Abstract
Acute liver injury results from the complex interactions of various pathological processes. The TGF-β superfamily plays a crucial role in orchestrating fibrogenic response. In contrast to TGF-β1, a role of TGF-β2 in hepatic fibrogenic response has not been fully investigated. In this study, we showed that TGF-β2 gene expression and secretion are induced in the liver of CCl4 (1 ml/kg)-treated WT mice. Studies with hepatocyte specific ERRγ knockout mice or treatment with an ERRγ-specific inverse agonist, GSK5182 (40 mg/kg), indicated that CCl4-induced hepatic TGF-β2 production is ERRγ dependent. Moreover, IL6 was found as upstream signal to induce hepatic ERRγ and TGF-β2 gene expression in CCl4-mediated acute toxicity model. Over-expression of ERRγ was sufficient to induce hepatic TGF-β2 expression, whereas ERRγ depletion markedly reduces IL6-induced TGF-β2 gene expression and secretion in vitro and in vivo. Promoter assays showed that ERRγ directly binds to an ERR response element in the TGF-β2 promoter to induce TGF-β2 transcription. Finally, GSK5182 diminished CCl4-induced fibrogenic response through inhibition of ERRγ-mediated TGF-β2 production. Taken together, these results firstly demonstrate that ERRγ can regulate the TGF-β2-mediated fibrogenic response in a mouse model of CC14-induced acute liver injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liver disease is a global health problem, especially acute liver injury is associated with high mortality. The causes of acute liver injury are known to include all different liver cell types and complex interactions of oxidative stress, apoptosis, autophagy and necrosis (Jaeschke et al. 2012; Shi et al. 2017). The mouse model of carbon tetrachloride (CCl4)-induced acute liver injury is widely used to delineate mechanisms of injury and repair, aiming at identifying potential treatment strategies, since it is comparable to chemical toxicity during acute liver injury in human (Chen et al. 2014; Shi et al. 2017; Torres et al. 2016; Zhang et al. 2017; Zhu et al. 2010). CCl4, like paracetamol, is metabolized by cytochrome P450 enzymes to form reactive intermediates, such as trichloro methyl-free radicals and peroxyl radicals, then initiate lipid peroxidation and cell injury (Slater et al. 1985). Previous studies suggested that CC14-injured hepatocytes secrete high mobility group box 1 as damage-associated molecular pattern, which activates hepatic stellate cells (HSCs), among others via autophagy induction (He et al. 2015), therewith initiating a fibrogenic response (Chen et al. 2014). In CCl4 toxicity, production of several cytokines, including interleukin-6 (IL6), is evident as a trigger of liver regeneration, including extra cellular matrix (ECM) production from activated HSCs (Dai et al. 2018).
The transforming growth factor β (TGF-β) family of multifunctional cytokines comprises three different mammalian isoforms (TGF-β1 to 3), which function through the same receptor signaling pathways. All three isoforms are expressed in the liver with cell type specific expression patterns, and play key roles in the regulation of various cellular processes like apoptosis, proliferation, differentiation and migration (Bissell et al. 1995; Dropmann et al. 2016; Leask and Abraham 2004; Massagué 1998). Among the TGF-β family ligands, TGF-β1 has been vastly studied in liver diseases, whereas the role of TGF-β2 has been less investigated. TGF-β2 is expressed in different cell types in the liver (De Bleser et al. 1997; Milani et al. 1991), and its dysfunction leads to developmental defects and facilitates fibrotic diseases in mice and human (Coker et al. 1997; Dropmann et al. 2020; Pinzani and Rombouts 2004; Serini and Gabbiana 1996). Recently, we showed that TGF-β2 silencing using antisense oligonucleotides ameliorated hepatic fibrogenesis in multidrug resistance gene 2-deficient mice without adverse effects on healthy livers (Dropmann et al. 2020). Additionally, TGF-β2 is important for regulation of immune tolerance (de Martin et al. 1987; Maheshwari et al. 2011; Zhang et al. 2008). In this aspect, recombinant TGF-β2 was found more effective than TGF-β1 and TGF-β3 in suppressing the macrophage inflammatory response in neonatal mice with inflammatory bowel necrosis (Maheshwari et al. 2011). Although the transcription factor cyclic AMP-responsive element-binding protein H (CREBH, encoded by Creb3l3) was reported to regulate TGF-β2 gene expression in hepatitis C virus infected hepatocytes (Chida et al. 2017), further regulatory mechanisms of TGF-β2 gene expression in liver parenchymal cells, to our knowledge have not been described yet.
Estrogen-related receptors (ERRs) are orphan nuclear receptors composed of ERRα (NR3B1), ERRβ (NR3B2) and ERRγ (NR3B3) (Giguère 2008). ERRs bind to classic estrogen response elements (ERE) as dimers, or to extended half-site core sequences (TCAAGGTCA) as monomers (Giguère et al. 1988; Luo et al. 2003; Razzaque et al. 2004). ERRγ, a third member of the ERR subfamily (Audet-Walsh and Giguére 2015; Huss et al. 2015), is found expressed in several organs, especially in key metabolic tissues such as heart, brain, skeletal muscle, adipose tissue and liver (Alaynick et al. 2007; Bookout et al. 2006), and its expression levels show dynamic changes, e.g., by peripheral circadian clock or various stimuli (Jung et al. 2016; Kim et al. 2012; Yang et al. 2006). ERRγ-dependent transactivation of target genes relies on interaction with co-activators or co-repressors, which enhance or inhibit the binding of ERRγ to target gene promoter sequences. Even though ERRγ is constitutively active in the absence of an endogenous ligand, transcriptional activity of ERRγ can be regulated by various synthetic compounds like, 4-hydroxytamoxifen (4-OHT) (Giguère 2008). GSK5182, a 4-OHT analogue, acts as a specific inverse agonist of ERRγ by enhancing the interaction of ERRγ with its co-repressor SHP-interacting leucine zipper protein (SMILE), and thereby inhibits the binding of ERRγ to its target gene promoter (Hentschke et al. 2002; Herzog et al. 2007; Hong et al. 1999). Upon bacterial infections, the pro-inflammatory cytokine IL6 activates the Janus kinase (JAK)/signal transducer and activator of transcription 3 (STAT3) signaling, which increases ERRγ gene expression in hepatocytes. Further, we recently reported that ERRγ mediates IL6-dependent transcriptional regulation of bone morphogenetic protein 6 and fibroblast growth factor 23 in liver (Radhakrishnan et al. 2020, 2021), therewith contributing to the regulation of iron homeostasis and mediating kidney liver communication in acute kidney injury. Besides this, ERRγ is involved in the regulation of glucose- (Kim et al. 2012; Misra et al. 2016), alcohol- (Jung et al. 2020; Kim et al. 2013), cholesterol- and lipid- (Kim et al. 2019) metabolism. However, a potential role of ERRγ upon chemical liver toxicity and its repair response remains to be elucidated.
In this study, we for the first time revealed a role of ERRγ in the hepatic fibrogenic response via upregulating TGF-β2 gene expression and secretion from hepatocytes in the CCl4-evoked acute liver injury mouse model.
Materials and methods
Chemicals
GSK5182 was synthesized as described previously (Chao et al. 2006) and used at a concentration of 40 mg/kg for in vivo experiments and 10 μM for in vitro experiments. Recombinant human IL6 (Cat # cyt-213; Prospec Protein Specialists, East Brunswick, NJ) was used at a concentration of 2.5 μg/kg for in vivo experiments and 20 ng/ml for in vitro experiments. Recombinant human tumor necrosis factor-α (TNFα) was used at a concentration of 20 ng/ml for in vitro experiments (R&D Systems, Minneapolis, MN, USA). CCl4 (Sigma-Aldrich Chemical Co., St Louis, MO, USA) was dissolved in corn oil to a 10% solution and injected as 1 ml/kg of body weight for in vivo experiments.
