Abstract
Nodular endophytes of drought-tolerant legumes are understudied. For this reason, we have isolated and studied non-symbiotic endophytic bacteria from nodules of Vachellia tortilis subsp. raddiana, a leguminous tree adapted to the harsh arid climate of Southern Morocco. Rep-PCR analysis followed by 16S rDNA sequencing revealed two main genera, Pseudomonas and Bacillus. Isolates responded variably to salt and water stresses, and mostly produced exopolysaccharides. Differences concerned also plant growth-promoting activities: phosphate, potassium, and zinc solubilization; biological nitrogen fixation; auxin, siderophore, ammonia, and HCN production; and ACC deaminase activity. Some strains exhibited antagonistic activities against phytopathogenic fungi (Fusarium oxysporum and Botrytis cinerea) and showed at least two enzymatic activities (cellulase, protease, chitinase). Four selected strains inoculated to vachellia plants under controlled conditions have shown significant positive impacts on plant growth parameters. These strains are promising bio-inoculants for vachellia plants to be used in reforestation programs in arid areas increasingly threatened by desertification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The effects of climate change are currently regarded as the most serious worldwide environmental problems, threatening natural ecosystems, and biodiversity (Bharali and Khan 2011). Arid zones constitute an excellent open natural laboratory to study the impact of aridity on species distribution, their survival strategies and adaptations they have acquired, their interactions with the other components of ecosystems, and so on. These areas host a diverse range of species that are mostly perfectly suited to the ecology of dry lands. This is the case of the Southern Madagascar, the Brazilian Cerrado, the California Chaparral, the Mediterranean Basin, and the Cape Florida Field that are considered among the high biodiversity areas in the world. Flowering plant species are particularly more diversified in dry sub-humid zones than in humid temperate zones (Boutaj et al. 2019).
However, arid areas are very vulnerable and can quickly be subjected to degradation, materialized by the loss of essential soil properties such as soil structure, plant nutrients availability, organic matter content, and microbial activities. At the end of this process, the natural plant communities are disrupted (Jeffries and Barea 2012). In these areas, leguminous plants appear to be good candidates for sustainable and ecological initiatives and programs to offset the negative effects of soil degradation and climate change. These species belong to one of the largest plant families in the world, with nearly 18,000 species. They are of great importance worldwide, and especially in countries with a Mediterranean climate like Morocco. Indeed, in addition to their importance as a source of plant proteins for animal and human use, legumes provide wood, industrial, and pharmaceutical products. They also have an important ecological role, linked to their ability to perform symbiotic nitrogen fixation in association with rhizobial bacteria, thus providing cheap and green N fertilizers (van Kessel and Hartley 2000). Besides their contribution to building soil fertility, legumes contribute to preventing erosion as they are also good colonizers of poor soils under extreme climatic conditions (Giller and Cadisch 1995).
Legume trees such as Acacia species play also an important role in arid ecosystem biodiversity and soil ecology by modifying solar radiation and soil moisture (Abdallah et al. 2012). They are also known to improve soil nutrient availability to plants and animals (Gedda 2003). Among these trees, Vachellia tortilis subsp. raddiana (V. t. subsp. raddiana) is known for its ability to withstand harsh conditions of seasonal waterlogging and climatic temperature variations (Malakootian et al. 2018). This tree can resist to severe drought conditions thanks to particular adaptations such a deep lateral roots and partial leaf repelling in the dry season (in the range of 20–200 mm). It is known as a bedrock species since it is the only forest tree that thrives on the edge of the desert (Abdallah et al. 2008). Under these unique bioclimatic circumstances, this tree has been regarded as a key component in ecosystem restoration and water conservation strategies (Mahamane and Mahamane 2005).
In addition to the morphological adaptations and physiological mechanisms that are implicated in plant’s adaptation to stressful conditions, soil microbial populations participate also in plant’s tolerance to biotic and abiotic stresses, particularly the beneficial bacteria inhabiting the rhizospheric soil known as plant growth-promoting rhizobacteria (PGPR). These bacteria contribute to soil fertility building and plants growth promotion through different biological mechanisms such as enhanced nutrient availability (N, P, K, Mg, etc.), growth stimulation (phytohormones production), improved stress tolerance (ACC deaminase and phytohormones activities), superior protection against phytopathogens (siderophores, antibiotics, lytic enzymes, and induced systemic resistance) (Chandran et al. 2021). Some of the rhizospheric bacteria can penetrate the root epidermis and colonize root tissues, and/or migrate to different parts and organs of the plant; these bacteria are known as endophytes (Strobel and Daisy 2003). Bary (1866) was the first to use the term endophytes to refer to microbes that persist in plant’s tissues, including bacteria, fungus, cyanobacteria, and actinobacteria (Rana et al. 2020).
Among bacterial endophytes, several species were isolated from legume nodules where they coexist with rhizobia. These bacteria are known as opportunists because they may infect nodules during nodule formation by rhizobia (Leite et al. 2017). They belong to different genera dominated by Bacillus, Pseudomonas, and Agrobacterium, and members of the Enterobacteriaceae family (Ibáñez et al. 2017). However, different other genera were reported as nodule endophytic bacteria (NEB) such as Arthrobacter, Acinetobacter, Micromonospora, Mycobacterium, and Stenotrophomonas (Velázquez et al. 2013), Aerobacter, Aeromonas, Chryseomonas, Curtobacterium, Erwinia, Flavimonas, and Sphingomonas that were isolated from nodules of pea cultivars (Elvira-Recuenco and van Vuurde 2000), Cupriavidus, Providencia, Staphylococcus, Kocuria, Micrococcus, Frondihabitans, Paracoccus, and Roseomonas that were obtained from cowpea nodules (Leite et al. 2017), and Agrobacterium, Klebsiella, Paenibacillus, Bacillus, Blastobacter, Dyadobacter, Phyllobacterium, Pseudomonas, Ensifer, and Enterobacter isolated from nodules of indigenous legumes in Flanders (Belgium) (De Meyer et al. 2015).
Some of these non-rhizobial endophytic bacteria were shown to possess diverse beneficial activities for plants such as synthesizing plant hormones, fixing atmospheric N2, and solubilizing inorganic phosphate (Peix et al. 2015), while others have in addition the catalytic activity of ACC deaminase or produce different compounds like siderophores (Kour et al. 2020; Rana et al. 2020), and others can promote their host plants by acquiring more restricted plant nutrients (Glick 2012). However, there is a lack of literature that deals with the endophytes of desert legume nodules, especially their diversity, their PGP activities, and their potential effects on plant's tolerance and growth in such stressful environments.
With the aim of knowing more about these particular endophytes, we have isolated bacteria from sterilized nodules of the leguminous tree species V. t. subsp. raddiana, grown in soils collected from the Guelmim region (south part of Morocco). Isolates that lacked nodC gene were considered as nodule endophytic bacteria (non-rhizobial strains). After identification by 16S rDNA sequencing, they were studied for their in vitro tolerance to salt and drought stresses, their plant growth-promoting activities, and their antagonistic activity against phytopathogens. Afterward, a set of selected nodule endophytic strains with multiple PGP traits were tested in planta in a pot experiment under greenhouse conditions. Strains were inoculated to V. t. subsp. raddiana seedlings and their impact on plant growth parameters was determined after six months. We found that four strains influenced positively plant growth; therefore, they can be considered promising potential candidates to formulate biofertilizers for V. t. subsp. raddiana.
Material and methods
Soil sampling and isolation of nodule endophytic bacteria
The soil samples were collected from the bulk soil surrounding 6 well-growing trees of V. t. subsp. raddiana, distant of 200–500 m, possessing higher coverage, and bordered by abundant vegetation. Sampling area was located near Taidalt, a small village near Guelmim in the extreme southwest part of the Anti-Atlas Mountains in Morocco. Seeds of the precedent year were collected from different trees in the same area to be used in the plant tests.
Due to its location in the pre-Saharan zone, the climate of Guelmim is rather Saharan with an influence from the Atlantic Ocean (semiarid Saharan), characterized by a hot dry summer and cold winter with 150 mm/year precipitation average (HCP 2021). The dominating tree species is V. t. subsp. raddiana, while the major shrub is Hammada scoparia (Pomel) Iljin. V. t. subsp. raddiana is locally associated with Panicum turgidum Forssk. in sandy riverbeds and Convolvulus trabutianus in terraces and Ziziphus lotus (L.) in the plain (Msanda et al. 2002).
For isolation of bacterial nodule inhabitants, V. t. subsp. raddiana seeds were scarified using 98% sulfuric acid for 2 h (Sakrouhi et al. 2016), washed several times with sterile water, transferred to Petri dishes containing 0.7% (w/v) Agar, and allowed to germinate in the dark at 28 °C for three to five days. Seedlings were then placed aseptically directly in disinfected plastic pots filled with the different soil samples (four seedlings per pot) for trapping the rhizobia present in the soil and eventually other nodule endophytes.
After 5 months of growth in the greenhouse, plants were gently pulled apart, and soil was carefully wiped off the roots with tap water. Nodules were separated and surface-sterilized by immersion in ethanol 70% (v/v) for one minute, then up to three minutes in 90% sodium hypochlorite. After several washing with sterilized distilled water, each nodule was crushed separately with a sterilized glass rod in a drop of sterilized distillated water and the resulting suspension streaked in a Petri dish of YEM agar medium (yeast extract—mannitol) (Vincent 1970). The efficacy of the sterilization protocol was checked by inoculating aliquots of the last rinsing water into Petri dishes of nutrient broth medium.
After 4 days of incubation at 28 °C, bacterial colonies differing in size, shape, and color were isolated and purified by repeated streaking to ensure the purity of each isolate. After purification, isolates were stored in glycerol 50% at − 86 °C.
Molecular identification of nodule endophytic isolates
DNA isolation
Bacterial genomic DNA was extracted following the method described by Chen and Kuo (1993). Fresh bacterial suspensions of each strain (previously grown at 28 °C in tryptone yeast extract (TY) for 4 days) were transferred to 1.5-ml sterile tubes and centrifuged at 13,000 g for 15 min. The resulting pellets were dissolved in 1 ml buffer (20% SDS, 2 M sodium acetate, 3 M Tris–acetate, 20 mM EDTA) and transferred to 1.5-ml sterile tubes, then treated with NaCl and incubated for 10 min at 65 °C. After that, each suspension was extracted with 1.2 ml phenol–chloroform–isoamyl alcohol (25:24:1) followed by extraction of the aqueous phase with 1.2 ml chloroform–isoamyl alcohol (24:1). The upper phase was collected and DNA was precipitated by adding an equivalent volume of isopropanol and stored at − 80 °C for 30 min or overnight at – 20 °C. After centrifugation, the pellet was washed twice with 500 µL of 96% and 70% ethanol. The pellet was dried at room temperature and dissolved in 50 µL of Tris–EDTA buffer and stored at – 20 °C, and the DNA was quantified at 260 nm with a NanoDrop spectrophotometer (Thermo Scientific).
nodC gene amplification
In order to distinguish between rhizobia and nodule endophytes, all isolates were screened for the presence of the nodC gene by performing selective amplification of this gene using primers (nodCFn/nodCi) as described by Laguerre et al. (2001). Each 25 μl of the PCR mixture contained the DNA template (20–100 ng), 1 × Taq reaction buffer, 25 pmol of each primer and 1.5 U of Taq DNA polymerase (Bioline). PCR amplifications were performed using the following temperature cycling program: initial denaturation at 94 °C for 5 min, 30 cycles of 94 °C (1 min), 56 °C (30 s), and 72 °C (1 min), followed by a final extension at 72 °C (10 min). All isolates that failed to amplify the nodC gene were considered as endophytes while those showing nodC amplification were left out.
