Abstract
Snake venoms are heterogeneous mixtures of proteins and peptides used for prey subjugation. With modern proteomics there has been a rapid expansion in our knowledge of snake venom composition, resulting in the venom proteomes of 30% of vipers and 17% of elapids being characterised. From the reasonably complete proteomic coverage of front-fanged snake venom composition (179 species—68 species of elapids and 111 species of vipers), the venoms of vipers and elapids contained 42 different protein families, although 18 were only reported in < 5% of snake species. Based on the mean abundance and occurrence of the 42 protein families, they can be classified into 4 dominant, 6 secondary, 14 minor, and 18 rare protein families. The dominant, secondary and minor categories account for 96% on average of a snake’s venom composition. The four dominant protein families are: phospholipase A2 (PLA2), snake venom metalloprotease (SVMP), three-finger toxins (3FTx), and snake venom serine protease (SVSP). The six secondary protein families are: L-amino acid oxidase (LAAO), cysteine-rich secretory protein (CRiSP), C-type lectins (CTL), disintegrins (DIS), kunitz peptides (KUN), and natriuretic peptides (NP). Venom variation occurs at all taxonomic levels, including within populations. The reasons for venom variation are complex, as variation is not always associated with geographical variation in diet. The four dominant protein families appear to be the most important toxin families in human envenomation, being responsible for coagulopathy, neurotoxicity, myotoxicity and cytotoxicity. Proteomic techniques can be used to investigate the toxicological profile of a snake venom and hence identify key protein families for antivenom immunorecognition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Snakes have been a source of fascination and symbolism since ancient times, being associated with rituals, good and evil mythology, fertility and healing. The snake has been on the medical emblem for over two millennia, based on the Greek God of healing and medicine Asclepius, who held a staff with a snake coiled around it (Antoniou et al. 2011). The advance of science has overtaken centuries of superstition about snakes, and snake venom research now spans the medical, pharmacological, ecological and molecular evolutionary domains (Chiappinelli 1983; Dutertre and Lewis 2010; Giorgianni et al. 2020; Oliveira et al. 2022a; Perry et al. 2022; Saviola et al. 2014; Whittington et al. 2018), with an exponential increase in publications over the last century (Sofyantoro et al. 2022).
The study of snake venom is a multi-disciplinary endeavour encompassing a diverse range of research areas from the therapeutic potential of toxins and drug discovery (Kalita et al. 2021; Li et al. 2018; Omidi et al. 2021; Urra and Araya-Maturana 2022), antivenom development and the treatment of snakebite (de Silva et al. 2016b, c, d, a; Fry et al. 2003; Gutiérrez et al. 2011; Harrison et al. 2011; Maduwage et al. 2015; Visser et al. 2008), pathophysiology of envenomation (Maduwage et al. 2013; Mamede et al. 2020; Silva et al. 2016b, 2016c), using toxins as physiological probes to better understand physiological mechanisms (Chiappinelli 1983; Harvey and Robertson 2005; Kerns et al. 1999; Wijeyewickrema et al. 2005; Yang et al. 2016), and venom characterisation, which includes venom composition and the sequence, structure and pharmacology of toxins (Bolt 2021; Kleiz-Ferreira et al. 2021; Tasoulis et al. 2022b; Yang et al. 2016). More recently the evolutionary biology of toxins and venom has been investigated, including both inter- and intra-specific venom variation and its evolutionary and ecological significance (Margres et al. 2021; Mora-Obando et al. 2020; Rautsaw et al. 2019; Zancolli et al. 2019). Venom is also being used as a model for understanding gene duplication, expression and regulation, and molecular evolution (Giorgianni et al. 2020; Hargreaves et al. 2014; Perry et al. 2022; Whittington et al. 2018).
The evolutionary history of how some snakes became venomous has only recently begun to be elucidated (Kardong 2002; Vidal 2002; Vonk et al. 2008). Snake venom is produced in a pair of post-orbitally located venom glands, and the venom glands of all snakes are homologous (Jackson et al. 2017). There are several tooth-bearing bones in the upper jaws of snakes, but venom-delivering fangs are only located on the marginal upper jawbone called the maxilla, with the location of the fang on the maxilla (front or rear), varying amongst different clades of snakes (Westeen et al. 2020). There are three families of front-fanged venomous snakes, Atractaspididae (Burrowing asps, 72 species), Elapidae (Elapids 389 species) and Viperidae (Vipers, 373 species) (data taken from www. Reptile-database. org) and also a number of rear-fanged venomous snake families and subfamilies (Colubriformes). The front-fanged elapids and vipers (Fig. 1) are responsible for almost all serious human envenoming and deaths globally (Gutiérrez et al. 2017), and venoms from all these groups have been the focus for most research in this field (Tasoulis and Isbister 2017).
Examples of the two most medically significant and speciose families of the front-fanged snakes. A An Elapid, an Australian coastal taipan (Oxyuranus scutellatus), and B a viper, a Sonoran desert sidewinder (Crotalus cerastes cercobombus). Note the large opening below and in front of the eye of the viper. This is an infra-red sensing pit, neurally integrated with the eye and allowing vision in the infra-red spectrum and distinguishes “pit vipers” Crotalinae, from “true vipers” Viperinae, the two major subfamilies of the family Viperidae. Photos courtesy of Shane Black (A), and Ross McGibbon (B)
Vipers are classified into three subfamilies, but 99% of viper species belong to just two of these: true vipers (Viperinae; 101 sp.), and pit vipers (Crotalinae; 271 sp.). Pit vipers are distinguished by the presence of infra-red sensitive facial pits (Fig. 1B), giving pit vipers the ability to see in the infra-red spectrum (Gower et al. 2019; Liu et al. 2016). A universally recognised group of vipers are the American rattlesnakes. Elapids are typically slender-bodied snakes and include many of the well-known species of medical significance, such as cobras, mambas, kraits, coral snakes, taipans, tiger snakes and sea snakes.
Snake venoms are heterogeneous mixtures of different toxic protein families with each protein family potentially containing many different toxins. Toxic proteins produced by different genes are classified as different toxins, whilst toxins with different sequences that are produced by the same gene are called proteoforms (Smith and Kelleher 2013); these can be the result of alternative splicing or post-translational modifications (Ogawa et al. 2019). Toxins have evolved to affect various physiological systems, and can be neurotoxic, haemotoxic, myotoxic or cytotoxic (Gutiérrez et al. 2017), depending on the site of action. Toxins are typically monomeric, but toxins can also oligomerise with themselves or other toxins to become multimeric. If they oligomerise with the same toxin, they are homomeric, and if they oligomerise with different toxins, they are heteromeric. Most snake toxins are in the mass range of 4 to 100 kDa (Olaoba et al. 2020; St Pierre et al. 2006); however, some multimeric toxins may have a total mass of 250 kDa; for example, Pseutarin, the procoagulant serine protease toxin from the Australian brown snake genus Pseudonaja (Rao and Kini 2002). Structurally, monomeric toxins can contain several domains, these are conspicuous parts of the protein that fold independently from the rest. In addition to toxin proteins, there are also believed to be several non-toxin protein modification enzymes present in the venom (Isomoto et al. 2022).
Different toxin protein families are mostly located on different chromosomes (Suryamohan et al. 2020), and multi-gene protein families occur as tandemly arrayed gene clusters (Giorgianni et al. 2020). These have expanded from single ancestral genes through repeated gene duplication events (Dowell et al. 2016; Shibata et al. 2018). The mechanisms for how these protein families may be integrated into regulatory networks for coordinated expression is an exciting new area of research (Perry et al. 2022).
Since 2004, there has been a rapid increase in the number of publications characterising snake venom proteomes, partly facilitated by the advent of transcriptomics, which has enabled far more rapid identification of toxins. A review in 2017 identified studies of the venom composition of 132 snake species (Tasoulis and Isbister 2017) and showed that snake venom protein families could be grouped using a combination of occurrence and abundance into a hierarchy of categories. There were four dominant protein families—phospholipase A2 (PLA2), snake venom metalloprotease (SVMP), snake venom serine protease (SVSP) and three-finger toxin (3FTx), six secondary protein families—L-amino acid oxidase (LAAO), cysteine-rich secretory protein (CRiSP), kunitz peptides (KUN), natriuretic peptides (NP), C-type lectins (CTL) and disintegrins (DIS) and nine minor protein families–nerve growth factor (NGF), nucleotidase (NUC), vascular endothelial growth factor (VEGF), phosphodiesterase (PDE), phospholipase B (PLB), hyaluronidase (HYAL), vespryn/ohanin, (VES/OHA) snake venom metalloprotease inhibitor (svMPi), acetylcholinesterase AChE). More recent analysis using a larger dataset suggests that there are in fact 14 minor families although some of these may not possess toxic activity. The remaining protein families were classified as rare.
Analytical techniques used to characterise snake venoms
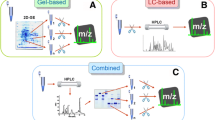
The first aim in characterising a snake’s venom proteome is toxin identification and quantification. This usually involves an initial venom separation (fractionation) step known as venom decomplexation. However, whole venom proteome characterisation is also often employed by researchers. Prior to the turn of the millennium, toxin identification was a laborious and time-consuming process involving Edman degradation (protein N-terminal sequencing). However, modern analytical techniques utilising workflows combining reverse-phase high-performance liquid chromatography (RP-HPLC), sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE), mass spectrometry (MS) and transcriptomics, have revolutionised the study of venom composition (Tan 2022).
There have been several recent excellent reviews comprehensively covering the methodologies of characterising snake venom proteomes (Sahyoun et al. 2022; Slagboom et al. 2022; Tan 2022), so they will only be briefly discussed here. All studies of snake venom investigations start with the collection of venom from accurately identified snakes either from licensed companies of snake venoms, or with clear information on the snake identification and location where the snake came from. After venom extraction, the venom from multiple individuals of the same species is usually pooled and then lyophilised by freeze drying. Lyophilised venom can then retain viability for at least eight decades (Jesupret et al. 2014). More recently, the importance of also studying the venom from individual snakes has been understood, and such investigations require identification and collection of data on all snake individuals involved. The characterisation (identification and quantification of constituent protein families and toxins) of snake venom proteomes can be achieved by a variety of workflows, all of which have different advantages and disadvantages. Several studies have indicated that combining different methodological approaches appears to maximise proteomic coverage (Chanda and Mukherjee 2020; Choudhury et al. 2017). A recent review which compared all the different workflows used over a 4-year period (2017 to 2021) (Tasoulis et al. 2022a), found that the strategy of choice was bottom-up proteomics, in particular involving a preliminary two-dimensional decomplexation of the venom, consisting of RP-HPLC, followed by SDS-PAGE, prior to in-gel trypsin digestion and MS analysis (Fig. 2). Other methods which are commonly used include shotgun proteomics, which uses digested crude venom without prior fractionation, top-down proteomics (TDP), which performs MS analysis directly on undigested crude venom or venom fractions, and bottom-up proteomics (BUP), which involves a sample preparation step of trypsin digestion, which can be either in-solution or in-gel, prior to MS. Several comparative studies have indicated that preliminary decomplexation of the venom by RP-HPLC followed by SDS-PAGE increases proteomic coverage, as the second decomplexation step of SDS-PAGE is useful for separating co-eluting toxins with similar hydrophobicity but often belonging to different protein families with different masses.
