Abstract
To prevent foodborne diseases and extend shelf life, antimicrobial agents may be used in food to inhibit the growth of undesired microorganisms. The present study was aimed to determine the antimicrobial and antifungal activities of the fermented medicinal plants extract using Lactobacillus acidophilus ATCC 4356. The fermentation kinetic parameters, biochemical composition and the volatile compounds of the fermented plant extract were assessed. The results showed that, the fermented plants extract exhibited high content in polyphenols, flavonoids, and tannins (152.7 mg AGE/L; 93.6 mg RE/L; and 62.1 mg CE/L, respectively) comparing to non-fermented the extract. The GC–MS headspace analyses showed the presence of 24 interesting volatile compounds. The richness of the fermented plants extracts in polyphenols and bioactive compound, such as Eucalyptol, Camphene, α-Phellandrene, α-Terpinene, improves their biological activity. In addition, the fermented plants extract exhibited a high antimicrobial potential against pathogenic bacteria and fungi determined by different methods. The maximum inhibition showed in the fermented plants extract against Escherichia coli 25922/3, Pseudomonas aeruginosa 27853 ATCC, Staphylococcus aureus 29213 ATCC, Enterococcus aerogenes 13048 ATCC, Phytophthora infestans P3 4/91 R + , P. infestans P4 20/01 R, P. infestans (GL-1). The obtained results support the hypothesis of using lactic fermentation as a functional ingredient to improve food preservation. The bioprocesses of fermentation technology enhance antimicrobial and antifungal activities which could be used in different industrial applications.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Aromatic and medicinal plants are particularly valuable for selective bioprocesses because they already contain many bioactive compounds, including phenolic compounds, carotenoids, anthocyanins and tocopherols (Naczk and Shahidi, 2006). In addition, the content of these compounds can be increased by the metabolic activity of the microorganisms involved in the fermentation process. In particular, plants can be used as an ideal substrate for the growth of LAB (Yoon et al. 2006; Andersen et al., 2012; Filho et al., 2017). More specifically, the fermentation technology breaks down or converts the substrates such as the medicinal plants into compatible components under the action of microbial enzymes, thereby improving the substrate properties via the production and enhancing the extraction of bioactive compounds (Parvez et al., 2006). Many of the changes occur during fermentation, leading to a modified the properties of products, such as bioactivity, digestibility and therefore the aromatic compounds changed, new compounds were generated from the fermentation (Zhang et al. 2012). Lactic acid bacteria (LAB) are commonly used in dairy and also nondairy fermentations because of their ability to metabolize different substrates (Freire et al. 2017; Luana et al. 2014). LAB produce metabolites, such as volatile and non-volatile compounds, during the fermentation process, which contribute to their use in the food industry. Fermentation-mediated bio-activation of the medicinal herbs results in improved therapeutic potencies and efficacies and decreased toxicities where the microbial population plays a pivotal role (Lin Wang Lee and Su, 2008; Miyake et al. 2005; Nakano et al. 2006; Wu et al. 2013). Fermentation improves the pharmacological properties of herbal medicines mainly through the modification of naturally occurring molecules, such as isoflavones, saponins, phytosterols, and phenols, that exerts beneficial health-promoting and disease-preventing effects, in keeping with the ‘theory of the oriental medicine’ (Choi and Kang, 2003). For the past few years, with the rapid progress in microbial fermentation technologies and in-depth research on the modernization of herbal medicines, the microbial fermentation and transformation of herbal drugs have gained considerable interest and appeared as new approaches to produce novel active compounds with potent medicinal values (Wu et al. 2013). Fermentation is considered to be one of the most useful techniques of biocatalytical process which is a feasible method for the production of new, active, and less toxic bioactive products that would be otherwise troublesome to generate from either biological systems or chemical synthesis (Rasor et al. 2001).
The rapid acidification of the food matrix occurs through conversion of fermentable carbohydrates, mainly into lactic acid. Reduced pH and the production of antimicrobial agents (such as bacteriocins) decrease the growth and development of pathogenic microorganisms and food spoilers, extending the shelf life and ensuring food safety of the final product (Smid et al. 2014). On another level as important as health, biological control is also a very promising field of action where fermented extracts will play a crucial role.
The fungi are the most destructive among all phytopathogens which can cause about 65% loss of plant host species (Fisher et al. 2012). According to FAOSTAT1, fungi affected most of the economically important crops globally (www. fao.org). Under the present climate change scenario, incidences of several fungal diseases have escalated (Garcia-Solache et al. 2010).
Therefore, the management of these fungal and microbial diseases is very crucial to ensure a constant supply of food to the growing world population. For example, Phytophthora infestans causes serious losses of potato crops worldwide and is probably the most important pathogen of potato and tomato today. To control the fungal diseases, a number of agrochemicals including synthetic fungicides are applied widely and repeatedly (Scarpino et al. 2015). Numerous studies have suggested the role of organic acid, such as lactic acid and acetic acid, in the antibacterial and antifungal activity of fermented extracts (Greenwalt et al. 1998; Blanc 1996; Velicanski et al. 2014; Steinkraus et al. 1996; Cetojevic Simin et al. 2008, Sreeramulu et al. 2001). Bacteriocins, enzymes, and other protein, volatile compounds, and compounds produced by bacteria have a role in the antimicrobial activity of this beverage. Bacteriocins are proteinaceous compounds that exhibit bactericidal activity against species closely related to the producer strain. Bacteriocins alter the membrane potential by corrupting the potassium ion and ATP and cause cell failure to balance intracellular pH (Sezer and Guven 2013). For the reasons, we are developing a new strategy using medicinal plants fermented by LAB as an alternative to the chemical agent against pathogenic microorganisms.
