Abstract
PlxyMNPV_LBIV-11 is an alphabaculovirus strain, isolated from Plutella xylostella larvae. This work characterized this strain at a biological, morphological, and molecular level to evaluate its similarity with other baculoviruses. Its ultrastructure showed a multiple arrangement of nucleocapsids within enveloped virions, all occluded within large cubical polyhedra. PlxyMNPV_LBIV-11 showed infectivity on the Hi5 and Sf9 cell lines, despite these being from heterologous origin. This in vitro infectivity was observed using either BVs or by transfection with genomic DNA. Restriction fragment patterns of PlxyMNPV_LBIV-11, using the enzymes EcoRI, BamHI and HindIII, showed a high relationship with those patterns shown by AcMNPV, except for one or two differential bands with each enzyme. Sequences of core genes lef-8 and lef-9 and the conserved polh gene showed identities ranging from 98 to 100% when compared with those of AcMNPV. Somewhat lower was the sequence identity of the gp64 gene (94%) as compared with those of AcMNPV and PlxyMNPV_CL3, which might be related to the difference in virulence. Besides, the presence of this gene in PlxyMNPV_LBIV-11 indicates that it belongs to group 1 of alphabaculoviruses. A phylogram was estimated with the core and conserved gene sequences, corroborating its high relationship with AcMNPV and PlxyMNPV_CL3. Bioassays were performed with P. xylostella larvae reared on a meridic diet, whose LC50 values indicated lower virulence than AcMNPV when tested against P. xylostella, Spodoptera frugiperda, and Trichoplusia ni larvae. Its virulence against S. frugiperda was only seven times lower than AcMNPV. Its potential as a biological control agent is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae), is a pest of cosmopolitan distribution. It feeds on a wide variety of cultivated and wild plants, though its main hosts are plants of the Brassicaceae family, such as broccoli (Brassica oleracea var. Italica), rapeseed (B. napus), cabbage (B. oleracea var. Capitata) and cauliflower (B. oleracea var. Botrytis) (Talekar and Shelton 1993). Its control is complicated because it has developed resistance to numerous synthetic and biological pesticides. In fact, it was the first pest to be detected to develop resistance to DDT in the 1950s (Capinera 2002) and Bacillus thuringiensis in 1990 (Heckel et al. 1999), under field conditions (Shelton et al. 1993).
In recent years, P. xylostella has become the most destructive pest of cruciferous crops in the world, estimating between $4–5 billion per year in pest damage and control expenses (Furlong et al. 2013; Zalucki et al. 2012). The minimal impact of its parasitoids and predators is one of the reasons for its success as a global pest. However, there are other biological control alternatives such as the use of entomopathogenic viruses (Thézé et al. 2018). These environmentally friendly strategies can allow better control of the pest and reduce environmental pollution and toxic products.
Baculoviruses belong to the family Baculoviridae which are generally highly selective pathogens of insects within the orders Lepidoptera (genera Alphabaculovirus and Betabaculovirus), Hymenoptera (genus Gammabaculovirus) and Diptera (genus Delatabaculovirus) (Fuxa 2004; Rohrmann 2019). They are a diverse group of viruses with circular, double-stranded DNA genomes, whose sizes range from 80 to 180 kb and encode between 90 and 180 genes. They present two virion phenotypes: the occlusion-derived virions (ODV) and budded virions (BV) (Herniou et al. 2011; Jehle et al. 2006a). Occlusion bodies (OBs) are proteinaceous particles that embed the virions and protect viruses to survive in the environment, composed of a crystalline matrix of protein (polyhedrin, in nucleopolyedroviruses or NPVs, and granulin, in granuloviruses or GVs).
The most studied baculovirus is the multiple NPV isolated from Autographa californica (AcMNPV), an alphabaculovirus that belongs to group I, which, opposite to most baculoviruses, it has a wide range of hosts (Rohrmann 2019). A baculovirus widely related to the AcMNPV strain is the virus isolated by Kariuki and McIntosch (1999), who studied an MNPV isolated from P. xylostella larvae labeled as PlxyMNPV-CL3. The genome of this strain is 134,417 bp long, 523 bp longer than AcMNPV genome, and its nucleotide sequence is co-linear with AcMNPV. These results indicate that this virus is a variant of the AcMNPV; however, little is known about the characterization and virulence of other strains of PlxyMNPVs, both in vivo and in vitro, which may show bioinsecticidal potential against this devastating pest.
On the other hand, baculoviruses are known for their complex infection mechanism, whose process are slightly different between whole insects and insect cell lines infections. The AcMNPV virus is known to be infective in cell lines derived from Spodoptera frugiperda (Vaughn et al. 1977) and Trichoplusia ni (Hink 1970). Recently, Ma et al. (2019) established six cell lines from P. xylostella embryonic tissues and evaluated AcMNPV baculovirus infection, showing high susceptibility in four of them. However, the susceptibility of PlxyMNPV strains was not tested. This is important, as the infectivity of a virus in cell lines can define its host range and can be an indication of the similarities or differences between strains or variants.
In Mexico, although natural enemies such as parasitoids have been used, in addition to entomopathogenic fungi and bacteria, the use and application of baculovirus against P. xylostella in broccoli, cauliflower, cabbage and lettuce crops has not been explored. In this work, a strain of baculovirus labeled as PlxyMNPV_LBIV-11 was studied at a biological and molecular level, phylogeny of conserved genes among baculoviruses, its infectivity in cell lines, and its virulence against P. xylostella, T. ni, and S. frugiperda. All this to analyze similarities and/or differences with AcMNPV and determine its bioinsecticide potential against these pests.