Simplified summary of published workflows used from 2017 to 2021 to characterise the venom proteomes of vipers and elapids, showing how almost half of the total studies used bottom-up proteomics (thick blue arrows), consisting of a two-dimensional decomplexation of the crude venom by reverse-phase high-performance liquid chromatography (RP-HPLC), followed by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE). The venom fractions are then subjected to in-gel trypsin digestion prior to mass spectrometric analysis. Diagram modified from Tasoulis et al. 2022a (colour figure online)
Snake venom composition
Snake venom has been shown to mostly consist of a relatively small number of protein families. We have previously attempted to classify these protein families in a hierarchical system based on a combination of occurrence across species, combined with relative abundance of these protein families as a percentage of the total venom. We graphed these values from 179 species of front-fanged snakes, 68 species of elapids, 26 species of true vipers, and 84 species of pit vipers, plus 1 species of viper from the monogeneric subfamily Azemiopinae (Fig. 3). These categories are discussed in more detail below.
-
1.
Dominant protein families (4): mean abundance > 10% of the whole venom and occur in > 33% of species.
-
2.
Secondary protein families (6): mean abundance > 3% but < 10% of the whole venom and occur in > 33% of species.
-
3.
Minor protein families (14): occur in > 5% of species with a mean abundance of < 3% (12) or are key toxins in one or a small number of species with an abundance > 3% (Fig. 3).
-
4.
Rare protein families (18): any protein family with likely toxic activity with a mean abundance < 5% and occur in < 5% of species. Many of the listed rare protein families may be non-toxin protein modification enzymes.
XY graph showing the criteria for our hierarchical classification system of the 42 protein families recorded from the venoms of 179 species of vipers and elapids (23.5% of all species), based on a combination of mean abundance as a percentage of the total venom (x-axis), and the number of species in which it is present (occurrence) (y-axis). Categories are: dominant protein families—present in > 33% of species, and with a mean abundance of > 10% of the venom (square icons above and to the right of dashed red lines). Secondary protein families—present in > 33% of species, and with a mean abundance of > 3% and < 10% of the venom (filled circles between the two vertical red dashed lines). Minor protein families—present in > 5% of species with a mean abundance of < 3% of the venom (triangles between the Y-axis and the vertical dashed red line line), with two exceptions; waglerin and crotamine. Rare protein families—present in < 5% of species and with a mean abundance of < 5% of the venom (empty black circles in lower left corner of graph). Details of the dominant protein family are given in Table 1 (colour figure online)
Phospholipase A 2 (PLA 2 )
PLA2s are one of the most important protein families in snake venoms globally, occurring in the majority of elapids and vipers. It has been recorded in all species of vipers except for some species of Palm vipers (Bothriechis), and in all species of elapids except for Senagalese cobra (Naja senegalensis), and east African green mamba (Dendroaspis angusticeps), although it is expressed in very low amounts in all mambas. The type of PLA2 differs between the two snake families, with elapids possessing pancreatic type (group I) PLA2 and vipers having synovial type (group II) PLA2 (Suranse et al. 2022). When averaged across all species that contain PLA2, the mean abundance of PLA2 is 26%, (31% in elapids and 23% in vipers). They are more abundant in the Australasian elapid clade (Hydrophiinae) and can make up 90% of the venoms of some of the species in this clade (e.g. Pseudechis papuanus (Pla et al. 2017)) and are likely the reason for systemic myotoxicity being an important clinical manifestation of Australian snake envenomation (Johnston and Isbister 2021). They have a diverse pharmacological profile and are responsible for pre-synaptic neurotoxicity from many elapids (Johnston et al. 2017a; Kuruppu et al. 2005), myotoxicity reported in Australasian elapids (Hart et al. 2014; Johnston et al. 2013), cytotoxicity, inflammation and anticoagulant effects (Johnston et al. 2013; Lane et al. 2011). They have a monomeric mass of 13 to 18.5 kDa (Cendron et al. 2012; Kang et al. 2011), and may oligomerise with other PLA2s (e.g. trimeric taipoxin) or with other toxin protein families such as kunitz peptides—(e.g. β-bungarotoxin in krait venoms) (Cendron et al. 2012; Rowan 2001). The most potent PLA2 neurotoxins are reported to be oligomers (e.g. taipoxin) (Montecucco and Rossetto 2008). In the viperid group II PLA2s, the 49th amino acid residue is aspartate (ASP49 or D49) and is in the active site (Suranse et al. 2022). This amino acid appears to be responsible for the catalytic activity of these PLA2. Interestingly, there is poor correlation between enzymatic activity and toxicity (Montecucco and Rossetto 2008; Tasoulis et al. 2020a). The presence of PLA2 in almost all venomous snakes means the detection of PLA2 activity may be a useful way to detect systemic envenoming in human snakebite patients. Preliminary studies have demonstrated that the detection of PLA2 activity in serum of snakebite patients distinguishes between patients with and without systemic envenoming (Isbister et al. 2020; Maduwage et al. 2014).
Three-finger toxins (3FTx)
3FTxs are one of the two most abundant protein families present in elapid venoms, with PLA2. The mean abundance of 3FTxs in all elapid species is 52%. They are extremely rare in viper venoms being only recorded in five species. In elapids, they have been recorded in all species and can make up 95% of the venom (e.g. Micrurus tschudii, M. surinamensis). These non-enzymatic toxins have a mass of between 6 and 9 kDa (Clarke et al. 2006), and are named after their characteristic spatial structure, as all have a similar highly conserved folding pattern consisting of three β-stranded loops (fingers), extending from a globular central core which is stabilised by four conserved disulfide bridges (Kini and Doley 2010). An important sub-group of this protein family are the α-neurotoxins, post-synaptic neurotoxins that act at the nicotinic acetylcholine receptors (nAChRs) (Nys et al. 2022). Alpha-neurotoxins competitively antagonise the ligand-gated ion channels at the acetylcholine binding site and prevent the opening of the channel. Alpha-neurotoxins are further sub-divided into two groups: 1) short-chain neurotoxins 60–62 amino acid residues and four disulfide bonds, and 2) long-chain 66–75 amino acid residues and five disulfide bonds, having an extra disulfide bond in the central finger (Kini 2002). It is important to note that human nAChR have an exceptionally low affinity for short-chain α-neurotoxins compared to long-chain α-neurotoxins (Ishikawa et al. 1985), and long-chain neurotoxins are likely to be more important in human envenomation (Silva et al. 2018). Other 3FTxs target other types of ion channels, such as muscarinic 3FTxs (Kini 2002). African mamba (Dendroaspis) venoms contain a further two types of specialised 3FTx neurotoxins—calciseptines, which selectively block L-type calcium channels, and fasciculins, which inhibit acetylcholinesterase (Casewell et al. 2020). The Malayan Blue Coral Snake (Calliophis bivirgata) contains the 3FTx neurotoxin, calliotoxin, which activates the voltage-gated sodium channel, Nav 1.4 (Yang et al. 2016). In some cobra venoms, particularly spitting cobras, cytotoxic 3FTxs are thought to be responsible for serious local dermonecrosis (Hiu and Yap 2022; Lin et al. 2022).
Snake venom metalloprotease (SVMP)
SVMPs are the most abundant toxin proteins in viper venoms, with a mean abundance of 34% across all viper species. SVMPs have been recorded in every viper species except Bothriechis nigroviridis. Some Bothrops spp. contain as much as 74% SVMPs in their venom. In elapids, SVMPs are far less abundant (mean abundance 5% across all species), although a recent study indicates that it may be more abundant in the currently understudied Australian elapid clades with Hoplocephalus stephensii containing 37% SVMPs (Tasoulis et al. 2022b). Metalloproteases are classified into three major classes, P-I, P-II and P-III, based on the domain structure (Olaoba et al. 2020). The P-III are the largest and believed to be the ancestral form, with P-II and P-I having lost some of the domains. P-III consist of a metalloprotease domain, a disintegrin domain, and a cysteine-rich domain (type P-III also contains a C-type lectin domain). P-II are only made up of a metalloprotease domain and a disintegrin domain, whilst P-I consist simply of a metalloprotease domain. Toxins belonging to this family typically, but not always, possess haemorrhagic activity, although some are procoagulants, such as the potent prothrombin activator Ecarin in Echis venom (Ainsworth et al. 2018). Ecarin is arguably one of the most medically important snake toxins in human envenoming, being responsible for venom-induced consumption coagulopathy (VICC) for the thousands of Echis envenoming cases (Mion et al. 2013).
Snake venom serine protease (SVSP)
This is the least abundant of the dominant protein families with a mean abundance of 14% in vipers but present in every species. SVSPs can make up as much as 53% of the venom in the pit viper Ovophis convictus and 26% in the true viper Bitis gabonica. SVSPs appear to be far less compositionally important in elapids with a mean abundance of only 1.3% and present in just over a third of the species. They appear to be a more important component in the venoms of the Australian clade, and the few published studies of Australian elapid venom proteomes have found higher than average values for elapids (6% for both Notechis scutatus and H. stephensii). This is consistent with venom-induced consumption coagulopathy VICC being the most important clinical manifestation in Australian snake envenomation (Johnston et al. 2017b). Pharmacologically SVSPs are procoagulants, disrupting haemostasis and triggering the clotting pathway by partial cleavage of the inactive clotting factor proteins (zymogens) to the active or partially active enzymes (Ainsworth et al. 2018; St Pierre et al. 2005). These toxins include factor X activators, factor V activators, prothrombin activators and thrombin-like enzymes (TLEs) (Isbister 2010). These toxins are medically important in some Australian elapids causing VICC (Isbister et al. 2010b), and responsible for significant coagulopathy in many vipers e.g. Calloselasma rhodostoma, and likely also Bothrops asper (Otero-Patiño 2009; Wongtongkam et al. 2005). This protein family also includes the anticoagulant protein C activator which are mainly found in the venoms of the pit viper Agkistrodon complex (Gempeler-Messina et al. 2001). Another class of toxins in this family are the kallikreins, which in addition to having fibrinogenolytic activity can also cleave kininogen to release bradykinin which can result in hypotensive effects (Felicori et al. 2003; Fry 2015).
L-Amino acid oxidase (LAAO)
This is the most ubiquitous of the secondary protein families, present in 93% of vipers and 69% of elapids (83% combined) (Table 2). LAAOs have a mean abundance of 4% and a maximum of 17% in vipers and a maximum of 11% in elapids. They are homodimers with monomeric masses of 50 to 70 kDa (Fox 2013). They have become a focus of research for their antimicrobial and anticancer potential (Cecchini et al. 2005; Ciscotto et al. 2009; Costa et al. 2014; Tan et al. 2018). Despite them being so widespread and extremely well characterised, their functional role in human envenomation remains unclear (Izidoro et al. 2014; Pawelek et al. 2000; Tasoulis et al. 2022b).
Cysteine-rich secretory protein (CRiSP)
This is the second most ubiquitous of the secondary protein families, present in 74% of species, with a mean abundance of 3% and a maximum of 14%. CRiSPs are more commonly recorded in vipers than elapids (86% versus 56%, respectively). They are monomeric with a molecular mass of approximately 25 kDa (Suntravat et al. 2019). They belong to the catabolite activator protein (CAP) superfamily (Tadokoro et al. 2020). CRiSPs from Australian elapids are the only known protein blockers of cyclic nucleotide-gated (CNG) ion channels which play pivotal roles in sensory transduction by retinal photoreceptors and olfactory neurons (Suzuki et al. 2008). Their role in the pathophysiology of human envenomation is still poorly understood (Boldrini-França et al. 2017), but a change or loss of smell has been reported for a number of Australian elapid bites (Churchman et al. 2010; Johnston et al. 2013).
C-type lectin (CTL)
CTLs are present in 69% of species, with a mean abundance of 6% and a maximum of 38%. They are more highly expressed in vipers than elapids (mean abundance 6.9% versus 0.8%) and are present in more species of vipers than elapids (89% versus 38%). They are haemotoxic, and can be anticoagulant, platelet aggregation inhibitors, or platelet aggregation agonists (Ogawa et al. 2005). There are two major groups; CTLs which are homodimeric (or higher multimers), and snaclecs (snake C-type lectins), which are heterodimeric (Clemetson et al. 2009).