Rep-PCR fingerprinting
Rep-PCR (repetitive extragenic palindromic polymerase chain reaction) was performed using primers REP1 R-1 and REP2-I (Table S1) as described by de Bruijn (1992), with the objective to avoid duplications and clonality and reduce the number of strains to be studied.
A dendrogram was built using the unweighted pair group with arithmetic mean (UPGMA) implemented in the GelCompar II software (version 2.5 Applied Maths, Belgium).
16S rDNA gene amplification and sequencing
A representative strain of each REP cluster was used for 16S rDNA gene sequencing. After isolation of total DNA from endophytic bacteria, the 16S rDNA gene was amplified using the universal primers fd1/rd1 (Table S1) (Weisburg et al. 1991). PCR amplification reaction was performed according to the following program (5 min 95 °C, 35 × (1 min 94 °C, 1 min 30 s 62 °C,10 min 72 °C), 10 min 72 °C). PCR products were sent to Genoscreen© (Lille, France) for purification and sequencing.
Phylogenetic analyses
The quality of all sequences was checked using Chromas Lite (version 2.1). All sequences were aligned using Ugene (version 2.7) (Okonechnikov et al. 2012) and then were treated with MEGA X for phylogenetic analyses (Kumar et al. 2018). Distances were calculated according to Kimura’s two-parameter model (Kimura 1980), and sequences were used to infer phylogenetic trees with the maximum likelihood phylogeny analysis. Strains possessing high similarity with our isolates after the BLAST using the NCBI platform and type strains of closely related species were included in the phylogenetic analysis.
The accession numbers of the nucleotide sequences used in this study are shown in the figure trees, and Bootstrap support for each node was evaluated with 1000 replicates.
Physiological characterization of isolated nodule endophytes
Plant growth-promoting activities
Auxin production
The production of indole acetic acid (IAA) was tested on a solid YEM agar medium supplemented with tryptophan (0.5 g L−1). The medium was inoculated with 5 μl of bacterial culture and incubated at 28 °C for 3 days. IAA production was detected by placing a Whatman filter paper soaked with Salkowski reagent on the bacterial culture. After 30 min in the dark, the appearance of pink halos around the colonies indicates the presence of IAA.
Quantification of the IAA produced by each isolate was determined by the Salkowski method (Ehmann 1977). Bacteria were cultivated in YEM tryptophane liquid medium tryptophan at 28° C and incubated in a shaking incubator at 180 rev min−1 for 48 h.
Cultures were centrifuged at 8000 rpm for 15 min and then passed through a 0.2 μm Millipore filter. Supernatants were placed in fresh test tubes, mixed with Salkowski's reagent, and then stored in the dark at room temperature for 25 min. The optical density (OD) of each reaction was then measured at 540 nm, and the auxin amount was determined based on a standard curve of IAA (Gordon and Weber 1951).
Phosphate solubilization
Solubilization of inorganic phosphate was determined according to the method of Pikovskaya and Pikovskaya (1948). Aliquots of strains’ fresh culture were spotted on Pikovskaya medium (PVK) agar (glucose C6H12O6 10 g; Ca3(PO4)2 (tricalcium phosphate (TCP) 5 g; yeast extract 0.5 g; ammonium sulfate (NH4)2SO4 0.5 g; potassium chloride (KCl) 0.2 g; sodium chloride (NaCl) 0.2 g; magnesium sulfate (MgSO4) 0.1 g; trace amounts of ferrous sulfates (FeSO4) and manganese sulfates (MnSO4); agar 15 g; distilled water 1 L; the pH value adjusted to 7.0 ± 0.2 before sterilization). Inoculated plates were incubated at 28 °C for 4 days. Bacteria that grow, dissolve, and use TCP as a source of phosphate are characterized by the presence of halo areas around bacterial colonies, which is considered a positive indicator of mineral phosphate solubilization. The ratio of halo diameter to colony diameter was calculated for each strain.
Quantitative estimation of phosphate solubilization was studied by growing the strains in Erlenmeyer flasks (250 ml) containing modified liquid PVK medium where 0.05 g of rock phosphate was used as the only P source instead of TCP. Bacterial cultures starting at a cell density (OD600 = 0.05) were incubated at 28 °C for 2 days in a rotary shaker. At each sampling time, pH and OD600 of the medium were measured. A sample of the culture was centrifuged at 8000 rpm for 15 min, and the phosphate content in the supernatant was determined according to the vanadium molybdenum phosphorus colorimetric method (Tandon et al. 1968). The specific absorbance of yellowing was measured at 400 nm, and the amount of the corresponding soluble phosphate was calculated from a standard curve of KH2PO4.
Siderophores production
The chrome azurol sulphonate (CAS) assay was used to measure siderophore production (Schwyn and Neilands 1987), following the procedure described by Arora and Verma (2017).
In a first screening test, 900 ml of sterilized LB agar medium and 100 ml of the CAS reagent were used to create the CAS agar plates. On each plate, four distinct bacterial strains were spot-inoculated. Negative controls consisted on non-inoculated plates. After 5–7 days incubation at 30 °C, plates were checked for the formation of a colorful halo around the bacterial colonies (Louden et al. 2011).
Siderophore production was then quantified for positive isolates through the same CAS methodology, performed according to the protocol proposed by (Arora and Verma 2017) and slightly modified to be adapted to 96-well microplate cultures. Modi medium was used (g L−1: K2HPO4 0.5; MgSO4 0.4; NaCl 0.1; mannitol 10; glutamine 1; NH4NO3 1) (Ahemad and Khan 2012). The control test consisted of a non-inoculated MODI medium. Three replicates were foreseen for each strain. After incubating at 28 °C for 1 week, bacterial cultures were centrifuged at 10,000 rpm for 10 min, the cell pellets were discarded, and the supernatants were used to estimate siderophores production. The supernatant (100 µL) of each bacterial culture was mixed with 100 µL of CAS reagent, and the optical density was measured at 630 nm for 30 min with a microplate reader (Biotekelx 808 spectrophotometer). CAS reagent was prepared as per Schwyn and Neilands (1987): 100 ml of pure water and 20 ml of a 1 mM ferric chloride (FeCl3 6H2O) solution prepared in 10 mM HCl were used to dissolve 121 mg of CAS. This solution was stirred while being added to a 20 ml solution of hexadecyl trimethyl ammonium bromide (HDTMA). To prepare an HDTMA solution, 729 mg of HDTMA were dissolved in 400 ml of distilled water. Prior to utilization, the CAS-HDTMA solution was sterilized. (MES is used as a buffer.)
The percent siderophore unit (psu) was calculated according to the following formula of Payne (1993):
Siderophore production = (Ar − As) × 100/Ar.
where Ar = absorbance of reference (CAS solution and uninoculated Modi medium), and As = absorbance of the sample (CAS solution and cell-free supernatant of a sample).
ACC deaminase activity
The 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase activity of the endophyte isolates was determined by using the method reported by Palaniyandi et al. (2014). Bacteria were grown in a minimal medium (Dworkin and Foster 1958). Three treatments were foreseen for each strain: minimal medium supplemented with ACC at a final concentration of 3 mmol L−1 for the test, without nitrogen source for the negative control, or supplemented with ammonium sulfate (NH4)2SO4 for the positive control. The test was performed in 96-well microplates, where each strain was tested in triplicate. As blanks, the outermost wells of the plate were filled with the culture medium free of bacteria. After 7 days at 28 °C, the OD was read at 630 nm. ACC deaminase positive strains exhibited similar growths when growing in ACC or (NH4)2SO4 media, whereas strains that could not utilize ACC presented a growth level similar to that recorded in the free nitrogen medium.
Zinc solubilization
The ability of selected endophytes to solubilize insoluble Zn compounds was measured on a Tris-mineral agar medium amended with 15.23 mM of Zn oxide (ZnO) (1.244 g L−1), 5.2 mM of Zn carbonate (ZnCO3) (1.728 g L−1) (Gandhi and Muralidharan 2016), and 0.1% (w/v) insoluble zinc in the form of zinc sulfate (ZnSO4) (Khanghahi et al. 2018; Sharma et al. 2012). The Tris-mineral agar medium contains D-glucose 10.0 g L−1; (NH4)2SO4 1.0 g L−1; KCl 0.2 g L−1; K2HPO4 0.1 g L−1; and MgSO4 0.2 g L−1; Agar 15 g L−1. The pH was adjusted to 7.00 ± 0.25 before autoclaving (Khanghahi et al. 2018). Isolates to be tested were spot-inoculated on each medium and incubated at 30 °C for 10 days; the solubilization of zinc is characterized by the presence of halo areas around bacterial colonies, which is considered a positive indicator of mineral zinc solubilization.
Potassium solubilization
Qualitative analysis of K solubilization was carried out using the Aleksandrov medium (pH 7.2 ± 0.2), containing 5.0 g L−1 glucose (C6H12O6), 0.5 g L−1 magnesium sulfate (MgSO4), 0.005 g L−1 ferric chloride (FeCl3), 0.1 g L−1 calcium carbonate (CaCO3), 2 g L−1 calcium phosphate (Ca3(PO4)2), and 2 g L−1 K-bearing minerals (Hu et al. 2006).
After adding the appropriate amount of the phenol red solution, the medium was autoclaved and then poured into Petri dishes. The control consisted in Aleksandrov medium plates without dye solution. Isolates were spot-inoculated and incubated at 30 °C during 72 h. The size of the halo zone was calculated as the difference between the total diameter and that of the colony (Rajawat et al. 2016).
Biological nitrogen fixation and nifH gene amplification
Isolates were streaked on Burk’s nitrogen (N)-free agar and incubated at 30 °C for 7–10 days. The medium consisted of 10 g L−1 glucose, 0.41 g L−1 KH2PO4, 0.52 g L−1 K2HPO4, 0.05 g L−1 Na2SO4, 0.2 g L−1 CaCl2, 0.1 g L−1 MgSO4·7H2O, 0.005 g L−1 FeSO4·7H2O, 0.0025 g L−1 Na2MoO4·2H2O, and 15 g L−1 agar (Park et al. 2005).
Each bacterial isolate was also streaked onto LB agar plates as a positive control. Growing isolates were repeated twice on new Burk’s N-free agar plates (Revillas et al. 2000). Only strains that developed in the third set of N-free medium plates are considered nitrogen-fixing bacteria.
To confirm the biological nitrogen capacity of positive strains, the nifH gene (781 bp) of each bacteria was amplified by PCR using two pairs of primers, respectively, nif H1 and nifH2 (Pandey et al. 2004), and nifHf/nifHi (Table S1) (Laguerre et al. 2001). Briefly, 25 μl of the PCR mixture contained the DNA template (20–100 ng) of each strain, 1 × Taq reaction buffer, 25 pmol of each primer and 1.5 U of Taq DNA polymerase (Bioline). PCR amplifications were performed using the following temperature cycling program: initial denaturation at 94 °C for 5 min, 30 cycles of 94 °C (1 min), 60 °C (30 s) and 72 °C (1 min), followed by a final extension at 72 °C (10 min).
