Abstract
Soy isoflavones (IF) are phytoestrogens, which interact with estrogen receptors. They are extensively metabolized by glucuronosyltransferases and sulfotransferases, leading to the modulation of their estrogenic activity. It can be assumed that this biotransformation also has a crucial impact on the uptake of IF by active or passive cellular transport mechanisms, but little is known about the transport of IF phase II metabolites into the cell. Therefore, transport assays for phase II metabolites of daidzein (DAI) were carried out using HEK293 cell lines transfected with five human candidate carriers, i.e., organic anion transporter OAT4, sodium-dependent organic anion transporter (SOAT), Na+-taurocholate cotransporting polypeptide (NTCP), apical sodium-dependent bile acid transporter ASBT, and organic anion transporting polypeptide OATP2B1. Cellular uptake was monitored by UHPLC-DAD. DAI monosulfates were transported by the carriers NTCP and SOAT in a sodium-dependent manner, while OAT4-HEK293 cells revealed a partly sodium-dependent transport for these compounds. In contrast, DAI-7,4′-disulfate was only taken up by NTCP-HEK293 cells. DAI-7-glucuronide, but not DAI-4′-glucuronide, was transported exclusively by OATP2B1 in a sodium-independent manner. DAI-7-glucuronide-4′-sulfate, DAI-7-glucoside, and DAI were no substrate of any of the tested carriers. In addition, the inhibitory potency of the DAI metabolites toward estrone-sulfate (E1S) uptake of the above-mentioned carriers was determined. In conclusion, human SOAT, NTCP, OATP2B1, and OAT4 were identified as carriers for the DAI metabolites. Several metabolites were able to inhibit carrier-dependent E1S uptake. These findings might contribute to a better understanding of the bioactivity of IF especially in case of hormone-related cancers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soy and soy protein are common foods (e.g., tofu, soy milk, and soy yogurt) and are also contained in many foodstuffs, such as bakery products and infant formulas (Ritchie et al. 2006). Soy is rich in phytoestrogenic isoflavones (IF), the consumption of which is being associated with many positive health effects, such as anti-osteoporotic effects, breast cancer prevention, or reduction in menopausal vasomotor symptoms (Mortensen et al. 2009). On the other hand, there is an ongoing discussion whether IF might stimulate the proliferation of existing breast cancer cells based on their estrogenic activity (Allred et al. 2001; Wietrzyk et al. 2004; Kijkuokool et al. 2006; Hilakivi-Clarke et al. 2010; Magee and Rowland 2012; Zhang et al. 2012; Zamora-Ros et al. 2013). IF undergo an extensive biotransformation by the gut microbiota as well as by xenobiotic-metabolizing enzymes. In human plasma, IF mainly exist as phase II conjugates, such as mono- and di-glucuronides, mono- and di-sulfates, and sulfoglucuronides (Rüfer et al. 2008; Bolca et al. 2010; Hosoda et al. 2011). Because of their polarity, these circulating metabolites are not able to pass the cell membrane via passive diffusion. Therefore, active transport mechanisms may play a role in the cellular uptake of IF conjugates.
It has been shown in rats that genistein (GEN) is excreted into the bile as GEN-7-glucuronide and reabsorbed in the intestine indicating an enterohepatic circulation (Sfakianos et al. 1997). However, the carriers involved are still unknown. The two carriers Na+-taurocholate cotransporting polypeptide NTCP (SLC10A1) and the apical sodium-dependent bile acid transporter ASBT (SLC10A2) are known to maintain the enterohepatic circulation of bile acids (Shneider et al. 1995; Meier and Stieger 2002; Trauner and Boyer 2003) and, therefore, represent promising candidate transporters for IF conjugates. NTCP transports bile acids, sulfoconjugated bile acids, and sulfoconjugated steroid hormones and is mainly expressed in the liver, whereas ASBT is expressed in the brush-border membrane of ileocytes, the apical membrane of cholangiocytes in the liver and in the apical domain of the proximal tubules in the kidney with a substrate pattern restricted to bile acids (Hagenbuch and Meier 1994; Wong et al. 1995; Craddock et al. 1998; Schroeder et al. 1998; Kramer et al. 1999).
The sodium-dependent organic anion transporter SOAT (SLC10A6) does not transport bile acids, but sulfoconjugated steroid hormones. SOAT is highly expressed in hormone-responsive tissues, such as testis, placenta, and mammary gland (Geyer et al. 2007; Fietz et al. 2013). Sulfoconjugated steroid hormones such as dehydroepiandrosterone sulfate (DHEAS), estrone-sulfate (E1S), and 17β-estradiol-3-sulfate (E2S) exhibit similar chemical structures compared with sulfated IF metabolites (Fig. 1). In plasma, sulfoconjugated steroid hormones are present in much higher concentrations than the corresponding free steroids. Sulfoconjugated steroid hormones are considered as reservoir for the biosynthesis of active free steroid hormones and may therefore contribute to the overall regulation of reproductive processes (Geisler 2003; Reed et al. 2005). Free steroid hormones are lipophilic and therefore capable of crossing the cell membrane by diffusion, whereas the hydrophilic conjugated steroid sulfates such as E1S can only get access to the intracellular compartment by carrier-mediated transport. In the cell, the steroid sulfatase can cleave the sulfate group from the sulfated conjugate, and the released steroid can participate in steroid regulation at nuclear estrogen and androgen receptors (Selcer et al. 2002; Pasqualini and Chetrite 2005). SOAT is considered to be involved in the cellular import of sulfoconjugated steroid hormones in reproductive tissues representing also target tissues for IF.
