Abstract
The effect of microplastic adsorption on marine microalgae Tetraselmis suecica, Amphora subtropica, and copepod Pseudodiaptomus annandalei was investigated in the present study. Fluorescence microscopic images were used to evaluate MP interactions with algae and copepods. T. suecica growth rate decreased with effects of 0.1 µm polystyrene exposure to 75 µl/100 ml (0.899 to 0.601 abs), 50 µl/100 ml (0.996 to 0.632 abs) and 25 µl/100 ml (0.996 to 0.632 abs), respectively. On the other hand, at 10th day of experiment, the control T. suecica showed the highest growth rate (0.965 abs), chlorophyll concentration (Chl-‘a' = 21.36 µg/L; Chl-‘b' = 13.65 µg/L), and cell density (3.3 × 106 cells/ml). A marine diatom A. subtropica absorbed 2.0 μm microplastics, and the maximal inhibition rate increased at higher MP concentration until 10th day. The highest MPs (75 μl/100 ml) treatment resulted in decreased growth rate of A. subtropica from 0.163 to 0.096 abs. A. subtropica (without MPs) had the highest lipid concentration of 27.15%, whereas T. suecica had the lowest lipid concentration of 11.2% (without MP). The maximum survival (80%) of P. annandalei was found in control on 15th day whereas on 12th day, the microplastics ingested copepod had the lowest survival rate (0%). On 15th day, the maximum Nauplii Production Rate (NPR) (19.33) female−1 was observed in control, whereas the minimum (17.33) female−1 NPR was observed in copepod ingested with MPs. The maximum lipid production (17.33% without MPs) was reported in control, whereas MPs fed copepods had the lowest lipid production (16%). Long-term exposure to polystyrene microplastics significantly reduced algae growth and chlorophyll concentration and also NPR and lipid concentration rate of copepod. We inferred that microplastic exposure of algae and copepods might results in persistent decreases in ingested carbon biomass over time.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plastic production has increased significantly since the large-scale industrial manufacturing started in early 1950s. Plastics are used in almost every aspects of daily life (Plastics Europe, 2016) and the majority of plastics are used in automotive industry (8.9%), electrical and electronic devices (5.8%), agriculture (3.3%), packaging (39.9%), building and construction (19.7%), and other sectors (22.4%). The plastic products would gradually break down into fragments over time, especially if they were continuously exposed to ultraviolet radiation (of sunlight) and high temperatures. This fragmentation would reduce the plastic material sizes from macroscopic to microscopic and finally nano-sizes. The increasing awareness of plastic pollution has resulted in the documentation of more scientific and societal concerns regarding the effects of microplastics (MPs) with diameter less than 5 mm (Gregory and Ryan, 1997; Kako et al. 2011, 2014; Yoon et al. 2010; Kubota, 1994; Zarfl and Matthies, 2010; Isobe et al. 2014; Maximenko et al. 2012). Microplastics can be categorized as either primary or secondary MPs based on their manufacturing process. Primary microplastics are tiny particles that are released into the environment directly or indirectly through domestic and industrial effluents, spills and sewage discharge. The most commonly found primary microplastics are fibres, pellets, film, fragments and spheres (Kang et al. 2015; Lusher 2015; Li et al. 2016). Secondary microplastics are produced by photo-oxidation of UV radiation, mechanical transformation and microbial degradation (Andrady and Neal, 2009; Cole et al. 2011). The smaller particles broken down into nanoplastics (1–100 nm) with toxicological characteristics (Koelmans et al. 2015; da Costa et al. 2016). Ingestion of microplastics by marine organisms including plankton, fish, benthic organisms that can be trophically transferred (Browne et al. 2008; Wright et al. 2013). Overall reported the impacts of microplastics on marine organisms such as growth delay, oxidative stress, reduction of feeding activity, genotoxicity, neurotoxicity and reduction of reproductive fitness (Mazurais et al. 2015; Li et al. 2018). Microplastics adsorption by microalgae influence the sinking rate of both microalgae and buoyant microplastics (Ballent et al. 2013). Biofouling of microplastics may also enhance their absorption by biota (Andrady 2011). Under stressful conditions such as a lack of light or nutritional shortage, microalgae produce polysaccharides molecules, known as exopolysaccharides which can subsequently coagulate, allowing algal cells to aggregate (Long et al. 2015; Staats et al. 2000). Copepods are secondary producer and popular model for ecotoxicological studies among zooplankton because they play an important role in aquatic ecosystem and are sensitive to environmental stressors (Ananth and Santhanam, 2011). Polystyrene is a transparent polymer contains styrene monomers with specific gravity of 1.04–1.07 g cm−3 and its soluble in various organic solvents such as esters, aromatic hydrocarbons and ketones. PS is resistant to salts, alkalis, mineral oils, organic acids and alcohols. Less weight PS foam provides many applications such as roofing, refrigerators, building walls and freezers. PS caused the gastrointestinal tract infections and toxic to the cellular level (Cole et al. 2015). According to Cai et al (2018), PS particles from laboratories, might be a source of main plastics particulate pollutants and their particles can create a variety of issues depending on their size, shape and functional groups. Polystyrene is a primary particle caused the delayed growth, feeding capacity and reproductive development in marine organisms (Alimi et al., 2018). The aggregation and attachment of polystyrene to marine organisms from microalgae to copepod were not investigated by earlier studies; hence, the present study was aimed to assess the effects of microplastics adsorption on marine microalgae Tetraselmis suecica, Amphora subtropica with effects on growth rate, chlorophyll concentration, lipid profile besides survival, nauplii hatching and lipid profile of copepod P. annandalei.
