome. All of the findings of these studies are summarized in Table 3.
Conclusion
Both acute and chronic PM exposure can affect the respiratory microbiome. Changes in microbial diversity and abundance have been associated with respiratory disease; therefore, it may be helpful in the future to use the respiratory microbiome as a predictor of respiratory diseases. PM exposure also caused pulmonary inflammation and pulmonary metabolism, which may interact or enhance with the respiratory microbiome to progress into respiratory diseases. Further studies should investigate the association between PM exposure, the respiratory microbiome, and various pulmonary parameters to understand more about the pathophysiology of respiratory diseases following exposure to air pollutants.
Data availability
Data will be made available on request.
References
Ahn J, Hayes RB (2021) Environmental influences on the human microbiome and implications for noncommunicable disease. Annu Rev Public Health 42:277–292. https://doi.org/10.1146/annurev-publhealth-012420-105020
Anderson JO, Thundiyil JG, Stolbach A (2012) Clearing the air: a review of the effects of particulate matter air pollution on human health. J Med Toxicol 8(2):166–175. https://doi.org/10.1007/s13181-011-0203-1
Bassis CM, Erb-Downward JR, Dickson RP et al (2015) Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. Mbio 6(2):e00037. https://doi.org/10.1128/mBio.00037-15
Bäumler AJ, Sperandio V (2016) Interactions between the microbiota and pathogenic bacteria in the gut. Nature 535:85–93
Bingula R, Filaire M, Radosevic-Robin N et al (2017) Desired turbulence? Gut-lung axis, immunity, and lung cancer. J Oncol 2017:5035371. https://doi.org/10.1155/2017/5035371
Bogaert D, De Groot R, Hermans PW (2004a) Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis 4(3):144–154. https://doi.org/10.1016/S1473-3099(04)00938-7
Bogaert D, van Belkum A, Sluijter M et al (2004b) Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet 363(9424):1871–1872. https://doi.org/10.1016/S0140-6736(04)16357-5
Bogaert D, Keijser B, Huse S et al (2011) Variability and diversity of nasopharyngeal microbiota in children: a metagenomic analysis. PLoS ONE 6(2):e17035. https://doi.org/10.1371/journal.pone.0017035
Bosch A, Levin E, van Houten MA et al (2016) Development of upper respiratory tract microbiota in infancy is affected by mode of delivery. EBioMedicine 9:336–345. https://doi.org/10.1016/j.ebiom.2016.05.031
Bosch A, de Steenhuijsen Piters WAA, van Houten MA et al (2017) Maturation of the infant respiratory microbiota, environmental drivers, and health consequences. A prospective cohort study. Am J Respir Crit Care Med 196(12):1582–1590. https://doi.org/10.1164/rccm.201703-0554OC
Burtscher H, Schuepp K (2012) The occurrence of ultrafine particles in the specific environment of children. Paediatr Respir Rev 13(2):89–94. https://doi.org/10.1016/j.prrv.2011.07.004
Cao C, Jiang W, Wang B et al (2014) Inhalable microorganisms in Beijing’s PM2.5 and PM10 pollutants during a severe smog event. Environ Sci Technol 48(3):1499–1507. https://doi.org/10.1021/es4048472
Chen J, Hoek G (2020) Long-term exposure to PM and all-cause and cause-specific mortality: a systematic review and meta-analysis. Environ Int 143:105974. https://doi.org/10.1016/j.envint.2020.105974
Chen YW, Li SW, Lin CD et al (2020) Fine particulate matter exposure alters pulmonary microbiota composition and aggravates Pneumococcus-induced lung pathogenesis. Front Cell Dev Biol 8:570484. https://doi.org/10.3389/fcell.2020.570484
Dai P, Shen D, Tang Q, Huang K, Li C (2020) PM2.5 from a broiler breeding production system: the characteristics and microbial community analysis. Environ Pollut 256:113368. https://doi.org/10.1016/j.envpol.2019.113368
