Abstract
GATA-1, a zinc finger transcription factor, has been demonstrated to play a key role in the progression of leukemia. In this study, we investigate the effects of wogonoside, a naturally bioactive flavonoid derived from Scutellaria baicalensis Georgi, on cell growth and cell cycle in chronic myeloid leukemia (CML) cells, and uncover its underlying mechanisms. The experimental design comprised CML cell lines K562, imatinib-resistant K562 (K562r) cells, and primary CML cells, treated in vitro or in vivo, respectively, with wogonoside; growth and cell cycle were then evaluated. We found that wogonoside could induce growth inhibition and G0/G1 cell cycle arrest in both normal and K562r cells. Wogonoside promotes the expression of GATA-1 and facilitates the binding to methyl ethyl ketone (MEK) and p21 promoter, thus inhibiting MEK/extracellular signal-regulated kinase signaling and cell cycle checkpoint proteins, including CDK2, CDK4, cyclin A, and cyclin D1, and increasing p21 expression. Furthermore, in vivo studies showed that administration of wogonoside decreased CML cells and prolonged survival in NOD/SCID mice with CML cell xenografts. In conclusion, these results clearly revealed the inhibitory effect of wogonoside on the growth in CML cells and suggested that wogonoside may act as a promising drug for the treatment of imatinib-resistant CML.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic myelogenous leukemia (CML) is the most common adults leukemia and represents a myeloproliferative disorder in consequence of a balanced, reciprocal translocation of chromosomes t (9; 22) (q34; q11) (Jemal et al. 2010; Melo and Barnes 2007). This chromosomal translocation leads to the generation of an oncogenic BCR-ABL fusion (Melo and Barnes 2007). BCR-ABL fusion oncogene is known as to be central to the pathogenesis of CML (Rowley 1973). The fusion gene encodes a BCR-ABL oncoprotein, which is a constitutively active tyrosine kinase and promotes the growth of leukemic cells and resistance to apoptosis (Menon 2013; Wei et al. 2013). Therefore, extensive efforts have been made to explore breakthrough drugs-tyrosine kinase inhibitors (Tojo 2014). Imatinib, a BCR-ABL tyrosine kinase inhibitor, possesses remarkable success in CML therapy and was widely used in clinical, which changes CML into a chronic disease (Gorre et al. 2001; Weisberg and Griffin 2000). Until now, imatinib also acts as a frontline drug against CML and carries a low risk of morbidity and mortality (Melo and Chuah 2008). However, the increasing emergence of drug resistance restricts the application of imatinib (Demidenko et al. 2005). Although several novel tyrosine kinase inhibitors have been designed to overcome imatinib resistance, they exhibit distinct response patterns relative to different CML patient characteristics, such as BCR-ABL mutational status and disease stage (Jabbour and Kantarjian 2014). Besides, dysregulation of protein phosphorylation, especially at the tyrosine residues, is important for the development of hematological malignancies (Tojo 2014). Several agents are designed to target other biomarkers of CML cells, including FLT3 and BTK, but they do not acquire a dramatically therapeutic effect (Tojo 2014). Therefore, a number of promising drugs for imatinib-resistant CML require further development.
Recently, cell type-specific transcription factor becomes a promising therapeutic target relying on its unique contributions of nuclear organization (Katsumura et al. 2013). GATA-1, a member of the GATA transcription factor family, is involved in cell growth of hematologic malignancies (Grass et al. 2003; Letting et al. 2004). GATA-1 is reported to be a crucial transcription factor for erythroid development by regulating the switch from fetal to adult hemoglobin, and mutations in GATA-1 gene have been associated with X-linked dyserythropoietic anemia and thrombocytopenia (Katsumura et al. 2013). Moreover, GATA-1 was found to be involved with the hematopoietic lineage specification control and differentiation (Crispino 2005). Particularly, a significant body of knowledge has been accumulated to describe that GATA-1 drives differentiation of megakaryocytes and erythrocytes (Crispino 2005; Nakajima 2011). In addition, cells that are forced to undergo differentiation, is always accompanied by growth inhibiting and cell cycle arrest (Huang et al. 2006b). Therefore, GATA-1-dependent transcriptional regulation plays an essential core in progression of hematologic malignancies and becomes a hotspot in current leukemia research.
Hematopoiesis is coordinated by a complex regulatory network, and GATA-1 is integral to successful hematopoiesis (Bresnick et al. 2012). GATA-1 not only acts as a master molecule, but also interacts with other protein, which is indispensable during hematopoietic cell fate decisions. Dysregulation of protein phosphorylation plays a key role in development and progression of hematological malignancies (Tojo 2014). Accumulating evidence suggests that phosphorylation of a variety of proteins, including MEK and extracellular signal-regulated kinase (ERK), is caused by the constitutively active of fusion tyrosine kinase in CML cells (Quintas-Cardama and Cortes 2009; Ren 2005). The phosphorylation of MEK and ERK resulted in deregulated proliferation and survival advantage of leukemia cells (Quintas-Cardama and Cortes 2009; Ren 2005). Moreover, there is clear a evidence that GATA-1 inhibits the phosphorylation of MEK by binding to MEK, thereby inhibiting cell proliferation via a non-transactivation mechanism (Tokunaga et al. 2010). Besides, the binding of GATA-1 to MEK inhibits downstream ERK signal, which is related to proliferation (Tokunaga et al. 2010). As a consequence, the binding between GATA-1 and MEK has been regarded as a promising drug target. The deregulated proliferation of leukemia cell could be reversed via the enhancement of the binding of GATA-1 and MEK. In addition, there are more evidence that the activation of GATA-1 also induced cell cycle arrest by binding to p21 promoter and promote its transcription (Matushansky et al. 2000; Papetti et al. 2010). p21, a crucially and extensively studied cyclin-dependent kinase inhibitor (CKI), exerts pivotal functions in cell cycle progression (Sherr and Roberts 1999). Moreover, the activation of p21 could further affect the expression of other cell cycle proteins, which trigger cell cycle arrest together.
Wogonoside is a naturally bioactive flavonoid derived from the root of Scutellaria baicalensis Georgi. It is known to possess a variety of biological activities including anti-inflammation and anti-angiogenesis (Chen et al. 2009; Lim 2003). Additionally, wogonoside is a kind of in vivo metabolite of wogonin which has been shown to be an anticancer candidate (Tai et al. 2005). It has recently been reported that wogonin could induce growth inhibition and cycle arrest in normal and imatinib-resistant K562 (K562r) cells (Yang et al. 2014). Our previous studies have elucidated that wogonoside possesses anti-leukemic properties with anti-proliferative and cycle arrest effects on acute myelogenous leukemia (AML) cells (Chen et al. 2013). However, whether wogonoside has the similar effect on the CML cell and the exact molecular mechanism remains unresolved. Our investigation showed that wogonoside induced growth inhibition and cycle arrest in CML cells via enhancing the binding ability of GATA-1 with MEK and p21. Our findings might provide a new sight for the exploitation of natural products for CML therapy.