The organic anion transporter OAT4 (SLC22A11) is mainly expressed in the kidney and the placenta, while the organic anion transporting polypeptide OATP2B1 is expressed in the liver, the intestine, and the placenta (Cha et al. 2000; Nishimura and Naito 2005; Kullak-Ublick et al. 2001; Kobayashi et al. 2003; St-Pierre et al. 2002). Transport activity for DHEAS and E1S has been determined for both OAT4 and OATP2B1 (Ugele et al. 2008; Tamai et al. 2000; Kullak-Ublick et al. 2001).
In the present study, transport assays for IF phase II metabolites were carried out with NTCP and ASBT, two candidate carriers for the enterohepatic circulation of IF metabolites, as well as with SOAT, OAT4, and OATP2B1, which are known to transport sulfoconjugated steroid hormones. In addition, the potential of the IF conjugates to inhibit E1S uptake was examined.
Materials and methods
Chemicals and radiochemicals
Daidzein (DAI) with a purity of >99 % was purchased from LC Laboratories (Woburn, MA, USA). Daidzein-4′-β-D-glucuronide (DAI-4′-GA) (96 %), daidzein-7-β-D-glucuronide (DAI-7-GA) (95 %), and daidzein-7-β-D-glucuronide-4′-sulfate (DAI-7-GA-4′-S) (98 %) were purchased from Toronto Research Chemicals (North York, Canada). Daidzein-7-β-D-glucoside (DAI-7-G) (>98 %) was purchased from Wako (Neuss, Germany). Daidzein-4′-sulfate (DAI-4′-S) (98 %), daidzein-7-sulfate (DAI-7-S) (99 %), and daidzein-7,4′-disulfate (DAI-7,4′-DS) (99 %) were synthesized as described elsewhere (Soukup et al. 2014). The chemical structures of the IF used in this study are depicted in Fig. 2. [3H]Estrone-sulfate ([3H]E1S, 45.6 Ci/mmol) was purchased from PerkinElmer Life Sciences (Boston, USA). Materials used for the cultivation of HEK293 cells were purchased from Gibco (via Life Technologies Carlsbad, CA, USA) and Sigma-Aldrich (Taufkirchen, Germany). All other chemicals and solvents used were of analytical grade. The standard stock and working solutions were prepared in DMSO.
Establishment of stably transfected SOAT-, NTCP-, OATP2B1-, ASBT-, and OAT4-HEK293 cell lines
SOAT-, NTCP-, and OATP2B1-HEK293 cells were generated using the commercially available Flp-In T-REx 293 cell line (Invitrogen), further referred to as HEK293 cells, according to the manufacturer’s protocol as reported before (Ugele et al. 2008; Geyer et al. 2007). Using the same approach, a stably transfected ASBT-HEK293 cell line was established with the human ASBT cDNA sequence according to GenBank accession number NM_000452. The tetracycline repressor that blocks expression of SOAT, NTCP, ASBT, or OATP2B1 in the absence of tetracycline is stably expressed in all of these cell lines. For induction of protein expression, 1 µg/ml tetracycline was added to the medium. OAT4-HEK293 cells were generated using Flp-In-HEK293 cells (Invitrogen) without tetracycline repressor and do not require tetracycline treatment for carrier expression (Ugele et al. 2008).
Transport studies in stably transfected SOAT-, NTCP-, OATP2B1-, ASBT-, and OAT4-HEK293 cells
Transport studies were performed as described before (Geyer et al. 2007) with the following modifications. Briefly, 12-well or 24-well plates with 1.25 × 105 cells per ml (2 ml in 12-well plates, 1 ml in 24-well plates) were plated and grown under standard conditions for 72 h. Protein expression was induced by pre-incubation with tetracycline (1 µg/ml). Non-transfected Flp-In HEK293 cells were used as control. In the sodium-free transport buffer, sodium chloride was substituted with equimolar concentrations of choline chloride (142.9 mM). After washing the cells three times with phosphate-buffered saline (PBS), cells were pre-incubated with sodium transport buffer. Uptake experiments were started by replacing the pre-incubation buffer by transport buffer containing the respective test compound (10 µM) and were performed at 37 °C. Non-radiolabeled compounds were used. After incubation of the cells with the substrate, the cells were washed five times with ice-cold PBS and lysed by adding demineralized H2O to the cell monolayer followed by five freeze–thaw cycles. An aliquot of the cell lysate was used to determine the cell-associated concentration of the tested compounds by UHPLC-DAD and another aliquot to measure the protein concentration by BCA assay (Novagen, Madison, WI, USA). For inhibition studies, transfected HEK293 cells were pre-incubated in 24-well plates with transport buffer containing the respective inhibitory compound (10 µM) for 5 min. Subsequently, transport measurements were started by adding the radiolabeled substrate (100 nM [3H]E1S) at 37 °C for 5 min and terminated by washing five times with ice-cold PBS. Cell monolayers were lysed in 1 N NaOH with 0.1 % SDS, and the cell-associated radioactivity was determined in a liquid scintillation counter. The protein content was determined according to Lowry using aliquots of the lysed cells with bovine serum albumin as a standard (Lowry et al. 1951).