PCR products were run in a 1% agarose gel electrophoresis using TBE buffer (0.089 M Tris, 0.089 M boric acid, and 0.002 M EDTA pH 8), stained with a solution of ethidium bromide (0.5 μg mL−1), and photographed under UV light using an automatic gel photography acquisition system (the ENDURO™ GDS) for digital recording.
Ammonium production
Ammonium production by the endophyte isolates was studied according to the method of Cappucino and Sherman (1992). Isolates cultures were introduced into 10 mL peptone water and incubated at 28 °C with shaking at 150 rpm. After a 48-h incubation period, each bacterial culture was supplied with 0.5 mL of Nessler reagent. Positive reactions consisted in the development of brown to yellow colors. As a control, an uninoculated medium was utilized.
Stress tolerance
Salt and drought tolerance
Endophyte isolates were tested for salt and drought tolerance on YEM medium containing amounts of NaCl between 0 and 14% (for salt stress) while for drought stress the media was adjusted to different osmotic potentials by adding PEG 6000 at concentrations of 10%, 20% and 30% (Michel and Kaufmann 1973; Sandhya et al. 2009). Media were inoculated with 1% of overnight YEM bacterial cultures. Three replicates of each isolate at each concentration were foreseen. Media were incubated for 48 h at 28 °C under shaking conditions (120 rpm) then growth was estimated by measuring the optical density at 630 nm using the microplate reader. Bacterial growth on the same media without a stressor was used as a reference.
Exopolysaccharides production
The method of Paulo et al. (2012) was used for studying EPS production. Briefly, bacterial liquid cultures (OD600 = 0.8) were inoculated on 5-mm-diameter sterile filter paper disks placed on a plate of mineral salts medium with (10% of sucrose) and incubated at 28 °C. After 24 h, EPS production was assessed based on the formation of a mucoid colony around the disks.
Biocontrol activities
Hydrogen cyanide (HCN) production
Selected bacteria among those that showed the best PGP activities were streaked on LB medium supplemented with 0.4% (w/v) glycine. A Whatman filter paper saturated with an alkaline picric acid solution (2% sodium carbonate in 0.5% picric acid) was placed on the upper lids of Petri plates sealed with parafilm and incubated at 28 °C for 4–5 days.
The development of a red–brown color from the initial yellow color of the filter paper indicated HCN production (Miller and Higgins 1970).
Antagonistic activity against fungal pathogens
Bacterial endophytes were examined for their ability to inhibit the growth of two fungal phytopathogens: Fusarium oxysporum and Botrytis cinerea. The strain of F. oxysporum was provided by the phytopathology team at the Faculty of Sciences of Rabat, Morocco, it was originally isolated from the roots of Lens culinaris. The B. cinerea strain was provided by ICARDA (Rabat) and was isolated from chickpea. These two fungi were chosen regarding their large host range, including many important crop species.
The protocol proposed by Zhou et al. (2014) was followed. In brief, the bacterial isolates was streaked onto plates in two parallel streaks (length = 6 cm), 3 cm apart from the fungal disk (diameter = 5 mm), in the center of an LB agar plate. Similar steps are taken to prepare a control plate without bacterial inoculation.
The potential production of volatile compounds by the bacteria that might have some effect on the growth of the fungal strains was assessed by following the protocol of Rahmansyah (2013). Fungal disks (diameter = 5 mm) were placed in the center of PDA Petri dishes (9 cm) and incubated for five days, while bacterial endophytes were grown in LB medium plates for 48 h. After incubation periods, plates containing the fungus and those containing the endophytic isolates were placed inverted on top of each other. The top was sealed with parafilm and adhesive tape to prevent the diffusion of volatiles.
A third technique was used to check for the presence and efficacy of diffusible compounds produced by the bacteria against the pathogenic agents. The endophytic isolates are pre-cultured for 48 h in LB tubes. PDA medium-containing Petri dishes were prepared. Sterile millipore-type nitrocellulose membranes (0.45 m porosity) were laid on the surface of the agar medium once it has solidified. After that, 5 µL of bacterial suspension was spotted on the membrane and left in place until the drops are absorbed.
After incubation for 48 h at 28 °C, the membrane (containing the bacterial culture) is removed from the surface of each agar culture medium, and a fungal disk (diameter = 5 mm) was placed in the center of the plate and re-incubated for an additional week. Finally, the fungal pathogen growth was measured and compared in both test and control plates (Liu et al. 2018).
Extracellular enzyme production
Overnight grown bacterial cultures were transferred aseptically by spot inoculation on LB agar plates supplemented with 1% carboxymethyl cellulose (CMC) for cellulase production (Liu et al. 2018) and skim milk agar plates for protease production (Kumar et al. 2005).
For cellulase activity, plates were stained with 0.1% Congo red for 15 min. After distaining (1 M NaCl for 15 min), positive results are indicated by the formation of yellowish zones around colonies. Protease production was indicated in the corresponding plates by a clear zone around the colonies after 4 days of incubation at 28 °C. For chitinolytic activity, colloidal chitin was prepared from crab shells (Sigma–Aldrich) according to Skujins et al. (1965). Chitin agar plates containing 1 g colloidal chitin, 0.1% K2HPO4, 0.05% MgSO47H2O, and 1.5% agar, and 2% phenol red, were inoculated with aliquots of bacterial suspensions and incubated at 30 °C for 7 days. Clear zones around inoculants’ spots indicate the presence of chitinase (Goswami et al. 2014).
Vachellia growth promotion by selected endophytes
Seed germination, seedlings inoculation, and growth conditions
For plants inoculation experiment, 8 strains were selected. Seven strains having at least one or more of the main four PGP activities: auxin and siderophores production, phosphate and potassium solubilization. The last strain was selected based on its stress tolerance (PEG and NaCl tolerance, and exopolysaccharides production).
The growing system adopted is constituted by seedling tanks with 45 alveoli. Each alveolus is filled with a mixture of autoclaved sand and vermiculite (2 V/1 V). V. t. subsp. raddiana seeds were prepared as described previously. Germinated seeds were transferred to alveoli and immediately inoculated at the rate of 109 cells seed−1 with 1 ml of bacterial inoculants that were previously grown for 48 h in a nutrient broth medium. A booster inoculation at the same rate was performed one week later while non-inoculated seedlings received the same volume of sterilized distilled water. Nine plants were provided for each treatment. They were watered once or twice a week with the nutritive solution of Hoagland and Arnon (1950). Plants were grown in a greenhouse for 6 months (April–October) under natural light (13.1–11.3 h daylight) and temperatures ranging between 12 and 15 °C (Tmin) and 21–24 °C (Tmax).
Plant harvest and measured parameters
At the end of the growing period, six plants per treatment were harvested for growth parameters measurements. Plants were carefully uprooted and roots were washed gently with tap water to remove the soil. Different parameters were measured: RDW: root dry weight (g plant−1), SDW: shoot dry weight (g plant−1), SL: average shoot length (cm), and RL: average root length (cm).
The estimation of photosynthetic pigments was determined according to protocols described by Pérez-Patricio et al. (2018) for chlorophyll a and b and Lichtenthaler and Wellburn (1983) for carotenoids.
Results
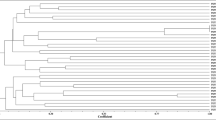
Among the collection of isolates obtained from root nodules of V. t. subsp. raddiana, 53 isolates were referred to as non-symbiotic nodule endophytic bacteria (NEB) according to the presence or absence of the nodC gene. They were subjected to REP-PCR fingerprinting for assessing their genetic diversity and grouping those showing a high percentage of similarity. In the present study, this parameter was fixed at 70%, which lead to the individualization of 23 different REP-PCR patterns among the isolates tested (Fig. 1).
Phylogenetic analysis of the 16S rRNA gene
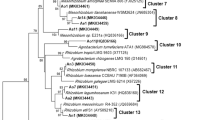
A total of 23 representative strains of the REP-PCR groups were identified by 16S rDNA sequencing. Sequences are available in GenBank database under the accession numbers shown in Table 1. Most of the sequences showed very high similarities (> 98%) with 16S rRNA gene sequences found in the NCBI database. Six strains were identified as closely related to 6 Bacillus species, 9 strains were linked to 4 Pseudomonas species, 2 strains were connected to 2 different Microbacterium species, 3 strains were related to Klebsiella sp., and one strain for each species Stenotrophomonas sp. and Streptomyces sp (Table 1).
A phylogenetic tree was constructed by using maximum likelihood from Mega. It shows that strains LMR700, LMR702, LMR703, LMR708, LMR715, LMR716, LMR717, LMR718, and LMR719 are related to the genus Pseudomonas, while strains LMR699, LMR701, LMR705, LMR706, LMR707, and LMR712 are linked to the Bacillus genus. Bacterial strains LMR714, LMR720, and LMR721 shared the same gene pool as Klebsiella pneumonaie (AB0Q4750). Similarly, LMR704, LMR709, and LMR711 and Microbacterium sp. strains were closely related to each other, while LMR713 and LMR710 represented each one a unique cluster (Fig. 2).
Maximum likelihood phylogeny of 16S rRNA gene sequences of endophytic strains isolated from Vachellia tortilis subsp. raddiana nodules. The analysis was based on 1461 nucleotides. Isolates are denoted in bold. Bootstrap values are indicated as percentages derived from 1000 replications. The tree is rooted with Planctomycetes bacterium strain Spb1
Plant beneficial properties and stress tolerance
PGP traits of nodule endophytic isolates
Among the 23 strains representing the NEB diversity, only some strains belonging to Pseudomonas and Bacillus genera showed higher results for the four main PGP activities (phosphate and potassium solubilization, auxin and siderophore production). The ones expressing at least three activities belonged to the genera Pseudomonas, Bacillus, Microbaterium, Klebsiella, Streptomyces, and Stenotrophomonas, while most isolates exhibited two important PGP traits: phosphate solubilization and IAA production. On the other hand, bacteria from the genus Klebsiella were the only ones able to solubilize two sources of zinc (zinc carbonate and zinc oxide) (Table 2).
The majority of the Pseudomonas isolates showed phosphate and potassium solubilization, auxin, and siderophores production, and only three isolates displayed just one activity (LMR710/LMR713/LMR715). Six Pseudomonas isolates out of nine fixed atmospheric nitrogen, a property that was confirmed by the presence of nifH gene in four isolates. Five isolates displayed ammonium production, and no isolate used ACC as a nitrogen source. Strains LMR700 and LMR701 showed a strong nitrogen fixation activity (NFB and nifH amplification), ammonia production, ACC deaminase, and chitinase activities. Only one isolate, Pseudomonas sp. LMR717, was able to solubilize zinc oxide.
Inside the Bacillus genus, half of the isolates solubilized phosphate, and two out of six showed positive potassium and auxin activities, whereas only one strain produced siderophores (LMR705) which were also able to solubilize zinc oxide. Two isolates (LMR706 and LMR712) had the ability to fix atmospheric nitrogen, but only the first one showed amplification of the nifH gene.