Disintegrin (DIS)
This protein family is present in 47% of species with a mean abundance of 5% and a maximum of 20% for vipers, and 6% for elapids. Disintegrins are problematic to quantify, as in many species they are only present as a domain of SVMP and are quantified as part of that protein family, so they may be underrepresented. They are classified into five different groups based on their polypeptide length and number of disulphide bonds (Calvete et al. 2003). In addition to occurring as a domain of SVMP, they may also occur as monomers or dimers released in the venom by proteolytic processing of P-II and P-III SVMPs or translated from short-coding mRNA (Arruda Macêdo et al. 2015; Calvete et al. 2005). They selectively block integrin receptors and are potent inhibitors of integrin–ligand interactions (Calvete et al. 2005). Disintegrins are being investigated as novel therapeutic leads for cancer treatments because of their ability to target specific integrins (Akhtar et al. 2021). They could conceivably interfere in important processes involved in carcinogenesis, tumour growth, invasion and migration (Arruda Macêdo et al. 2015).
Natriuretic peptide (NP)
This protein family has been recorded in 36% of species. They are more commonly occurring and more highly expressed in vipers than elapids and are most widespread and highly expressed in pit vipers. The mean abundance values in pit vipers are 12% in the 51% of species that they occur, and in true vipers are 7% in the 46% of species that they occur. They are far less common in elapids with a mean abundance of only 1% in the 21% of species that they occur. The maximum abundance of NPs is 39% (Bothriechis supraciliaris) and it is a prominent protein family in the venoms of Bushmasters (Lachesis), and palm vipers (Bothriechis), but is absent in the genus Agkistrodon. They have a molecular mass of approximately 4 kDa (St Pierre et al. 2006). NPs also contain both bradykinin-potentiating peptides (BPP), and bradykinin-inhibiting peptides (BIP) (Fry 2015). In some studies NPs are recorded as a different protein family, “snake venom metalloprotease inhibitors” (svMPi), but the svMPis reported in this review either lacked sufficient sequence information to confirm they belonged to a different protein family or they matched directly to a NP (Giribaldi et al. 2020; Neri-Castro et al. 2020). Tripeptide svMPis have previously been shown to be encoded by precursor NPs (Boldrini-França et al. 2017), so we have merged svMPi and NP in Fig. 3. The drug captopril which is widely used for the treatment of hypertension and heart disease was developed from a BPP toxin isolated from the venom of the South American pit viper Bothrops jararaca (Bryan 2009; Vonk et al. 2011). It inhibits angiotensin-converting enzyme (ACE), and numerous drugs in the same family have been subsequently developed.
Kunitz peptide (KUN)
This protein family is present in 36% of species but is unequally distributed across snake families as it has been recorded in 65% of elapid and true viper species, but only in 4% of pit vipers. KUN are also more highly expressed in elapids than vipers, with a mean abundance of 6.4% in elapids versus 3.3% in true vipers, and only 0.06% in pit vipers. In elapids, they are highly expressed in African mambas (Dendroaspis), and desert cobras (Walterinnesia), and also occurs in Asian kraits (Bungarus), and some Australian elapids. In black mamba Dendroaspis polylepis, a KUN makes up 50% of the venom (dendrotoxin), a neurotoxin which blocks neuronal Kv1 ion channels (Harvey and Robertson 2005; Owen et al. 1997). Dendrotoxin appears to preferentially block inactivating forms of K + current, and can induce repetitive firing in neurons and facilitate neurotransmitter release (Harvey 1997).
Vascular endothelial growth factor (VEGF)
VEGFs are most common in true vipers, being present in 77% of species (mean abundance 3%), then pit vipers, 42% of species (mean abundance 1.5%), and only 13% of elapids (mean abundance 0.06%) (Table 3). They have been recorded as being 25 kDa homodimeric heparin-binding proteins (Ferreira et al. 2021), but one toxin isolated from a pit viper was heterodimeric with a molecular mass of 37 kDa (monomeric masses of 13 and 26 kDa) (Nakamura et al. 2014).
Family and subfamily comparisons of the abundance of the 22 most ubiquitous protein families in snake venoms. Y-axis is % of whole venom and X-axis is the different protein families. Coloured boxes show median and interquartile range Please move Figure 4 to the section on Variation in snake families (colour figure online)
Nucleotidase (NUC)
This protein family contains 5’ nucleotidase, ATPase and ADPase (Boldrini-França et al. 2017). They are one of the two most widespread of the minor protein families along with NGF, occurring in 39% of species, and are more commonly reported in vipers than elapids. They have a mean abundance of 0.7% and a maximum of 4.6%. There are few studies concerning the isolation and characterisation of snake venom NUCs, but they have been shown to inhibit platelet aggregation in both true and pit vipers (Ouyang and Huang 1983; Trummal et al. 2005).
Phosphodiesterase (PDE)
This protein family is present in just over one third of species and had a mean abundance of 0.7% and a maximum of 3.6%. They are mono or multimeric with a molecular mass of 90 to 160 kDa (Oliveira et al. 2022b). They may facilitate venom diffusion through the prey’s tissues (Oliveira et al. 2022b) and PDEs isolated from rattlesnake venoms (Crotalus spp.) were found to produce an immediate profound hypotensive crisis but did not interfere with neurotransmission (Russell et al. 1963).
Nerve growth factor (NGF)
This protein family is one of the two most widespread of the minor protein families along with NUC, occurring in 39% of species. They have a mean abundance of 0.6% and a maximum of 3.6% and belong to the class of structurally related proteins known as neurotrophins (Sunagar et al. 2013). They have a molecular mass of 12 to 37 kDa (Islam et al. 2021).
Phospholipase B (PLB)
This protein family was present in 30% of species and had a mean abundance of 0.5% and a maximum of 3%. They have a molecular mass of 35 to 55 kDa (Bernheimer et al. 1987; Ullah and Masood 2020). There have been limited investigations into this protein family, and their function and role in human envenomation is unclear.
Cobra venom factor (CVF)
This protein family has only been recorded from cobra venoms with two exceptions, the sea snake Hydrophis cyanocinctus (0.2%), and the pit viper Trimeresurus insularis (1%) (Jones et al. 2019; Wang et al. 2020). The largest amounts recorded were in king cobra Ophiophagus hannah (2.8 to 5.5%) (Tan et al. 2015; Vonk et al. 2013). It has a molecular mass of 149 kDa and is composed of three chains (Liu et al. 2018; Vogel and Fritzinger 2010). It continuously activates the complement system resulting in a depletion of complement activity (Van den Berg et al. 1991). As such it has attracted much experimental research to study the role of the complement system in host defence and immune response, as well as the pathogenesis of diseases (Vogel and Fritzinger 2010).
Vespryn/ohanin (VES/OHA)
Vespryns were most common in elapid venoms, being recorded in 25% of species and with a mean abundance of 1.6%. They were recorded in 7% of pit vipers with a mean abundance of 0.6%, but there were no records from true vipers. It is most abundantly expressed in the venom of the Malayan Blue Coral Snake Calliophis bivirgata flaviceps (15%) (Tan et al. 2016).
Acetylcholinesterase (AChE)
AChEs is present in almost a quarter of elapid species, but no vipers except the true viper Echis (Ghezellou et al. 2021). They are particularly prevalent in krait (Bungarus spp.) venoms, and almost completely absent from South American Coral Snakes (Micrurus). They are monomeric with a molecular mass of 59 kDa (Tan and Tan 1988). Although the function of these enzymes in hydrolysing acetylcholine is well described, it is unclear what their role is in snake venom or in human envenoming. There are several nomenclatural taxonomies used for AChE, but we are here treating it as belonging to the cholinesterase subfamily of type-B carboxylesterases (Wheelock et al. 2005).
Hyaluronidase (HYAL)
Hyaluronidases are recorded in 16% of species and have a mean abundance of 0.3% and a maximum abundance of 1.9%. They are less common in the venoms of pit viper venoms. They have a molecular mass of 28 to 70 kDa (Boldrini-França et al. 2017). They are commonly regarded as a “spreading factor”, and a non-toxic enzyme that degrades hyaluronic acid, the main component of extracellular matrix, so potentially allows the spread of other toxins (Bala et al. 2018). It has been shown to increase the diffusion of crotoxin and PLA2 through mouse tissue in Crotalus durissus, thus potentiating crotoxin (Bordon et al. 2012).
Cystatin (CYS)
Cystatins have been recorded in 15% of true vipers and 12% of elapids, but there are no records in pit vipers. A study has shown that they inhibit tumour angiogenesis and that they may have therapeutic potential as antiangiogenic and anti-metastatic agents (Xie et al. 2013). Their role in envenomation is currently unknown (Boldrini-França et al. 2017).
Glutaminyl cyclase
Glutaminyl cyclases have only been recorded in viper venoms and are one of the least understood protein families in snake venoms (Wang et al. 2014). They may not possess toxic activity, but instead may play a role in N-terminal modifications of other toxins (Pawlak and Manjunatha Kini 2006). These enzymes belong to the family of transferases, sometimes referred to as aminotransferases or acyltransferases (Huang et al. 2005).
Aminopeptidase
Aminopeptidases were one of the least commonly reported of the minor protein families, reported in 27% of true vipers with a mean abundance of 0.3%, in 6% of pit vipers with a mean abundance of 0.4%, and in 1.5% of elapids with a mean abundance of 0.004%.
Crotamine
This toxin belongs to the protein family β-defensins but is typically referred to as crotamine in venom proteome studies (Fry 2015). It has only been recorded from the venom of some species of rattlesnakes (Crotalus), and is a 42 residue peptide with a molecular mass of 4.9 kDa (Mancin et al. 1998). Crotamine makes up 37% of the venom of Crotalus viridis (Saviola et al. 2015). It is a myoneurotoxin with multiple activities and has been reported to be a cell-penetrating peptide, antitumoral agent and a potent Kv channel inhibitor (Kerkis et al. 2014; Peigneur et al. 2012).
Waglerin
This protein family has only been recorded from the Oriental pit viper genus Tropidolaemus (Tan et al. 2017). It is 22 residues in length with a proline-rich sequence and a molecular mass of 2.5 kDa (Lin et al. 1995; Weinstein et al. 1991). It is a neurotoxin that competitively antagonises muscle nicotinic acetylcholine receptors (nAChRs) (Molles et al. 2002). They are the only characterised toxin from viper venoms that target nicotinic receptors (Molles et al. 2002). It makes up 38% of the venom.
Rare protein families
This category contains 18 protein families (Table 4). The classification criteria for inclusion in this group were: present in < 5% of species, and with a mean abundance of < 5% (Fig. 3). The number of protein families in this category has been reduced from the 36 listed in the 2017 review, as many of the protein families included therein are now known to be either impurities, non-toxins, synonyms, or protein subfamilies (Supplementary file 1. Tables 3, 4).
Venom variation
Types and levels of venom variation
Snake venom can vary in composition at all taxonomic levels; families, clades, genera, species (inter-specific variation) and within species (intra-specific or inter-population variation (Saviola et al. 2017)). The expression of different protein families can also vary within populations (intra-population variation) (Tasoulis et al. 2020b). Venom composition can also vary in a number of ways, including different relative amounts of each protein family, different numbers of toxins within each protein family and different types of toxins with each protein family (amino acid sequence differences).
Variation in snake families
Elapid venoms are overwhelmingly dominated by just two protein families, 3FT and PLA2 (Fig. 4A), making up 82% of the venom when averaged across all elapid species. The remainder of their venoms are mostly comprised of small amounts of SVMP, and three of the six secondary protein families, LAAO, CRiSP and KUN. Viper venoms are mainly comprised of three of the dominant protein families, SVMP, PLA2 and SVSP (Figs. 4B, C), which make up 69% of their venoms when averaged across all viper species. Viper venoms contain more of the secondary protein families than elapids (combined average of 23% versus 8%). The differences between the venoms of true and pit vipers are minor, with true vipers containing less SVMP (median 22% versus 35%), and much more of the secondary protein family KUN and the minor protein family VEGF.
