Abstract
The spread of biodegradable plastic films (BDFs) not only increases grain yield but also reduces environmental pollution from plastic film to a large extent. Soil microbes are considered to be involved in biodegradation processes. However, the study of microbe diversity in soil mulched with biodegradable plastic film remains limited. Here, we compared the diversity of microbes between soils with biodegradable film and nonbiodegradable film (NBDF) mulch. The results showed that BDFs affected total C, P and NH4+-N, especially organism C content, as well as microbe species richness (ACE; Chao1) and diversity (Simpson index; Shannon index). In terms of dominant phyla and genera, BDFs and NBDF can influence the abundance of disparate species. Furthermore, BDFs could also contribute to improving the richness of the important functional bacterial groups in soil, e.g., Pedomicrobium and Comamonas, both of which are involved in the degradation of plastic residues in soil. Finally, we found that BDFs improved the transformation of nitrogen by significantly increasing the abundances of Nitrobacter and Nitrospira. Our results highlight the impact of BDF mulch on the abundance of functional bacteria in the soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the increasing human population, the yield and quality of grain have become the most serious problems. The emergence of plastic film mulching (PFM) has solved a problem, as precipitation and other water resources are used more efficiently to attain the required food production. PFM is primarily used to protect shoots and seedlings and maintain or increase soil humidity and temperature through insulation and evaporation prevention (Steinmetz et al. 2016). Furthermore, PFM may increase yields; extend the growing season; conserve soil moisture and water-use efficiency; increase fertilizer use efficiency; prevent soil erosion with weed growth; and reduce weed pressure, as well as consequently reduce herbicide and fertilizer use. In recent years, the usage of PFM has been increasing worldwide (Cuello et al. 2015). For example, in China, approximately 14.7 × 105 tons PFM has been used on crops (Gao et al. 2018), most of which is distributed in 19.8 million hectares of agricultural land, while in Europe, areas of PMF cover approximately 162,000 ha (Briassoulis et al. 2010), and in the United States, more than 130,000 metric tons of plastics are used only in vegetable production (Li et al. 2014). PFM has significantly improved crop yield and greatly solved the food crisis but also results in a series of issues. For example, plastic residues in the soil have become one of the major environmental pollution sources. The residues include microplastics, phthalates and agrochemicals, all of which have been found to lead to substantial amounts of plastic waste residue accumulation and may possibly liberate toxic additives into the soil and promote soil water repellency and soil degradation (Steinmetz et al. 2016). At present, countries all over the world are developing and studying technologies to reduce plastic film residues, such as alternative technologies of agricultural film, production and promotion of biodegradable film (BDF) and encouraging the recovery of agricultural film residues after harvest. Among them, the use of biodegradable plastic films has attracted increasing attention.
Biodegradable film (BDF), first synthesized in the mid-1970s (Briassoulis et al. 2010; Li et al. 2014), consists of either BDF plastics (i.e., plastics produced from fossil materials) or biobased plastics (i.e., plastics synthesized from biomass or renewable resources) (Tokiwa et al. 2009; Li et al. 2014). BDF primarily consists of biobased plastics that will eventually biodegrade into the soil in the form of CO2 and H2O (Ashley et al. 2012; Sintim et al. 2017; Bandopadhyay et al. 2020) after the end of their effective process without leaving toxic and polluting remains in the soil (Briassoulis et al. 2010; Li et al. 2014). The advantages of BDFs are as follows: improved tensile strength, flexibility and controllability of rupture and degradation; pollution free; enhanced ability to increase the soil temperature and preserve the soil moisture and satisfy the demand of crop production with plastic film mulching (Yan et al. 2016). However, some shortcomings of BDFs are also exposed as the service time increases. For instance, there is perceived uncertainty about the weak market transparency, product properties, a lack of uniformity in the product and a discouraging cost of biodegradable plastic mulch compared to conventional mulch in certain applications (Briassoulis et al. 2010). In addition, for some reason, bewilderment still exists regarding the capability of these materials under real soil conditions. Studies show that the incomplete breakdown of BDFs could lead to an accumulation of plastic fragments and particulates in soils (Sintim et al. 2017; Zhao et al. 2021). Therefore, whether BDFs may become a desirable alternative to traditional PFM in solving agricultural plastic residues, in addition to the above advantages, more evaluation parameters should be provided.
Microbes are an important part of the soil ecosystem and play a key role in carbon, phosphorus, nitrogen and potassium cycling in the soil (Kennedy et al. 1995; Qian et al. 2018). In soil, the diversity and function of microbes could be affected by agricultural management practices such as fertilization, film mulch, pesticides and crop rotation (Hassan et al. 2021). Thus, their abundance and activity, as well as the community structure, may act as a prime indicator of soil fertility and quality (Puglisi et al. 2012). Obviously, the maintenance of soil microbial communities is important for the sustainability of soil agroecosystems (Hill et al. 2005; Sreejata et al. 2018; Qi et al. 2020). Recent reports have found that PFM, including BDF and NBDF, and microplastics can also influence the activities and composition of soil microbes (Subrahmaniyan K et al. 2006; Brodhagen et al. 2015; Sreejata et al. 2018; Bandopadhyay et al. 2020). Furthermore, as expected, the BDF residue fragments in soil could be degraded by soil microorganisms; for example, some bacterial strains, especially some microorganism communities in the soil, have been identified to be involved in microplastic degradation (Bandopadhyay et al. 2020), suggesting that the abundance and activity of soil microbes may change with the disposal of BDFs. The study of abundance and diversity could contribute to screening microbes that could be involved in plastic residues and provide a method to evaluate the feasibility of biodegradable plastic films.
In this study, we evaluated the effects on microbe diversity and abundance in soils mulched by three potentially biodegradable films (BDFs: BDF1, BDF2, BDF3) and one nonbiodegradable film (NBDF, also named polyethylene film). We also quantified the abundance of some functional microbes that can improve soil fertility as well as soil properties or accelerate the degradation of BDFs. Our results highlight the effect of BDF mulch on soil microbe diversity, which might be involved in the degradation of BDFs.