Animal studies
Eight-week-old male C57BL/6 J mice were used for all experiments. C57BL/6 J wild-type (WT) mice were obtained from Korea Research Institute of Biosciences and Biotechnology (KRIBB, Daejeon, Korea). C57BL/6 J mice containing floxed ERRγ exon 2 (ERRγ f/f) were obtained from PHENOMIN-iCS, PHENOMIN, the French National Infrastructure in Biology and Health (Illkirch, France). To produce the hepatocyte-specific ERRγ knockout line (ERRγ-LKO), ERRγ f/f animals were crossbred with C57BL/6 J-Alb-Cre transgenic mice, which express Cre recombinase in hepatocytes under the control of the albumin promoter (Jackson Laboratories, Bar Harbor, ME, USA) (Radhakrishnan et al. 2020, 2021). Prior to the experiments, mice were acclimatized to a 12 h light/dark cycle at 22 ± 2 °C for 2 weeks with unlimited food and water in a specific pathogen-free facility. WT or ERRγ-LKO mice were treated with a single dose of CCl4 (10% CCl4 dissolved in corn oil as 1 ml/kg of body weight) or IL6 (2.5 μg/kg in PBS) via intraperitoneal injection for the indicated time points. Where indicated, GSK5182 (40 mg/kg in 30% PEG400) was administered intraperitoneally (i.p) into mice. Ad-GFP or Ad-ERRγ were injected into WT mice (3 × 109 PFU/mouse) via the tail vein, and mice were sacrificed 5 days after adenovirus injection. Ad-US and Ad-shERRγ were injected into WT mice (5 × 109 PFU/mouse) via the tail vein for the indicated time points, and IL6 (2.5 μg/kg) was given one time by intraperitoneal injection 3 h before sacrifice. All animal procedures were approved by the Institutional Animal Care and Use Committee of KRIBB (KRIBB-AEC-20135). All animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
Histopathology and immunohistochemistry
Liver samples were fixed in 10% neutral buffered formalin, embedded in paraffin, cut into 5-μm-thick sections and stained with hematoxylin and eosin (H&E) staining. To detect expression of fibrogenic factor, liver sections were stained with anti-alpha smooth muscle actin (αSMA) antibody (ab5694; Abcam, Cambridge, MA, USA) and visualized using 3,3'-diaminobenzidine (SK4100; Vector Lab, Burlingame, CA, USA). Images were captured using a light microscope (BX51; Olympus Corporation, Tokyo, Japan).
Blood analysis
Plasma alanine aminotransferase (ALT) and aspartate transaminase (AST) were determined using an automated blood chemistry analyzer (AU480; Beckman Coulter, Krefeld, Germany).
Plasmids and DNA constructs
The promoters of mouse TGF-β2 (−2904 bp/ + 93 bp) was cloned into the SacI/BglIII site of the PGL3-basic vector. TGF-β2 ERR response element mut-Luc (−1686bpTTAGGTCAGC−1676 bp to −763 bp TTAAATCAGC−753 bp) was generated using the QuikChange II site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA). FLAG-ERRα, FLAG-ERRβ, and FLAG-ERRγ constructs were described previously (Sanyal et al. 2002). All plasmids used were confirmed by complete sequence analysis.
Recombinant adenoviruses
Ad-GFP and Ad-US were described previously (Kim et al. 2012). In brief, the cDNA encoding ERRγ was cloned into a pAd-YC2 shuttle vector, containing a bovine growth hormone polyadenylation signal sequence under the control of the cytomegalovirus (CMV) promoter. Adenovirus short hairpin ERRγ (Ad-shERRγ) was generated with the pAd-easy system. The target sequence of shERRγ is GAACGGACTGGACTCGCCACCTCTCTA (Jeong et al. 2009). All virus constructs were purified by cesium chloride density gradient ultracentrifugation. Ad-GFP and Ad-FLAG-ERRγ were used to infect cells (1 × 108 PFU) and mice (3 × 109 PFU/mouse). For ERRγ knockdown experiments, cells were infected with Ad-US and Ad-shERRγ (1 × 108 PFU) for 48 h, and mice were infected with Ad-shERRγ (5 × 109 PFU/mouse) for 5 days, before the indicated treatment protocol.
Cell culture and transient transfection assays
Huh7 (human hepatoma cells), 293 T (human embryonic kidney cells) and AML12 (mouse immortalized hepatocytes) cells were obtained as described previously (Radhakrishnan et al. 2020). The cells were maintained in a humidified atmosphere containing 5% CO2 at 37 °C and used for experiments at 75% confluence. Transient transfections were performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The cells were treated with 10 μM GSK5182 unless noted otherwise. After 48 h of transfection, the cells were harvested, and luciferase activity was measured and normalized to β-galactosidase activity. All cell culture experiments were performed as three independent biological replicates.
RNA isolation and analysis
Total RNA was isolated using the TRIzol reagent (Invitrogen, CA, USA) according to the manufacturer’s instructions, and used as template to synthesize cDNA with TOPscript RT DryMix (dT18 plus; Enzynomics, Daejeon, Korea). Reverse transcription was performed with temperature set as 37 °C for 5 min, 45 °C for 60 min and 95 °C for 5 min on a thermocycler (TaKaRa, Shiga, Japan). cDNAs were analyzed by the Applied Biosystems StepOnePlus real-time PCR system (Applied Biosystems, Foster City, CA, USA) using Power SYBR Green PCR Master Mix (Applied Biosystems). From the obtained CT values, mRNA expressions were calculated by the 2−ΔΔCT method. The following primers were used in this study: ERRγ (mouse/human), 5′-AAGATCGACACATTGATTCCAGC-3′ (Forward) and 5′-CATGGTTGAACTGAATTCCCAC-3′ (Reverse); TGF-β2 (mouse), 5′-CTTCGACGTGACAGACGCT-3′ (Forward) and 5′-GCAGGGGCAGTGTAAACTTATT-3′ (Reverse); TGF-β2 (human), 5′-CAGCACACTCGATATGGACCA-3′ (Forward) and 5′-CCTCGGGCTCAGGATAGTCT-3′ (Reverse); IL6 (mouse), 5′-CTGCAAGAGACTTCCATCCAG-3′ (Forward) and 5′- AGTGGTATAGACAGGTCTGTTGG -3′ (Reverse); αSMA (mouse), 5′-AACGCCTTCCGCTGCCC-3′ (Forward) and 5′-CGATGCCCGCTGACTCC-3′ (Reverse); COL1A1 (mouse), 5′-GCTCCTCTTAGGGGCCACT-3′ (Forward) and 5′-CCACGTCTCACCATTGGGG-3′ (Reverse); PDGFR (mouse) 5′-TTCCAGGAGTGATACCAGCTT-3′ (Forward) and 5′-AGGGGGCGTGATGACTAGG-3′ (Reverse); TIMP1 (mouse), 5′-GCAACTCGGACCTGGTCATAA-3′ (Forward) and 5′-CGGCCCGTGATGAGAAACT-3′ (Reverse). Relative target gene expression levels in adenovirus infected mice and cells were normalized to L32 (mouse/human) 5′-TCTGGTGAAGCCCAAGATGG-3′ (Forward) and 5′-CTCTGGGTTTCCGCCAGT-3′ (Reverse). Relative target gene expression levels in CCl4-treated mice were normalized to GUSB (mouse) 5′-GGCTGGTGACCTACTGGATTT-3′ (Forward) and 5′-GGCACTGGGAACCTGAAGT-3′ (Reverse) expression. Relative target gene expression levels in IL6-treated mice and cells were normalized to HPRT1 (human) 5′-CCTGGCGTCGTGATTAGTGAT-3′ (Forward) and 5′-AGACGTTCAGTCCTGTCCATAA-3′ (Reverse); HPRT1 (mouse) 5′-TCAGTCAACGGGGGACATAAA-3′ (Forward) and 5′-GGGGCTGTACTGCTTAACCAG-3′ (Reverse) expression.
