Abstract
Coronaviruses are a diverse family of viruses, and new strains can emerge. While the majority of coronavirus strains cause mild respiratory illnesses, a few are responsible for severe diseases such as Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS). SARS-CoV-2, the virus responsible for COVID-19, is an example of a coronavirus that has led to a pandemic. Coronaviruses can mutate over time, potentially leading to the emergence of new variants. Some of these variants may have increased transmissibility or resistance to existing vaccines and treatments. The emergence of the COVID-19 pandemic in the recent past has sparked innovation in curbing virus spread, with sanitizers and disinfectants taking center stage. These essential tools hinder pathogen dissemination, especially for unvaccinated or rapidly mutating viruses. The World Health Organization supports the use of alcohol-based sanitizers and disinfectants globally against pandemics. However, there are ongoing concerns about their widespread usage and their potential impact on human health, animal well-being, and ecological equilibrium. In this ever-changing scenario, metal nanoparticles hold promise in combating a range of pathogens, including SARS-CoV-2, as well as other viruses such as norovirus, influenza, and HIV-1. This review explores their potential as non-alcoholic champions against SARS-CoV-2 and other pandemics of tomorrow. This extends beyond metal nanoparticles and advocates a balanced examination of pandemic control tools, exploring their strengths and weaknesses. The manuscript thus involves the evaluation of metal nanoparticle-based alternative approaches as hand sanitizers and disinfectants, providing a comprehensive perspective on this critical issue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since time immemorial, infectious diseases with the capacity for pandemic spread have recurrently emerged. Instances such as the Antonine plague, the Black Death, cholera, yellow fever, the severe acute respiratory syndrome coronavirus, and the swine flu exemplify major pandemics and epidemics that have impacted humanity. Coronaviruses constitute a diverse viral family, with the majority typically causing mild respiratory ailments. However, a subset of coronaviruses has been linked to more severe clinical conditions, including Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS). One illustrative case is SARS-CoV-2, which exemplifies a coronavirus species with the potential to trigger a global pandemic. In the latest historical context, SARS-CoV-2 was responsible for the emergence of a highly transmissible and severe acute respiratory syndrome in 2019, designated as Coronavirus Disease 2019 (COVID-19) which infected millions of individuals worldwide. The COVID-19 pandemic posed a significant public health challenge, and there is concern that a potential resurgence may occur in the future owing to new strains of the coronavirus. The presence of an infected host causes rapid transmission in confined spaces and crowds. When an infected individual sneezes or coughs, the agent is released into the air. SARS-CoV-2 can persist on commonly touched surfaces for an extended period before infecting the host through hand-to-face contact (Chin et al. 2020). The virus can persist for up to 48 h after being wrapped in droplets for hours (Chan et al. 2020) and remain infectious on surfaces for up to 9–14 days (Abiodun et al. 2020; Jing et al. 2020). SARS-CoV-2 has a unique mechanism in which the virus binds to ACE-2 receptors in the human body via its spike protein (Watanabe et al. 2020a, b; Naresh and Guruprasad 2023). ACE-2 receptor also permits fusion between the host cell membrane and viral envelope, enabling the viral life cycle to persist (Sportelli et al. 2020). Because important extracellular viral components, such as spike proteins, are expected to be relatively amenable to destruction. It's important to note that the potential for SARS-CoV-2 or other novel coronaviruses to pose future pandemics remains a concern. The risk of future pandemics depends on various factors, including the following:
-
1.
Virus mutations: viruses like coronaviruses can mutate over time, potentially leading to the emergence of new variants. Some of these variants may have increased transmissibility, virulency, or resistance to existing vaccines and treatments.
-
2.
Zoonotic spillover: zoonotic diseases occur when a virus jumps from animals to humans. Continued human interaction with wildlife and the global trade in animals can increase the risk of such spillovers. Several pandemics, including COVID-19, originated from animal populations.
-
3.
Global travel and connectivity: increased global travel and interconnectedness make it easier for infectious diseases to spread rapidly across borders, contributing to pandemic potential.
-
4.
Healthcare and public health preparedness: the ability to detect and respond to new infectious diseases, as well as the availability of vaccines and treatments, plays a crucial role in determining the outcome of potential pandemics.
Ongoing endeavors focus on monitoring and readiness for the potential emergence of new viruses that could lead to pandemics, including coronaviruses. These initiatives involve activities such as surveillance, research, vaccine development, and the implementation of public health measures, potentially including the creation of a new class of sanitizers or disinfectants. It is important to stay informed through reliable sources and support public health efforts to mitigate the risk of future pandemics, whether caused by coronaviruses or other pathogens. Public health measures such as vaccination, hygiene practices, and early detection are essential tools in preventing and controlling the spread of infectious diseases.
Disinfection is the key technique for preventing the virulent spread of bacterial and viral pathogens including SARS-CoV-2. According to the World Health Organization, Alcohol-based hand sanitizers and disinfectants are currently the most effective formulations to inactivate viruses and bacteria therefore these disinfectants have been recommended as effective agents against microbial infections. Moreover, Alcohol-based hand sanitizers (ABHS) are recommended because of their quick action and broad spectrum microbicidal activity in ensuring protection against bacteria and viruses. The effectiveness against non-enveloped viruses, on the other hand, is still uncertain (van Doremalen et al. 2020).
Although alcohol-based (hand sanitizers and disinfectants) are mildly flammable when the alcohol level is excessively high, they can cause skin poisoning (Toresdahl and Asif 2020). Skin toxicity when ethanol concentrations are quite high and unregulated due to the proliferation of fraudulent goods on the market is among the rare side effects of alcohol-based hand sanitizers (Siddharta et al. 2017; Kampf 2018). Therefore, hand sanitizers and disinfectants based on nanoparticles could be an effective and beneficial alternative against pathogens including SARS-CoV-2 (Tang et al. 2020; Bull et al. 2020). Moreover, excessive use of disinfectants and alcohol-based sanitizers can result in bioaccumulation and biomagnification, leading to toxicity, mutations, and the development of antibiotic-resistant bacteria. The use of sanitizers and disinfectants plays a crucial role in pandemic management, extending beyond COVID-19 to address the challenges posed by various epidemics and pandemics. Nevertheless, this review highlights several notable gaps and concerns that warrant attention to foster a more comprehensive understanding of the subject and to explore potential remedies:
-
1.
Effectiveness: The World Health Organization (WHO) recommends ABHS as an effective and convenient method for ensuring hand hygiene to combat the spread of the COVID-19 pandemic. However, it is crucial to carefully design and formulate ABHS to achieve the desired quality, efficacy, and safety. The inactive ingredients used in ABHS may have unforeseen effects on product performance, emphasizing the need for thorough consideration in their formulation. Additionally, safety features should be incorporated into ABHS products to minimize risks, including concerns such as flammability and the potential for exposure through ingestion.
-
2.
Adverse effects: while the text briefly mentions concerns about the adverse effects of alcohol-based sanitizers and disinfectants on human and animal health, as well as the environment, it does not delve into these concerns in detail. A more comprehensive exploration is necessary to understand the nature and extent of these adverse effects, considering their potential impact on various epidemics and pandemics.
-
3.
Lack of alternative solutions: while the text discusses the potential use of metal nanoparticles as an alternative to alcohol-based sanitizers and disinfectants, it lacks sufficient information on their mechanism of action, safety profile, and availability. A more in-depth examination of this alternative solution is essential, as it may hold relevance for a wide range of infectious disease scenarios.
-
4.
Environmental impact: the text briefly acknowledges the negative effects on the environment and ecological balance resulting from the use of sanitizers and disinfectants. It is imperative to provide a detailed account of how these products can harm the environment and suggest potential solutions or alternatives that are environmentally friendly, considering their application in various epidemic and pandemic settings.
-
5.
Regulatory and safety considerations: the review overlooks regulatory considerations for sanitizers and disinfectants, a critical aspect of their usage. It is vital to discuss how these products are regulated and the safety standards they must meet to ensure their responsible use during epidemics and pandemics on a global scale.
-
6.
Global perspective: while the discussion primarily focuses on the global response to COVID-19 and future epidemics and pandemics, it should also acknowledge regional disparities in the availability and utilization of sanitizers and disinfectants. Access to these essential products can vary significantly between countries and regions, and understanding these variations is vital for comprehensive pandemic preparedness.
Nonetheless, while alcohol-based disinfectants and sanitizers have played pivotal roles in mitigating the impact of COVID-19 including other pandemics, and protecting public health, there are substantial concerns surrounding their widespread use. These concerns range from potential adverse effects on human and animal health to the disruption of ecological balance and environmental harm. Amidst these apprehensions, metal nanoparticles emerge as a ray of hope, offering a promising alternative to combat not only SARS-CoV-2 but also a range of other virulent pathogens, including norovirus, influenza, and HIV-1, etc. Their broad-spectrum antiviral properties position them as formidable contenders in the array of pandemic control strategies.
To enrich the discourse and address these gaps, this review will encompass more comprehensive insights into metal nanoparticles as alternative potential non-alcoholic disinfectants and sanitizers effective against SARS-CoV-2 and future pandemics. Furthermore, it will provide a balanced examination of the advantages and disadvantages of various pandemic control measures, alongside potential alternatives, to offer a more holistic perspective on this critical topic.
Structure and stability of SARS CoV-2 in different environments
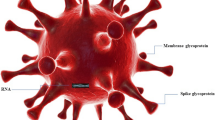
Coronaviruses (CoVs) are members of the Coronaviridae family, which comprises the α-coronavirus, β-coronavirus, γ-coronavirus, and δ-coronavirus (Yin and Wunderink 2018). Alpha- and beta-coronaviruses (CoVs) can infect mammals, whereas gamma- and delta-coronaviruses can also infect birds. So far, seven CoVs including (1) Human coronavirus 229E (HCoV-229E), (2) Human coronavirus NL63 (HCoV-NL63), (3) Human coronavirus OC43 (HCoV-OC43), (4) Human coronavirus HKU1 (HCoV-HKU1), (5) Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV), (6) Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and (7) Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), have been found to infect humans. First four cause a mild upper respiratory tract infections (Tosta 2022). These infections are usually self-limiting and manifest as symptoms similar to common cold such as cough, congestion and mild respiratory symptoms. On the other hand the other three corona viruses have been associated with more severe respiratory illnesses such as pneumonia and Acute Respiratory Distress Syndrome (ARDS) (Kane et al. 2023). SARS-CoV-2 exhibits a spherical morphology with a diameter ranging from 50 to 140 nm. Its genetic material consists of a 30 kb positive-stranded RNA viral genome, which undergoes transcription to produce both structural and non-structural proteins. Enclosed by a lipid bilayer, the virus envelope is adorned with glycoproteins that extend outward as spikes and transmembrane proteins. These specialized proteins play a crucial role in facilitating the virus's attachment to the host cell surface and mediating its entry into infected cells. Within the lipid membrane, the genetic RNA code of the virus is enveloped, subsequently undergoing replication once inside the host cell. The spike glycoprotein is a homotrimer found on the surface of coronaviruses that serves a critical function in recognizing the human host cell surface receptor angiotensin-converting enzyme-2 (ACE-2) (Guruprasad 2021). This recognition is essential for the fusion of the viral and host cellular membranes, which allow the viral nucleocapsid to be transferred into the host cells. SARS-CoV-2 is thought to have started in bats and then spread to humans via pangolins as an intermediate host (Li et al. 2020; Han 2020). The viral genome undergoes multiple modifications in the spike proteins to be able to leap species and infect a new mammalian host. An N-terminal S1 subunit and a C-terminal membrane-proximal S2 subunit make up the spike protein. S1A, S1B, S1C, and S1D domains make up the S1 subunit. The N-terminal domain (NTD) of the S1A domain detects carbohydrates, such as sialic acid, which is essential for virus attachment to the host cell membrane (Ahmadi et al. 2023). The structure of SARS CoV-2 has been depicted in Fig. 1.
The SARS-CoV-2 spike protein's S1B domain, also known as the receptor-binding domain (RBD), interacts with the human ACE-2 receptor (Wang et al. 2020; Guruprasad 2021). Three-length helices, several helical segments, prolonged twisted sheets, a membrane-spanning helix, and an intracellular cysteine-rich section make up the structural constituents of the S2 subunit. In SARS-CoV-2, the PRRA sequence motif between the S1 and S2 subunits contains a furin-cleavage site (Ou et al. 2020). A second proteolytic cleavage site S2′ is present in the S2 subunit, ahead of the fusion peptide. Both of these cleavage sites are involved in viral infection of host cells. At room temperature, it is stable throughout a wide pH range (pH 3.0–10.0) and is particularly stable in a favorable environment (Chin et al. 2020; Van Doremalen et al. 2020). In diverse conditions, SARS-CoV-2 can survive for 4–7 days on smooth surfaces like paper, glass, plastic, and stainless steel and up to 7 days on the external part of surgical masks (Chin et al. 2020). SARS-CoV-2 was not found on printing or tissue sheets after 3 h, and treated wood and cloth tested negative for the virus after two days (Chin et al. 2020) SARS-CoV-2 seems to be more persistent on stainless steel and plastic than on copper and paperboard, with active virus detected up to 72 h result of exposure to these surfaces (Van Doremalen et al. 2020). The infectivity of the virus relies on critical factors such as the structural integrity of the viral membrane, the defined topology and tertiary structure of membrane proteins, and the conserved structure and activity of the virion genome. Damage or disruption to key components like glycoproteins, lipid envelope, spike proteins, and the virion genome can lead to virus inactivation or exert effects on its infectivity (Al-Sayah 2020). SARS-CoV-2 has demonstrated resistance to a broad spectrum of antimicrobials (Chin et al. 2020). Figure 2 depicts the survival time of SARS-CoV-2 on various surfaces.
Coronavirus has a unique genomic sequence, similar to Severe Acute Respiratory Syndrome because they belong to the same genus Beta-Coronavirus, and have nearly the same shape (Yin and Wunderink 2018; Naresh and Guruprasad 2023). They are single-stranded RNA viruses with a positive strand. The good news is that a solvent like propanol, isopropanol, or ethanol can be used to eliminate, deactivate, or render these viruses redundant. Certain disinfectants, chloroforms, and antiseptics can also stop them from growing (Jing et al. 2020). Alcohol is a powerful disinfectant against lipophilic and hydrophilic viruses at concentrations of 60–80% (Jing et al. 2020). Within the range of 60–85%, ethanol demonstrates higher efficacy against viruses when contrasted with isopropanol (60–80%) and n-propanol (60–80%) (Gold and Avva 2018). To date, most of the effective hand sanitizer products are alcohol-based formulations containing 62–95% of alcohol as it can denature the proteins of microbes and has the ability to inactivate viruses (Jing et al. 2020). A study done with WHO demonstrated the efficiency of alcohol as a potent virucidal against emerging pathogens such as Ebola Virus, Zika Virus, SARS-CoV, and MERS-CoV. Another study conducted in Germany discovered ethanol at a concentration of 42.6% eliminates SARS and MERS coronaviruses. These studies proved the efficacy of alcohol-based hand sanitizers varied depending on concentration levels (Dexter et al. 2020). Non-ionic (uncharged) detergents are favored among anionic detergents because they are superior hydrocolloids, have lower surface tension, produce less foam, and do not suffer from the formation of complexes with hard water, which promotes microbial buildup in the leftover (Dhama et al. 2021).