Materials and methods
Sampling location and collection
The sampling site is located in the potato planting area of Shandan County (38° 47′ 1.90″ N, 101° 05′ 11.33 E, Arid area of Yellow River, Gansu Province, China), where potatoes (Solanum tuberosum L. Daxiyang) are cultivated in double ridge furrows with different mulches(The time span of mulching is 6 months). The laying method of plastic film adopts machine tiling, artificial seeding cultivation technology. Five soil samples (CK, NBDF and BDFs, including BDF1, BDF2, BDF3) were collected using a soil columnar sampler 15 cm above the ridge. CK refers to soil without plastic mulches, NBDF samples were covered with nonbiodegradable mulches (polyethylene), and three BDF samples present the three types of biodegradable mulches, which are different in thickness, color and manufacturer. Furthermore, among the five sampling locations, there was no significant difference in field management measures such as watering and fertilization. In this study, the PE film was white and 0.008 mm thick. Three biodegradable plastic films used in this study are BDF1 (black, 0.008 mm thickness), BDF2 (white, 0.008 mm thickness) and BDF3 (black, 0.008 mm thickness, manufactured by Xinjiang, and Lanzhou of China). Starch is the main synthetic raw material of these degradable membranes, which have the characteristics of large tensile strength, can be degraded into H2O and CO2 by microbes in soil, and is absolutely friendly to the environment.
Before potato harvesting, the soil samples were evenly collected from 10 to 15 cm above the planting ridge, where the BDFs cracked and began to degrade, while the NBDF was intact. Each sample was collected from at least five points (the distance between two points was not less than 30 m), and three biological replicates (samples from the same site were collected three times, and the samples were mixed in equal proportion) were used for statistical analysis. All collected samples were packed and transported back to the laboratory at 4 °C, and all of the samples were stored at − 20 °C. Soil DNA was extracted and sequenced from three biological replicates of each sample.
Soil available nutrient measurements
The soil samples used for soil property determination were dried in air. Conspicuous plants, stones and other substances were removed from the soil samples and transported back to a clean and ventilated indoor environment with dehumidified air after the soil samples were collected. For the soil property analyses, the soil samples were sieved (to 5 mm and 2 mm) and stored at 4 °C until analyses were performed within the next 2–3 weeks (Acosta-Martínez et al. 2011). Two copies of 5 mm screened soil samples, each 200 g, were finely ground, and 1 part was thoroughly processed at 2 mm for soil pH determination (DELTA320 pH). The rapid determination of total N in the soil was performed by an automatic Kjeldahl apparatus (DDY9820). NH4+-N (indophenol blue colorimetry), NO3–-N (colorimetry of phenoldisulfonic acid) and total P (digestion colorimetry) were determined from air-dried soil by spectrophotometry. The organic C was determined by TOC-V (series SSM-5000A) solid sample measurement. The conductivity was determined by a conductometer.
DNA extraction from the soil samples and polymerase chain reaction (PCR) amplification
DNA was isolated from at least 2 g of mixed soil using a MoBio High-throughput PowerSoil DNA Isolation Kit (Young et al. 2015) and purified on agarose gels. The 16S rRNA gene fragment (V3-V4) (Sui et al. 2013) of the appropriate size and sequence was amplified using universal primers 1492R (5ʹ-GGTTACCTTGTTACGACTT-3ʹ) and 27F (5ʹ-AGAGTTTGATCCTGGCTCAG-3ʹ) (Krumins et al. 2009). The PCR conditions were as follows: 94 °C for 5 min; then 25 cycles of 45 s of denaturation at 94 °C, 60 s of annealing at 55 °C, and 60 s of extension at 72 °C, followed by 10 min at 72 °C (Sharma et al. 2009; Sabine and Ralf 1997).
16S rRNA gene fragment analysis
The purified PCR products were sequenced by the Illumina HiSeq2500 platform at Novogene Technologies Corporation (Beijing, China). The high-quality reads with 97% similarity were clustered into operational taxonomic units (OTUs) using UCLUST (Edgar 2010) in QIIME (version 1.8.0) (Caporaso et al. 2010). The bioinformatics analysis, including the creation of Venn diagrams (Chen and Boutros 2011) and calculation of alpha diversity indexes (Grice et al. 2009), was performed on the biocloud platform (www.biocloud.org). Species richness was projected by utilizing the Chao 1 and ACE methods, while quantitative species richness or evenness was projected via nonparametric Simpson and Shannon’s indexes in Mothur (Version v.1.30). We created UPGMA dendrogram (an unweighted pair group method with arithmetic mean) using Fast UniFrac analysis (Amin et al. 2017). UPGMA was used to analyze the correlations between the five samples.
Functional analysis by KEGG
Picrust software was used to compare species composition information obtained from 16S sequencing data to predict the functional genetic composition of the samples and to analyze functional differences between different samples or groups (Ma et al. 2020). First, the generated OTU table needs to be standardized (different species contain different copies of 16S). Then, through the Greengene ID corresponding to each OTU, the KEGG family information corresponding to OTUs can be obtained (Schfer et al. 2009). Thus, the KEGG abundance was calculated, and pathway, EC information and OTU abundance were obtained from KEGG database information to calculate the abundance of each functional category.
Determining the abundance of soil Nitrobacter and Nitrospira
Total DNA was extracted from the soil samples using a DNeasy Power Soil kit (Qiagen GmbH, Germany). The nitrifier communities from all the soil samples were examined by qRT-PCR-related analyses using specific primers (Table S1). The number of nitrifying bacteria in the samples was analyzed based on the data from the different fragments obtained from specific amplifications (Li et al. 2018).
Data statistics
A two-sided p value < 0.05 was considered statistically significant; the confidence intervals were set at 95%. Statistical analyses were performed using SPSS 19.0 software. Normally distributed continuous variables are expressed as the means ± standard errors of the mean (SEM), and nonnormally distributed continuous variables are expressed as the median (interquartile range). One-way analysis of variance was used to compare multiple groups.
