Abstract
Listeriosis is an emerging bacterial disease of animals and humans worldwide, caused by Listeria monocytogenes. The infected dairy cows continuously shed the microbes in their milk, a human being’s concern. This study was designed to molecular characterize the Listeria monocytogenes isolated from symptomatic cow's milk of tehsils Samundri, Gujar khan, and Alipur of Punjab. A total of 175 milk samples were collected, pre-enriched and cultured on PALCAM agar. The affirmation of the hlyA gene of Listeria monocytogenes was performed by polymerase chain reaction (PCR) and 3.43% of isolates were found positive. The phylogenetic analysis showed a resemblance of our isolates of Listeria monocytogenes with India (KP965733), the USA (DQ812484), and 3 of our isolates made a clade. The leucocytes and neutrophils count were found significantly increased in listeriosis affected cows. The Chi-square test showed a significant association between poor quality silage feeding and listeriosis. The presence of L.monocytogenes in cow’s milk indicates a potential threat to humans. It is further recommended that it should be consistently monitored to ensure food safety.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Listeriosis is a global bacterial disease, and it affects a variety of animals and human beings. Listeria monocytogenes is a Gram-positive, facultatively anaerobic, non-spore-forming, motile, and intracellular pathogen (Mary and Shrinithivihahshini 2017). L.monocytogenes can withstand a wide range of pH, temperatures and salt concentrations, making it a possible hazard in raw milk (Owusu-Kwarteng et al. 2018). The bacterium has been reported as a significant public health issue with high mortality than other food-borne microbes (Saha et al. 2015). L.monocytogenes has been identified in raw cow's milk and dairy products (Santorum et al. 2012; Jamali et al. 2013). Most instances of listeriosis emerge from the ingestion of contaminated food and silage feeding in ruminants. In cattle, the main forms of listeriosis are abortion, encephalitis, and septicemia (Pohl et al. 2006). The case mortality rate due to listeriosis may vary from 20 to 100% (Bundrant et al. 2011).
At present, L. monocytogenes has four lineages (I, II, III, and IV), lineage I and II are primarily found in human cases. Lineage II is frequently isolated from foods and animal cases. Lineages III and IV are present rarely in animals (Orsi et al. 2011). L. monocytogenes has 13 serotypes and about 95% of human listeriosis occurred due to serotypes 1/2a, 1/2b, 1/2c, and 4b (Alía et al. 2020). The bacterium contains various virulence factors such as listeriolysin O, internalins, phosphatidylinositol-phospholipase C, actin, invasion-associated protein, and virulence regulator (Vera et al. 2013). Among these factors, listeriolysin O (hlyA) is the major virulent marker of L.monocytogenes (Hamon et al. 2012).
The diagnosis of listeriosis by the conventional culture methods has the drawback of being time-consuming with the probability of false-positive results (Gasanov et al. 2005). However, the molecular technique for the confirmation of virulence genes of L.monocytogenes has been revealed to be more reliable (Rawool et al. 2007b).
In Pakistan, the first human listeriosis was reported with an incidence rate of 1.66% (Nasim and Vahidy 1993). The prevalence of L. monocytogenes in cow’s milk was 2.25% to 6% in Pakistan (Chandio et al. 2007; Usman and Mukhtar 2014). In the South Asian region, previous studies have been reported that contaminated milk acts as a vehicle for the transmission of L. monocytogenes (Jayamanne and Samarajeewa 2001; Sharma et al. 2012). Globally, the occurrence of L. monocytogenes in cow’s milk samples was stated 5.3%, 4%, 1.66%, and 7.5% (Yakubu et al. 2012; Nayak et al. 2015; Dalzini et al. 2016; Obaidat and Stringer 2019), respectively. In Pakistan, cow’s milk is mostly consumed by people at risk of L. monocytogenes. There is limited data available in Pakistan, particularly Punjab, on the risk posed by cow's milk. Hence, the current study was conducted (1) to find out the L. monocytogenes from cows' milk samples by cultural and molecular techniques and (2) to compare the hematological profile of L. monocytogenes infected cows with healthy ones to fill a breach in an understanding of this emerging bacterial disease.