Intra-specific venom variation
There has been little uniformity in the results of studies investigating intra-specific venom variation in snakes. Inter-population expression variation has been found in eastern diamondback rattlesnake Crotalus adamanteus (Margres et al. 2015), Malayan pit viper Calloselasma rhodostoma (Daltry et al. 1997), jararaca Bothrops jararaca (Goncalves-Machado et al. 2016), and Lancehead Bothrops asper (Mora-Obando et al. 2020). Conversely, it was not found in the elapid, eastern coral snake Micrurus fulvius (Margres et al. 2015), and only minimally in the coastal taipan Oxyuranus scutellatus (Tasoulis et al. 2020b). A study on the sidewinder rattlesnake Crotalus cerastes (Rautsaw et al. 2019), found little evidence for toxin coding sequence differentiation across populations, suggesting that their venoms are not under strong positive selection pressures. A study on strongly dichotomous venom expression in populations of the Mojave rattlesnake Crotalus scutulatus found that the venom variation could not be explained by diet (Zancolli et al. 2019). Likewise, the combined results of three studies on the trophic generalist sea snake Hydrophis curtus from three localities spanning a distance of almost 5000 km, suggest a general cline in venom variation rather than positive selection shaping venom composition for local differences in diet (Neale et al. 2017; Tan et al. 2019; Wang et al. 2020). The venom of this species varied from PLA2 dominant at the southern end of its distribution (QLD, northern Australia), to almost equal parts PLA2 and 3FTx in the centre of its distribution (Penang Malaysia), to 3FTx dominant in the northern part of its range (Hainan, China). Interestingly, studies on insular populations of the Australian tiger snakes have shown that there has been strong selective pressure on snake body size imposed by the size of available prey items, so venom may not always be the trait under strongest selection pressure (Aubret 2015; Keogh et al. 2005). This raises the question about whether selection can simultaneously optimise venom and non-venom traits.
Although numerous studies have found an association between venom variation and diet, others have not found such an association. One possibility is that geographical differences in diet generally drive venom variation, but there are circumstances in which non-venom traits are under stronger selection pressure than venom. Perhaps circumstances like these mean that venom expression may alter in ways not associated with diet. Such geographic differences in venom expression could then easily be misinterpreted as being the result of positive selection for diet. Another possibility is that the dominant protein families are sufficiently efficacious at prey subjugation that selection cannot detect differences in venom composition below a certain toxicity range. This would allow for initial random venom variation between fragmented or newly founded populations to be retained in some circumstances. Such initial random variation could then potentially reset the venom composition of these populations onto radically new evolutionary trajectories. Like all traits, venom evolution would therefore be the result of the interplay between pre-existing composition, chance and ecological drivers.
Medical aspects of venom composition and variation
Until recently, a large proportion of research on snake venom composition has focussed on medically important toxins, either as potential drug leads or for their functional role in human envenomation. What is interesting is that the four dominant protein families appear to be the most important toxin families in human envenomation, being responsible for VICC, pre- and post-synaptic neurotoxicity, myotoxicity and cytotoxicity. Potentially this is because these protein families likely possess broad spectrum toxicity and lethality across all classes of vertebrate prey, and therefore have similar toxicity in humans (Barua and Mikheyev 2019). This would explain why toxins in sea snake venom, that may have been selected to kill fish, are also highly toxic in humans. Clearly there are differences in the effects of different toxins in humans compared to animals, such as the relative resistance of humans to short-chain neurotoxins (Silva et al. 2018), or differences in the effects of snake procoagulants on different animal toxins. (Maduwage et al. 2016) Possibly, the actual lethality of the toxins in these four dominant families across vertebrates is more important than the specific toxic effect.
There have been numerous reviews on the potential value of toxins for the development of novel drugs (Oliveira et al. 2022a; Saviola et al. 2014), following the success of captopril being developed from the discovery of the bradykinin-potentiating peptides (C-type natriuretic peptides) in B. jararaca venom (Ferreira 1965). However, despite a multitude of studies attempting to isolate novel toxins over the last few decades, there have been very few drugs developed from toxins that are now used in clinical practice. An example is ancrod, the purified thrombin-like enzyme from Calloselasma rhodostoma (Malayan Pit Viper), which was trialled in the treatment of acute ischaemic stroke (Hennerici et al. 2006). However, it was shown to not improve outcomes and increased bleeding in patients (Levy et al. 2009).
The role of snake venom variation in human envenomation is somewhat contentious. There are clearly differences in the clinical effects of snake venoms, based on the occurrence and predominance of the different toxin protein families, as discussed above. The effects of toxins from the four dominant toxin families are well described in humans, and different relative abundances of these toxin families in different snakes correlate well with the range of different clinical effects. Vipers have a predominance of SVSPs and SVMPs consistent with haemotoxic and local effects being more common in human envenomation (Warrell 2010). Conversely, neurotoxicity is far more common in elapids, such as kraits, cobras and coral snakes (Silva et al. 2017), in which PLA2s and 3FTx are the most abundant toxin families. The difference in venom composition for different genera also appears to be associated with different clinical effects, for example in the medically important Australasian elapids (Johnston et al. 2017b). It is less clear whether variation in the individual toxins within toxin families is important in human envenomation.
An understanding of venom composition is also central to developing efficacious antivenoms. Antivenoms are mixtures of antibodies raised against multiple toxins in a snake venom in large mammals (e.g. sheep, horse) (Silva and Isbister 2020). For an antivenom to be efficacious, it needs to contain antibodies to all medically important toxins in a snake venom. In the past, this has been difficult, without a complete understanding of venom composition. More recently, proteomic techniques have been used to investigate the toxicological profile of a snake venom and hence identify which protein families are most important for antivenom immunorecognition (Gutiérrez et al. 2009; Laustsen 2018).
Currently antivenoms are only effective against snakes in certain geographical regions, because only venoms from the specific geographical region are used in producing the antivenom (Silva and Isbister 2020). Worldwide this has meant that numerous antivenoms are required, and an antivenom produced in one country is highly unlikely to be useful in another country, unless a close neighbour with similar snake fauna. This has contributed to an ongoing crisis in antivenom supply to resource poor countries, many which cannot produce their own antivenoms (Lalloo et al. 2002).
A different approach to antivenom development is to consider the similarity in venom composition between snakes worldwide, rather than the differences. It appears that most medically important effects in humans are due to the four dominant toxin families, with a few important exceptions, such as natriuretic peptides in vipers (Péterfi et al. 2019; Souza et al. 2007), crotamine in South American pit vipers and dendrotoxins in mambas (Erulu et al. 2018; Harvey 2001; Quarch et al. 2017). A universal antivenom could be developed that contained antibodies against the four dominant toxin families and other key toxin groups. Such an antivenom would then be efficacious across a much larger geographical range, potentially covering continents or multiple continents.
There is considerable support for a universal antivenom in the many studies of antivenom cross-neutralisation (Huynh et al. 2022; Isbister et al. 2014, 2010a; Kornhauser et al. 2013; Silva et al. 2016a). For example, commercial antivenoms developed in Asia cross-neutralise the neurotoxic effects of Australasian elapids, and conversely those developed in Australia cross-neutralise the effects of Asian elapids (Silva et al. 2016a). In the case of pit vipers, an antivenom developed against American pit vipers appeared to be efficacious for an Asian pit viper envenomation (Isbister et al. 2014). To develop a universal antivenom requires designing toxin-specific antivenoms using bioinformatics and multi-epitope DNA immunisation. One study showed that antiserum raised against a single synthetic multi-epitope DNA immunogen (epitope string) from SVMPs in E. ocellatus, cross neutralised haemorrhage induced by both E. ocellatus and Cerastes cerastes venoms (Wagstaff et al. 2006).
Conclusion
There has been a rapid expansion in our knowledge of snake venom composition with modern proteomics. Studies of hundreds of snake species have shown that the venoms of vipers and elapids are made up of 42 different protein families, but 24 of these make up 96% of the venom on average. There are 4 dominant, 6 secondary, 14 minor and 18 rare families. From the four dominant protein families, 3FTx and PLA2 make up 82% of elapid venoms on average, and SVMP, PLA2 and SVSP make up 69% of viper venoms on average. Venom variation occurs at all taxonomic levels including within populations, and venom variation cannot always be explained as being the result of positive selection for local differences in diet. With only a few exceptions, human envenomation syndromes appear to result from the toxic effects of the four dominant protein families, suggesting broad ranging toxic effects across all vertebrates. The use of proteomic techniques will also help to improve antivenom therapy worldwide.