Materials and methods
Reagents and antibodies
Wogonoside (98 % purity, Langze Pharmaceutical Co, Ltd., Nanjing, China) was dissolved in dimethylsulfoxide (DMSO) as a stock solution at 0.5 M and stored at −20 °C. Cells treated with the highest concentration of DMSO were used as control in the corresponding experiments. MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] was purchased from Sigma Chemical Co. (St. Louis, MO). A nuclear/cytosol fractionation kit (KeyGEN, Nanjing, China) was used according to the manufacturer’s directions. Primary antibodies against β-actin, GATA-1, MEK, and GATA-1 siRNA were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against p21, ERK, p-ERK (Thr 177/160), and p-MEK (Ser 217/221) were products from Cell Signaling Technology (Danvers, MA). Primary antibodies against cyclin A, cyclin D1, and CDK4 were products from Bioworld (OH, USA). IRDyeTM 800-conjugated secondary antibodies were purchased from (Rockland Inc., Philadelphia, PA).
Cell culture
Human CML cell line K562 cells were purchased from Cell Bank of Shanghai Institute of Biochemistry & Cell Biology. The imatinib-resistant K562 cells (K562r) were provided by Professor Junia V. Melo, Department of Pathophysiology, Chemical Biology Division of Shanghai Universities E-Institutes, Key Laboratory of Cell Differentiation and Apoptosis of the National Ministry of Education. Primary leukemic cells from newly diagnosed CML blast crisis patients without prior therapy (DrumTower Hospital of Nanjing University Medical School, Nanjing, China) were collected using lymphocyte–monocyte separation medium (Jingmei, Nanjing, China). The protocol of collection of cells from patients complied with guidelines in the Declaration of Helsinki and was approved by the Institutional Review Board of DrumTower Hospital. Cells were cultured in RPMI-1640 medium (Gibco, Invitrogen Corporation, Carlsbad, CA) supplemented with 10 % FBS, 100 U/ml of penicillin, and 100 mg/ml of streptomycin in a humidified environment with 5 % CO2 at 37 °C.
Animal model
Animal studies were conducted in accordance with the regulations of the China Food and Drug Administration (CFDA) on Animal Care. Female NOD/SCID immunodeficient mice (aged 6–9 weeks) were purchased from Beijing HFK bioscience Company Limited. The animals were maintained under controlled temperature (23 ± 2, 55 ± 5 % humidity) and under conditions of controlled lighting (12-h light/day) pathogen-free room. Animals were fed a standard diet of laboratory food and water throughout the experimental period. Survival was represented using a Kaplan–Meier survival plot.
Cell viability assay
Cells were seeded into 96-well plates (Corning, New York, NY) at, respectively, indicated density in 100 μL fresh RPMI-1640 medium and then treated with wogonoside at different concentrations for 24 h and 48 h. Each group consisted of five parallel wells. Subsequently, 20 μL/well of MTT dye (5 mg/ml) was added, and then the plates were incubated for another 4 h at 37 °C in a 5 % CO2 atmosphere. Then the precipitation of formazan crystals was collected by centrifugation at 4000 rpm/15 min. The supernatants were removed and DMSO (100 μL) was added to dissolve the precipitation. Subsequently, the plates were shocked for 2 min, and then absorbance was read at 570 nm on an automated microtiter plate reader (EL800, BIO-TEK Instruments Inc.). The inhibitory effect of wogonoside on cells was calculated according to the following equation:
The concentration that caused 50 % inhibition of cell viabilities (IC50) values was calculated by GraphPad Software. All assays were performed in triplicate.
Cell proliferation assays in vivo
In vivo investigations were performed using immunodeficient (NOD/SCID) mice were sublethally irradiated (2.4Gy) and were engrafted with K562, K562r cells and primary CML cells via the tail vein (2–5 × 106 cells per mouse, n = 6 per group) in 24 h following the radiation treatment. The next day, the mice were injected intraperitoneally (i.p.) with or without wogonoside (80 mg/kg) every other day for 14 days. Twenty days later, blood of the NOD/SCID mice was collected and the expression of CD13, a marker of K562, and CD45, a marker of human primary CML cells, were examined by flow cytometry with a FACSCalibur flow cytometer (Becton, Dickinson, San Jose, CA, USA).
Analysis of cell cycle
The cell cycle was analyzed by propidium iodide staining and quantified by using a BD FACSCalibur flow cytometer (Becton, Dickinson, San Jose, CA) (Tian et al. 2010). The percentage of cells in S, G0–G1 and G2-M phases of the cell cycle was discriminated by the PI fluorescence signal peak versus the integral and quantitated with Modfit software (Becton, Dickinson,San Jose, CA, USA).
Preparation of nuclear extracts
The cells were collected at various time points after wogonoside (200 μM) treatment or at indicated concentrations for 48 h. A nuclear–cytosol fractionation kit (BioVision, Mountain View, CA) was used the nuclear and cytosolic protein extraction preparations according to the modified method as described (Huang et al. 2006a). Then the nuclear extract was subjected to western blot analysis and EMSA assay.
Western blot analysis
Cells were collected and lysed in lysis buffer (50 mM Tris–Cl, pH 7.6, 150 mM NaCl, 1 mM EDTA, 1 % (m/v) NP-40, 0.2 mM phenylmethanesulfonyl fluoride (PMSF), 0.1 mM NaF and 1.0 mM dithiothreitol). The lysates were clarified by centrifugation at 4 °C for 15 min at 13,000×g. The concentration of protein in the supernatants was measured using bicinchoninic acid (BCA) assay kit (Pierce, Rockford, IL, USA) with a Varioskan multimode microplate spectrophotometer (Thermo Waltham, MA, USA). Then equal amounts of extracts (50 μg) were separated by 8–12 % sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto the PVDF membranes (Millipore, Boston, MA, USA). The blots were incubated with specific antibodies against indicated primary antibodies overnight at 4 °C followed by IRDyeTM 800-conjugated secondary antibody for 1 h at 37 °C. Detection was performed using the Odyssey Infrared Imaging System (LI-COR Inc, Lincoln, NE, USA).
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts (8 mg/sample) were prepared as previously described, and electrophoretic mobility shift assay was conducted according to the manufacturer’s instructions (EMSA kit, Beyotime, Nanjing, China). The results were photographed using a Bio-Rad phosphorimager and analyzed with Image Lab™ Software version 3.0 (Bio-Rad).