Materials and methods
Inclusion and exclusion criteria of the study
The inclusion criteria of the study comprised demographic characteristics of cows (female, lactation stage, breed local or exotic, and tehsils Samundri, Gujar khan, and Alipur) and clinical indicators of listeriosis such as anorexia, motor incoordination, tilting of the head, circling, unilateral facial hypalgesia, ear drooping, lips paralysis, jaw muscles paresis, abortion and mastitis (Erdogan et al. 2001). Those dairy cows were excluded for samples that did not fall in the defined inclusion criteria.
Sampling and design
The study was conducted at different veterinary hospitals and dairy farms of tehsils Samundri (31° 03′ 45″ N, 72° 57′15″ E), Gujar khan (33° 15′ 15.6″ N, 73° 18′ 23.7″ E) and Alipur (29° 23′ 0″ N, 70° 55′ 0″ E) of Punjab Pakistan (Fig. S1). One hundred seventy-five (175) milk samples comprising 44 Sahiwal, 53 Friesian, 38 Cross-bred, 23 Cholistani, and 17 Non-descriptive dairy cows (25 ml of milk from each) were collected (Meyer-Broseta et al. 2003) from teats aseptically in sterilized vials from October 2019 to June 2021. Simultaneously, 6 listeriosis suspected cows and 6 healthy cows were selected for hematological analysis. 3 ml of blood samples from each selected cattle were collected from the external jugular vein using 5 ml disposable syringes (Jiangsu Zhiyu Medical Instrument Co. China) and shifted to EDTA.K3 coated vacutainers (Ali et al. 2011). These vacutainers were kept in an icebox and transported to the University of Veterinary and Animal Sciences, Lahore (Biosafety level-2). These vacutainers were stored at − 20 °C until further analysis. The dairy cows’ data of environmental and management factors were recorded in our questionnaires.
Isolation and identification of L. monocytogenes
As per the protocol of ISO 11290 method, 25 ml of milk sample was added in 225 ml Listeria half Fraser broth base ISO (Cat # 1183, Condalab Spain) and incubated at 30 °C for 24 h. 0.1 ml of pre-enriched broth was poured into 10 ml Listeria Fraser broth base and incubated at 37 °C for 48 h. After that, the inoculum was streaked onto Polymyxin Acriflavin Lithium-chloride Ceftazidime Esculin Mannitol (PALCAM) Listeria Agar Base IS0 11290-2 (Cat # 1141.55, Condalab) with added PALCAM Listeria Selective Supplements (Cat # 6004, Condalab) and incubated at 37 °C for 48 h. The apparent Listeria colonies (3/plate) were examined by the Gram staining. The biochemical tests comprising catalase, methyl red, oxidase, urease, citrate, nitrate, Voges Proskauer, and motility were performed. The positive isolates were cultured on 5% sheep blood agar (Cat # 1108, Condalab), then subjected to sugar fermentation (mannitol, maltose, rhamnose, xylose, and glucose) and Christie–Atkins–Munch-Peterson (CAMP) tests (Rahimi et al. 2014).
Extraction of genomic DNA and primer designing
According to the manufacturer’s instructions, genomic DNA was extracted using a GeneJet Genomic DNA purification kit (Cat # k 0721, Thermo Fisher Scientific, USA). The pure culture (1 ml) of L. monocytogenes was used for genomic DNA extraction. These isolates were centrifuged at 5000×g for 10 min at 37 °C. A Nanodrop 1000 spectrophotometer checked the genomic DNA concentration at 260 and 280 nm (Thermo Scientific, USA).
The oligonucleotide primers were purchased commercially (Cat # EO 6400-05, GeneLink™, USA). The forward primer (5′- GCA GTT GCA AGC GCT TGG AGT GAA-3′) and reverse primers (5'- GCA ACG TAT CCT CCA GAG TGA TCG-3′) targeting hlyA gene of L. monocytogenes for a 456 bp product size (Paziak-Domańska et al. 1999; Rawool et al. 2007b).
Amplification of hlyA gene
The primers for a specific gene (hlyA) were optimized by adjusting the annealing temperature, the concentration of primers, DNA template, master mix volume, and cycling conditions. The protocol for detecting the hlyA gene of L. monocytogenes was followed with slight modification (Laximan et al. 2016).