Materials and methods
Biological material
The strain PlxyMNPV_LBIV-11 is a part of the stock collection of entomopathogens at the Laboratory of Bioinsecticides in CINVESTAV-Irapuato, Mexico, which was originally isolated in Oxford (England) from a P. xylostella colony originated in Japan (Biever and Andrews 1984). For the amplification of the strain and virulence tests, a native colony of P. xylostella was used. Eggs oviposit on broccoli leaves hatched and larvae of second instar were transferred to plastic containers with meridic diet, and incubated under insectary conditions: 25 °C, 80% RH and 16:8 h of photoperiod, until pupation. The meridic diet was slightly modified from Carpenter and Bloem (2002): 500 ml distilled water, 2.5 g bacteriological agar, 5 g kale, 1.22 g oil, 0.2 g cysteine, 1.42 g methylparaben, 2 g sucrose, 2 g ascorbic acid, 1 g sorbic acid, 0.50 mg streptomycin; 3.2 ml formaldehyde 10% and 18.75 g Vanderzant vitamin mixture. Pupae were transferred to breeding cages for adult emergence, which were kept with 10% corn honey. Fresh broccoli leaves were changed daily for oviposition. The colonies of S. frugiperda and T. ni were maintained according to the methodology reported by Rangel-Núñez et al. (2014) and Del Rincón-Castro and Ibarra (1997). In vitro infection tests were performed on two commercial cell lines (Gibco™): High-Five™ BTI-TN-5B1-4 (Hi5) and Sf9. These were maintained in Sf-900™ III SFM medium (Thermo Fisher Scientific) by adding 5% fetal bovine serum (Gibco™). Cell lines were incubated at 28 °C and subcultures were performed every 72 h.
Amplification of PlxyMNPV_LBIV-11 in P. xylostella, S. frugiperda, and T. ni larvae
Amplification of the viral strains was carried out in Petri dishes with artificial diet, which were surface inoculated with 500 μl of a 1 × 106 OBs/ml suspension. Twenty 2nd instar P. xylostella larvae and 20 1st instar S. frugiperda and T. ni larvae were transferred to each inoculated dish. In all cases, larvae were incubated under insectary conditions for 5 days. Dead or dying larvae were collected and homogenized in sterile porcelain mortars, with 4 ml sterile distilled water (SDW). Homogenates were filtered through an organza mesh, and the filtrates were centrifuged (Hermle Z216M) at 13,000 rpm for 15 min at 4 °C. The resulting pellet was resuspended in SDW to finally store the suspensions at 4 °C.
Ultrastructural morphology of PlxyMNPV_LBIV-11
Scanning electron microscopy (SEM)
Drops of PlxyMNPV_LBIV-11 OB suspensions (1 × 106 OB/ml) were thoroughly dried on SEM aluminum slides. Samples were covered with gold in an E. F. Fullam ionizer (EMS-76 M), and subsequently observed and photographed in a JEOL JSM-35C scanning electron microscope run at 15 kV voltage.
Transmission electron microscopy (TEM)
OB pellets from a suspension (1 × 106 OB/ml) were embedded in 250 μl 1% agarose. Subsequently, samples were fixed in 3% glutaraldehyde in phosphate buffer, dehydrated in serial dilutions of ethanol (10 to 90%) and embedded in low viscosity Epoxy resins. Obtained blocks were subjected to ultrathin sectioning in a NOVA LKB ultramicrotome, contrasted with lead citrate and uranyl acetate, and examined and photographed in a JEOL JEM-2000 EX transmission electron microscope, run at 80 kV voltage. In both techniques, AcMNPV OBs were used as a morphological reference.
Purification and quantification of occlusion bodies
OBs (polyhedra) amplified in P. xylostella larvae were purified in continuous sucrose gradients (40–66% weight/weight), centrifuged at 24,000 rpm and 4 °C for 1.5 h, using a SW28 swinging bucket rotor (Beckman Coulter Ultracentrifuge, Optima L100XP). Once bands of the purified polyhedra were extracted, sucrose was removed with three washes in SDW at 15,000 rpm and 4 °C for 15 min. Purification was corroborated under phase contrast microscopy and resulting pellets were resuspended in 3 ml SDW and stored at 4 °C. Concentrations of viral polyhedra were quantified with a hemacytometer and stored in aliquots of 500 μl SDW at 4 °C, until required.
Cell line infection with PlxyMNPV_LBIV-11
For the infection of the Hi5 and Sf-9 cell lines, 50 2nd instar P. xylostella larvae were infected with 500 μl of 1 × 106 OB/ml suspension dispersed on the diet. These were incubated for 72 h, and hemolymph was extracted from the infected larvae to obtain BVs by excising the last pair of prolegs under a stereomicroscope with microdissection scissors and squeezing the larvae with sterilized tweezers on a container with medium. Hemolymph was initially mixed with 200 μl Sf-900TM III SFM medium, to bring it then to 3 ml with the same medium and then sterilized by filtration (Millipore filters 0.22 μm). Each cell line was inoculated with 1 ml of the BV filtrate, when cell cultures reached a concentration of 1 × 106 cells/ml. Extent of the infection was corroborated 5 days post-infection.
DNA extraction of PlxyMNPV_LBIV-11 and AcMNPV, and restriction analysis
DNA from PlxyMNPV_LBIV-11 and AcMNPV was extracted from purified OBs from 1 ml of 7 × 1010 OB/ml and 8 × 1010 OBs/ml suspensions, respectively (AcMNPV was amplified in T. ni larvae, as described above). Suspensions were pelleted at 14,000 rpm for 10 min and resuspended in 300 μl alkaline buffer (0.1 M NaCO3 and 1 M NaCl pH 11), bringing the suspension to 400 μl with proteinase K buffer (0.01 M Tris, 0.005 M EDTA, 0.5% SDS). Ten μl proteinase K (10 mg/ml) was added and incubated at 60 °C for 30 min. Then, 500 μl of phenol–chloroform-isoamyl alcohol (25:24:1) was added, homogenized, and centrifuged again under the same conditions. The aqueous phase was mixed with one volume of cold isopropanol and centrifuged at 14,000 rpm for 10 min. Pelleted DNA was dried and solubilized in SDW. Viral DNA (100 ng/μl) was digested with 3 restriction enzymes: EcoRI, BamHI and HindIII. Resulting restriction patterns were electrophoresed in 0.7% agarose gels carried out at 20 V for 16 h and visualized in a GelDoc Bio-Rad system with the Image Lab software. Restriction patterns of AcMNPV were used as a reference.