Western blot analysis
Mouse tissues were lysed with RIPA buffer (Elpis-Biotech, Daejeon, Korea) and subjected to immunoblot analysis as described previously (Lee et al. 2008). Proteins from liver, brain and heart lysates were separated by 10% SDS-PAGE and transferred to nitrocellulose membranes. Membranes were probed with anti-β-actin (AbFrontier, Seoul, Korea; diluted 1:5000) (Kim et al. 2013), anti-ERRγ (Cell Signaling Technology, Danvers, MA, USA; diluted 1:1000) (Jung et al. 2020) and anti-α-tublin (AbFrontier; diluted 1:5000). Immunoreactive proteins were visualized using an Amersham ECL kit (GE Healthcare, Piscataway, NJ, USA), or using an iBright CL1000 imaging system (Invitrogen) according to the manufacturer’s instructions.
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) assays were performed according to the manufacturer’s protocol of SimpleChIP Plus Enzymatic Chromatin IP kit (Upstate Biotechnology, Lake Placid, NY, USA). Immunoprecipitation was performed using Primary antibodies used include mouse monoclonal anti-ERRγ (Clone # H6812, Cat # PP-H6812-00; Perseus Proteomics, Tokyo, Japan) or IgG as a negative control. After recovery of DNA, real-time quantitative PCR analysis was performed using primers encompassing the TGF-β2 promoter region. The primers used for PCR were as follows: TGF-β2 −2.8 kb/−2.6 kb, 5′- AAGAAAATTTACAAAAGATT- 3′ (Forward) and 5′-GCTGCAGAATAATCTACTGC-3′ (Reverse); and −1.7 kb/−1.5 kb, 5′-TGTGGAATCCCGGAGAACTC- 3′ (Forward) and 5′-CCCTGAGTCCCTCCTGTGTT-3′ (Reverse).
Measurement of TGF-β2 levels
TGF-β2 levels from mice plasma and cell culture media were determined using an enzyme-linked immunosorbent assay (ELISA) kit, according to the manufacturer’s protocol (MBS703270 for mouse plasma and medium; MyBioSource, San Diego, CA, USA; DB250 for human plasma, R&D Systems) (Takahashi et al. 2019).
Statistical analysis
Data were analyzed with Prism 8 (GraphPad Software, La Jolla, CA, USA) and presented as mean ± SD. Comparison between two groups was performed using the two-tailed Student’s t-test, whereas comparison between multiple groups was performed by ordinary one-way ANOVA with Tukey’s multiple comparison test. Differences were considered statistically significant at p < 0.05.
Results
CCl 4 toxicity increases ERRγ to regulate TGF-β2 gene expression and secretion in mouse liver
We treated WT mice with CCl4 for different time periods, as indicated and confirmed acute liver injury, as evidenced by elevated plasma levels of liver damage markers (ALT and AST) and increased parenchymal degeneration in histopathological analysis of liver sections (Supplementary Fig. 1). In CCl4-injected mouse liver, ERRγ, TGF-β1 and TGF-β2, but not TGF-β3 mRNA levels were increased at 3 h, reaching maximum levels at 6 h (Fig. 1A). Moreover, ERRγ protein levels were induced at 6 h after CCl4 injection (Fig. 1B), and plasma levels of TGF-β2 were increased at the 3 h time point, reaching maximum levels after 6 h (Fig. 1C). We hypothesized that ERRγ is an upstream regulator of CCl4-induced TGF-β1 and TGF-β2 expression. Therefore, we generated hepatocyte-specific ERRγ knockout (ERRγ-LKO) mice (Supplementary Fig. 2) (Radhakrishnan et al. 2020), and treated those with CCl4 for 6 h for a comparative investigation. As expected, ERRγ protein levels were only increased in CCl4-injected (6 h) WT mice, but not in ERRγ-LKO mice (Fig. 1E). In WT mice, CCl4 treatment for 6 h increased hepatic TGF-β1 and TGF-β2 mRNA levels, whereas TGF-β2, but not TGF-β1 mRNA levels were significantly reduced in ERRγ-LKO mice (Fig. 1D). TGF-β2 plasma levels were also significantly reduced after CCl4 treatment (6 h) in ERRγ-LKO compared to WT mice (Fig. 1F). We further verified the role of ERRγ in CCl4-mediated hepatic TGF-β2 gene upregulation using GSK5182, an ERRγ-specific inverse agonist. GSK5182 treatment specifically and significantly inhibited TGF-β2 mRNA levels and ERRγ protein levels in CCl4-treated (6 h) mouse liver (Fig. 1G and H). Plasma levels of TGF-β2 was increased by CCl4 treatment, whereas significantly inhibited in the GSK5182 administration group (Fig. 1I). These results suggest that ERRγ is the key regulator for TGF-β2, but not TGF-β1 or TGF-β3 gene expression, in CCl4-damaged mouse livers.
CCl4-induced ERRγ regulates TGF-β2 gene expression and secretion. A–C WT mice were treated with CCl4 for different time intervals as indicated. A Schematic representation of the treatment protocol (left panel) and RT-qPCR analysis of total RNA isolated from mouse livers (right panel) (n = 5 per groups). B Western blot analysis of liver tissues (control n = 2 and CCl4 n = 3). C ELISA measurement of plasma TGF-β2 levels (n = 5 per group). D–F WT and ERRγ-LKO mice were treated with CCl4 and sacrificed after 6 h. D Schematic representation of the treatment protocol (left panel) and RT-qPCR analysis of total RNA isolated from mouse livers (right panel) (n = 6 per group). E Western blot analysis of liver tissues (n = 3 per group). (F) ELISA measurement of plasma TGF-β2 levels (n = 6 per group). G–I WT mice were treated with CCl4 in presence or absence of GSK5182 and sacrificed after 6 h. G Schematic representation of the treatment protocol (left panel) and RT-qPCR analysis of total RNA isolated from mouse livers (right panel) (n = 5 per group). H Western blot analysis of total protein isolated from liver tissues (n = 3 per group). (I) ELISA measurement of plasma TGF-β2 levels (n = 5 per group). Data represent mean ± S.D. Data in A and C were analyzed by two-tailed Student t-test as *p < 0.05; **p < 0.01; ***p < 0.001 compared with the control group. Data in D, F, G and I were analyzed by ordinary one-way ANOVA with Tukey’s multiple comparisons test as ***p < 0.001; not significant (n.s)
IL6 is an upstream regulator of hepatic ERRγ and TGF-β2 gene expression
A previous study suggests that IL6 and TNFα levels are increased in CCl4-induced acute liver injury (Dai et al. 2018), and we previously reported that IL6 can induce ERRγ gene expression in the liver (Kim et al. 2014; Radhakrishnan et al. 2020). Therefore, we hypothesized that IL6 (or TNFα) may be upstream signal to induce hepatic ERRγ and TGF-β2 gene expression in response to CCl4 toxicity. To test our hypothesis, we firstly treated AML12 cells in vitro with TNFα for different time points as indicated, and found that ERRγ and TGF-β2 mRNA levels were unchanged (Fig. 2A), suggesting that TNFα does not modulate ERRγ and TGF-β2 expression in hepatocytes. Next, we treated AML12 cells with recombinant IL6 in a time-dependent manner. Intriguingly, IL6 increased ERRγ and TGF-β2 mRNA levels that both reached maximum levels at 3 h. These data could also be reproduced in Huh7 cells (Fig. 2B). Moreover, TGF-β2 secretion was significantly increased in AML12 cells, reaching maximum levels after 3 h of IL6 treatment (Fig. 2C). To confirm this result in vivo, we firstly measured mRNA and secretion levels of IL6 in CCl4-treated WT mice, and found that both, IL6 mRNA and secreted levels were significantly increased and reached maximum levels at 3 h CCl4 treatment (Fig. 2D). Next, we injected recombinant IL6 for into WT mice and analyzed the mice at different time points as indicated. In accordance with in vitro results, IL6 treatment significantly increased hepatic ERRγ and TGF-β2 mRNA levels at the 1 h time point, reaching maximum levels at 3 h (Fig. 2E). Finally, plasma TGF-β2 levels were markedly increased 3 h after IL6 injection, and reached maximum levels at 6 h (Fig. 2F). These results indicate that IL6 is the upstream regulator of ERRγ induced hepatic TGF-β2 gene expression and secretion in mice.