Results
Soil available nutrient contents
To evaluate the effects of BDFs and NBDF on soil environmental factors, the relevant parameters were determined. The organic C value was significantly different (P = 0.005 < 0.05) between BDFs and NBDF. The total P value was significantly different (P = 0.045 < 0.05) between BDFs with NBDF (Table 1, Fig S1a). Additionally, pH, total N, NH4+-N, NO3–-N and conductivity were not significantly different among CK, BDFs and NBDF (P = 0.294 > 0.05, P = 0.344 > 0.05, P = 0.294 > 0.05, P = 0.339 > 0.05, and P = 0.119 > 0.05, respectively) (Table 1). The results suggested that the presence or absence of mulch does not affect these factors in the soil. Nevertheless, some variation in soil factors was obvious between BDFs and NBDF, e.g., conductivity was significantly different between BDF3 and NBDF (Duncan’s test). The pH of all samples was slightly alkaline; the quantity of total N was higher in NBDF (1.94 ± 0.81) than BDFs. However, the maximum values of NH4+-N and NO3–-N were found in BDFs (BDF2 83.87 ± 51.69; BDF3, 4.06 ± 1.28) (Table 1). The BDF samples showed significant differences in NO3–-N and conductivity. In addition, different changes exist in BDFs for soil physical and chemical factors. Total P and organic C were higher in BDFs than CK, and the NBDF had the maximum value of total P and conductivity and the minimum value of organic C. In other words, PFM (NBDF and BDFs) can change the value (rise or fall) of physical and chemical factors in the soil. There is no obvious rule in the two types of mulch, so it is impossible to distinguish which type of mulch is more suitable for soil planting.
Diversity of bacteria
A total of 782,586 clean reads were obtained for all samples after removing the low-quality and chimeric reads. Approximately 50% of the effective reads were paired-end reads (Table S2). At the 97% OTU level, the quality reads could be distributed into 1655 OTUs, consisting of 437 microorganism genera in 28 phyla (Table S3) (Schneider et al. 2015). CK lacks one phylum compared with those in other samples, with the least number of genera. There were 1555 OTUs shared as a core group of all samples, and NBDF had no unique OTUs (Fig. 1a). The results of the rarefaction curve showed that the data size of all samples was sufficient to support the response of species diversity and richness (Fig S1b). For the five sample bacterial communities, the CK had the lowest ACE (1609) and Chao1 (1617) indexes (Table S4), and BDFs (BDF3) had the highest species richness (ACE 1637 and Chao1 1644). BDFs (except BDF2) had higher ACE and Chao 1 indexes than NBDF. BDF3 showed the richest species diversity (Simpson index: 0.0034 and Shannon index: 6.44), and CK had the lowest species diversity (Simpson index: 0.0045 and Shannon index: 6.23). Each index of the NBDF sample was slightly different than that of the BDFs, with no significant differences. In addition, a ternary phase diagram can visually show the proportion and relationship of different species in CK, NBDF and BDFs (Fig. 1b).
Venn diagram and ternary phase diagram of all samples. At the 97% similarity level, the number of OTUs in each sample was obtained. A Venn diagram was used to display the number of common and unique OTUs in the samples. Among the five groups of samples, except NBDF, the other four groups of samples all have non-overlapping parts that is the number of unique OUTs contained in each group of samples (a). Three corners of the triangle represent three samples, three samples are represented by three colors, three edges are used to measure the species abundance of the corresponding color of the sample, and the circles in the triangle represent all species at the level of a phylum (b)
Correlations of the bacterial taxa
The UPGMA dendrogram created based on the UniFrac distance matrix exhibited three major clades (Fig. 2). The CK sample formed one clade relatively independent from the other samples, and it also had the farthest genetic distance from the other four samples. The BDF clade was basically identical to the NBDF clade, and there was little genetic distance between BDF2 and NBDF. NBDF and BDFs samples were different with CK. In three clades, BDFs (BDF1 and BDF3) formed a single closed, independent cluster; among all samples, the highest similarity was found between BDF1 and BDF3. In addition, we drew a graph NMDS to support the result, which showed that in CK as the control group, the distance between BDF1 and BDF3 was closer in the coordinate axis, and the distance between BDF2 and NBDF was closer, which was consistent with the results of UPGMA dendrogram, and further confirmed that there were different correlations among the soils with four kinds of mulching films (Fig. 3, Fig S2). Overall, the reciprocal similarity of the three BDF samples was the highest, while that of the CK sample was the lowest. There were obvious effects of mulching on soil microbial species composition, and the similarity distances of the CK, NBDF and BDFs ranged from far to near.
UPGMA dendrogram based on the UniFrac data distance matrix for the comparison of all the samples. The phylogenetic tree generated by the UPGMA (unweighted pair group method with arithmetic mean) is a simple embodiment of the species tree. After each divergence, the length of the branches from the common ancestor node to the two OTUs is the same. The closer the samples were, the shorter the branches, indicating that the species composition of the two samples was more similar
NMDS of all samples. Non-metric Multi-Dimensional Scaling (NMDS) is a ranking method suitable for ecological research. Points in the figure represent each sample, different colors represent different groups (CK, NBDF, BDFs), and the distance between points represents the degree of difference. The closer the sample is in the coordinate map, the similarity is higher
Bacterial community composition
Abundance of phyla
Amplicon sequencing of bacterial 16S rRNA genes (V3–V4 region) was used to assess the influence of applying four different films on the soil bacterial community (Dawson et al. 2017). The five soil samples were dominated by bacteria belonging to the phyla Proteobacteria (Batzke et al. 2007), Acidobacteria, Bacteroidetes, Gemmatimonadetes, Chloroflexi, Verrucomicrobia, Nitrospirae, Armatimonadetes and Fusobacteria, with each of these phyla constituting more than 0.1% of the relative abundance of the detected 16S rRNA genes (Fig. 4a, Table S5).
Soil bacterial community composition of all the samples at the a phylum level and b genus level. Each color represents a species, and the length of the color block (histogram) indicates the relative abundance of the species. We show only the top 10 species at the phylum level; the remaining species were combined and displayed as others (a). The top 20 different taxonomic genera were selected from the five samples (b). As shown in the figure, unclassified represents the species that have not been taxonomically annotated
The dominant phylum Proteobacteria was the most abundant in BDFs (BDF1: 37.01%, BDF2: 36.28%), followed by the NBDF sample (32.23%) and CK (31.12%). The abundance of Acidobacteria in the four film mulches (NBDF: 20.79%, BDF1: 17.39%, BDF2: 20.55%, and BDF3: 22.27%) was lower than that in the CK (24.07%). The species abundances of the most dominant bacterial phyla Verrucomicrobia and Armatimonadetes were slightly higher in BDFs than in CK. There was no significant difference in species abundance between the NBDF and BDFs, for example, Gemmatimonadetes (NBDF: 11.63%, BDF1: 9.05%, BDF2: 9.61%, and BDF3: 10.87%) and Chloroflexi (NBDF: 6.46%, BDF1: 5.70%, BDF2: 6.13%, and BDF3: 6.56%).