Cell line transfection with PlxyMNPV_LBIV-11 DNA
For viral DNA transfection, both Hi5 and Sf9 cell lines were first quantified in an automated cell counter (Bio-Rad TC10) to fit a concentration of 1 × 106 cells/culture bottle (95% survival). They were kept at 28 °C for 24 h. DNA extracted from PlxyMNPV_LBIV-11 and AcMNPV was prepared at concentrations from 100 to 2000 ng/culture bottle using HBS buffer (20 mM HEPES, 1 mM NaHPO4, 5 mM KCL, 140 mM NaCl, 10 mM glucose, pH 7.05), then 50 μl 125 mM CaCl2 were added until precipitation was observed. Treated cells were incubated for 1 h at 28 °C before the transfection mixture was removed and 2 ml of Sf-900TM III SFM medium was added, without fetal bovine serum. Both cell lines were examined every 24 h until 72 and 96 hpi or until signs of viral infection were observed.
Detection and sequencing of lef-8, lef-9, polh, and gp64 genes
The presence of three core and conserved genes, such as the late expressed factor 8 gene (lef-8), the late expressed factor 9 gene (lef-9), and the polyhedrin gene (polh) in the DNA of PlxyMNPV_LBIV-11 was determined by PCR, using primers described in Table 1. Reaction mixtures consisted of 5 μl 10X PCR buffer, 2.5 μl 50 mM MgCl2, 1 μl dNTP's 1 mM, 1 μl 10 mM primers, 1 U Taq DNA polymerase Platinum SuperFi II (Invitrogen), 100 ng DNA and total volume adjusted to 50 μl with SDW. Thermocycler (BioRad) was set to the following amplification conditions: lef-8 and lef-9 genes fragments were performed by touchdown PCR (initial denaturalization at 95 °C for 3 min; 15 cycles of 95 °C for 30 s, 55 °C for 30 s (decreasing the annealing temperature by 1 °C each cycle), 72 °C for 30 s; plus 20 cycles at 95 °C for 30 s, 60 °C for 30 s, 72 °C for 30 s; and final extension at 72 °C for 7 min. polh gene was amplified by conventional PCR (initial denaturalization 95 °C for 4 min; 35 cycles of 95 °C for 30 s, 55 °C for 1 min, 72 °C for 1 min; with a final extension at 72 °C for 10 min).
Additionally, the presence of the envelope glycoprotein 64 gene (gp64) in the PlxyMNPV_LBIV-11 was also determined using the primers described in Table 1. The PCR reaction was prepared to a final volume of 50 μl, containing 5 μl 10X PCR buffer, 2.5 μl 50 mM MgCl2, 1 μl dNTP ́s 1 mM, 1 μl primers 10 mM, 0.2 μl (1 U) TaqDNApolymerase Platinum SuperFi II (Invitrogen), 1 μl 100 ng/μl DNA and brought to 50 μl with SDW. The same thermocycler was set to one cycle of 95 °C for 3 min, plus 35 cycles of 95 °C for 30 s, 50 °C for 30 s, 72 °C for 30 s and a final cycle of 72 °C for 10 min.
Once amplicons of the lef-8, lef-9, polh, and gp64 genes were obtained, they were purified with the Pure Link PCR Kit (Invitrogen) and sent to sequence by the pyrosequencing method of the Illumina HiSeq SBS platform at Macrogen company (USA).
Phylogenetic analysis
Nucleotide sequences of lef-8, lef-9 and polh genes were downloaded from the NCBI GenBank from group I of alphabaculoviruses (Jehle et al. 2006b) reported genomes. Nucleotide sequences were compared to the partial sequences of the corresponding genes in PlxyMNPV_LBIV-11.
SeqMan 5.0 software was used to assemble the sequences (DNASTAR Inc.). The nucleotide sequence alignment was performed in the Mega X program (Kumar et al. 2018) using the Muscle algorithm and fitting to the size of the fragments obtained from the sequencing, to be later concatenated in the Mesquite software (version 3.5.1). The phylogenetic analysis was completed in the Mega X software using the neighbor-joining method (Saitou and Nei 1987). The nucleotide substitution model applied was p-distance. Gaps were treated as missing data. Bootstrap analyses (using 1000 replications) were used to assess the confidence in the branching order.
Virulence of PlxyMNPV_LBIV-11 on P. xylostella, S. frugiperda and T. ni
Bioassays were performed with purified polyhedra from PlxyMNPV_LBIV-11 and using AcMNPV as a reference. Second instar larvae of P. xylostella and 1st larvae of S. frugiperda and T. ni were used to estimate their corresponding LC50s. In each replicate, six concentrations of each viral strain were used, which were adjusted with preliminary bioassays. For the bioassays of the PlxyMNPV_LBIV-11 against P. xylostella, a concentration of 1 × 105 OB/ml was the highest using a dilution factor 0.5 for the remaining five concentrations. For the bioassays against S. frugiperda and T. ni, PlxyMNPV_LBIV-11 at 5 × 106 OB/ml and 5 × 105 OB/ml, respectively, were used as the highest concentrations of the virus (dilution factors of 0.4 and 0.5, respectively, were used to prepare the remaining concentrations). In the case of bioassays with AcMNPV against P. xylostella, 2.7 × 103 OB/ml was used as the highest concentration (with a dilution factor 0.5), and against S. frugiperda and T. ni, highest concentrations of 5 × 105 OB/ml and 3 × 104 OB/ml were used, respectively, using dilution factor of 0.5 and 0.3, for the remaining concentrations.