Materials and methods
Experimental setup
Pre-cultured T. suecica (PSBDU002) and A. subtropica (KM099276) at the logarithmic growth phase were taken from microalgae culture collection of marine planktonology and aquaculture laboratory of Department of Marine science, Bharathidasan University, and added into 100 ml of Conway’s and TMRL-enriched seawater medium was used as a control, of which 10% inoculum was added. Depending on the size of the microalgae, two distinct Polystyrene microplastics (0.1 μm (Lot# MKCH2797) amine modified fluorescent orange beads for T. suecica and 2.0 μm (Lot#MKCJ4402) fluorescent yellow green beads for A. subtropica) were obtained from Sigma-Aldrich and individually injected to each algal culture to achieve concentrations of 25, 50 and 75 μl/100 ml, respectively. All the experiments were incubated under controlled laboratory conditions that remained constant throughout the investigation (23 ± 1 °C, 50 μmol photons/(m2 sec−1) and a 12 h/12 h (light/dark) cycle). The aggregation and attachment of MPs to algae were evaluated over an entire culture cycle, from seeding to stationary growth phase for each algal species. The microalgal physiology was examined through the analysis of their growth rates, cell density and chlorophyll for assessing the photosynthetic ability which is used to identify the potential impacts of micro-PS on phytoplankton community. All groups were maintained as triplicate.
Determination of photosynthetic pigment
Chlorophyll was extracted by taking 0.5 g of algae to which 5 ml 90% acetone was added. It was kept undisturbed overnight and the optical density of the chlorophyll was measured next day with a UV/Vis spectrophotometer (Spectro 20D plus, U.S.A). The amount of chlorophyll was calculated according to the equations of Jeffrey and Humphrey (1975).
Chlorophyll analysis for green algae
Chlorophyll ‘a’ (µg/ml) = 11.93 E664–1.93 E647.
Chlorophyll ‘b’ (µg/ml) = 20.36 E647–5.50 E664.
Chlorophyll analysis for diatom
Chlorophyll ‘a’ (µg/ml) = 11.47 E664–0.40 E630.
Chlorophyll ‘c’ (µg/ml) = 24.36 E630–3.73 E664.
Determination of microalgae growth rate
Algal growth was measured by taking absorbance. Absorbance was measured every 2 days once for the period of 10 days by recording the changes in optical density at 680 nm with a UV/Vis spectrophotometer according to Lichtenthaler (1987).
Determination of algal cell density
Microalgal cell concentration was determined through cell counting using haemocytometer. The haemocytometer is a slide glass size 30 mm × 70 mm × 4 mm thick. It has 2 chambers and cells counts were performed at the center part. The specific growth rate of microalgae was calculated using Eq. 1 derives formula from Andersen (2005).
Extraction of total lipids from microalgae
The total lipids of the algae were extracted by mixing chloroform–methanol (4:2 v/v) by adopting the standard procedure of Folch et al. (1957). A mixture of 2 ml methanol and 1 ml chloroform was made and added to 1 g algal biomass. It was kept for 24 h at room temperature to dissolve the lipids properly. The mixture was centrifuged at 3000 rpm for 10 min. Supernatant was separated, 2 ml of chloroform was again added to the pellets and shaken properly. It was again centrifuged at 3000 rpm for 5 min and supernatant was separated. After adding 2 ml of 1% KCL to the supernatant, two layers were formed. Lower layer was pipette out and weighed.
Collection and identification of copepods
The zooplankton samples were collected from the Vettar estuary (10°46' N Lat. 79°12' E Lon.) using plankton net with 158 μm mesh. The collected samples were immediately transported to laboratory by providing with vigorous aeration using battery aerator. The zooplankton samples were thoroughly rinsed to reduce the contamination from other zooplankters. From the samples, P. annandalei was isolated with fine brush, needle and stempel pipette and identified under microscope using the key of Davis (1955); Kasturirangan (1963). Based on the keys provided by the authors, the species was confirmed for their taxonomy and used for culture.
Copepod culture
About 50 individuals of P. annandalei were isolated and stocked in 1000 ml beaker contained seawater. The marine microalga Isochrysis galbana at the concentration of 25,000 to 30,000 cells/ml. was given as feed for copepod P. annandalei. The water quality parameters, viz., temperature (23–26° C), salinity (28–30 PSU) and pH (7–8.5) were maintained at on optimized condition. The generation time of P. annandalei under optimal conditions was about 10–12 days and having 6 nauplii and 6 copepodite stages including the adult. Finally, the adult gravid female copepods were used to restart the stock culture.