UHPLC analysis of cell lysate
A 90-µl aliquot of the cell lysate was mixed with 10 µl of methanol and stirred on a vortex mixer. The samples were filtered through a syringe filter (Phenomenex, PTFE 0.2 µm, 4 mm), and an aliquot of the filtrate was analyzed by UHPLC-DAD. The analyses were performed on a Shimadzu LC system, which consisted of a controller (CBM-20A), a degasser (DGU-20A5), two pumps (LC-30AD), an autosampler (SIL-30AC), a column oven (CTO-20AC), and a DAD (SPD-M20A). The LC system was controlled by the software LabSolution 5.32. Separation of the analytes was performed on a Waters Acquity HSS T3 column (2.1 mm internal diameter, 100 mm length, 1.8 µm) with an oven temperature of 40 °C. Solvent A was a 40 mM ammonium formate buffer (pH 3), and solvent B was an acetonitrile/methanol mixture (1/2.5, v/v). A flow rate of 0.5 ml/min was adjusted, and 40 µl of the samples was injected. The elution profile was as follows: 0–2.6 min isocratic with 3 % B, 2.6–16.7 min from 3 to 56 % B, 16.7–17.3 min from 56 to 95 % B, 17.3–19.9 min isocratic with 95 % B, 19.9–20.5 min from 95 to 3 % B, and 20.5–24.7 min isocratic with initial conditions. Chromatograms were monitored at 250 and 300 nm for the analytes, and UV spectra were recorded between 200 to 400 nm. For each compound, external calibration curves were prepared in 10 % (v/v) methanolic solution in the range of 5–3,125 nM. The coefficients of correlation were 0.99965– 0.99998 (1/C weighted linear regression). The identity of each compound was confirmed by the retention time and the UV–Vis spectra. The limit of detection (LOD) was 0.2 pmol on column for all target analytes, except for DAI-7,4′-DS and DAI-7-G which had an LOD of 1 pmol on column.
Statistical analysis
Statistical significance for uptake measurements with radiolabeled substrates was calculated using Student’s t test (GraphPad Prism Software 6.03).
Results
Transport studies with DAI and DAI phase II metabolites
Uptake experiments were performed with DAI and DAI metabolites (Fig. 2) in stably transfected OAT4-, SOAT-, NTCP-, OATP2B1-, and ASBT-HEK293 cells using non-transfected FlpIn-HEK293 as control. Specific uptake was determined by analyzing the cell lysates by UHPLC-DAD. The quantification of the analytes was done by an external calibration using the corresponding standard compound. Representative UHPLC-DAD chromatograms are illustrated in Fig. 3. There was no metabolic transformation activity of the cells since no metabolites beside the respective test compound could be detected by UHPLC-DAD (data not shown).
Representative UHPLC-DAD chromatograms of cell lysates. NTCP-HEK293 cells incubated with DAI-7-S (a), DAI-4′-S (b) and DAI-7,4′-DS (c) monitored at 300 nm. d OATP2B1-HEK293 cells (gray and black line) and Flp-In-HEK293 control cells (dotted line) incubated with DAI-7-GA monitored at 250 nm. e NTCP-HEK293 cells (gray and black line) and Flp-In-HEK293 control cells (dotted line) incubated with DAI monitored at 300 nm
Sodium-dependent transport was observed in NTCP- and SOAT-HEK293 cells for 10 µM DAI-4′-S and DAI-7-S after an incubation time of 10 min (Fig. 4). The transport activity for DAI-4′-S detected in NTCP-HEK293 cells was 20-fold higher compared with SOAT-HEK293 cells, and fourfold higher for DAI-7-S. OAT4-HEK293 cells revealed a partly sodium-dependent uptake with a ratio of 1.3 for DAI-4′-S and 1.8 for DAI-7-S by comparison of experimental conditions with or without sodium. Transport of DAI-7-S by OAT4-HEK293 cells was as high as the uptake by NTCP-HEK293 cells, whereas the uptake of DAI-4′-S by NTCP-HEK293 cells was threefold lower compared with OAT4-HEK293 cells. In contrast, OATP2B1- and ASBT-HEK293 cells showed no uptake activity for any of these compounds. However, only OATP2B1 cells were able to transport DAI-7-GA with and without sodium. DAI-7,4′-DS transport was observed in NTCP-HEK293 cells under sodium conditions only. In contrast to the carrier-specific transport of DAI conjugates, the parent DAI was taken up in all carrier-transfected cell lines including non-transfected HEK-293 control cells under sodium and sodium-free conditions. Therefore, DAI transport was considered to be non-specific and to occur by passive diffusion. For validation, the specific uptake of DAI-4′-S and DAI-7-S by NTCP, SOAT, and OAT4 as well as of DAI-7-GA by OATP2B1, and of DAI-7,4′-DS by NTCP, was completely inhibited in a competitive manner by adding a 100-fold molar excess (1 mM) of the already known substrates E1S (for SOAT, OAT4, OATP2B1) or taurocholic acid (for NTCP) prior to co-incubation with the above-mentioned compounds (data not shown). Finally, DAI-7-GA-4′-S, DAI-4′-GA, and DAI-7-G were not transported by any of the tested carriers, since no analytes could be detected by UHPLC-DAD in all carrier-transfected cell lines including non-transfected HEK-293 control cells under sodium and sodium-free conditions. The results of the transport studies are summarized in Table 1.