For each concentration, a volume of 1000 μl was prepared, which was evenly spread in two volumes of 500 μl on each petri dish with meridic diet. For each bioassay, a control group was included, using 500 μl of SDW. A total of 20 larvae were used for each concentration, placing 10 larvae per petri dish. Each of these bioassays was repeated at least three times. Bioassays were incubated under insectary conditions and mortality was quantified at 7 d.p.i. LC50s were estimated by Probit analysis, using only valid replicates that fit the statistical parameters established by Ibarra and Federici (1987).
Results
Standardization of the P. xylostella colony
Under the conditions described in the previous section, the colony of P. xylostella maintained a monthly average production of 4000 larvae of 2nd instar on artificial diet, approximately 1500 pupae and 1000 adults per entomological cage, in a total of 3 cages. These yields increased by 20%, when compared with a colony maintained on broccoli leaves. However, more important than production levels was the use of a meridic diet, instead broccoli leaves, as it was vital to standardize a more precise bioassay procedure.
Amplification of the viral inoculum in larvae
PlxyMNPV_LBIV-11 was efficiently amplified in P. xylostella 2nd instar larvae. To obtain a larger volume and concentration of the virus, S. frugiperda and T. ni 3rd instar larvae were also used. Concentrations obtained, according to the insect, were: 7 × 1010 OBs/ml for P. xylostella and 8 × 1010 OBs/ml for S. frugiperda and T. ni. These results indicate the ability of this virus to produce large amounts of OBs in insects of a different family (Noctuidae) to that of the original host (Plutellidae), which is an indicator of a wide host range for this virus.
Ultrastructural characterization of PlxyMNPV-LIBV-11
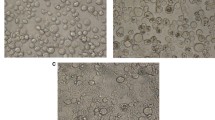
TEM analysis of OBs corroborated that PlxyMNPV_LBIV-11 showed a phenotype of multiple nucleocapsids per viral envelope (MNPV), with multiple infective units per OB (Fig. 1A) very similar to AcMNPV (Fig. 1B). Virions showed an average size of 220 × 25 nm for both strains (Fig. 1A and B). On the other hand, OBs of PlxyMNPV_LBIV-11 presented an almost perfect cubic shape (Fig. 1C). Its average size was 1.98 ± 0.04 μm, without significant differences in its morphology and size (2.03 ± 0.04 μm), when compared with the OBs of AcMNPV (Fig. 1D).
Ultrastructural characterization of PlxyMNPV_LBIV-11, as compared with AcMNPV. A Morphology of a PlxyMNPV_LBIV-11 polyhedron (occlusion body) under TEM microscopy. B Morphology of a AcMNPV polyhedron under TEM microscopy. C Morphology of PlxyMNPV_LBIV-11 polyhedra under SEM microscopy. D Morphology of AcMNPV olyhedral under SEM microscopy
Infections and transfection of cell lines with PlxyMNPV_LBIV-11
Both Hi5 and Sf9 cell lines were efficiently infected and transfected with BVs and DNA from the PlxyMNPV_LBIV-11 and AcMNPV. Although both cell lines were susceptible to both baculoviruses, when transfected with PlxyMNPV_LBIV-11 DNA, a greater amount of OBs was observed within the nuclei of the Sf9 and Hi5 cells, when using 2000 ng of DNA per culture bottle (Fig. 2A), while fewer OBs were observed within the nuclei of both cell lines (Fig. 2B) with the AcMNPV DNA, but they were transfected only with 100 ng of DNA (Fig. 2B). In both cases, the presence of OBs was observed after 36 hpi. When BVs from AcMNPV and PlxyMNPV_LBIV-11 were used to infect both cell lines, OBs were observed within the cell nuclei 72 hpi (2 × 106 OB/ml of culture with AcMNPV BVs and 2 × 104 OB/ml of culture for PlxyMNPV_LBIV-11 BVs). In all cases, restriction patterns were analyzed after infection or transfection of each cell line (as it was made in any characterization procedure), to corroborate the identity of each strain and rule out contamination of lines with the AcMNPV virus.
PlxyMNPV_LBIV-11 restriction fragment patterns
As a first approach to molecularly characterize PlxyMNPV_LBIV-11, restriction pattern analyses were carried out with DNA extracted from purified OBs amplified in infected larvae and from infected cell lines. These patterns were compared with those obtained with the AcMNPV strain. Restriction patterns shown in Fig. 3 were obtained from DNA extracted from PlxyMNPV_LBIV-11 and AcMNPV and digested with the enzymes EcoRI (Fig. 3A), BamHI (Fig. 3B) and HindIII (Fig. 3C). Figure 3A (lane 2), 3B (lane 2) and 3C (lane 9) shows DNA from AcMNPV digested with the same enzymes, respectively. Figure 3A, lanes 3, 6, 7, 4, and 5 show PlxyMNPV_LBIV-11 DNA digested with EcoRI from viruses amplified in vivo in larvae of P. xylostella, T. ni and S. frugiperda and in vitro in the cell lines Sf9 and Hi5, respectively, detecting identical patterns between each other and highly similar to the AcMNPV restriction pattern, except for a fragment of around 1,800 bp in PlxyMNPV_LBIV-11 which is absent in AcMNPV.
Genomic restriction analysis of the PlxyMNPV_LBIV-11 strain. A Genomic DNA digested with EcoRI. B BamHI and C HindIII. DNA was extracted from olyhedral amplified in vivo in P. xylostella, T. ni and S. frugiperda larvae and in vitro in the cell lines Sf9 and Hi5. See text to identify each lane. Genomic AcMNPV DNA was used as a reference patter, shown in A lane 2, B lane 2, and C lane 9
Figure 3B shows the same distribution of samples as in Fig. 3A, in the agarose gel, but DNAs were digested with BamHI. In this restriction pattern, only two fragments of approximately 10,000 and 6000 bp were observed, present in PlxyMNPV_LBIV-11 and absent in AcMNPV. Finally, Fig. 3C shows the same distribution of samples in the agarose gel, but DNA was digested with HindIII. In these restriction patterns, only two fragments of approximately 12,000 and 10,000 bp were observed, present in AcMNPV and absent in PlxyMNPV_LBIV-11. After these restriction analyses with three different enzymes, it was clear that AcMNPV and PlxyMNPV_LBIV-11 are very similar viruses.