However, there was a slight difference in species abundance among the three BDF treatments, the highest species abundance of Proteobacteria (37.01%) and Bacteroidetes (20.34%) in BDF1, and Chloroflexi (6.56%) in BDF3. The abundances of species in the following phyla increased in NBDF relative to the CK: Gemmatimonadetes (from 10.04% in CK to 11.63% in NBDF), Chloroflexi (from 6.08 to 6.46%), Actinobacteria (from 3.83 to 3.94%), Verrucomicrobia (from 1.22 to 1.62%), and Nitrospirae (from 0.55 to 0.91%). The exception was Bacteroidetes, which decreased from 20.11% in the CK sample to 18.72% in the NBDF sample. Mulching (with BDFs or NBDF) changed the abundance of the different species in the CK soil, especially the dominant phyla.
In addition, Proteobacteria, Acidobacteria and Bacteroidetes were the most common dominant bacterial phyla in all samples. It is interesting to note that these three kinds of bacteria showed different states in CK compared with the samples from the four mulching treatments. The lowest difference abundances of different species between the samples were found in Proteobacteria, the highest difference was found in the Acidobacteria, and almost no difference was found in Bacteroidetes. In NBDF, the species abundances of the three phyla showed an increasing trend compared with BDF1 and BDF2. The species abundances of Proteobacteria and Bacteroidetes were higher in the BDF3 treatment than in the other two BDF treatments. There was no significant difference in species abundances of the three dominant bacteria in all samples, and the species abundance value was between the lowest and the highest values. Gemmatimonadetes and Nitrospirae are two phyla that can play an important role in soils. The species abundances of Gemmatimonadetes and Nitrospirae were higher in the NBDF than in the other samples, and their abundances in the CK were between those in the NBDF and BDFs. In addition, the abundances of species from these two important phyla in BDF2 and BDF3 were lower than those in BDF1.
Abundance of genera
In this study, the top 20 different taxonomic genera were examined from the 5 samples. The five dominant genera were uncultured_bacterium_c_Subgroup_6 (from 5.53 to 7.93%), uncultured_bacterium_f_Gemmatimonadaceae (from 4.42 to 6.0%), uncultured_bacterium_f_Saprospiraceae (from 3.89 to 4.83%), uncultured_bacterium_o_Subgroup_7 (from 2.31 to 4.18%) and Sphingomonas (from 2.24 to 3.06%), which were common in all the samples (Fig. 4b, Table S6).
Dominant bacterial genera were generally more abundant in CK than in NBDF and BDFs, while the functional genera (Sphingomonas, Haliangium, Lysobacter, Arenimonas) were generally lower in CK than in NBDF and BDFs. There was no significant difference in species abundances between the NBDF and BDFs. The abundances of uncultured_bacterium_f_Gemmatimonadaceae, Haliangium and Lysobacter were the highest in BDF1, and BDF2 and BDF3 had the highest species abundances of Sphingomonas and Arenimonas, respectively. It is worth noting that in NBDF, there were two functional genera, Uncultured_bacterium_f_Gemmatimonadaceae and Arenimonas, with maximum values, and one group that reached the lowest value (Sphingomonas).
In addition, the species abundances of the other four important functional bacterial genera in CK were lower than those in all plastic film treatments (except for Lysobacter). Interestingly, the increases or decreases in relative abundance were driven not only by the normalized sequence counts of abundant OTUs (≥ 3% of the relative abundance per OTU) (Qian et al. 2018) but also by the normalized sequence counts of the low-abundance OTUs (< 3% relative abundance per OTU). For example, this pattern is seen in the relative abundances of the low-abundance OTUs in the genera uncultured_bacterium_c_S0134_terrestrial_group (1.40–2.11%), uncultured_bacterium_f_Blastocatellaceae (1.21–2.53%), Arenimonas (1.25–2.51%), Bryobacter (1.10–1.83%) and so on. The low-abundance genera account for approximately 45.28% of all the OTU sequences in the five samples. The results indicate that the abundance of species does not completely determine the importance of species in the population and, in most cases, it plays a role in regulating population function (Ren W et al. 2015; Brackin R et al. 2013).
Functional genera
The metabolic pathways of the five groups of samples were analyzed by KEGG, among which two metabolic pathways, xenobiotic biodegradation and metabolism, and biosynthesis of other secondary metabolites, should be given more attention (Fig. 5, Fig S3a, b). The presence of these two metabolism pathways in BDFs suggests that microbial communities are involved in both biodegradation and biosynthesis metabolism pathways. However, in the CK and NBDF groups, the biosynthesis of other secondary metabolites was not found. The results suggest that biodegradable membrane mulching could increase the relative abundance of the functional microbial communities that extensively participate in the functional metabolism pathways, especially the biodegradable and biosynthetic metabolism pathways.
Differential analysis of KEGG metabolism pathway. Through the difference analysis of KEGG metabolic pathway, we can observe the differences and changes of functional genes in metabolic pathway of microbial community between BDF2 and BDF3. The figure shows the proportion of the abundance of different functions in the two samples, the middle shows the proportion of the difference in the abundance of different functions in the 95% confidence interval, and the right-most value is the P value
Previous study shows that the bacterial genera Pedomicrobium and Comamonas have participated in the degradation of soil plastics (Shah et al. 2008). In this study, compared to CK, the abundance of Pedomicrobium species in the BDFs and NBDF samples have increased significantly, and which are higher in the BDFs than that in NBDF (except BDF1 sample) (Fig. 6a). Furthermore, there was no significant difference in the abundance of Comamonas species between the CK and NBDF samples; the abundance levels were almost uniform, although there were significant differences among BDFs, CK and NBDF. The abundances of Comamonas species were higher in all BDF treatments than in the CK and NBDF treatments.