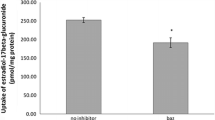
Uptake studies in stably transfected NTCP-, ASBT-, SOAT-, OAT4-, and OATP2B1-HEK293 cells. For uptake of 10 µM DAI-4′-S, DAI-7-S, DAI-7-GA, DAI-7,4′-DS, and DAI, the respective stably transfected HEK293 cells were incubated for 10 min in transport medium with (black bars) or without (white bars) sodium chloride at 37 °C together with the indicated compound. Cell-associated uptake was determined by UHPLC-DAD. Non-transfected Flp-In HEK293 cells were used as control. The values represent mean ± SD of triplicate analyses. Non-detectable values (n.d.) are below the LOD (0.2 pmol on column for all target analytes except for DAI-7,4′-DS and DAI-7-G with 1 pmol on column)
Inhibition studies with DAI and DAI phase II metabolites
In order to analyze the interaction of DAI and DAI phase II metabolites with the investigated transporters, inhibition experiments were performed on stably transfected OAT4-, OATP2B1-, SOAT-, and NTCP-HEK293 cells (Fig. 5). Cis-inhibitory effects of the indicated compounds (all at 10 µM) were examined on the carrier-mediated uptake of 100 nM [3H]E1S. DAI-7-G and DAI-7-GA-4′-S did not inhibit carrier-mediated [3H]E1S transport at all. In contrast, the carrier-mediated [3H]E1S uptake was significantly reduced by DAI-4′-S, DAI-7-GA, and DAI-4′-GA in all stably transfected cell lines tested. DAI-4′-S was the compound with the highest inhibitory potency for OAT4 and SOAT, reducing the uptake of [3H]E1S by 74 % and by 57 % for OAT4 and SOAT, respectively. DAI-7-S was identified as potent inhibitor of OAT4-, OATP2B1-, and SOAT-mediated transport activity, but not for NTCP. For OATP2B1, DAI-7-S was the strongest inhibitor of [3H]E1S transport. Similar to DAI-7-S, DAI itself was not an inhibitor of [3H]E1S uptake for NTCP, but for OAT4, OATP2B1, and SOAT. Interestingly, DAI-7,4′-DS inhibited the [3H]E1S transport by OAT4, OATP2B1, and SOAT cells, but slightly stimulated the NTCP-mediated uptake of [3H]E1S to 116 %. The results of the inhibition studies are summarized in Table 2.
Inhibition of [3H]E1S transport by different isoflavone metabolites and DAI in OAT4-, OATP2B1-, SOAT-, and NTCP-HEK293 cells. Uptake of 100 nM [3H]E1S was measured in the presence of 10 µM inhibitory compound. Cells incubated without inhibitor served as a positive control (set to 100 %), while Flp-In HEK293 cells served as a negative control (set to 0 %). The values represent the percentage of E1S transport activity in the presence of the indicated inhibitor relative to the positive and negative control and are expressed as mean ± SD of quadruplicate analyses. The values were significantly different from positive controls with *p < 0.05; **p < 0.01; ***p < 0.001
Discussion
The human SOAT, the member of the solute carrier family SLC10, is highly expressed in testis, placenta, and mammary gland. SOAT selectively transports the sulfated steroids E1S, E2S, DHEAS, 16α-hydroxy-DHEAS, androstenediol-3-sulfate, and pregnenolone sulfate in a sodium-dependent manner (Geyer et al. 2007; Fietz et al. 2013; Schweigmann et al. 2014) and, therefore, was a promising candidate carrier for the uptake of the DAI monosulfates due to their similar chemical structure (Fig. 1). Our in vitro results indicate an uptake of DAI-4′-S and DAI-7-S by SOAT in the above-mentioned hormone-dependent tissues. It is under debate whether IF might stimulate the proliferation of existing breast cancer cells based on their estrogenic activity (Allred et al. 2001; Wietrzyk et al. 2004; Kijkuokool et al. 2006; Hilakivi-Clarke et al. 2010; Magee and Rowland 2012; Zhang et al. 2012; Zamora-Ros et al. 2013). Since SOAT is present in the human mammary gland (Geyer et al. 2007) and sulfated DAI is actively transported by SOAT, this might lead to elevated IF concentrations in breast and breast cancer cells capable to stimulate proliferation. On the other hand, uptake of the endogenous sulfated steroid hormones by SOAT might be inhibited by the two sulfated DAI metabolites and, to a lesser degree, by DAI-7,4′-DS, DAI-7-GA, DAI-4′-GA, and DAI. This could indicate a protective effect, because estrogens yield a much higher estrogenic activity compared to IF (Kinjo et al. 2004; Pugazhendhi et al. 2008).