Disinfectants and sanitizers: types and their mode of action against pathogens including SARS-CoV
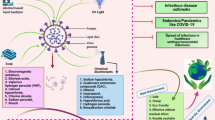
Detergents, alcohols, acids, oxidizing agents, halogens, phenols, aldehydes, and quaternary ammonium compounds, are all examples of disinfectants and sanitizers (Choi et al. 2021). They may be classified into two categories: non-alcohol-based and alcohol-based. Both of these categories have varying degrees of efficiency against germs and viruses. These have a variety of action mechanisms, however, the majority of them target the outer lipid layer of coronaviruses (CoVs) to inactivate the viral particles (Choi et al. 2021). Duarte and Santana (2020) explored various disinfection measures and control of SARS-COV-2 transmission and reported that virus can be inactivated by heating at 56 °C for 30 min and by using lipid solvents such as ethanol (> 75%), isopropanol (> 70%), formaldehyde (> 0.7%), povidone iodine (> 0.23%), sodium hypochlorite (> 0.21%), or hydrogen peroxide (> 0.5%), but not chlorhexidine. Of the various types of disinfectants and sanitizers, alcohol-based counterparts have been most commonly used against a variety of pathogens including SARS-CoV. Microorganisms are harmed by alcohol because it denatures proteins, causing membrane disintegration and cell destruction (Al-Sayah 2020). Figure 3 depicts how sanitizers and disinfectants prevent viral dissemination. A few recent studies have given a comprehensive overview of the use of hand sanitizers in COVID-19 prevention (Vuppu et al. 2023; Ma et al. 2023). The modes of action of various sanitizers and disinfectants, against various pathogens including SARS CoV-2, are enlisted in Table 1.
Non-alcohol-based hand sanitizers and disinfectants
Alcohol-free are those that do not contain alcohol but do contain detergents such as Benzalkonium chloride. Antiseptics, soaps, and gels are examples of alcohol-free sanitizers, which have a wide range of applications and modes of action (Erasmus et al. 2010; Ma et al. 2023). Some examples of non-alcoholic commonly used disinfectants are discussed hereafter.
Sodium hypochlorite
Sodium hypochlorite (NaOCl) stands as the food industry's primary disinfectant of choice. It boasts potent oxidizing capabilities, a wide-ranging antimicrobial effectiveness, and a remarkable history of application spanning more than a century. Alkalis and acids facilitate their antiviral activity through OH− and H+ ions, which disrupt nucleic acid bonds, precipitate proteins, alter cytoplasmic pH, and hydrolyze lipids (Morris and Esseili 2023).
Hydrogen peroxide (H2O2)
It catalyzes the conformational changes and oxidation of lipids and proteins, ensuing in membrane disarray and swelling as a result of H+ ion saturation (Al-Sayah 2020). H2O2 has the potential to degrade membrane proteins, enzymes, and nucleic acids, resulting in viral inactivation (Stuart et al. 2020) whereas; Peroxides induce acute toxicity in greater quantities (Holm et al. 2019). H2O2 has virucidal action against SARS-CoV at concentrations of 1–3%, It takes one min to destroy the virus, although the gaseous version is even more potent (Goyal et al. 2014). H2O2-based non-touch eradication techniques, particularly in healthcare settings and critical care units with potential pathogens (Huttner and Harbarth 2015), can help prevent environmental contamination after regular sanitization (Huttner and Harbarth 2015). In clinical settings, airborne H2O2 in the form of vapor and Dry mist has been used as an infection control agent in the environment (Falagas et al. 2011).
Quaternary ammonium (QA) compounds
Quaternary ammonium compounds are characterized by four alkyl groups attached to a central nitrogen atom. Common examples include benzalkonium chloride, benzethonium chloride, and cetylperidium chloride. These compounds exert their function by adsorbing the cytoplasmic membrane and inducing leakage of cellular components. They exhibit higher susceptibility towards Gram-positive bacteria and lipophilic viruses. In contrast, fungi, mycobacteria, and Gram-negative bacteria show relatively lower responses to these compounds. The coronavirus's protein and lipid structures are impacted by quaternary ammonium (QA) disinfectants, thereby constraining the virus's propagation (Baker et al. 2020). Benzalkonium chloride, a frequently used quaternary ammonium compound, has been associated with causing dermatitis (Holm et al. 2019; Okeke et al. 2023).
Phenols and phenol-derivatives
Compounds commonly derived from substituted phenols and bisphenols, where the hydrogen atom on the aromatic ring is substituted by either an alkyl group or a halogen (McDonnell and Russell 1999), exhibit notable efficacy against various pathogens including viruses. Phenol derivatives, within a concentration range of 0.5–5%, have demonstrated the ability to rapidly deactivate viruses, including HIV, and other hydrophilic viruses. The deactivation mechanism involves inducing membrane damage, resulting in the leakage of intracellular components and the denaturation of proteins (Al-Sayah 2020).
Aldehydes
Formaldehyde and glutaraldehyde are recognized as high-level disinfectants for medical devices and surgical equipment. These function as disinfectants against bacteria and viruses by alkylating their proteins and nucleic acids. They exhibit activity against coronaviruses within a concentration range of 0.5–3% within a 2-min exposure period (Rabenau et al. 2005a, b; Kariwa et al. 2006; Al-Sayah 2020).
Iodine-based compounds
Povidone-iodine, has a longstanding application as an antiseptic on skin and tissues, exhibiting effectiveness against a broad spectrum of bacteria and viruses including SARS-CoV at a concentration of 1% or less (Wood and Payne 1999; Kariwa et al. 2006; Eggers et al. 2015, 2018). The liberated elemental iodine can permeate cell membranes, targeting proteins at sulfuryl and disulfide bonds, along with causing damage to nucleic acids (Kariwa et al. 2006; Eggers et al. 2015, 2018).
Alcohol-based hand sanitizers and disinfectants
Alcohol-based hand Sanitizers (ABHS), contain ethanol, isopropanol, or n-propanol. Alcohols have broad activity against a wide range of microorganisms, although they are less efficient against protozoa (Carling 2021). It is recommended that the alcohol concentration ranges from 60 to 95% by volume to achieve optimal bactericidal activity (Centers for Disease Control and Prevention 2016). Some reports indicate 60–80% of alcohol concentration to be optimal for antimicrobial activity (Manaye et al. 2021; Ma et al. 2023). The antimicrobial properties of alcohols stem from their capacity to dissolve lipid membranes and denature proteins in microbes. Alcohols exhibit a broad-spectrum antimicrobial effect against most vegetative forms of bacteria, including Mycobacterium tuberculosis, fungi, and enveloped viruses like the human immunodeficiency virus (HIV) and herpes simplex virus. However, they do not effectively combat bacterial spores commonly present in raw materials. Addressing this limitation, the addition of hydrogen peroxide (3%) is proposed as a potential solution, although careful handling during production is necessary due to its corrosive nature (World Health Organisation 2009). Ethanol is superior to isopropanol against hydrophilic viruses, such as rotavirus, human immunodeficiency virus (HIV), and coronaviruses, while isopropanol is more active against lipophilic viruses, such as poliovirus and hepatitis A virus (HAV) (Al-Sayah 2020). Ethanol and isopropanol are capable of destroying coronavirus at 70–90% concentrations within 30 s (Kampf et al. 2020). It is believed that the alcohol causes membrane damage and denaturing of virus proteins in addition to damaging the RNA. The strong ability of these alcohols to form hydrogen bonding and their amphoteric nature allow them to disrupt the tertiary structure of proteins by disrupting the intramolecular hydrogen bonds within the structure. If used correctly, they are quite successful in preventing SARS-CoV-2-based infections. When alcohol-based hand sanitizers are utilized, the procedure becomes easier because time is not wasted. Various components of alcohol-based hand sanitizers and disinfectants are discussed hereafter.
Ethanol
Ethanol is a chemical substance that is a volatile, flammable, colorless liquid with a distinct odor (Winnefeld et al. 2000). Alcoholic beverages, such as beer, wine, and distilled spirits, contain ethanol as an intoxicating element. At a concentration of less than 75% ethanol works as a powerful virucidal agent, inactivating all lipophilic viruses such as herpes, influenza, and vaccinia as well as some hydrophilic viruses such as enterovirus, adenovirus, rotaviruses, and rhinovirus.
Isopropanol
Isopropyl alcohol is a colorless, flammable, and pungent chemical substance. The simplest example of secondary alcohol is an isopropyl group connected to a hydroxyl group. It's a white liquid with antibacterial qualities. It's used to make acetone and its derivatives, as well as a solvent (Oluwatuyi et al. 2020a, b). It has a high level of antiviral activity against lipid viruses (McMullan et al 2016). Apart from its lipid solvent characteristics, the principal method of action is protein coagulation and denaturation.
Propanol
Propanol is the most common kind of alcohol. As the lipid membrane of microorganisms is altered, about 65–90% of the alcohol concentration in a particular hand sanitizer is efficient to destroy or act against microbes (Song et al. 2019). The World Health Organization (WHO) has endorsed two formulations for alcohol-based hand sanitizers, distinguished solely by their alcohol constituents, and is widely adopted globally. Formulation 1 comprises Ethanol 80% v/v, glycerol 1.45% v/v, and hydrogen peroxide (H2O2) 0.125% v/v. Formulation 2 consists of Isopropyl alcohol 75% v/v, glycerol 1.45% v/v, and hydrogen peroxide 0.125% v/v (Singh et al. 2020). Recognizing the inherent variability of raw materials and the volatility of alcohol, in response to the COVID-19 pandemic, the United States Pharmacopeia has revised WHO Formulation 2 by elevating the concentration of isopropanol to 91% v/v. The consideration of an n-propanol-based formulation has not been put forth due to the absence of safety data regarding human use (Singh et al. 2020).
Most hand sanitizers contain alcohol as the active ingredient, effectively destroying microorganisms. Available in supermarkets and pharmacies, these sanitizers are portable and can be stored in various locations such as desks, bags, vehicles, and other frequently visited places. Offering both advantages and disadvantages in the context of skin hygiene, these sanitizers are applied to the skin, specifically the epidermis, dermis, and hypodermis, constituting the three layers of the integumentary system. The skin acts as a formidable barrier against potential microbial intruders, employing innate defense mechanisms (Myung et al. 2021). Prolonged use of hand sanitizers and frequent handwashing have been empirically linked to alterations in the composition of the skin microbiota (Oughton et al. 2009). Maintaining a harmonious microbial community on the skin is crucial for reducing virulence (Kampf et al. 2010). The natural process of skin regeneration takes approximately twenty-eight days, involving mitotic divisions in the basal epithelium and culminating in desquamation (Stebbins et al. 2011). This continuous shedding of dead keratinocytes effectively diminishes the presence of microorganisms that might have colonized it, significantly mitigating the risk of bacterial infiltration while preserving a balanced microbial population (Coronado et al. 2012).
Adverse effects associated with alcohol-based disinfectants and sanitizers
Due to their high flammability, alcohol-based hand sanitizers can be dangerous when used irresponsibly near a fire source (Myung et al. 2021). Skin burns have been recorded as a result of using these sanitizers, particularly when handling heat sources soon after application. It is recommended to stay away from a fire source for at least 15 min or until the skin surface is dry after using them. Despite their effectiveness, alcohol-based hand sanitizers are ineffective against non-lipophilic viruses, bacterial spores, and protozoa. Individuals unaware of this fact may mistakenly believe they are safe from non-lipophilic viruses after exceeding prescribed limits. using these sanitizers (Fahimipour et al. 2018). Skin poisoning can occur when alcohol concentrations The proliferation of unregulated brands in markets poses a risk of selling products with high alcohol concentrations that may cause skin toxicity. To maintain hand cleanliness and avoid practices that may cause or worsen skin irritation, it is recommended to choose products with fewer irritating components and moisturizing properties (Wickett and Visscher 2006).
Healthcare professionals can opt for products that are not only effective but also safe for all skin types. Research has explored the use of benzethonium chloride, enhancing antiviral efficacy while mitigating adverse effects on the skin and addressing flammability concerns associated with alcohol-based hand sanitizers (Clayton et al. 2017). Triclosan, a common active ingredient in hand sanitizers known to destroy microorganisms on hands, may contribute to the development of antibiotic-resistant bacteria. Personal protective equipment, regular handwashing, and surface disinfection have all been linked to an elevated risk of irritation or allergic contact dermatitis (Babino et al. 2022). First-line COVID-19 healthcare workers at the epicenter in China reported a high incidence of hand hygiene-induced skin damage. Among healthcare workers in Hubei province, 76.6% who performed hand hygiene more than ten times per day reported experiencing hand skin damage with dermatitis symptoms (MacGibeny and Wassef 2021). Hand sanitizers have several adverse effects, including poisoning by alcohol-based hand sanitizers containing concentrations to ensure hand hygiene. Table 2 enlists some merits and demerits of the most commonly used sanitizers and disinfectants, while Fig. 4 depicts various adverse effects associated with alcohol-based sanitizers.
Dermal effects
Prolonged and frequent use of alcohol-based sanitizers has the potential to disrupt the natural pH balance of the skin, resulting in issues such as dryness, flakiness, and a weakened skin barrier (Imran et al. 2023). Regular use of these sanitizers may cause the skin to become dry, flaky, and more susceptible to damage. Additionally, individuals applying alcohol-based sanitizers may experience a burning or stinging sensation, particularly if their skin is sensitive or already compromised. This discomfort can be more pronounced for those with delicate skin. Moreover, individuals with heightened sensitivity to the chemicals in alcohol-based sanitizers may develop allergic reactions, including conditions like contact urticaria (Yuindartanto et al. 2023).
Respiratory effects
The use of alcohol-based sanitizers, especially in poorly ventilated areas, has the potential to irritate the respiratory tract. This irritation may manifest as symptoms such as coughing, throat discomfort, and difficulty breathing. Individuals, especially healthcare workers, who regularly use sanitizers in settings with inadequate air circulation may face an increased risk of respiratory issues due to prolonged exposure to sanitizer vapors (Hasanien et al. 2023).
Systemic effects
The consumption of alcohol-based sanitizers, particularly those with high alcohol content, can lead to digestive issues, alcohol poisoning, central nervous system depression, and other systemic complications. Children are particularly susceptible to accidental ingestion, as they may mistake sanitizers for beverages. To prevent such incidents, it is crucial to keep alcohol-based sanitizers out of reach of children, closely monitor their usage, and adhere to safety guidelines (Alwan et al. 2023).
Role of hand sanitizers in controlling SARS-CoV-2 transmission
In a global context grappling with the complexities of infectious diseases, researchers across the world are diligently engaged in the development of efficacious treatments, vaccines, and preventative measures. COVID-19, predominantly disseminated through aerosols and droplets, has underscored the pivotal role of meticulous hand hygiene in mitigating community transmission. Governments, collaborating with public health agencies and organizations, actively support and endorse this indispensable practice (Dhama et al. 2021; Abuga and Nyamweya 2021; Prajapati et al. 2022). Epidemiological investigations have accentuated the significance of specific preventative measures, considering the duration and nature of exposure, in averting infections. A study indicates that the practice of hand hygiene can diminish infection transmission by approximately 16%, establishing it as a crucial element in our combat against infectious diseases (Natnael et al. 2022). Recognizing the practicality of hand sanitizers, the World Health Organization advocates for their use as a convenient alternative to handwashing, particularly when soap and water are unavailable. In Germany, a noteworthy behavioral shift was observed, with hand hygiene compliance surging from 47% to nearly 95% during the COVID-19 pandemic. This substantial increase underscores the significance of this practice in our daily lives. Hand sanitizers have emerged as effective tools in the battle against SARS-CoV-2, with their efficacy influenced by various factors, including viral strains, sanitizer composition, and environmental conditions (Natnael et al. 2022).
A comprehension of the scientific principles governing hand sanitizers reveals that their effectiveness stems from their ability to disrupt the lipid layer protecting the virus's core. This lipid layer is susceptible to commonly used disinfectants such as alcohol and soaps. However, the inclusion of specific compounds in sanitizer formulations, such as emollients and fragrances, can occasionally compromise their antimicrobial activity. Natural emollients like Aloe vera exhibit promise, possessing inherent antimicrobial properties. Hydrogen peroxide, another compound with antimicrobial properties, acts as an oxidant, releasing highly reactive hydroxyl free radicals that interact with various components, including lipids, proteins, and nucleic acids. Despite its effectiveness, hydrogen peroxide cannot serve as an active ingredient in hand sanitizers due to its potential harm to the skin (Marumure et al. 2022). It is essential to note that commercially available sanitizers have been associated with adverse effects on both the environment and human health. The primary ingredients in alcohol-based sanitizers, as recommended by the World Health Organization, involve combinations of ethanol, isopropyl alcohol, and hydrogen peroxide. The misuse and abuse of these components can result in toxic consequences for both human health and the environment. Methanol contamination has been identified as a source of toxicity in alcohol-based hand sanitizers, and excessive use of such sanitizers can elevate the risk of skin infections due to damage. Moreover, the release of alcoholic components into the environment poses a more significant hazard to aquatic life compared to terrestrial organisms (Mahmood et al. 2020).