Immunoprecipitation
Cells were treated with 0 and 200 μM of wogonoside for 48 h. Immunoprecipitation was analyzed as previously described (Gu et al. 2008). The proteins were precipitated from the supernatants of cell lysates by the incubation of Trx-1 antibody. Supernatants of cell lysates were incubated with GATA-1 antibody for 1 h at 4 °C, and then added 20 μL of protein G/A agarose beads (Santa Cruz Biotechnology, St. Louis Park, Minnesota, USA) overnight at 4 °C. Beads were washed with cell lysis buffer four times, and bound proteins were eluted with 2× loading sample buffer and analyzed by western blot with indicated antibodies.
Chromatin immunoprecipitation assay (CHIP)
The CHIP assay was performed as described previously (Hui et al. 2014). Cells were cross-linked with formaldehyde, quenched with glycine, sonicated on ice, and centrifuged at 4 °C. Next, cells were incubated with preimmune IgG and then anti-GATA-1 with rotation at 4 °C overnight and then incubated with protein A/G agarose at 4 °C for 6 h. Finally, immune complexes were captured by protein A/G agarose and eluted with elution buffer (1 % SDS and 0.1 M NaHCO3) at 37 °C for 30 min. Cross-linking was reversed at 65 °C for 4 h in a high salt buffer (0.2 M NaCl, 50 mM Tris, pH 6.5, 10 mM EDTA and 0.2 mg/ml proteinase K). Extracted and dissolved immunoprecipitated DNA was quantified by real-time PCR with primers encompassing the GATA-1-binding sites. The primers for the GATA-1 region in p21 promoter (Papetti et al. 2010) quantification were forward: (−2931 bp relative to TATA box): TGC AAG GCT GCA TCA GTC CT, and reverse: (−2826 bp relative to TATA box): TAG TCC CCA CCC AGG ACT GAA. Next, the PCR analyses were performed with a real-time PCR kit (TaKaRa Biotechnology, Dalian, China).
Transient transfection with small interfering RNA (siRNA)
Cells were plated in six-well plates with fresh RPMI 1640 medium. GATA-1 siRNA transfection was performed using Lipofectamine 2000 reagent according to the manufacturer’s instructions (Mu et al. 2009).
Statistical analysis
Data were expressed as the mean ± standard error of the mean (SEM). The values were obtained from at least three independent experiments. Statistical analysis of multiple-group comparisons was performed by one-way analysis of variance (ANOVA) followed by the Bonferroni post hoc test. Comparisons between two groups were analyzed using the two-tailed Student’s t test. P value <0.05 was considered statistically significant.
Results
Wogonoside inhibits the viability of K562, imatinib-resistant K562 cells and primary CML cells
To assess the effect of wogonoside on leukemia cell viability, MTT assay was performed. K562, imatinib-resistant K562 (K562r), and primary CML cells were treated with various concentrations of wogonoside (4–400 μM) for 24 or 48 h. As shown in Fig. 1a, cell viability of K562 cells was markedly reduced by wogonoside at 24 and 48 h. Moreover, with exposure time up to 48 h, wogonoside shows more obviously growth inhibition than 24 h on K562 cells. The IC50 (the concentration of drug inhibiting 50 % of cells) values of K562 cells at 24 h and 48 h were 271.5 ± 15.49 and 123.5 ± 10.99 μM, respectively. Similar results were also observed in imatinib-resistant K562 and primary CML cells. As the result shows, IC50 values of imatinib-resistant K562 cells were 191.4 ± 14.67 and 117.7 ± 12.32 μM at 24 and 48 h (Fig. 1b). In primary CML cells, IC50 values were 441.0 ± 25.32 and 320.8 ± 17.52 μM at 24 and 48 h, respectively (Fig. 1c). These results indicated that K562, imatinib-resistant K562, and primary CML cells exhibited time- and concentration-dependent sensitivity to wogonoside. Therefore, K562 and imatinib-resistant K562 cell lines were chosen for all subsequent experiments with treatment of 50, 100, and 200 μM wogonoside for 48 h.
Growth inhibition effects of wogonoside on CML cells. a K562 cells in logarithmic growth phase were incubated in 96-well plates with 1 × 104/well, 0.8 × 104/well in 100 μL culture medium, then were treated with 100 μL various concentration of wogonoside for 24 h and 48 h, respectively. The inhibitory effect of wogonoside on K562 cells was detected by MTT assay. b K562r cells in logarithmic growth phase were incubated in 96-well plates with 1.2 × 104/well, 1 × 104/well in 100 μL culture medium and then were treated with 100 μL various concentration of wogonoside for 24 and 48 h, respectively. Cell viability was determined using MTT assay. Data were shown as mean ± SD (n = 3). c Primary CML cells were incubated in 96-well plates with 5 × 104/well in 100 μL culture medium and then were treated with 100 μL various concentration of wogonoside for 24 and 48 h, respectively. Cell viability was determined using MTT assay. Data were shown as mean ± SD (n = 3)
Wogonoside induces cell cycle arrest in K562 and K562r cells
In order to measure the effects of wogonoside on cell cycle progression in K562 and K562r cells, cells were treated with 50, 100, and 200 μM wogonoside for 48 h. Next, fluorescence-activated cell sorting (FACS) was used to analyze the cell cycle status. As shown in Fig. 2a, c, with the increased concentration of wogonoside, the number of K562 cells in the G1 phase increased and the percentage of K562 cells in the S phase decreased accordingly in a concentration-depended manner. This suggested that the wogonoside-treated K562 cells were arrested in the transition from G1 to S phase.
Effects of wogonoside on cell cycle progression in K562 and K562r cells. a, b Representative cell cycle analysis performed by flow cytometry of K562 and K562r cells treated with 50, 100, and 200 μM wogonoside for 48 h. c, d The percentages of cells in the G0/G1 phases of cell cycle following wogonoside treatment for 48 h are shown. Data represent mean ± SEM from three independent experiments. Asterisks denote statistically significant (P < 0.05) differences compared with controls by one-way ANOVA
Consistent with the effect on K562 cells, our findings revealed that wogonoside similarly induced the cycle arrest of imatinib-resistant K562 cells (K562r). The BCR-ABL tyrosine kinase inhibitor imatinib is highly effective in CML treatment (Dan et al. 1998). However, drug-resistant problem restricts the clinical efficacy of imatinib (Demidenko et al. 2005). We found that wogonoside arrested the cell cycle at the G0/G1 phase in K562r cells (Fig. 2b, d). It may provide a new therapeutic strategy for treating CML and reversing CML drug resistance with a different mechanism from imatinib, suggesting the possible sensitivity of wogonoside to K562r cells.