Similar to SOAT, stably transfected OAT4-HEK293 cells showed an uptake for DAI-4′-S and DAI-7-S. This partly sodium-dependent uptake by OAT4 has already been demonstrated for DHEAS, 16α-hydroxy-DHEAS, and E1S in the same in vitro system (Cha et al. 2000; Ugele et al. 2008; Schweigmann et al. 2014). Transport by OAT4 is coupled to the Na+/H+ exchanger NHE3 which shows similar expression pattern compared with OAT4 (Brant et al. 1995; Lang et al. 2003; Hagos et al. 2007). OAT4 is mainly localized in the apical membrane of proximal tubule cells of the kidney, indicating a role in the reabsorption of sulfated steroids from the primary urine (Cha et al. 2000; Nishimura and Naito 2005; Ugele et al. 2008). In a similar manner, OAT4 might play a role in the reuptake of sulfated IF metabolites in the kidney. If so, this would explain results of own (data not yet published) and other human intervention studies, showing that DAI sulfates are abundant in plasma (20–38 % of the total concentration of DAI conjugates), but exhibit low abundance (4–5 %) in urine (Hosoda et al. 2011).
In contrast to OAT4 and SOAT, OATP2B1 revealed no transport activity for DAI-4′-S and DAI-7-S in transfected cells. However, this carrier was previously shown to transport E1S in stably transfected OATP2B1-HEK293 cells in a sodium-independent manner (Ugele et al. 2008). Therefore, this carrier could contribute to supply a basal level of active estrogens via the sulfatase pathway, which was described before for breast cancer cells (Santner et al. 1984; Ugele et al. 2008). OATP2B1 is highly expressed in normal breast tissue as well as in breast tumors, whereby its expression increased with tumor grade severity (Pizzagalli et al. 2003; Al Sarakbi et al. 2006). DAI-4′-S and DAI-7-S were identified as potential inhibitors for E1S uptake by OATP2B1 into the cell. Therefore, these IF might act in a protective way as discussed for SOAT. On the other hand, DAI-7-GA was transported by OATP2B1, which may lead to IF concentration in breast and breast cancer cells capable to stimulate proliferation.
OATP2B1 is also highly expressed in the liver. Here, OATP2B1 is localized at the basolateral membrane of the hepatocytes, whereas in the gut, it is expressed in the apical membrane domain of enterocytes (Tamai et al. 2000; Kullak-Ublick et al. 2001; Kobayashi et al. 2003). For OATP2B1, we were able to show transport of DAI-7-GA indicating its potential role in the enterohepatic circulation of DAI-7-GA. Interestingly, DAI-4′-GA was not transported by OATP2B1. DAI-4′-GA presents a similar structure to 17β-E2-glucuronide that was also not taken up by OATP2B1 (Grube et al. 2007). Thus, the position of the glucuronic acid residue seems to play a crucial role whether glucuronides are transported by OATP2B1 or not.
Besides SOAT, OAT4, and OATP2B1, the carriers NTCP and ASBT were tested for IF phase II metabolite transport. Both transporters are essentially involved in the maintenance of the enterohepatic circulation of bile acids (Shneider et al. 1995; Meier and Stieger 2002; Trauner and Boyer 2003). In the present study, ASBT showed no transport activity for the tested DAI metabolites at all, whereas NTCP revealed a transport activity for DAI-4′-S, DAI-7-S, and DAI-7,4′-DS and thus may participate in the enterohepatic circulation of sulfated DAI metabolites. DAI-7,4′-DS slightly stimulated the uptake of E1S in NTCP-HEK293 cells. This effect was observed for different carriers before, where the uptake of sulfoconjugated steroids was enhanced by progesterone (Grube et al. 2006; Grosser et al. 2013). Grube et al. suggest a possibly important modulation of the sulfoconjugated steroid uptake by progesterone in the placenta. For OATP2B1, this effect was demonstrated to be substrate-specific. For example, DHEAS and E1S but not bromosulfophthalein uptake was stimulated by progesterone (Koenen et al. 2012).
Less is known about active transport mechanism for IF into the cell. Wong et al. showed that GEN-4′-S is transported by OAT1, whereas DAI-7-GA, GEN-7-GA, and GLY-7-GA are transported by OAT3 (Wong et al. 2011). In addition, GEN was identified as a substrate of the breast cancer resistance protein (BCRP) and competitively inhibited BCRP-mediated drug efflux, confirming the potential inhibitory effects of IF shown in the present study (Imai et al. 2004).
Our aim was to investigate the uptake of DAI and its conjugative metabolites by the human transporters SOAT, NTCP, OAT4, and OATP2B1. Except for OAT4, in rodents, the corresponding orthologous carriers were identified (Geyer et al. 2006; Hagenbuch and Meier 1994; Roth et al. 2012). Therefore, the results presented here are also relevant for animal studies and vice versa, and outcomes from rodent studies in which SOAT, NTCP, and OATP2B1 are included might be transferable to humans.