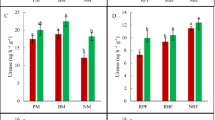
Functional microorganisms in all samples. The two functional genera have been shown to be involved in the degradation of plastics in the soil. The two colors bars represent the two genera, Pedomicrobium and Comamonas, and the height of the bars represents the abundance of species in both genera in the five samples (a). The number of nitrifying microorganisms in the different samples is shown in the figure, and the three different colors represent Nitrobacter nxrA, Nitrobacter nxrB, and Nitrospira nxrB (b). Statistical analysis was performed with Student’s t test (*P < 0.05, **P < 0.01)
The abundance of Nitrobacter and Nitrospira in soils
To investigate whether mulching with BDFs or NBDFs affected the functional microbial population in soils, we detected the number of nitrifying bacteria in the soil samples using qRT-PCR methods. The number of nitrifying bacteria was determined by the number of two important functional genes, which were amplified by three pairs of specific primers. The results showed that the number of nxrA in the PFM (NBDF and BDFs) was significantly higher than that in the CK sample, and there were significant differences between BDF1 and CK. The number of nxrB in the NBDF and BDF samples (except BDF1) was higher than that in the CK sample, and both BDF2 and BDF3 were significantly different from that in the CK sample. Therefore, compared with the CK sample, the number of Nitrobacter communities (Nitrobacter nxrA and Nitrobacter nxrB) (Li et al. 2018) was increased in the BDF and NBDF samples. The content of Nitrobacter in the BDFs was higher than that in the NBDF sample (Fig. 6b). It can be found from the data that the number of Nitrobacter communities in the three treatment groups gradually increased in the order of CK, NBDF and BDFs, indicating that soil mulch could contribute to increasing the abundance of Nitrobacter while reducing the abundance of Nitrospira.
Discussion
BDF mulch could improve the soil available nutrient contents
Soil quality indicators can reflect changes in soil intuitively and effectively (Li et al. 2008). Soil pH affects the availability of nutrients, plant growth and microbial activity (Domagała-Świątkiewicz and Siwek 2013). Here, in the soils mulched with different films, the pH was stable at 8.20 ± 0.14 (no significant difference), and similar results were also found in terms of the total P, NH4+-N, or NO3− -N. However, the contents of total N, NO3− -N and total P in NBDF and BDF2 were higher than those in CK. It is suggested that film mulch could improve soil quality (Arutchelvi et al. 2008). PFM increases soil nutrients; for example, PFM maximizes soil moisture savings and protects soil nutrients from loss (Kader et al. 2017). The difference in organic C in each sample may be due to the activities of soil organisms, which influence C retention and, consequently, aggregation and soil structure (Domagała-Świątkiewicz and Siwek 2013) or improve the ability of organic matter degradation by soil microorganisms (Liu et al. 2012). Meanwhile, BDFs have a higher retention effect on soil organic C than NBDF, which had the maximum conductivity value. A possible explanation is that the NBDF leaches toxic substances into the soil for a long time, leading to an increase in soil conductivity (Carteau et al. 2014). In addition, the differences among BDFs in thickness, color and composition could affect soil structure, quality and activity (Alkayssi and Alkaraghouli 1991; Sun et al. 2015; Zhao et al. 2021).
BDFs could increase the diversity and richness of functional microbe species in the soil
Plastic film mulch can lead to changes in soil properties, such as solar radiant intensity, temperature, humidity and microbes (Fan and Liu 2003; Yadav et al. 2013). In terms of microbes, the species diversity and richness in the NBDF and BDF soil samples were all higher than those in the CK. A more reasonable explanation is that the NBDF residue period increases phthalate concentrations, which leads to significant decreases in soil microbial carbon and nitrogen, enzyme activities and microbial diversity (Wang et al. 2016), while the biodegradable film can degrade in 1 year and its residue does not remain in the soil for a long time (Barragán et al. 2016).
Furthermore, the dominant bacterial phyla, such as Proteobacteria, Acidobacteria, Bacteroidetes and Gemmatimonadetes, and common bacterial phyla, including Actinobacteria, Chloroflexi, Verrucomicrobia and Nitrospirae (Farmer et al. 2016), were all found in our study. Among them, the abundance of Proteobacteria was not significantly different among CK, BDFs and NBDF. Gemmatimonas has the beneficial effect of fixing atmospheric nitrogen under low oxygen pressure conditions (DeBruyn et al. 2013). With the exception of BDF3, the PFM systems showed a higher abundance of uncultured_bacterium_f_Gemmatimonadaceae species, suggesting that PFM can enhance Gemmatimonas activity and promote nitrogen fixation. PFM could also enhance the abundance of Actinobacteria, Nitrospirae and Alphaproteobacteria, in which Actinobacteria can secrete antibiotics over long periods to inhibit disease (Landwehr et al. 2016), while Nitrospirae (Zhang et al. 2015) and Alphaproteobacteria (Trujillo et al. 2015) play an important role in nitrogen fixation and soil nitrification. Collectively, mulch with film could contribute to resistance to disease and soil nitrification.
BDF mulch could increase the abundance of microbes involved in plastic degradation
Microbes, such as bacteria and fungi in the soil, are involved in the degradation of BDFs and plastic film residue (Shah et al. 2008). For example, plastic film phthalate esters take up 20–60% of the NBDF, which contaminates the environment and affects the soil microbial communities and enzymatic activities (Qian et al. 2018). However, soil microbes prefer polymers, especially plastics, as substrates. For example, the genus Sphingomonas can degrade the toxic substance bromine acid and participate in the nitrogen cycle in soil (Vasilyeva and Strijakova 2007). Samples CK and BDF2 have higher Sphingomonas abundance than NBDF and BDF 1 and BDF 3). Due to possible competition between Gemmatimonas and Sphingomonas, PFM increased Gemmatimonas abundance and inhibited Sphingomonas development. Haliangium is a common saprophyte in soil, and it plays an important role in the nutrient cycle of soil (Tayyab et al. 2018). The Haliangium abundance in the BDF and NBDF samples was higher than that in the CK samples in this study. It is obvious that NBDF and BDFs could improve soil microbial activities by enhancing the abilities of saprophytic bacteria and soil nutrient recycling. Moreover, Pedomicrobium and Comamonas are bacteria that have been reported to have the ability to degrade plastic residues (Shah et al. 2008). In this study, the numbers of Pedomicrobium and Comamonas were significantly higher in BDFs than in CK and NBDF, suggesting that both microbes might be involved in the degradation of BDFs.