The aim of this study was to explore the kinetics and biochemical composition (content of total phenolic compounds, sugar and proteins) and volatile compounds of fermented sugarcane molasses supplemented with medicinal plant extracts using Lactobacillus acidophilus ATCC 4356, to evaluate their potential to inhibit pathogenic bacteria and fungi using different tests.
Materials and methods
Plant materiel
The plant materials used in this study ficus-indica, Linum usitatissimum, Lavandula multifida, Periploca laevigata, Foeniculum Vulgare and Thymus algeriensis collected from Orbata National Park Mountain (Gafsa, Tunisia) with coordinates: N 34° 22′49.8′′and E 9° 3′23.4′′, Nigella sativa, Linum usitatissimum, Zingiber officinalis, and Vitis vinifera were purchased from commercial central market of Tunisia (Gadhoumi et al. 2021). A voucher specimen was deposited in the Laboratory of Extremophile plants, Center of Biotechnology at the Ecopark of Borj-cédria, and identified by prof. Abderrazak Smaoui. The selected plant materials were harvested and mixed with 30 g/l of sugarcane molasses dissolved in sterile distillated water (Table 1).
Microorganisms
The fungi P. infestans (GL-1: 01,114 mold type from Julius Kühn Institute (Federal Institute for Crop Research, Groß Lüsewitz), German; P3: R + producent, Meppen, German; P4:20,101 R- wild type (unknown) obtained from Julius Kühn Institute in Braunschweig, Germany), Colletotrichum higginsianum (originally isolated in Japan, via the Department of Biology, Friedrich-Alexander-Universität (Erlangen, Germany)); Fusarium oxysporum 39/1201 (St.9336 from the Technische Universität Berlin in Germany); Aspergilus niger DSM 246 from Leibniz-Institute DSMZ-German Collection of Microorganisms and Cell Cultures.
The bacteria strains Escherichia coli ATCC 25,922, E. coli K12, E. coli ATCC 8739, E. coli ATCC 25,922/3, Pseudomonas aeruginosa DSM 1128, P. aeruginosa ATCC 27,853, Staphylococcus aureus ATCC 25,923, S. aureus ATCC 29,213, Serratia marcescens (Enterobacteriaceae) ATCC 13,880, Enterococcus aerogenes ATCC 13,048, E. faecalis ATCC 29,212 were obtained from the microbiology Laboratory of the Department of Agriculture and Food Sciences, University of Neubrandenburg, Germany.
The culture of Lactobacillus acidophilus ATCC 4356 obtained from the microbiology Laboratory of the Department of Agriculture and Food Sciences, University of Neubrandenburg, Germany.
Fermentation condition
Two equivalent batches were prepared using plants raw material. The selected plant materials were crushed and mixed with sugarcane molasses in sterile distillated water were sterilized by pasteurization (80 °C in 15 min) (Table 1). After pasteurization, the first batches were filtered through 0.2-μm filters and concentrated using freeze-drying and preserved at + 4 °C until analysis. The second batches were fermented using lactic acid bacteria (LAB) cultures: Lactobacillus acidophilus ATCC 4356. Before fermentation, For the purpose of fermentation, a ready-to-use (RTU), the LAB cultures were activated to the stationary phase by cultivation at 37 °C for 24 h and used for the inoculation with Log 6 – 8 CFU/ml at pH = 3.5 were added to each batch at the rate of 10 ml/l. After two weeks of fermentation, the fermented extract was sterilized by filtration through 0.2-μm filters and concentrated using freeze-drying and preserved at + 4 °C until analysis (Gadhoumi et al. 2021).
Fermentative parameters
Acidity was measured by titration using 0.1 M NaOH solution and phenolphthalein as indicator. The pH was measured using a calibrated Hanna pH meter. Total sugars were assessed by an ATC refractometer (Brix 0–32%). The determination of reducing sugars was assessed using the DNS method described by Song et al. (2016). The total proteins were determined according to the Bradford method (1976). The results were expressed as the means of three replicates.
Total Phenolic content
The polyphenols content was determinate using the Folin–Ciocalteu reagent, using the method described by Velioglu et al. (1998). 0.1 ml of extract was added to 1 ml of distilled water and 0.2 ml of the Folin–Ciocalteu reagent (diluted 1/10). The mixture solution was shaken and allowed to stand for 5 min. After that, 1 ml of Na2CO3 (6%) was added to the mixture. After 30 min of incubation in dark, the absorbance was read at 760 nm. Total polyphenol content was expressed in µg gallic acid equivalents per g dry residue (mg GAE/ L).
Flavonoid content
The flavonoids content was determinate using the method described by Tlili et al. (20,013), using the AlCl3 reagent. A volume of 1.5 ml of diluted extract was mixed with 1.5 ml of AlCl3 solution (10% in methanol). After incubation for 30 min at room temperature, the absorbance was read at 415 nm. The results were expressed in μg rutin equivalents per g dry residue (mg RE/L).
Condensed tannins contents
The determination of the condensed tannins content in the different extracts was according to the method described by Tlili et al. (2013). A volume of 400 μl of diluted extract or standard, 3 ml of vanillin (4%) dissolved in methanol and 1.5 ml of concentrated sulfuric acid. After 15 min of incubation at ambient temperature, the absorbance is measured at 500 nm. The concentration of condensed tannins was expressed in µg catechin equivalent per gram dry residue (mg CE / L).