Microplastic beads
The latex beads, carboxylate-modified polystyrene, fluorescent yellow-green were purchased from (Sigma-Aldrich, USA) L4530 (Lot # MKCF0821) aqueous suspension with 2.0 μm mean particle size was used for the present experiment.
Experimental setup
The copepod, P. annandalei was cultured in filtered seawater. The 2 μm green fluorescent microplastic beads were used for the experimental study. The control copepods were fed with I. galbana with the concentration of 25,000 cells/ml and 2 μl/100 ml MPs was ingested in the experimental copepod. Control and experimental groups were triplicated. Survival rate, nauplii production rate and total lipid was determined in control and experimental copepod.
Effect of MPs ingestion on survival rate of P. annandalei
The survival rate of copepods was analyzed for the period of 15 days. 10 numbers of healthy P. annandalei were picked up from the stock culture by using fine brush and then transferred to a 100 ml beaker filled with sterile seawater and fed with I. galbana and inoculated with 2.0 μm fluorescence yellow green MPs. The number of live copepod specimens remaining in each chamber was recorded daily. Dead copepods were removed from treatments. The survival rates of copepods in respect to experimental and control treatments were measured by subtracting the final density of copepods from initial density of copepods.
Assessment of MP ingestion on nauplii hatching of P. annandalei
For determining the nauplii producing capacity of microplastic-ingested P. annandalei and control P. annandalei five individual female with viable egg sacs were stocked in a glass test tube containing 20 ml of filtered seawater. Copepod P. annandalei was stocked in the beaker filled with seawater and 2 μl of MPs. The copepod stocked with only microalgae I. galbana was control. The copepod was examined at regular intervals (every 2 h) for the release of nauplii. Once the nauplii were released, the adult female was carefully removed from the test tube and the nauplii were counted under the microscope.
Determination of total lipids from P. annandalei fed with MPs
The copepod P. annandalei was cultured with control (I. galbana) and fed with MPs was harvested using the 48 μm mesh. The harvested copepod sample was first washed with filled seawater followed by distilled water. After removing the excess moisture using blotting paper copepod sample was subjected to drying in an oven. Lipid content of copepod was estimated by the method of Folch et al. (1957). In brief, a known amount of dried copepod sample was homogenized in 10 ml of chloroform–methanol mixture (2/1 v/v). The homogenate was centrifuged at 2000 rpm. The supernatant was washed with 0.9% saline solution (KCl) to remove the non-lipid contaminants and allowed to separate. The upper phase was discarded by siphoning. The lower phase was allowed to dry in a hot air oven and the weight was taken.
Statistical analyses
The statistical analysis of the obtained data was assessed with one-way analysis of variance (ANOVA) with help of SPSS by using Regression Analysis Test (DMRT).
Results
Effect of MPs adsorption on algal photosynthesis
The green algae T. suecica (0.1 μm MPs) were subjected to three different concentrations of microplastics (25, 50, and 75 μl/100 ml) and 2.0 μm of green fluorescent MPs were injected in to A. subtropica culture flask with three different concentrations (25, 50 and 75 μl/100 ml). The impacts on their photosynthesis and growth rate were monitored every 48 h for 10 days.
Effect of MPs (0.1 µm) on growth rate, cell density and chlorophyll concentration of T. suecica
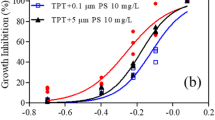
The growth rate, cell density and chlorophyll concentration of T. suecica was increased in microplastics suspension culture and no homo-aggregates were formed from day 1 to day 4. In MPs exposure treatment homo-aggregation was observed from 6th day onwards. However, the rate of growth and chlorophyll content decreased from 8th day to 10th day under 75 µl/100 ml (0.899 to 0.601 abs) MPs exposure followed by 50 µl/100 ml (0.996 to 0.632 abs) and 25 µl/100 ml (0.792 to 0.52 abs). The control experiment showed maximum growth rate (0.965 abs), chlorophyll concentration (Chl-‘a’ = 21.36 µg/L; Chl-‘b’ = 13.65 µg/L) and cell density (3.3 × 106 cells/ml) on the 10th day of experiment. Only the microalgae (in suspension) were taken into account for the estimation of growth rate and chlorophyll (Fig. 1). T. suecica exposed to 0.1 µm polystyrene for different exposure time revealed a positive correlation and significantly affected chlorophyll ‘a' (R2 = 0.94; P < 0.01) and ‘b' (R2 = 0.89; P < 0.02) concentrations when compared to control (R2 = 0.59; P0.05). At different exposure periods, the growth rate of T. suecica was not significantly different from the control (R2 = 0.34; P > 0.081). Cell density was decreased in experimental algae as compared with control showed non-significance and reduced maximum cell density concentration (R2 = 0.140; P < 0.496).