Most Microbacterium isolates displayed auxin, phosphate, and siderophore activities, whereas only one was able to fix nitrogen and possessed the nifH gene, as well as the ability to use ACC as a nitrogen source and to solubilize zinc oxide.
Klebsiella sp. strains showed generally positive results for siderophore and auxin production, phosphate and potassium solubilization, growth in NFB medium, and ACC deaminase activity. The isolate LMR720 was the only one in the collection that was able to solubilize two types of zinc (zinc oxide and zinc carbonate).
Quantification of phosphate solubilization activity demonstrated that all tested NEB isolates solubilized more than 20 µg m1−1, with LMR700 (Pseudomonas sp.) having the highest phosphate production (155.05 µg ml−1 equivalent K2HPO4), followed by Klebsiella sp. LMR714 (145.27 µg ml−1) (Table 2).
Quantification of IAA production showed that 7 isolates from the whole collection of NEB produced more than 20 µg ml−1 IAA, with isolate LMR709 (Microbacterium sp.) producing the highest amount (183.2 µg ml−1) (Table 2). It was followed by strain LMR700 (Pseudomonas sp.) with 75.64 µg ml−1 AIA.
Stress tolerance of nodule endophytic isolates
All Pseudomonas isolates were able to grow in the presence of 10% PEG. Reduction of growth ranged between 34.30% for LMR700 at 30% PEG and 94.30% for LMR708 at 10% PEG (Table 3). Pseudomonas isolates tolerated NaCl concentrations ranging between 4 and 6% and only one isolate was able to produce EPS (LMR703). Bacillus strains were less tolerant to osmotic stress, with a reduction of growth ranging from 70.91% to 92.48% at 10% PEG, but they were more tolerant to salt as they were able to tolerate NaCl concentrations ranging from 6 to 14%. Moreover, the majority of isolates (5 out of 6) produced EPS (Table 3).
All isolates of Klebsiella and Stenotrophomonas grew at the higher concentration of salt tested (14% NaCl). Under water stress Klebsiella isolates showed tolerance to 10 and 20% PEG with, respectively, 92.96% and 88.75% reduction of growth, while Stenotrophomonas strains were less tolerant with 88.39% of growth reduction at 10% PEG (Table 3).
Biocontrol activities
Evaluation of the ability of NEB isolates to inhibit the mycelial growth of two phytopathogenic fungi, Fusarium oxysporum and Botrytis cinerea, showed that isolates LMR701, LMR703, and LMR712 exhibited antagonistic activity against the 2 fungal species tested, whatever the testing method used (volatile substances, direct contact, and diffusible substances). Another strain, LMR713, had a similar potential except for direct contact antagonism activity against Fusarium oxysporum. In addition, LMR701 and LMR712 revealed at least two lytic enzyme activities, while LMR703 did not show any positive result (Table 4).
Global analysis of bacterial beneficial activities
The Venn diagram of PGP activities (Fig. 3a) showed that only one isolate possessed the 5 activities analyzed; however, the concerned strain (LMR702) had in general low levels of activity, as it is recorded in Table 2. The diagram showed also that 6 other isolates presented 4 PGP activities. The second Venn diagram grouping extracellular enzyme production and HCN activities (Fig. 3b) showed that no isolate possessed the whole activities analyzed, and only three strains presented 3 activities: isolates LMR720, LMR721, and LMR714 that presented cellulase and chitinase activities and produced HCN (additional information is provided in Table 4).
Venn diagrams showing the PGP activities (a), extracellular enzyme production and HCN (b) of a collection of 23 endophytic bacterial isolates obtained from Vachellia tortilis subsp. raddiana nodules (http://bioinformatics.psb.ugent.be/webtools/Venn/)
Effect of inoculation with selected strains on V. t. subsp. raddiana growth
Generally, inoculation with the 8 selected NEB strains mostly increased the dry biomass of the shoot and root parts of Vachellia plants compared to non-inoculated plants, with significant differences registered between some inoculation treatments. Moreover, each bacterial isolate promoted one or more growth parameters of the tested plants.
Strains LMR707 (Bacillus sp.) and LMR700 (Pseudomonas sp.) promoted significantly the dry weights of shoots (respectively, 87.19% and 75% increase), while only the second one displayed a significant increase in root dry weights by 89.52%. At the opposite, no significant differences were observed among the growth parameters of plants inoculated with the other isolates. Concerning shoot length, strains LMR713 (Streptomyces sp.), LMR700 (Pseudomonas sp.), and LMR712 (Bacillus sp.) exhibited a significant increase compared to the uninoculated plants. For root length, no significant differences were recorded between the different inoculants or between inoculated and uninoculated plants (Table 5).
Chlorophyll content measurements indicated that the majority of the bacterial inoculants induced a significant difference in chlorophyll a, chlorophyll b, and total chlorophyll. At the opposite they did not have any effect on the amount of carotenoids.
Based on the analysis of the two Heatmap diagrams of Fig. 4, it appears that there is no general positive correlation between the strain’s plant growth-promoting activities levels (phosphate and potassium solubilization, auxin and siderophores production) and the impact they had on plant growth under our experimental conditions. Only two higher PGP isolates exhibited a significant effect on the dry biomass of the shoots and/or roots, LMR700 (Pseudomonas sp.) that produced in vitro the highest amount of phosphate and an important amount of auxin, and LMR709 (Microbacterium sp.) that produced the highest amount of auxin.
Heatmap diagrams representing the plant growth-promoting traits (phosphate and potassium solubilization, auxin and siderophores production) of nodule endophytic bacteria of Vachellia tortilis subsp. raddiana and their effect on shoot and root dry weights and lengths (clustering and heat map generated by TBtools (Chen et al. 2020)
Discussion
The present research concerns the bacterial endophytes inhabiting root nodules of the desert leguminous tree V. t. subsp. raddiana. This type of endophytes was reported to coexist with rhizobia in nodules of many legume species, but little is known about those associated with Vachellia species. It is of particular interest to study and characterize these bacteria, especially their diversity, their tolerance to abiotic stresses, their PGP activities, and their possible effects on plant growth. However, isolation of the bacteria inhabiting root nodules from wild V. t. subsp. raddiana trees is hard, because nodules are not easily accessible and it is rare to find tree nodules in nature, and extremely difficult to provide proof that nodules highlighted in a soil belong really to the nearby tree (De Lajudie et al. 2003). In addition V. t. subsp. raddiana trees was reported to possess an immense root system which can reach depths of up to 30 m (Bensaid et al. 1996) and several nodulating strains were isolated from soils collected at − 32 m by De Lajudie et al. (2003). For this reasons, we adopted a trapping procedure, although this constitutes a bias compared to direct isolation from nodules taken from the field (De Lajudie et al. 2003). This method consisted in bringing soil samples taken around wild trees back to the laboratory, and to use seedlings obtained from sterilized seeds, taken also from the same desert area, for trapping the bacteria present in the soil. After several months of growth under greenhouse conditions, root nodules were collected and sterilized and the bacteria inside the nodules were isolated.
To distinguish between nodule endophytic bacteria and symbiotic rhizobia, we adopted nodC gene amplification as a filter; all isolates that failed to amplify this gene were considered as endophytes. Based on this criterion a total of 53 non-symbiotic isolates were obtained in pure culture from surface sterilized nodules of V. t. subsp. raddiana grown in soils of the Guelmim region in the southwest of Morocco. The genetic diversity of these isolates was studied by REP-PCR fingerprinting, a highly discriminating method that enables grouping the bacteria at the intraspecies level (Laguerre et al. 1997). A total of 23 different REP-PCR patterns were obtained and further identified by 16S rDNA sequencing. NEB strains were examined for their in vitro tolerance to salt and drought stresses, plant growth-promoting activities (auxin and siderophores synthesis, phosphate, zinc and potassium solubilization, and ACC deaminase activity), and biocontrol activities (antagonism against phytopathogenic fungi, HCN and lytic enzymes production). Finally, the beneficial impact of inoculation with elite selected strains on V. t. subsp. raddiana growth was studied under greenhouse conditions.
The phylogenetic tree constructed from 16S rDNA sequencing data indicated that the NEB isolated in this study belonged to three different phyla: Pseudomonadota, Actinomycetota, and Bacillota. The first phylum contained 13 isolates that belonged to the γ-class of the Pseudomonadota (Pseudomonas, Klebsiella, and Stenotrophomonas). Six strains were grouped within the Bacillota (Bacillus) and four within the Actinomycetota (Streptomyces and Microbacterium). However, affiliation at the species level did not confirm all the BLAST results. It was relevant that the majority of strains are closely related to different species of Pseudomonas and Bacillus, but their identification at the species level needs sequencing of more marker genes in particular housekeeping genes (Mulet et al. 2010; Rajendhran and Gunasekaran 2011).
It is relevant that the three phyla identified in this study are commonly found in the rhizosphere of plants (Roesch et al. 2007; Mendes et al. 2013). On the other hand, they were also reported to be predominant in different seed microbiomes such as rice (Matsumoto et al. 2021), wild cabbage (Tyc et al. 2020), and pumpkin (Adam et al. 2018). Thus, it is impossible to know with certainty whether nodule endophytes originated from the soil microbiota or from seed’s endophytes.
All the genera identified in our study were previously reported as nodule endophytes of different leguminous species. Bacillus species were isolated from nodules of chickpea (Joseph et al. 2007), pigeon pea (Rajendran et al. 2008), Lespedeza sp. (Palaniappan et al. 2010), peanut (Wang et al. 2013), and soybean (Zhao et al. 2018). Pseudomonas species were isolated from nodules of peanut, soybean, and bean (Aserse et al. 2013; Wang et al. 2013; Zhao et al. 2018; Dinić et al. 2015). Klebsiella was reported as NEB by Pandya et al. (2013), Streptomyces by Schrey and Tarkka (2008) and Sreevidya et al. (2016), Microbacterium by Hakim et al. (2020) and Maheshwari et al. (2022), and Stenotrophomonas by Velázquez et al. (2013), Boukhatem et al. (2016), Hakim et al. (2020), Muindi et al. (2021) and Rahal and Chekireb (2021).
PGP activities of Vachellia nodule endophytic bacteria
To our knowledge and until today, the study of Boukhatem et al. (2016) is the only one that reported some results about nodule endophytic bacteria isolated from desert Acacia species. They have identified the isolates and studied their tolerance to temperature and salinity, but they did not study their PGP activities and their potential impact on plants’ growth. The importance of these activities on plant growth and tolerance under harsh environmental conditions is of great importance. In fact, endophytic bacteria possess a direct plant growth-promoting potential through improving nutrient acquisition or mobilization, regulating or producing phytohormones, enhancing the antioxidant system, etc. (White et al. 2019). Thus, inoculation with these bacteria was reported as one of the most important sustainable practices to improve agricultural productivity (Sánchez-Cruz et al. 2019).