Daniel S, Phillippi D, Schneider LJ, Nguyen KN, Mirpuri J, Lund AK (2021) Exposure to diesel exhaust particles results in altered lung microbial profiles, associated with increased reactive oxygen species/reactive nitrogen species and inflammation, in C57Bl/6 wildtype mice on a high-fat diet. Part Fibre Toxicol 18(1):3. https://doi.org/10.1186/s12989-020-00393-9
Dickson RP, Huffnagle GB (2015) The lung microbiome: new principles for respiratory bacteriology in health and disease. PLoS Pathog 11(7):e1004923. https://doi.org/10.1371/journal.ppat.1004923
Dickson RP, Erb-Downward JR, Freeman CM et al (2015) Spatial Variation in the healthy human lung microbiome and the adapted island model of lung biogeography. Ann Am Thorac Soc 12(6):821–830. https://doi.org/10.1513/AnnalsATS.201501-029OC
Doiron D, de Hoogh K, Probst-Hensch N et al (2019) Air pollution, lung function and COPD: results from the population-based UK Biobank study. Eur Respir J. https://doi.org/10.1183/13993003.02140-2018
Donaldson K, Stone V, Clouter A, Renwick L, MacNee W (2001) Ultrafine particles. Occup Environ Med 58(3):211–216. https://doi.org/10.1136/oem.58.3.211
Dy R, Sethi S (2016) The lung microbiome and exacerbations of COPD. Curr Opin Pulm Med 22(3):196–202. https://doi.org/10.1097/MCP.0000000000000268
Enaud R, Prevel R, Ciarlo E et al (2020) The gut-lung axis in health and respiratory diseases: a place for inter-organ and inter-kingdom crosstalks. Front Cell Infect Microbiol 10:9. https://doi.org/10.3389/fcimb.2020.00009
Gisler A, Korten I, de Hoogh K et al (2021) Associations of air pollution and greenness with the nasal microbiota of healthy infants: a longitudinal study. Environ Res 202:111633. https://doi.org/10.1016/j.envres.2021.111633
Hamra GB, Guha N, Cohen A et al (2014) Outdoor particulate matter exposure and lung cancer: a systematic review and meta-analysis. Environ Health Perspect 122(9):906–911. https://doi.org/10.1289/ehp.1408092
Hapfelmeier S, Lawson MA, Slack E et al (2010) Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science 328(5986):1705–1709. https://doi.org/10.1126/science.1188454
Hiemstra PS, McCray PB Jr, Bals R (2015) The innate immune function of airway epithelial cells in inflammatory lung disease. Eur Respir J 45(4):1150–1162. https://doi.org/10.1183/09031936.00141514
Hosgood HD 3rd, Mongodin EF, Wan Y et al (2019) The respiratory tract microbiome and its relationship to lung cancer and environmental exposures found in rural china. Environ Mol Mutagen 60(7):617–623. https://doi.org/10.1002/em.22291
Jungnickel C, Wonnenberg B, Karabiber O et al (2015) Cigarette smoke-induced disruption of pulmonary barrier and bacterial translocation drive tumor-associated inflammation and growth. Am J Physiol Lung Cell Mol Physiol 309(6):L605–L613. https://doi.org/10.1152/ajplung.00116.2015
Koppen IJN, Bosch A, Sanders EAM, van Houten MA, Bogaert D (2015) The respiratory microbiota during health and disease: a paediatric perspective. Pneumonia (nathan) 6:90–100. https://doi.org/10.15172/pneu.2015.6/656
Kozyrskyj AL, Ernst P, Becker AB (2007) Increased risk of childhood asthma from antibiotic use in early life. Chest 131(6):1753–1759. https://doi.org/10.1378/chest.06-3008
Laufer AS, Metlay JP, Gent JF, Fennie KP, Kong Y, Pettigrew MM (2011) Microbial communities of the upper respiratory tract and otitis media in children. Mbio 2(1):e00245-e310. https://doi.org/10.1128/mBio.00245-10
Leeming ER, Johnson AJ, Spector TD, Le Roy CI (2019) Effect of diet on the gut microbiota: rethinking intervention duration. Nutrients. https://doi.org/10.3390/nu11122862
Leikauf GD, Kim SH, Jang AS (2020) Mechanisms of ultrafine particle-induced respiratory health effects. Exp Mol Med 52(3):329–337. https://doi.org/10.1038/s12276-020-0394-0
Lepeule J, Laden F, Dockery D, Schwartz J (2012) Chronic exposure to fine particles and mortality: an extended follow-up of the Harvard Six Cities study from 1974 to 2009. Environ Health Perspect 120(7):965–970. https://doi.org/10.1289/ehp.1104660