Phylogenetic relationship of PlxyMNPV_LVB-11 with other baculoviruses
Presence of two core and one conserved baculovirus genes (lef-8, lef-9 and polh) in the genome of PlxyMNPV_LBIV-11, as well as the presence of the gp64 gene, was corroborated by PCR amplification (Fig. 4A and B). Obtained amplicons matched the expected sizes for each gene: 702 bp for lef-8, 540 bp for polh, 295 bp for lef-9 and 1,500 bp for gp64. Once the amplicons were sequenced, they were compared with their homologous counterparts in PlxyMNPV_CL3 and AcMNPV. The PlxyMNPV_LBIV-11 lef-8 gene showed an identity of 99.58% with its homologous gene in AcMNPV and PlxyMNPV_CL3. The lef-9 gene showed an identity of 98.98% and 100% when compared to those of AcMNPV and PlxyMNPV_CL3, respectively. On the other hand, the polh gene showed an identity of 98.9% and 99.4% with those of AcMNPV and PlxyMNPV_CL3, respectively. Additionally, the presence of the gp64 gene in PlxyMNPV_LBIV-11, indicated its association to the group I of alphabaculoviruses, and showed a lower identity with gp64 genes in AcMPNV and PlxyMNPV_CL3 (94%).
Sequences of the three core and conserved genes in PlxyMNPV_LBIV-11 were used to create a phylogram, to compare their homologous genes in 55 alphabaculoviruses (Fig. 5). Evidently, the PlxyMNPV_LBIV-11 showed the highest similarity to its PlxyMNPV_CL3 counterpart, although the AcMNPV strain is found in the same clade, along the widely known strain BmNPV, and that of Maruca vitrata, MaviMPNV.
Virulence of PlxyMNPV_LBIV-11 against P. xylostella, S. frugiperda and T. ni
Bioassays carried out with PlxyMNPV_LBIV-11 against larvae of P. xylostella, S. frugiperda and T. ni showed important levels of virulence against these insect species; however, when these LC50s were compared with those obtained with AcMNPV against the same species, its virulence was significantly lower against each of the three insect species (Table 2). Virulence of PlxyMNPV_LBIV-11 was 45 times lower than that of AcMNPV, when tested against larvae of P. xylostella; seven times lower, when tested against S. frugiperda larvae; and 49 times lower when tested against T. ni larvae. Probit analyses from all replicates met the acceptable statistical parameters (Table 2) (Ibarra and Federici 1987).
Discussion
In this work, the baculovirus PlxyMNPV_LBIV-11 strain, originally isolated in England from a P. xylostella colony original from Japan, was characterized at a biological, morphological, and molecular level to evaluate its similarity with the AcMNPV strain, and a strain also isolated from P. xylostella. Its infectivity was studied both in vivo and in vitro, against three different species of lepidopteran insects (P. xylostella, S. frugiperda and T. ni), which are highly susceptible to AcMNPV, and on two cell lines derived from T. ni (Hi5) and S. frugiperda (Sf9). Subsequently, to determine its morphology at OB level, images from transmission and scanning electron microscopies were obtained, followed by the comparison of its restriction patterns with those of AcMNPV and the estimation of its phylogenetic relationship with other alphabaculoviruses, using core and conserved gene sequences.
First, it was important to standardize an artificial diet for the P. xylostella, to facilitate its manipulation, maintenance, amplification of the virus, and bioassays, all under controlled conditions. This insect is normally reared on natural diet (fresh broccoli leaves), which involves many uncontrolled factors in bioassays.
The infective ability of PlxyMNPV_LBIV-11 and AcMNPV, on the Hi5 and Sf9 insect cell lines, was similar, despite the great difference of cell lines origin and the PlxyMNPV_LBIV-11 original host. Insect cell lines have demonstrated great versatility, due to their relative ease of cultivation, their wide use in obtaining recombinant baculoviruses, and their ability to express heterologous proteins, which make them an attractive platform to generate viral particles (Puente-Massaguer et al. 2020). In a recent study (Ma et al. 2019) six cell lines derived from P. xylostella were tested, showing that they were highly susceptible to a PlxyMNPV strain, as expected. However, PlxyMNPV_LBIV-11 was infective to two cell lines derived from heterologous hosts, indicating its wide host range. Kariuki et al. (2000) also observed high virulence of a PlxyMNPV strain on heterologous insect cell lines (H. virescens and T. ni) but, unexpectedly, AcMNPV was not infective on these cell lines. This result contrasts with our results as PlxyMNPV_LBIV-11 and AcMNPV showed high infectivity towards the Sf9 and Hi5 cell lines. These results may indicate that the in vitro specificity of baculoviruses can be genetically multifactorial, a phenomenon that should be studied with more detail.
Morphological characterization of PlxyMNPV_LBIV-11 polyhedra indicated that this strain shows quasi cubic polyhedra in SEM microscopy and multiple arrangement of virions in each enveloped infective unit. Size and morphology of OBs are identical to those shown by AcMNPV, which indicates a primary similarity between both strains.