Minerals solubilization. In the present study, most strains of the genus Pseudomonas showed interesting levels of phosphate and potassium solubilization, with Pseudomonas sp. LMR700 producing the higher amount of soluble phosphate, followed by the strains of the genera Bacillus and Klebsiella. Isolates of Microbacterium solubilized inorganic phosphate moderately, but did not affect mica silicates (K source), while Streptomyces and Stenotrophomonas strains did not show any phosphate, potassium, or zinc solubilization activities. Different species of NEB were reported to be P solubilizers, such asBacillus megaterium LNL6 a root nodule bacterium isolated from Lespedeza sp. (Palaniappan et al. 2010). More generally bacterial strains belonging to Bacillus species (Biswas et al. 2018), Pseudomonas (Hussein and Joo 2015), Klebsiella and Microbacterium (Zhang et al. 2017), have been reported to be active inorganic phosphate solubilizers. At the opposite, K solubilization activity seems to be less present in endophytic rhizobacteria (Dhiman et al. 2019), and almost absent in nodule endophytes bacteria. For instance, in a study by Patel et al. (2017), none of over 50 endophytic banana rhizobacteria was associated with K solubilization despite the fact that strains showed solubilization abilities for other important plant nutrients. At the contrary, many authors reported that potassium solubilization is a common property of many rhizobacterial strains belonging to the genera Pseudomonas, Bacillus, and Klebsiella (Zhang and Kong 2014; Sattar et al. 2019), which is partially confirmed by our results.
The third mineral element tested for bacterial solubilization activity was zinc, an important microelement for plants nutrition. However, its availability in soils may differ according to the sources of this element present in soils and microbial solubilization activity. At this respect, different Zn sources were used in our study. Zinc oxide was solubilized by one isolate of each genus Bacillus, Pseudomonas, Microbacterium, and Klebsiella, while only strain LMR720 of Klebsiella sp. solubilized also zinc carbonate and none of the tested strains solubilized zinc sulfate. Similar results were reported for strains of these genera, like the endophytic soybean strain 1 J (Klebsiella sp.) and the summer mung bean rhizosphere Pseudomonas sp. strain 19D (Ramesh et al. 2014). Other authors reported that strains of Bacillus sp. and Pseudomonas fluorescens isolated from garden and paddy soils (Saravanan et al. 2011) and Pseudomonas aeruginosa isolated from wheat (Verma et al. 2015) were able to solubilize different Zn sources (ZnO, ZnSO4, and ZnCO3).
Auxin, siderophores, and ACC deaminase production. The synthesis of auxin by endophytic microbes is well known. For example, 34% of the endophytic bacteria isolated from two soybean cultivars produced IAA (Kuklinsky-Sobral et al. 2004) and the best producers were strains of Bacillus megaterium, Enterobacter asburiae, Pantoea agglomerans, and Rhizobium sp. In our study, the majority of the Pseudomonas isolates produced IAA while just two Bacillus isolates and two Microbacterium isolates possessed this activity. Similar results were reported for endophytic Pseudomonas and Bacillus strains isolated from soybean nodules (Kumawat et al. 2019) and rice endophytic bacteria (Ji et al. 2014).
Endophytic Pseudomonas sp. isolates of V. t. subsp. tortillis nodules were also excellent in producing siderophores (five out of nine strains). Similar results were reported for nodule endophytes of chickpea (Wani and Khan 2010) and soybean (Zhao et al. 2018). Other endophytic bacteria isolated from different plant species were also able to produce siderophores such as Pseudomonas strains isolated from rice (Yasmin et al. 2016), Pseudomonas and Bacillus wheat endophytes (Verma et al. 2015), and a Stenotrophomonas maltophilia endophytic wheat isolate (Youseif 2018). Siderophores producing strains belonging to the three precedent genera were also isolated from the rhizome of Zingiber officinale (Jasim et al. 2014).
Salt and osmotic stress tolerance, and exopolysaccharides production by vachellia nodule endophytic bacteria
All isolates tested were tolerant to water deficiency as they were able to grow in the presence of 10% PEG that mimics water stress; however, bacterial growth was reduced between 34.30% and 94.30%. The best tolerating strains to water stress were LMR701 (Bacillus sp), followed by LMR712 (Bacillus sp.) and LMR700 (Pseudomonas sp.). The endophytic strains tested were also able to tolerate different levels of salinity, and the higher one was 14% NaCl recorded for 6 strains belonging to three different genera: Stenotrophomonas sp. LMR710, Klebsiella strains LMR714, LMR720, and LMR721, and Bacillus strains LMR699 and LMR707. The majority of Bacillus isolates produced also exopolysaccharides that are known to be implicated in strain’s tolerance to different stresses. Among the other genera only one isolate of Pseudomonas (LMR703) and another of Stenotrophomonas (LMR710) possessed this property.
These results are promising, since this kind of strains can be used for plants inoculation under water and/or saline stress. In fact, it is known that stress-tolerant bacterial endophytes can provide tolerance and adaptation to plants under different environmental stresses such as drought, extreme temperatures, or high salinity (Chandra et al. 2018). Under salinity stress, it was reported that plant-associated salt-tolerant endophytic bacteria have the ability to alleviate the negative impacts of salt, even where salt-tolerant crop varieties and genotypes have not achieved much success (Kushwaha et al. 2020). Different Klebsiella strains were reported to confer enhanced tolerance to salinity and plant growth promotion in different species (Triticum aestivum L. Singh et al. (2015) and Avena sativa L. seedlings (Sapre et al. 2018)). Concerning non-rhizobial endophytic bacteria, the Klebsiella sp. A36 was found to be more tolerant to salt stress than rhizobial strains, and it increased the nitrogen content of alfalfa to a greater extent than the symbiotic bacteria Ensifer meliloti ARh29 (Noori et al. 2018). Relevant results were also reported for Bacillus strains isolated from chickpea nodules (Bacillus cereus NUU1, Achromobacter xylosoxidans NUU2, Bacillus thuringiensis, NUU3, and Bacillus subtilis NUU4) that induced a good improvement of chickpea–rhizobia symbiotic performance and plant growth in pots under saline soil conditions (Egamberdieva et al. 2017).
Antagonistic activity of vachellia nodule endophytic bacteria
Since endophytes share their ecological niches with many microorganisms colonizing plants, direct antagonism may happen in cells and tissues with different degrees of association. In vitro proof of the phenomenon is constant, with few direct inhibitions of pathogens that are mainly mediated by the synthesis of antibiotics, production of volatile hydrogen cyanide (HCN), and antifungal metabolites (Raaijmakers et al. 2010). Among the bacterial endophytes tested in our study, isolates, LMR701 (Bacillus sp.), LMR703 (Pseudomonas sp.), LMR712 (Bacillus sp.), and LMR713 (Streptomyces sp.) exhibited antagonistic activities against both fungal species tested (F. oxysporum and B. cinerea). Similar results were found with different Paenibacillus strains that displayed antagonistic activities against five phytopathogenic fungi (F. graminearum, Magnaporthe oryzae, Rhizoctonia solani, Sclerotinia sclerotiorum, and B. cinerea) and were able to synthesize in vitro many hydrolytic enzymes, siderophores, and the lipopeptide fusaricidin (Ali et al. 2021).
This type of trains may reduce considerably plant disease, as demonstrated by the B. subtilis strain NUU4, a non-rhizobial endophytic bacterium isolated from the root nodules of chickpea that reduced diseased plants by 8%, whereas Fusarium-infected control plants showed 27% diseased plants (Egamberdieva et al. 2017). Other studies indicated that endophytic bacterial strains with significant lytic enzymes activities may also play an important role in protecting plants against pathogenic fungi such as B. cinerea, F. oxysporum, Pythium ultimum, Phytophthora sp., and Rhizoctonia solani (Yasmin et al. 2016; Chen et al. 2020).
Effect of inoculation with nodule endophytic selected strains on Vachellia plants growth
The characterization of the collection of NEB isolated from V. t. subsp. raddiana nodules allowed the selection of eight strains that were inoculated to seedlings of this tree species grown under greenhouse conditions. Among them, strains LMR707 (Bacillus sp.), LMR700 (Pseudomonas sp.), LMR712 (Bacillus sp.), and LMR709 (Microbacterium sp.) induced important increases in shoot dry weights ranging from 87.19% for the first strain to 40.59% for the last one. The improvement of growth parameters was confirmed by the plants chlorophyll content with significant differences in chlorophyll a, chlorophyll b, and total chlorophyll.
Similar increases in growth parameters were reported by different authors and especially when using Bacillus strains. This genus is among the most studied regarding its ubiquity and the multiple growth-promoting traits shown by the strains (Kohler et al. 2007; Ramírez and Kloepper 2010). For example, increases in plant’s growth parameters of cowpea (Vigna unguiculata) inoculated by Bacillus sp. were reported by (Minaxi et al. 2012). Similarly, inoculation with Bacillus aryabhattai strains MDSR7 and MDSR14 significantly increased shoot and seed weights, Zn uptake/assimilation as compared to un-inoculated control in soybean and wheat crops (Ramesh et al. 2014). Bacillus thuringiensis imparts also good effects on plant development as a PGP bacteria, especially an increase in dry matter in peanut plants (Wang et al. 2014), in addition to its valuable known biotechnological applications (Jouzani et al. 2017).
Strains belonging to different species of Pseudomonas were also reported to be very performing when inoculated to different plants. P. fluorescens and P. putida strains were reported to be effective in increasing nutrient uptake in paddy fields of rice (Lavakush et al. 2014). A P. reinekei strain also showed a positive effect on root parameters (roots diameter and biomass) which influenced growth and chlorophyll content in durum wheat (Elhaissoufi et al. 2020). In another study, the strain MAIIF2a of Microbacterium imperiale induced increased shoot and root growth of Cassava (Manihot esculenta) by over 100% compared to uninoculated controls (Freitas et al. 2019).
To our knowledge, this is the first report on the impact of Vachellia nodule endophytic strains on the growth of their original host plant. On the other hand, our finding confirms previous results concerning the potential of endophytic bacteria to improve plant growth (Egamberdieva et al. 2017; Abd Allah et al. 2018). Moreover, endophytes isolated from the same plants or closely related plants seem to be superior in promoting plant growth (Long et al. 2008). The better performance of endophytes compared to rhizospheric bacteria may be due to the fact that they have the ability to communicate or interact more effectively with their host plants (Coutinho et al. 2015). However, in the case of nodule endophytic bacteria, even if they are considered nowadays as normal inhabitants of many leguminous species nodules (Zhao et al. 2018), numerous questions still to be resolved, like mechanisms by which they penetrate nodules, their possible impact on nodule functioning, or their possible role in plant growth especially under stressful conditions prevailing in desert regions. A deep genomic analyses of these bacteria (genes functional analysis) and genome comparison (pangenome and phylogeny of core genes) may provide insights into the mechanisms of action of nodule endophytic bacteria that confer benefits to rhizobia–legume symbiosis, as well as their involvement in legume plant growth and adaptation.
Conclusion
The present study showed that the aridity-tolerant tree V. t. subsp. raddiana, a leguminous species largely distributed in desert and Saharan regions of Africa, harbor diverse endophytic bacteria in its root nodules, in addition to symbiotic rhizobia. These endophytes belong to three phyla: Pseudomonadota, Actinomycetota, and Bacillota, and at least six genera. The dominating genera are Pseudomonas and Bacillus, while Klebsiella, Microbacterium, Stenotrophomonas, and Streptomyces were less represented. Most of the isolated endophytes exhibited in vitro important PGP traits, high tolerance to salt and drought stresses, and/or interesting biocontrol potential. Four elite strains displaying a good potential for improving the growth of vachellia plants under controlled conditions were identified. These strains should be considered promising candidates for developing biofertilizers for V. t. subsp. raddiana to be reintroduced under the increasingly stressful conditions prevailing in the natural areas of distribution of this tree species.