Effect of MPs (2.0 µm) on growth rate, cell density and chlorophyll concentration of A. subtropica
Three growth phases (the lag phase, the logarithmic phase and the stationary phase) of A. subtropica over a 10 days of incubation period were clearly distinguished in the control groups and algal growth rate, cell density and chlorophyll continuously increased from 0.012 to 0.596 abs, 4.02 × 104 to 2.102 × 105 cells/ml and Chl-a = 3.23 to 15.23 μg/L and Chl-b = 1.56 to 7.15 µg/L from initial day to 10th day, respectively. Then the algae was exposed to 2.0 μm microplastics and the maximum inhibition rate was recorded with increased MPs concentration until 10th day. In the present study, the highest concentration (75 µl/100 ml PS) of microplastic exposure treatment flask inhibit the cell density from 6th day to 10th day. Under 75 µl/100 ml MPs treatments growth rate decreased from 0.163 to 0.096 abs at the end of the study (as compared to the control). Similarly, the chlorophyll concentration was also reduced under highest MP exposure with homo aggregates starting from 6th day to 10th day (Fig. 2). 2.0 μm polystyrene-ingested A. subtropica with different exposure days are significantly affected the chlorophyll concentration ‘a’ & ‘b’ as compared with control (R2 = 0.98; P < 0.03) and (R2 = 0.98; P < 0.01). A. subtropica growth rate was not significantly different with control at different exposure days (R2 = 0.22; P < 0.454). Maximum cell density concentration decreased in ingested one against control (R2 = 0.76; P < 0.496).
Effect of MPs on lipid concentration of T. suecica and A. subtropica
While many microalgal strains were found to naturally have high lipid content. Lipid accumulation refers to increased concentration of lipids within the micro algae cells. In the present investigation the lipid concentration of T. suecica and A. subtropica was analyzed in relation to control (Without MPs) and different concentrations of MP exposed algae. In the present study, maximum lipid concentration of 27.15% was observed in control A. subtropica (without MPs) and the minimum (11.2%) was observed in T. suecica (Without MPs). Only minimal changes were observed in the lipid content MPs exposed microalgae (Fig. 3). Lipid concentration was significantly lower in Polystyrene-exposed T. suecica compared to control (R2 = 0.82; P < 0.04). Similarly, when A. subtropica was exposed to 2.0 μm PS, its lipid level reduced in comparison to the control (R2 = 0.92; P < 003).
Effect of MPs on survival rate (a), nauplii hatching rate (b), lipids of P. annandalei (c). The means values shown of three triplicates ± standard deviation and standard deviations were calculated from replications and lipids of T. suecica and A. subtropica showed significantly (P < 0.04; P < 003) (d)
Survival rate of P. annandalei
In the present study, the maximum survival rate was observed in copepod fed with I. galbana (80%) whereas microplastics ingested copepods were died at the 12th day of experiment and the survival rate of MPs ingested P. annandalei was 0% (Fig. 3).
Nauplii hatching rate of P. annandalei
The observed results revealed that the microplastic feeding adversely impacts the production of nauplii in copepods. The highest nauplii production rate was observed in the control copepod diet with an average of 19.33 nauplii female−1 whereas the lowest nauplii production was observed in MPs ingested P. annandalei with 4 nauplii female−1 (Fig. 3).
Lipids
In adult P. annandalei, approximately 40% of energy derived from their food goes toward the buildup of their lipid store. The presence of lipid concentration in copepod was based on the mass culture production. In the present investigation, micro-algae fed copepod showed highest lipid production (17.3%) whereas the MPs ingested copepods result the lowest lipid profile (Fig. 3). Furthermore, we concluded that a feeding may result in an altered lipid profile. Yet, there was no difference in the total (Plate 1) lipid concentration of copepods at the end of the exposure period (Plate 2).
Discussion
Polystyrene (PS), a high-volume polymer with global market worth more than $30 billion dollars, is one of the plastics often found in oceans (Transparency Market Research, 2014). Because marine microalgae are the major producers at the bottom of the food chain, there is concern about the potentially adverse effects of nano- and micro-sized plastic particles (Kaiser et al., 2011). According to Bhattacharya et al. (2010) the interactions between microplastics and microalgae are expected to alter cell properties such as size and shape. Additionally, algal cell walls act as barriers to particle penetration and the cell wall characteristics may consequently influence particle sorption. Therefore, the present study was conducted in marine diatom, A. subtropica (with a silicate cell wall) and the marine alga T. suecica without a cell wall.