For PCR reaction, a total volume of 25 μL mixture consisting of 12.5 μL PCR master mix (2X) (Cat # K1081, ThermoFisher Scientific, USA), 1 μL forward primer, 1 μL reverse primer, 1.5 μL DNA template and 9 μL nuclease-free water (Cat # RO581, ThermoFisher Scientific, USA). The DNA amplification was carried out in SimpliAmp™ Thermal Cycler (Cat # A24811, ThermoFisher Scientific, USA). The PCR cycling conditions included an initial denaturation of DNA at 94 °C for 1 min followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 53 °C for 1 min and extension at 72 °C for 45 s. The final extension was conducted at 72 °C for 2 min and held at 4 °C. The PCR products (5 µl) were run on 1.5% agarose gel against a 100 bp molecular ladder (Cat # DM001-R500, GeneDirex, USA) for the presence of the band, and then visualized under a gel imaging system (Omega fluor plus ™, Aplegen Inc, USA). The PCR products (456 bp) were purified by a GeneJet Gel extraction kit (Cat # 0691, Thermo Scientific, USA).
DNA sequencing, editing, and phylogenetic analysis
Six isolates were sent to the 1st Base biological technology (Malaysia) and the primers for sequencing. The BioEdit 7.2 software was used for editing and sequence alignment. The hlyA gene sequences were aligned against recognized sequences in the GenBank and analyzed through NCBI nucleotide BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The Neighbour-Joining method constructed the phylogenetic tree (Saitou and Nei 1987). The level of duplicate trees in which the related taxa bunched together in the bootstrap test (1000 replicates) is displayed close to the branches (Felsenstein 1985). The evolutionary distances were calculated by utilizing the Tamura-Nei method (Tamura and Nei 1993) and are in the units of the number of base substitutions per site. All ambiguous positions were taken out for each arrangement pair. Evolutionary analyses were conducted in MEGA X version 10.2.6 (Kumar et al. 2018).
Hematological analysis
A hematology analyzer analyzed the 6 positive cases and 6 healthy dairy cows for hematological parameters like RBCs, hemoglobin, hematocrit, neutrophils, etc., by a hematology analyzer (Abacus junior Vet, Vienna, Austria) (Schweizer et al. 2006).
Statistical analysis
The study data were shifted to a Microsoft Excel sheet for compiling and analyzed using SPSS 16.0 (IBM, USA). The Chi-square test was applied to determine the association of risk factors with listeriosis in dairy cows (Boerlin et al. 2003; Strawn et al. 2013; Scobie et al. 2019). Independent samples t test was performed on the data of hematological parameters. The compiled data were normally distributed and homogenized by Levene’s test for equality of variances. The values of p < 0.05 were considered significant.
Accession numbers of nucleotide sequences
The nucleotide sequences of the hlyA gene of L. monocytogenes were retrieved from the NCBI GenBank database under the accession numbers OK492161-OK492166.
Results
Isolation and identification of L. monocytogenes
The growth on the PALCAM agar plate showed distinctive colonies of Listeria. These colonies were grey-green with blackening of surrounding media. These colonies appeared shining and pointed. On 5% sheep blood agar, small colonies of listeria and β-hemolysis were observed. The β-hemolysis was limited to the edge of colonies. The microscopic characteristics revealed the coccobacilli shape, arranged in single or pair form on Gram staining.
Biochemical tests
Biochemical tests indicated catalase, methyl red positive, and indole negative. The maltose and glucose fermentation tests were positive. The nitrate reduction, citrate utilization and urease test were found negative. The motility test on semi-solid media described umbrella-like growth and exhibited a positive motility test at 25 °C.
Out of 175 symptomatic cows samples processed, 9 isolates presumptively were found L.monocytogenes positive based on phenotypic characteristics. For confirmation, these isolates were further tested by molecular technique.
Polymerase chain reaction (PCR)
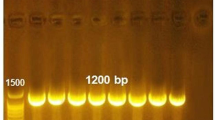
The isolates were analyzed for confirmation of virulence-associated genes. 6 isolates showed the product size of 456 bp (hlyA gene) against the molecular weight marker of 100 bp (Cat # DM001-R500, GeneDirex, USA) (Fig. S2).
Phylogenetic analysis
The evolutionary tree was created by the hlyA gene sequence of L. monocytogenes accessible in GenBank and the sequence of our isolates. The nucleotides identity of our sequences of L. monocytogenes with GenBank sequences was retrieved from NCBI GenBank (Table 1). This information showed that our isolates had a high level of the hlyA gene sequences percent identity (97–100%) with other reported sequences of the hlyA gene.