Effects of wogonoside on nuclear transcription factor GATA-1 in K562 and K562r cells
GATA-1 is known as a crucial nuclear transcription factor that regulates cell proliferation, differentiation, and erythroid cell development (Chou et al. 2009; Rylski et al. 2003). Our previous study also demonstrated that GATA-1 plays a key role in wogonin-induced growth inhibition and cycle arrest of CML cells (Yang et al. 2014). To determine whether the growth inhibitory effect of wogonoside on K562 and K562r cells is related to activation of GATA-1, we first fractionated nuclei and cytoplasm/membrane proteins and detected nuclear proteins expression of the CML cells in the presence of 200 μM wogonoside for 0, 12, 24, 36, and 48 h. As shown in Fig. 3a, b and d, e, wogonoside elevated the expression level of nuclear GATA-1 in a time-dependent manner in both K562 and K562r cells. Next, to further confirm the mechanism and the inhibition efficiency of different concentrations of wogonoside on K562 and K562 cells, cells were exposed to 50, 100, and 200 μM wogonoside for 48 h. Results of western blot analyses showed that levels of nuclear GATA-1 increased in a concentration-dependent manner (Fig. 3a, b and d, e). Moreover, EMSA results showed a concentration-dependent increase in DNA binding of GATA-1(Fig. 3c, f). Therefore, it is concluded that wogonoside significantly upregulated the expression of GATA-1 when induced growth inhibition and cycle arrest in CML cells, suggesting that GATA-1 might be a potential target in wogonoside-treated CML cells.
Wogonoside regulates the expression of GATA-1 in K562 and K562r cells. a, d Expression levels of GATA-1 of the nuclear fractions of K562 and K562r cells were analyzed by western blotting. Lamin A was used as a nuclear loading control. b, e Data represent the mean ± SEM of three different experiments. Asterisks denote statistically significant (P < 0.05) differences compared with controls by one-way ANOVA. c, f EMSA assay to detect GATA-1 binding to its consensus site is shown. K562 and K562r cells were incubated with wogonoside (200 μM) for 48 h, and DNA binding was determined in nuclear extracts using EMSA. To determine the composition of the DNA-binding complex, the anti-GATA-1 antibody was used for supershift experiments. Data are representative of three separate experiments
Wogonoside facilitates the interaction between GATA-1 and MEK and inhibits phosphorylation of MEK and ERK in K562, K562r, and primary CML cells
In the above results, we found that wogonoside promoted GATA-1 activation and induced proliferation inhibition in K562 and K562r cells. We speculate that the upregulation of GATA-1 is responsible for the proliferation inhibition. Next, we further investigate the specific molecular mechanism of wogonoside-induced proliferation inhibition. Previous studies have demonstrated that CML is characterized by accumulation and proliferation of myeloid cells and precursors (Cai et al. 2012), which is associated with several downstream proteins phosphorylation, including MEK and ERK (Quintas-Cardama and Cortes 2009; Ren 2005). Moreover, in view of the binding of GATA-1 to MEK is involved with the inhibition of MEK pathway (Tokunaga et al. 2010), we next detected the effects of wogonoside on the binding ability between GATA-1 and MEK by immunoprecipitation assay. The two CML cells were treated with or without 200 μM wogonoside for 48 h respectively. As shown in Fig. 4a, b and c, d, wogonoside enhanced the binding of GATA-1 to MEK in both K562 and K562r cells.
Wogonoside facilitates the interaction between GATA-1 and MEK and influences the expression of MEK-related proteins. a, c K562 and K562r cells incubated with or without 200 μM wogonoside for 36 h were immunoprecipitated with anti-GATA-1 antibody, followed by western blot analysis with anti-MEK antibodies. b, d Quantification of the precipitated MEK in K562 and K562r cells. Data represented the mean ± SEM of three different experiments. Asterisks denote statistically significant (P < 0.05) differences compared with controls by one-way ANOVA. e, g K562 and K562r cells were treated with 200 μM wogonoside for 0, 12, 24, 36, and 48 h. Expression levels of MEK, p-MEK, ERK, and p-ERK were analyzed by western blotting using indicated antibodies. β-Actin was used as a loading control. f, h Quantification of MEK/ERK phosphorylation inhibition. Data represented the mean ± SEM of three different experiments. Asterisks denote statistically significant (P < 0.05) differences compared with controls by one-way ANOVA
To certify whether the phosphorylation of MEK and ERK in CML cells could be inhibited following wogonoside induced binding of GATA-1 to MEK, we detected the protein expression of K562 and K562r cells in the presence of 200 μM wogonoside for 0, 12, 24, 36, and 48 h. As shown in Fig. 4e–h, wogonoside markedly downregulated the expression level of p-MEK and p-ERK in a time-dependent manner in both K562 and K562r cells. Moreover, similar to the results of CML cell lines, primary CML cells treated with wogonoside for 48 h also showed notable reduction in phosphorylated MEK and ERK, and the expression level of GATA-1 and p21 is also upregulated (Fig. 5g). Taken together, the results suggested that wogonoside facilitated the interaction between GATA-1 and MEK, inhibited phosphorylation of MEK and ERK, and then caused the proliferation inhibition of CML cells.
Wogonoside increases GATA-1 binding to p21 promoter and promotes the expression of cell cycle-related proteins in K562 and K562r cells. a, b Activity of GATA-1 binding to p21 promoter in K562 and K562r cells were detected by CHIP assay. The bars indicate the percentages of input DNA fragments present in specific immunoprecipitates. c, e Expression levels of cyclin A, CDK2, CDK4, cyclin D1, and p21 were analyzed by western blotting after K562 and K562r cells treated with 50, 100, 200 μM wogonoside for 48 h. β-Actin was used as a loading control. Results are representative of 3 independent experiments. d, f Quantification of the data shown in c, e. Data represented the mean ± SEM of 3 different experiments. Asterisks denote statistically significant (P < 0.05) differences compared with controls by one-way ANOVA. g Expression levels of GATA-1, MEK, p-MEK, ERK and p-ERK were analyzed by western blotting using indicated antibodies. β-actin was used as a loading control in primary CML cells
Effects of GATA-1 on wogonoside-induced cell cycle in K562 and K562r cells
To further investigate the molecular mechanism of wogonoside on the cell cycle arrest in K562 and K562r cells, we examined expression level of several G0/G1-related proteins in these two CML cell lines after 0, 50, 100, and 200 μM wogonoside treatment 48 h. Results showed that wogonoside significantly upregulated the expression of p21, which is a crucially and extensively studied CKI (Sherr and Roberts 1999). Furthermore, wogonoside downregulated cyclin-dependent kinase 4 (CDK4), CDK2, cyclin A, and cyclin D1 in both cell lines (Fig. 5c–f). It is reported that CDK4 and cyclin D1 is indispensable for transition through the early G1 phase, whereas CDK2 and cyclin A are necessary for the completion of the G1 phase and the initiation of the S phase (Vermeulen et al. 2003). These results indicated that wogonoside could arrest cell cycle at G0/G1 phase by regulating several cell cycle checkpoints in human CML cells.