Microplastics debris, as an emerging environmental pollutant, is prevalent globally now. Therefore, it is crucial to have a basic understanding of the interactions between microplastics and the organisms. In the present study, the effects of microplastics on microalgae (T. suecica) was exposed to 0.1 µm size MPs and then the effects on their photosynthesis and growth were determined every 48 h for the period of 10 days. All the tests were performed under identical temperature, illumination and photoperiod conditions with the exponentially grown cultures at a start density of (5 × 105 cells/ml) (allowing for exponential growth during the experiment). In contrast to photosynthesis, a clear effect of the MPs beads on the growth of T. suecica was observed. The highest MP concentration of 75 µl/100 ml showed the lowest microalgal photosynthesis, growth rate and cell density. The growth rate of T. suecica was higher on the 6th of the experiment, which might be owing to the homo-aggregation of microalgal cells. The reduction in cell density, algal photosynthesis and growth rate were exposed to 0.1 µm size with concentration of 25 µl/100 ml MPs beads was clearly reduced from 8 to 10th day when compared to the control.
When compared to other species, diatoms like A. subtropica expelled large amounts of sticky transparent exo-polymeric particles, and these diatoms possess biogenic silica (Passow, 2002). A. subtropica is known to easily aggregate for these reasons. The influence of microplastics on the marine diatom A. subtropica was studied from the logarithmic to stationary phases in this study. Diatom (A. subtropica) was exposed to 2.0 µm size of MPs beads and their effects on photosynthesis and growth were determined every 48 h once for the period of 10 days. All tests were performed under controlled conditions with a start density (4.02 × 104 cells/ml) allowing for exponential growth during the experiment. Among these four different treatments, the highest MPs concentration of 75 µl/100 ml showed the lowest microalgal photosynthesis, growth rate and cell density followed by 50 and 25 µl/100 ml microplastics exposure treatments at the mid stationary phase. The growth rate, cell density and chlorophyll concentration were found to be increased from day 2 to day 6 after it was declined. The minimum of cell density, algal photosynthesis and growth rate were observed with the MP concentration of 25 μl /100 ml. Besseling et al. (2014) demonstrated that PS particles of a similar size (0.07 µm) inhibited the growth of the microalga Scenedesmus obliquus. The adsorption of the MPs beads on algae could block light from reaching the photosynthesis, similar studies were earlier carried out by Yufeng Mao et al. (2018) and Marc long et al. (2015). Sjollema et al. (2016) suggested that the nanoscale, particles (0.1 µm) become more easily undergo aggregation (Gigault et al. 2018) and (Galloway et al. 2017), which would cause more interaction between microplastics and algae. These results confirmed that the effect on microalgal growth decreased with increasing beads concentration and size. During the present investigation period, T. suecica showed the highest homo aggregation compared to A. subtropica. The role of MPs in the fate of aggregates may vary depending on the MPs size, density and composition (Cózar et al. 2014).
Microalgae generate a wide range of lipids with nutritional value. As a result, microalgae play an important role in the aquaculture food chain, primarily as live feeds for larval culture (Brown 1997; Martínez-Fernández et al. 2006). Lipid composition and productivity depend on growth conditions such as growth phase (Xu et al. 2008), medium composition (Valenzuela-Espinoza et al. 2002), irradiance rate (Thompson et al. 1993), and temperature conditions (Renaud et al. 2002). In the present investigation, the total lipid was analyzed in T. suecica and A. subtropica (with and without MPs). The maximum concentration of lipid was observed in A. subtropica without MPs (Control) which may be due to the nitrogen depletion (Valenzuela 2013). The MPs containing microalgae not showed any variation in total lipid concentration. Similar findings were earlier reported in mussels by Smolders and Degryse (2002). Furthermore, we hypothesized that the aggregated microalgae may alter the lipid profile. However, there was a substantial change in total lipid mass of microalgae at end of the exposure period, but the lipid profiles of the control were significantly altered.
Microplastics have been found to be present in many parts of the marine food web, especially in the water column with various impacts on the zooplankton (Cole et al. 2015). This study investigated the effects of microplastic beads of 2 μm that might have on the filtration ability in copepod P. annandalei. The present results showed that the copepod can ingest microplastics (2.0 μm diameter) in the absence of natural food. Microplastics were unknowingly ingested via filter-feeding and later egested in faecal pellets, typically within a matter of hours. Microplastics were found to be trapped between the external appendages of copepods owing to its accumulation on the external surface. The microscopic images of copepod clearly showed that 2.0 μm polystyrene beads clustered within the alimentary canal and aggregated between the setae and joints of external appendages. The presence of 2.0 μm polystyrene beads reduced the algal ingestion rate, nauplii production and survival rates of copepod P. annandalei. Similarly, Cole et al. (2013) reported Temora longicornis and Centropages typicus ingested microplastics with different sizes 7.3, 20.6 and 30.6 μm beads and Acartia clausi, Calanus helgolandicus ingested 7.3 μm beads affected the feeding and survival rates of the animals. The present study demonstrated that the minimum survival and nauplii production rate was found in microplastic ingested copepod. Prolonged exposure to the microplastics resulted in less egg production with reduced hatching success and survival rate was also reduced within 4–5 days in P. annandalei. The present findings shows that microplastics can impede copepod feeding and reduce the nauplii production rate and survival. These effects were most noticeable at 3–4 days after the ingestion of microplastics into the organisms. Lee et al. (2013) found that when exposed to 0.5 and 6 μm microplastics, the number of nauplii which hatched from eggs produced by the benthic copepod Tigriopus japonicus was reduced. Similar results have been observed by Bonnet et al. (2005), Cole et al. (2013, 2015). In adult P. annandalei, approximately 40% of energy derived from their food goes towards the buildup of their lipid store. (Marshall and Orr, 1955). The present study results concluded that the maximum concentration of lipid was shown in algae fed copepod (19%) and lipid contents were observed in the MPs exposure copepod (18%). Furthermore, there were no differences found in the total lipid concentration of copepods at end of the exposure period.