As we navigate the challenges presented by infectious diseases, it becomes imperative to conduct comprehensive research to formulate safe, cost-effective hand sanitizers that are accessible and affordable, particularly for individuals in low-income countries. This endeavor entails a thorough understanding of the science behind these sanitizers, including their benefits, potential risks, and their impact on both human health and the environment.
Nanotechnology: shield against SARS-CoV-2 and future pandemics
Nanotechnology-based sensors and disinfectants have demonstrated utility in detecting and combatting SARS-CoV-2. The antiviral attributes of nanoparticles present an opportunity to enhance respiratory personal protective equipment (PPE) with self-sterilization capabilities against viruses like SARS-CoV-2 and influenza (Talebian et al. 2020). Antiviral agents with prolonged efficacy that can be aerosolized are valuable for preserving the cleanliness of human habitats and protective equipment. Broad-spectrum nanoparticles mimicking biological heparan sulfate can be employed to bind viral glycoproteins and disrupt their interaction with host cell receptors (Cagno et al. 2018; Dey et al. 2018). Zinc, as a nanoparticle, hampers viral entry while concurrently inhibiting viral RNA polymerase and viral reproduction through metal ion release (Zoghi et al. 2021). Silver, gold, titanium dioxide, zinc oxide, copper, and iron oxide represent potential nanoparticles, as illustrated in Fig. 5. These nanoparticles have the potential to interfere with viral infections, particularly by inhibiting adhesion and compromising integrity. Processes such as limiting receptor binding sites, reactive oxygen species (ROS) degradation to cellular components, and nullifying key viral components exemplify the virucidal actions of these nanomaterials (Imani et al. 2020).
Metal nanoparticles provide diverse inactivation mechanisms, offering a broader range of applications compared to narrow-spectrum antivirals and antibiotics. This complexity makes it challenging for bacteria and viruses to develop numerous defensive mutations, thereby reducing the likelihood of resistance (Galdiero et al. 2011). With a large specific surface area and distinctive chemical properties, metal nanoparticles are at the forefront of nanomaterial exploration, making them suitable for various biomedical applications, from antiviral surface coatings to disease treatment. The efficient antiviral activity of nanoparticles is facilitated by their large surface area-to-volume ratio, allowing for potent antiviral effects with minimal metal content. Additionally, their small size enables easy incorporation into medications, polymers, and various surfaces (Rai et al. 2016). Metals such as copper, silver, and gold exhibit oligodynamic effects with well-established biocidal properties persisting on the nanoscale (Prasher et al. 2018). This approach has the potential to enhance the inhibition of viral penetration into cells, solvation of the lipid bilayer envelope, and ROS production, thereby augmenting the effectiveness of disinfectants (Jamshidinia and Mohammadipanah 2022).
Metal and metal oxide-based nanoparticles as potential non-alcoholic disinfectants and sanitizer
Disinfectants and sanitizers play an increasingly pivotal role in daily life, serving as somewhat effective preventive measures against the novel Coronavirus. However, their long-term use poses risks to overall health. In the context of the coronavirus disease 2019 (COVID-19) pandemic, these agents were crucial for prevention; unfortunately, excessive anticipation led to uncontrolled use. Despite their potential to prevent COVID-19, widespread application raised severe issues, including detrimental effects on animal and human health, along with negative environmental consequences. Highly toxic chemical disinfectants are employed for surface decontamination in various settings, necessitated for safety. Nonetheless, alternative procedures with minimal contaminants and toxicity are being developed. Safe, affordable, and effective disinfectants, especially those based on eco-friendly nanotechnology and nanomedicine, are crucial innovations for mitigating these impacts.
Copper, copper oxide, silver, and zinc antiviral nanoparticles offer a feasible alternative to chemical procedures, applicable to PPE materials and surfaces (Ruiz-Hitzky et al. 2020; Valdez-Salas et al. 2021). Recent studies confirm nano disinfectants as reliable strategies for decontamination, recycling, and antimicrobial enhancement of medical protective clothing (Valdez-Salas et al. 2021). Advanced nanomedicine and nanotechnology techniques address the global increase in infection cases, particularly viral illnesses (Nikaeen et al. 2020). Nanoparticles, with prolonged antiviral activity (Campos et al. 2020; Ruiz-Hitzky et al. 2020), are proposed as attractive disinfection alternatives (Fig. 6), offering the potential for environmentally friendly and long-lasting disinfectants and sanitizers.
The antiviral properties and mechanisms of various metals and their oxides have undergone extensive research. The action of nanoparticles against pathogens involves multiple steps, as outlined below:
-
1.
Prevention of attachment and penetration: binding or destroying viral surface features, such as spike glycoproteins, hinders attachment and penetration into host cells (Almasi and Mohammadipanah 2021) (Fig. 7a).
-
2.
Degradation of viral components: reactive oxygen species (ROS) and metal ions play a role in breaking down nucleic acid, glycan shield, protein capsid, and lipid envelope of the virus (Imani et al. 2020) (Fig. 7b).
-
3.
Direct interaction with virus components: metal ions and nanoparticle permeation enable direct interaction with proteins, genetic material, and surfaces of the virus. This compromises viral unification and inhibits processes like genome replication and protein translation (Imani et al. 2020) (Fig. 7c).
-
4.
Disruption of disulfide bonds: breakdown of disulfide bond(s) between cysteine amino acid residues in viral glycoproteins leads to their denaturation and deactivation (Akhtar et al. 2019) (Fig. 7d).
Silver-based nanoparticles
Silver, a noble metal with a rich history of medical use in various cultures and widespread applications in modern biomedical technology, possesses notable microbicidal action, making it an excellent sterilizing agent in wound care products, surgical equipment, cleaning filters, and touch surfaces. For sustained microbicidal properties, plastics are commonly infused with silver thiosulfate in silica gel microparticles, as observed in Japan across items ranging from children's toys to toilet seats (Morones et al. 2005). Additionally, Ag nanoparticles find utility in biomedical devices, wound dressing, textiles, and keyboards (Zhang et al. 2016).
Breakthroughs in nanotechnology have resulted in the availability of pure AgNPs with actionable and chemical activity for antimicrobial applications. These nanoparticles offer novel solutions to challenges in modern medicine, particularly against pandemic viruses and antibiotic-resistant bacteria. In a study, 14 nm AgNPs were employed against the African swine fever virus (ASFV) due to the absence of vaccination for ASFV, emphasizing the role of disinfection in preventing the spread of this highly contagious disease among domestic pig herds (Dung et al. 2020). Similarly, nanoparticle-based disinfectants and sanitization hold promise in combating SARS-CoV-2, especially in locations where immunization efforts face challenges. Researchers determined that 0.78 ppm AgNPs represented the optimal non-toxic concentration for porcine alveolar macrophage (PAM) cells, exhibiting significant antiviral activity against ASFV. AgNPs were found to disable ASFV's membrane proteins, inhibit viral entry into host cells, and demonstrate biocidal activity against fungi and bacteria such as E. coli, Salmonella enterica, Coliforms, and Vibrio cholerae (Dung et al. 2020). AgNPs were also shown to bind to H1N1 influenza A, inactivating membrane proteins (Mori et al. 2013).
Silver, extensively explored in nanoparticle form, proves effective against a broad spectrum of viruses and bacteria, offering potential applications in self-sterilizing materials. The antiviral mechanisms of silver nanoparticles focus on their ability to bind and interact directly with chemical groups on protein structures and viral lipid membranes, impeding viral adherence to host cells. These mechanisms involve reactions with phosphate, imidazole groups, sulfhydryl, amino, and carboxyl, leading to the destruction of membranes and inactivation of enzymes (Xu et al. 2012; Chen et al. 2016).
Penicillium oxalicum GRS-1 and Aspergillus terreus N4 emerged as a sustainable tool for producing AgNPs with antibacterial and disinfectant qualities (Rose et al. 2020; 2023). The biosynthesized Ag nanoparticles using P. oxalicum GRS-1 were found to be effective against common food-borne pathogens, including S. aureus, E. coli and S. typhimurium with MBC values of 32, 16 and 32 μg/ml, respectively. AgNPs from A. terreus N4 also demonstrated effective disinfectant properties. These AgNPs hold promise as alternatives to alcoholic disinfectants due to their superior bactericidal efficacy at low concentrations. Nevertheless, further research is imperative to comprehensively investigate their antiviral properties before endorsing them as versatile sanitizers and disinfectants suitable for diverse applications.
Nanoparticle size and concentration are crucial factors influencing antimicrobial activity, with a higher percentage frequently associated with greater effectiveness within the optimal 5–15 nm size range (Mori et al. 2013; Dung et al. 2020). Silver nanoparticles exhibit an antiviral mechanism against various viruses such as PEDV, HIV-1, influenza A (Kim et al. 2020), and poliovirus (Huy et al. (2017). Silver inhibits the growth of SARS-CoV-2 by breaking down spike protein bonds. The bactericidal effect of silver nanoparticles against challenging multi-drug resistant bacteria, such as MRSA, enhances their potential for broad-spectrum antiseptic surface coating (Hulme 2022). Spherical-shaped Ag nanomaterials exceeding 20 nm in size demonstrate anti-coronavirus activity (Carvalho and Conte‐Junior 2021). A study comparing Kaolinite-supported silver nanocomposites with a commercially available disinfectant (Aqua Tab) yielded similar results (Woldegebreal et al. 2022).
Gold-based nanoparticles
Gold is recognized for its antiviral capabilities and its antiviral effects and possibilities as a harmless disinfecting substance are aided by its ability to form interactions with biomolecules. One of the main techniques for limiting viral dissemination as a nanoparticle is to bind Binding receptors on viral surfaces that are targeted and inhibited, impairing binding and host cell incorporation (Meléndez-Villanueva et al. 2019). One study observed this antiviral mechanism against the measles virus (MeV) using 11 nm gold nanoparticles (Kimling et al. 2006) synthesized using the Turkevich method.
Gold nanoparticles are effective antiviral drugs that work by attaching to viral surface structures and inhibiting their function, similar to how Copper and silver work. One study established that if gold nanoparticles have a positive surface charge, they will be attracted towards oppositely charged SARS-CoV-2 virion (Rosa et al. 2021). Vonnemann et al. (2014) Increased interaction of gold nanoparticles to the viral envelope and membrane proteins is facilitated by oppositely charged molecule attraction, which can result in functional inhibition via cluster formation and receptor obstruction. Rai et al. (2016) also showed that if the viral pathogenicity and inhibitory mechanism are well understood, surface modification can boost the virucidal effectiveness of metal and metal oxide nanoparticles against a specific virus. SARS-CoV-2 virions might be precisely incorporated into the interface of gold nanoparticles (AuNPs) for targeted suppression, based on the previous investigation into SARS-CoV-2 pathogenesis via inhibition of spike protein binding to ACE-2 receptor, and gold nanoparticles may interact with ACE-2 receptor and spike protein (Lan et al. 2020). One study reported that peptide-functionalized gold nanoparticles can be used as antiviral agents (Carvalho and Conte‐Junior 2021). However, compared to other metal nanoparticles, there is a scarcity of information on AuNPs for commercial processes such as personal protective equipment or coating materials. This is likely due to gold nanoparticles are higher cost, which limits their applicability.
Titanium dioxide-based nanoparticles
Titanium dioxide has several advantages, including minimal human toxicity and high UV-activated viral suppression (Liu et al. 2016). When exposed to oxygen, moisture, and UVA photons, it produces hydroxyl radicals (OH), neutral hydroxide ions, and superoxide (O–2) as a photocatalytic reaction and is an example of ROS (reactive oxygen species) (Park et al. 2014). Unpaired electrons in ROS are particularly unstable, and they react quickly with biomolecules in electron-exchange processes. ROS can disrupt proteins, nucleic acids, and polysaccharides and thus are harmful to a wide range of species (Sun and Ostrikov 2020).
Several studies have demonstrated the effectiveness of TiO2 as an active antiviral coating material, against feline calicivirus (FCV), bacteriophage MS2, murine norovirus, and human norovirus (Park et al. 2014), Both non-enveloped viruses like noroviruses and enveloped viruses like HSV-1 can be inactivated by TiO2 photodegradation mechanism (Park et al. 2014). SARS-CoV-2, like HSV-1, is an encapsulated virus with a basic lipid bilayer shell that could be sensitive to TiO2 surface coatings.
Given that SARS-CoV-2 spreads as aerosolized aqueous droplets that deposit on surfaces in the availability of moisture and UV wavelengths, the photodegradation of TiO2 is well suited to inactivating the virus (Chin et al. 2020). Even under ordinary indoor lighting, through photocatalysis processes, to initiate the process of photocatalysis moisture could be used so there is a generation of reactive oxygen species in TiO2 nanoparticle-based surface coatings. Interactions with nanoparticles and their generated ROS may damage spike protein is the example of surface, because SARS-CoV-2 is an enveloped virus with a surface glycoprotein and outer lipid bilayer, for fusing with the human host, they are anchored in and rely on an uncompromised membrane (Bianchi et al. 2020). SARS-CoV-2 suppression would then be done by reactive free radicals causing oxidative damage to its nucleic acids, base proteins, and lipids. Furthermore, in a rat model of hepatitis, reactive oxygen species (ROS) were found to be able to disrupt and change the oligosaccharides of glycoproteins in the case of copper ions (Yasuda et al. 2006). Cation interactions with hydrogen peroxide, for example, can easily form hydroxyl radicals, which disrupt N-linked glycosidic linkages between proteins, oligosaccharides, and glycoprotein glycosylation is reduced as a result of this interaction, which changes biological function.
To protect susceptible epitopes from immunological antibodies, the spike SARS-CoV-2 protein has a lot of glycosylation again from the human cell that synthesized it (Casalino et al. 2020). This glycan coating is important for SARS-CoV-2 infectivity, as it aids immune evasion and allows the virus's ability to penetrate the body's host cells As a result, the glycosylation of the viral spike protein is significantly reduced caused by ROS potentially making the virus less virulent before it is exposed to the host and easier to kill by the host's immune response. A surface coating, possibly based on TiO2, could be used to preemptively modify SARS-CoV-2 oligosaccharide moieties, as the TiO2 photocatalytic process also produces hydrogen peroxide and hydroxyl radicals.
Further research into a composite covering containing TiO2 and Cu to see whether the spike glycoprotein may be destroyed by ROS, resulting in SARS-CoV-2 suppression is also worthwhile. The concept is that TiO2 nanoparticles can directly engage with SARS-CoV-2 virions and cause physical harm (Akhtar et al. 2019). They showed that TiO2 nanoparticles (NPs) inactivate Newcastle and influenza viruses by destroying their membranes, an activity that could be transferred to SARS-CoV-2 because all are enclosed RNA viruses with comparable structures. This possibility appears to be supported by preliminary studies (Khaiboullina et al. 2020). Another intriguing development is TiO2 nanoparticles' virucidal activity against enveloped viruses, norovirus surrogates, and human norovirus.