The BDFs and NBDF form a bottleneck on the soil surface that impacts soil temperature, moisture and soil-air gas exchange, indirectly altering the microorganism communities (Bandopadhyay et al. 2018). Nitrobacteria are involved in regulating the balance of inorganic nitrogen (N forms) in soils (Attard et al. 2010) by facilitating nitrification, which is a vital step of the nitrogen cycle (Poly et al. 2008). In this study, the abundance of three functional nitrobacteria was determined by quantitative real-time PCR. Both oxidizing bacteria (NOB), Nitrobacteria and Nitrospira, are involved in soil nitrification from ammonia to nitrate and play a crucial role in nitrogen biogeochemical cycling and plant nutrition (Li et al. 2018). The results showed that the number of nitrobacteria in BDFs was higher than that in CK and NBDF. BDFs changed the species abundance of the nitrobacterial community in soil and selectively increased two of the species (Nitrobacter nxrA and nxrB). The decrease in Nitrospira quantity in BDFs and NBDF may have less of an effect than CK on the degradation process of plastics. The increase in the number of these two nitrobacteria also shows that the cover of biodegradable film used in this study has an effect on the nitrobacteria community in the soil.
Conclusions and perspectives
PFM (plastic film mulch) could significantly increase the diversity and richness of the soil microbial species. The results suggested that PFM is beneficial for increasing the richness of the soil microbial community and improving the diversity and richness of soil microbial species without changing the dominant community. In particular, BDFs could improve the soil quality and the species abundance of functional genera (e.g., Pedomicrobium, Comamonas and nitrifying microbes), which would be involved in the degradation of BDFs or nitrification in soil.
The degradation of BDFs requires a soil microbial community that could be influenced by various environmental factors, including sunlight, rainfall, soil moisture and temperature, and film mulch. To date, there are still some problems that need to be solved in the use of biodegradable films, e.g., long degradation times, incomplete degradation and the possibility of residual particles in the soil. Therefore, in the future, biodegradable films should be particularly improved in many ways, such as material innovation, degradation process optimization and agronomic technology in sustainable agriculture.
Availability of data and materials
The datasets used or analyzed in the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Acosta-Martínez V, Calderón F, Booker JD, Zobeck TM, Dan RU (2011) Dryland cropping systems influence the microbial biomass and enzyme activities in a semiarid sandy soil. Biol Fertil Soils 47:655–667. https://doi.org/10.1007/s00374-011-0565-1
Alkayssi AW, Alkaraghouli AA (1991) Influence of different colour plastic mulches used for soil solarization on the effectiveness of soil heating. Fao Plant Prod Prot Pap 109:297–308
Amin A, Ahmed I, Salam N, Kim BY, Singh D, Zhi XY, Xiao M, Li WJ (2017) Diversity and distribution of thermophilic bacteria in hot springs of Pakistan. Microb Ecol 74:1–12. https://doi.org/10.1007/s00248-017-0930-1
Arutchelvi J, Sudhakar M, Arkatkar A, Doble M, Uppara PV (2008) Biodegradation of polyethylene and polypropylene. Indian J Biotechnol 7(1):9–22. https://doi.org/10.1089/hum.2007.086
Ashley S, Jo H (2012) Beyond the Venn diagram: the hunt for a core microbiome. Environ Microbiol 14:4–12. https://doi.org/10.1111/j.1462-2920.2011.02585.x
Attard E, Poly F, Commeaux C, Laurent F, Terada A, Smets BF, Roux RSXL (2010) Shifts between Nitrospira-and Nitrobacter-like nitrite oxidizers underlie the response of soil potential nitrite oxidation to changes in tillage practices. Environ Microbiol 12:315–326. https://doi.org/10.1111/j.1462-2920.2009.02070.x
Bandopadhyay S, Martin-Closas L, Pelacho AM, DeBruyn JM (2018) Biodegradable plastic mulch films: impacts on soil microbial communities and ecosystem functions. Front Microbiol 9:819–823. https://doi.org/10.3389/fmicb.2018.00819
Bandopadhyay S, Sintim HY, DeBruyn JM (2020) Effects of biodegradable plastic film mulching on soil microbial communities in two agroecosystems. PeerJ 8:1–27. https://doi.org/10.7717/peerj.9015
Barragán DH, Pelacho AM, Martinclosas L (2016) Degradation of agricultural biodegradable plastics in the soil under laboratory conditions. Soil Res 54:216–224. https://doi.org/10.1071/SR15034
Batzke A, Engelen B, Sass H, Cypionka H (2007) Phylogenetic and physiological diversity of cultured deep-biosphere bacteria from equatorial Pacific Ocean and Peru Margin sediments. Geomicrobiol J 24:261–273. https://doi.org/10.1080/01490450701456453
Brackin R, Robinson N, Lakshmanan P, Schmidt S (2013) Microbial function in adjacent subtropical forest and agricultural soil. Soil Biol Biochem 57:68–77. https://doi.org/10.1016/j.soilbio.2012.07.015
Briassoulis D, Dejean C (2010) Critical review of norms and standards for biodegradable agricultural plastics part Ι. Biodegrad Soil 18:384–400. https://doi.org/10.1007/s10924-010-0168-1
Brodhagen M, Peyron M, Miles C, Inglis DA (2015) Biodegradable plastic agricultural mulches and key features of microbial degradation. Appl Microbiol Biotechnol 99:1039–1056. https://doi.org/10.1007/s00253-014-6267-5
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. https://doi.org/10.1038/nmeth.f.303
Carteau D, Vallée-Réhel K, Linossier I, Quiniou F, Davy R, Compère C, Delbury M, Faÿ F (2014) Development of environmentally friendly antifouling paints using biodegradable polymer and lower toxic substances. Prog Org Coat 77:485–493. https://doi.org/10.1016/j.porgcoat.2013.11.012
Chen H, Boutros PC (2011) VennDiagram: a package for the generation of highly-customizable Venn and Euler diagrams in Bmc. Bioinformatics 12:35. https://doi.org/10.1186/1471-2105-12-35