References
Ainsworth S, Slagboom J, Alomran N et al (2018) The paraspecific neutralisation of snake venom induced coagulopathy by antivenoms. Commun Biol 1(1):34. https://doi.org/10.1038/s42003-018-0039-1
Akhtar B, Muhammad F, Sharif A, Anwar MI (2021) Mechanistic insights of snake venom disintegrins in cancer treatment. Eur J Pharmacol 899:174022. https://doi.org/10.1016/j.ejphar.2021.174022
Antoniou SA, Antoniou GA, Learney R, Granderath FA, Antoniou AI (2011) The rod and the serpent: history’s ultimate healing symbol. World J Surg 35(1):217–221. https://doi.org/10.1007/s00268-010-0686-y
Arruda Macêdo JK, Fox JW, de Souza CM (2015) Disintegrins from snake venoms and their applications in cancer research and therapy. Curr Protein Pept Sci 16(6):532–548. https://doi.org/10.2174/1389203716666150515125002
Aubret F (2015) Island colonisation and the evolutionary rates of body size in insular neonate snakes. Heredity 115(4):349–356. https://doi.org/10.1038/hdy.2014.65
Bala E, Hazarika R, Singh P, Yasir M, Shrivastava R (2018) A biological overview of hyaluronidase: a venom enzyme and its inhibition with plants materials. Mater Today 5(2):6406–6412. https://doi.org/10.1016/j.matpr.2017.12.252
Barua A, Mikheyev AS (2019) Many options, few solutions: over 60 my snakes converged on a few optimal venom formulations. Mol Biol Evol 36(9):1964–1974. https://doi.org/10.1093/molbev/msz125
Bernheimer AW, Linder R, Weinstein SA, Kim KS (1987) Isolation and characterization of a phospholipase B from venom of collett’s snake. Pseudechis Colletti Toxicon 25(5):547–554. https://doi.org/10.1016/0041-0101(87)90290-x
Boldrini-França J, Cologna CT, Pucca MB et al (2017) Minor snake venom proteins Structure function and potential applications. General Subjects. https://doi.org/10.1016/j.bbagen.2016.12.022
Bolt HM (2021) New aspects in snake venom toxicology. Arch Toxicol 95(6):1865–1866. https://doi.org/10.1007/s00204-021-03066-4
Bordon KCF, Perino MG, Giglio JR, Arantes EC (2012) Isolation, enzymatic characterization and antiedematogenic activity of the first reported rattlesnake hyaluronidase from crotalus durissus terrificus venom. Biochimie 94(12):2740–2748. https://doi.org/10.1016/j.biochi.2012.08.014
Bryan J (2009) From snake venom to ACE inhibitor the discovery and rise of captopril. Pharm J 282:455–456
Calvete JJ, Moreno-Murciano MP, Theakston RD, Kisiel DG, Marcinkiewicz C (2003) Snake venom disintegrins: novel dimeric disintegrins and structural diversification by disulphide bond engineering. Biochem J 372(Pt 3):725–734. https://doi.org/10.1042/BJ20021739
Calvete JJ, Marcinkiewicz C, Monleón D et al (2005) Snake venom disintegrins: evolution of structure and function. Toxicon 45(8):1063–1074. https://doi.org/10.1016/j.toxicon.2005.02.024
Casewell NR, Jackson TNW, Laustsen AH, Sunagar K (2020) Causes and consequences of snake venom variation. Trend Pharmacol Sci 41(8):570–581. https://doi.org/10.1016/j.tips.2020.05.006
Cecchini AL, Marcussi S, Silveira LB et al (2005) Biological and enzymatic activities of Micrurus sp (Coral) snake venoms. Comp Biochem Physiol A Mol Integr Physiol 140(1):125–134. https://doi.org/10.1016/j.cbpb.2004.11.012
Cendron L, Mičetić I, Polverino de Laureto P, Paoli M (2012) Structural analysis of trimeric phospholipase A2 neurotoxin from the Australian taipan snake venom. FEBS J 279(17):3121–3135. https://doi.org/10.1111/j.1742-4658.2012.08691.x
Chanda A, Mukherjee AK (2020) Quantitative proteomics to reveal the composition of Southern India spectacled cobra (Naja naja) venom and its immunological cross-reactivity towards commercial antivenom. Int J Biol Macromol 160:224–232. https://doi.org/10.1016/j.ijbiomac.2020.05.106
Chang CC, Tseng KH (1978) Effect of crotamine, a toxin of South American rattlesnake venom, on the sodium channel of murine skeletal muscle. Br J Pharmacol 63(3):551–559. https://doi.org/10.1111/j.1476-5381.1978.tb07811.x
Chiappinelli VA (1983) Kappa-Bungarotoxin: a probe for the neuronal nicotinic receptor in the avian ciliary ganglion. Brain Res 277(1):9–22. https://doi.org/10.1016/0006-8993(83)90902-2
Choudhury M, McCleary RJR, Kesherwani M, Kini RM, Velmurugan D (2017) Comparison of proteomic profiles of the venoms of two of the ‘Big Four’ snakes of India, the Indian cobra (Naja naja) and the common krait (Bungarus caeruleus), and analyses of their toxins. Toxicon 135:33–42. https://doi.org/10.1016/j.toxicon.2017.06.005
Churchman A, O’Leary MA, Buckley NA et al (2010) Clinical effects of red-bellied black snake (Pseudechis porphyriacus) envenoming and correlation with venom concentrations: Australian Snakebite Project (ASP-11). Med J Aust 193(11–12):696–700. https://doi.org/10.5694/j.1326-5377.2010.tb04108.x
Ciscotto P, Machado de Avila RA, Coelho EAF et al (2009) Antigenic, microbicidal and antiparasitic properties of an l-amino acid oxidase isolated from Bothrops jararaca snake venom. Toxicon 53(3):330–341. https://doi.org/10.1016/j.toxicon.2008.12.004
Clarke C, Kuruppu S, Reeve S, Ian Smith A, Hodgson WC (2006) Oxylepitoxin-1, a reversible neurotoxin from the venom of the inland taipan (Oxyuranus microlepidotus). Peptides 27(11):2655–2660. https://doi.org/10.1016/j.peptides.2006.06.003
Clemetson KJ (2010) Snaclecs (snake C-type lectins) that inhibit or activate platelets by binding to receptors. Toxicon 56(7):1236–1246. https://doi.org/10.1016/j.toxicon.2010.03.011
Clemetson KJ, Morita T, Manjunatha Kini R (2009) Scientific and standardization committee communications: classification and nomenclature of snake venom C-type lectins and related proteins. J Thromb Haemost 7(2):360. https://doi.org/10.1111/j.1538-7836.2008.03233.x
Costa T, Burin S, Menaldo D, de Attié F, Sampaio S (2014) Snake venom L-amino acid oxidases: An overview on their antitumor effects. Journal Venom Animal Tox Includ Trop Diseases 20:23. https://doi.org/10.1186/1678-9199-20-23
Daltry JC, Wuster W, Thorpe RS (1997) The role of ecology in determining venom variation in the Malayan pitviper, Calloselasma rhodostoma. Venomous Snakes: Ecology, Evolution and Snakebite: Symposia of the Zoological Society of London No.70, Pp. 155-171. Clarendon Press, Oxford
de Silva HA, Ryan NM, de Silva HJ (2016a) Adverse reactions to snake antivenom, and their prevention and treatment. Br J Clin Pharmacol 81(3):446–452. https://doi.org/10.1111/bcp.12739
Dowell NL, Giorgianni MW, Kassner VA, Selegue JE, Sanchez EE, Carroll SB (2016) The Deep origin and recent loss of venom toxin genes in rattlesnakes. Curr Biol 26(18):2434–2445. https://doi.org/10.1016/j.cub.2016.07.038
Dutertre S, Lewis RJ (2010) Use of venom peptides to probe ion channel structure and function. J Biol Chem 285(18):13315–13320. https://doi.org/10.1074/jbc.R109.076596
Erulu VE, Okumu MO, Ochola FO, Gikunju JK (2018) Revered but poorly understood: a case report of dendroaspis polylepis (black mamba) envenomation in watamu, malindi kenya, and a review of the literature. Trop Med Infect Dis. https://doi.org/10.3390/tropicalmed3030104
Felicori LF, Souza CT, Velarde DT et al (2003) Kallikrein-like proteinase from bushmaster snake venom. Protein Expr Purif 30(1):32–42. https://doi.org/10.1016/S1046-5928(03)00053-6
Ferreira SH (1965) A bradykinin-potentiating factor (bpf) present in the venom of bothrops jararca. Br J Pharmacol Chemother 24(1):163–169. https://doi.org/10.1111/j.1476-5381.1965.tb02091.x
Ferreira IG, Pucca MB, Oliveira ISd, Cerni FA, Jacob BdCdS, Arantes EC (2021) Snake venom vascular endothelial growth factors (svVEGFs): Unravelling their molecular structure, functions, and research potential. Cytokine Growth Factor Rev 60:133–143. https://doi.org/10.1016/j.cytogfr.2021.05.003
Fox JW (2013) A brief review of the scientific history of several lesser-known snake venom proteins: l-amino acid oxidases, hyaluronidases and phosphodiesterases. Toxicon 62:75–82. https://doi.org/10.1016/j.toxicon.2012.09.009
Fry BG (2015) Venomous reptiles and their toxins. Oxford University Press, New York
Fry BG, Winkel KD, Wickramaratna JC, Hodgson WC, Wüster W (2003) Effectiveness of snake antivenom: species and regional venom variation and its clinical impact. J Toxicol Toxin Review 22(1):23–34. https://doi.org/10.1081/TXR-120019018
Gempeler-Messina PM, Volz K, Bühler B, Müller C (2001) Protein c activators from snake venoms and their diagnostic use. Pathophysiol Haemost Thromb 31(3–6):266–272. https://doi.org/10.1159/000048072
Ghezellou P, Albuquerque W, Garikapati V et al (2021) Integrating top-down and bottom-up mass spectrometric strategies for proteomic profiling of iranian saw-scaled viper, echis carinatus sochureki. Venom J Prot Res 20(1):895–908. https://doi.org/10.1021/acs.jproteome.0c00687
Giorgianni MW, Dowell NL, Griffin S, Kassner VA, Selegue JE, Carroll SB (2020) The origin and diversification of a novel protein family in venomous snakes. Proc Natl Acad Sci 117(20):10911–10920. https://doi.org/10.1073/pnas.1920011117
Giribaldi J, Kazandjian T, Amorim FG et al (2020) Venomics of the asp viper vipera aspis aspis from France. J Proteomics 218:103707. https://doi.org/10.1016/j.jprot.2020.103707
Goncalves-Machado L, Pla D, Sanz L et al (2016) Combined venomics, venom gland transcriptomics, bioactivities, and antivenomics of two bothrops jararaca populations from geographic isolated regions within the brazilian Atlantic rainforest. J Proteomics 135:73–89. https://doi.org/10.1016/j.jprot.2015.04.029
Gower DJ, Sampaio FL, Peichl L et al (2019) Evolution of the eyes of vipers with and without infrared-sensing pit organs. Biol J Lin Soc 126(4):796–823. https://doi.org/10.1093/biolinnean/blz003
Gutiérrez JM, Lomonte B, León G et al (2009) Snake venomics and antivenomics: proteomic tools in the design and control of antivenoms for the treatment of snakebite envenoming. J Proteomics 72(2):165–182. https://doi.org/10.1016/j.jprot.2009.01.008
Gutiérrez JM, León G, Burnouf T (2011) Antivenoms for the treatment of snakebite envenomings: The road ahead. Biologicals 39(3):129–142. https://doi.org/10.1016/j.biologicals.2011.02.005
Gutiérrez JM, Calvete JJ, Habib AG, Harrison RA, Williams DJ, Warrell DA (2017) Snakebite Envenoming. Nat Rev Dis Primers 3(1):17063. https://doi.org/10.1038/nrdp.2017.63
Hargreaves AD, Swain MT, Hegarty MJ, Logan DW, Mulley JF (2014) Restriction and recruitment—gene duplication and the origin and evolution of snake venom toxins. Genome Biol Evol 6(8):2088–2095
Harrison RA, Cook DA, Renjifo C, Casewell NR, Currier RB, Wagstaff SC (2011) Research strategies to improve snakebite treatment: Challenges and progress. J Proteomics 74(9):1768–1780. https://doi.org/10.1016/j.jprot.2011.06.019