In relation to the genetic characterization of PlxyMNPV_LBIV-11, the straightforward restriction fragment pattern (RFP) technique was used to detect differences and similarities between strains. Although this technique is already amply surpassed by genome sequencing, RFP can serve as a first approach to detect genetic differences between virus species (Marsberg et al. 2018). In this work, once the RFP obtained with the restriction enzymes EcoRI, HindIII and BamHI were analyzed, subtle differences were found between the strains PlxyMNPV_LBIV-11 and AcMNPV, which led to the assumption that they were two highly related strains. Marsberg et al. (2018) analyzed the restriction fragments of an alphabaculovirus isolated from Cryptophlebia peltastica in South Africa, demonstrating with this technique that, also, there were a few genotypic differences between this strain and the standard AcMNPV strain. In this work, few RFP differences were also found between the strains PlxyMNPV_LBIV-11 and AcMNPV; but unlike the work mentioned above, significantly different levels of virulence were observed in the present work, between both strains towards the three insects tested, implying that the strains, despite their high similarity in the restriction fragments, they are significantly different, at least in their virulence. In an investigation carried out by Del Rincón-Castro and Ibarra (1997) a great genotypic diversity between different strains of baculovirus isolated from T. ni was demonstrated, showing that this technique is highly efficient to discriminate between strains isolated from the same host. Kariuki and McIntosch (1999) also found few differences in restriction fragment patterns between the AcMNPV and PlxyMNPV_CL3 strains, which confirmed that both strains were slightly different. In the present work, the differences observed in the RFPs together with the extremely different levels of virulence allow us to infer that strains PlxyMNPV_LBIV-11 and AcMNPV are highly related, but there are specific genetic components, associated to the great difference in virulence.
The three core and conserved genes used to establish a phylogenetic relationship among baculoviruses were previously validated by Jehle et al. (2006b) and Lange et al. (2004), to be used with that purpose, instead of using the 38core genes used previously. Our results showed that PlxyMNPV_LBIV-11, PlxyMNPV-CL3, and AcMNPV are very similar among them. However, despite this genetic similarity, a gene involved in the infection of the insect cells, the gp64 gene, showed lower identity (94%) with its homologous genes in AcMNPV and PlxyMNPV_CL3. This difference might explain, at least in part, the virulence variability among strains. Additionally, the presence of this gene indicates that PlxyMNPV_LBIV-11 belongs to group I of the genus Alphabaculovirus, like the other two strains. However, its only presence is not a guarantee of replication of the virus in host cells, as it is the case of BmN cells which showed infection and expression of early AcMNPV genes, but the synthesis of infectious BV particles or expression of very late genes was not detected (Rahman and Gopinathan 2003). Still, PlxyMNPV_LBIV-11 showed an important virulence to the Sf9 and Hi5 cell lines, and the presence of this gp64 gene, in this case, is considered to favor the entry of virions into cells grown in vitro and contribute to their virulence in vivo (Westenberg et al. 2007).
Despite this difference, the slight genetic variation found between the two PlxyMNPVs and AcMNPV indicates that both are variants of the latter, but with characteristics that distinguish them in terms of their virulence levels, both in vivo and in vitro. Therefore, it is necessary to continue their study, using more precise tools such as the comparison of genomes, focusing on those genes which may explain different virulence properties.
Once the P. xylostella colony was established on a meridic diet, more precise bioassays with inocula spread on the surface of the diet was possible to perform (Hughes and Woods 1986). Virulence between both tested strains was significantly different, always showing AcMNPV with higher virulence than PlxyMNPV_LBIV-11, against the three tested insect species. Interestingly, AcMNPV was 45, 44 and 7.4 times more virulent than PlxyMNPV_LBIV-11 against P. xylostella, T. ni and S. frugiperda, respectively, indicating three interesting phenomena: a) PlxyMNPV_LBIV-11 is less virulent than AcMNPV towards its original host, b) both strains were more virulent against P. xylostella than against the other two species of lepidopteran insects, and c) the virulence of both strains is very high towards S. frugiperda, which interestingly shows great variability in its susceptibility towards their own NPVs (Zanella-Saenz et al. 2022).
LC50 values similar with those obtained for PlxyMNPV_LBIV-11 against P. xylostella were reported by Kalantari et al. (2019) when testing a PlxyNPV native to Iran against this pest (3.8 × 104 OB/ml). On the other hand, Kariuki and McIntosch (1999) performed bioassays with AcMNPV and PlxyMNPV_CL3 on different insect species; however, they obtained very different results from those obtained in the present work (once our results were converted to OB/cm2 units), as PlxyMNPV_CL3 was 21 times more virulent than the PlxyMNPV_LBIV-11, while AcMNPV was 4,400 times less virulent than PlxyMNPV_CL3, when compared with our results on P. xylostella. Likewise, these authors estimated LC50 values much lower against S. frugiperda and T. ni than those found in our work, estimating that the PlxyMNPV_CL3 strain, is 5 and 78 times more virulent against S. frugiperda and T. ni, respectively, when compared with our results with PlxyMNPV_LBIV-11. All these contrasting results indicate that strains PlxyMNPV_LBIV-11 and PlxyMNPV_CL3 may be very similar, genetically, as they are with the AcMNPV strain, but a genomic comparison is required to answer the enormous inconsistencies and differences observed in both works, in terms of virulence.
It should be noted both AcMNPV and PlxyMNPV_LBIV-11 showed a wide range of hosts, as they were able to infect two noctuid species (S. frugiperda and T. ni), significantly different to the P. xylostella’s family. Also, PlxyMNPV_LBIV-11 virulence towards S. frugiperda was only 7 times lower than that caused by AcMNPV. This is of particular importance, as Wan et al. (2021) reported the invasion of S. frugiperda in more than 47 African and 18 Asian countries since 2016, when it was first detected in Nigeria and Ghana. This is known to be a pest with a wide range of habitats, strong migration ability, high fecundity, and rapid development of resistance to synthetic insecticides; so, it is important to consider PlxyMNPV_LBIV-11, as an alternative baculovirus with potential to be integrated into IPM programs, for the control of S. frugiperda, despite its lower virulence shown in this study, which is compensated with its wide host range. In general, more detailed studies are required to have more bases to be able to select the best candidate to be used in a biological control program against P. xylostella in the world.