Cuello JP, Hwang HY, Gutierrez J, Sang YK, Kim PJ (2015) Impact of plastic film mulching on increasing greenhouse gas emissions in temperate upland soil during maize cultivation. Appl Soil Ecol 91:48–57. https://doi.org/10.1016/j.apsoil.2015.02.007
Dawson W, Hör J, Egert M, Van KM, Pester M (2017) A small number of low-abundance bacteria dominate plant species-specific responses during rhizosphere colonization. Front Microbiol 8:975. https://doi.org/10.3389/fmicb.2017.00975
DeBruyn JM, Fawaz MN, Peacock AD, Dunlap JR, Nixon LT, Cooper KE, Radosevich M (2013) Gemmatirosa kalamazoonesis gen. nov., sp. nov., a member of the rarely-cultivated bacterial phylum Gemmatimonadetes. J Gen Appl Microbiol 59:305–312. https://doi.org/10.2323/jgam.59.305
Domagała-Świątkiewicz I, Siwek P (2013) The effect of direct covering with biodegradable nonwoven film on the physical and chemical properties of soil. Pol J Environ Stud 22:667–674. https://doi.org/10.1080/10962247.2013.755406
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460. https://doi.org/10.1093/bioinformatics/btq461
Fan AW, Liu W (2003) Simulation of the daily change of soil temperature under different conditions. Heat Transf Asian Res 32:533–544. https://doi.org/10.1002/htj.10106
Farmer J, Zhang B, Jin X, Peng Z, Wang J (2016) Long-term effect of plastic film mulching and fertilization on bacterial communities in a brown soil revealed by high through-put sequencing. Arch Agron Soil Sci 63:230–241. https://doi.org/10.1080/03650340.2016.1193667
Gao H, Yan C, Liu Q, Ding W, Chen B, Li Z (2018) Effects of plastic mulching and plastic residue on agricultural production: a meta-analysis. Sci Total Environ 651:484–492. https://doi.org/10.1016/j.scitotenv.2018.09.105
Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, Bouffard GG, Blakesley RW, Murray PR (2009) Topographical and temporal diversity of the human skin microbiome. Science 324:1190–1192. https://doi.org/10.1126/science.1171700
Hassan MA, Shirai Y, Husni M, Zainudin M, Mustapha NA (2021) Effect of inorganic fertilizer application on soil microbial diversity in an oil palm plantation. BioResources 18:2279–2203
Hill J (2005) Recycling biosolids to pasture-based animal production systems in Australia: a review of evidence on the control of potentially toxic metals and persistent organic compounds recycled to agricultural land. Aust J Agric Res 56(8):753–773. https://doi.org/10.1071/AR04264
Kader M, Senge M, Mojid M, Ito K (2017) Recent advances in mulching materials and methods for modifying soil environment. Soil Tillage Res 168:155–166. https://doi.org/10.1016/j.still.2017.01.001
Kennedy AD (1995) Antarctic terrestrial ecosystem response to global environmental change. Annu Rev Ecol Syst 26(1):683–704. https://doi.org/10.1146/annurev.es.26.110195.003343
Krumins JA, Dighton J, Gray D, Franklin RB, Morin PJ, Roberts MS (2009) Soil microbial community response to nitrogen enrichment in two scrub oak forests. For Ecol Manage 258:1380–1390. https://doi.org/10.1016/j.foreco.2009.06.046
Landwehr W, Wolf C, Wink J (2016) Actinobacteria and myxobacteria-two of the most important bacterial resources for novel antibiotics. Curr Top Microbiol Immunol. https://doi.org/10.1007/82-2016-503
Li J, Zhao B, Li X (2008) Effects of long-term combined application of organic and mineral fertilizers on soil microbiological properties and soil fertility. Sci Agric Sin 41:144–152. https://doi.org/10.3724/SP.J.1005.2008.01083
Li C, Moore-Kucera J, Lee J, Corbin A, Brodhagen M, Miles C (2014) Effects of biodegradable mulch on soil quality. Appl Soil Ecol 79:59–69. https://doi.org/10.1016/j.apsoil.2014.02.012
Li Y, Chapman SJ, Nicol GW, Yao H (2018) Nitrification and nitrifiers in acidic soils. Soil Biol Biochem 116:290–301. https://doi.org/10.1016/j.soilbio.2017.10.023
Liu Y, Mao L, He X, Cheng G, Ma X, An L, Feng H (2012) Rapid change of AM fungal community in a rain-fed wheat field with short-term plastic film mulching practice. Mycorrhiza 22:31–39. https://doi.org/10.1007/s00572-011-0378-y
Ma Q, Qu H, Meng N, Li S, Sun Y (2020) Biodegradation of skatole by burkholderia sp. ido3 and its successful bioaugmentation in activated sludge systems. Environ Res 182:109123. https://doi.org/10.1016/j.envres.2020.109123
Poly F, Wertz S, Brothier E, Degrange V (2008) First exploration of Nitrobacter diversity in soils by a PCR cloning-sequencing approach targeting functional gene nxrA. FEMS Microbiol Ecol 63:132–140. https://doi.org/10.1111/j.1574-6941.2007.00404.x
Puglisi E, Vasileiadis S, Demiris K, Bassi D, Karpouzas DG, Capri E, Cocconcelli PS, Trevisan M (2012) Impact of fungicides on the diversity and function of non-target ammonia-oxidizing microorganisms residing in a litter soil cover. Microb Ecol 64:692–701. https://doi.org/10.1007/s00248-012-0064-4
Qi Y, Ossowicki A, Yang X, Huerta Lwanga E, Dini-Andreote F, Geissen V, Garbeva P (2020) Effects of plastic mulch film residues on wheat rhizosphere and soil properties. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2019.121711
Qian H, Meng Z, Liu G, Tao L, Qian Q, Du B, Pan X (2018) Effects of soil residual plastic film on soil microbial community structure and fertility. Water Air Soil Pollut 229:261. https://doi.org/10.1007/s11270-018-3916-9
Ren W, Ren G, Teng Y, Li Z, Li L (2015) Time-dependent effect of graphene on the structure, abundance, and function of the soil bacterial community. J Hazard Mater 297:286–294. https://doi.org/10.1016/j.jhazmat.2015.05.017
Sabine L, Ralf C (1997) Detection in soil of aerobic hydrogen-oxidizing bacteria related to Alcaligenes eutrophus by PCR and hybridization assays targeting the gene of the membrane-bound (NiFe) hydrogenase. FEMS Microbiol Ecol 22:193–206. https://doi.org/10.1111/j.1574-6941.1997.tb00371.x