In conclusion, SOAT, NTCP, OATP2B1, and OAT4 were identified as carriers for DAI metabolites. In addition, several of the DAI metabolites were able to inhibit the carrier-dependent E1S uptake. The results of this study are a further step to better understand the mechanism of enterohepatic circulation of IF, their tissue distribution, and their role in hormone-dependent breast cancer.
Abbreviations
- ASBT:
-
Apical sodium-dependent bile acid transporter
- DAI:
-
Daidzein
- DHEAS:
-
Dehydroepiandrosterone sulfate
- DS:
-
Disulfate
- E1S:
-
Estrone-sulfate
- E2S:
-
17β-Estradiol-3-sulfate
- G:
-
Glucoside
- GA:
-
Glucuronide
- GEN:
-
Genistein
- GLY:
-
Glycitein
- IF:
-
Isoflavones
- NTCP:
-
Na+-taurocholate cotransporting polypeptide
- OAT:
-
Organic anion transporter
- OATP:
-
Organic anion transporting polypeptide
- S:
-
Sulfate
- SOAT:
-
Sodium-dependent organic anion transporter
- STS:
-
Steroid sulfatase
References
Al Sarakbi W, Mokbel R, Salhab M, Jiang WG, Reed MJ, Mokbel K (2006) The role of STS and OATP-B mRNA expression in predicting the clinical outcome in human breast cancer. Anticancer Res 26:4985–4990
Allred CD, Ju YH, Allred KF, Chang J, Helferich WG (2001) Dietary genistin stimulates growth of estrogen-dependent breast cancer tumors similar to that observed with genistein. Carcinogenesis 22:1667–1673
Bolca S, Urpi-Sarda M, Blondeel P, Roche N, Vanhaecke L, Possemiers S, Al-Maharik N, Botting N, De Keukeleire D, Bracke M, Heyerick A, Manach C, Depypere H (2010) Disposition of soy isoflavones in normal human breast tissue. Am J Clin Nutr 91:976–984
Brant SR, Yun CH, Donowitz M, Tse CM (1995) Cloning, tissue distribution, and functional analysis of the human Na+/N+ exchanger isoform, NHE3. Am J Physiol 269:C198–C206
Cha SH, Sekine T, Kusuhara H, Yu E, Kim JY, Kim DK, Sugiyama Y, Kanai Y, Endou H (2000) Molecular cloning and characterization of multispecific organic anion transporter 4 expressed in the placenta. J Biol Chem 275:4507–4512
Craddock AL, Love MW, Daniel RW, Kirby LC, Walters HC, Wong MH, Dawson PA (1998) Expression and transport properties of the human ileal and renal sodium-dependent bile acid transporter. Am J Physiol 274:G157–G169
Fietz D, Bakhaus K, Wapelhorst B, Grosser G, Gunther S, Alber J, Doring B, Kliesch S, Weidner W, Galuska CE, Hartmann MF, Wudy SA, Bergmann M, Geyer J (2013) Membrane transporters for sulfated steroids in the human testis—cellular localization, expression pattern and functional analysis. PLoS One 8:e62638
Geisler J (2003) Breast cancer tissue estrogens and their manipulation with aromatase inhibitors and inactivators. J Steroid Biochem Mol Biol 86:245–253
Geyer J, Wilke T, Petzinger E (2006) The solute carrier family SLC10: more than a family of bile acid transporters regarding function and phylogenetic relationships. Naunyn Schmiedebergs Arch Pharmacol 372:413–431
Geyer J, Doring B, Meerkamp K, Ugele B, Bakhiya N, Fernandes CF, Godoy JR, Glatt H, Petzinger E (2007) Cloning and functional characterization of human sodium-dependent organic anion transporter (SLC10A6). J Biol Chem 282:19728–19741
Grosser G, Fietz D, Gunther S, Bakhaus K, Schweigmann H, Ugele B, Brehm R, Petzinger E, Bergmann M, Geyer J (2013) Cloning and functional characterization of the mouse sodium-dependent organic anion transporter Soat (Slc10a6). J Steroid Biochem Mol Biol 138:90–99
Grube M, Kock K, Karner S, Reuther S, Ritter CA, Jedlitschky G, Kroemer HK (2006) Modification of OATP2B1-mediated transport by steroid hormones. Mol Pharmacol 70:1735–1741
Grube M, Reuther S, Meyer Zu Schwabedissen H, Kock K, Draber K, Ritter CA, Fusch C, Jedlitschky G, Kroemer HK (2007) Organic anion transporting polypeptide 2B1 and breast cancer resistance protein interact in the transepithelial transport of steroid sulfates in human placenta. Drug Metab Dispos 35:30–35
Hagenbuch B, Meier PJ (1994) Molecular cloning, chromosomal localization, and functional characterization of a human liver Na+/bile acid cotransporter. J Clin Investig 93:1326–1331
Hagos Y, Stein D, Ugele B, Burckhardt G, Bahn A (2007) Human renal organic anion transporter 4 operates as an asymmetric urate transporter. J Am Soc Nephrol 18:430–439
Hilakivi-Clarke L, Andrade JE, Helferich W (2010) Is soy consumption good or bad for the breast? J Nutr 140:2326S–2334S