Conclusion
In the present study, two different microplastic beads were induced in a two-microalga, viz., T. suecica and A. subtropica over the 10 days experimental period. The significant PS beads aggregation was observed in T. suecica (than in the A. subtropica). The findings on the effect of MP beads absorption in total lipid production of T. suecica and A. subtropica showed not much variations observed in lipid production of control and MPs ingested microalgae. The microplastic ingested P. annandalei, showed less survival and nauplii production further, microplastic adsorption and consumed copepods showed minimal changes in the lipid production. Higher trophic species that rely on the high lipid content of microalgae and copepods for their own sustenance may be affected by energetic deficits and decreased lifespan of microplastic exposed marine microalgae and copepods. The results concluded that better understand the density of bioavailable microplastics in biota-rich waters, as well as to test whether environmentally relevant concentrations of plastic litter can impact keystone species such as T. suecica, A. subtropica and P. annandalei, as well as the consequences for commercially important predators.
References
Alimi OS, Farner Budarz J, Hernandez LM, Tufenkji N (2018) Microplastics and nanoplastics in aquatic environments: aggregation, deposition, and enhanced contaminant transport. Environ Sci & Tech 52:1704–1724. https://doi.org/10.1021/acs.est.7b05559
Ananth S, Santhanam P (2011) Laboratory culture and biochemical profile of marine copepod, Macrosetella gracilis (Dana). Aquaculture 12(1):49–55
Andrady AL (2011) Microplastics in the marine environment. Mar Pollut Bll 62(8):1596–1605. https://doi.org/10.1016/j.marpolbul.2011.05.030
Andrady AL, Neal MA (2009) Applications and societal benefits of plastics. Phil Trans R Soc b 364(1526):1977–1984. https://doi.org/10.1098/rstb.2008.0304
Ballent A, Pando S, Purser A, Juliano MF, Thomsen L (2013) Modelled transport of benthic marine microplastic pollution in the Nazaré Canyon. Biogeosciences 10(12):7957–7970. https://doi.org/10.5194/bg-10-7957-2013
Besseling E, Wang B, Lürling M, Koelmans AA (2014) Nanoplastic affects growth of S. obliquus and reproduction of D. magna. Environ Sci Technol 48(20):12336–12343. https://doi.org/10.1021/es503001d.
Bhattacharya P, Lin S, Turner JP, Ke PC (2010) Physical adsorption of charged plastic nanoparticles affects algal photosynthesis. J Phys Chem 114(39):16556–16561. https://doi.org/10.1021/jp1054759
Bonnet D, Richardson A, Harris R, Hirst A, Beaugrand G, de Edwards M, Puelles MLF (2005) An overview of Calanus helgolandicus ecology in European waters. Prog in Oceano 65(1):1–53. https://doi.org/10.1016/j.pocean.2005.02.002
Brown KS (1997) Diversity, disturbance, and sustainable use of Neotropical forests: insects as indicators for conservation monitoring. J Insect Conserv 1(1):25–42
Browne MA, Galloway TS, Thompson RC (2008) Spatial patterns of plastic debris along estuarine shorelines. Environ Sci Technol 44(9):3404–3409. https://doi.org/10.1021/es903784e
Cai L, Hu L, Shi H, Ye J, Zhang Y, Kim H (2018) Effects of inorganic ions and natural organic matter on the aggregation of nanoplastics. Chemosphere 197:142–151. https://doi.org/10.1016/j.chemosphere.2018.01.052
Cole M, Lindeque P, Halsband C, Galloway TS (2011) Microplastics as contaminants in the marine environment. A Review Mar Pollut Bll 62(12):2588–2597. https://doi.org/10.1016/j.marpolbul.2011.09.025
Cole M, Lindeque P, Fileman E, Halsband C, Goodhead R, Moger J, Galloway TS (2013) Microplastic ingestion by zooplankton. Environ Sci Technol 47(12):6646–6655. https://doi.org/10.1021/es400663f
Cole M, Lindeque P, Fileman E, Halsband C, Galloway TS (2015) The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod Calanus helgolandicus. Environ Sci & Tech 49(2):1130–1137
Costa JP, Santos PS, Duarte AC, Rocha-Santos T (2016) Nano plastics in the environment-sources, fates and effects. Sci Total Environ 566:15–26. https://doi.org/10.1016/j.scitotenv.2016.05.041
Cózar A, Echevarría F, González-Gordillo JI, Irigoien X, Úbeda B, Hernández-León S, Palma ÁT, Navarro S, García-de-Lomas J, Ruiz A, Fernández-de-Puelles ML (2014) Plastic debris in the open ocean. P Natl Acad Sci-Biol 111(28):10239–10244. https://doi.org/10.1073/pnas.1314705111
Davis C. C 1955 The marine and freshwater plankton. Michigan State University.