Data availability statement
All gene sequences used are available in Genbank database, while the accession numbers and the other data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Abd Allah EF, Alqarawi AA, Hashem A et al (2018) Endophytic bacterium Bacillus subtilis (BERA 71) improves salt tolerance in chickpea plants by regulating the plant defense mechanisms. J Plant Interact 13:37–44. https://doi.org/10.1080/17429145.2017.1414321
Abdallah F, Noumi Z, Touzard B et al (2008) The influence of Acacia tortilis (Forssk.) Subsp. raddiana (Savi) and livestock grazing on grass species composition, yield and soil nutrients in arid environments of south Tunisia. Flora Morphol Distrib Funct Ecol Plants 203:116–125. https://doi.org/10.1016/j.flora.2007.02.002
Abdallah F, Noumi Z, Ouled-Belgacem A et al (2012) The influence of Acacia tortilis (Forssk.) ssp. raddiana (Savi) Brenan presence, grazing, and water availability along the growing season, on the understory herbaceous vegetation in southern Tunisia. J Arid Environ 76:105–114. https://doi.org/10.1016/j.jaridenv.2011.06.002
Adam E, Bernhart M, Müller H, Winkler J, Berg G (2018) The Cucurbita pepo seed microbiome: genotype-specific composition and implications for breeding. Plant Soil 422:35–49
Ahemad M, Khan M (2012) Assessment of plant growth promoting activities of rhizobacterium Pseudomonas putida under insecticide-stress. Ann Microbiol 62:1531–1540. https://doi.org/10.1007/s13213-011-0407-2
Ali MA, Lou Y, Hafeez R et al (2021) Functional analysis and genome mining reveal high potential of biocontrol and plant growth promotion in nodule-inhabiting bacteria within Paenibacillus polymyxa complex. Front Microbiol 11:618601. https://doi.org/10.3389/fmicb.2020.618601
Arora N, Verma M (2017) Modified microplate method for rapid and efficient estimation of siderophore produced by bacteria. 3 Biotech. https://doi.org/10.1007/s13205-017-1008-y
Aserse AA, Räsänen LA, Aseffa F, Hailemariam A, Lindström K (2013) Diversity of sporadic symbionts and nonsymbiotic endophytic bacteria isolated from nodules of woody, shrub, and food legumes in Ethiopia. Appl Microbiol Biotechnol 97(23):10117–10134. https://doi.org/10.1007/s00253-013-5248-4
Bensaid S, Ait Mohand L, Echaib B (1996) Evolution spatio-temporelle des peuplements d’Acacia tortilis (Forsk.) Hayne raddiana (Savi) Brenan dans les monts Ougarta (Sahara nord-occidental). Sécheresse 7(3):173–178
Bharali S, Khan ML (2011) Climate change and its impact on biodiversity; some management options for mitigation in Arunachal Pradesh. Curr Sci 101(7):855–860
Biswas JK, Banerjee A, Rai MK et al (2018) Exploring potential applications of a novel extracellular polymeric substance synthesizing bacterium (Bacillus licheniformis) isolated from gut contents of earthworm (Metaphire posthuma) in environmental remediation. Biodegradation 29:323–337. https://doi.org/10.1007/s10532-018-9835-z
Boukhatem ZF, Merabet C, Bekki A, Sekkour S, Domergue O, Dupponois R, Galiana A (2016) Nodular bacterial endophyte diversity associated with native Acacia spp. in desert region of Algeria. Afr J Microbiol Res 10(18):634–645
Boutaj H, Moumni A, Nassiri O, Aitouna AO (2019) Climate change impacts on biodiversity in arid and semi-arid areas: biodiversity under climate change. In: Research anthology on environmental and societal impacts of climate change. IGI Global, pp 578–602. Accessed 16 Aug 2022
Cappucino JG, Sherman N (1992) Microbiology: a laboratory manual. The Benyamin/Cummings Publ Co Inc, New York
Chandra D, Srivastava R, Glick BR, Sharma AK (2018) Drought-tolerant Pseudomonas spp. improve the growth performance of finger millet (Eleusine coracana (L.) Gaertn.) under non-stressed and drought-stressed conditions. Pedosphere 28:227–240. https://doi.org/10.1016/S1002-0160(18)60013-X
Chandran H, Meena M, Swapnil P (2021) Plant growth-promoting rhizobacteria as a green alternative for sustainable agriculture. Sustainability 13:10986. https://doi.org/10.3390/su131910986
Chen W, Kuo T-T (1993) A simple and rapid method for the preparation of gram-negative bacterial genomic DNA. Nucleic Acids Res 21:2260. https://doi.org/10.1093/nar/21.9.2260
Chen C, Chen H, Zhang Y et al (2020) TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant 13:1194–1202. https://doi.org/10.1016/j.molp.2020.06.009
Coutinho BG, Licastro D, Mendonça-Previato L et al (2015) Plant-influenced gene expression in the rice endophyte Burkholderia kururiensis M130. MPMI 28:10–21. https://doi.org/10.1094/MPMI-07-14-0225-R
de Bary A (1866) Morphologie und Physiologie der Pilze, Flechten und Myxomyceten. Hofmeister’s handbook of physiological botany, vol 2. W. Engelmann, Leipzig
De Bruijn FJ (1992) Use of repetitive (repetitive extragenic palindromic and enterobacterial repetitive intergeneric consensus) sequences and the polymerase chain reaction to fingerprint the genomes of Rhizobium meliloti isolates and other soil bacteria. Appl Environ Microbiol 58:2180–2187. https://doi.org/10.1128/aem.58.7.2180-2187.1992
De Lajudie P, Dreyfus B, Boivin C, Ba S, N’diaye A, Lorquin J, Neyra M, Detrez C, Willems A, Gillis M, Jeder H, Promé J-C (2003) Diversité taxonomique et propriétés symbiotiques des rhizobia nodulant Acacia raddiana au nord et au sud du. Sahara In Un arbre au désert Acacia raddiana. Editeurs Grouzis M Le Floch E IRD Editions, Paris, p 148
De Meyer SE, De Beuf K, Vekeman B, Willems A (2015) A large diversity of non-rhizobial endophytes found in legume root nodules in Flanders (Belgium). Soil Biol Biochem 83:1–11. https://doi.org/10.1016/j.soilbio.2015.01.002
Dhiman S, Dubey RC, Baliyan N et al (2019) Application of potassium-solubilising Proteus mirabilis MG738216 inhabiting cattle dung in improving nutrient use efficiency of Foeniculum vulgare Mill. Environ Sustain. https://doi.org/10.1007/s42398-019-00088-8
Dinić Z, Ugrinović M, Bosnic P et al (2015) Solubilization of inorganic phosphate by endophytic Pseudomonas sp. from French bean nodules. Ratar Povrt 51:100–105. https://doi.org/10.5937/ratpov51-6222
Dworkin M, Foster J (1958) Experiments with some microorganisms which utilize ethane and hydrogen. J Bacteriol 75:592–603. https://doi.org/10.1128/jb.75.5.592-603.1958
Egamberdieva D, Wirth SJ, Shurigin VV et al (2017) Endophytic bacteria improve plant growth, symbiotic performance of chickpea (Cicer arietinum L.) and induce suppression of root rot caused by Fusarium solani under salt stress. Front Microbiol 8:1887. https://doi.org/10.3389/fmicb.2017.01887
Ehmann AK (1977) The van urk-Salkowski reagent–a sensitive and specific chromogenic reagent for silica gel thin-layer chromatographic detection and identification of indole derivatives. J Chromato 132(2):267–276. https://doi.org/10.1016/S0021-9673(00)89300-0
Elhaissoufi W, Khourchi S, Ibnyasser A et al (2020) Phosphate solubilizing rhizobacteria could have a stronger influence on wheat root traits and aboveground physiology than rhizosphere P solubilization. Front Plant Sci 11:979. https://doi.org/10.3389/fpls.2020.00979
Elvira-Recuenco M, van Vuurde JWL (2000) Natural incidence of endophytic bacteria in pea cultivars under field conditions. Can J Microbiol 46:1036–1041. https://doi.org/10.1139/w00-098
Freitas MA, Medeiros FHV, Melo IS et al (2019) Stem inoculation with bacterial strains Bacillus amyloliquefaciens (GB03) and Microbacterium imperiale (MAIIF2a) mitigates Fusarium root rot in cassava. Phytoparasitica 47:135–142. https://doi.org/10.1007/s12600-018-0706-2
Gandhi A, Muralidharan G (2016) Assessment of zinc solubilizing potentiality of Acinetobacter sp. isolated from rice rhizosphere. Eur J Soil Biol 76:1–8. https://doi.org/10.1016/j.ejsobi.2016.06.006
Gedda AE (2003) Rangeland evaluation in relation to pastoralists' perceptions in the middle Awash valley of Ethiopia. Thesis, University of the Free State. https://hdl.handle.net/11660/6302. Accessed 16 Aug 2022
Giller KE, Cadisch G (1995) Future benefits from biological nitrogen fixation: an ecological approach to agriculture. In: Ladha JK, Peoples MB (eds) Management of biological nitrogen fixation for the development of more productive and sustainable agricultural systems. Springer, Netherlands, Dordrecht, pp 255–277
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Scientifica 2012:1–15. https://doi.org/10.6064/2012/963401
Goswami D, Dhandhukia P, Patel P, Thakker JN (2014) Screening of PGPR from saline desert of Kutch: Growth promotion in Arachis hypogea by Bacillus licheniformis A2. Microbiol Res 169:66–75. https://doi.org/10.1016/j.micres.2013.07.004
Gordon SA, Weber RP (1951) Colorimetric estimation of indoleacetic acid. Plant Physiol 26:192–195. https://doi.org/10.1104/pp.26.1.192
Hakim S, Mirza BS, Imran A, Zaheer A, Yasmin S, Mubeen F, Mclean JE, Mirza MS (2020) Illumina sequencing of 16S rRNA tag shows disparity in rhizobial and non-rhizobial diversity associated with root nodules of mung bean (Vigna radiata L.) growing in different habitats in Pakistan. Microbiol Res 231:126356. https://doi.org/10.1016/j.micres.2019.126356
[HCP] High Commission for the Moroccan Plan (2021) Monographie de la region Guelmim Essemara [The monograph of the region Guelmim Essemara]. http://www.Hcp.ma. Accessed 14 Apr 2021
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Circular 347, california agricultural experiment station. The college of Agriculture University of California, Berkley
Hu X, Chen J, Guo J (2006) Two phosphate- and potassium-solubilizing bacteria isolated from Tianmu mountain, Zhejiang, China. World J Microbiol Biotechnol 22:983–990. https://doi.org/10.1007/s11274-006-9144-2
Ibáñez F, Tonelli ML, Muñoz V, Figueredo MS, Fabra A (2017) Bacterial endophytes of plants: diversity, invasion mechanisms and effects on the host. In: Maheshwari D (ed) Endophytes: biology and biotechnology. Sustainable development and biodiversity, vol 15. Springer, Cham, pp 25–40