Hart AJ, Hodgson WC, O’Leary M, Isbister GK (2014) Pharmacokinetics and pharmacodynamics of the myotoxic venom of Pseudechis australis (mulga snake) in the anesthetised rat. Clin Toxicol (phila) 52(6):604–610. https://doi.org/10.3109/15563650.2014.914526
Harvey AL (1997) Recent studies on dendrotoxins and potassium ion channels. General Pharmacol 28(1):7–12. https://doi.org/10.1016/S0306-3623(96)00173-5
Harvey AL (2001) Twenty years of dendrotoxins. Toxicon 39(1):15–26. https://doi.org/10.1016/S0041-0101(00)00162-8
Harvey A, Robertson B (2005) Dendrotoxins: structure-activity relationships and effects on potassium ion channels. Curr Med Chem 11:3065–3072. https://doi.org/10.2174/0929867043363820
Hennerici MG, Kay R, Bogousslavsky J, Lenzi GL, Verstraete M, Orgogozo JM (2006) Intravenous ancrod for acute ischaemic stroke in the European stroke treatment with ancrod trial: a randomised controlled trial. Lancet 368(9550):1871–1878. https://doi.org/10.1016/s0140-6736(06)69776-6
Hiu JJ, Yap MKK (2022) The myth of cobra venom cytotoxin: More than just direct cytolytic actions. Toxicon X 14:100123. https://doi.org/10.1016/j.toxcx.2022.100123
Huang K-F, Liu Y-L, Cheng W-J, Ko T-P, Wang AH-J (2005) Crystal structures of human glutaminyl cyclase, an enzyme responsible for protein N-terminal pyroglutamate formation. Proc Natl Acad Sci 102(37):13117–13122. https://doi.org/10.1073/pnas.0504184102
Huynh TM, Hodgson WC, Isbister GK, Silva A (2022) The Effect of Australian and Asian Commercial Antivenoms in Reversing the Post-Synaptic Neurotoxicity of O hannah N naja and N kaouthia Venoms In Vitro. Toxins. https://doi.org/10.3390/toxins14040277
Isbister GK (2010) Snakebite doesn’t cause disseminated intravascular coagulation: coagulopathy and thrombotic microangiopathy in snake envenoming. Semin Thromb Hemost 36(4):444–451. https://doi.org/10.1055/s-0030-1254053
Isbister GK, O’Leary MA, Hagan J et al (2010a) Cross-neutralisation of Australian brown snake, taipan and death adder venoms by monovalent antibodies. Vaccine 28(3):798–802. https://doi.org/10.1016/j.vaccine.2009.10.055
Isbister GK, Scorgie FE, O’Leary MA, Seldon M, Brown SG, Lincz LF (2010b) Factor deficiencies in venom-induced consumption coagulopathy resulting from Australian elapid envenomation: Australian Snakebite Project (ASP-10). J Thromb Haemost 8(11):2504–2513. https://doi.org/10.1111/j.1538-7836.2010.04050.x
Isbister GK, Maduwage K, Page CB (2014) Antivenom cross neutralisation in a suspected Asian pit viper envenoming causing severe coagulopathy. Toxicon 90:286–290. https://doi.org/10.1016/j.toxicon.2014.08.071
Isbister GK, Mirajkar N, Fakes K, Brown SGA, Veerati PC (2020) Phospholipase A2 (PLA2) as an early indicator of envenomation in australian elapid snakebites (ASP-27). Biomedicines 8(11):459
Ishikawa Y, Kano M, Tamiya N, Shimada Y (1985) Acetylcholine receptors of human skeletal muscle: a species difference detected by snake neurotoxins. Brain Res 346(1):82–88. https://doi.org/10.1016/0006-8993(85)91097-2
Islam T, Madhubala D, Mukhopadhyay R, Mukherjee AK (2021) Transcriptomic and functional proteomics analyses to unveil the common and unique pathway(s) of neuritogenesis induced by Russell’s viper venom nerve growth factor in rat pheochromocytoma neuronal cells. Expert Rev Proteomics 18(6):463–481. https://doi.org/10.1080/14789450.2021.1941892
Isomoto A, Shoguchi E, Hisata K et al (2022) Active expression of genes for protein modification enzymes in habu venom glands. Toxins 14(5):300
Izidoro LF, Sobrinho JC, Mendes MM et al (2014) Snake venom L-amino acid oxidases: trends in pharmacology and biochemistry. Biomed Res Int 2014:196754. https://doi.org/10.1155/2014/196754
Jackson TNW, Young B, Underwood G et al (2017) Endless forms most beautiful: the evolution of ophidian oral glands, including the venom system, and the use of appropriate terminology for homologous structures. Zoomorphology 136(1):107–130. https://doi.org/10.1007/s00435-016-0332-9
Jesupret C, Baumann K, Jackson TNW et al (2014) Vintage venoms: Proteomic and pharmacological stability of snake venoms stored for up to eight decades. J Proteomics 105:285–294. https://doi.org/10.1016/j.jprot.2014.01.004
Johnston CI, Isbister GK (2021) Australian snakebite myotoxicity (ASP-23). Clin Toxicol 59(7):611–618. https://doi.org/10.1080/15563650.2020.1836377
Johnston CI, Brown SGA, O’Leary MA et al (2013) Mulga snake (Pseudechis australis) envenoming: a spectrum of myotoxicity, anticoagulant coagulopathy, haemolysis and the role of early antivenom therapy – Australian Snakebite Project (ASP-19). Clin Toxicol 51(5):417–424. https://doi.org/10.3109/15563650.2013.787535
Johnston CI, Ryan NM, Page CB et al (2017b) The australian snakebite project, 2005–2015 (ASP-20). Med J Aust 207(3):119–125. https://doi.org/10.5694/mja17.00094
Johnston CI, Ryan NM, O’Leary MA, Brown SG, Isbister GK (2017a) Australian taipan (Oxyuranus spp) envenoming clinical effects and potential benefits of early antivenom therapy-Australian snakebite project. Clin Toxicol. https://doi.org/10.1080/15563650.2016.1250903
Jones BK, Saviola AJ, Reilly SB et al (2019) Venom composition in a phenotypically variable pit viper (trimeresurus insularis) across the lesser sunda archipelago. J Proteome Res 18(5):2206–2220. https://doi.org/10.1021/acs.jproteome.9b00077
Kalita B, Saviola AJ, Mukherjee AK (2021) From venom to drugs: a review and critical analysis of Indian snake venom toxins envisaged as anticancer drug prototypes. Drug Discovery Today 26(4):993–1005. https://doi.org/10.1016/j.drudis.2020.12.021
Kang TS, Georgieva D, Genov N et al (2011) Enzymatic toxins from snake venom: structural characterization and mechanism of catalysis. FEBS J 278(23):4544–4576. https://doi.org/10.1111/j.1742-4658.2011.08115.x
Kardong KV (2002) COLUBRID SNAKES AND DUVERNOY’S “VENOM” GLANDS. J Toxicol 21(1–2):1–19. https://doi.org/10.1081/TXR-120004739
Keogh JS, Scott IA, Hayes C (2005) Rapid and repeated origin of insular gigantism and dwarfism in Australian tiger snakes. Evolution 59(1):226–233. https://doi.org/10.1111/j.0014-3820.2005.tb00909.x
Kerkis I, Hayashi MAF, Prieto da Silva ARB et al (2014) State of the Art in the Studies on Crotamine, a cell penetrating peptide from south american rattlesnake. Biomed Res Int 2014:675985. https://doi.org/10.1155/2014/675985
Kerns RT, Kini RM, Stefansson S, Evans HJ (1999) Targeting of venom phospholipases: the strongly anticoagulant phospholipase a2 from naja nigricollis venom binds to coagulation factor xa to inhibit the prothrombinase complex. Arch Biochem Biophys 369(1):107–113. https://doi.org/10.1006/abbi.1999.1345
Kini RM (2002) Molecular moulds with multiple missions: functional sites in three-finger toxins. Clin Exp Pharmacol Physiol 29(9):815–822. https://doi.org/10.1046/j.1440-1681.2002.03725.x
Kini RM (2005) Serine Proteases Affecting Blood Coagulation and Fibrinolysis from Snake Venoms. Pathophysiol Haemost Thromb 34(4–5):200–204. https://doi.org/10.1159/000092424
Kini RM, Doley R (2010) Structure, function and evolution of three-finger toxins: mini proteins with multiple targets. Toxicon 56(6):855–867. https://doi.org/10.1016/j.toxicon.2010.07.010
Kleiz-Ferreira JM, Cirauqui N, Trajano EA, Almeida MdS, Zingali RB (2021) Three-finger toxins from brazilian coral snakes: from molecular framework to insights in biological function. Toxins 13(5):328
Kornhauser R, Isbister GK, O’Leary MA, Mirtschin P, Dunstan N, Hodgson WC (2013) Cross-neutralisation of the neurotoxic effects of Egyptian cobra venom with commercial tiger snake antivenom. Basic Clin Pharmacol Toxicol 112(2):138–143. https://doi.org/10.1111/j.1742-7843.2012.00925.x
Kuruppu S, Reeve S, Banerjee Y, Kini RM, Smith AI, Hodgson WC (2005) Isolation and pharmacological characterization of cannitoxin, a presynaptic neurotoxin from the venom of the papuan taipan (Oxyuranus scutellatus canni). J Pharmacol Exp Ther 315(3):1196–1202. https://doi.org/10.1124/jpet.105.093641
Lalloo DG, Theakston RD, Warrell DA (2002) The African challenge. Lancet 359(9316):1527. https://doi.org/10.1016/s0140-6736(02)08456-8
Lane J, O’Leary MA, Isbister GK (2011) Coagulant effects of black snake (Pseudechis spp) venoms and in vitro efficacy of commercial antivenom. Toxicon 58(3):239–246. https://doi.org/10.1016/j.toxicon.2011.05.020
Laustsen AH (2018) Guiding recombinant antivenom development by omics technologies. N Biotechnol 45:19–27. https://doi.org/10.1016/j.nbt.2017.05.005
Laustsen AH, Gutierrez JM, Lohse B et al (2015) Snake venomics of monocled cobra (Naja kaouthia) and investigation of human IgG response against venom toxins. Toxicon 99:23–35. https://doi.org/10.1016/j.toxicon.2015.03.001
Levy DE, del Zoppo GJ, Demaerschalk BM et al (2009) Ancrod in acute ischemic stroke: results of 500 subjects beginning treatment within 6 hours of stroke onset in the ancrod stroke program. Stroke 40(12):3796–3803. https://doi.org/10.1161/strokeaha.109.565119
Li L, Huang J, Lin Y (2018) Snake venoms in cancer therapy: past. Pres Future Toxins 10(9):346
Lin WW, Smith LA, Lee CY (1995) A study on the cause of death due to waglerin-I, a toxin from trimeresurus wagleri. Toxicon 33(1):111–114. https://doi.org/10.1016/0041-0101(94)00134-t
Lin J-H, Sung W-C, Mu H-W, Hung D-Z (2022) Local cytotoxic effects in cobra envenoming: a pilot study. Toxins 14(2):122
Lippa E, Török F, Gómez A et al (2019) First look into the venom of Roatan Island’s critically endangered coral snake Micrurus ruatanus: Proteomic characterization, toxicity, immunorecognition and neutralization by an antivenom. J Proteomics 198:177–185. https://doi.org/10.1016/j.jprot.2019.01.007
Liu Y, Chen Q, Papenfuss TJ, Lu F, Tang Y (2016) Eye and pit size are inversely correlated in crotalinae: implications for selection pressure relaxation. J Morphol 277(1):107–117. https://doi.org/10.1002/jmor.20483
Liu CC, Lin CC, Hsiao YC, Wang PJ, Yu JS (2018) Proteomic characterization of six taiwanese snake venoms: identification of species-specific proteins and development of a siscapa-mrm assay for cobra venom factors. J Proteomics 187:59–68. https://doi.org/10.1016/j.jprot.2018.06.003
Maduwage K, Isbister GK, Silva A, Bowatta S, Mendis S, Gawarammana I (2013) Epidemiology and clinical effects of hump-nosed pit viper (Genus: Hypnale) envenoming in Sri Lanka. Toxicon 61:11–15. https://doi.org/10.1016/j.toxicon.2012.10.013
Maduwage K, O’Leary MA, Isbister GK (2014) Diagnosis of snake envenomation using a simple phospholipase A2 assay. Sci Rep 4:4827. https://doi.org/10.1038/srep04827