Noroviruses exhibit substantial genetic variability and a rapid mutation rate, posing challenges for vaccine development and constraining the duration of immunity post-recovery. Additionally, a minimal infectious dose of viral particles (Teunis et al. 2008), resistance to alcohol sanitizers due to a non-enveloped structure (Liu et al. 2010; Park et al. 2010), and sustained viability on surfaces for several weeks (Liu et al. 2009) collectively contribute to their heightened transmissibility (Bartsch et al. 2016). Consequently, in terms of persistence and environmental stability on cross-contaminated materials, enteric viruses closely resemble SARS-CoV-2 over extended periods, displaying a proclivity for rapid mutation (Chin et al. 2020). Significantly advancing our capacity to inhibit the transmission of both non-enveloped and enveloped infectious viruses, particularly in vulnerable settings such as hospitals and eateries, would necessitate the implementation of swift disinfection strategies. A recent investigation demonstrated the efficacy of predominantly spherical-shaped nanoparticles at a concentration of 300 mg/ml on self-cleaning surfaces (Carvalho and Conte‐Junior 2021).
Copper-based nanoparticles
The Smith Papyrus, Egyptian medical literature dating between 2600 and 2200 B.C. (Grass et al. 2011), contains the first reported use of copper for its antibacterial qualities. It tells the story of how people discovered the benefits of copper in cleaning chest wounds and keeping safe drinking water. In 1832 and following outbreaks in Paris, France, copper miners were shown to be significantly less vulnerable to cholera (Grass et al. 2011) making copper's medical potential more generally acknowledged in the nineteenth century. Subsequently, scientific investigation on copper has allowed for a greater knowledge of copper's action as a natural microbicide, and it has become more widely used as a valuable metal for a variety of uses, including sterile touch surfaces and medicines (Grass et al. 2011; Rai et al. 2018; Ermini and Voliani 2021). CuSNPs showed low bioavailability and low toxicity to fish embryos because of their low solubility (Kong et al. 2022; Palencia and García-Quintero 2023). Because of their versatility, affordability, and low toxicity, copper and numerous of its compounds are currently intriguing prospects in nanomaterials research. copper compounds have a higher antiviral activity than cupric oxide because of their oxidation state. On solid-state surfaces and in solution, Cu2O and CuI compounds have been shown to potentially suppress bacteriophages, FCV, influenza, HCV, and HSV (Mazurkow et al. 2019).
Free radical formation, disulfide bond breaking, direct membrane contact, and competitive inhibition of receptors are just some of the antiviral mechanisms used by copper-based nanoparticles and chemicals (Broglie et al. 2015; Tavakoli and Hashemzadeh 2020). One study discovered that the size range of 40–160 nm copper nanoparticles was excellent against enveloped viruses and had greater nanoparticles in proportion than the ideal sizes for zinc and silver nanoparticles (Tavakoli and Hashemzadeh 2020). Copper is typically considered safe for human contact in real-world applications, as unless ingested, it is non-irritating to the skin and extremely non-cytotoxic (Hostynek and Maibach 2004).
Copper nanoparticles' antiviral action involves oxidative damage to biomolecules induced by reactive oxygen species (ROS). Copper as a catalyst facilitates the formation of ROS, especially hydroxyl radicals, in Fenton and Haber-Wiess-like processes (Fujimori et al. 2012). Lipid peroxidation, protein misformation, and genetic damage are all caused by hydroxyl radicals quickly bonding with biomolecules to resolve unpaired electrons copper nanoparticles and reactive oxygen species (ROS) can cause oxidative damage to SARS-CoV-2 since it is a lipid-enclosed RNA virus with spike and capsid proteins (Schoeman and Fielding 2019; Bianchi et al. 2020; Imani et al. 2020). Furthermore, the antiviral mechanism involving the SARS-CoV-2 spike protein, copper, and free radicals can preferentially target oligosaccharide glycosylation. In their investigation, Eguchi et al. found that copper ions and hydrogen peroxide produce free radicals that can rupture glycosidic linkages (Eguchi et al. 2002).
In oligosaccharide monomers and glycoproteins in a rat model of hepatitis (Yasuda et al. 2006). The N-acetyl group of N-Acetylglucosamine residues was degraded by free radicals, which they discovered using reversed-phase high-performance liquid chromatographic analysis. Although severe acute respiratory syndrome -2 viral glycan shields are mainly linked with 22 sites via N- linkage this targeting of N-linked glycosylation is noteworthy. Many key oligosaccharides on the spike protein, including oligomannose (Man5GlcNAc2 and Man9GlcNAc2), connect to the protein via GlcNAc at their base at these places (Watanabe et al. 2020a, b). Oligomannose structures protect SARS-CoV-2 and utilize the spike glycoprotein receptor-binding region to contact ACE-2 sites, as part of the glycan shield (Watanabe et al. 2020a, b). Spike glycoprotein of the SARS-CoV-2 may become highly susceptible to antibodies by preventing it from adhering to host cells, whether copper nanoparticles can produce hydroxyl radicals that cleave the N-linked linkages in oligomannose and other glycans and degrade those (Watanabe et al. 2019). Furthermore, the potential of copper ions to disrupt disulfide bonds has been established in SARS-CoV-2, which has S–S bonds that keep the spike protein in the correct fold (Minoshima et al. 2016).
The virus would be unable to infect host cells through ACE-2 if the spike protein was inactivated. Copper-based nanomaterials could prophylactically inhibit SARS-CoV-2 via ROS-mediated glycosylation and disulfide breakdown processes could be explained by these inactivation processes (Van Doremalen et al. 2020). SARS-CoV-2 was immediately inactivated by copper coatings, with a half-life of a drop of 0.774 h and a full reduction with no active virus found after 4 h, according to their findings. Copper outperformed stainless steel and polypropylene polymer in terms of efficacy (Van Doremalen et al. 2020). The virucidal activity of different Cu-based nanoparticles, which are similar to TiO2, against human norovirus (Broglie et al. 2015). Another avenue to broad-spectrum disinfecting surfaces could be encapsulated viruses such as influenza A, key breakthrough would be rapid disinfection methods that deactivate both enveloped and non-enveloped viral domains. With the EPA's approval of copper surfaces for use against coronavirus (Michels 2021) and greater studies into Copper-based nanoparticles (NPs) are required to evaluate their disinfection potential for SARS-CoV-2 and their durability for usage in self-sterilizing floors, as well as the inclusion of copper compounds into industrial textile goods.
Zinc oxide-based nanoparticles
Zinc oxide has been used as an antiseptic for at least 4000 years when it was used in ointments to treat Egyptian Civilization, diseased hair follicles manifested as carbuncles and boils (York et al. 2009). ZnO and its nanoparticles (ZnO NPs) have been shown to have antibacterial activities against a wide variety of bacteria, including E. coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Pseudomonas vulgaris, and Campylobacter jejuni (Siddiqi et al. 2018). At the same time, zinc oxide is regarded as safe for human contact, even in nanoparticle form. Zinc nanoparticles are used in sunscreen, textiles, hair dye, lipsticks, dressing material, and nanofibers (Fytianos et al. 2020).
Through Zn2+ suppresses coronavirus and antivirus RNA polymerase activity, zinc ionophores prevent virus reproduction. Zn2+ ions and pyrithione inhibit SARS-CoV, and arterivirus RNA replication at low doses (Ishida 2020). For disinfection, Zinc oxide nanoparticles (ZnO NPs), like other metal oxide nanoparticles, improved with greater concentration and exposure. El-Megharbel et al. (2021) found that zinc oxide nanoparticles (ZnO NPs) suppress SARS-CoV-2 at low nanoparticle levels (IC50 of 526 ng/ml) via direct targeting. Enveloped viruses, like SARS-CoV-2, also have a lipophilic outer membrane that nanoparticles might rupture and penetrate in a similar way to that bacteria are inactivated mechanism (Schoeman and Fielding 2019; Bianchi et al. 2020; El-Megharbel et al. 2021). SARS-CoV-2 virus particles were demonstrated to be destroyed by bifunctional soap by degrading their lipid bilayer outer membrane, suggesting that nanoparticles like ZnO could target the same structures to achieve viral inactivation (Chin et al. 2020). Moreover, due to its photocatalytic function, it has a similar effect as TiO2 and ZnO may share inactivation mechanisms, such as hydroxyl radical-mediated breakage of oligosaccharides from the glycan shield and ROS-mediated viral integrity degradation (Yasuda et al. 2006). ZnO-NPs, on the other hand, are most suited for somewhere other than the body, such as in cleaning products and surface coating, where the antiviral effect can be utilized with the least amount of danger of exposed live healthy cells (El-Megharbel et al. 2021).
Iron oxide-based nanoparticles
Due to their high biocompatibility and magnetic properties, iron–gold-based hybrid nanoparticles are a ubiquitous nanomaterial employed in medical appliances such as biosensors, imaging contrast agents, and cancer nanotherapy (Tarkistani et al. 2021; Mondal et al. 2023). IO-NPs, for example, can be regulated by an external magnetic field and used to target drugs or produce local hyperthermia for tumor treatment (Chang et al. 2018). Various European Union agencies and the US Food and Drug Administration (FDA) have approved iron oxide nanoparticles (IO-NPs) for anemia treatment (Bobo et al. 2016), which, when combined with antibacterial (Yu et al. 2020) and antiviral activity against influenza subtypes (Qin et al. 2019; Kumar et al. 2019) and Dengue virus (Murugan et al. 2017). Iron oxide nanoparticles have a variety of antiviral properties, including the formation of reactive oxygen species (ROS), lipid peroxidation, and binding to viral surface proteins to prevent viral attachment to host cells. SARS-CoV-2 has a lipid membrane envelope making it sensitive to lipid peroxidation, and ROS and nanoparticle binding can block its integral membrane proteins. Other encapsulated viruses may be susceptible to iron oxide such as SARS-CoV-2, by attacking the envelope of the virus, which is a widely stable structure between viruses that are encapsulated (Qin et al. 2019). Abo-zeid et al. (2020) published a detailed theoretical molecular docking analysis demonstrating the unique interactions of IO-NPs with SARS-COV-2 viral glycoproteins and found that both Fe3O4 and Fe2O3 significantly interact with the HCV glycoproteins E1 and E2 and SARS-CoV-2 S1-RBD. Fe2O3 preferred to form a complex with HCV E1 and E2 whereas, Fe3O4 forms a more stable complex S1-RBD.
In the case of the spike glycoprotein, the binding affinity region of SARS-CoV-2 may be prevented from sticking to the host cell receptor (ACE-2 receptors) by iron oxide nanoparticles (IO-NPs), preventing the virus from infecting cells. Furthermore, the formation of reactive oxygen species by IO-NPs is thought to cause inactivating oxidative damage to viral lipid envelopes (Abo-Zeid et al. 2020). Studies with various metal nanoparticles support this mechanism of oxidative inactivation, as did a study by Qin et al. in which lipid peroxidation of the influenza lipid envelope killed virions (Qin et al. 2019). If iron oxide (IO-NPs) are combined with other metal nanomaterials, such as silver nanoparticles (Ag-NPs), new properties emerge. Antibacterial and synergistic antiviral action can be produced, indicating that they have possibilities to be used in surface coatings and cleaners in general. Iron oxide nanoparticles are also biocompatible, and because they already have FDA permission, they might be quickly repurposed into antiviral nanomaterials to protect human surroundings and personal protective equipment (Singh et al. 2023). Different types of metal and metal oxide nanoparticles and their mode of action are summarized in Table 3.
Green synthesis of metal nanoparticles
The green synthesis of metal nanoparticles, a sustainable and environmentally friendly approach, has gained significant attention in recent years. Various biological resources, such as plants, bacteria, fungi, and algae, have been utilized in this process (Soltys et al. 2021).
Bacterial synthesis
The green synthesis of metal nanoparticles using bacteria and other microorganisms is a promising and environmentally friendly approach, as highlighted by Jeevanandam et al. (2022) and Ali et al. (2015). This method offers reduced toxicity and lower production costs compared to traditional synthesis methods. The use of agriculture waste as a bioresource for nanoparticle synthesis is also discussed (Jeevanandam et al. 2022). The potential of microorganisms as a source for nanoparticle production is further emphasized by Ali et al. (2015).
Bacteria showcase a remarkable proficiency in the synthesis of nanoparticles (NPs), specifically in the case of gold (Au) and silver (Ag) NPs. Pseudomonas stutzeri, an Ag-resistant bacterium, assumes a pivotal role in the biosynthesis of Ag NPs. These NPs materialize in concentrated AgNO3 media, with cells aggregating Ag significantly, resulting in the bulk accumulation of Ag in the form of 200 nm diameter NPs, exhibiting sizes ranging from 35 to 46 nm (Klaus et al. 1999). Waghmare et al. (2017) reported the synthesis of manganese (Mn) and zinc (Zn) NPs using Streptomyces HBUM171191. This synthesis was accomplished through the reduction of MnSO4 and ZnSO4 in the presence of actinomycetes biomass in rotator shakers, resulting in NPs within the range of 10–20 nm.
Nair and Pradeep (2002) uncovered that subjecting lactobacilli bacteria to highly concentrated metal solutions induces the formation of small-sized Au, Ag, and Ag–Au hybrid NPs, characterized by excellent shape and size. This process occurs through an intracellular method. In the exploration conducted by Shivaji et al. (2011), silver nanoparticles (AgNPs) were synthesized utilizing cell-free culture supernatants derived from five psychrophilic bacteria, namely Pseudomonas antarctica, Pseudomonas proteolytica, Pseudomonas meridiana, Arthrobacter kerguelensis, and Arthrobacter gangotriensis, alongside two mesophilic bacteria, Bacillus indicus, and Bacillus cecembensis. The resultant AgNPs exhibited sizes ranging between 6 and 13 nm and maintained stability over a remarkable duration of 8 months in the absence of light. Interestingly, the synthesis and stability of AgNPs were found to be contingent upon factors such as temperature, pH, and the specific bacterial species (P. antarctica or A. kerguelensis) contributing to the supernatant. A noteworthy observation indicated that the supernatant from A. kerguelensis failed to produce AgNPs under conditions conducive for P. antarctica-mediated synthesis. This study underscores a crucial revelation that the constituents within the cell-free culture supernatants, facilitating the synthesis of AgNPs, exhibit variability among different bacterial species. Moreover, the AgNPs synthesized through this process demonstrated bacteriocidal properties. Notably, this report unveils, for the first time, insights into the generation of AgNPs through the utilization of culture supernatants from psychrophilic bacteria. Additionally, it establishes that culture supernatants from species of Arthrobacter, a genus hitherto unexplored, possess the capability to synthesize AgNPs, thereby broadening the scope of nanoparticle synthesis methods,
Further investigations have unveiled that the temporal alignment of NP development does not invariably coincide with the duration of culture preparation. Experiments often involve an incubation period of 2 h, yielding surprisingly diverse morphologies that facilitate a wide array of applications (Vigneshwaran et al. 2006). He et al. (2007) delved into the extracellular method for Au NP formation, developing NPs of varying morphologies, encompassing shape and size, with the aid of bacteria Rhodopseudomonas capsulata. These NPs exhibit stability over three months, underscoring the bacteria's resistance to Au ions and Au NPs.
Fungal synthesis
In the realm of metal nanoparticle (NP) formation, fungi, akin to bacteria, demonstrate notable potential, highlighting their adept bearing, metal agglomeration propensity, and impressive linking capability. Aspergillus sp. and Penicillium emerge as particularly favorable candidates for synthesizing metal NPs, owing to their convenient manipulation in laboratory environments. Fungi unleash a substantial quantity of enzymes, expediting the reduction of silver ions and consequent NP generation (Mandal et al. 2006). The roots of fungal involvement in NP fabrication trace back to the early twentieth century, exemplified by the development of silver NPs measuring approximately 25 ± 12 nm in diameter, courtesy of the fungus Verticillium (Mukherjee et al. 2001).
A spectrum of fungi, including Aspergillus niger, Fusarium solani, Aspergillus oryzae, Pleurotus sajor-caju, and Trichoderma viride, has been enlisted in the extracellular green synthesis of silver NPs, yielding varied morphologies. Fusarium oxysporum, when exposed to silver ions, stands out for producing stabilized silver hydrosol (Gade et al. 2008; Ingle et al. 2009; Nithya & Ragunathan 2009; Binupriya et al. 2010; Thakkar et al. 2010). Furthermore, investigations into the antimicrobial activities of Ag NPs developed by Phoma glomerata against Escherichia coli and Staphylococcus aureus have added valuable insights to this dynamic field of study (Birla et al. 2009).