In addition, a literature search revealed that GATA-1 binds to the promoter of p21 and triggers its transcriptional activation (Matushansky et al. 2000; Papetti et al. 2010). Our previous studies also revealed that wogonin enhanced the binding ability between GATA-1 and p21 promoter in both K562 and K562r cells (Yang et al. 2014). We next investigated the effects of wogonoside on p21 expression. CHIP assay demonstrated that 48 h wogonoside treatment could significantly facilitate the binding of GATA-1 to p21 promoter in K562 and K562r cells (Fig. 5a, b). Also, wogonoside increased the expression of p21 in both K562 and K562r cells (Fig. 5c, e).
As a whole, these results indicated that wogonoside promoted the binding of GATA-1 to p21, triggered the activation of p21, regulated the downstream cell cycle-related proteins, and finally caused cell cycle arrest in K562 and K562r cells. Similar to GATA-1/MEK signaling, GATA-1-p21 pathway also plays a key role in wogonoside-induced CML cells cycle arrest (Fig. 5).
GATA-1 deficiency suppresses wogonoside-induced growth inhibition and cycle arrest effects on K562 and K562r cells
To investigate whether the growth inhibition and cycle arrest effects of wogonoside on CML cells is dependent on GATA-1, cells were transfected with GATA-1 siRNA and the efficacy of transfection monitored using western blot (Fig. 6a). Upon silencing of GATA-1,MTT assay showed that GATA-1 siRNA partially impaired wogonoside-induced CML cells proliferation inhibition (Fig. 6b). Moreover, transfection of CML cells with GATA-1 siRNA led to significant inhibition of wogonoside-induced cell cycle arrest (Fig. 6c, d). Meanwhile, the upregulation of cell cycle regulators induced by wogonoside was also reduced (Fig. 6e, f). Collectively, these results confirm that growth inhibition and cell cycle arrest induced by wogonoside is mediated by GATA-1 and supported the requirement of GATA-1 in CML treatment.
GATA-1 is involved in wogonoside-modulated growth inhibition and cell cycle arrest. K562 and K562r cells were transfected with nonspecific siRNA and GATA-1 siRNA treated with or without 200 μM wogonoside for 48 h. a Confirmation of the silencing of GATA-1 expression was detected by western blotting with β-actin as a loading control. b Growth inhibition effect of wogonoside was assessed by MTT assay. c, d The percentages of cells in G0/G1 phases of the cell cycle are shown. Data represent the mean ± SEM of three different experiments. Asterisks denote statistically significant (P < 0.05) differences compared with controls by one-way ANOVA. e, f Effect of silenced GATA-1 on the expression of growth-related proteins and cell cycle checkpoint proteins
Anti-proliferative effects of wogonoside on CML cells in vivo
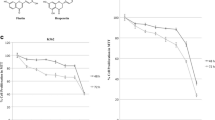
We next examined the effects of wogonoside in vivo. Twenty days after injection with K562, K562r and primary CML cells, blood samples from mice randomly picked in each group were examined for CD13 expression, a surface marker of K562 cells, and CD45 expression, a surface marker of human primary CML cells. The results showed that mice inoculated K562 and K562r cells expressed more CD13 than normal mice and wogonoside significantly decreased the expression of CD13 in K562 and K562r group (Fig. 7a). Mice inoculated primary CML cells expressed more CD45 than normal mice and wogonoside significantly decreased the expression of CD45 in primary CML cell group (Fig. 7b). Wogonoside also extended the survival of CML-bearing mice compared with mice in the control group (Fig. 7c). The median survival time of mice injected with K562, K562r cells, and primary CML cells were 36.5 ± 6.5, 26 ± 2.3, and 21.5 ± 3.2 days in the control groups and were 53.5 ± 9.6, 44 ± 7.8, and 26.5 ± 10.1 days in the wogonoside-treated groups. These results demonstrated that wogonoside suppressed proliferation of CML cells in vivo.
Effects of wogonoside on CML-bearing NOD/SCID mice. a CD13 expression was examined in blood samples from three mice of each group in CML cell lines-bearing groups. Data represent the mean ± SEM of three different experiments. Asterisks denote statistically significant (P < 0.05) differences compared with controls by one-way ANOVA. b CD45 expression was examined in blood samples from three mice of each group in primary CML cells-bearing group. Data represent the mean ± SEM of three different experiments. Asterisks denote statistically significant (P < 0.05) differences compared with controls by one-way ANOVA. c A Kaplan–Meier survival plots of CML-bearing NOD/SCID mice are shown. K562, K562r and primary CML cells were injected into the tail vein 24 h (2–5 × 106 cells per mouse, 6 mice per group) after sublethally irradiation (2.4 Gy). Starting the next day, mice were injected intraperitoneally with or without wogonoside (80 mg/kg) every other day for 14 days. The blank animal group, without CML cells treated with solvent, was used to evaluate survival ability. Data are representative of two separate experiments. Animals were observed for 80 days after cell injection. The survival curves differed significantly between the wogonoside-treated group and the control group. Wogonoside prolonged survival in mice compared with controls (P < 0.001; log-rank test)
Discussion
Although our previous studies clarify that wogonin could induce proliferative inhibition and cell cycle arrest in CML cells and wogonoside, a metabolite of wogonin, possesses remarkable anti-leukemic properties in AML cells (Chen et al. 2013; Yang et al. 2014), the anti-CML effects of wogonoside and its underlying mechanism remain unclear. This study demonstrated that wogonoside exerted inhibitory effects on cell growth and cell cycle in K562 and imatinib-resistant K562 cells, which suggest that wogonoside has a potential anti-leukemia effect in CML cells. Moreover, our investigation confirmed that nuclear transcription factor GATA-1 is important in wogonoside-induced blockade of cell cycle progression and proliferative inhibition (Fig. 6). The binding of GATA-1 to MEK and p21 promotor contributed to growth inhibition and cycle arrest in CML cells.