The phylogenetic analysis showed a resemblance of our isolate L. monocytogenes-8 with strain 4H hemolysin A (hlyA) gene of India (accession number KP965733). Three of our isolates (LS2, P1, and P21) made a clade and showed a resemblance with strain VC2 hlyA gene of India (KJ504140). Our isolate L. monocytogenes LS1 revealed resemblance with strain NRRL 33313 hlyA gene of USA (DQ812484). The isolate P2 showed a non-significance resemblance with our and other reported sequences (Fig. 1).
Risk factor analysis
The data obtained on questionnaires from each experimental unit of dairy farmers showed a higher occurrence of listeriosis in Samundri (4.84%) as compared to Alipur (3.07%) and Gujar khan (2.08%) tehsils. Non-descriptive (5.88%), cross-bred (5.26%), Frisian (3.78%) were found highly susceptible to infection as compared to Sahiwal (2.27%) and Cholistani cows (0%). The mostly silage feeding was significantly (p = 0.002) associated with listeriosis. The cows > 3 years of age (3.87%) were found more prone to listeriosis as compared to cows < 3 year of age (2.32%). Other risk factors are non-significantly (p > 0.05) associated with listeriosis (Table 2).
Hematological profiles
The hematological parameters were examined in diseased and healthy dairy cows. There was an association of increased neutrophils and leucocytes levels with listeriosis in dairy cows. The leucocytes and neutrophils values showed a significant increase (p < 0.05) in diseased cows as compared to healthy ones. The values of lymphocytes, monocytes, and eosinophils were non-significantly decreased (p > 0.05) in diseased cows. The plasma protein and plasma fibrinogen values showed a non-significant increase (p > 0.05) in diseased cows as compared to healthy ones (Table 3).
Discussion
Listeriosis was first recognized as a sporadic disease in many animals in Germany. Infectious agent L. monocytogenes is a cryophiles bacterium with a vast virulence potential equal to the epizootic occurrence in domestic animals (Seeliger 1988). Ongoing perceptions show that milk is a vehicle for the transmission of listeriosis. At present, the limited information on the occurrence of listeriosis in dairy cows has hindered listeriosis control and protective strategies. The clinical manifestations of listeriosis in cows were described as unilateral facial paralysis, incoordination, tilting of the head, etc., (Low and Donachie 1997). In this study, similar signs/symptoms were observed.
The pre-enrichment with Fraser broth facilitated the isolation and identification of Listeria spp. The blacking of Listeria selective broth due to esculin hydrolysis (Fraser and Sperber 1988). Listeria grows slowly and could be outgrown by other micro-organisms, so the bacteriostatic drugs were added in broth/media (Gasanov et al. 2005b); a similar pattern was followed in this study.
The present study has reported the occurrence of L. monocytogenes 3.43% in cows milk. This is supported by the occurrence of L. monocytogenes 4.4%, 1.9%, 2.04%, 1.66%, 2.5%, and 2.92% in raw milk (Baek et al. 2000; Rahimi et al. 2014; Seyoum et al. 2015; Dalzini et al. 2016; El Hag et al. 2021; Kumar and Ph 2021), respectively. 6% and 8.8% occurrence of L. monocytogenes in raw cow's milk in India and Ghana (Biswas et al. 2018; Owusu-Kwarteng et al. 2018). This study's findings were similar to the 5.8% occurrence in cow's milk (Soni et al. 2013). On the contrary, the high frequency (40%) of L. monocytogenes in raw milk (Zafar 2020). A study reported a 22.5% occurrence of L. monocytogenes in raw cow milk (Jamali et al. 2013). These variations in findings might be due to poor hygienic conditions at the farm level, different farming practices, and altered weather conditions in different countries.
In this study, the risk factors associations between low-quality silage feeding and listeriosis cases were found significant. Infected animals at the farm, poor quality silage, and fecal contamination during milking were the main sources of listeriosis (Rodriguez et al. 2021). The occurrence of listeria was 8.5% on farms fed on silage. The risk factors reported earlier included low quality of feed, fecal contamination of raw milk, and unhygienic conditions at farms (Sanaa et al. 1993). The milk, fecal, and silage samples showed 6.1%, 9.3%, and 6.0% occurrence of L. monocytogenes (Vilar et al. 2007). A preceding study elaborated a significant association between silage feeding and the occurrence of L. monocytogenes. It was documented that silage feeding increased the risk of listeriosis from 3 to 7 times more than no silage-fed animals (Schoder et al. 2011). Listeria spp. in raw milk was mostly due to contamination during milking and poor quality silage (Bemrah et al. 1998). Listeriosis due to poorly fermented silage feeding cattle was reported (Nightingale et al. 2004). These results are in congruence with this study.