Hosoda K, Furuta T, Ishii K (2011) Metabolism and disposition of isoflavone conjugated metabolites in humans after ingestion of kinako. Drug Metab Dispos 39:1762–1767
Imai Y, Tsukahara S, Asada S, Sugimoto Y (2004) Phytoestrogens/flavonoids reverse breast cancer resistance protein/ABCG2-mediated multidrug resistance. Cancer Res 64:4346–4352
Kijkuokool P, Parhar IS, Malaivijitnond S (2006) Genistein enhances N-nitrosomethylurea-induced rat mammary tumorigenesis. Cancer Lett 242:53–59
Kinjo J, Tsuchihashi R, Morito K, Hirose T, Aomori T, Nagao T, Okabe H, Nohara T, Masamune Y (2004) Interactions of phytoestrogens with estrogen receptors a and b (III). Estrogenic activities of soy isoflavone aglycones and their metabolites isolated from human urine. Biol Pharm Bull 27:185–188
Kobayashi D, Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I (2003) Involvement of human organic anion transporting polypeptide OATP-B (SLC21A9) in pH-dependent transport across intestinal apical membrane. J Pharmacol Exp Ther 306:703–708
Koenen A, Kock K, Keiser M, Siegmund W, Kroemer HK, Grube M (2012) Steroid hormones specifically modify the activity of organic anion transporting polypeptides 2852. Eur J Pharm Sci 47:774–780
Kramer W, Stengelin S, Baringhaus KH, Enhsen A, Heuer H, Becker W, Corsiero D, Girbig F, Noll R, Weyland C (1999) Substrate specificity of the ileal and the hepatic Na(+)/bile acid cotransporters of the rabbit. I. Transport studies with membrane vesicles and cell lines expressing the cloned transporters. J Lipid Res 40:1604–1617
Kullak-Ublick GA, Ismair MG, Stieger B, Landmann L, Huber R, Pizzagalli F, Fattinger K, Meier PJ, Hagenbuch B (2001) Organic anion-transporting polypeptide B (OATP-B) and its functional comparison with three other OATPs of human liver. Gastroenterology 120:525–533
Lang K, Wagner C, Haddad G, Burnekova O, Geibel J (2003) Intracellular pH activates membrane-bound Na(+)/H(+) exchanger and vacuolar H(+)-ATPase in human embryonic kidney (HEK) cells. Cell Physiol Biochem 13:257–262
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Magee PJ, Rowland I (2012) Soy products in the management of breast cancer. Curr Opin Clin Nutr Metab Care 15:586–591
Meier PJ, Stieger B (2002) Bile salt transporters. Annu Rev Physiol 64:635–661
Mortensen A, Kulling SE, Schwartz H, Rowland I, Ruefer CE, Rimbach G, Cassidy A, Magee P, Millar J, Hall WL, Birkved FK, Sorensen IK, Sontag G (2009) Analytical and compositional aspects of isoflavones in food and their biological effects. Mol Nutr Food Res 53:S266–S309
Nishimura M, Naito S (2005) Tissue-specific mRNA expression profiles of human ATP-binding cassette and solute carrier transporter superfamilies. Drug Metab Pharmacokinet 20:452–477
Pasqualini JR, Chetrite GS (2005) Recent insight on the control of enzymes involved in estrogen formation and transformation in human breast cancer. J Steroid Biochem Mol Biol 93:221–236
Pizzagalli F, Varga Z, Huber RD, Folkers G, Meier PJ, St-Pierre MV (2003) Identification of steroid sulfate transport processes in the human mammary gland. J Clin Endocrinol Metab 88:3902–3912
Pugazhendhi D, Watson KA, Mills S, Botting N, Pope GS, Darbre PD (2008) Effect of sulphation on the oestrogen agonist activity of the phytoestrogens genistein and daidzein in MCF-7 human breast cancer cells. J Endocrinol 197:503–515
Reed MJ, Purohit A, Woo LW, Newman SP, Potter BV (2005) Steroid sulfatase: molecular biology, regulation, and inhibition. Endocr Rev 26:171–202
Ritchie MR, Cummings JH, Morton MS, Steel CM, Bolton-Smith C, Riches AC (2006) A newly constructed and validated isoflavone database for the assessment of total genistein and daidzein intake. Br J Nutr 95:204–213
Roth M, Obaidat A, Hagenbuch B (2012) OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol 165:1260–1287
Rüfer CE, Bub A, Moseneder J, Winterhalter P, Sturtz M, Kulling SE (2008) Pharmacokinetics of the soybean isoflavone daidzein in its aglycone and glucoside form: a randomized, double-blind, crossover study. Am J Clin Nutr 87:1314–1323
Santner SJ, Feil PD, Santen RJ (1984) In situ estrogen production via the estrone sulfatase pathway in breast tumors: relative importance versus the aromatase pathway. J Clin Endocrinol Metab 59:29–33