Schfer H, Dr MW, Wink M (2009) Medicinally important secondary metabolites in recombinant microorganisms or plants: progress in alkaloid biosynthesis. Biotechnol J 4(12):1684–1703. https://doi.org/10.1002/biot.200900229
Schneider D, Engelhaupt M, Allen K, Kurniawan S, Krashevska V, Heinemann M, Nacke H, Wijayanti M, Meryandini A, Corre MD (2015) Impact of lowland rainforest transformation on diversity and composition of soil prokaryotic communities in Sumatra (Indonesia). Front Microbiol 6:1339. https://doi.org/10.3389/fmicb.2015.01339
Shah AA, Hasan F, Hameed A, Ahmed S (2008) Biological degradation of plastics: a comprehensive review. Biotechnol Adv 26:246–265. https://doi.org/10.1016/j.biotechadv.2007.12.005
Sharma M, Ingram DT, Patel JR, Millner PD, Wang XL, Hull AE, Donnenberg MS (2009) A novel approach to investigate the uptake and internalization of Escherichia coli O157:H7 in spinach cultivated in soil and hydroponic medium. Journal of Food Protuction 72:1513–1520. https://doi.org/10.1080/08821127.1996.10731809
Sintim HY, Flury M (2017) Is biodegradable plastic mulch the solution to agriculture’s plastic problem? Environ Sci Technol 51:1068–1069. https://doi.org/10.1021/acs.est.6b06042
Sreejata B, Lluis MC, Pelacho AM, DeBruyn JM (2018) Biodegradable plastic mulch films: impacts on soil microbial communities and ecosystem functions. Front Microbiol 9:1–7. https://doi.org/10.3389/fmicb.2018.00819
Steinmetz Z, Wollmann C, Schaefer M, Buchmann C, David J, Tröger J, Muñoz K, Frör O, Schaumann GE (2016) Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci Total Environ 550:690–705. https://doi.org/10.1016/j.scitotenv.2016.01.153
Subrahmaniyan K, Kalaiselvan P, Balasubramanian TN, Zhou W (2006) Crop productivity and soil properties as affected by polyethylene film mulch and land configurations in groundnut (Arachis hypogaea L.). Arch Agron Soil Sci 52(1):79–103. https://doi.org/10.1080/03650340500421786
Sui X, Feng F, Lou X, Zheng J, Han S, Dai G (2013) Effects of birch forest at different developmental stages on soil microorganism community structure. J Bionanosci 7:78–83. https://doi.org/10.1166/jbns.2013.1085
Sun T, Zhang Z, Ning T, Mi Q, Zhang X, Zhang S, Liu Z (2015) Colored polyethylene film mulches on weed control, soil conditions and peanut yield. Plant Soil Environ 61:79–85
Tayyab M, Islam W, Arafat Y, Pang Z, Zhang C, Lin Y, Waqas M, Lin S, Lin W, Zhang H (2018) Effect of sugarcane straw and goat manure on soil nutrient transformation and bacterial communities. Sustainability 10:2361. https://doi.org/10.3390/su10072361
Tokiwa Y, Calabia BP, Ugwu CU, Aiba S (2009) Biodegradability of plastics. Int J Mol Sci 10(9):3722–3742. https://doi.org/10.3390/ijms10093722
Trujillo F, Caballero S, Correa-Cárdenas CA (2015) Population structure and genetic diversity of the endangered south american giant otter (pteronura brasiliensis) from the orinoco basin in colombia: management implications and application to current conservation programs. J Hered 106:469–477. https://doi.org/10.1093/jhered/esv049
Vasilyeva G, Strijakova E (2007) Bioremediation of soils and sediments contaminated by polychlorinated biphenyls. Microbiology 76:725–741. https://doi.org/10.1134/S002626170706001X
Wang J, Lv S, Zhang M, Chen G, Zhu T, Zhang S, Teng Y, Christie P, Luo Y (2016) Effects of plastic filmresidues on occurrence of phthalates and microbial activity in soils. Chemosphere 151:171–177. https://doi.org/10.1016/j.chemosphere.2016.02.076
Yadav A, Yadav K (2013) Seasonal population dynamics of rhizosphereand non-rhizosphere soil microorganisms of chir pine seedlings (Pinus roxburghii Sarg.). Br Microbiol Res J 3:664–677. https://doi.org/10.9734/BMRJ/2013/4206
Yan C, He W, Xue Y, Liu E, Liu Q (2016) Application of biodegradable plastic film to reduce plastic film residual pollution in Chinese agriculture. Chin J Biotechnol 32:748–760
Young JM, Weyrich LS, Clarke LJ, Cooper A (2015) Residual soil DNA extraction increases the discriminatory. power between samples. Forensic Sci Med Pathol 11:268–272. https://doi.org/10.1007/s12024-015-9662-z
Zhang J, Lan T, Müller C, Cai Z (2015) Dissimilatory nitrate reduction to ammonium (DNRA) plays an important role in soil nitrogen conservation in neutral and alkaline but not acidic rice soil. J Soils Sedim 15:523–531. https://doi.org/10.1007/s11368-014-1037-7
Zhao Z, Wang P, Wang Y, Zhou R, Koskei K, NdoloMunyasya A, Liu S, Wang W, Su Y, Xiong Y (2021) Fate of plastic film residues in agro—ecosystem and its effects on aggregate—associated soil carbon and nitrogen stocks. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2021.125954
Acknowledgements
This work was supported by the monitoring and evaluation of the environmental impact of plastic film pollution in the Gansu region (2019–125A0701) and funding from the central government for agricultural production and development in 2018 and projects on recycling machinery for plastic film and plastic film reduction technology.
Author information
Authors and Affiliations
Contributions
YX, TJ, and CG provided the site for the experiment, provided the right to sample and played an important role in writing articles. CG, CL and TZ, sample collection and processing, PFM collection, soil screening, experimental data statistics. MY and DW, microbial sample processing and sequencing analysis. MY and DW, overall experimental design including chart drawing review.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
All authors agree to participate in this study.
Consent for publication
All authors agree to publish this article in the journal of Archives of Microbiology.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xue, Y., Jin, T., Gao, C. et al. Effects of biodegradable film mulching on bacterial diversity in soils. Arch Microbiol 204, 195 (2022). https://doi.org/10.1007/s00203-022-02799-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-02799-9