Headspace solid-phase micro-extraction of volatile compounds
The volatile compounds were analyzed by headspace solid-phase micro-extraction (HS-SPME) coupled with gas chromatography–mass spectrometry (GC–MS) as previously described Navarini et al. (1999). The GC–MS headspace analysis was performed with an iron sources temperature of 240 °C and an ionization voltage of 70 eV. The mass spectrometer was operated in scan mode from m/z 50 to 350. Peak areas were determined for each compound by integrating a selected ion unique to that compound. The volatile compounds were identified by matching their mass spectra with those in the NIST1.l Library of MS spectra. The Kovats retention index (RI) was calculated with a homologous series of n-alkanes (C6-C28) under the same conditions applied for the sample analyses. The volatile compounds (OAV > 1) are considered to contribute to the aroma of fermented extracts (You et al. 2013; Tian et al. 2017). The different fermented extracts were analyzed in triplicate.
Antimicrobial activity and antifungal activity determinate by Disk diffusion methods
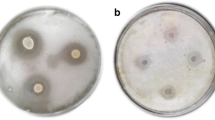
The antimicrobial and antifungal activity of the different extracts was determined by the diffusion method in agar medium cited by Celiktas et al. (2007) with a slight modification. This method was employed to determine inhibition diameter of fermented extract against pathogen bacteria strains. In the square Petri dish surface, Mueller–Hinton agar MHA solid plate was swabbed with 100 µL of the culture bacterial suspension or spores 105–7 CFU/mL with each tested microorganism and kept 1 h at 4 °C for bacterial fixation. Sterile disks of extracts at the rate of 20 µl per disk of concentration were deposited sterile on the agar surface. The dishes were incubated for 24 h at 37 °C for the antimicrobial activity, in a normal atmosphere for not demanding bacteria, and in an atmosphere containing 5% CO2 for bacteria demanding. The inhibition diameter was measured (mm) three times in triplicate. 50 µg/disk of streptomycin (sigma chemical) was used as positive control.
Antimicrobial and antifungal susceptibility test
Antifungal and antibacterial susceptibility testing was performed by a broth microdilution method in accordance with the National Committee for Clinical Laboratory Standards (NCCLS, 1997). The final concentrations of the antifungal and antimicrobial agents were 0.1 to 20 μg/ml. Test strains were suspended in NB to give a final density (bacteria or spores) of Log 6–7 CFU/ml and these were confirmed by viable counts. Plates were incubated at 35 °C for 48 h for the bacteria and 3–6 days for the fungal. MICs of all the samples were defined as the lowest concentrations resulting in 80% inhibition of growth compared to that of the growth control. The wells were then examined for evidence of growth and MICs values were determined as the lowest extracts concentration that inhibited visible growth of the tested microorganism which was indicated by the presence of a white “pellet” on the well bottom. MIC ranges were obtained for each species–extract combination tested. MICs for 50 and 90% of the isolates of each species were tested (MIC50 and MIC90, respectively).
Antifungal activity: inhibitory testing using growth assays
The antifungal activity was determined by inhibitory testing using growth assays using the method described by (Thanusu et al. 2010). This method can be used to generally test growth inhibitory effects of samples toward fungi. The spore solution was prepared from the different fungal strain as follows: streak spores from a single colony on a CM agar plate and incubated until the plate was abundantly covered with sporulated mycelium (3–6 days, 25–37°). The spores were harvested from CM agar plate, by adding 10 mL of saline solution to the plate and carefully released spores by scraping over the surface plate with a sterile cotton stick. Pipetted spore solution from the plate into a sterile 15-mL tube; if required, removed mycelial debris (vegetative mycelium, conidiophores) by filtration through a sterile miracloth filter. The spore’s count was done by microscope counting chamber to prepare different concentration of a spore solution. The test of growth inhibition assays with letting the spores germinate first was started. Filled each well of a flat-bottom 96-well plate with 300 µL sterile medium, 100 µL 2 × MM and 50 µL spore solution. Prepared a stock plate for efficient and fast addition of the fermented plants extract using a V-bottom 96-well plate and add 20 µg per well for each extract with three replications of each treatment, and all experiments were conducted twice; pipetted also 50 µL of water or any other solvent used as negative control to at least three wells. The different plates were incubated at 30 °C. The antifungal activities were visualized in the plate with 96-well after 4–7 days of each condition. After incubation, a drop from each well was taken and spread on the slide and improved with methyl blue and observations by microscopy light imaging (JPG) (bright field microscopic morphological examination (Olympus BH-2 microscope; magnification × 400).
Statistical analyses
The data were expressed as mean values and the standard deviations (SD) were calculated. The comparison between the average variables was performed by Duncan's multiple range tests and the differences considered significant when p < 0.05. The Statistica version 7.0 software was used for data processing.
Results
Monitoring of biochemical and fermentation parameters
As reported in Figs. 1 and 2, all samples obtained from sugarcane molasses and plants raw material, fermented by LAB, showed a substantial decrease of the pH and a subsequent increase of the titratable acidity due to the liberation of organic acids, such as lactic, acetic, formic, malic …. However, there was an increase in pH values up to the end of fermentation (pH = 3). In addition, the results illustrated in the Fig. 2 showed that the level of the total sugar and reducing sugar, total proteins and free amino acid decreased (38 mg/l, 18 mg/l, 20 mg/l, and 8 mg/l, respectively) after fermentation in the fermented plants extract to compare with the plant extract without fermentation. In this study, we used the lactic fermentation technology applied for fermenting medicinal plants to enhance the extract of bioactive metabolite, which has a high antimicrobial and antifungal activity. The lactic fermentation process influenced on physical–chemical parameters, the pH decreased and acidity increased on the obtained fermented plants extract. The biochemical analyses flavonoids, tannins and total polyphenols content of the obtained fermented plants extract (Fig. 2) showed that, the fermented extract using LAB after two weeks of fermentation increased the levels of total phenolics, flavonoids contents and tannin content (152.7; 93.6; and 62.1, respectively) to compare the levels of total phenolics, flavonoids contents and tannin content in the extract before fermentation (93.2; 38.4; and 28.11, respectively).