The green synthesis pathway of NPs involving fungi enjoys global recognition across diverse scientific domains, manifesting both extracellular and intracellular occurrences. Notably, Aspergillus sp. and Penicillium exhibit a proclivity for generating silver and gold NPs (Shankar et al. 2003; Vigneshwaran et al. 2006). Fungi-mediated NP synthesis gains preference over its bacterial counterpart for various reasons, including the augmented release of proteins and enzymes, fostering heightened NP production (Narayanan and Sakthivel 2011). There is evidence suggesting fungi produce smaller-sized NPs by accumulating a greater metal content intracellularly (Mukherjee et al. 2001).
The conditions of the medium play a pivotal role in influencing the green synthesis of metal NPs. For instance, Trichothecium sp. orchestrates the extracellular reduction of gold NPs, with non-agitation conditions enhancing the secretion of proteins and enzymes, while agitation impedes the process (Ahmad et al. 2005). Fungi-mediated NP synthesis encompasses both intra and extracellular methods, each exhibiting distinct characteristics. Intracellular approaches lead to downstream processes with diminished yields, while extracellular methods result in NP formation beyond the cell, often regenerating under low-flow conditions (Dhillon et al. 2012).
In a study by Rose et al. (2018), a natural variant of Penicillium oxalicum GRS-1 showed the capability of reducing Ag ions into Ag nanoparticles extracellularly with sizes ranging from 10 to 40 nm. They also utilized a cultured strain of Aspergillus terreus N4, known for its dual nitrate reductase activity in both periplasmic and intracellular regions as a potent reducing agent, converting Ag+ ions into Ag0, ultimately leading to the creation of AgNPs (Rose et al. 2023).
Plant extract synthesis
The current research focus extends to the synthesis of metal ion nanoparticles from plant extracts, harnessing phytoconstituents like saponins, alkaloids, flavonoids, and terpenoids. This green synthesis approach yields non-toxic, high-yield, scalable, and well-defined nanoparticles, proving effective and easily manageable nanoparticles (Ramadhan et al. 2012; Bethke et al. 2018). Saratale et al. (2018) comprehensively reviewed diverse green synthesis practices, emphasizing applications in biomedicine and agriculture. Natural medicinal plants contribute to diverse nanoparticles, exhibiting properties such as anticancer, antibacterial, antioxidant, anti-inflammatory, and antiviral effects (Bordiwala 2023). Green synthesis of AgNPs from Aloe vera leaf extract showed enhanced antibacterial properties against gram-negative (Escherichia coli, Pseudomonas aeruginosa, Enterobacter spp.) and gram-positive (Staphylococcus aureus) bacteria, which suggests possible bio-medical applications (Anju et al. 2021). Larger nanoparticles of 30 nm and 50 nm were also found to be effective, although smaller Ag nanoparticles had a broader spectrum of action and lesser cytotoxicity, probably due to their capacity to more easily bind virions (Park et al. 2014). The Ives cultivar pomace extract can generate stable AgNPs for use in wastewater disinfection (Raota et al. 2019). In vitro analysis affirms the antimicrobial potential of Ixora brachypoda leaf aqueous extract-synthesized AgNPs, suggesting broad-spectrum antimicrobial properties against multi-drug resistant microbial pathogens (Bhat et al. 2021). AgNPs synthesized using E. billardieri extract provide cost-effective technology for industrial manufacturing, with potential applications in various industries (Allafchian et al. 2022). Gold nanaoparticles are also reported using garlic extract (Allium sativum) as a reducing agent in combination with chloroauric acid (HAuCl4) (Meléndez-Villanueva et al. 2019).
Contemporary scientific inquiry is systematically investigating the medicinal properties inherent in naturally occurring plants to elucidate their potential applications. Empirical evidence indicates that nanoparticles synthesized from various plant-derived materials, such as gold (AuNPs), silver (AgNPs), copper (CuNPs), zinc (ZnNPs), platinum (PtNPs), iron (FeNPs), nickel (NiNPs), and cobalt (CoNPs), manifest noteworthy attributes including anticancer, antibacterial, antioxidant, anti-inflammatory, and antiviral properties (Bordiwala 2023). The amalgamation of bacteria, fungi, and plant extracts in the green synthesis of metal nanoparticles presents a holistic and sustainable approach. Each microorganism brings unique attributes, contributing to the versatility and effectiveness of this innovative methodology. As research progresses, the potential applications of green-synthesized nanoparticles continue to diversify across various scientific fields.
The use of green-synthesized metal nanoparticles for disinfection against SARS-CoV-2 is a promising area of research. The metal nanoparticles, particularly ZnO and silver, have shown promising results in disinfection against SARS-CoV-2. Copper nanoparticles, synthesized from copper sulfate, have been found to significantly reduce the infectivity of the virus on coated surfaces (Purniawan et al. 2022). Sportelli et al. (2022) demonstrated the efficacy of ZnO nanoparticles in reducing viral load by 70–90%, while Baselga et al. (2022) and Lam et al. (2022) both reported the antiviral activity of silver nanoparticles in coatings and composites, with inactivation yields greater than 99.9%. These studies highlight the potential of these nanoparticles in preventing the spread of SARS-CoV-2, particularly in high-risk environments such as hospitals and public places. Karthik et al. (2022) further support this, emphasizing the biocompatibility and antiviral potential of silver nanoparticles synthesized through green methods. Rose et al. (2023) demonstrated the antimicrobial activity of green synthesized AgNPs against common foodborne pathogens, including Staphylococcus aureus and Salmonella typhimurium, indicating their potential as non-alcoholic disinfectants. da Silva et al. (2023) demonstrated the effectiveness of PVC nanocomposites with silver nanoparticles in reducing the virucidal activity of SARS-CoV-2. Mbatha et al. (2023) provided a broader perspective on the application of green-synthesized metal nanoparticles in COVID-19 therapies, emphasizing the need for eco-friendly materials and safety evaluation. These studies collectively underscore the potential of green-synthesized metal nanoparticles in disinfection for SARS-CoV-2.
Advancing understanding of metal NPs for adoption as disinfectants: addressing gaps and challenges with potential solutions
The widespread adoption of metal nanoparticles (NPs) as disinfectants necessitates further research to establish their direct efficacy against SARS-CoV-2, coupled with comprehensive safety assessments. Moreover, overcoming manufacturing challenges is paramount before the large-scale utilization of inorganic NPs. Given the structural resemblances between SARS-CoV-2 and other enveloped viruses, it is imperative to seriously consider the incorporation of metal nanoparticles into self-sterilizing surfaces as a means of sanitation. These metal and metal oxide nanoparticles harbor significant potential as alternatives in the ongoing battle against future pandemics, concurrently safeguarding human health through their distinctive antibacterial and antiviral properties. Notwithstanding their potential, there exist specific gaps and challenges in harnessing metal nanoparticles as non-alcoholic disinfectants and sanitizers, which are subsequently delineated along with potential solutions.
Gaps and challenges
-
1.
Efficacy and safety: there is a lack of comprehensive research on the efficacy and safety of metal nanoparticles as disinfectants against SARS-CoV-2 and other pathogens. Studies need to be conducted to determine the minimum effective concentration of metal nanoparticles required for disinfection while ensuring safety for human contact.
-
2.
Mechanism of action: understanding the precise mechanism by which metal nanoparticles interact with viruses like SARS-CoV-2 is crucial. Research should focus on elucidating these mechanisms to optimize nanoparticle properties for better disinfection.
-
3.
Environmental impact: the environmental impact of using metal nanoparticles, especially in large-scale applications, needs to be thoroughly assessed. This includes evaluating their potential for bioaccumulation and toxicity in ecosystems.
-
4.
Stability and longevity: investigating the stability and longevity of metal nanoparticles as disinfectants is essential. Questions about the degradation of these nanoparticles over time and their effectiveness in real-world conditions need to be addressed.
-
5.
Resistance development: it is important to study whether pathogens can develop resistance to metal nanoparticles over time. This could have implications for long-term use.
-
6.
Regulatory approval: the regulatory approval process for using metal nanoparticles as disinfectants can be complex and varies by region. Streamlining this process and establishing clear guidelines for their use is necessary.
-
7.
Cost-effectiveness: assessing the cost-effectiveness of metal nanoparticles compared to traditional disinfection methods is crucial, especially for widespread adoption in resource-limited settings.
Exploring potential pathways for solutions
-
1.
Rigorous research: comprehensive in vitro and in vivo research studies are required to determine the efficacy, safety, and optimal concentrations of different metal nanoparticles (e.g., silver, copper, zinc) against SARS-CoV-2 and other pathogens.
-
2.
Mechanistic studies: in-depth mechanistic studies are required to understand how metal nanoparticles interact with viruses at the molecular level. This can guide the design of nanoparticles with enhanced antiviral properties.
-
3.
Environmental assessment: evaluation of the environmental impact of using metal nanoparticles as disinfectants is required. This may involve conducting ecotoxicity studies and developing strategies for responsible nanoparticle disposal.
-
4.
Stability testing: long-term stability testing of metal nanoparticles is required to assess their shelf life and effectiveness under various environmental conditions.
-
5.
Resistance monitoring: surveillance programs are to be carried out to monitor the development of resistance to metal nanoparticles by pathogens. This can inform strategies to mitigate resistance.
-
6.
Regulatory streamlining: collaboration with regulatory agencies is required to expedite the approval process for metal nanoparticles as disinfectants, ensuring that safety standards are met.
-
7.
Cost–benefit analysis: cost-effectiveness analyses are required for comparing metal nanoparticles to traditional disinfection methods, considering factors such as initial costs, longevity, and environmental impact.
-
8.
Education and training: education and training for healthcare workers and the public are required on the proper use of metal nanoparticles as disinfectants to maximize their effectiveness and safety.
-
9.
Scaling production: research and development to scale up the production of metal nanoparticles for widespread use, especially in regions with limited access to traditional disinfectants is needed.
-
10.
International collaboration: international collaboration is needed to share research findings, best practices, and regulatory guidelines related to the use of metal nanoparticles as disinfectants in the context of pandemics. This can accelerate progress and ensure global preparedness.
This review paper has comprehensively assessed the gaps and concerns associated with the use of sanitizers and disinfectants in pandemic control, offering insights into the need for in-depth data, safety considerations, environmental valuations, and equitable distribution strategies. Furthermore, we have explored the potential of metal nanoparticles as a viable alternative to alcohol-based sanitizers and disinfectants, underlining their promising role in enhancing the global response to SARS-CoV-2 and future pandemics.
Conclusion
In conclusion, while metal nanoparticles are being considered as a potential substitute, it is imperative to acknowledge that, akin to alcoholic disinfectants with concomitant side effects, nanoparticles may also pose potential risks to human health. Therefore, the exploration of nanoparticles as an alternative should be approached judiciously, considering potential side effects and necessitating comprehensive safety assessments before widespread implementation. Although, silver nanoparticles have undergone extensive research for their antiviral properties, but the effectiveness of metal nanoparticles in combating viruses varies and is influenced by factors such as the particular virus, nanoparticle size and shape, and the method of application. This emphasizes the importance of a thorough investigation into safety and biocompatibility before considering their clinical applications. Persistent research in this domain is essential, as novel findings may continue to refine our comprehension of the potential advantages and risks associated with the utilization of metal nanoparticles for antiviral therapies.
Data availability
Not applicable.
References
Abiodun D, Olatunji F, Abimbola O, Michael A, Taye O, Kayode O, Victor O (2020) A review into the clinical characteristics of 2019-Ncov and its potential impact on African economy. Int J Public Health 5(3):47–56. https://www.researchgate.net/publication/343749177
Abo-Zeid Y, Ismail NS, McLean GR, Hamdy NM (2020) A molecular docking study repurposes FDA approved iron oxide nanoparticles to treat and control COVID-19 infection. Eur J Pharm Sci 153:105465. https://doi.org/10.1016/j.ejps.2020.105465
Abuga K, Nyamweya N (2021) Alcohol-based hand sanitizers in COVID-19 prevention: A multidimensional perspective. Pharmacy 2021(9):64. https://doi.org/10.3390/pharmacy9010064
Ahmad A, Senapati S, Khan MI, Kumar R, Sastry M (2005) Extra-/intracellular, biosynthesis of gold nanoparticles by an alkalotolerant fungus, Trichothecium sp. J Biomed Nanotechnol 1:47–53
Ahmadi S, Bazargan M, Elahi R, Esmaeilzadeh A (2023) Immune evasion of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2); molecular approaches. Mol Immunol 156:10–19. https://doi.org/10.1016/j.molimm.2022.11.020
Akhtar S, Shahzad K, Mushtaq S, Ali I, Rafe MH, Fazal-ul-Karim SM (2019) Antibacterial and antiviral potential of colloidal Titanium dioxide (TiO2) nanoparticles suitable for biological applications. Mater Res Express 6(10):105409. https://doi.org/10.1088/2053-1591/ab3b27
Ali A, Zainab S, Ali N (2015) Green Synthesis of Metal nanoparticles by microorganisms; a current prospective. J Nanoanalysis 2(1):32–38
Ali J, Ali N, Wang L, Waseem H, Pan G (2019) Revisiting the mechanistic pathways for bacterial mediated synthesis of noble metal nanoparticles. J Microbiol Methods 159:18–25. https://doi.org/10.1016/j.mimet.2019.02.010
Allafchian A, Balali F, Vahabi MR, Jalali SAH (2022) Antibacterial and cytotoxic effects of silver nanoparticles fabricated by Eryngium billarderi Delar. Chem Phys Lett 791:139385. https://doi.org/10.1016/j.cplett.2022.139385
Almasi F, Mohammadipanah F (2021) Hypothetical targets and plausible drugs of coronavirus infection caused by SARS-CoV-2. Transbound Emerg Dis 68(2):318–332. https://doi.org/10.1111/tbed.13734
Al-Sayah MH (2020) Chemical disinfectants of COVID-19: an overview. J Water Health 18(5):843–848. https://doi.org/10.2166/wh.2020.108
Alwan N, Almazrouei S, AlMazrouei M, Aldhaheri J, Alismaili F, Ghach W (2023) Evaluation of public awareness and performance toward the safe use of household disinfectants-cleaners to prevent COVID-19 in the Emirate of Abu Dhabi. Front Public Health. https://doi.org/10.3389/fpubh.2023.1214240
Anju TR, Parvathy S, Veettil MV, Rosemary J, Ansalna TH, Shahzabanu MM, Devika S (2021) Green synthesis of silver nanoparticles from Aloe vera leaf extract and its antimicrobial activity. Mater Today: Proc 43:3956–3960. https://doi.org/10.1016/j.matpr.2021.02.665
Babino G, Argenziano G, Balato A (2022) Impact in Contact Dermatitis during and after SARS-CoV2 Pandemic. Curr Treat Options Allergy 9:19–26. https://doi.org/10.1007/s40521-022-00298-2
Baker N, Williams AJ, Tropsha A, Ekins S (2020) Repurposing quaternary ammonium compounds as potential treatments for COVID-19. Pharm Res 37(6):104. https://doi.org/10.1007/s11095-020-02842-8
Baram-Pinto D, Shukla S, Gedanken A, Sarid R (2010) Inhibition of HSV-1 attachment, entry, and cell-to-cell spread by functionalized multivalent gold nanoparticles. Small 6:1044–1050
Bartsch SM, Lopman BA, Ozawa S, Hall AJ, Lee BY (2016) Global economic burden of norovirus gastroenteritis. PLoS One 11(4):e0151219. https://doi.org/10.1371/journal.pone.0151219
Baselga M, Uranga-Murillo I, de Miguel D, Arias M, Sebastián V, Pardo J, Arruebo M (2022) Silver nanoparticles-polyethyleneimine-based coatings with antiviral activity against SARS-CoV-2: A new method to functionalize filtration media. Materials (Basel) 15(14):4742. https://doi.org/10.3390/ma15144742
Bethke K, Palantöken S, Andrei V, Roß M, Raghuwanshi VS, Kettemann F, Greis K, Ingber TTK, Stückrath JB, Valiyaveettil S, Rademann K (2018) Functionalized cellulose for water purification, antimicrobial applications, and sensors. Adv Funct Mater 28(23):1800409. https://doi.org/10.1002/adfm.201800409
Bhat M, Chakraborty B, Kumar RS, Almansour AI, Arumugam N, Kotresha D, Nayaka S (2021) Biogenic synthesis, characterization and antimicrobial activity of Ixora brachypoda (DC) leaf extract mediated silver nanoparticles. J King Saud Univ Sci 33(2):101296. https://doi.org/10.1016/j.jksus.2020.101296
Bianchi M, Benvenuto D, Giovanetti M, Angeletti S, Ciccozzi M, Pascarella S (2020) SARS-CoV-2 envelope and membrane proteins: structural differences linked to virus characteristics? Biomed Res Int 4389089. https://doi.org/10.1155/2020/4389089
Binupriya AR, Sathishkumar M, Yun S (2010) Myco-crystallization of silver ions to nanosized particles by live and dead cell filtrates of Aspergillus oryzae var. viridis and its bactericidal activity toward Staphylococcus aureus KCCM 12256. Ind Eng Chem Res 49:852–858
Birla SS, Tiwari VV, Gade AK, Ingle AP, Yadav AP, Rai MK (2009) Fabrication of silver nanoparticles by Phoma glomerata and its combined effect against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Lett Appl Microbiol 48(2):173–179. https://doi.org/10.1111/j.1472-765X.2008.02510.x. (Epub 2008 Jan 3 PMID: 19141039)
Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR (2016) Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res 33(10):2373–2387. https://doi.org/10.1007/s11095-016-1958-5
Bordiwala RV (2023) Green synthesis and applications of metal nanoparticles - A review article. Results Chem 5(2023):100832. https://doi.org/10.1016/j.rechem.2023.100832
Broglie JJ, Alston B, Yang C, Ma L, Adcock AF, Chen W, Yang L (2015) Antiviral activity of gold/copper sulfide core/shell nanoparticles against human norovirus virus-like particles. PLoS ONE 10(10):e0141050. https://doi.org/10.1371/journal.pone.0141050
Bull RA, Adikari TN, Ferguson JM, Hammond JM, Stevanovski I, Beukers AG, Deveson IW (2020) Analytical validity of nanopore sequencing for rapid SARS-CoV-2 genome analysis. Nat Commun 11(1):1–8. https://doi.org/10.1038/s41467-020-20075-6
Cagno V, Andreozzi P, D’Alicarnasso M, Silva PJ, Mueller M, Galloux M, Stellacci F (2018) Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nat Mater 17(2):195–203. https://doi.org/10.1038/nmat5053
Campos EV, Pereira AE, De Oliveira JL, Carvalho LB, Guilger-Casagrande M, De Lima R, Fraceto LF (2020) How can nanotechnology help to combat COVID-19? Opportunities and urgent need. J Nanobiotechnology 18(1):1–23. https://doi.org/10.1186/s12951-020-00685-4
Carling PC (2021) Health care environmental hygiene: New insights and centers for disease control and prevention guidance. Clin Infect Dis 35(3):609–629. https://doi.org/10.1016/j.idc.2021.04.005
Carvalho APA. Conte‐Junior CA (2021) Recent advances on nanomaterials to COVID‐19 management: a systematic review on antiviral/virucidal agents and mechanisms of SARS‐CoV‐2 inhibition/inactivation. Global chall 5(5):2000115. https://doi.org/10.1002/gch2.202000115
Casalino L, Gaieb Z, Goldsmith JA, Hjorth CK, Dommer AC, Harbison AM, Amaro RE (2020) Beyond shielding: the roles of glycans in the SARS-CoV-2 spike protein. ACS Cent Sci 6(10):1722–1734. https://doi.org/10.1021/acscentsci.0c01056
Centers for Disease Control and Prevention. (2016). Chemical disinfectants—Guideline for disinfection and sterilization in healthcare facilities. Centers for Disease Control and Prevention; Atlanta, GA, USA.