Maduwage KP, Scorgie FE, Lincz LF, O’Leary MA, Isbister GK (2016) Procoagulant snake venoms have differential effects in animal plasmas: Implications for antivenom testing in animal models. Thromb Res 137:174–177. https://doi.org/10.1016/j.thromres.2015.12.002
Maduwage K, Buckley NA, de Silva HJ, Lalloo DG, Isbister GK (2015) Snake antivenom for snake venom induced consumption coagulopathy. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD011428.pub2
Mamede CCN, de Sousa Simamoto BB, da Cunha Pereira DF, de Oliveira CJ, Ribeiro MSM, de Oliveira F (2020) Edema, hyperalgesia and myonecrosis induced by brazilian bothropic venoms: overview of the last decade. Toxicon 187:10–18. https://doi.org/10.1016/j.toxicon.2020.08.016
Mancin AC, Soares AM, Andrião-Escarso SH et al (1998) The analgesic activity of crotamine, a neurotoxin from crotalus durissus terrificus (south american rattlesnake) venom: a biochemical and pharmacological study. Toxicon 36(12):1927–1937. https://doi.org/10.1016/S0041-0101(98)00117-2
Margres MJ, McGivern JJ, Seavy M, Wray KP, Facente J, Rokyta DR (2015) Contrasting modes and tempos of venom expression evolution in two snake species. Genetics 199(1):165–176. https://doi.org/10.1534/genetics.114.172437
Margres MJ, Wray KP, Sanader D et al (2021) Varying Intensities of Introgression Obscure Incipient Venom-Associated Speciation in the Timber Rattlesnake (Crotalus horridus). Toxins. https://doi.org/10.3390/toxins13110782
Mion G, Larréché S, Benois A, Petitjeans F, Puidupin M (2013) Hemostasis dynamics during coagulopathy resulting from echis envenomation. Toxicon 76:103–109. https://doi.org/10.1016/j.toxicon.2013.09.003
Molles BE, Tsigelny I, Nguyen PD, Gao SX, Sine SM, Taylor P (2002) Residues in the epsilon subunit of the nicotinic acetylcholine receptor interact to confer selectivity of waglerin-1 for the alpha-epsilon subunit interface site. Biochemistry 41(25):7895–7906. https://doi.org/10.1021/bi025732d
Montecucco C, Rossetto O (2008) On the quaternary structure of taipoxin and textilotoxin: The advantage of being multiple. Toxicon 51(8):1560–1562. https://doi.org/10.1016/j.toxicon.2008.03.020
Mora-Obando D, Salazar-Valenzuela D, Pla D et al (2020) Venom variation in Bothrops asper lineages from North-Western South America. J Proteomics 229:103945. https://doi.org/10.1016/j.jprot.2020.103945
Mukherjee AK, Dutta S, Kalita B, Jha DK, Deb P, Mackessy SP (2016) Structural and functional characterization of complex formation between two Kunitz-type serine protease inhibitors from Russell’s Viper venom. Biochimie 128–129:138–147. https://doi.org/10.1016/j.biochi.2016.08.005
Nakamura H, Murakami T, Imamura T et al (2014) Discovery of a novel vascular endothelial growth factor (VEGF) with no affinity to heparin in Gloydius tsushimaensis venom. Toxicon 86:107–115. https://doi.org/10.1016/j.toxicon.2014.05.003
Neale V, Sotillo J, Seymour JE, Wilson D (2017) The Venom of the Spine-Bellied Sea Snake (Hydrophis curtus): proteome, toxin diversity and intraspecific variation. Int J Mol Sci 18(12):2695. https://doi.org/10.3390/ijms18122695
Neri-Castro E, Sanz L, Olvera-Rodríguez A, Bénard-Valle M, Alagón A, Calvete JJ (2020) Venomics and biochemical analysis of the black-tailed horned pitviper, Mixcoatlus melanurus, and characterization of melanurutoxin, a novel crotoxin homolog. J Proteomics 225:103865. https://doi.org/10.1016/j.jprot.2020.103865
Nys M, Zarkadas E, Brams M et al (2022) The molecular mechanism of snake short-chain α-neurotoxin binding to muscle-type nicotinic acetylcholine receptors. Nat Commun 13(1):4543. https://doi.org/10.1038/s41467-022-32174-7
Ogawa T, Chijiwa T, Oda-Ueda N, Ohno M (2005) Molecular diversity and accelerated evolution of C-type lectin-like proteins from snake venom. Toxicon 45(1):1–14. https://doi.org/10.1016/j.toxicon.2004.07.028
Ogawa Y, Murayama N, Fujita Y, Yanoshita R (2007) Characterization and cDNA cloning of aminopeptidase A from the venom of Gloydius blomhoffi brevicaudus. Toxicon 49(8):1172–1181. https://doi.org/10.1016/j.toxicon.2007.02.012
Ogawa T, Oda-Ueda N, Hisata K et al (2019) Alternative mRNA Splicing in Three Venom Families Underlying a Possible Production of Divergent Venom Proteins of the Habu Snake Protobothrops flavoviridis. Toxins (basel). https://doi.org/10.3390/toxins11100581
Olaoba OT, Santos PKd, Selistre-de-Araujo HS, Souza DHFd (2020) Snake venom metalloproteinases (SVMPs) A structure-function update. Toxicon X. https://doi.org/10.1016/j.toxcx.2020.100052
Oliveira AL, Viegas MF, da Silva SL, Soares AM, Ramos MJ, Fernandes PA (2022a) The chemistry of snake venom and its medicinal potential. Nat Rev Chem 6(7):451–469. https://doi.org/10.1038/s41570-022-00393-7
Oliveira ISd, Pucca MB, Ferreira IG et al (2022b) State-of-the-art review of snake venom phosphodiesterases (svPDEs). Toxicon 217:121–130. https://doi.org/10.1016/j.toxicon.2022.08.004
Omidi S, Mehrpouya M, Oladnabi M, Azadmehr A, Kazemi-Lomedasht F, Yardehnavi N (2021) Evaluation of venom as a promising tool for drug discovery [focusing on Neurological disorders]. Venom Toxins. https://doi.org/10.2174/2666121701666211124151529
Otero-Patiño R (2009) Epidemiological, clinical and therapeutic aspects of bothrops asper bites. Toxicon 54(7):998–1011. https://doi.org/10.1016/j.toxicon.2009.07.001
Ouyang C, Huang T-F (1983) Inhibition of platelet aggregation by 5′-nucleotidase purified from Trimeresurus gramineus snake venom. Toxicon 21(4):491–501. https://doi.org/10.1016/0041-0101(83)90127-7
Owen DG, Hall A, Stephens G, Stow J, Robertson B (1997) The relative potencies of dendrotoxins as blockers of the cloned voltage-gated K channel when stably expressed in Chinese hamster ovary cells. Br J Pharmacol 120(6):1029–1034
Pawelek PD, Cheah J, Coulombe R, Macheroux P, Ghisla S, Vrielink A (2000) The structure of L-amino acid oxidase reveals the substrate trajectory into an enantiomerically conserved active site. EMBO J 19(16):4204–4215. https://doi.org/10.1093/emboj/19.16.4204
Pawlak J, Manjunatha Kini R (2006) Snake venom glutaminyl cyclase. Toxicon 48(3):278–286. https://doi.org/10.1016/j.toxicon.2006.05.013
Peigneur S, Orts DJB, Prieto da Silva AR et al (2012) Crotamine pharmacology revisited: novel insights based on the inhibition of k<sub>v</sub> channels. Mol Pharmacol 82(1):90–96. https://doi.org/10.1124/mol.112.078188
Perry BW, Gopalan SS, Pasquesi GIM et al (2022) Snake venom gene expression is coordinated by novel regulatory architecture and the integration of multiple co-opted vertebrate pathways. Genome Res 32(6):1058–1073
Péterfi O, Boda F, Szabó Z, Ferencz E, Bába L (2019) Hypotensive snake venom components—a mini-review. Molecules 24(15):2778
Pla D, Bande BW, Welton RE et al (2017) Proteomics and antivenomics of papuan black snake (pseudechis papuanus) venom with analysis of its toxicological profile and the preclinical efficacy of australian antivenoms. J Proteomics 150:201–215. https://doi.org/10.1016/j.jprot.2016.09.007
Quarch V, Brander L, Cioccari L (2017) An Unexpected case of black mamba (dendroaspis polylepis) bite in switzerland. Case Rep Crit Care 2017:5021924. https://doi.org/10.1155/2017/5021924
Rao VS, Kini RM (2002) Pseutarin C, a prothrombin activator from pseudonaja textilis venom: its structural and functional similarity to mammalian coagulation factor xa-va complex. J Thromb Haemost 88(4):611–619
Rautsaw RM, Hofmann EP, Margres MJ et al (2019) Intraspecific sequence and gene expression variation contribute little to venom diversity in sidewinder rattlesnakes ( Crotalus cerastes). Proc Biol Sci 286(1906):20190810–20190810. https://doi.org/10.1098/rspb.2019.0810
Richards R, St Pierre L, Trabi M et al (2011) Cloning and characterisation of novel cystatins from elapid snake venom glands. Biochimie 93(4):659–668. https://doi.org/10.1016/j.biochi.2010.12.008
Rowan EG (2001) What does β-bungarotoxin do at the neuromuscular junction? Toxicon 39(1):107–118. https://doi.org/10.1016/s0041-0101(00)00159-8
Russell FE, Buess FW, Woo MY (1963) Zootoxicological properties of venom phosphodiesterase. Toxicon 1(3):99–108. https://doi.org/10.1016/0041-0101(63)90070-9
Sahyoun C, Rima M, Mattei C, Sabatier J-M, Fajloun Z, Legros C (2022) Separation and Analytical Techniques Used in Snake Venomics: A Review Article. Processes 10(7):1380
Saviola AJ, Pla D, Sanz L, Castoe TA, Calvete JJ, Mackessy SP (2015) Comparative venomics of the Prairie Rattlesnake (Crotalus viridis viridis) from Colorado: Identification of a novel pattern of ontogenetic changes in venom composition and assessment of the immunoreactivity of the commercial antivenom CroFab(R). J Proteomics 121:28–43. https://doi.org/10.1016/j.jprot.2015.03.015
Saviola AJ, Gandara AJ, Bryson RW Jr, Mackessy SP (2017) Venom phenotypes of the Rock Rattlesnake (Crotalus lepidus) and the Ridge-nosed Rattlesnake (Crotalus willardi) from Mexico and the United States. Toxicon 138:119–129. https://doi.org/10.1016/j.toxicon.2017.08.016
Saviola AJ, Peichoto ME, Mackessy SP (2014) Rear-fanged snake venoms: an untapped source of novel compounds and potential drug leads. Toxin Rev 33(4):185–201
Shibata H, Chijiwa T, Oda-Ueda N et al (2018) The habu genome reveals accelerated evolution of venom protein genes. Sci Rep 8(1):1–1. https://doi.org/10.1038/s41598-018-28749-4
Silva A, Isbister GK (2020) Current research into snake antivenoms, their mechanisms of action and applications. Biochem Soc Trans 48(2):537–546. https://doi.org/10.1042/bst20190739
Silva A, Hodgson WC, Isbister GK (2016b) Cross-neutralisation of in vitro neurotoxicity of asian and australian snake neurotoxins and venoms by different antivenoms. Toxins 8(10):302
Silva A, Maduwage K, Sedgwick M et al (2016c) Neurotoxicity in Russell’s viper (Daboia russelii) envenoming in Sri Lanka: a clinical and neurophysiological study. Clin Toxicol (phila) 54(5):411–419. https://doi.org/10.3109/15563650.2016.1143556
Silva A, Cristofori-Armstrong B, Rash LD, Hodgson WC, Isbister GK (2018) Defining the role of post-synaptic α-neurotoxins in paralysis due to snake envenoming in humans. Cell Mol Life Sci 75(23):4465–4478. https://doi.org/10.1007/s00018-018-2893-x
Silva A, Maduwage KP, Sedgwick MEE et al (2016d) Neuromuscular Effects of Common Krait (Bungarus caeruleus) Envenoming in Sri Lanka. PLoS Negl Trop Dis. https://doi.org/10.1371/journal.pntd.0004368
Silva A, Hodgson WC, Isbister GK (2017) Antivenom for Neuromuscular Paralysis Resulting From Snake Envenoming. Toxins (basel). https://doi.org/10.3390/toxins9040143
Slagboom J, Kaal C, Arrahman A et al (2022) Analytical strategies in venomics. Microchem J 175:107187. https://doi.org/10.1016/j.microc.2022.107187