The hematological parameters had slight diagnostic importance and leukocytosis was not a specific feature of listeriosis. But, these could be helpful for treatment purposes (Braun et al. 2002; Schweizer et al. 2006). However, leukocytosis and neutrophilia were reported in the present study. The difference in findings might be due to neutrophils’ protective response for intracellular bacteria, i.e., L. monocytogenes has been recognized recently. Owing to disease with L.monocytogenes, neutrophils conscripted from the bone marrow to sites of contagion. These neutrophils played a vital role in phagocytosis and bactericidal effect (Wa et al. 2021).
The phylogenetic analysis showed a resemblance of Pakistani isolate of L. monocytogenes-8 with strain 4H hemolysin A (hlyA) gene of India (accession number KP965733) (Laximan et al. 2016). Likewise, a PCR assay was performed to detect virulence genes (Rawool et al. 2007), one of the virulence-associated genes (hlyA) identified resembled this study’s findings. The phylogenetic tree based on the sequence of the hlyA gene was recognized to distinguish between L. monocytogenes, L. seeligeri, and L. ivanovii. The sequences were aligned by CLUSTAL-W and similarity was assessed by the neighbor-joining method (Soni et al. 2013). Similar alignment and methods were used in this study; however, MEGA-X was used for phylogenetic analysis. Based on the virulence-associated genes, previous research reported that the hlyA gene was found 3.2% in raw milk. An association of L.monocytogenes lineage-II (1/2a and 3a serogroup) with animal milk and its products might be a public health risk (Swetha et al. 2015). The application of PCR to detect virulence markers of L.monocytogenes was a rapid technique (Vázquez-Boland et al. 2001). In the present study, Pakistani isolate of L. monocytogenes LS1 revealed resemblance with strain NRRL 33313 hlyA gene of USA (DQ812484). Three Pakistani isolates (LS2, P1, and P21) made a clade and showed a resemblance with strain VC2 hlyA gene of India (KJ504140). Pakistani isolate P2 showed non-significance resemblance with our and other reported sequences. L. monocytogenes is an exceptionally diverse species with two different phylogenetic heredities varying in their developmental history; lateral gene transfer and positive selection played a role in the evolution of L. monocytogenes (Nightingale et al. 2005). Although there is no direct movement of domestic animals between India and Pakistan, but listeriosis can be transmitted through infected wild animals and birds crossing the borders, contaminated crops, and soil. Farm animals may get this infection either through grazing on infected pastures or by feeding on contaminated silage (Nightingale et al., 2004).
Different researchers recognized the virulence of L. monocytogenes to be linked with lineage (Su et al. 2016). 1/2a and 3a were reported as the main serovars of L. monocytogenes (Shakuntala et al. 2019). This study's findings are in line as 1/2a, and 3a (lineage-II) were the most abundant serotype in animal listeriosis cases. The lineage II strains might be adjusted to ecological conditions than lineage I strain. Four lineages of L. monocytogenes will urge prospective research (Orsi et al. 2011).
Conclusions
The current study has depicted the occurrence of L. monocytogenes in the raw milk of dairy cows in Punjab, Pakistan. The feeding of poor quality silage has been identified as a substantial risk factor for listeriosis in dairy cows. Exotic breeds have been observed as more vulnerable to listeriosis than native dairy breeds. The elevated level of hematological parameters like neutrophils and leucocytes may helpful in the preliminary diagnosis of listeriosis in dairy cows. The study findings provide benchmark data for future research on listeriosis in dairy cows.
Data availability
The accession numbers of the hlyA gene of L. monocytogenes retrieved from NCBI GenBank were described in Table 1.
Code availability
The software used for statistical and phylogenetic analysis is freely available on the internet.