Biochemical composition before and after fermentation of the medicinal plants. + mg GAE/l: mg gallic acid equivalents per l of beverages. + + mg RE/l: mg of rutin equivalent per l of beverages. + + + mg CE/l: mg catechin equivalent per l of beverages. Results are expressed as mean of 3 experiments. **Data bearing different lowercase letters indicate a significant difference between the beverages before and after fermentation (p < 0.05)
Volatile compounds
During the lactic acid fermentation process, the selected LAB strains could not only reduce the content of carbohydrate, total protein and free amino acid but also to improve the flavor of the fermented plants extract. These metabolisms and biotransformation in the extract can prove the presence of new volatile compounds. The results indicated in Table 2 has shown twenty-four volatile compounds and identified with remarkable differences between the fermented plants extract and the plant extract without fermentation (Table 2). The GC–MS profiling showed that some compounds were detected only in fermented plants extract, such as 2-methyl-butyraldehyde, n-propyl acetate, 1-butanol. 3-methyl- formate, propanoic acid, propanoic acid-propyl ester, 2-oxopentanedioic acid, and lactic acid, other compounds, and detected only in non-fermented plants extract, such as α-phellandrene, D-limonene, fenchone, bornyl acetate, and caryophyllene. Among the volatile compounds detected some are typically produced by plants and other compounds are generated by the LAB metabolism. There were great variations in the composition of aroma components. The major compounds detected were (Eucalyptol (61%); Terpinen-4-ol, endo-Borneol, and p-Cimene were found in the two extracts. The results showed that the fermentation technology enhances the secondary metabolite extraction which gives new aromatic compounds to compare at the results before fermentation. In addition, our results suggest that the volatile compounds produced after fermentation by LAB could give positive flavor attributes to the flavor of the fermented plants extract. On the other hand, the primary metabolic actions of the used selected strain in fermented plants extract include their ability to predominantly ferment carbohydrates and, to a lesser degree, degrade proteins and fats in the substrate. This leads to the production of a broad range of bioactive metabolites, mainly organic acids (for example, lactic, acetic, malic, formic, propionic), peptides (bacteriocin), free amino acids, along with many volatile and non-volatile low molecular mass compounds, such as ketones and esters. During this study, the aromatic compounds changed after fermentation new volatile compound generated by the lactic acid bacteria, such as 2-methyl-butyraldehyde, n-propyl acetate, 1-butanol. 3-methyl-formate, propanoic acid, propanoic acid propyl ester, 2-oxopentanedioic acid, camphene, α-phellandrene, α-terpinene, p-cimene, D-limonene, Eucalyptol, gamma -Terpinene, and lactic acid, Table 2.
Antimicrobial activity and antifungal activity determinate by Disk diffusion methods
In this study, we have tested the fermented plants’ extract for their antimicrobial activity against resistant strains. The antimicrobial activity tested by disk diffusion method of obtaining extracts against resistance strains, presented in Table 3. The result showed the high antimicrobial activity with a maximum zone of inhibition against E. coli ATCC 25,922, E. coli ATCC 8739, E. coli ATCC 25,922/3, P. aeruginosa DSM 1128, S. aureus ATCC 29,213, E. aerogenes ATCC 13,048, E. faecalis ATCC 29,212 (13.4 ± 2, 14.4 ± 3, 16 ± 3, 10 ± 2, 14.3 ± 1, 14.5 ± 5, and 12 ± 4 mm, respectively). Results indicate that the fermented extract exhibited a great potential of antibacterial activity against all tested strains to compare with the extract without fermentation. In addition, the antifungal activity tested by disk diffusion method of obtaining extracts against resistance fungi strains, presented in Table 2. The result showed that the fermented plants’ extract exhibited high antifungal activity with a maximum zone of inhibition against Fusarium oxysporum, Aspergillus niger, P. infestans (GL-1), P. infestans P3 4/91 R+, and P. infestans P4 20/01 R (8 ± 2, 9 ± 3, 14 ± 1, 10 ± 2, and 8 ± 2.5, respectively) to compare with the plants’ extract without fermentation. However, the fermentation showed a significant (p ≤ 0.05) increase in antifungal activity of plants. This high antimicrobial activity due to the effect of lactic acid bacteria increased and enhanced the extraction of bioactive metabolites from the medicinal plants that have a high antimicrobial activity.
Antimicrobial and antifungal susceptibility testing
Susceptibility testing was done by broth microdilution in accordance with the National Committee for Clinical Laboratory Standards (CLSI). Table 4 summarizes the MIC50 and MIC90 values against the resistance bacterial and fungal. The results showed that the fermented plants’ extract exhibited highest antimicrobial and antifungal activity more than the plants extract without fermentation. The antimicrobial test showed that the MIC50 and MIC90 values against resistance bacteria (1.8 µg to 10 µg, respectively, from the fermented plants extract and 4 µg to > 20 µg, respectively, for the plants extract without fermentation), were lower or equivalent to the corresponding values of the control antibiotics.