Smith LM, Kelleher NL (2013) Proteoform: a single term describing protein complexity. Nat Methods 10(3):186–187. https://doi.org/10.1038/nmeth.2369
Sofyantoro F, Yudha DS, Lischer K et al (2022) Bibliometric analysis of literature in snake venom-related research worldwide (1933–2022). Animals 12(16):2058
Souza R, Lima T, Luiz J, Cardoso CN (2007) The Enigma of the North Margin of the Amazon River: Proven Lachesis Bites in Brazil, Report of Two Cases, General Considerations about the Genus and Bibliographic Review. In 42(7):105–115
St Pierre L, Masci PP, Filippovich I et al (2005) Comparative analysis of prothrombin activators from the venom of Australian elapids. Mol Biol Evol 22(9):1853–1864. https://doi.org/10.1093/molbev/msi181
St Pierre L, Flight S, Masci PP et al (2006) Cloning and characterisation of natriuretic peptides from the venom glands of Australian elapids. Biochimie 88(12):1923–1931. https://doi.org/10.1016/j.biochi.2006.06.014
Sunagar K, Fry BG, Jackson TN et al (2013) Molecular evolution of vertebrate neurotrophins: co-option of the highly conserved nerve growth factor gene into the advanced snake venom arsenalf. PLoS ONE 8(11):e81827. https://doi.org/10.1371/journal.pone.0081827
Suntravat M, Cromer WE, Marquez J et al (2019) The isolation and characterization of a new snake venom cysteine-rich secretory protein (svCRiSP) from the venom of the Southern Pacific rattlesnake and its effect on vascular permeability. Toxicon 165:22–30. https://doi.org/10.1016/j.toxicon.2019.04.006
Suranse V, Jackson TNW, Sunagar K (2022) Contextual Constraints: Dynamic Evolution of Snake Venom Phospholipase A2. Toxins 14(6):420
Suryamohan K, Krishnankutty SP, Guillory J et al (2020) The Indian cobra reference genome and transcriptome enables comprehensive identification of venom toxins. Nat Genet 52(1):106–117. https://doi.org/10.1038/s41588-019-0559-8
Suzuki N, Yamazaki Y, Brown RL, Fujimoto Z, Morita T, Mizuno H (2008) Structures of pseudechetoxin and pseudecin, two snake-venom cysteine-rich secretory proteins that target cyclic nucleotide-gated ion channels: implications for movement of the C-terminal cysteine-rich domain. Acta Crystallogr D Biol Crystallogr 64(Pt 10):1034–1042. https://doi.org/10.1107/s0907444908023512
Tadokoro T, M. Modahl C, Maenaka K, Aoki-Shioi N, (2020) Cysteine-Rich Secretory Proteins (CRISPs) from Venomous Snakes: An Overview of the Functional Diversity in a Large and Underappreciated Superfamily. Toxins 12(3):175
Tan N-H, Tan C-S (1988) Partial purification of acetylcholinesterase from the venom of the shore pit viper (Trimeresurus purpureomaculatus). Toxicon 26(5):505–508. https://doi.org/10.1016/0041-0101(88)90190-0
Tan CH, Tan KY, Fung SY, Tan NH (2015) Venom-gland transcriptome and venom proteome of the Malaysian king cobra (Ophiophagus hannah). BMC Genomics 16(1):687. https://doi.org/10.1186/s12864-015-1828-2
Tan CH, Fung SY, Yap MK, Leong PK, Liew JL, Tan NH (2016) Unveiling the elusive and exotic: Venomics of the Malayan blue coral snake (Calliophis bivirgata flaviceps). J Proteomics 132:1–12. https://doi.org/10.1016/j.jprot.2015.11.014
Tan CH, Tan KY, Yap MK, Tan NH (2017) Venomics of Tropidolaemus wagleri, the sexually dimorphic temple pit viper: Unveiling a deeply conserved atypical toxin arsenal. Sci Rep 7:43237. https://doi.org/10.1038/srep43237
Tan KK, Bay BH, Gopalakrishnakone P (2018) L-amino acid oxidase from snake venom and its anticancer potential. Toxicon 144:7–13. https://doi.org/10.1016/j.toxicon.2018.01.015
Tan CH, Tan KY, Ng TS, Sim SM, Tan NH (2019) Venom Proteome of Spine-Bellied Sea Snake (Hydrophis curtus) from Penang, Malaysia: Toxicity Correlation, Immunoprofiling and Cross-Neutralization by Sea Snake Antivenom. Toxins 11(1):3
Tan CH (2022) Snake Venomics: Fundamentals, Recent Updates, and a Look to the Next Decade. Toxins (basel). https://doi.org/10.3390/toxins14040247
Tasoulis T, Isbister GK (2017) A Review and Database of Snake Venom Proteomes. Toxins (basel) 9(9):290. https://doi.org/10.3390/toxins9090290
Tasoulis T, Lee MSY, Ziajko M, Dunstan N, Sumner J, Isbister GK (2020a) Activity of two key toxin groups in Australian elapid venoms show a strong correlation to phylogeny but not to diet. BMC Evol Biol 20(1):9. https://doi.org/10.1186/s12862-020-1578-x
Tasoulis T, Silva A, Veerati PC et al (2020b) Intra-Specific Venom Variation in the Australian Coastal Taipan Oxyuranus scutellatus. Toxins 12(8):485
Tasoulis T, Pukala TL, Isbister GK (2022a) Investigating Toxin Diversity and Abundance in Snake Venom Proteomes. Front Pharmacol. https://doi.org/10.3389/fphar.2021.768015
Tasoulis T, Wang CR, Sumner J, Dunstan N, Pukala TL, Isbister GK (2022b) The Unusual Metalloprotease-Rich Venom Proteome of the Australian Elapid Snake Hoplocephalus stephensii. Toxins 14(5):314
Trummal K, Tonismagi K, Siigur E et al (2005) A novel metalloprotease from Vipera lebetina venom induces human endothelial cell apoptosis. Toxicon 46(1):46–61. https://doi.org/10.1016/j.toxicon.2005.03.008
Ullah A, Masood R (2020) The Sequence and Three-Dimensional Structure Characterization of Snake Venom Phospholipases B. Front Mol Biosci 7:175. https://doi.org/10.3389/fmolb.2020.00175
Urra FA, Araya-Maturana R (2022) Putting the brakes on tumorigenesis with snake venom toxins: New molecular insights for cancer drug discovery. Semin Cancer Biol 80:195–204. https://doi.org/10.1016/j.semcancer.2020.05.006
Vaiyapuri S, Wagstaff SC, Watson KA, Harrison RA, Gibbins JM, Hutchinson EG (2010) Purification and Functional Characterisation of Rhiminopeptidase A, a Novel Aminopeptidase from the Venom of Bitis gabonica rhinoceros. PLoS Negl Trop Dis 4(8):e796. https://doi.org/10.1371/journal.pntd.0000796
Van den Berg CW, Aerts PC, Van Dijk H (1991) In vivo anti-complementary activities of the cobra venom factors from Naja naja and Naja haje. J Immunol Methods 136(2):287–294. https://doi.org/10.1016/0022-1759(91)90015-8
Vidal N (2002) Colubroid systematics: Evidence for an early appearance of the venom apparatus followed by extensive evolutionary tinkering. Journal of Toxicology-Toxin Reviews 21(1–2):21–41. https://doi.org/10.1081/Txr-120004740
Visser LE, Kyei-Faried S, Belcher DW, Geelhoed DW, van Leeuwen JS, van Roosmalen J (2008) Failure of a new antivenom to treat Echis ocellatus snake bite in rural Ghana: the importance of quality surveillance. Trans R Soc Trop Med Hyg 102(5):445–450. https://doi.org/10.1016/j.trstmh.2007.11.006
Vogel C-W, Fritzinger DC (2010) Cobra venom factor: Structure, function, and humanization for therapeutic complement depletion. Toxicon 56(7):1198–1222. https://doi.org/10.1016/j.toxicon.2010.04.007
Vonk FJ, Admiraal JF, Jackson K et al (2008) Evolutionary origin and development of snake fangs. Nature 454(7204):630–633. https://doi.org/10.1038/nature07178
Vonk FJ, Jackson K, Doley R, Madaras F, Mirtschin PJ, Vidal N (2011) Snake venom: From fieldwork to the clinic: Recent insights into snake biology, together with new technology allowing high-throughput screening of venom, bring new hope for drug discovery. BioEssays 33(4):269–279. https://doi.org/10.1002/bies.201000117
Vonk FJ, Casewell NR, Henkel CV et al (2013) The king cobra genome reveals dynamic gene evolution and adaptation in the snake venom system. Proc Natl Acad Sci U S A 110(51):20651–20656. https://doi.org/10.1073/pnas.1314702110
Wagstaff SC, Laing GD, Theakston RD, Papaspyridis C, Harrison RA (2006) Bioinformatics and multiepitope DNA immunization to design rational snake antivenom. PLoS Med 3(6):e184. https://doi.org/10.1371/journal.pmed.0030184
Wang Y-M, Huang K-F, Tsai I-H (2014) Snake venom glutaminyl cyclases: Purification, cloning, kinetic study, recombinant expression, and comparison with the human enzyme. Toxicon 86:40–50. https://doi.org/10.1016/j.toxicon.2014.04.012
Wang B, Wang Q, Wang C et al (2020) A comparative analysis of the proteomes and biological activities of the venoms from two sea snakes, Hydrophis curtus and Hydrophis cyanocinctus, from Hainan, China. Toxicon 187:35–46. https://doi.org/10.1016/j.toxicon.2020.08.012
Warrell DA (2010) Snake bite. Lancet 375(9708):77–88. https://doi.org/10.1016/s0140-6736(09)61754-2
Weinstein SA, Schmidt JJ, Bernheimer AW, Smith LA (1991) Characterization and amino acid sequences of two lethal peptides isolated from venom of Wagler’s pit viper. Trimeresurus Wagleri Toxicon 29(2):227–236. https://doi.org/10.1016/0041-0101(91)90107-3
Westeen EP, Durso AM, Grundler MC, Rabosky DL, Davis Rabosky AR (2020) What makes a fang? Phylogenetic and ecological controls on tooth evolution in rear-fanged snakes. BMC Evol Biol 20(1):80. https://doi.org/10.1186/s12862-020-01645-0
Wheelock C, Shan GM, Ottea J (2005) Overview of Carboxylesterases and Their Role in the Metabolism of Insecticides. J Pesticide Sci 30:75–83. https://doi.org/10.1584/jpestics.30.75
Whittington AC, Mason AJ, Rokyta DR (2018) A Single Mutation Unlocks Cascading Exaptations in the Origin of a Potent Pitviper Neurotoxin. Mol Biol Evol 35(4):887–898. https://doi.org/10.1093/molbev/msx334
Wijeyewickrema LC, Berndt MC, Andrews RK (2005) Snake venom probes of platelet adhesion receptors and their ligands. Toxicon 45(8):1051–1061. https://doi.org/10.1016/j.toxicon.2005.02.025
Wongtongkam N, Wilde H, Sitthi-Amorn C, Ratanabanangkoon K (2005) A Study of 225 Malayan Pit Viper Bites in Thailand. Mil Med 170(4):342–348. https://doi.org/10.7205/milmed.170.4.342
Xie Q, Tang N, Wan R, Qi Y, Lin X, Lin J (2013) Recombinant snake venom cystatin inhibits tumor angiogenesis in vitro and in vivo associated with downregulation of VEGF-A165, Flt-1 and bFGF. Anti Agent Med Chem 13(4):663–671. https://doi.org/10.2174/1871520611313040015
Yang DC, Deuis JR, Dashevsky D et al (2016) The Snake with the Scorpion’s Sting: Novel Three-Finger Toxin Sodium Channel Activators from the Venom of the Long-Glanded Blue Coral Snake (Calliophis bivirgatus). Toxins 8(10):303
Zancolli G, Calvete JJ, Cardwell MD et al (2019) When one phenotype is not enough: divergent evolutionary trajectories govern venom variation in a widespread rattlesnake species. Proc Biol Sci 286(1898):20182735. https://doi.org/10.1098/rspb.2018.2735
Acknowledgements
The authors would like to thank Shane Black and Ross McGibbon for allowing the use of their stunning photographs.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to the writing of the manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tasoulis, T., Isbister, G.K. A current perspective on snake venom composition and constituent protein families. Arch Toxicol 97, 133–153 (2023). https://doi.org/10.1007/s00204-022-03420-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-022-03420-0