Chan JFW, Yuan S, Kok KH, To KKW, Chu H, Yang J, Yuen KY (2020) A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. The Lancet 395(10223):514–523. https://doi.org/10.1016/S0140-6736(20)30154-9
Chang D, Lim M, Goos JA, Qiao R, Ng YY, Mansfeld FM, Kavallaris M (2018) Biologically targeted magnetic hyperthermia: potential and limitations. Front Pharmacol 9:831. https://doi.org/10.3389/fphar.2018.00831
Chen YN, Hsueh YH, Hsieh CT, Tzou DY, Chang PL (2016) Antiviral activity of graphene–silver nanocomposites against non-enveloped and enveloped viruses. Int J Environ Res Public Health 13(4):430. https://doi.org/10.3390/ijerph13040430
Chin AW, Chu JT, Perera MR, Hui KP, Yen HL, Chan MC, Poon LL (2020) Stability of SARS-CoV-2 in different environmental conditions. The Lancet Microbe 1(1):e10. https://doi.org/10.1016/S2666-5247(20)30003-3
Choi H, Chatterjee P, Lichtfouse E, Martel JA, Hwang M, Jinadatha C, Sharma VK (2021) Classical and alternative disinfection strategies to control the COVID-19 virus in healthcare facilities: a review. Environ Chem Lett. https://doi.org/10.1007/s10311-021-01180-4
Clayton K, Vallejo AF, Davies J, Sirvent S, Polak ME (2017) Langerhans cells—programmed by the epidermis. Front Immunol 8:1676. https://doi.org/10.3389/fimmu.2017.01676
Coronado GD, Holte SE, Vigoren EM, Griffith WC, Barr DB, Faustman EM, Thompson B (2012) Do workplace and home protective practices protect farm workers? Findings from the For Healthy Kids Study. J Occup Environ Med 54(9):1–17. https://doi.org/10.1097/JOM.0b013e31825902f5
da Silva DJ, Gramcianinov GB, Jorge PZ, Malaquias VB, Mori AA, Hirata MH, Lopes SAM, Bueno LA, Champeau M, Carastan DJ (2023) PVC containing silver nanoparticles with antimicrobial properties effective against SARS-CoV-2. Front Chem 11:1083399. https://doi.org/10.3389/fchem.2023.1083399
Dexter F, Parra MC, Brown JR, Loftus RW (2020) Perioperative COVID-19 defense: an evidence-based approach for optimization of infection control and operating room management. Anesth Analg 1(1):37–42. https://doi.org/10.1213/ANE.0000000000004829
Dey P, Bergmann T, Cuellar-Camacho JL, Ehrmann S, Chowdhury MS, Zhang M, Azab W (2018) Multivalent flexible nanogels exhibit broad-spectrum antiviral activity by blocking virus entry. ACS Nano 12(7):6429–6442. https://doi.org/10.1021/acsnano.8b01616
Dhama K, Patel SK, Kumar R, Masand R, Rana J, Yatoo MI, Harapan H (2021) The role of disinfectants and sanitizers during COVID-19 pandemic: Advantages and deleterious effects on humans and the environment. Environ Sci Pollut Res 28(26):34211–34228. https://doi.org/10.1007/s11356-021-14429-w
Dhillon GS, Brar SK, Kaur S, Verma M (2012) Green approach for nanoparticle biosynthesis by fungi: current trends and applications. Crit Rev Biotechnol 32(1):49–73. https://doi.org/10.3109/07388551.2010.550568
Dicastillo CL, Correa MG, Martínez FB, Streitt C, Galotto MJ (2020) Antimicrobial effect of titanium dioxide nanoparticles. Antimicro Res. https://doi.org/10.5772/intechopen.90891
Duarte P, Santana VT (2020) Disinfection measures and control of SARS-COV-2 transmission. Global Biosecurity. https://doi.org/10.31646/gbio.64
Dung TTN, Nam VN, Nhan TT, Ngoc TTB, Minh LQ, Nga BTT, Quang DV (2020) Silver nanoparticles as potential antiviral agents against African swine fever virus. Mater Res Exp 6(12):1250g9. https://doi.org/10.1088/2053-1591/ab6ad8
Eggers M, Eickmann M, Zorn J (2015) Rapid and effective virucidal activity of povidone-iodine products against Middle East Respiratory Syndrome coronavirus (MERS-CoV) and modified vaccinia virus Ankara (MVA). Infect Dis Ther 4(4):491–501
Eggers M, Koburger-Janssen T, Ward LS, Newby C, Muller S (2018) Bactericidal and virucidal activity of povidone-iodine and chlorhexidine gluconate cleansers in an in vivo hand hygiene clinical simulation study. Infect Dis Ther 7(2):235–247
Eguchi H, Ikeda Y, Koyota S, Honke K, Suzuki K, Gutteridge JM, Taniguchi N (2002) Oxidative damage due to copper ion and hydrogen peroxide induces GlcNAc-specific cleavage of an Asn-linked oligosaccharide. J Biochem 131(3):477–484. https://doi.org/10.1093/oxfordjournals.jbchem.a003124
El-Megharbel SM, Alsawat M, Al-Salmi FA, Hamza RZ (2021) Utilizing of (zinc oxide nano-spray) for disinfection against “SARS-CoV-2” and testing its biological effectiveness on some biochemical parameters during (COVID-19 pandemic)—” ZnO nanoparticles have antiviral activity against (SARS-CoV-2)”. Coatings 11(4):388. https://doi.org/10.3390/coatings11040388
Erasmus V, Daha TJ, Brug H, Richardus JH, Behrendt MD, Vos MC, van Beeck EF (2010) Systematic review of studies on compliance with hand hygiene guidelines in hospital care. Infect Control Hosp Epidemiol 31(3):283–294. https://doi.org/10.1086/650451
Ermini ML, Voliani V (2021) Antimicrobial nano-agents: The copper age. ACS Nano 15(4):6008–6029. https://doi.org/10.1021/acsnano.0c10756
Fahimipour AK, Ben Maamar S, McFarland AG, Blaustein RA, Chen J, Glawe AJ, Hartmann EM (2018) Antimicrobial chemicals associate with microbial function and antibiotic resistance indoors. mSystems 3(6):e00200–18. https://doi.org/10.1128/mSystems.00200-18
Falagas ME, Thomaidis PC, Kotsantis IK, Sgouros K, Samonis G, Karageorgopoulos DE (2011) Airborne hydrogen peroxide for disinfection of the hospital environment and infection control: a systematic review. J Hosp Infect 78(3):171–177. https://doi.org/10.1016/j.jhin.2010.12.006
Fujimori Y, Sato T, Hayata T, Nagao T, Nakayama M, Nakayama T, Suzuki K (2012) Novel antiviral characteristics of nanosized copper (I) iodide particles showing inactivation activity against 2009 pandemic H1N1 influenza virus. Appl Environ Microbiol 78(4):951–955. https://doi.org/10.1128/AEM.06284-11
Fytianos G, Rahdar A, Kyzas GZ (2020) Nanomaterials in Cosmetics: Recent Updates Nanomaterials 10(5):979. https://doi.org/10.3390/nano10050979
Gade AK, Bonde P, Ingle AP, Marcato PD, Durán N, Rai M (2008) Exploitation of Aspergillus niger for synthesis of silver nanoparticles. J Biobased Mater Bioenergy 2:243–247
Galdiero S, Falanga A, Vitiello M, Cantisani M, Marra V, Galdiero M (2011) Silver nanoparticles as potential antiviral agents. Molecules 16(10):8894–8918. https://doi.org/10.3390/molecules16108894
Ghaffari H, Tavakoli A, Moradi A, Tabarraei A, Bokharaei-Salim F, Zahmatkeshan M, Farahmand M, Javanmard D, Kiani SJ, Esghaei M (2019) Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: another emerging application of nanomedicine. J Biomed Sci 26(1):1–10. https://doi.org/10.1186/s12929-019-0563-4
Gold NA, Avva U (2018) Alcohol Sanitizer. StatPearls Publishing; St, Petersburg, FL, USA
Goyal SM, Chander Y, Yezli S, Otter JA (2014) Evaluating the virucidal efficacy of hydrogen peroxide vapour. J Hosp Infect 86(4):255–259. https://doi.org/10.1016/j.jhin.2014.02.003
Grass G, Rensing C, Solio z, M. (2011) Metallic copper as an antimicrobial surface. Appl Environ Microbiol 77(5):1541–1547. https://doi.org/10.1128/AEM.02766-10
Guruprasad L (2021) Human SARS CoV-2 spike protein mutations. Proteins 89(5):569–576. https://doi.org/10.1002/prot.26042
Han GZ (2020) Pangolins harbor SARS-CoV-2-related coronaviruses. Trends Microbiol 28(7):515–517. https://doi.org/10.1016/j.tim.2020.04.001
Hasanien YA, Mosleh MA, Abdel-Razek AS, El-Sayyad GS, El-Hakim EH, Borai EH (2023) Green synthesis of SiO2 nanoparticles from Egyptian white sand using submerged and solid-state culture of fungi. Biomass Convers Biorefin 1–14. https://doi.org/10.1007/s13399-023-04586-y
He S, Guo Z, Zhang Y, Zhang S, Wang J, Gu N (2007) Biosynthesis of gold nanoparticles using the bacteria Rhodopseudomonas capsulata. Mater Lett 61(18):3984–3987
Holm SM, Leonard V, Durrani T, Miller MD (2019) Do we know how best to disinfect child care sites in the United States? A review of available disinfectant efficacy data and health risks of the major disinfectant classes. Am J Infect Control 47(1):82–91. https://doi.org/10.1016/j.ajic.2018.06.013
Hostynek JJ, Maibach HI (2004) Copper hypersensitivity: dermatologic aspects. Dermatol Ther 17(4):328–333. https://doi.org/10.1111/j.1396-0296.2004.04035.x
Hulme J (2022) Application of nanomaterials in the prevention, detection, and treatment of methicillin-resistant Staphylococcus aureus (MRSA). Pharmaceutics 14(4):805. https://doi.org/10.3390/pharmaceutics14040805
Huttner BD, Harbarth S (2015) Hydrogen peroxide room disinfection–ready for prime time? Crit Care 19(1):1–3. https://doi.org/10.1186/s13054-015-0915-8
Huy TQ, Thanh NTH, Thuy NT, Van Chung P, Hung PN, Le AT, Hanh NTH (2017) Cytotoxicity and antiviral activity of electrochemical–synthesized silver nanoparticles against poliovirus. J Virol Methods 241:52–57. https://doi.org/10.1016/j.jviromet.2016.12.015
Imani SM, Ladouceur L, Marshall T, Maclachlan R, Soleymani L, Didar TF (2020) Antimicrobial nanomaterials and coatings: Current mechanisms and future perspectives to control the spread of viruses including SARS-CoV-2. ACS Nano 14(10):12341–12369. https://doi.org/10.1021/acsnano.0c05937
Imran M, Jin X, Ali M, Tapfumaneyi P, Lelasseur P, Carlo L, Mohammed Y (2023) The Pandemic and your skin—direct and indirect impact of COVID-19. Cosmetics 10(1):34. https://www.mdpi.com/2079-9284/10/1/34#
Ingle A, Rai M, Gade A, Bawaskar M (2009) Fusarium solani: a novel biological agent for the extracellular synthesis of silver nanoparticles. J Nanopart Res 11:2079–2085. https://doi.org/10.1007/s11051-008-9573-y
Ishida, S. T. (2020). Zinc (II)-immune pediatric virucidal activities for 2019-nCoV prevention and therapeutic effects of COVID-19 Bonchitis and Pneumonia. Int J Med Sci Public Health 5(6), 21–33. https://doi.org/10.31579/2692-9406/012
Jamshidinia N, Mohammadipanah F (2022) Nanomaterial-augmented formulation of disinfectants and antiseptics in controlling SARS CoV-2. Food Environ Virol 1–15. https://doi.org/10.1007/s12560-022-09517-0
Jeevanandam J, Kiew SF, Boakye-Ansah S, Lau SY, Barhoum A, Danquah MK, Rosrigues J (2022) Green approaches for the synthesis of metal and metal oxide nanoparticles using microbial and plant extracts. Nanoscale 14:2534–2571
Jing JLJ, Pei Yi T, Bose RJ, McCarthy JR, Tharmalingam N, Madheswaran T (2020) Hand sanitizers: a review on formulation aspects, adverse effects, and regulations. Int J Environ Res Public Health 17(9):3326. https://doi.org/10.3390/ijerph17093326
Kampf, G. (2018). Efficacy of ethanol against viruses in hand disinfection. J Hosp Infect 98(4), 331–338. https://doi.org/10.1016/j.jhin.2017.08.025
Kampf G, Marschall S, Eggerstedt S, Ostermeyer C (2010) Efficacy of ethanol-based hand foams using clinically relevant amounts: a cross-over controlled study among healthy volunteers. BMC Infect Dis 10(1):1–5. https://doi.org/10.1186/1471-2334-10-78
Kampf G, Todt D, Pfaender S, Steinmann E (2020) Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect 104(3):246–251
Kane Y, Wong G, Gao GF (2023) Animal models, zoonotic reservoirs, and cross-species transmission of emerging human-infecting coronaviruses. Annu Rev Anim Biosci 11:1–31
Kariwa H, Fujii N, Takashima I (2006) Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions and chemical reagents. Dermatology 212(Suppl. 1):119–123
Karthik C, Punnaivalavan KA, Prabha SP, Caroline DG (2022) Multifarious global flora fabricated phytosynthesis of silver nanoparticles: a green nanoweapon for antiviral approach including SARS-CoV-2. Int Nano Lett 12:313–344. https://doi.org/10.1007/s40089-022-00367-z
Khaiboullina S, Uppal T, Dhabarde N, Subramanian V, Verma SC (2020) In vitro inactivation of human coronavirus by titania nanoparticle coatings and UVC radiation: Throwing light on SARS-CoV-2. Viruses 13(1):19. https://doi.org/10.3390/v13010019
Kim J, Yeom M, Lee T, Kim HO, Na W, Kang A, Haam S (2020) Porous gold nanoparticles for attenuating infectivity of influenza A virus. J Nanobiotechnology 18(1):1–11. https://doi.org/10.1186/s12951-020-00611-8
Kimling J, Maier M, Okenve B, Kotaidis V, Ballot H, Plech A (2006) Turkevich method for gold nanoparticle synthesis revisited. J Phys Chem 110(32):15700–15707. https://doi.org/10.1021/jp061667w
Klaus T, Joerger R, Olsson E, Granqvist CG (1999) Silver-based crystalline nanoparticles, microbially fabricated. Proc Natl Acad Sci USA 96(24):13611–13614. https://doi.org/10.1073/pnas.96.24.13611.PMID:10570120;PMCID:PMC24112