References
Ali A, Derar R, Hussein H, Abd Ellah M, Abdel-Razek AK (2011) Clinical, hematological, and biochemical findings of uterine torsion in buffaloes (Bubalus bubalis). Anim Reprod Sci 126:168–172
Alía A, Andrade MJ, Córdoba JJ, Martín I, Rodríguez A (2020) Development of a multiplex real-time PCR to differentiate the four major Listeria monocytogenes serotypes in isolates from meat processing plants. Food Microbiol 87:103367
Baek SY, Lim SY, Lee DH, Min KH, Kim CM (2000) Incidence and characterization of Listeria monocytogenes from domestic and imported foods in Korea. J Food Prot 63:186–189. https://doi.org/10.4315/0362-028X-63.2.186
Bemrah N, Sanaa M, Cassin MH, Griffiths MW, Cerf O (1998) Quantitative risk assessment of human listeriosis from consumption of soft cheese made from raw milk. Prev Vet Med 37:129–145. https://doi.org/10.1016/S0167-5877(98)00112-3
Biswas P, Deka D, Dutta T, Motina E, Roychoudhury P (2018) Conventional and molecular detection of Listeria monocytogenes and its antibiotic sensitivity proβile from cattle sources of Aizawl, Mizoram (India). Int J Curr Microbiol Appl Sci 7:2284–2829
Boerlin P, Boerlin-Petzold F, Jemmi T (2003) Use of listeriolysin O and internalin A in a seroepidemiological study of listeriosis in Swiss dairy cows. J Clin Microbiol 41:1055–1061
Braun U, Stehle C, Ehrensperger F (2002) Clinical findings and treatment of listeriosis in 67 sheep and goats. Vet Rec 150:38–42. https://doi.org/10.1136/vr.150.2.38
Bundrant BN, Hutchins T, den Bakker HC, Fortes E, Wiedmann M (2011) Listeriosis outbreak in dairy cattle caused by an unusual Listeria monocytogenes serotype 4B strain. J Vet Diagn Investig 23:155–158. https://doi.org/10.1177/104063871102300130
Chandio TH, Soomro AH, Bhutto MB, Dewani P, Shah G (2007) Occurrence of Listeria monocytogenes in bovine milk in Hyderabad, Pakistan. Ann Microbiol 57:341–344
Dalzini E, Bernini V, Bertasi B, Daminelli P, Losio M-N, Varisco G (2016) Survey of prevalence and seasonal variability of Listeria monocytogenes in raw cow milk from Northern Italy. Food Control 60:466–470
El Hag MMA, El Zubeir IEM, Mustafa NEM (2021) Prevalence of Listeria species in dairy farms in Khartoum State (Sudan). Food Control 123:107699. https://doi.org/10.1016/j.foodcont.2020.107699
Erdogan H, Cripps P, Morgan K, Cetinkaya B, Green L (2001) Prevalence, incidence, signs and treatment of clinical listeriosis in dairy cattle in England. Vet Rec 149:289–293
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Fraser JA, Sperber WH (1988) Rapid detection of Listeria spp. in Food and environmental samples by esculin hydrolysis. J Food Prot 51:762–765
Gasanov U, Hughes D, Hansbro PM (2005) Methods for the isolation and identification of Listeria spp. and Listeria monocytogenes: a review. FEMS Microbiol Rev 29:851–875. https://doi.org/10.1016/j.femsre.2004.12.002
Hamon MA, Ribet D, Stavru F, Cossart P (2012) Listeriolysin O: the Swiss army knife of Listeria. Trends Microbiol 20:360–368. https://doi.org/10.1016/j.tim.2012.04.006
Jamali H, Radmehr B, Thong KL (2013) Prevalence, characterisation, and antimicrobial resistance of Listeria species and Listeria monocytogenes isolates from raw milk in farm bulk tanks. Food Control 34:121–125
Jayamanne V, Samarajeewa U (2001) Incidence and detection of Listeria monocytogenes in milk and milk products of Sri Lanka
Kumar S, Ph D (2021) Genetic diversity, virulence and distribution of antimicrobial resistance among Listeria monocytogenes isolated from milk, beef, and bovine farm environment. Iran J Vet Res 22:1–8
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547
Laximan S, Kaur S, Aulakh RS, Gill JPS (2016) Molecular characterization of Listeria monocytogenes in bovine milk and evaluating the sensitivity of PCR for direct detection in milk. Indian J Anim Sci 86:512–517
Low JC, Donachie W (1997) A review of Listeria monocytogenes and listeriosis. Vet J 153:9–29. https://doi.org/10.1016/S1090-0233(97)80005-6
Mary M, Shrinithivihahshini N (2017) Pervasiveness of Listeria monocytogenes in milk and dairy products. J Food Microbiol Saf Hyg 2:2476–2059