Folch J, Lees M, Stanley GS (1957) A simple method for the isolation and purification of total lipids from animal tissues. J of Bio Chem 226(1):497–509
Galloway TS, Cole M, Lewis C (2017) Interactions of microplastic debris throughout the marine ecosystem. Nat Ecol Evol 1(5):0116. https://doi.org/10.1038/s41559-017-0116
Gigault J, Ter Halle A, Baudrimont M, Pascal PY, Gauffre F, Phi TL, El Hadri H, Reynaud GB, S, (2018) Current opinion: What is a nanoplastic? Environ Pollut 235:1030–1034. https://doi.org/10.1016/j.envpol.2018.01.024
Gregory MR, Ryan PG (1997) pelagic plastics and other seaborne persistent synthetic debris. A review of Southern Hemisphere perspectives. In Marine Debris (49–66). Springer. New York. NY.49–66. https://doi.org/10.1007/978-1-4613-8486-1_6.
Isobe A, Uchiyama-Matsumoto K, Uchida K, Tokai T (2014) Microplastics in the Southern Ocean. Mar Pollut Bull 114(1):623–626. https://doi.org/10.1016/j.marpolbul.2016.09.037
Jeffrey ST, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem Phy 167(2):191–194. https://doi.org/10.1016/S0015-3796(17)30778-3
Kaiser MJ, Attrill MJ, Jennings S, Thomas DN, Barnes DK (2011) Marine ecology processes systems, and impacts. Oxford University Press
Kako S, Isobe A, Magome S, Hinata H, Seino S, Kojima A (2011) Establishment of numerical beach litter hindcast/forecast models: an application to Goto Islands. Japan Mar Pollut Bull 62:293–302. https://doi.org/10.1016/j.marpolbul.2010.10.011
Kako S, Isobe A, Kataoka T, Hinata H (2014) A decadal prediction of the quantity of plastic marine debris littered on beaches of the East Asian marginal seas. Mar Pollut Bull 81:174–184. https://doi.org/10.1016/j.marpolbul.2014.01.057
Kang JK, Kwon OY, Lee KW, Song YK, Shim WJ (2015) Marine neustonic microplastics around the south eastern coast of Korea. Mar Pollut Bull 96(1–2):304–312. https://doi.org/10.1016/j.marpolbul.2015.04.054
Kasturirangan LR (1963) A Key for the Identification of the More Common Planktonic Copepoda: Of Indian Coastal Waters (No. 2). Council of Scientific & Industrial Research.
Koelmans AA, Besseling E, Shim WJ (2015) Nanoplastics in the aquatic environment. Critical review. In Marine Anthropogenic Litter (325–340). Springer, Cham. https://doi.org/10.1007/978-3-319-16510-3.
Kubota M (1994) A mechanism for the accumulation of floating marine debris north of Hawaii. J Phys Oceanogr 24:1059–1064. https://doi.org/10.1175/1520-0485(1994)024%3c1059:AMFTAO%3e2.0.CO;2
Lee KW, Shim WJ, Kwon OY, Kang JH (2013) Size-dependent effects of micro polystyrene particles in the marine copepod Tigriopus japonicus. Environ Sci & Tech 47(19):11278–11283. https://doi.org/10.1021/es401932b
Li J, Qu X, Su L, Zhang W, Yang D, Kolandhasamy P, Shi H (2016) Microplastics in mussels along the coastal waters of China. Environ Pollut 214:177–184. https://doi.org/10.1016/j.envpol.2016.04.012
Li J, Liu H, Chen JP (2018) Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res 137:362–374. https://doi.org/10.1016/j.watres.2017.12.056
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Method Enzymol 148:350–382. https://doi.org/10.1016/0076-6879(87)48036-1
Long M, Moriceau B, Gallinari M, Lambert C, Huvet A, Raffray J, Soudant P (2015) Interactions between microplastics and phytoplankton aggregates: Impact on their respective fates. Mar Chem 175:39–46. https://doi.org/10.1016/j.marchem.2015.04.003
Lusher A (2015) Microplastics in the marine environment: distribution, interactions and effects. In Marine Anthropogenic Litter (245–307): Springer Cham 245–307. https://doi.org/10.1007/978-3-319-16510-3.
Mao Y, Ai H, Chen Y, Zhang Z, Zeng P, Kang L, Li W, Gu W, He Q, Li H (2018) Phytoplankton response to polystyrene microplastics: perspective from an entire growth period. Chemosphere 208:59–68. https://doi.org/10.1016/j.chemosphere.2018.05.170
Marshall S. M, Orr A. P (1955) Experimental feeding of the copepod Calanus finmarchicus (Gunner) on phytoplankton cultures labelled with radioactive carbon (14C). Deep-Sea Research (1953) (Supplement).