IL6-induced ERRγ regulates TGF-β2 gene expression. A RT-qPCR analysis of total RNA isolated from AML12 cells treated with TNFα for different time intervals as indicated. B RT-qPCR analysis of total RNA isolated from AML12 and Huh7 cells treated with IL6 for different time intervals as indicated. C Measurement of TGF-β2 levels in culture supernatant IL6-treated AML12 cells. D RT-qPCR analysis of total RNA isolated from mouse livers (left panel), and measurement of plasma IL6 levels from mice treated with vehicle or CCl4 for the indicated times (right panel) (n = 5 per group). E, F WT mice were treated with IL6 for different times as indicated (n = 5 per group). E Schematic representation of the treatment protocol (left panel), and RT-qPCR analysis of total RNA isolated from mouse livers (right panel). F ELISA measurement of plasma TGF-β2 levels. All cell culture experiments were performed as three independent biological replicates. Data represent mean ± SD. All data were analyzed by two-tailed Student’s t-test as *p < 0.05; **p < 0.01; ***p < 0.001 compared with control groups
ERRγ directly regulates TGF-β2 gene expression
As we found ERRγ required for CCl4-mediated upregulation of hepatic TGF-β2 gene expression and secretion (Fig. 1), we intended to test whether ERRγ is sufficient to increase TGF-β2 expression. For this purpose, we overexpressed ERRγ using adenovirus (Ad-ERRγ) in AML12 and Huh7 cells, which induces TGF-β2 mRNA expression in both cell types (Fig. 3A). Upregulated TGF-β2 secretion was also shown in the supernatant of ERRγ overexpressing AML12 cells (Fig. 3B). To confirm this result in vivo, we injected Ad-ERRγ via the tail-vein into WT mice and sacrificed these after 5 days. TGF-β2 mRNA levels were significantly increased in Ad-ERRγ infected mice compared to the Ad-GFP infected control group (Fig. 3C), and ERRγ overexpression also increased plasma TGF-β2 levels. (Fig. 3D). The participation of ERRγ in IL6-induced hepatic TGF-β2 mRNA expression and secretion was examined by knockout experiments using adenoviral shERRγ (Ad-shERRγ) infection. IL6 (3 h)-induced TGF-β2 mRNA levels were significantly reduced in Ad-shERRγ-infected AML12 and Huh7 cells, compared to Ad-US infected cells (Fig. 3E). Moreover, depletion of ERRγ expression in AML12 cells significantly inhibited IL6-induced TGF-β2, as measured in the supernatant (Fig. 3F). Next, we infected WT mice with Ad-US or Ad-shERRγ for 5 days, and then treated them or not with IL6 for 3 h. IL6-induced hepatic TGF-β2 mRNA and plasma TGF-β2 levels were significantly reduced in Ad-shERRγ infected mice (Fig. 3G and H). These results confirm that (1) overexpression of ERRγ is sufficient to increase hepatic TGF-β2 gene expression and (2) ERRγ is required for IL6-induced TGF-β2 gene expression and secretion.
ERRγ directly regulates TGF-β2 gene expression. A RT-qPCR analysis of total RNA isolated from AML12 and Huh7 cells infected with Ad-GFP or Ad-ERRγ. B Measurement of TGF-β2 levels in Ad-GFP- or Ad-ERRγ-infected AML12 cell supernatant. C, D WT mice were infected with Ad-GFP or Ad-ERRγ and sacrificed after 5 days (n = 4 per group). C Schematic representation of the treatment protocol (left panel) and RT-qPCR analysis of total RNA isolated from mouse livers (right panel). D ELISA measurement of plasma TGF-β2 levels. E RT-qPCR analysis of total RNA isolated from AML12 and Huh7 cells infected with Ad-US or Ad-shERRγ for 36 h, and then treated with IL6 for 3 h. F Measurement of TGF-β2 levels in the supernatant of AML12 cells infected with Ad-US or Ad-shERRγ for 36 h, and then treated with IL6 for 3 h. G, H WT mice were infected with Ad-US or Ad-shERRγ for 5 days, and then treated or not with IL6 for 3 h (n = 4 per group). G Schematic representation of the treatment protocol (left panel), and RT-qPCR analysis of total RNA isolated from mouse livers (right panel). (H) ELISA measurement of plasma TGF-β2 levels. All cell culture experiments were performed as three independent biological replicates. Data represent mean ± SD. Data in A–D were analyzed by two-tailed Student’s t-test as ***p < 0.001. Data in E–H were analyzed by ordinary one-way ANOVA with Tukey’s multiple comparison test as *p < 0.05; ***p < 0.001; not significant (n.s)
ERRγ activates the TGF-β2 gene promoter
To explore the molecular mechanism of ERRγ regulated TGF-β2 gene expression, we performed gene promoter studies after modulating ERRγ expression. Knockdown of ERRγ by Ad-shERRγ infection decreased IL6-induced TGF-β2 promoter activity in AML12 cells (Fig. 4A). Overexpression of ERRγ, but not ERRα or ERRβ, enhanced TGF-β2 promoter activity in 293 T cells (Fig. 4B). As mention above, in silico sequence analysis suggested a putative ERRγ binding site (AGGTCA) in the TGF-β2 gene promoter, which we mutated by site directed mutagenesis. ERRγ overexpression mediated upregulation of TGF-β2 promoter activity was significantly decreased, when analyzing the construct with the TGF-β2 promoter ERRγ binding site mutation (Fig. 4C). Moreover, IL6 treatment increased WT TGF-β2 promoter activity, but failed to activate the ERRγ binding site mutant TGF-β2 promoter in AML12 cells (Fig. 4D). To confirm binding of ERRγ to the endogenous TGF-β2 promoter in AML12 cells, we performed ChIP assays. IL6-induced endogenous ERRγ was recruited to the ERRγ consensus binding site in the TGF-β2 promoter (Fig. 4E). Collectively, these results indicate that ERRγ directly binds and activates the TGF-β2 promoter in response to IL6.