Meyer-Broseta S, Diot A, Bastian S, Rivière J, Cerf O (2003) Estimation of low bacterial concentration: Listeria monocytogenes in raw milk. Int J Food Microbiol 80:1–15
Nasim RB, Vahidy R (1993) Human listerial meningitis: reported from Karachi, Pakistan. J Islamic World Acad Sci 6:253–258
Nayak DN, Savalia C, Kalyani I, Kumar R, Kshirsagar D (2015) Isolation, identification, and characterization of Listeria spp. from various animal origin foods. Vet World 8:695
Nightingale KK et al (2004) Ecology and transmission of Listeria monocytogenes infecting ruminants and in the farm environment. Appl Environ Microbiol 70:4458–4467. https://doi.org/10.1128/AEM.70.8.4458-4467.2004
Nightingale K, Windham K, Wiedmann M (2005) Evolution and molecular phylogeny of Listeria monocytogenes isolated from human and animal listeriosis cases and foods. J Bacteriol 187:5537–5551
Obaidat MM, Stringer AP (2019) Prevalence, molecular characterization, and antimicrobial resistance profiles of Listeria monocytogenes, Salmonella enterica, and Escherichia coli O157: H7 on dairy cattle farms in Jordan. J Dairy Sci 102:8710–8720
Orsi RH, Bakker HCd, Wiedmann M (2011) Listeria monocytogenes lineages: genomics, evolution, ecology, and phenotypic characteristics. Int J Med Microbiol 301:79–96. https://doi.org/10.1016/j.ijmm.2010.05.002
Owusu-Kwarteng J, Wuni A, Akabanda F, Jespersen L (2018) Prevalence and characteristics of Listeria monocytogenes isolates in raw milk, heated milk and nunu, a spontaneously fermented milk beverage, in Ghana. Beverages 4:40
Paziak-Domańska B et al (1999) Evaluation of the API test, phosphatidylinositol-specific phospholipase C activity and PCR method in identification of Listeria monocytogenes in meat foods. FEMS Microbiol Lett 171:209–214. https://doi.org/10.1016/S0378-1097(98)00607-7
Pohl MA, Wiedmann M, Nightingale KK (2006) Associations among Listeria monocytogenes genotypes and distinct clinical manifestations of listeriosis in cattle. Am J Vet Res 67:616–626
Rahimi E, Momtaz H, Behzadnia A, Baghbadorani ZT (2014) Incidence of Listeria species in bovine, ovine, caprine, camel and water buffalo milk using cultural method and the PCR assay. Asian Pac J Trop Dis 4:50–53. https://doi.org/10.1016/S2222-1808(14)60313-3
Rawool D, Malik S, Shakuntala I, Sahare A, Barbuddhe SJIJOFM (2007) Detection of multiple virulence-associated genes in Listeria Monocytogenes isolated from bovine mastitis cases. Int J Food Microbiol 113:201–207
Rodriguez C, Taminiau B, García-fuentes E, Daube G, Korsak N (2021) Listeria monocytogenes dissemination in farming and primary production: sources, shedding and control measures. Food Control 120:107540. https://doi.org/10.1016/j.foodcont.2020.107540
Saha M, Debnath C, Pramanik A (2015) Listeria monocytogenes: an emerging food borne pathogen. Int J Curr Microbiol Appl Sci 4:52–72
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sanaa M, Poutrel B, Menard JL, Serieys F (1993) Risk factors associated with contamination of raw milk by Listeria monocytogenes in dairy farms. J Dairy Sci 76:2891–2898. https://doi.org/10.3168/jds.S0022-0302(93)77628-6
Santorum P, Garcia R, Lopez V, Martínez-Suárez JV (2012) Dairy farm management and production practices associated with the presence of Listeria monocytogenes in raw milk and beef. Span J Agric Res 2:360–371
Schoder D, Melzner D, Schmalwieser A, Zangana A, Winter P, Wagner M (2011) Important vectors for Listeria monocytogenes transmission at farm dairies manufacturing fresh sheep and goat cheese from raw milk. J Food Prot 74:919–924. https://doi.org/10.4315/0362-028X.JFP-10-534
Schweizer G, Ehrensperger F, Torgerson PR, Braun U (2006) Clinical findings and treatment of 94 cattle presumptively diagnosed with listeriosis. Vet Rec 158:588–592. https://doi.org/10.1136/vr.158.17.588
Scobie A et al (2019) Mortality risk factors for listeriosis—A 10 year review of non-pregnancy associated cases in England 2006–2015. J Infect 78:208–214
Seeliger H (1988) Listeriosis—history and actual developments. Infection 16:S80–S84