Li N, He F, Liao B, Zhou Y, Li B, Ran P (2017) Exposure to ambient particulate matter alters the microbial composition and induces immune changes in rat lung. Respir Res 18(1):143. https://doi.org/10.1186/s12931-017-0626-6
Li X, Sun Y, An Y et al (2019) Air pollution during the winter period and respiratory tract microbial imbalance in a healthy young population in Northeastern China. Environ Pollut 246:972–979. https://doi.org/10.1016/j.envpol.2018.12.083
Li J, Hu Y, Liu L, Wang Q, Zeng J, Chen C (2020) PM2.5 exposure perturbs lung microbiome and its metabolic profile in mice. Sci Total Environ 721:137432. https://doi.org/10.1016/j.scitotenv.2020.137432
Li H, Xu D, Li H et al (2021) Exposure to ultrafine particles and oral flora, respiratory function, and biomarkers of inflammation: a panel study in children. Environ Pollut 273:116489. https://doi.org/10.1016/j.envpol.2021.116489
Liu C, Chen R, Sera F et al (2019) Ambient particulate air pollution and daily mortality in 652 cities. N Engl J Med 381(8):705–715. https://doi.org/10.1056/NEJMoa1817364
Man WH, de Steenhuijsen Piters WA, Bogaert D (2017) The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat Rev Microbiol 15(5):259–270. https://doi.org/10.1038/nrmicro.2017.14
Mariani J, Iodice S, Cantone L et al (2021) Particulate matter exposure and allergic rhinitis: the role of plasmatic extracellular vesicles and bacterial nasal microbiome. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph182010689
Marsland BJ, Yadava K, Nicod LP (2013) The airway microbiome and disease. Chest 144(2):632–637. https://doi.org/10.1378/chest.12-2854
Marsland BJ, Trompette A, Gollwitzer ES (2015) The gut-lung axis in respiratory disease. Ann Am Thorac Soc 12(Suppl 2):S150–S156. https://doi.org/10.1513/AnnalsATS.201503-133AW
McAleer JP, Kolls JK (2018) Contributions of the intestinal microbiome in lung immunity. Eur J Immunol 48(1):39–49. https://doi.org/10.1002/eji.201646721
Morris A, Beck JM, Schloss PD et al (2013) Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am J Respir Crit Care Med 187(10):1067–1075. https://doi.org/10.1164/rccm.201210-1913OC
Mulders RJ, de Git KCG, Schele E, Dickson SL, Sanz Y, Adan RAH (2018) Microbiota in obesity: interactions with enteroendocrine, immune and central nervous systems. Obes Rev 19(4):435–451. https://doi.org/10.1111/obr.12661
O’Dwyer DN, Dickson RP, Moore BB (2016) The lung microbiome, immunity, and the pathogenesis of chronic lung disease. J Immunol 196(12):4839–4847. https://doi.org/10.4049/jimmunol.1600279
Olszak T, An D, Zeissig S et al (2012) Microbial exposure during early life has persistent effects on natural killer T cell function. Science 336(6080):489–493
Ophir N, Shai AB, Korenstein R, Kramer MR (2019) Functional, inflammatory and interstitial impairment due to artificial stone dust ultrafine particles exposure. Occup Environ Med 76:875–879. https://doi.org/10.1136/oemed-2019-105711
Orellano P, Quaranta N, Reynoso J, Balbi B, Vasquez J (2017) Effect of outdoor air pollution on asthma exacerbations in children and adults: Systematic review and multilevel meta-analysis. PLoS ONE 12(3):e0174050. https://doi.org/10.1371/journal.pone.0174050
Pettigrew MM, Laufer AS, Gent JF, Kong Y, Fennie KP, Metlay JP (2012) Upper respiratory tract microbial communities, acute otitis media pathogens, and antibiotic use in healthy and sick children. Appl Environ Microbiol 78(17):6262–6270. https://doi.org/10.1128/AEM.01051-12
Qin T, Zhang F, Zhou H et al (2019) High-level PM2.5/PM10 exposure is associated with alterations in the human pharyngeal microbiota composition. Front Microbiol 10:54. https://doi.org/10.3389/fmicb.2019.00054