Conclusions and prospects
According to the results obtained in this work, due to its morphology, the PlxyMNPV_LBIV-11 strain is a multiple nucleopolyhedrovirus within the genus Alphabaculovirus, highly similar to AcMNPV. The presence of the gp64 gene includes this virus within the group 1 of the genus, despite a somewhat low identity with other homologous genes. PlxyMNPV_LBIV-11 is infective in vivo towards P. xylostella, S. frugiperda, and T. ni larvae; as well as in vitro, either by transfection or by BV infection on Hi5 and Sf9 cell lines. However, AcMNPV is more virulent towards these insect species. Sequences of lef-8, lef-9 and polh core and conserved genes indicate that PlxyMNPV_LBIV-11 is highly related to PlxyMNPV-C13 and AcMNPV strains. Its significant difference in virulence when compared with AcMNPV may be related to specific gene content. Still, PlxyMNPV_LBIV-11 show enough potential to be considered as an alternative control agent against P. xylostella and S. frugiperda.
It is highly advisable to sequence the PlxyMNPV_LBIV-11 genome to analyze its content and to compare it with other related strains. A thorough analysis may identify possible factors related to its virulence and specificity. Variation in virulence may be related, at least in part, to its difference in identity shown by the gp64 gene, as this gene is involved directly in the virion infection of cells, but there might be some other factors involved in this phenomenon. Also, it might be advisable to perform some greenhouse and filed tests to define the real potential of this strains as a control agent against these three pests. Infection tests should be performed on other important pests, such as Helicoverpa spp., Heliothis spp., other Spodoptera spp., among others.
References
Biever KD, Andrews PL (1984) Susceptibility of lepidopterous larvae to Plutella xylostella nuclear polyhedrosis virus. J Invertebr Pathol 44:117–119. https://doi.org/10.1016/0022-2011(84)90055-7
Capinera JL (2002) Diamondback moth, Plutella xylostella (Linnaeus) (Insecta: Lepidoptera: Plutellidae). The Institute of food and agricultural sciences (IFAS). https://doi.org/10.32473/edisin276-2000
Carpenter EJ, Bloem S (2002) Interaction between insect strain and artificial diet in diamondback moth development and reproduction. Entomol Exp Appl 102(3):283–294. https://doi.org/10.1023/A:1019546605422
Del Rincón-Castro MC, Ibarra JE (1997) Genotypic divergence of three single nuclear polyhedrosis virus (SNPV) strains from the cabbage looper, Trichoplusia Ni. Biochem Syst Ecol 25(4):287–295. https://doi.org/10.1016/S0305-1978(97)00002-1
Furlong MJ, Wright DJ, Dosdall LM (2013) Diamondback moth ecology and management: problems, progress, and prospects. Annu Rev Entomol. 58:517–541. https://doi.org/10.1146/annurev-ento-120811-153605
Fuxa JR (2004) Ecology of insect nucleopolyhedroviruses. Agric Ecosyst Environ 103:27–43. https://doi.org/10.1016/j.agee.2003.10.013
Heckel DG, Gahan LJ, Liu YB, Tabashnik BE (1999) Genetic mapping of resistance to Bacillus thuringiensis toxins in diamondback moth using biphasic linkage analysis. Proc Natl Acad Sci USA 96(15):8373–8377. https://doi.org/10.1073/pnas.96.15.8373
Herniou EA, Arif BM, Becnel JJ, Blissard GW, Bonning BC, Harrison R, Jehle JA, Theilmann DA, Vlak JM (2011) Baculoviridae. In: Adams MJ, King AMQ, Cartsens EB, Lefkowitz EJ (eds) Virus taxonomy, Ixth report of the international committee on taxonomy of viruses. Elsevier, Oxford, pp 163–174
Hink WF (1970) Established insect cell line from the cabbage looper, Trichoplusia ni. Nature 226:466–467. https://doi.org/10.1038/226466b0
Hughes PR, Woods HA (1986) In vivo and in vitro bioassay methods for baculovirus. In: Granados RR, Federici BA (eds) The biology of baculovirus. CRC Press, Florida, pp 1–30
Ibarra JE, Federici BA (1987) An alternative bioassay employing neonate larvae for determining the toxicity of suspended particles to mosquitoes. J Am Mosq Control Assoc. 2:187–191
Jehle JA, Blissard GW, Bonning BC, Cory JS, Herniou EA, Rohrmann GF, Theilmann DA, Thiem SM, Vlak JM (2006) On the classification and nomenclature of baculoviruses: a proposal for revision. Arch Virol. 151:1257–1266. https://doi.org/10.1007/s00705-006-0763-6
Jehle JA, Lange M, Wang H, Hu Z, Wang Y, Hauschild R (2006b) Molecular identification and phylogenetic analysis of baculoviruses from Lepidoptera. Virology 346(1):180–193. https://doi.org/10.1016/j.virol.2005.10.032
Kalantari MZ, MagholiFhard Z, Marzban R (2019) Virulence determination nuclear polyhedrosis virus on cotton bollworm Helicoverpa armigera and diamond black moth, Plutella xylostella. J Appl Res Plant Prot 7(4):37–47
Kariuki CW, McIntosch AH (1999) Infectivity studies of a new baculovirus isolate for the control of the diamondback moth (Plutellidae: Lepidoptera). J Econ Entomol 92(5):1093–1098. https://doi.org/10.1093/jee92.5.1093
Kariuki CW, McIntosh AH, Goodman CL (2000) In vitro host range studies with a new baculovirus isolate from the diamondback moth Plutella xylostella (L) (Plutellidae: Lepidoptera). In Vitro Cell Dev Biol Anim. 36(4):271–276
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35(6):1547–1549. https://doi.org/10.1093/molbev/msy096
Lange M, Wang H, Zhihong H, Jehle JA (2004) Towards a molecular identification and classification system of lepidopteran-specific baculoviruses. Virology 325(1):36–47. https://doi.org/10.1016/j.virol.2004.04.023