The antifungal activity tested against fungi showed significant difference between the fermented plant extract and the plants extract without fermentation (p < 0.05) to compare with the control antibiotics. In addition, similar results for the susceptibility antifungal activity testing are also observed in Table 4. The MIC50 and MIC90 values of the fermented plants extract exhibited high activity against Fusarium oxysporum, Aspergillus niger, P. infestans (GL-1), P. infestans P3 4/91 R+, and P. infestans P4 20/01 R (9 to 16.5, 8 to 16, 10 to 18, 13 to 17 and 13 to 17.6 µg, respectively) to compare with the plants extract without fermentation (9 µg to 18 µg, respectively, for the fermented plants extract) to compared with the plants extract without fermentation (14 µg to > 20 µg, respectively).
Antifungal activity: inhibitory testing using growth assays
In this study, the antifungal activity of the fermented plant extract was tested against five stains having a high antifungal resistance: Fusarium oxysporum, Aspergillus niger, P. infestans (GL-1), P. infestans P3 4/91 R+, and P. infestans P4 20/01 R. The screening of the antifungal activity evaluated using growth assays illustrated in Table 5 showed that, the fermented plants extract exhibited high inhibition of fungal at different concentrations of spores to compare with plant extract without fermentation as in Table 2. In addition, the fermented plants’ extract exhibited total inhibition of the different fungi at the concentration of spore’s log (8) CFU/ml, to compare with the plants extract the fungal inhibition showed at log (4) CFU/ml. These data were confirmed by the morphological study illustrated in Fig. 3. That is, the microscopic observation showed the fermented plants’ extract total inhibition of fungi growth, Fig. 3, to compare with the plants’ extract without fermentation. This study confirmed the other data obtained by disk diffusion methods and the susceptibility test.
Microscopic pictures taken with an inverted microscope after 72 h of growth incubation with different samples. FO Fusarium oxysporum, AN Aspergillus niger, GL-1 P. infestans (GL-1), P3 P. infestans P3 4/91 R + , and P4: P. infestans P4 20/01 R. Control; without treatment, FP-ext; treated with fermented plants extract, P-ext; treated with plants extract without fermentation, Fungicide; trailed by fungicide
Discussion
During the fermentation process, the soluble components of protein, free amino acid, carbohydrate, free sugars were readily available, and the consumption of these nutrients by microorganisms led pH levels to drop (Di Cagno et al. 2017). As shown in Fig. 2, lactic acid fermentation is a metabolic process carried out by LAB to convert carbohydrates from plants into lactic acid or a mixture of lactic acid, acetic acid, ethanol and CO2. During the fermentation process, the decrease of sugar was due to their biotransformation inorganic acid. The production of these compounds can extend the shelf life by limiting the growth of contaminating and pathogenic microorganisms. In this sense, the growth and subsequent disappearance of some bacteria is common due to intra- and interspecific competition, as well as competition regarding the substrate, hence involving production of organic acids (Stoyanova et al. 2012). In this way, the fluctuation of pH values is a recurrent finding by several authors (Di Cagno et al. 2011; Begunova et al. 2020). Numerous studies have suggested the role of organic acid, such as lactic acid and acetic acid, in the antibacterial and antifungal activity of fermented extracts (Greenwalt et al. 1998; Sreeramulu et al. 2001).
In addition, polyphenols constitute one of the most preponderate groups of secondary metabolites in plants, including a wide variety of bioactive molecules that contain at least one aromatic ring with one or more hydroxyl groups in addition to other substitutes with large spectra of biological activities (Tlili et al. 2013). They are divided into different groups, such as flavonoids, tannins and phenolic acids (Tlili et al. 2013). After fermentation, the results indicated in Fig. 2 showed the content of total polyphenols, flavonoids and tannins increased to compare with the plants extract without fermentation, which confirms that lactic fermentation increases the extraction of secondary metabolites such as polyphenols compounds. During fermentation, many changes of composition occur, leading to a modified ratio of nutrients and anti-nutrients and therefore the properties of the product, such as bioactivity and digestibility, are modified (Barba et al. 2015). Several authors indicated that the lactic fermentation of plants has been shown to increase the concentration of several phenolic compounds (Teplova et al. 2018; Lukianov et al. 2018; Gadhoumi et al. 2021). We reported that the richness of our fermented medicinal plants extracts with phenolic compound could be an important starting point to explore their biological activities such as antioxidant activity (Gadhoumi et al. 2021).
Our results report that the microbial activity during the fermentation processes of the aromatic compound profiles of fermented plants extract changed to compare with plants extract without fermentation. Several authors suggested that, among the aromatic compounds, organic acids are regarded as the predominant compounds in the aromatic profile of fermented plants extract and are a common terminal end product in the catabolism of the sugar and the free amino acids (Gadhoumi et al. 2021; Di Cagno et al. 2017). Also, the aromatic compound characterization of each extract is a result of the diverse routes used by microorganism to metabolize the substrate releasing volatile compounds. Esters are considered to be important volatile components in fermented plants extract. The ester compounds are generated by the esterification of free acids with alcohol (Lukianov et al. 2018), in our results; in the fermented plants extract, we find the propanoic acid and propyl ester are produced by the esterification reaction. The major compound detected by GC–MS from the plant’s materiel, such as α-PINENE, Camphene, α-Phellandrene, α-Terpinene, and Eucalyptol, has a high antimicrobial and antifungal activity (Lukianov et al. 2018). The formation of medicinal plants enhances the extraction of bioactive compounds such as the volatile compounds produced by the plants (Gadhoumi et al. 2021).
The richness of the fermented plants extracts in bioactive compound, such as Eucalyptol, Camphene, α-Phellandrene, α-Terpinene, Bacteriocins, enzymes, and other polyphenol compounds, improves their biological activity. Several proteinaceous compounds exhibit bactericidal activity against pathogenic bacteria species closely related to the producer strain such as bacteriocins. These compounds alter the membrane potential and cause cell failure to balance intracellular pH (Sezer et al. 2013).