Ran Z, An Y, Zhou J et al (2021) Subchronic exposure to concentrated ambient PM2.5 perturbs gut and lung microbiota as well as metabolic profiles in mice. Environ Pollut 272:115987. https://doi.org/10.1016/j.envpol.2020.115987
Rylance J, Fullerton DG, Scriven J et al (2015) Household air pollution causes dose-dependent inflammation and altered phagocytosis in human macrophages. Am J Respir Cell Mol Biol 52(5):584–593. https://doi.org/10.1165/rcmb.2014-0188OC
Rylance J, Kankwatira A, Nelson DE et al (2016) Household air pollution and the lung microbiome of healthy adults in Malawi: a cross-sectional study. BMC Microbiol 16(1):182. https://doi.org/10.1186/s12866-016-0803-7
Shen D, Guo Z, Huang K et al (2022) Inflammation-associated pulmonary microbiome and metabolome changes in broilers exposed to particulate matter in broiler houses. J Hazard Mater 421:126710. https://doi.org/10.1016/j.jhazmat.2021.126710
Silva Figueiredo P, Carla Inada A, Marcelino G et al (2017) Fatty acids consumption: the role metabolic aspects involved in obesity and its associated disorders. Nutrients. https://doi.org/10.3390/nu9101158
Smit LAM, Boender GJ, de Steenhuijsen Piters WAA et al (2017) Increased risk of pneumonia in residents living near poultry farms: does the upper respiratory tract microbiota play a role? Pneumonia (nathan) 9:3. https://doi.org/10.1186/s41479-017-0027-0
Sokolowska M, Frei R, Lunjani N, Akdis CA, O’Mahony L (2018) Microbiome and asthma. Asthma Res Pract 4:1. https://doi.org/10.1186/s40733-017-0037-y
Sommariva M, Le Noci V, Bianchi F et al (2020) The lung microbiota: role in maintaining pulmonary immune homeostasis and its implications in cancer development and therapy. Cell Mol Life Sci 77(14):2739–2749. https://doi.org/10.1007/s00018-020-03452-8
U.S. EPA (2021) Particulate matter (PM) basics. In: https://www.epa.gov/pm-pollution/particulate-matter-pm-basics Accessed 23 May 2022
Wang L, Cheng H, Wang D et al (2019) Airway microbiome is associated with respiratory functions and responses to ambient particulate matter exposure. Ecotoxicol Environ Saf 167:269–277. https://doi.org/10.1016/j.ecoenv.2018.09.079
Wu Y, Li H, Xu D et al (2021) Associations of fine particulate matter and its constituents with airway inflammation, lung function, and buccal mucosa microbiota in children. Sci Total Environ 773:145619. https://doi.org/10.1016/j.scitotenv.2021.145619
Wypych TP, Marsland BJ, Ubags NDJ (2017) The impact of diet on immunity and respiratory diseases. Ann Am Thorac Soc 14(Supplement_5):S339–S347. https://doi.org/10.1513/AnnalsATS.201703-255AW
Yang B, Xiao C (2018) PM2.5 exposure significantly improves the exacerbation of A549 tumor-bearing CB17-SCID mice. Environ Toxicol Pharmacol 60:169–175. https://doi.org/10.1016/j.etap.2018.04.025
Yang JW, Shen YC, Lin KC et al (2020) Organ-on-a-chip: opportunities for assessing the toxicity of particulate matter. Front Bioeng Biotechnol 8:519. https://doi.org/10.3389/fbioe.2020.00519
Zheng D, Liwinski T, Elinav E (2020) Interaction between microbiota and immunity in health and disease. Cell Res 30(6):492–506. https://doi.org/10.1038/s41422-020-0332-7
Acknowledgements
This work was supported by the Distinguished Research Professor Grant from the National Research Council of Thailand (SCC); the NSTDA Research Chair grant from the National Science and Technology Development Agency Thailand (NC); the Chiang Mai University Center of Excellence Award (NC); and the National Research Council of Thailand [NRCT: N41A640146 (WP)].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Panumasvivat, J., Pratchayasakul, W., Sapbamrer, R. et al. The possible role of particulate matter on the respiratory microbiome: evidence from in vivo to clinical studies. Arch Toxicol 97, 913–930 (2023). https://doi.org/10.1007/s00204-023-03452-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-023-03452-0