Jasim B, Joseph AA, John CJ et al (2014) Isolation and characterization of plant growth promoting endophytic bacteria from the rhizome of Zingiber officinale. 3 Biotech 4:197–204. https://doi.org/10.1007/s13205-013-0143-3
Jeffries P, Barea JM (2012) Arbuscular mycorrhiza: a key component of sustainable plant–soil ecosystems. In: Hock B (ed) Fungal associations. Springer, Berlin Heidelberg, pp 51–75. https://doi.org/10.1007/978-3-642-30826-0_4
Ji SH, Gururani MA, Chun S-C (2014) Isolation and characterization of plant growth promoting endophytic diazotrophic bacteria from Korean rice cultivars. Microbiol Res 169:83–98. https://doi.org/10.1016/j.micres.2013.06.003
Joseph B, Ranjan Patra R, Lawrence R (2007) Characterization of plant growth promoting rhizobacteria associated with chickpea (Cicer arietinum L.). Int J Plant Prod 1(2)141–152. https://doi.org/10.22069/ijpp.2012.532
Jouzani GS, Valijanian E, Sharafi R (2017) Bacillus thuringiensis: a successful insecticide with new environmental features and tidings. Appl Microbiol Biotechnol 101:2691–2711. https://doi.org/10.1007/s00253-017-8175-y
Khanghahi MY, Ricciuti P, Allegretta I et al (2018) Solubilization of insoluble zinc compounds by zinc solubilizing bacteria (ZSB) and optimization of their growth conditions. Environ Sci Pollut Res 25:25862–25868. https://doi.org/10.1007/s11356-018-2638-2
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120. https://doi.org/10.1007/BF01731581
Kohler J, Caravaca F, Carrasco L, Roldán A (2007) Interactions between a plant growth-promoting rhizobacterium, an AM fungus and a phosphate-solubilising fungus in the rhizosphere of Lactuca sativa. Appl Soil Ecol 35:480–487. https://doi.org/10.1016/j.apsoil.2006.10.006
Kour D, Kaur T, Devi R et al (2020) Biotechnological applications of beneficial microbiomes for evergreen agriculture and human health. New and future developments in microbial biotechnology and bioengineering. Elsevier, Amsterdam, pp 255–279. https://doi.org/10.1016/B978-0-12-820528-0.00019-3
Kuklinsky-Sobral J, Araujo WL, Mendes R et al (2004) Isolation and characterization of soybean-associated bacteria and their potential for plant growth promotion. Environ Microbiol 6:1244–1251. https://doi.org/10.1111/j.1462-2920.2004.00658.x
Kumar V, Pathak D, Dudeja SS et al (2013) Legume nodule endophytes more diverse than endophytes from roots of legumes or non legumes in soil. J Microbiol Biotech Res 3:383–392
Kumar S, Stecher G, Li M et al (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Kumawat KC, Sharma P, Sirari A et al (2019) Synergism of Pseudomonas aeruginosa (LSE-2) nodule endophyte with Bradyrhizobium sp. (LSBR-3) for improving plant growth, nutrient acquisition and soil health in soybean. World J Microbiol Biotechnol 35:47. https://doi.org/10.1007/s11274-019-2622-0
Kushwaha P, Kashyap PL, Kuppusamy P et al (2020) Functional characterization of endophytic bacilli from pearl millet (Pennisetum glaucum) and their possible role in multiple stress tolerance. Plant Biosyst Int J Deal Asp Plant Biol 154:503–514. https://doi.org/10.1080/11263504.2019.1651773
Laguerre G, van Berkum P, Amarger N, Prévost D (1997) Genetic diversity of rhizobial symbionts isolated from legume species within the genera Astragalus, Oxytropis, and Onobrychis. Appl Environ Microbiol 63:4748–4758. https://doi.org/10.1128/aem.63.12.4748-4758.1997
Laguerre G, Nour SM, Macheret V et al (2001) Classification of rhizobia based on nodC and nifH gene analysis reveals a close phylogenetic relationship among Phaseolus vulgaris symbionts. Microbiology 147:981–993. https://doi.org/10.1099/00221287-147-4-981
Lavakush YJ, Verma JP et al (2014) Evaluation of PGPR and different concentration of phosphorus level on plant growth, yield and nutrient content of rice (Oryza sativa). Ecol Eng 62:123–128. https://doi.org/10.1016/j.ecoleng.2013.10.013
Leite J, Fischer D, Rouws L et al (2017) Cowpea nodules harbor non-rhizobial bacterial Communities that are shaped by soil type rather than plant genotype. Front Plant Sci. https://doi.org/10.3389/fpls.2016.02064
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592. https://doi.org/10.1042/bst0110591
Liu J, Jia R, Zhou E et al (2018) Antimicrobial Cu-bearing 2205 duplex stainless steel against MIC by nitrate reducing Pseudomonas aeruginosa biofilm. Int Biodeterior Biodegrad 132:132–138. https://doi.org/10.1016/j.ibiod.2018.03.002
Long HH, Schmidt DD, Baldwin IT (2008) Native bacterial endophytes promote host growth in a species-specific manner; phytohormone manipulations do not result in common growth responses. PLoS ONE 3:e2702. https://doi.org/10.1371/journal.pone.0002702
Louden BC, Haarmann D, Lynne AM (2011) Use of blue agar CAS assay for siderophore detection. J Microbiol Biol Educ 12:51–53. https://doi.org/10.1128/jmbe.v12i1.249
Mahamane L, Mahamane S (2005) Biodiversity of ligneous species in semi-arid to arid zones of southwestern Niger according to anthropogenic and natural factors. Agric Ecosyst Environ 105:267–271. https://doi.org/10.1016/j.agee.2004.03.004
Maheshwari R, Kumar P, Bhutani N, Suneja P (2022) Exploration of plant growth-promoting endophytic bacteria from Pisum sativum and Cicer arietinum from South-West Haryana. J Gen Microbiol 62(7):857–874. https://doi.org/10.1002/jobm.202100575
Malakootian M, Mahvi AH, Mansoorian HJ, Khanjani N (2018) Agrowaste based ecofriendly bio-adsorbent for the removal of phenol: adsorption and kinetic study by Acacia Tortilis Pod Shell. Chiang Mai J Sci 45(1):355–368
Matsumoto H, Fan X, Wang Y, Kusstatscher P, Duan J, Wu S, Chen S, Qiao K, Wang Y, Ma B (2021) Bacterial seed endophyte shapes disease resistance in rice. Nat Plants 7:60–72
Mendes R, Garbeva P, Raaijmakers JM (2013) The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol Rev 37:634–663. https://doi.org/10.1111/1574-6976.12028
Michel BE, Kaufmann MR (1973) The osmotic potential of polyethylene glycol 6000 1. Plant Physiol 51:914–916. https://doi.org/10.1104/pp.51.5.914
Miller RL, Higgins BB (1970) Association of cyanide with infection of birdsfoot trefoil by Stemphylium loti. Phytopathology 60:104. https://doi.org/10.1094/Phyto-60-104
Minaxi NL, Yadav RC, Saxena J (2012) Characterization of multifaceted Bacillus sp. RM-2 for its use as plant growth promoting bioinoculant for crops grown in semi arid deserts. Appl Soil Ecol 59:124–135. https://doi.org/10.1016/j.apsoil.2011.08.001
Msanda F, El Aboudi A, Peltier JP (2002) Originalité de la flore et de la végétation de l’Anti-Atlas sud-occidental (Maroc). Feddes Repert 113:603–615. https://doi.org/10.1002/fedr.200290008
Muindi MM, Muthini M, Njeru EM, Maingi J (2021) Symbiotic efficiency and genetic characterization of rhizobia and non rhizobial endophytes associated with cowpea grown in semi-arid tropics of Kenya. Heliyon 7(4):e06867. https://doi.org/10.1016/j.heliyon.2021.e06867
Mulet M, Lalucat J, García-Valdés E (2010) DNA sequence-based analysis of the Pseudomonas species. Environ Microbiol 12(6):1513–1530. https://doi.org/10.1111/j.1462-2920.2010.02181.x
Noori F, Etesami H, Zarini HN, Khoshkholgh-Sima NA, Salekdeh GH, Alishahi F (2018) Mining alfalfa (Medicago sativa L.) nodules for salinity tolerant non-rhizobial bacteria to improve growth of alfalfa under salinity stress. Ecotoxicol Environ Saf 162:129–138
Okonechnikov K, Golosova O, Fursov M (2012) Unipro UGENE: a unified bioinformatics toolkit. Bioinformatics 28:1166–1167. https://doi.org/10.1093/bioinformatics/bts091
Palaniappan P, Chauhan PS, Saravanan VS et al (2010) Isolation and characterization of plant growth promoting endophytic bacterial isolates from root nodule of Lespedeza sp. Biol Fertil Soils 46(8):807–816. https://doi.org/10.1007/s00374-010-0485-5
Palaniyandi SA, Damodharan K, Yang SH, Suh JW (2014) Streptomyces sp. strain PGPA39 alleviates salt stress and promotes growth of ‘Micro Tom’ tomato plants. J Appl Microbiol 117:766–773. https://doi.org/10.1111/jam.12563
Pandey P, Sahgal M, Maheshwari D, Johri BN (2004) Genetic diversity of rhizobia isolated from medicinal legumes growing in the sub-Himalayan region of Uttaranchal. Curr Sci 86:202–207
Pandya M, Naresh Kumar G, Rajkumar S (2013) Invasion of rhizobial infection thread by non-rhizobia for colonization of Vigna radiata root nodules. FEMS Microbiol Lett 348(1):58–65. https://doi.org/10.1111/1574-6968.12245
Park M, Kim C, Yang J et al (2005) Isolation and characterization of diazotrophic growth promoting bacteria from rhizosphere of agricultural crops of Korea. Microbiol Res 160:127–133. https://doi.org/10.1016/j.micres.2004.10.003
Patel SV, Sharma H, Vaja S (2017) Effect of Banana Pseudostem sap and P. fluorescens on mango diseases in field condition. Int J Curr Microbiol Appl Sci 6:514–521. https://doi.org/10.20546/ijcmas.2017.611.062
Paulo EM, Boffo EF, Branco A et al (2012) Production, extraction and characterization of exopolysaccharides produced by the native Leuconostoc pseudomesenteroides R2 strain. An Acad Bras Cienc 84:495–508. https://doi.org/10.1590/s0001-37652012000200018
Payne SM (1993) Iron acquisition in microbial pathogenesis. Trends Microbiol 1:66–69. https://doi.org/10.1016/0966-842x(93)90036-q
Peix A, Ramírez-Bahena MH, Velázquez E, Bedmar EJ (2015) Bacterial associations with legumes. Crit Rev Plant Sci 34:17–42. https://doi.org/10.1080/07352689.2014.897899
Pérez-Patricio M, Camas-Anzueto J, Sanchez-Alegría A et al (2018) Optical method for estimating the chlorophyll contents in plant leaves. Sensors 18:650. https://doi.org/10.3390/s18020650
Pikovskaya RI, Pikovskaya RI (1948) Mobilization of phosphorus in soil in connection with the vital activity of some microbial species. Microbiology 17:362–370
Raaijmakers JM, De Bruijn I, Nybroe O, Ongena M (2010) Natural functions of lipopeptides from Bacillus and Pseudomonas : more than surfactants and antibiotics. FEMS Microbiol Rev 34:1037–1062. https://doi.org/10.1111/j.1574-6976.2010.00221.x