Recently, flavonoids are widely investigated in cancer research for its unique pharmacological properties (Chen et al. 2013; Hui et al. 2014; Ikemoto et al. 2000; Yang et al. 2014). Wogonin, one of flavonoids extracted from Scutellariae radix, has been reported to have several biological effects including anti-tumor activity in various cancer cells (Ikemoto et al. 2000; Li-Weber 2009). In particular, previous studies have provided evidence that wogonin exerts therapeutic potential in treating hematological malignancies (Baumann et al. 2008; Yang et al. 2014). The research of flavonoid metabolism showed that flavonoid aglycones undergo rapid and extensive metabolism after either oral or intravenous administration. Moreover, glucuronide or sulfate conjugates is the main form of flavonoid aglycones into the blood stream, and when flavonoid aglycones enter the blood stream, unchanged aglycone became barely detectable, but the levels of glucuronic acid conjugates are high. In addition, the glucuronidation altered the physicochemical properties of flavonoids and then affected the biological activity of aglycones. The metabolic process was regard as a detoxification process (Li et al. 2011; Walle 2004). Therefore, the glucuronic acid conjugates may contribute to the safe biological activities of flavonoid and become an important modification site. Wogonoside is just such a flavonoid, which is a metabolite of wogonin and is considered as a safe candidate of anticancer drugs. Indeed, it has been reported that wogonoside possess anti-leukemic properties (Chen et al. 2013). Our data also demonstrated that the wogonoside-treated mice possess a longer survival compared to wogonin group (Fig. 7).
Hematologic malignancies is characterized by disrupted actions of physiological regulators of blood cells, and transcription factor GATA has been shown to be a master regulator of hematopoietic cells and play a pivotal role in hematopoiesis (Bresnick et al. 2012). In particular, an increasing evidence demonstrated that GATA-2, a member of GATA family, and its target genes are implicated in human hematologic pathophysiologies and leukemogenesis (Bresnick et al. 2012). It is instructive to consider the underlying therapeutic potential by thwarting GATA-2-dependent signaling. Here, we highlighted the function of GATA-1 in the treatment of CML. GATA-1 has been reported to promote erythrocyte, megakaryocyte, mast cell, and eosinophil development, and was considered to affect the cell fate (Migliaccio et al. 2003; Yu et al. 2002). GATA-1 is a zinc finger transcription factor and the zinc finger closest to the amino terminus (N-finger), which mediates an important and extensive protein–protein interaction (Tsang et al. 1997). Moreover, GATA-1 with its broad N-terminus can enhance activation of the target gene (Johnson et al. 2006). Previous studies demonstrated that GATA-1 suppresses a number of genes transcription, including GATA-2 (Grass et al. 2003; Letting et al. 2004). Furthermore, the interaction of GATA factors has been found to be a general mechanism for controlling developmental processes of blood cells (Bresnick et al. 2010). It reveals unique opportunities for seeking drugs targeting GATA-1, then to achieve inhibition of GATA-2-dependent processes and enhancement of protein–protein interaction.
Our previous research showed that wogonin displays anti-CML properties via regulating the function of GATA-1. Therefore, further studies focused on the effect of wogonoside in CML cells by regulating GATA-1. In this study, we found that wogonoside has the similar effect to wogonin on cell growth inhibition (Fig. 1). Moreover, mechanism investigation demonstrated that wogonoside significantly enhanced the expression of GATA-1 in a concentration- and time-dependent manner in both K562 and imatinib-resistant K562 cells (Fig. 3). Drug-resistant problem restricts the application of imatinib for CML patients (Wei et al. 2013), K562r cells responding to wogonoside might indicate that new agent except tyrosine kinase inhibitor is feasible for CML therapy. As research continued to deepen, the mechanism behind growth inhibition mediated by GATA-1 has become much clearer. Phosphorylations of MEK and ERK contribute to the leukemic malignancy (Quintas-Cardama and Cortes 2009; Ren 2005). Moreover, it is reported that GATA-1 inhibits the phosphorylation of MEK by binding to MEK, thereby inhibiting the cell proliferation via a non-transactivation mechanism (Tokunaga et al. 2010). Our data showed that 200 μM wogonoside markedly facilitated the binding ability of GATA-1 to MEK (Fig. 4a, c) and then inhibited MEK and its downstream ERK phosphorylation in both K562 and K562r cells (Fig. 4e, g). Therefore, we confirmed the key role of GATA-1/MEK/ERK signal pathway in wogonoside-induced growth inhibition of K562 and K562r cells.
It is well known that tumorigenesis is accompanied by deregulated cell proliferation and unlimited cell cycle; therefore, cell cycle arrest induction is regarded as an effective strategy for cancer therapy (Buolamwini 2000; Hajduch et al. 1999). Here, we found that wogonoside induced G0/G1 phase arrest in both K562 and imatinib-resistant K562 cells after treatment for 48 h (Fig. 2). Moreover, the mechanism investigation suggested that wogonoside enhanced the binding ability of GATA-1 to p21 promoter, which activated its expression (Fig. 5e). G1/S transition, an important checkpoint, is tightly controlled by cyclin/cyclin-dependent kinase (Cdks) complexes and plays crucial role in maintaining cell proliferation. For instance, CDK4 and cyclin D1 is indispensable for transition through the early G1 phase, whereas CDK2 and cyclin A are necessary for the completion of the G1 phase and the initiation of the S phase (Vermeulen et al. 2003). In addition, p21 exerts pivotal function in regulation of cell cycle-related proteins (Sherr and Roberts 1999). Our further investigation revealed that these G0/G1 transition regulatory proteins were involved with wogonoside-induced cell cycle arrest in K562 and K562r cells and that activation of p21 could further downregulated the expression of downstream cycle-related proteins, which trigger the cell cycle arrest together (Fig. 5a, c). These results indicated that wogonoside-induced GATA-1 played a significant role in regulation of cell cycle in human CML cells.
The further investigation revealed that wogonoside also inhibited the viability of primary CML cells and upregulated the GATA-1 expression. Meanwhile, wogonoside exhibited an inhibitory effect on phosphorylation of MEK and ERK in primary CML cells (Fig. 5g). However, we found that wogonoside showed no effect on the cell cycle arrest of primary CML cells, even the expression level of p21 was increased. It might be due to the fact that the proliferation of primary cells is slow in vitro and the cycle change is non-detectable. Moreover, it has been reported that p21 exerts a function in development of cancer and uncoupled from cell cycle, and the upregulation effect of wogonoside on p21 may involve in growth inhibition of primary CML cells (Abbas and Dutta 2009). Thus, wogonoside-induced GATA-1 activation was involved with two distinct signal pathways, one linked to MEK/ERK and the other was associated with p21, both exhibiting anti-CML function (Fig. 8). Our findings proposed that wogonoside could be a potential candidate for treating CML with high efficiency and low toxicity.