Seyoum ET, Woldetsadik DA, Mekonen TK, Gezahegn HA, Gebreyes WA (2015) Prevalence of Listeria monocytogenes in raw bovine milk and milk products from central highlands of Ethiopia. J Infect Dev Ctries 9:1204–1209. https://doi.org/10.3855/jidc.6211
Shakuntala I et al (2019) Prevalence, characterization, and genetic diversity of Listeria monocytogenes isolated from foods of animal origin in North East India. Food Biotechnol 33:237–250
Sharma D, Sharma PK, Saharan B, Malik A (2012) Isolation, identification and antibiotic susceptibility profiling of antimicrobial resistant Listeria monocytogenes from dairy milk. Int J Microbiol Res Tech 1:1–4
Soni DK, Singh RK, Singh DV, Dubey SK (2013) Characterization of Listeria monocytogenes isolated from Ganges water, human clinical and milk samples at Varanasi, India. Infect Genet Evol 14:83–91. https://doi.org/10.1016/j.meegid.2012.09.019
Strawn LK, Gröhn YT, Warchocki S, Worobo RW, Bihn EA, Wiedmann M (2013) Risk factors associated with Salmonella and Listeria monocytogenes contamination of produce fields. Appl Environ Microbiol 79:7618–7627
Su X et al (2016) Molecular characterization and antimicrobial susceptibility of Listeria monocytogenes isolated from foods and humans. Food Control 70:96–102
Swetha CS, Babu AJ, Rao TM (2015) Detection of Listeria monocytogenes in milk and ice cream by polymerase chain reaction. RRJoMV 5
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10:512–526
Usman M, Mukhtar N (2014) Prevalence of Listeria monocytogenes in raw milk in Faisalabad, Pakistan. J Agric Vet Sci 7:08–11
Vázquez-Boland JA et al (2001) Listeria pathogenesis and molecular virulence determinants. Clin Microbiol Rev 14:584–640
Vera A, Gonzalez G, Dominguez M, Bello H (2013) Main virulence factors of Listeria Monocytogenes and its regulation. Rev Chilena De Infectol Org of De La Soc Chil De Infectol 30:407–416
Vilar MJ, Yus E, Sanjuán ML, Diéguez FJ, Rodríguez-Otero JL (2007) Prevalence of and risk factors for Listeria species on dairy farms. J Dairy Sci 90:5083–5088. https://doi.org/10.3168/jds.2007-0213
Wa R, Okunnu BM, Berg RE, Alerts E (2021) The essential role of neutrophils during infection with the intracellular bacterial pathogen Listeria monocytogenes. J Immunol. https://doi.org/10.4049/jimmunol.1600599
Yakubu Y et al (2012) Prevalence and antibiotic susceptibility of Listeria monocytogenes in raw milk from cattle herds within Sokoto Metropolis, Nigeria. Sokoto J Vet Sci 10:13–17
Zafar N (2020) Prevalence, molecular characterization and antibiogram study of Listeria monocytogenes isolated from raw milk and milk products. Pure Appl Biol 9:1982–1987. https://doi.org/10.19045/bspab.2020.90211
Acknowledgements
The authors are grateful to Dr. Sarfraz ul Rehman, Ph.D. scholar, UVAS Lahore for proofreading and Dr. Muhammad Tariq Naveed, Assistant Professor, National University of Medical Sciences, Rawalpindi for guidance.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
JAK and MI conceived and planned the study. MZM contributed to sample preparation, performed the experiments, data analysis, writing of the manuscript. FA contributed to primer designing, performed phylogenetic analysis and interpretation. All authors provided critical feedback and helped shape the research and manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest in the publication of this study.
Ethical approval
The study was approved by the ethical review committee of the University of Veterinary and Animal Sciences, Lahore Pakistan (Number. DR/946 Dated 13-09-2019).
Consent to participate
Consent from animals owners was obtained to participate in this study.
Consent for publication
All the authors have read and approved the final manuscript to be published.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Munir, M.Z., Khan, J.A., Ijaz, M. et al. Molecular characterization and hematological analysis of Listeria monocytogenes infection in dairy cows in Punjab (Pakistan). Arch Microbiol 204, 201 (2022). https://doi.org/10.1007/s00203-022-02804-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-02804-1