Moreover, the three tests used during this study; Disk diffusion methods, susceptibility testing, and inhibitory testing using growth assays confirmed the high antimicrobial and fungal activity of the fermented plants extract against pathogenic bacteria and fungi. Several authors mention that different bacteria and fungi process high resistance to antibiotic, and fungicide such as P. infestans exhibited high resistance to the fungicide and has harmful effects on plants, such as tomatoes and potatoes (Fry et al. 2008). In our study, the fermented plants extract exhibited a high antimicrobial and antifungal activity against resistant bacteria and fungi which is in accordance with the results reported by Jiang et al. (2021). The fermentation technology based on lactic fermentation and medicinal plants can be used as a solution for the pathogenic bacteria and fungi. It has been proposed that the antimicrobial effects involve the inhibition of different mechanism cellular, followed by an increase in plasma membrane permeability and finally ion leakage from the cells which causes pH imbalance (Kang et al. 2006). The antimicrobial and antifungal potency of the fermented plants extract is believed to be due to the bioactive compounds, such as tannins, saponins, phenolic compounds and flavonoids (Omafuvbe et al. 2009). It is interesting to note that even crude extracts of the medicinal plants improved by the fermentation technology showed good activity against resistant strains where modern antibiotic therapy has failed. As per our results, it suggests that this fermented extract inhibited growth of the test microorganisms at lower concentrations. The fermentation would be an opportunity for improving antimicrobial and antifungal activity of medicinal plants, which would open more applications. However, further studies are required to elucidate the biochemical composition and the mechanism of action.
Conclusion
To conclude, this study has demonstrated for the first time the effects of fermented plants extract using LAB against resistant pathogenic bacteria and fungal determined by inhibitory testing using growth assays, disk diffusion methods and susceptibility testing (MICs) according to National Committee for Clinical Laboratory Standards. In our study, the fermented plants extract revealed an enhanced level of polyphenols, flavonoids and tannins. In addition, the detection of aromatic compounds in the fermented plants extract showed interesting, volatile compounds, which could give pleasant-positive aroma attributes to the flavor of the extract and high antimicrobial activity. It is recognized that medicinal plants, as well as LAB, can be sources of antimicrobial compounds which can be applied for food preservation. The results showed that, the lactic fermentation enhances the extraction of polyphenols content, aromatic compound and increases the antimicrobial and antifungal activity potential against resistance pathogenic microorganism such as P. infestans (GL-1).
References
Anderson DM, Cembella AD, Hallegraeff GM (2012) Progress in understanding harmful algal blooms: paradigm shifts and new technologies for research, monitoring, and management. Ann Rev Mar Sci 4:143–176. https://doi.org/10.1146/annurev-marine-120308-081121
Barba FJ, Parniakov O, Pereira SA, Wiktor A, Grimi N, Boussetta N, Saraiva JA, Raso J, Martin-Belloso O, Witrowa-Rajchert D, Lebovka N (2015) Current applications and new opportunities for the use of pulsed electric fields in food science and industry. Int Food Res 77:773–798. https://doi.org/10.1016/j.foodres.2015.09.015
Begunova AV, Savinova OS, Rozhkova IV, Krysanova YI, Fedorova TV (2020) In vitro assessment of probiotic potential and functional properties of lactobacillus reuteri LR1. Appl Biochem Microbiol 56:544–552. https://doi.org/10.1134/S000368382005004X
Blanc PJ (1996) Characterization of the tea fungus metabolites. Biotech Lett 18(2):139–142. https://doi.org/10.1007/BF00128667
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Celiktas OY, Kocabas EH, Bedir E, Sukan FV, Ozek T, Baser KH (2007) Antimicrobial activities of methanol extracts and essential oils of Rosmarinus officinalis, depending on location and seasonal variations. Food Chem 100(2):553–559. https://doi.org/10.1016/j.foodchem.2005.10.011
Cetojevic-Simin DD, Bogdanovic GM, Cvetkovic DD, Velicanski AS (2008) Antiproliferative and antimicrobial activity of traditional Kombucha and Satureja montana L. Kombucha J BUON 13(3):395–401
Ciliao Filho M, Bertéli MBD, Valle JS, Paccola-Meirelles LD, Linde GA, Barcellos FG, Colauto NB (2017) Genetic diversity and pectinolytic activity of epiphytic yeasts from grape carposphere. Genet Mol Res. https://doi.org/10.4238/gmr16029698
Di Cagno R, Minervini G, Rizzello CG, De Angelis M, Gobbetti M (2011) Effect of lactic acid fermentation on antioxidant, texture, color and sensory properties of red and green smoothies. Food Microbiol 28(5):1062–1071. https://doi.org/10.1016/j.fm.2011.02.011
Di Cagno R, Filannino P, Gobbetti M (2017) Lactic acid fermentation drives the optimal volatile flavor-aroma profile of pomegranate juice. Int J Food Microbiol 248:56–62. https://doi.org/10.1016/j.ijfoodmicro.2017.02.014
Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, Gurr SJ (2012) Emerging fungal threats to animal, plant and ecosystem health. Nature 484(7393):186–194. https://doi.org/10.1038/nature10947
Freire AL, Ramos CL, da Costa Souza PN, Cardoso MG, Schwan RF (2017) Nondairy beverage produced by controlled fermentation with potential probiotic starter cultures of lactic acid bacteria and yeast. Int J Food Microbiol. https://doi.org/10.1016/j.ijfoodmicro.2017.02.011
Fry W (2008) Phytophthora infestans: the plant (and R gene) destroyer. Mol Plant Pathol 9(3):385–402. https://doi.org/10.1111/j.1364-3703.2007.00465.x
Gadhoumi H, Gullo M, De Vero L, Martinez-Rojas E, Saidani Tounsi M, Hayouni EA (2021) Design of a new fermented beverage from medicinal plants and organic sugarcane molasses via lactic fermentation. Appl Sci 11(13):6089. https://doi.org/10.3390/app11136089
Garcia-Solache MA, Casadevall A (2010) Global warming will bring new fungal diseases for mammals. Mbio. https://doi.org/10.1128/mBio.00061-10
Greenwalt CJ, Ledford RA, Steinkraus KH (1998) Determination and characterization of the antimicrobial activity of the fermented tea kombucha. LWT-Food Sci Technol 31(3):291–296. https://doi.org/10.1006/fstl.1997.0354
Henwood CJ, Livermore DM, James D, Warner M, Pseudomonas Study Group T (2001) Antimicrobial susceptibility of Pseudomonas aeruginosa: results of a UK survey and evaluation of the British Society for Antimicrobial Chemotherapy disc susceptibility test. J Antimicrob Chemother 47(6):789–799
Jiang S, Zhang J, Yang Q, Sun D, Pu X, Shen H, Li Q, Wang Z, Lin B (2021) Antimicrobial activity of natural plant compound carvacrol against soft rot disease agent Dickeya zeae. Curr Microbiol 78(9):3453–3463. https://doi.org/10.1007/s00284-021-02609-3
Kang HJ, Hwang IK, Kim KS, Choi HC (2003) Comparative structure and physicochemical properties of Ilpumbyeo, a high-quality japonica rice, and its mutant, Suweon 464. J Agric Food Chem 51(22):6598–6603. https://doi.org/10.1021/jf0344946
Kang CI, Kim SH, Bang JW, Kim HB, Kim NJ, Kim EC, Oh MD, Choe KW (2006) Community-acquired versus nosocomial Klebsiella pneumoniae bacteremia: clinical features, treatment outcomes, and clinical implication of antimicrobial resistance. J Korean Med Sci 21(5):816–822. https://doi.org/10.3346/jkms.2006.21.5.816
Lim YS, Kim WR (2008) The global impact of hepatic fibrosis and end-stage liver disease. Clin Liver Dis. https://doi.org/10.1016/j.cld.2008.07.007
Luana N, Rossana C, Curiel JA, Kaisa P, Marco G, Rizzello CG (2014) Manufacture and characterization of a yogurt-like beverage made with oat flakes fermented by selected lactic acid bacteria. Int J Food Microbiol 18(185):17–26. https://doi.org/10.1016/j.ijfoodmicro.2014.05.004
Lukianov DA, Debabov VG (2018) Obtainment of succinic acid and higher alcohols (C 8–C 10) diesters by biphasic esterification. Appl Biochem Microbiol 54(9):863–868. https://doi.org/10.1134/S0003683818090053
Miyake Y, Fukumoto S, Okada M, Sakaida K, Nakamura Y, Osawa T (2005) Antioxidative catechol lignans converted from sesamin and sesaminol triglucoside by culturing with Aspergillus. J Agric Food Chem. https://doi.org/10.1021/jf048743h
Naczk M, Shahidi F (2006) Phenolics in cereals, fruits and vegetables: occurrence, extraction and analysis. J Pharm Biomed Anal. https://doi.org/10.1016/j.jpba.2006.04.002
Nakano D, Kwak CJ, Fujii K, Ikemura K, Satake A, Ohkita M, Takaoka M, Ono Y, Nakai M, Tomimori N, Kiso Y (2006) Sesamin metabolites induce an endothelial nitric oxide-dependent vasorelaxation through their antioxidative property-independent mechanisms: possible involvement of the metabolites in the antihypertensive effect of sesamin. J Pharmacol Exp Thera 318(1):328–335. https://doi.org/10.1124/jpet.105.100149
Navarini L, Gilli R, Gombac V, Abatangelo A, Bosco M, Toffanin R (1999) Polysaccharides from hot water extracts of roasted Coffea arabica beans: isolation and characterization. Carbohydr Polym 40(1):71–81. https://doi.org/10.1016/S0144-8617(99)00032-6
Omafuvbe BO, Akanbi OO (2009) Microbiological and physico-chemical properties of some commercial Nigerian honey. Afr J Microbiol Res 3(12):891–896
Parvez S, Malik KA, Ah Kang S, Kim HY (2006) Probiotics and their fermented food products are beneficial for health. J Appl Microbiol 100(6):1171–1185. https://doi.org/10.1111/j.1365-2672.2006.02963.x
Pino JA, Moncayo-Molina L, Spengler I, Pérez JC (2021) Chemical composition and antibacterial activity of the leaf essential oil of Eucalyptus globulus Labill. from two highs of the canton Cañar Ecuador. Revista CENIC Ciencias Químicas 52:026–033
Rasor JP, Voss E (2001) Enzyme-catalyzed processes in pharmaceutical industry. Appl Catal A 221(1–2):145–158. https://doi.org/10.1016/S0926-860X(01)00804-3
Scarpino V, Reyneri A, Sulyok M, Krska R, Blandino M (2015) Effect of fungicide application to control Fusarium head blight and 20 Fusarium and Alternaria mycotoxins in winter wheat (Triticum aestivum L.). World Mycotoxin J 8(4):499–510. https://doi.org/10.3920/WMJ2014.1814
Sezer Ç, Güven A, Oral NB, Vatansever L (2013) Detoxification of aflatoxin B_1 by bacteriocins and bacteriocinogenic lactic acid bacteria. Turkish J Vet Anim Sci 37(5):594–601. https://doi.org/10.3906/vet-1301-31