Abbreviations
- GATA-1:
-
GATA binding protein 1
- CML:
-
Chronic myeloid leukemia
- ERK:
-
Extracellular signal-regulated kinase
- MEK:
-
Methyl ethyl ketone
- CKI:
-
Cyclin-dependent kinase inhibitor
- DMSO:
-
Dimethyl sulfoxide
- NOD/SCID:
-
Non-obese diabetic/severe combined immunodeficiency
References
Abbas T, Dutta A (2009) p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer 9(6):400–414. doi:10.1038/nrc2657
Baumann S, Fas SC, Giaisi M et al (2008) Wogonin preferentially kills malignant lymphocytes and suppresses T-cell tumor growth by inducing PLCgamma1- and Ca2+ -dependent apoptosis. Blood 111(4):2354–2363. doi:10.1182/blood-2007-06-096198
Bresnick EH, Lee HY, Fujiwara T, Johnson KD, Keles S (2010) GATA switches as developmental drivers. J Biol Chem 285(41):31087–31093. doi:10.1074/jbc.R110.159079
Bresnick EH, Katsumura KR, Lee HY, Johnson KD, Perkins AS (2012) Master regulatory GATA transcription factors: mechanistic principles and emerging links to hematologic malignancies. Nucleic Acids Res 40(13):5819–5831. doi:10.1093/nar/gks281
Buolamwini JK (2000) Cell cycle molecular targets in novel anticancer drug discovery. Curr Pharm Des 6(4):379–392
Cai A, Keskin DB, DeLuca DS et al (2012) Mutated BCR-ABL generates immunogenic T-cell epitopes in CML patients. Clin Cancer Res 18(20):5761–5772. doi:10.1158/1078-0432.ccr-12-1182
Chen Y, Lu N, Ling Y et al (2009) Wogonoside inhibits lipopolysaccharide-induced angiogenesis in vitro and in vivo via toll-like receptor 4 signal transduction. Toxicology 259(1–2):10–17. doi:10.1016/j.tox.2009.01.010
Chen Y, Hui H, Yang H et al (2013) Wogonoside induces cell cycle arrest and differentiation by affecting expression and subcellular localization of PLSCR1 in AML cells. Blood 121(18):3682–3691. doi:10.1182/blood-2012-11-466219
Chou ST, Khandros E, Bailey LC et al (2009) Graded repression of PU.1/Sfpi1 gene transcription by GATA factors regulates hematopoietic cell fate. Blood 114(5):983–994. doi:10.1182/blood-2009-03-207944
Crispino JD (2005) GATA1 in normal and malignant hematopoiesis. Semin Cell Dev Biol 16(1):137–147. doi:10.1016/j.semcdb.2004.11.002
Dan S, Naito M, Tsuruo T (1998) Selective induction of apoptosis in Philadelphia chromosome-positive chronic myelogenous leukemia cells by an inhibitor of BCR–ABL tyrosine kinase, CGP 57148. Cell Death Differ 5(8):710–715. doi:10.1038/sj.cdd.4400400
Demidenko ZN, An WG, Lee JT, Romanova LY, McCubrey JA, Blagosklonny MV (2005) Kinase-addiction and bi-phasic sensitivity-resistance of Bcr-Abl- and Raf-1-expressing cells to imatinib and geldanamycin. Cancer Biol Ther 4(4):484–490
Gorre ME, Mohammed M, Ellwood K et al (2001) Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science 293(5531):876–880. doi:10.1126/science.1062538
Grass JA, Boyer ME, Pal S, Wu J, Weiss MJ, Bresnick EH (2003) GATA-1-dependent transcriptional repression of GATA-2 via disruption of positive autoregulation and domain-wide chromatin remodeling. Proc Natl Acad Sci USA 100(15):8811–8816. doi:10.1073/pnas.1432147100
Gu H, Wang X, Rao S et al (2008) Gambogic acid mediates apoptosis as a p53 inducer through down-regulation of mdm2 in wild-type p53-expressing cancer cells. Mol Cancer Ther 7(10):3298–3305. doi:10.1158/1535-7163.mct-08-0212
Hajduch M, Havlieek L, Vesely J, Novotny R, Mihal V, Strnad M (1999) Synthetic cyclin dependent kinase inhibitors. New generation of potent anti-cancer drugs. Adv Exp Med Biol 457:341–353
Huang C, Cao J, Huang KJ et al (2006a) Inhibition of STAT3 activity with AG490 decreases the invasion of human pancreatic cancer cells in vitro. Cancer Sci 97(12):1417–1423. doi:10.1111/j.1349-7006.2006.00340.x
Huang Y, Zhao Q, Zhou CX et al (2006b) Antileukemic roles of human phospholipid scramblase 1 gene, evidence from inducible PLSCR1-expressing leukemic cells. Oncogene 25(50):6618–6627. doi:10.1038/sj.onc.1209677
Hui H, Chen Y, Yang H et al (2014) Oroxylin A has therapeutic potential in acute myelogenous leukemia by dual effects targeting PPARgamma and RXRalpha. Int J Cancer 134(5):1195–1206. doi:10.1002/ijc.28435
Ikemoto S, Sugimura K, Yoshida N et al (2000) Antitumor effects of Scutellariae radix and its components baicalein, baicalin, and wogonin on bladder cancer cell lines. Urology 55(6):951–955
Jabbour E, Kantarjian H (2014) Chronic myeloid leukemia: 2014 update on diagnosis, monitoring, and management. Am J Hematol 89(5):547–556. doi:10.1002/ajh.23691
Jemal A, Siegel R, Xu J (2010) Ward E (2010) Cancer statistics. CA Cancer J Clin 60(5):277–300. doi:10.3322/caac.20073
Johnson KD, Kim SI, Bresnick EH (2006) Differential sensitivities of transcription factor target genes underlie cell type-specific gene expression profiles. Proc Natl Acad Sci USA 103(43):15939–15944. doi:10.1073/pnas.0604041103
Katsumura KR, DeVilbiss AW, Pope NJ, Johnson KD, Bresnick EH (2013) Transcriptional mechanisms underlying hemoglobin synthesis. Cold Spring Harb Perspect Med 3(9):a015412. doi:10.1101/cshperspect.a015412
Letting DL, Chen YY, Rakowski C, Reedy S, Blobel GA (2004) Context-dependent regulation of GATA-1 by friend of GATA-1. Proc Natl Acad Sci USA 101(2):476–481. doi:10.1073/pnas.0306315101
Li C, Lin G, Zuo Z (2011) Pharmacological effects and pharmacokinetics properties of Radix Scutellariae and its bioactive flavones. Biopharm Drug Dispos 32(8):427–445. doi:10.1002/bdd.771
Lim BO (2003) Effects of wogonin, wogonoside, and 3,5,7,2′,6′-pentahydroxyflavone on chemical mediator production in peritoneal exduate cells and immunoglobulin E of rat mesenteric lymph node lymphocytes. J Ethnopharmacol 84(1):23–29
Li-Weber M (2009) New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat Rev 35(1):57–68. doi:10.1016/j.ctrv.2008.09.005