ERRγ activates mouse TGF-β2 gene promoter activity. A AML12 cells were transfected with a mouse TGF-β2 promoter luciferase construct and stimulated with IL6 for 3 h. B 293 T cells were transfected with the mouse TGF-β2 promoter luciferase construct, along with expression vectors for ERRα, ERRβ or ERRγ. C The alignment of potential ERRE sequences in the mouse TGF-β2 promoter is shown. 293 T cells were co-transfected with vectors expressing WT or ERRE mutant TGF-β2 promoter luciferase constructs and ERRγ overexpression plasmids. D AML12 cells were transfected with WT or ERRE mutant TGF-β2 promoter luciferase constructs and stimulated with IL6 for 3 h. E ChIP assays, showing binding of ERRγ to the TGF-β2 gene promoter. AML12 cells were treated with IL6 for 3 h, and soluble chromatin was immunoprecipitated with an ERRγ antibody. Purified DNA samples were used for PCR with primers that are embedding the ERREs in the TGF-β2 gene promoter (-1.7 kb to -1.5 kb). All cell culture experiments were performed as three independent biological replicates. Data represent mean ± SD. All data were analyzed by ordinary one-way ANOVA with Tukey’s multiple comparison test as ***p < 0.001; not significant (n.s)
An inverse agonist of ERRγ abolishes IL6-induced hepatic TGF-β2 gene expression and protein secretion
GSK5182, an ERRγ-specific inverse agonist, suppresses ERRγ target gene expression by inhibiting ERRγ transactivation (Chao et al. 2006; Kim et al. 2012). In the present study, IL6-mediated TGF-β2 promoter activity was significantly inhibited by GSK5182 in AML12 cells (Fig. 5A). In line, AML12 and Huh7 cells treated with IL6 in presence or absence of GSK5182, both showed dramatically decreased IL6-stimulated TGF-β2 mRNA levels in the GSK5182 setting (Fig. 5B). GSK5182 also decreased IL6 mediated TGF-β2 secretion into the supernatant of AML12 cell (Fig. 5C). To test the effect of GSK5182 in vivo, we treated WT mice with IL6 (3 h) with or without GSK5182 pretreatment. GSK5182 markedly decreased IL6-induced hepatic TGF-β2 mRNA and plasma TGF-β2 levels (Fig. 5D and E). These results suggest that inhibition of ERRγ transcriptional activity by GSK5182 administration represses IL6-mediated hepatic TGF-β2 mRNA expression and plasma TGF-β2 levels.
An inverse agonist of ERRγ inhibits IL6-induced TGF-β2 gene expression. A AML12 cells were transfected with the mouse TGF-β2 promoter luciferase construct and stimulated with IL6 for 3 h, in presence or absence of GSK5182. B RT-qPCR analysis of total RNA isolated from AML12 and Huh7 cells treated with IL6 for 3 h with or without GSK5182. C Measurement of TGF-β2 levels in the supernatant of AML12 cells stimulated with IL6 for 3 h in presence or absence of GSK5182. D, E WT mice were treated with IL6 in presence or absence of GSK5182 and sacrificed after 3 h (n = 5 per groups). D Schematic representation of the treatment protocol (left panel), and RT-qPCR analysis of total RNA isolated from mouse livers (right panel). E ELISA measurement of plasma TGF-β2 levels. All cell culture experiments were performed as three independent biological replicates. Data represent mean ± SD. All data were analyzed by ordinary one-way ANOVA with Tukey’s multiple comparison test as *p < 0.05; ***p < 0.001; not significant (n.s)
Hepatic ERRγ expression is required for the fibrogenic response in CCl 4 -induced acute liver injury
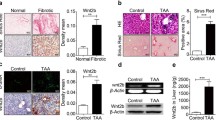
We examined the effect of GSK5182 and liver-specific ERRγ knockout on expression of fibrogenic factors in CCl4-induced acute liver injury. WT or ERRγ-LKO mice were treated with CC14 for 72 h, where a maximum fibrogenic tissue response including activation of HSC is evident (data not shown). CC14-mediated upregulation of plasma TGF-β2 levels are significantly decreased in ERRγ-LKO mice, compared to WT mice (Fig. 6A). Gene expression levels of all tested fibrogenic factors (αSMA, COL1a1, PDGFR and TIMP1) were also markedly reduced in liver of CCl4-injected ERRγ-LKO mice compared to WT mice (Fig. 6B). Consistent with mRNA levels, αSMA protein levels were reduced as a result of the hepatocyte-specific ERRγ knockout (Fig. 6E). Next, we treated WT mice with CC14 for 72 h in presence or absence of GSK5182. Plasma TGF-β2 levels are increased after 72 h of CCl4 intoxication, whereas GSK5182 treatment effectively inhibited CCl4-mediated upregulation of TGF-β2 plasma levels (Fig. 6C). Elevated mRNA levels of fibrogenic factors (αSMA, COL1a1, PDGFR and TIMP1) after CCl4 administration were also significantly reduced upon GSK5182 treatment (Fig. 6D). In line with gene expression results, immunohistochemistry of CCl4-treated mouse liver sections shows that αSMA expression was drastically reduced in the GSK5182 co-treatment group compared to the CCl4 only group, indicating a significant reducing effect towards HSC activation (Fig. 6F). These results propose that ERRγ, besides other signals, is a modulator of the fibrogenic response by inducing TGF-β2 expression and secretion from hepatocytes upon CCl4-acute toxicity in mice.
An inverse agonist of ERR inhibits expression of fibrogenic factors and HSC activation in CCl4-induced acute liver injury. A, B and E WT and ERRγ-LKO mice were treated with CCl4 and sacrificed after 72 h (n = 5 per groups). A Schematic representation of the treatment protocol (left panel) and ELISA measurement of plasma TGF-β2 levels (right panel). B RT-qPCR analysis of total RNAs isolated from mouse livers. C, D and F WT mice were treated with CCl4 in the presence or absence of GSK5182 and sacrificed after 72 h (n = 5 per groups). C Schematic representation of the treatment protocol (left panel) and ELISA measurement of plasma TGF-β2 levels (right panel). D RT-qPCR analysis of total RNAs isolated from mouse livers. E, F Representative images of αSMA immunohistochemical analysis in liver sections. Data represent mean ± S.D. All data analyzed by one-way ANOVA with Tukey’s multiple comparisons test as ***p < 0.001; not significant (n.s). G Schematic representation of CCl4-IL6 axis-induced fibrogenic response through ERRγ regulated TGF-β2 gene upregulation
Discussion
As a results of this study, we suggest that ERRγ mediated induction of hepatic TGF-β2 gene expression contributes to the fibrogenic response in the CCl4-mediated acute liver injury in mice. We have evidence from effects of GSK5182, a selective ERRγ inverse agonist, and from data obtained in hepatocyte specific ERRγ knockout mice, which both lead to attenuated TGF-β2 gene expression and fibrogenic response in CCl4-treated mice.