Smid EJ, Kleerebezem M (2014) Production of aroma compounds in lactic fermentations. Annu Rev Food Sci Technol 5:313–326. https://doi.org/10.1146/annurev-food-030713-092339
Song W, Han X, Qian Y, Liu G, Yao G, Zhong Y, Qu Y (2016) Proteomic analysis of the biomass hydrolytic potentials of Penicillium oxalicum lignocellulolytic enzyme system. Biotechnol Biofuels 9(1):1–15. https://doi.org/10.1186/s13068-016-0477-2
Sreeramulu G, Zhu Y, Knol W (2001) Characterization of antimicrobial activity in Kombucha fermentation. Acta Biotechnol 21(1):49–56. https://doi.org/10.1002/1521-3846(200102)21:13.0.CO;2-G
Steinkraus KH, Shapiro KB, Hotchkiss JH, Mortlock RP (1996) Investigations into the antibiotic activity of tea fungus/kombucha beverage. Acta Biotechnol 16(2–3):199–205. https://doi.org/10.1002/abio.370160219
Stoyanova LG, Ustyugova EA, Netrusov AI (2012) Antibacterial metabolites of lactic acid bacteria: their diversity and properties. Appl Biochem Microbiol 48(3):229–243. https://doi.org/10.1134/S0003683812030143
Teplova VV, Isakova EP, Klein OI, Dergachova DI, Gessler NN, Deryabina YI (2018) Natural polyphenols: biological activity, pharmacological potential, means of metabolic engineering. Appl Biochem Microbiol 54(3):221–237. https://doi.org/10.1134/S0003683818030146
Thanusu J, Kanagarajan V, Gopalakrishnan M (2010) Synthesis, spectral characterization, and in vitro antibacterial and antifungal activities of novel 1, 3-thiazine-2-amines comprising morpholine nucleus. J Enzyme Inhib Med Chem 25(6):756–764. https://doi.org/10.3109/14756360903389898
Tian H, Shen Y, Yu H, He Y, Chen C (2017) Effects of 4 probiotic strains in coculture with traditional starters on the flavor profile of yogurt. J Food Sci 82(7):1693–1701. https://doi.org/10.1111/1750-3841.13779
Tlili N, Elfalleh W, Hannachi H, Yahia Y, Khaldi A, Ferchichi A, Nasri N (2013) Screening of natural antioxidants from selected medicinal plants. Int J Food Prop 16(5):1117–1126. https://doi.org/10.1080/10942912.2011.576360
Velićanski AS, Cvetković DD, Markov SL, Tumbas Šaponjac VT, Vulić JJ (2014) Antioxidant and antibacterial activity of the beverage obtained by fermentation of sweetened lemon balm (Melissa offi cinalis L.) tea with symbiotic consortium of bacteria and yeasts. Food Technol Biotechnol. 52(4):420–429. https://doi.org/10.17113/b.52.04.14.3611
Wu T, Wang N, Zhang Y, Xu X (2013) Advances in the study on microbial fermentation and transformation of traditional Chinese medicine. Afr J Microbiol Res 7(17):1644–1650. https://doi.org/10.5897/AJMRx12.012
Yoon KY, Woodams EE, Hang YD (2006) Production of probiotic cabbage juice by lactic acid bacteria. Bioresour Technol 97(12):1427–1430. https://doi.org/10.1016/j.biortech.2005.06.018
You L, Gao Q, Feng M, Yang B, Ren J, Gu L, Cui C, Zhao M (2013) Structural characterisation of polysaccharides from Tricholoma matsutake and their antioxidant and antitumour activities. Food Chem 138(4):2242–2249. https://doi.org/10.1016/j.foodchem.2012.11.140
Zhang Z, Lv G, Pan H, Fan L, Soccol CR, Pandey A (2012) Production of powerful antioxidant supplements via solid-state fermentation of wheat (Triticum aestivum Linn.) by Cordyceps militaris. Food Technol Biotechnol 50(1):32–39
Acknowledgements
We are grateful and provide sincere thanks to honorable Vice Chancellor University of Applied Sciences. Neubrandenburg, Germany for the infrastructure and facilities. This study supported by a grant from the Ministry of Tunisia, Laboratory of Aromatic and Medicinal Plants, Center of Biotechnology (LR15CBBC06) at the Ecopark of Borj-cédria. BP-901, 2050 Hammam-Lif. Tunisia.
Funding
This study supported by University of Tunis EL Manar, University of Applied Sciences, Neubrandenburg, Germany, for the infrastructure and facilities, and Laboratory of Aromatic and Medicinal Plants, Center of Biotechnology (LR15CBBC06) at the Ecopark of Borj-cédria. BP-901, 2050 Hammam-Lif. Tunisia.
Author information
Authors and Affiliations
Contributions
HG and AelH, M-RE, and MST designed the study and performed the experiments. HG and M-R Enriqueta performed the experiment and carried out data analyses. HG and AelH contributed to drafting the manuscript. All authors have read and approved the final version of submitted manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors of this manuscript certify that they have no affiliation with or involvement in any organization or entity with any financial interest. I certify that I am submitting the manuscript on behalf of all the authors.
Ethics approval
This article does not contain any studies with human participants performed by any of the authors.
Consent for publication
All authors have seen the manuscript and approved to submit Archives of Microbiology.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gadhoumi, H., Hayouni, E.L.A., Martinez-Rojas, E. et al. Biochemical composition, antimicrobial and antifungal activities assessment of the fermented medicinal plants extract using lactic acid bacteria. Arch Microbiol 204, 374 (2022). https://doi.org/10.1007/s00203-022-02985-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-02985-9