Rahal S, Chekireb D (2021) Diversity of rhizobia and non-rhizobia endophytes isolated from root nodules of Trifolium sp. growing in lead and zinc mine site Guelma Algeria. Arch Microbiol 203(7):3839–3849. https://doi.org/10.1007/s00203-021-02362-y
Rahmansyah M (2013) Endophytic fungi isolated from Mangrove plant and have antagonism role against Fusarium Wilt. J Agric Biol Sci 8(3):251–257
Rajawat MVS, Singh S, Tyagi SP, Saxena AK (2016) A modified plate assay for rapid screening of potassium-solubilizing bacteria. Pedosphere 26:768–773. https://doi.org/10.1016/S1002-0160(15)60080-7
Rajendhran J, Gunasekaran P (2011) Microbial phylogeny and diversity: small subunit ribosomal RNA sequence analysis and beyond. Microbiol Res 166(2):99–110. https://doi.org/10.1016/j.micres.2010.02.003
Rajendran G, Sing F, Desai AJ, Archana G (2008) Enhanced growth and nodulation of pigeon pea by co-inoculation of Bacillus strains with Rhizobium spp. Biores Technol 99(11):4544–4550. https://doi.org/10.1016/j.biortech.2007.06.057
Ramesh A, Sharma SK, Sharma MP et al (2014) Inoculation of zinc solubilizing Bacillus aryabhattai strains for improved growth, mobilization and biofortification of zinc in soybean and wheat cultivated in Vertisols of central India. Appl Soil Ecol 73:87–96. https://doi.org/10.1016/j.apsoil.2013.08.009
Ramírez CA, Kloepper JW (2010) Plant growth promotion by Bacillus amyloliquefaciens FZB45 depends on inoculum rate and P-related soil properties. Biol Fertil Soils 46:835–844. https://doi.org/10.1007/s00374-010-0488-2
Rana KL, Kour D, Kaur T et al (2020) Endophytic fungi from medicinal plants: biodiversity and biotechnological applications. Microbial Endophytes. Elsevier, Amsterdam, pp 273–305
Revillas JJ, Rodelas B, Pozo C et al (2000) Production of B-group vitamins by two Azotobacter strains with phenolic compounds as sole carbon source under diazotrophic and adiazotrophic conditions. J Appl Microbiol 89:486–493. https://doi.org/10.1046/j.1365-2672.2000.01139.x
Roesch LFW, Fulthorpe RR, Riva A, Casella G, Hadwin AKM, Kent AD, Daroub SH, Camarg FAO, Farmerie WG, Triplett EW (2007) Pyrosequencing enumerates and contrasts soil microbial diversity. ISME J 1:283–290. https://doi.org/10.1038/ismej.2007.53
Sakrouhi I, Belfquih M, Sbabou L et al (2016) Recovery of symbiotic nitrogen fixing acacia rhizobia from Merzouga Desert sand dunes in south east Morocco—identification of a probable new species of Ensifer adapted to stressed environments. Syst Appl Microbiol 39:122–131. https://doi.org/10.1016/j.syapm.2016.01.001
Sánchez-Cruz R, Vázquez IT, Batista-García RA et al (2019) Isolation and characterization of endophytes from nodules of Mimosa pudica with biotechnological potential. Microbiol Res 218:76–86. https://doi.org/10.1016/j.micres.2018.09.008
Sandhya V, Sk ZA, Grover M et al (2009) Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-P45. Biol Fertil Soils 46:17–26. https://doi.org/10.1007/s00374-009-0401-z
Sapre S, Gontia-Mishra I, Tiwari S (2018) Klebsiella sp. confers enhanced tolerance to salinity and plant growth promotion in oat seedlings (Avena sativa). Microbiol Res 206:25–32. https://doi.org/10.1016/j.micres.2017.09.009
Saravanan VS, Kumar MR, Sa TM (2011) Microbial zinc solubilization and their role on plants. In: Maheshwari DK (ed) Bacteria in agrobiology: plant nutrient management. Springer, Berlin, pp 47–63. https://doi.org/10.1007/978-3-642-21061-7_3
Sattar A, Naveed M, Ali M, Zahir ZA, Nadeem SM, Yaseen M, Meena VS, Farooq M, Singh R, Rahman M, Meena HN (2019) Perspectives of potassium solubilizing microbes in sustainable food production system: a review. Appl Soil Ecol 133:146–159. https://doi.org/10.1016/j.apsoil.2018.09.012
Schrey SD, Tarkka MT (2008) Friends and foes: streptomycetes as modulators of plant disease and symbiosis. Antonie Van Leeuwenhoek 94(1):11–19. https://doi.org/10.1007/s10482-008-9241-3
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56. https://doi.org/10.1016/0003-2697(87)90612-9
Sharma P, Kumawat KCK, Kaur S, Kaur N (2012) Assessment of zinc solubilization by endophytic bacteria in legume rhizosphere. IJAR 4(6):439–441. https://doi.org/10.15373/2249555X/June2014/137
Singh RP, Jha P, Jha PN (2015) The plant-growth-promoting bacterium Klebsiella sp. SBP-8 confers induced systemic tolerance in wheat (Triticum aestivum) under salt stress. J Plant Physiol 184:57–67. https://doi.org/10.1016/j.jplph.2015.07.002
Skujins JJ, Potgieter HJ, Alexander M (1965) Dissolution of fungal cell walls by a streptomycete chitinase and β-(1→ 3) glucanase. Arch Biochem Biophys 111:358–364
Sreevidya M, Gopalakrishnan S, Kudapa H, Varshney RK (2016) Exploring plant growth-promotion actinomycetes from vermicompost and rhizosphere soil for yield enhancement in chickpea. Braz J Microbiol 47(1):85–95. https://doi.org/10.1016/j.bjm.2015.11.030
Strobel G, Daisy B (2003) Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol Rev 67:491–502. https://doi.org/10.1128/MMBR.67.4.491-502.2003
Tandon HLS, Cescas MP, Tyner EH (1968) An acid-free vanadate-molybdate reagent for the determination of total phosphorus in soils. Soil Sci Soc Am J 32:48–51. https://doi.org/10.2136/sssaj1968.03615995003200010012x
Tyc O, Putra R, Gols R, Harvey JA, Garbeva P (2020) The ecological role of bacterial seed endophytes associated with wild cabbage in the United Kingdom. Microbiol Open 9:e00954. https://doi.org/10.1002/mbo3.954
van Kessel C, Hartley C (2000) Agricultural management of grain legumes: has it led to an increase in nitrogen fixation? Field Crops Res 65:165–181. https://doi.org/10.1016/S0378-4290(99)00085-4
Velázquez E, Martínez-Hidalgo P, Carro L et al (2013) Nodular endophytes: an untapped diversity. In: Belén Rodelas González M, Gonzalez-Lopez J (eds) Beneficial plant-microbial interactions. Ecology and applications. CRC Press, Boco Raton, pp 214–236. https://doi.org/10.1201/B15251-11
Verma P, Yadav AN, Khannam KS et al (2015) Assessment of genetic diversity and plant growth promoting attributes of psychrotolerant bacteria allied with wheat (Triticum aestivum) from the northern hills zone of India. Ann Microbiol 65:1885–1899. https://doi.org/10.1007/s13213-014-1027-4
Vincent JM (1970) A manual for the practical study of the root-nodule bacteria. IBP handbook 15 Oxford and Edinburgh. Blackwell Scientific Publications, London
Wang S, Wang W, Jin Z et al (2013) Screening and diversity of plant growth promoting endophytic bacteria from peanut. Afr J Microbiol Res 7:875–884. https://doi.org/10.5897/AJMR12.1500
Wang P, Zhang C, Guo M et al (2014) Complete genome sequence of Bacillus thuringiensis YBT-1518, a typical strain with high toxicity to nematodes. J Biotechnol 171:1–2. https://doi.org/10.1016/j.jbiotec.2013.11.023
Wani PA, Khan MS (2010) Bacillus species enhance growth parameters of chickpea (Cicer arietinum L.) in chromium stressed soils. Food Chem Toxicol 48:3262–3267. https://doi.org/10.1016/j.fct.2010.08.035
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703. https://doi.org/10.1128/jb.173.2.697-703.1991
White JF, Kingsley KL, Zhang Q et al (2019) Review: endophytic microbes and their potential applications in crop management. Pest Manag Sci 75:2558–2565. https://doi.org/10.1002/ps.5527
Yasmin S, Zaka A, Imran A et al (2016) Plant growth promotion and suppression of bacterial leaf blight in rice by inoculated bacteria. PLoS ONE 11:e0160688. https://doi.org/10.1371/journal.pone.0160688
Youseif SH (2018) Genetic diversity of plant growth promoting rhizobacteria and their effects on the growth of maize plants under greenhouse conditions. Ann Agric Sci 63:25–35. https://doi.org/10.1016/j.aoas.2018.04.002
Zhang C, Kong F (2014) Isolation and identification of potassium-solubilizing bacteria from tobacco rhizospheric soil and their effect on tobacco plants. Appl Soil Ecol 82:18–25. https://doi.org/10.1016/j.apsoil.2014.05.002
Zhang C, Xu S-H, Ma B-L et al (2017) New derivatives of ursolic acid through the biotransformation by Bacillus megaterium CGMCC 1.1741 as inhibitors on nitric oxide production. Bioorg Med Chem Lett 27:2575–2578. https://doi.org/10.1016/j.bmcl.2017.03.076
Zhao L, Xu Y, Lai X (2018) Antagonistic endophytic bacteria associated with nodules of soybean (Glycine max L.) and plant growth-promoting properties. Braz J Microbiol 49:269–278. https://doi.org/10.1016/j.bjm.2017.06.007
Zhou JY, Zhao XY, Dai CC (2014) Antagonistic mechanisms of endophytic Pseudomonas fluorescens against Atheliarolfsii. J Appl Microbiol 117:1144–1158. https://doi.org/10.1111/jam.12586
Acknowledgements
The authors thank the Faculty of sciences, Mohammed V University in Rabat, for their support, and the Moroccan Ministry of Higher Education, Research and Innovation that funded the PhD thesis of Mohamed Hnini.
Funding
The Faculty of sciences, Mohammed V University in Rabat, supported this research, and the Moroccan Ministry of Higher Education, Research and Innovation funded the PhD thesis of Mohamed Hnini.
Author information
Authors and Affiliations
Contributions
Conceptualization, supervision, resources, and funding acquisition were performed by JA. Investigation and data curation were done by MH and KT. Writing the original draft was done by MH. All authors participated in the methodology, writing - review & editing. They have read and agreed on the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial or non-financial interests, nor personal relationships that could have influenced the work reported in this paper.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hnini, M., Taha, K. & Aurag, J. Molecular identification and characterization of phytobeneficial osmotolerant endophytic bacteria inhabiting root nodules of the Saharan tree Vachellia tortilis subsp. raddiana. Arch Microbiol 205, 45 (2023). https://doi.org/10.1007/s00203-022-03358-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-03358-y