Previous reports suggest that ERRγ gene expression is increased by fasting (via glucagon receptor) (Kim et al. 2012), upon alcohol consumption (via endocannabinoid 1receptor) (Kim et al. 2013), during inflammation (via IL6 receptor) (Kim et al. 2014). We previously reported that ERRγ upregulates the transcription of a number of target genes by direct binding at ERRE regions and thereby plays an important role in various biological processes, like glucose (Kim et al. 2012) and lipid metabolism (Kim et al. 2019) or iron homeostasis (Kim et al. 2014). Studies with knockout mice showed that the general depletion leads to lethality during the first week of life (Alaynick et al. 2007). To overcome this dramatic phenotype, we generated hepatocyte specific ERRγ-deficient mice to perform depletion studies and found that ERRγ is a key mediator for IL6-induced hepatic BMP6 gene expression (Radhakrishnan et al. 2020). In this study, we also used hepatocyte-specific ERRγ-deficient mice to examine the role of ERRγ in regulating CCl4-induced hepatic TGF-β2 expression.
Previous studies dealing with TGF-β2 regulation suggest that TGF-β2 gene expression is induced by cyclic AMP in the human androgen-independent prostate carcinoma cell line PC-3 (Bang et al. 1992), or by CREBH in hepatitis C virus infected mouse liver (Chida et al. 2017). Moreover, miR-30b modulates TGF-β2 production via regulating the activating transcription factor (ATF) 2 activity (Howe et al. 2017). However, other membrane receptor signaling pathways regulating hepatic TGF-β2 gene expression remain elusive. In this study, we provide evidence for the transcriptional upregulation of hepatic TGF-β2 gene expression through ERRγ by direct binding at the ERRE motif in the TGF-β2 gene promoter. Indeed, TGF-β2 gene expression is upregulated in various pathological conditions, including developmental defects and fibrotic diseases in mice (Coker et al. 1997; Pinzani and Rombouts 2004; Serini and Gabbiana 1996). We have recently shown that TGF-β2 is the predominant driver of liver fibrogenesis in biliary liver disease (Dropmann et al. 2020). Here, we show that ERRγ upregulates hepatic TGF-β2 gene expression and secretion, as well as increases the expression of fibrogenic factors in CC14-treated mice. It is known that in CCl4-mediated chronic liver damage with multiple CCl4 injections, TGF-β1 gene expression is upregulated, mediating fibrogenesis (Shrestha et al. 2016). We now found that TGF-β2 mRNA levels are increased much stronger compared to TGF-β1 mRNA levels upon 1 × CCl4 treatment, after 6 h (Fig. 1A). CCl4-mediated TGF-β2 mRNA levels, but not those of TGF-β1, were significantly reduced in ERRγ-LKO mice and in a setting of GSK5182 pretreatment, an inverse agonist of ERRγ (Fig. 1D and 1G). Therefore, we suggest that TGF-β2, induced by CCl4-via ERRγ, plays an important role for the fibrogenic response in acute liver injury.
Previous studies reported that IL6 signaling cascades are activated during the liver response on acute injury, both by CCl4 injection or partial hepatectomy (PHx), and the importance in liver regeneration was shown (Trautwein et al. 1992, 1994; Zhang et al. 1996). IL6 serum levels are increased early time while hepatocyte proliferation begins after 24 h of liver resection in mice. Moreover, targeted disruption of IL6 results in impaired liver regeneration, as characterized by necrosis in PHx mice (Cressman et al. 1996). CCl4 toxicity involves an inflammatory response and upregulates several pro-inflammatory cytokines, whereby in particular plasma IL6 levels were significantly increased 6 h after CCl4 injection (Dai et al. 2018). Moreover, helicid treatment (4-formylphenyl-O-β-d-allopyranoside, a main active constituent from seeds of the Chinese herb Helicia nilagirica) protected CCl4-induced acute liver injury in mice including anti-inflammatory and anti-oxidative effects (Chen et al. 2020). Here, we identified IL6 as upstream signal to induce hepatic ERRγ expression, which in turn upregulates TGF-β2 gene expression in response to CCl4 toxicity. IL6 mRNA levels are increased already at 1 h in mouse liver upon LPS injection. In addition, FGF23 mRNA levels and secretion are maximally increased after 3 h in LPS-treated mice (Agoro et al. 2021). We confirmed gene expression and protein secretion of IL6 early in CCl4-treated mice, and showed subsequent ERRγ and TGF-β2 expression at the 6-h time point, all in all a fast tissue response upon CC14 intoxication. Treatment with GSK5182 or hepatocyte-specific ERRγ depletion significantly reduced CCl4-induced TGF-β2 plasma levels at least for 3 days after CCl4 injection (Fig. 6A-D). In line, the fibrogenic response at day 3 is also blunted, as evident from reduced expression of fibrogenic factors, including αSMA, COL1a1, PDGFR and TIMP1, or significantly decreased numbers of activated HSC/αSMA staining (Fig. 6B, D, E, and F). A previous study suggests that TGF-β2 contributes to induce TGF-β1/2 gene expression and secretion in an autocrine manner through its own signaling pathway (Chida et al. 2017), which may have impact on initiating the above described TGF-β1 mediated fibrogenic response in the chronic setting. Taken together, upregulation of TGF-β2 gene expression is a fast tissue response after CC14 intoxication, acting as fibrogenic hepatokine, upon induction by IL6 via ERRγ.
In conclusion, we showed a novel mechanism of ERRγ-mediated TGF-β2 gene upregulation in hepatocytes, and demonstrated that hepatic ERRγ could modulate the fibrogenic response in CCl4-intoxicated mice.
References
Agoro R, Park MY, Le Henaff C, Jankauskas S, Gaias A, Chen G et al (2021) C-FGF23 peptide alleviates hypoferremia during acute inflammation. Haematologica 106(2):391–403. https://doi.org/10.3324/haematol.2019.237040
Alaynick WA, Kondo RP, Xie W, He W, Dufour CR, Downes M et al (2007) ERRgamma directs and maintains the transition to oxidative metabolism in the postnatal heart. Cell Metab 6(1):13–24. https://doi.org/10.1016/j.cmet.2007.06.007
Audet-Walsh É, Giguére V (2015) The multiple universes of estrogen-related receptor α and γ in metabolic control and related diseases. Acta Pharmacol Sin 36(1):51–61. https://doi.org/10.1038/aps.2014.121
Bang YJ, Kim SJ, Danielpour D, O’Reilly MA, Kim KY, Myers CE et al (1992) Cyclic AMP induces transforming growth factor beta 2 gene expression and growth arrest in the human androgen-independent prostate carcinoma cell line PC-3. Proc Natl Acad Sci USA 89(8):3556–3560. https://doi.org/10.1073/pnas.89.8.3556
Bissell DM, Wang SS, Jarnagin WR, Roll FJ (1995) Cell-specific expression of transforming growth factor-beta in rat liver. Evidence for autocrine regulation of hepatocyte proliferation. J Clin Invest 96(1):447–455. https://doi.org/10.1172/jci118055
Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ (2006) Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell 126(4):789–799. https://doi.org/10.1016/j.cell.2006.06.049
Chao EY, Collins JL, Gaillard S, Miller AB, Wang L, Orband-Miller LA et al (2006) Structure-guided synthesis of tamoxifen analogs with improved selectivity for the orphan ERRgamma. Bioorgan Med Chem Lett 16(4):821–824. https://doi.org/10.1016/j.bmcl.2005.11.030
Chen M, Huang W, Wang C, Nie H, Li G, Sun T et al (2014) High-mobility group box 1 exacerbates CCl4-induced acute liver injury in mice. Clin Immunol 153(1):56–63. https://doi.org/10.1016/j.clim.2014.03.021
Chen S, Zhang CL, Zhou XF, Gao Y, Chen H, Fu BD et al (2020) Anti-inflammatory and antioxidative properties of helicid protect against CCl(4) induced acute liver injury in mice. Biotech Histochem 95(7):483–489. https://doi.org/10.1080/10520295.2020.1718210
Chida T, Ito M, Nakashima K, Kanegae Y, Aoshima T, Takabayashi S et al (2017) Critical role of CREBH-mediated induction of transforming growth factor β2 by hepatitis C virus infection in fibrogenic responses in hepatic stellate cells. Hepatology 66(5):1430–1443. https://doi.org/10.1002/hep.29319
Coker RK, Laurent GJ, Shahzeidi S, Lympany PA, du Bois RM, Jeffery PK et al (1997) Transforming growth factors-beta 1, -beta 2, and -beta 3 stimulate fibroblast procollagen production in vitro but are differentially expressed during bleomycin-induced lung fibrosis. Am J Pathol 150(3):981–991
Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V et al (1996) Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science (new York, NY) 274(5291):1379–1383. https://doi.org/10.1126/science.274.5291.1379
Dai C, Xiao X, Li D, Tun S, Wang Y, Velkov T et al (2018) Chloroquine ameliorates carbon tetrachloride-induced acute liver injury in mice via the concomitant inhibition of inflammation and induction of apoptosis. Cell Death Dis 9(12):1164. https://doi.org/10.1038/s41419-018-1136-2
De Bleser PJ, Niki T, Rogiers V, Geerts A (1997) Transforming growth factor-beta gene expression in normal and fibrotic rat liver. J Hepatol 26(4):886–893. https://doi.org/10.1016/s0168-8278(97)80257-7
de Martin R, Haendler B, Hofer-Warbinek R, Gaugitsch H, Wrann M, Schlüsener H et al (1987) Complementary DNA for human glioblastoma-derived T cell suppressor factor, a novel member of the transforming growth factor-beta gene family. EMBO J 6(12):3673–3677
Dropmann A, Dediulia T, Breitkopf-Heinlein K, Korhonen H, Janicot M, Weber SN et al (2016) TGF-β1 and TGF-β2 abundance in liver diseases of mice and men. Oncotarget 7(15):19499–19518. https://doi.org/10.18632/oncotarget.6967
Dropmann A, Dooley S, Dewidar B, Hammad S, Dediulia T, Werle J et al (2020) TGF-β2 silencing to target biliary-derived liver diseases. Gut 69(9):1677–1690. https://doi.org/10.1136/gutjnl-2019-319091
Giguère V (2008) Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocrine Rev 29(6):677–696. https://doi.org/10.1210/er.2008-0017
Giguère V, Yang N, Segui P, Evans RM (1988) Identification of a new class of steroid hormone receptors. Nature 331(6151):91–4. doi: https://doi.org/10.1038/331091a0
He Y, Jin L, Wang J, Yan Z, Chen T, Zhao Y (2015) Mechanisms of fibrosis in acute liver failure. Liver Int 35(7):1877–1885. https://doi.org/10.1111/liv.12731
Hentschke M, Süsens U, Borgmeyer U (2002) PGC-1 and PERC, coactivators of the estrogen receptor-related receptor gamma. Biochem Biophys Res Commun 299(5):872–879. https://doi.org/10.1016/s0006-291x(02)02753-5
Herzog B, Hallberg M, Seth A, Woods A, White R, Parker MG (2007) The nuclear receptor cofactor, receptor-interacting protein 140, is required for the regulation of hepatic lipid and glucose metabolism by liver X receptor. Mol Endocrinol (baltimore, Md) 21(11):2687–2697. https://doi.org/10.1210/me.2007-0213
Hong H, Yang L, Stallcup MR (1999) Hormone-independent transcriptional activation and coactivator binding by novel orphan nuclear receptor ERR3. J Biol Chem 274(32):22618–22626. https://doi.org/10.1074/jbc.274.32.22618
Howe GA, Kazda K, Addison CL (2017) MicroRNA-30b controls endothelial cell capillary morphogenesis through regulation of transforming growth factor beta 2. PLoS ONE 12(10):e0185619. https://doi.org/10.1371/journal.pone.0185619
Huss JM, Garbacz WG (1852) Xie W (2015) Constitutive activities of estrogen-related receptors: Transcriptional regulation of metabolism by the ERR pathways in health and disease. Biochem Biophys Acta 9:1912–1927. https://doi.org/10.1016/j.bbadis.2015.06.016
Jaeschke H, McGill MR, Ramachandran A (2012) Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev 44(1):88–106. https://doi.org/10.3109/03602532.2011.602688
Jeong BC, Lee YS, Park YY, Bae IH, Kim DK, Koo SH et al (2009) The orphan nuclear receptor estrogen receptor-related receptor gamma negatively regulates BMP2-induced osteoblast differentiation and bone formation. J Biol Chem 284(21):14211–14218. https://doi.org/10.1074/jbc.M808345200
Jung YS, Lee JM, Kim DK, Lee YS, Kim KS, Kim YH et al (2016) The orphan nuclear receptor ERRγ regulates hepatic CB1 receptor-mediated fibroblast growth factor 21 gene expression. PLoS ONE 11(7):e0159425. https://doi.org/10.1371/journal.pone.0159425
Jung YS, Kim YH, Radhakrishnan K, Kim J, Kim DK, Lee JH et al (2020) An inverse agonist of estrogen-related receptor γ regulates 2-arachidonoylglycerol synthesis by modulating diacylglycerol lipase expression in alcohol-intoxicated mice. Arch Toxicol 94(2):427–438. https://doi.org/10.1007/s00204-019-02648-7
Kim DK, Kim YH, Lee JH, Jung YS, Kim J, Feng R et al (1864) (2019) Estrogen-related receptor γ controls sterol regulatory element-binding protein-1c expression and alcoholic fatty liver. Biochim Biophys Acta 12:158521. https://doi.org/10.1016/j.bbalip.2019.158521
Kim DK, Ryu D, Koh M, Lee MW, Lim D, Kim MJ et al (2012) Orphan nuclear receptor estrogen-related receptor γ (ERRγ) is key regulator of hepatic gluconeogenesis. J Biol Chem 287(26):21628–21639. https://doi.org/10.1074/jbc.M111.315168
Kim DK, Kim YH, Jang HH, Park J, Kim JR, Koh M et al (2013) Estrogen-related receptor γ controls hepatic CB1 receptor-mediated CYP2E1 expression and oxidative liver injury by alcohol. Gut 62(7):1044–1054. https://doi.org/10.1136/gutjnl-2012-303347
Kim DK, Jeong JH, Lee JM, Kim KS, Park SH, Kim YD et al (2014) Inverse agonist of estrogen-related receptor γ controls Salmonella typhimurium infection by modulating host iron homeostasis. Nat Med 20(4):419–424. https://doi.org/10.1038/nm.3483
Leask A, Abraham DJ (2004) TGF-beta signaling and the fibrotic response. FASEB J 18(7):816–827. https://doi.org/10.1096/fj.03-1273rev
Lee YS, Kim DK, Kim YD, Park KC, Shong M, Seong HA et al (2008) Orphan nuclear receptor SHP interacts with and represses hepatocyte nuclear factor-6 (HNF-6) transactivation. Biochem J 413(3):559–569. https://doi.org/10.1042/bj20071637