Schroeder A, Eckhardt U, Stieger B, Tynes R, Schteingart CD, Hofmann AF, Meier PJ, Hagenbuch B (1998) Substrate specificity of the rat liver Na(+)-bile salt cotransporter in Xenopus laevis oocytes and in CHO cells. Am J Physiol 274:G370–G375
Schweigmann H, Sanchez-Guijo A, Ugele B, Hartmann K, Hartmann MF, Bergmann M, Pfarrer C, Doring B, Wudy SA, Petzinger E, Geyer J, Grosser G (2014) Transport of the placental estriol precursor 16alpha-hydroxy-dehydroepiandrosterone sulfate (16alpha-OH-DHEAS) by stably transfected OAT4-, SOAT-, and NTCP-HEK293 cells. J Steroid Biochem Mol Biol 143:259–265. doi:10.1016/j.jsbmb.2014.03.013
Selcer KW, Kabler H, Sarap J, Xiao Z, Li PK (2002) Inhibition of steryl sulfatase activity in LNCaP human prostate cancer cells. Steroids 67:821–826
Sfakianos J, Coward L, Kirk M, Barnes S (1997) Intestinal uptake and biliary excretion of the isoflavone genistein in rats. J Nutr 127:1260–1268
Shneider BL, Dawson PA, Christie DM, Hardikar W, Wong MH, Suchy FJ (1995) Cloning and molecular characterization of the ontogeny of a rat ileal sodium-dependent bile acid transporter. J Clin Investig 95:745–754
Soukup ST, Al-Maharik N, Botting N, Kulling SE (2014) Quantification of soy isoflavones and their conjugative metabolites in plasma and urine: an automated and validated UHPLC-MS/MS method for use in large-scale studies. Anal Bioanal Chem 406:6007–6020
St-Pierre MV, Hagenbuch B, Ugele B, Meier PJ, Stallmach T (2002) Characterization of an organic anion-transporting polypeptide (OATP-B) in human placenta. J Clin Endocrinol Metab 87:1856–1863
Tamai I, Nezu J, Uchino H, Sai Y, Oku A, Shimane M, Tsuji A (2000) Molecular identification and characterization of novel members of the human organic anion transporter (OATP) family. Biochem Biophys Res Commun 273:251–260
Trauner M, Boyer JL (2003) Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev 83:633–671
Ugele B, Bahn A, Rex-Haffner M (2008) Functional differences in steroid sulfate uptake of organic anion transporter 4 (OAT4) and organic anion transporting polypeptide 2B1 (OATP2B1) in human placenta. J Steroid Biochem Mol Biol 111:1–6
Wietrzyk J, Mazurkiewicz M, Madej J, Dzimira S, Grynkiewicz G, Radzikowski C, Opolski A (2004) Genistein alone or combined with cyclophosphamide may stimulate 16/C transplantable mouse mammary cancer growth. Med Sci Monitor 10:BR414–BR419
Wong MH, Oelkers P, Dawson PA (1995) Identification of a mutation in the ileal sodium-dependent bile acid transporter gene that abolishes transport activity. J Biol Chem 270:27228–27234
Wong CC, Botting NP, Orfila C, Al-Maharik N, Williamson G (2011) Flavonoid conjugates interact with organic anion transporters (OATs) and attenuate cytotoxicity of adefovir mediated by organic anion transporter 1 (OAT1/SLC22A6). Biochem Pharmacol 81:942–949
Zamora-Ros R, Ferrari P, González C, Tjønneland A, Olsen A, Bredsdorff L, Overvad K, Touillaud M, Perquier F, Fagherazzi G, Lukanova A, Tikk K, Aleksandrova K, Boeing H, Trichopoulou A, Trichopoulos D, Dilis V, Masala G, Sieri S, Mattiello A, Tumino R, Ricceri F, Bueno-de-Mesquita HB, Peeters PM, Weiderpass E, Skeie G, Engeset D, Menéndez V, Travier N, Molina-Montes E, Amiano P, Chirlaque M-D, Barricarte A, Wallström P, Sonestedt E, Sund M, Landberg R, Khaw K-T, Wareham N, Travis R, Scalbert A, Ward H, Riboli E, Romieu I (2013) Dietary flavonoid and lignan intake and breast cancer risk according to menopause and hormone receptor status in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Breast Cancer Res Treat 139:163–176
Zhang Y, Kang H, Li B, Zhang R (2012) Positive effects of soy isoflavone food on survival of breast cancer patients in China. Asian Pac J Cancer Prev 13:479–482
Acknowledgments
We want to thank Klaus Schuh for his skillful technical support. Parts of the work were funded by the German Research Foundation (DFG), Grant KU-1079/10-1. The project is part of the collaborative research project entitled IsoCross “Isoflavones: Cross-species comparison on metabolism, estrogen sensitivity, epigenetics, and carcinogenesis”.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grosser, G., Döring, B., Ugele, B. et al. Transport of the soy isoflavone daidzein and its conjugative metabolites by the carriers SOAT, NTCP, OAT4, and OATP2B1. Arch Toxicol 89, 2253–2263 (2015). https://doi.org/10.1007/s00204-014-1379-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-014-1379-3