Matushansky I, Radparvar F, Skoultchi AI (2000) Reprogramming leukemic cells to terminal differentiation by inhibiting specific cyclin-dependent kinases in G1. Proc Natl Acad Sci USA 97(26):14317–14322. doi:10.1073/pnas.250488697
Melo JV, Barnes DJ (2007) Chronic myeloid leukaemia as a model of disease evolution in human cancer. Nat Rev Cancer 7(6):441–453. doi:10.1038/nrc2147
Melo JV, Chuah C (2008) Novel agents in CML therapy: tyrosine kinase inhibitors and beyond. Hematology Am Soc Hematol Educ Prog 427–435. doi:10.1182/asheducation-2008.1.427
Menon H (2013) Issues in current management of chronic myeloid leukemia: importance of molecular monitoring on long term outcome. South Asian J Cancer 2(1):38–43. doi:10.4103/2278-330x.105893
Migliaccio AR, Rana RA, Sanchez M et al (2003) GATA-1 as a regulator of mast cell differentiation revealed by the phenotype of the GATA-1low mouse mutant. J Exp Med 197(3):281–296
Mu R, Qi Q, Gu H et al (2009) Involvement of p53 in oroxylin A-induced apoptosis in cancer cells. Mol Carcinog 48(12):1159–1169. doi:10.1002/mc.20570
Nakajima H (2011) Role of transcription factors in differentiation and reprogramming of hematopoietic cells. Keio J Med 60(2):47–55
Papetti M, Wontakal SN, Stopka T, Skoultchi AI (2010) GATA-1 directly regulates p21 gene expression during erythroid differentiation. Cell Cycle 9(10):1972–1980
Quintas-Cardama A, Cortes J (2009) Molecular biology of bcr-abl1-positive chronic myeloid leukemia. Blood 113(8):1619–1630. doi:10.1182/blood-2008-03-144790
Ren R (2005) Mechanisms of BCR-ABL in the pathogenesis of chronic myelogenous leukaemia. Nat Rev Cancer 5(3):172–183. doi:10.1038/nrc1567
Rowley JD (1973) Letter: a new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature 243(5405):290–293
Rylski M, Welch JJ, Chen YY et al (2003) GATA-1-mediated proliferation arrest during erythroid maturation. Mol Cell Biol 23(14):5031–5042
Sherr CJ, Roberts JM (1999) CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 13(12):1501–1512
Tai MC, Tsang SY, Chang LY, Xue H (2005) Therapeutic potential of wogonin: a naturally occurring flavonoid. CNS Drug Rev 11(2):141–150
Tian K, Yang S, Ren Q et al (2010) p38 MAPK contributes to the growth inhibition of leukemic tumor cells mediated by human umbilical cord mesenchymal stem cells. Cell Physiol Biochem 26(6):799–808. doi:10.1159/000323973
Tojo A (2014) Kinase inhibitors against hematological malignancies. Nihon Rinsho 72(6):1118–1124
Tokunaga M, Ezoe S, Tanaka H et al (2010) BCR-ABL but not JAK2 V617F inhibits erythropoiesis through the Ras signal by inducing p21CIP1/WAF1. J Biol Chem 285(41):31774–31782. doi:10.1074/jbc.M110.118653
Tsang AP, Visvader JE, Turner CA et al (1997) FOG, a multitype zinc finger protein, acts as a cofactor for transcription factor GATA-1 in erythroid and megakaryocytic differentiation. Cell 90(1):109–119
Vermeulen K, Van Bockstaele DR, Berneman ZN (2003) The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif 36(3):131–149
Walle T (2004) Absorption and metabolism of flavonoids. Free Radic Biol Med 36(7):829–837. doi:10.1016/j.freeradbiomed.2004.01.002
Wei W, Huang H, Zhao S et al (2013) Alantolactone induces apoptosis in chronic myelogenous leukemia sensitive or resistant to imatinib through NF-kappaB inhibition and Bcr/Abl protein deletion. Apoptosis 18(9):1060–1070. doi:10.1007/s10495-013-0854-2
Weisberg E, Griffin JD (2000) Mechanism of resistance to the ABL tyrosine kinase inhibitor STI571 in BCR/ABL-transformed hematopoietic cell lines. Blood 95(11):3498–3505
Yang H, Hui H, Wang Q et al (2014) Wogonin induces cell cycle arrest and erythroid differentiation in imatinib-resistant K562 cells and primary CML cells. Oncotarget 5(18):8188–8201
Yu C, Cantor AB, Yang H et al (2002) Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J Exp Med 195(11):1387–1395
Acknowledgments
This work was supported by the Project Program of State Key Laboratory of Natural Medicines, China Pharmaceutical University (No. JKGZ201101, SKLNMZZ201210, SKLNMZZCX201303 SKLNMZZJQ201302 and No. G140042), Science Fund for Distinguished Young Scholars of Jiangsu province (BK20130024), the National Science & Technology Major Project (No. 2012ZX09304-001, 2012ZX09103101-050), the National Natural Science Foundation of China (Nos. 81300379, 81373449, 91029744, and 81173086), Natural Science Foundation of Jiangsu province (No. BK20140668), the Key Project supported by medical science and technology development Foundation of Nanjing Department of Health (No. ZKX14015), Six big talent peak in Jiangsu province project (2014-WSN-049), Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT-IRT1193), and Huahai Graduate Innovation Fund (CX13B-006HH), and the Priority Academic Program Development of Jiangsu Higher Education Institutions and the Fundamental Research Funds for the Central Universities (PY2014YX0001; ZL2014YX0034).
Conflict of interest
All the authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Hui Li and Hui Hui have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
204_2015_1552_MOESM1_ESM.tif
Viable cells were counted using a hemocytometer after trypan blue staining to assess anti-proliferation activity. (TIFF 1483 kb)
Rights and permissions
About this article
Cite this article
Li, H., Hui, H., Xu, J. et al. Wogonoside induces growth inhibition and cell cycle arrest via promoting the expression and binding activity of GATA-1 in chronic myelogenous leukemia cells. Arch Toxicol 90, 1507–1522 (2016). https://doi.org/10.1007/s00204-015-1552-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-015-1552-3