Kong L, Wang X, Li X, Liu J, Huang X, Qin Y, Yan B (2022) Aggravated toxicity of copper sulfide nanoparticles via hypochlorite-induced nanoparticle dissolution. Environ Sci Nano 9:1439–1452. https://doi.org/10.1039/D1EN01203G
Kratzel A, Todt D, V’kovski P, Steiner S, Gultom M, Thao TTN, Pfaender S, (2020) Inactivation of severe acute respiratory syndrome coronavirus 2 by WHO-recommended hand rub formulations and alcohols. Emerg Infect Dis 26(7):1592. https://doi.org/10.3201/eid2607.200915
Kumar R, Nayak M, Sahoo GC, Pandey K, Sarkar MC, Ansari Y, Das V, Topno R, Madhukar M, Das P (2019) Iron oxide nanoparticles based antiviral activity of H1N1 influenza A virus. J Infect Chemother 25(5):325–329. https://doi.org/10.1016/j.jiac.2018.12.006
Kumar GD, Mishra A, Dunn L, Townsend A, Oguadinma IC, Bright KR, Gerba CP (2020) Biocides and novel antimicrobial agents for the mitigation of coronaviruses. Front Microbiol 11:1351. https://doi.org/10.3389/fmicb.2020.01351
Lam WT, Babra TS, Smith JHD, Bagley MC, Spencer J, Wright E, Greenland BW (2022Oct 4) (2022) Synthesis and evaluation of a silver nanoparticle/polyurethane composite that exhibits antiviral activity against SARS-CoV-2. Polymers (basel) 14(19):4172. https://doi.org/10.3390/polym14194172
Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, Wang X (2020) Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 581(7807):215–220. https://doi.org/10.1038/s41586-020-2180-5
Lara HH, Ayala-Nunez NV, Ixtepan-Turrent L, Rodriguez-Padilla C. (2010). Mode of antiviral action of silver nanoparticles against HIV-1. J Nanobiotechnol 8, 1 (2010). https://doi.org/10.1186/1477-3155-8-1.
Li Y, Wang H, Tang X, Fang S, Ma D, Du C, Zhong G (2020) SARS-CoV-2 and three related coronaviruses utilize multiple ACE2 orthologs and are potently blocked by an improved ACE2-Ig. J Virol 94(22):e01283-e1320. https://doi.org/10.1128/JVI.01283-20
Lingayya H, Aayushi H, Alisha M, Jacqueline A, Ryna SS, Sriraksha BK (2020) Biocides for textiles against SARS-CoV 2. J Text Sci Eng 10(7):1–6. https://doi.org/10.37421/jtese.2020.10.424
Liu P, Chien YW, Papafragkou E, Hsiao HM, Jaykus LA, Moe C (2009) Persistence of human noroviruses on food preparation surfaces and human hands. Food Environ Virol 1(3):141–147. https://doi.org/10.1007/s12560-009-9019-4
Liu P, Yuen Y, Hsiao HM, Jaykus LA, Moe C (2010) Effectiveness of liquid soap and hand sanitizer against Norwalk virus on contaminated hands. Appl Environ Microbiol 76(2):394–399. https://doi.org/10.1128/AEM.01729-09
Liu Z, Qu Y, Wang J, Wu R (2016) Selenium deficiency attenuates chicken duodenal mucosal immunity via activation of the NF-κb signaling pathway. Biol Trace Elem Res 172(2):465–473. https://doi.org/10.1007/s12011-015-0589-8
Ma Y, Yi J, Ma J, Yu H, Luo L, Wu W, Cao M (2023) Hand sanitizer gels: Classification, challenges, and the future of multipurpose hand hygiene products. Toxics 11(8):687. https://doi.org/10.3390/toxics11080687
MacGibeny MA, Wassef C (2021) Preventing adverse cutaneous reactions from amplified hygiene practices during the COVID-19 pandemic: how dermatologists can help through anticipatory guidance. Arch Dermatol Res 313(6):501–503. https://doi.org/10.1007/s00403-020-02086-x
Mahmood A, Eqan M, Pervez S, Alghamdi HA, Tabinda AB, Yasar A, Pugazhendhi A (2020) COVID-19 and frequent use of hand sanitizers; human health and environmental hazards by exposure pathways. Sci Total Environ 742:140561. https://doi.org/10.1016/j.scitotenv.2020.140561
Malik A, Tahir Butt T, Zahid S, Zahid F, Waquar S, Rasool M, Qazi AM (2017) Use of magnetic nanoparticles as targeted therapy: theranostic approach to treat and diagnose cancer. J Nanotechnol 1–8:1098765. https://doi.org/10.1155/2017/1098765
Manaye G, Muleta D, Henok A, Asres A, Mamo Y, Feyissa D, Niguse W (2021) Evaluation of the efficacy of alcohol-based hand sanitizers sold in southwest Ethiopia. Infect Drug Resist 14:547–554. https://doi.org/10.2147/IDR.S288852
Mandal D, Bolander ME, Mukhopadhyay D, Sarkar G, Mukherjee P (2006) The use of microorganisms for the formation of metal nanoparticles and their application. Appl Microbiol Biotechnol 69(5):485–492. https://doi.org/10.1007/s00253-005-0179-3
Marumure J, Makuvara Z, Alufasi R, Chapungu L, Gufe C (2022) Effectiveness of hand sanitizers in the prevention of COVID-19 and related public health concerns: A review. Cogent Public Health 9:2060904. https://doi.org/10.1080/27707571.2022.2060904
Mazurkova NA, Spitsyna YE, Shikina NV et al (2010) Interaction of titanium dioxide nanoparticles with influenza virus. Nanotechnol Russia 5:417–420
Mazurkow JM, Yüzbasi NS, Domagala KW, Pfeiffer S, Kata D, Graule T (2019) Nano-sized copper (oxide) on alumina granules for water filtration: effect of copper oxidation state on virus removal performance. Environ Sci Technol 54(2):1214–1222. https://doi.org/10.1021/acs.est.9b05211
Mbatha LS, Akinyelu J, Chukwuma CI, Mokoena MP, Kudanga T (2023) Current Trends and Prospects for Application of Green Synthesized Metal Nanoparticles in Cancer and COVID-19 Therapies. Viruses 15:741. https://doi.org/10.3390/v15030741
McDonnell G, Russell AD (1999) Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev 12(1):147–179
McMullan LK, Flint M, Dyall J, Albariño C, Olinger GG, Foster S, Spiropoulou CF (2016) The lipid moiety of brincidofovir is required for in vitro antiviral activity against Ebola virus. Antiviral Res 125:71–78. https://doi.org/10.1016/j.antiviral.2015.10.010
Meléndez-Villanueva MA, Morán-Santibañez K, Martínez-Sanmiguel JJ, Rangel-López R, Garza-Navarro MA, Rodríguez-Padilla C, Trejo-Ávila LM (2019) Virucidal activity of gold nanoparticles synthesized by green chemistry using garlic extract. Viruses 11(12):1111. https://doi.org/10.3390/v11121111
Michels HT (2021) Materials science and coronavirus series epa officially says copper surfaces help fight COVID-19: The story behind the environmental protection agency's registration of copper surfaces against the virus that causes COVID-19. Adv Mater 179(3):27–29. https://doi.org/10.1101/2021.01.02.424974
Minoshima M, Lu Y, Kimura T, Nakano R, Ishiguro H, Kubota Y, Sunada K (2016) Comparison of the antiviral effect of solid-state copper and silver compounds. J Hazard Mater 312:1–7. https://doi.org/10.1016/j.jhazmat.2016.03.023
Mondal A, Nayak AK, Chakraborty P, Banerjee S, Nandy BC (2023) Natural polymeric nanobiocomposites for anti-cancer drug delivery therapeutics: A recent update. Pharmaceutics 15(8):2064. https://doi.org/10.3390/pharmaceutics15082064
Mori Y, Ono T, Miyahira Y, Nguyen VQ, Matsui T, Ishihara M (2013) Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Res Lett 8(1):1–6. https://doi.org/10.1186/1556-276X-8-93
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, Yacaman MJ (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16(10):2346. https://doi.org/10.1088/0957-4484/16/10/059
Morris JN, Esseili MA (2023) The effect of water hardness and pH on the efficacy of peracetic acid and sodium hypochlorite against SARS-CoV-2 on Food-Contact Surfaces. Foods 12(16):2981. https://doi.org/10.3390/foods12162981
Mukherjee P, Ahmad A, Mandal D, Senapati S, Sainkar SR, Khan MI, Parishcha R, Ajaykumar P, Alam M, Kumar R (2001) Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: A novel biological approach to nanoparticle synthesis. Nano Lett 1:515–519
Murugan K, Wei J, Alsalhi MS, Nicoletti M, Paulpandi M, Samidoss CM, Benelli G (2017) Magnetic nanoparticles are highly toxic to chloroquine-resistant Plasmodium falciparum, dengue virus (DEN-2), and their mosquito vectors. Parasitol Res 116(2):495–502. https://doi.org/10.1007/s00436-016-5310-0
Myung SK, Moskowitz JM, Choi YJ, Hong YC (2021) Reply to comment on Choi, Y.-J., et al. Cellular phone use and risk of tumors: Systematic review and meta-analysis. Int J Environ Res Public Health 17:8079. Int J Environ Res Public Health 18(6):3326. https://doi.org/10.3390/ijerph17218079
Nair B, Pradeep T (2002) Coalescence of nanoclusters and formation of submicron crystallites assisted by Lactobacillus strains. Cryst Growth Des 2(4):293–298. https://doi.org/10.1021/cg0255164
Narayanan KB, Sakthivel N (2011) Green synthesis of biogenic metal nanoparticles by terrestrial and aquatic phototrophic and heterotrophic eukaryotes and biocompatible agents. Adv Colloid Interface Sci 169(2):59–79. https://doi.org/10.1016/j.cis.2011.08.004. (Epub 2011 Sep 8 PMID: 21981929)
Naresh GK, Guruprasad L (2023) Mutations in the receptor-binding domain of human SARS CoV-2 spike protein increases its affinity to bind human ACE-2 receptor. J Biomol Struct 41(6):2368–2381. https://doi.org/10.1080/07391102.2022.2032354
Natnael, T.; Adane, M.; Goraw, S(2022). Hand hygiene practices during the COVID-19 pandemic and associated factors among barbers and beauty salon workers in Ethiopia. PLoS One 17, e0269225. https://doi.org/10.1371/journal.pone.0269225
Nikaeen G, Abbaszadeh S, Yousefinejad S (2020) Application of nanomaterials in treatment, anti-infection and detection of coronaviruses. Nanomedicine 15(15):1501–1512. https://doi.org/10.2217/nnm-2020-0117
Nithya R, Ragunathan R (2009) Synthesis of silver nanoparticle using Pleurotus sajor caju and its antimicrobial study. Dig J Nanomater Bios 4:623–629
Okeke CA, Khanna R, Ehrlich A (2023) Quaternary ammonium compounds and contact dermatitis: A review and considerations during the COVID-19 pandemic. Clin Cosmet Investig Dermatol 16:1721–1728. https://doi.org/10.2147/CCID.S410910
Oluwatuyi SV, Agbele AT, Ogunrinde ME, Ayo ATV, Ayo AM, Fayoke AB, Deborah AA (2020) Alcohol-based hand sanitizers: review of efficacy and adverse effect. Alcohol 81. https://doi.org/10.7176/JHMN/81-01
Oluwatuyi VS, Okunade RA, Oluwatuyi MF, Tolulope A, Agbele OISI, Bello M (2020b) Covid-19 in Ekiti State Nigeria: Why should we worry. Int J Res Sci Innov 7(6):146–149
Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, Qian Z (2020) Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun 11(1):1–12. https://doi.org/10.1038/s41467-020-15562-9
Oughton MT, Loo VG, Dendukuri N, Fenn S, Libman MD (2009) Hand hygiene with soap and water is superior to alcohol rub and antiseptic wipes for removal of Clostridium difficile. Infect Control Hosp Epidemiol 30(10):939–944. https://doi.org/10.1086/605322
Palencia M, García-Quintero A (2023) Green synthesis of nanomaterials and their use in bio-and nanoremediation. In Bio and Nanoremediation of Hazardous Environmental Pollutants (pp 195–229). CRC Press
Park GW, Barclay L, Macinga D, Charbonneau D, Pettigrew CA, Vinjé J (2010) Comparative efficacy of seven hand sanitizers against murine norovirus, feline calicivirus, and GII. 4 norovirus. J Food Prot 73(12):2232–2238. https://doi.org/10.4315/0362-028X-73.12.2232
Park GW, Cho M, Cates EL, Lee D, Oh BT, Vinjé J, Kim JH (2014) Fluorinated TiO2 as an ambient light-activated virucidal surface coating material for the control of human norovirus. J Photochem Photobiol B Biol 140:315–320. https://doi.org/10.1016/j.jphotobiol.2014.08.009
Prajapati P, Desai H, Chandarana C (2022) Hand sanitizers as a preventive measure in COVID-19 pandemic, its characteristics, and harmful effects: A review. J Egypt Public Health Assoc 97:6. https://doi.org/10.1186/s42506-021-00094-x
Prasher P, Singh M, Mudila H (2018) Oligodynamic effect of silver nanoparticles: a review. BioNanoScience 8(4):951–962. https://doi.org/10.1007/s12668-018-0552-1
Purniawan A, Lusida MI, Pujiyanto RW, Nastri AM, Permanasari AA, Harsono AAH, Oktavia NH, Wicaksono ST, Dewantari JR, Prasetya RR, Rahardjo K, Nishimura M, Mori Y, Shimizu K (2022) Synthesis and assessment of copper-based nanoparticles as a surface coating agent for antiviral properties against SARS-CoV-2. Sci Rep 12:4835. https://doi.org/10.1038/s41598-022-08766-0
Qin T, Ma R, Yin Y, Miao X, Chen S, Fan K, Gao L (2019) Catalytic inactivation of influenza virus by iron oxide nanozyme. Theranostics 9(23):6920. https://doi.org/10.7150/thno.35826
Rabenau HF, Cinatl J, Morgenstern B, Bauer G, Preiser W, Doerr HW (2005a) Stability and inactivation of SARS coronavirus. Med Microbiol Immunol 194(1–2):1–6
Rabenau HF, Kampf G, Cinatl J, Doerr HW (2005b) Efficacy of various disinfectants against SARS coronavirus. J Hosp Infect 61(2):107–111
Rai M, Deshmukh SD, Ingle AP, Gupta IR, Galdiero M, Galdiero S (2016) Metal nanoparticles: The protective nanoshield against virus infection. Crit Rev Microbiol 42(1):46–56. https://doi.org/10.3109/1040841X.2013.879849