Lung OY, Cruz-Alvarez M, Blissard GW (2003) Ac23, an envelope fusion protein homolog in the baculovirus Autographa californica multicapsid nucleopolyhedrovirus, is a viral pathogenicity factor. J Virol 77(1):328–329. https://doi.org/10.1128/JVI.77.1.328.339.2003
Ma XL, He WY, Wang P, You MS (2019) Cell lines from diamondback moth exhibiting differential susceptibility to baculovirus infection and expressing midgut genes. Insect Sci 26(2):251–262. https://doi.org/10.1111/1744-7917.12533
Marsberg T, Jukes MD, Krejmer-Rabalska M, Rabalski L, Knox CM, Moore SD, Hill MP, Boguslaw S (2018) Morphological, genetic and biological characterization of a novel alphabaculovirus isolated from Cryptphlebia peltastica (Lepidoptera: Tortricidae). J Invertebr Pathol 157:3–34. https://doi.org/10.1016/j.jip.2018.08.006
Puente-Massaguer E, Gòdia F, Lecina M (2020) Development of a non-viral platform for rapid virus-like particle production in Sf9 cells. J Biotechnol 322:43–53. https://doi.org/10.1016/j.jbiotec.2020.07.009
Rahman MM, Gopinathan KP (2003) Analysis of host specificity of two closely related baculoviruses in permissive and nonpermissive cell lines. Virus Res 93:13–23. https://doi.org/10.1016/S0168-1702(03)00046-7
Rangel-Núñez JC, Del Rincón-Castro MC, Vázquez-Ramírez MF (2014) Caracterización biológica y molecular de cepas exóticas de baculovirus SfNPV, con actividad bioinsecticida hacia una población mexicana del gusano cogollero del maíz Spodoptera frugiperda (Lepidoptera: Noctuidae). Interciencia 39:320–332
Rohrmann GF (2019) Baculovirus molecular biology, 4 edn. Bethesda (MD), National center for biotechnology information, USA https://www.ncbi.nlm.nih.gov/books/NBK543458/. Accessed 11 May 2022
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4(4):406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Shelton AM, Robertson JL, Tang JD, Perez C, Eigenbrode SD, Preisler HK, Wilsey WT, Cooley RJ (1993) Resistance of diamondback moth (Lepidoptera: Plutellidae) to Bacillus thuringiensis subspecies in the field. J Econ Entomol 86:697–705. https://doi.org/10.1093/jee/86.3.697
Talekar NS, Shelton AM (1993) Biology, ecology, and management of the diamondback moth. Annu Rev Entomol 38:275–301. https://doi.org/10.1146/annurev.en.38.010193.001423
Thézé J, Lopez-Vaamonde C, Cory JS, Herniou EA (2018) Biodiversity, evolution and ecological specialization of baculoviruses: a treasure trove for future applied research. Viruses 10(7):366. https://doi.org/10.3390/v10070366
Vaughn JL, Goodwin RH, Tompkins GJ, McCawley P (1977) The establishment of two cell lines from the insect Spodoptera frugiperda (Lepidoptera: Noctuidae). In Vitro 13(4):213–217. https://doi.org/10.1007/BF02615077
Wan J, Huang C, Chang-you L, Hong-xu Z, Yong-lin R, Zai-yuan L, Long-sheng X, Bin Zh, Bo L, Cong-hui L, Wan-xue L, Wen-kai W, Wan-Qiang Q, Mckirdy S, Fang-Hao W (2021) Biology, invasion and management of the agricultural invader: fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). J Integr Agric 20(3):646–663. https://doi.org/10.1016/S2095-3119(20)63367-6
Westenberg M, Uijtdewilligen P, Vlak JM (2007) Baculovirus envelope fusion proteins F and GP64 exploit distinct receptors to gain entry into cultured insect cells. J Gen Virol 88:3302–3306. https://doi.org/10.1099/vir.083240-0
Zalucki MP, Shabbir A, Silva R, Adamson D, Sheng L, Furlong MJ (2012) Estimating the economic cost of one of the world’s major insect pests, Plutella xylostella (Lepidoptera: Plutellidae): just how long is a piece of string? J Econ Entomol 105(4):1115–1129. https://doi.org/10.1603/EC12107
Zanella-Saenz I, Herniou EA, Ibarra JE, Huerta-Arredondo IA, Del Rincón-Castro MC (2022) Virulence and genetic characterization of six baculovirus strains isolated from different populations of Spodoptera frugiperda (Lepidoptera: Noctuidae). Arch Microbiol 204(1):1–11. https://doi.org/10.1007/s00203-021-02722-8
Acknowledgements
Authors thank Ma. Fernanda Vázquez-Ramírez and Javier Luévano Borroel for their technical assistance with all experiments and to CONACYT for supporting SYJH with a graduate student fellowship (No. 583555).
Funding
This work was supported by Secretaría de Desarrollo Agroalimentario y Rural del Estado de Guanajuato (SDAyR) grant No. DS/SDCA/DGA/DSV/02/2020, and Consejo Nacional de Ciencia y Tecnología (CONACYT México) grant No. 140615.
Author information
Authors and Affiliations
Contributions
Bioassays, gene analysis, cell line infections [SYJH]; data analysis of manuscript [JCRN]; data analysis, correction, and revision of manuscript [JEI]; project leader, design, correction, and revision of manuscript [MCDRC].
Corresponding author
Ethics declarations
Conflict of interests
Authors declare they have no conflicts of interest nor competing interest.
Ethical statement
This manuscript is in compliance with scientific ethical standards. This manuscript does not contain any studies with human participants or laboratory vertebrates performed by any of the authors.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiménez-Hernández, S.Y., Rangel-Núñez, J.C., Ibarra, J.E. et al. Biological, morphological, and molecular characterization of the baculovirus PlxyMNPV_LBIV-11, and its virulence towards Plutella xylostella, Trichoplusia ni, and Spodoptera frugiperda larvae. Arch Microbiol 204, 598 (2022). https://doi.org/10.1007/s00203-022-03222-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-03222-z