Luo J, Sladek R, Carrier J, Bader JA, Richard D, Giguère V (2003) Reduced fat mass in mice lacking orphan nuclear receptor estrogen-related receptor alpha. Mol Cell Biol 23(22):7947–7956. https://doi.org/10.1128/mcb.23.22.7947-7956.2003
Maheshwari A, Kelly DR, Nicola T, Ambalavanan N, Jain SK, Murphy-Ullrich J et al (2011) TGF-β2 suppresses macrophage cytokine production and mucosal inflammatory responses in the developing intestine. Gastroenterology 140(1):242–253. https://doi.org/10.1053/j.gastro.2010.09.043
Massagué J (1998) TGF-beta signal transduction. Annu Rev Biochem 67:753–791. https://doi.org/10.1146/annurev.biochem.67.1.753
Milani S, Herbst H, Schuppan D, Stein H, Surrenti C (1991) Transforming growth factors beta 1 and beta 2 are differentially expressed in fibrotic liver disease. Am J Pathol 139(6):1221–1229
Misra J, Kim DK, Jung YS, Kim HB, Kim YH, Yoo EK et al (2016) O-GlcNAcylation of orphan nuclear receptor estrogen-related receptor γ promotes hepatic gluconeogenesis. Diabetes 65(10):2835–2848. https://doi.org/10.2337/db15-1523
Pinzani M, Rombouts K (2004) Liver fibrosis: from the bench to clinical targets. Digest Liver Dis 36(4):231–242. https://doi.org/10.1016/j.dld.2004.01.003
Radhakrishnan K, Kim YH, Jung YS, Kim J, Kim DK, Cho SJ et al (2020) Orphan nuclear receptor ERRγ is a novel transcriptional regulator of IL-6 mediated hepatic BMP6 gene expression in mice. Int J Mol Sci. https://doi.org/10.3390/ijms21197148
Radhakrishnan K, Kim YH, Jung YS, Kim DK, Na SY, Lim D et al (2021) Orphan nuclear receptor ERR-γ regulates hepatic FGF23 production in acute kidney injury. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.2022841118
Razzaque MA, Masuda N, Maeda Y, Endo Y, Tsukamoto T, Osumi T (2004) Estrogen receptor-related receptor gamma has an exceptionally broad specificity of DNA sequence recognition. Gene 340(2):275–282. https://doi.org/10.1016/j.gene.2004.07.010
Sanyal S, Kim JY, Kim HJ, Takeda J, Lee YK, Moore DD et al (2002) Differential regulation of the orphan nuclear receptor small heterodimer partner (SHP) gene promoter by orphan nuclear receptor ERR isoforms. J Biol Chem 277(3):1739–1748. https://doi.org/10.1074/jbc.M106140200
Serini G, Gabbiana G (1996) Modulation of alpha-smooth muscle actin expression in fibroblasts by transforming growth factor-beta isoforms: an in vivo and in vitro study. Wound Repair Regener 4(2):278–287. https://doi.org/10.1046/j.1524-475X.1996.40217.x
Shi H, Han W, Shi H, Ren F, Chen D, Chen Y et al (2017) Augmenter of liver regeneration protects against carbon tetrachloride-induced liver injury by promoting autophagy in mice. Oncotarget 8(8):12637–12648. https://doi.org/10.18632/oncotarget.14478
Shrestha N, Chand L, Han MK, Lee SO, Kim CY, Jeong YJ (2016) Glutamine inhibits CCl4 induced liver fibrosis in mice and TGF-β1 mediated epithelial-mesenchymal transition in mouse hepatocytes. Food Chem Toxicol 93:129–137. https://doi.org/10.1016/j.fct.2016.04.024
Slater TF, Cheeseman KH, Ingold KU (1985) Carbon tetrachloride toxicity as a model for studying free-radical mediated liver injury. Philos Trans R Soc Lond Ser B Biol Sci 311(1152):633–645. https://doi.org/10.1098/rstb.1985.0169
Takahashi H, Alves CRR, Stanford KI, Middelbeek RJW, Pasquale N, Ryan RE et al (2019) TGF-β2 is an exercise-induced adipokine that regulates glucose and fatty acid metabolism. Nat Metab 1(2):291–303. https://doi.org/10.1038/s42255-018-0030-7
Torres LR, Santana FC, Torres-Leal FL, Melo IL, Yoshime LT, Matos-Neto EM et al (2016) Pequi (Caryocar brasiliense Camb.) almond oil attenuates carbon tetrachloride-induced acute hepatic injury in rats: antioxidant and anti-inflammatory effects. Food Chem Toxicol 97:205–216. https://doi.org/10.1016/j.fct.2016.09.009
Trautwein C, Ramadori G, Gerken G, Meyer zum Büschenfelde KH, Manns M (1992) Regulation of cytochrome P450 IID by acute phase mediators in C3H/HeJ mice. Biochem Biophys Res Commun 182(2):617–623. https://doi.org/10.1016/0006-291x(92)91777-n
Trautwein C, Böker K, Manns MP (1994) Hepatocyte and immune system: acute phase reaction as a contribution to early defence mechanisms. Gut 35(9):1163–1166. https://doi.org/10.1136/gut.35.9.1163
Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M et al (2006) Nuclear receptor expression links the circadian clock to metabolism. Cell 126(4):801–810. https://doi.org/10.1016/j.cell.2006.06.050
Zhang D, Sun M, Samols D, Kushner I (1996) STAT3 participates in transcriptional activation of the C-reactive protein gene by interleukin-6. J Biol Chem 271(16):9503–9509. https://doi.org/10.1074/jbc.271.16.9503
Zhang H, Yang P, Zhou H, Meng Q, Huang X (2008) Involvement of Foxp3-expressing CD4+ CD25+ regulatory T cells in the development of tolerance induced by transforming growth factor-beta2-treated antigen-presenting cells. Immunology 124(3):304–314. https://doi.org/10.1111/j.1365-2567.2007.02769.x
Zhang DG, Zhang C, Wang JX, Wang BW, Wang H, Zhang ZH et al (2017) Obeticholic acid protects against carbon tetrachloride-induced acute liver injury and inflammation. Toxicol Appl Pharmacol 314:39–47. https://doi.org/10.1016/j.taap.2016.11.006
Zhu RZ, Xiang D, Xie C, Li JJ, Hu JJ, He HL et al (2010) Protective effect of recombinant human IL-1Ra on CCl4-induced acute liver injury in mice. World J Gastroenterol 16(22):2771–2779. https://doi.org/10.3748/wjg.v16.i22.2771
Acknowledgements
Y.S.J. is supported by basic science research program (NRF-2020R1A6A3A01096145) through the NRF funded by Korean government (Ministry of Science and ICT). Y.-H.K. is supported by the NRF grant funded by the Korean government (MSIT) (NRF-2019R1C1C1005319). I.-K.L. is supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea. (Grant Number: HI16C1501). S.D. is supported by the Federal Ministry of Education and Research-Liver Systems Medicine Program, Grant PTJ-031L0043, and by the Stiftung für Biomedizinische Alkoholforschung. C.-H.L. is supported by the NRF grant funded by the Korean government (MSIT) (NRF-2020R1A2C3006952). H.-S.C. is supported by National Research Foundation (NRF) of Korea (NRF-2021R1A2C3004923) and National Creative Research Initiatives Grant (20110018305) funded by the Korean government, Ministry of Science and ICT (MIST).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest to declare.
Ethical standards
The manuscript does not contain clinical studies or patient data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jung, Y.S., Kim, YH., Radhakrishnan, K. et al. Orphan nuclear receptor ERRγ regulates hepatic TGF-β2 expression and fibrogenic response in CCl4-induced acute liver injury. Arch Toxicol 95, 3071–3084 (2021). https://doi.org/10.1007/s00204-021-03112-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-021-03112-1