Rai M, Ingle AP, Pandit R, Paralikar P, Shende S, Gupta I, da Silva SS (2018) Copper and copper nanoparticles: Role in management of insect-pests and pathogenic microbes. Nanotechnol Rev 7(4):303–315. https://doi.org/10.1515/ntrev-2018-0031
Ramadhan LOAN, Radiman CL, Suendo V, Wahyuningrum D, Valiyaveettil S (2012) Synthesis and characterization of polyelectrolyte complex N-succinylchitosan-chitosan for proton exchange membranes. Procedia Chem 4:114–122
Raota CS, Cerbaro AF, Salvador M, Delamare APL, Echeverrigaray S, da Silva Crespo J, Giovanela M (2019) Green synthesis of silver nanoparticles using an extract of Ives cultivar (Vitis labrusca) pomace: Characterization and application in wastewater disinfection. J Environ Chem Eng 7(5):103383. https://doi.org/10.1016/j.jece.2019.103383
Rosa V, Ho D, Sabino-Silva R, Siqueira WL, Silikas N (2021) Fighting viruses with materials science: Prospects for antivirus surfaces, drug delivery systems and artificial intelligence. Dent Mater 37(3):496–507. https://doi.org/10.1016/j.dental.2020.12.004
Rose GK, Thakur B, Soni R, Soni SK (2023) Biosynthesis of silver nanoparticles using nitrate reductase from Aspergillus terreus N4 and their potential use as a non-alcoholic disinfectant. J Biotechnol 373:49–62. https://doi.org/10.1016/j.jbiotec.2023.07.002
Rose G, Soni R, Rishi P, Soni S (2018) Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens. Green Process Synth 8(1):144–156. https://doi.org/10.1515/gps-2018-0042
Ruiz-Hitzky E, Darder M, Wicklein B, Ruiz-Garcia C, Martín-Sampedro R, Del Real G, Aranda P (2020) Nanotechnology responses to COVID-19. Adv Healthc Mater 9(19):2000979. https://doi.org/10.1002/adhm.202000979
Safety WP, World Health Organization (2009) WHO guidelines on hand hygiene in health care (No. WHO/IER/PSP/2009/01). World Health Organization
Saratale RG, Karuppusamy I, Saratale GD, Pugazhendhi A, Kumar G, Park Y, Shin HS (2018) A comprehensive review on green nanomaterials using biological systems: recent perception and their future applications. Colloids Surf B 170:20–35
Schoeman D, Fielding BC (2019) Coronavirus envelope protein: current knowledge. Virol J 16(1):1–22. https://doi.org/10.1186/s12985-019-1182-0
Shankar SS, Ahmad A, Pasricha R, Sastry M (2003) Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J Mater Chem 13:1822–1826. https://doi.org/10.1039/b303808b
Shivaji S, Madhu S, Singh S (2011) Extracellular synthesis of antibacterial silver nanoparticles using psychrophilic bacteria. Process Biochem 46(9):1800–1807. https://doi.org/10.1016/j.procbio.2011.06.008
Siddharta A, Pfaender S, Vielle NJ, Dijkman R, Friesland M, Becker B, Steinmann E (2017) Virucidal activity of World Health Organization–recommended formulations against enveloped viruses, including Zika, Ebola, and emerging coronaviruses. J Infect Dis 215(6):902–906. https://doi.org/10.1093/infdis/jix046
Siddiqi KS, ur Rahman A, Husen A (2018) Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res Lett 13(1):1–13. https://doi.org/10.1186/s11671-018-2532-3
Singh D, Joshi K, Samuel A, Patra J, Mahindroo N (2020) Alcohol-based hand sanitisers as first line of defence against SARS-CoV-2: a review of biology, chemistry and formulations. Epidemiol Infect 148:e229. https://doi.org/10.1017/S0950268820002319
Singh P, Ali SW, Kale RD (2023) Antimicrobial nanomaterials as advanced coatings for self-sanitizing of textile clothing and personal protective equipment. ACS Omega 8(9):8159–8171. https://doi.org/10.1021/acsomega.2c06343
Soltys L, Olkhovyy O, Tatarchuk T, Naushad M (2021) Green synthesis of metal and metal oxide nanoparticles: Principles of green chemistry and raw materials. Magnetochemistry 7:145. https://doi.org/10.3390/magnetochemistry7110145
Song X, Vossebein L, Zille A (2019) Efficacy of disinfectant-impregnated wipes used for surface disinfection in hospitals: a review. Antimicrob Resist Infect Control 8(1):1–14. https://doi.org/10.1186/s13756-019-0595-2
Sportelli MC, Izzi M, Kukushkina EA, Hossain SI, Picca RA, Ditaranto N, Cioffi N (2020) Can nanotechnology and materials science help the fight against SARS-CoV-2? Nanomaterials 10(4):802. https://doi.org/10.3390/nano10040802
Sportelli MC, Izzi M, Loconsole D, Sallustio A, Picca RA, Felici R, Chironna M, Cioffi N (2022) On the Efficacy of ZnO Nanostructures against SARS-CoV-2. Int J Mol Sci 23(6):3040. https://doi.org/10.3390/ijms23063040
Stebbins S, Cummings DA, Stark JH, Vukotich C, Mitruka K, Thompson W, Burke DS (2011) Reduction in the incidence of influenza A but not influenza B associated with use of hand sanitizer and cough hygiene in schools: a randomized controlled trial. J Pediatr Infect Dis 30(11):921. https://doi.org/10.1097/INF.0b013e3182218656
Stuart J, Chewins J, Tearle J (2020) Comparing the efficacy of formaldehyde with hydrogen peroxide fumigation on infectious bronchitis virus. Appl Biosaf 25(2):83–89. https://doi.org/10.1177/1535676020909998
Sun Z, Ostrikov KK (2020) Future antiviral surfaces: Lessons from COVID-19 pandemic. Sustain Mater Technol 25:e00203. https://doi.org/10.1016/j.susmat.2020.e00203
Talebian S, Wallace GG, Schroeder A, Stellacci F, Conde J (2020) Nanotechnology-based disinfectants and sensors for SARS-CoV-2. Nat Nanotechnol 15(8):618–621. https://doi.org/10.1038/s41565-020-0751-0
Tang Z, Kong N, Zhang X, Liu Y, Hu P, Mou S, Tao W (2020) A materials-science perspective on tackling COVID-19. Nat Rev Mater 5(11):847–860. https://doi.org/10.1038/s41578-020-00247-y
Tarkistani MAM, Komalla V, Kayser V (2021) Recent advances in the use of iron–gold hybrid nanoparticles for biomedical applications. Nanomaterials 11(5):1227. https://doi.org/10.3390/nano11051227
Tavakoli A, Hashemzadeh MS (2020) Inhibition of herpes simplex virus type 1 by copper oxide nanoparticles. J Virol Methods 275:113688. https://doi.org/10.1016/j.jviromet.2019.113688
Teunis PF, Moe CL, Liu P, Miller SE, Lindesmith L, Baric RS, Pendu JL, Calderon RL (2008) Norwalk virus: How infectious is it? J Med Virol 80(8):1468–1476. https://doi.org/10.1002/jmv.21237
Thakkar KN, Mhatre SS, Parikh RY (2010) Biological synthesis of metallic nanoparticles. Nanomedicine 6(2):257–262. https://doi.org/10.1016/j.nano.2009.07.002. (Epub 2009 Jul 16 PMID: 19616126)
Toresdahl BG, Asif IM (2020) Coronavirus disease 2019 (COVID-19): considerations for the competitive athlete. Sports health 12(3):221–224. https://doi.org/10.1177/1941738120918876
Tosta E (2022) The adaptation of SARS-CoV-2 to humans. Mem Inst Oswaldo Cruz 116:e210127. https://doi.org/10.1590/0074-02760210127
Valdez-Salas B, Beltran-Partida E, Nelson Cheng JSC, Valdez-Salas EA, Curiel-Alvarez M, Ibarra-Wiley R (2021) Promotion of surgical masks antimicrobial activity by disinfection and impregnation with disinfectant silver nanoparticles. Int J Nanomedicine 16:2689. https://doi.org/10.2147/IJN.S301212
van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, Munster VJ (2020) Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med 382(16):1564–1567. https://doi.org/10.1056/NEJMc2004973
Vigneshwaran N, Nachane RP, Balasubramanya RH, Varadarajan PV (2006) A novel one-pot “green” synthesis of stable silver nanoparticles using soluble starch. Carbohydr Res 341(12):2012–2018. https://doi.org/10.1016/j.carres.2006.04.042
Vonnemann J, Sieben C, Wolff C, Ludwig K, Böttcher C, Herrmann A, Haag R (2014) Virus inhibition induced by polyvalent nanoparticles of different sizes. Nanoscale 6(4):2353–2360. https://doi.org/10.1039/C3NR04449A
Vuppu S, Mishra T, Chinamgari A (2023) Use of hand sanitizers in COVID-19 prevention: A comprehensive overview. Pharmacoepidemiology 2(3):257–271. https://doi.org/10.3390/pharma2030022
Waghmare S, Deshmukh RR, Shrishrimal P, Waghmare VB, Janvale GB, Sonawane BS (2017) A comparative study of recognition technique used for development of automatic stuttered speech dysfluency Recognition system. Ind J Sci Technol 10:1–10
Wang Q, Zhang Y, Wu L, Niu S, Song C, Zhang Z, Qi J (2020) Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell 181(4):894–904. https://doi.org/10.1016/j.cell.2020.03.045
Watanabe Y, Allen JD, Wrapp D, McLellan JS, Crispin M (2020a) Site-specific glycan analysis of the SARS-CoV-2 spike. Science 369(6501):330–333. https://doi.org/10.1126/science.abb9983
Watanabe Y, Berndsen ZT, Raghwani J, Seabright GE, Allen JD, Pybus OG, Crispin M (2020b) Vulnerabilities in coronavirus glycan shields despite extensive glycosylation. Nat Commun 11(1):1–10. https://doi.org/10.1038/s41467-020-16567-0
Watanabe Y, Bowden TA, Wilson IA, Crispin M (2019) Exploitation of glycosylation in enveloped virus pathobiology. Biochim Biophys Acta Gen Subj 1863(10):1480–1497. https://doi.org/10.1016/j.bbagen.2019.05.012
Wickett RR, Visscher MO (2006) Structure and function of the epidermal barrier. Am J Infect Control 34(10):S98–S110. https://doi.org/10.1016/j.ajic.2006.05.295
Winnefeld MA, Richard MA, Drancourt M, Grob JJ (2000) Skin tolerance and effectiveness of two hand decontamination procedures in everyday hospital use. Br J Dermatol 143(3):546–550. https://doi.org/10.1111/j.1365-2133.2000.03708.x
Woldegebreal T, Teju E, Kebede A, Taddesse A, Bezu Z (2022) Water disinfection using kaolinite supported magnetic silver nanoparticle. Chem Data Collect 39:100857. https://doi.org/10.1016/j.cdc.2022.100857
Wood A, Payne D (1999) The action of three antiseptics/ disinfectants against enveloped and non-enveloped viruses. J Hosp Infect 38(4):283–295
World Health Organisation. (2009). WHO guidelines on hand hygiene in health care: First global patient safety challenge: Clean care is safer care. World Health Organisation; Geneva, Switzerland.
Xu H, Qu F, Xu H, Lai W, Wang YA, Aguilar ZP, Wei H (2012) Role of reactive oxygen species in the antibacterial mechanism of silver nanoparticles on Escherichia coli O157: H7. Biometals 25(1):45–53. https://doi.org/10.1007/s10534-011-9482-x
Yari S, Moshammer H, Asadi AF (2020) Side effects of using disinfectants to fight COVID-19. Asian Pac J Environ Cancer 3(1):9–13. https://doi.org/10.31557/apjec.2020.3.1.9-13
Yasuda J, Eguchi H, Fujiwara N, Ookawara T, Kojima S, Yamaguchi Y, Suzuki K (2006) Reactive oxygen species modify oligosaccharides of glycoproteins in vivo: a study of a spontaneous acute hepatitis model rat (LEC rat). Biochem Biophys Res Commun 342(1):127–134. https://doi.org/10.1016/j.bbrc.2006.01.118
Yin Y, Wunderink RG (2018) MERS, SARS and other coronaviruses as causes of pneumonia. Respirology 23(2):130–137. https://doi.org/10.1111/resp.13196
York GK, Steinberg DA (2009) Neurology in ancient Egypt. Handbook of clinical neurology, 95, 29–36. Elsevier. https://doi.org/10.1016/S0072-9752(08)02103-9
Yu DG, Wang M, Li X, Liu X, Zhu LM, Annie Bligh SW (2020) Multifluid electrospinning for the generation of complex nanostructures. Wiley Interdiscip Rev Nanomed Nanobiotechnol 12(3):e1601. https://doi.org/10.1002/wnan.1601
Yuindartanto A, Prakoeswa CRS, Anggraeni S, Umborowati MA, Zulkarnain I, Listiawan MY, Hidayati AN (2023) Epidemiology of occupational contact dermatitis (OCD) on health workers in Covid-19. J Pak Assoc Dermatol 33(1):220–234. https://www.jpad.com.pk/index.php/jpad/article/view/2000
Zhang XF, Liu ZG, Shen W, Gurunathan S (2016) Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci 17(9):1534. https://doi.org/10.3390/ijms17091534
Zoghi S, Khamirani HJ, Dastgheib SA, Dianatpour M, Ghaffarieh A (2021) An analysis of inhibition of the severe acute respiratory syndrome coronavirus 2 RNA-dependent RNA polymerase by zinc ion: an in silico approach. Future Virol 16(5):331–339. https://doi.org/10.2217/fvl-2020-0369
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
SKS: conceptualization, writing—review and editing, supervision; TM: writing—original draft preparation, writing—review and editing; AS: writing—original draft preparation, writing—review and editing; BT: writing—review and editing; RS: writing—original draft preparation, writing—review and editing, supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Institutional review board statement
Not applicable.
Informed consent
Not applicable.
Additional information
Communicated by Yusuf Akhter.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Soni, S.K., Marya, T., Sharma, A. et al. A systematic overview of metal nanoparticles as alternative disinfectants for emerging SARS-CoV-2 variants. Arch Microbiol 206, 111 (2024). https://doi.org/10.1007/s00203-023-03818-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-023-03818-z