Martínez-Fernández E, Acosta-Salmón H, Southgate PC (2006) The nutritional value of seven species of tropical microalgae for black-lip pearl oyster (Pinctada margaritifera, L.) larvae. Aquaculture. 257: (1–4)491–503. https://doi.org/10.1016/j.aquaculture.2006.03.022.
Maximenko N, Hafner J, Niiler P (2012) Pathways of marine debris derived from trajectories of Lagrangian drifters. Mar Pollut Bull 65(1–3):51–62. https://doi.org/10.1016/j.marpolbul.2011.04.016
Mazurais D, Ernande B, Quazuguel P, Severe A, Huelvan C, Madec L, Mouchel O, Soudant P, Robbens J, Huvet A, Zambonino-Infante J (2015) Evaluation of the impact of polyethylene microbeads ingestion in European sea bass (Dicentrarchus labrax) larvae. Mar Environ Res 112:78–85. https://doi.org/10.1016/j.marenvres.2015.09.009
Passow U (2002) Production of transparent exopolymer particles (TEP) by phyto- and bacterioplankton. Mar Ecol Prog Ser 236:1–12. https://doi.org/10.3354/meps236001
Renaud SM, Thinh LV, Lambrinidis G, Parry DL (2002) Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture 211(1–4):195–214. https://doi.org/10.1016/S0044-8486(01)00875-4
Sjollema SB, Redondo-Hasselerharm P, Leslie HA, Kraa MH, Vethaak AD (2016) Do plastic particles affect microalgal photosynthesis and growth? Aquat Toxicol 170:259–261. https://doi.org/10.1016/j.aquatox.2015.12.002
Smolders E, Degryse F (2002) Fate and effect of zinc from tire debris in soil. Environ Sci & Tech 36(17):3706–3710. https://doi.org/10.1021/es025567p
Staats N, Stal LJ, Mur LR (2000) Exopolysaccharide production by the epipelic diatom Cylindrotheca closterium: effects of nutrient conditions. J Exp Mar Biol Ecol 249(1):13–27. https://doi.org/10.1016/S0022-0981(00)00166-0
Thompson PA, Guo M, Harrison PJ (1993) The influence of irradiance on the biochemical composition of three phytoplankton species and their nutritional value for larvae of the Pacific oyster (Crassostrea gigas). Mar Biol 117(2):259–268. https://doi.org/10.1016/j.marpolbul.2010.05.026
Valenzuela S (2013) Unpacking the use of social media for protest behavior: The roles of information, opinion expression, and activism. Am Behav Sci 57(7):920–942. https://doi.org/10.1177/0002764213479375
Valenzuela-Espinoza E, Millán-Núñez R, Núñez-Cebrero F (2002) Protein, carbohydrate, lipid and chlorophyll a content in Isochrysis aff. galbana (clone T-Iso) cultured with a low cost alternative to the f/2 medium. Aquac Eng.25(4), 207–216.
Wright SL, Thompson RC, Galloway TS (2013) The physical impacts of microplastics on marine organisms: a review. Environ Pollut 178:483–492. https://doi.org/10.1016/j.envpol.2013.02.031
Xu ZB, Yan XJ, Pei LQ, Luo QJ, Xu JL (2008) Changes in fatty acids and sterols during batch growth of Pavlova viridis in photobioreactor. J Appl Phycol 20:237–243
Yoon TP, Ischay MA, Du J (2010) Visible light photocatalysis as a greener approach to photochemical synthesis. Nat Chem 2(7):527
Zarfl C, Matthies M (2010) Are marine plastic particles transport vectors for organic pollutants to the Arctic? Mar Pollut Bull 60(10):1810–1814
Acknowledgements
The authors are extremely thankful to the Bharathidasan University for providing the necessary facilities. The Ministry of Environment, Forest and Climate Change (MoEF & CC), Govt. of India, New Delhi, for providing financial support through a research Project (F. No. 220180/06/2015-RE (Tax), 05.10.2016). The Department of Biotechnology (DBT), Govt. of India, is gratefully acknowledged with thanks for the copepod culture facility provided (BT/PR 5856/AAQ/3/598/2012).
Funding
Ministry of Environment, Forest and Climate Change (F. No. 220180/06/2015-RE (Tax), 5th October 2016) to Santhanam Perumal.
Author information
Authors and Affiliations
Contributions
PR: conceptualization, methodology, resources, writing—original draft, writing—review and editing. PS: conceptualization, methodology, writing—review and editing. SSP, MD, and AA: data analysis. KND, SA, and JR: writing—review and editing and PP: writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Raju, P., Santhanam, P., Pandian, S.S. et al. Impact of polystyrene microplastics on major marine primary (phytoplankton) and secondary producers (copepod). Arch Microbiol 204, 84 (2022). https://doi.org/10.1007/s00203-021-02697-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-021-02697-6