Abstract
Murraya paniculata (L.) Jack is commonly cultivated as ornamental plant in Assam and has been used as spice and phytomedicine traditionally for many healthcare purposes. The therapeutic potential and chemical constituents of the essential oil of M. paniculata leaf was investigated against several pathogenic microbial species and human cancer cell lines. 29 chemical compounds were identified by GC–MS analysis from the essential oil representing 97.62% of the oil. The major compound identified was caryophyllene (20.93%). Leaf essential oil exhibited promising antibacterial activity against Mycobacterium smegmatis (MIC = 4 µg/mL) and Pseudomonas aeruginosa (MIC = 4 µg/mL). Best anticancer activity of the oil was observed for HeLa cells (IC50 = 6.28 μg/mL). Further, scanning electron microscopic studies revealed that the oil kills micro-organisms with the deformation of cellular morphology on treatment of the oil. Thus, the essential oil of M. paniculata leaf can be an excellent alternative for development of new antimicrobials and anticancer chemotherapeutic agents for the pharmaceutical industries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antimicrobial resistance (AMR) as well as cancer is a life-threatening disease in today’s world. Microorganisms develop resistance mechanism so fast and spread globally which bring complexity to fight against common infectious diseases. In 2019, Centers for Disease Control and Prevention (CDC) reported that every year above 2.8 million microbial infections occur in United States due to antibiotic resistance, as a result almost 35,000 people die (CDC 2019). World Health Organization (WHO) identified and listed the names of such antibiotic resistant bacteria to prioritize research for the development of new and effective alternative drugs (WHO 2017). Mycobacterium tuberculosis, Staphylococcus aureus and Pseudomonas aeruginosa occupied top categories in the list as they become resistant to existing antibiotics like rifampicin, vancomycin, methicillin, carbapenem, etc. bringing threat to the human population. Moreover, cancer is the 2nd deadliest disease all over the world which is responsible for almost 9.6 million deaths in 2018. It was expected to double the number by 2040, mostly in low- and middle-income countries (WHO 2020).
It has been a challenging task to find out new antimicrobials for the microbes that become resistant to the established drugs. As plants are alternative source of treatment to the conventional antibiotics which can fight as resistant modifying agents (RMAs) function through different mechanisms against pathogenic micro-organisms (Abreu et al. 2012; Chassagne et al. 2021). Moreover, plant kingdom is the richest reservoir of medicines in the form of natural compounds that can be easily obtained to replace the chemotherapeutic drugs (Russo et al. 2015). Among the plant secondary metabolites essential oils have rich medicinal values due to the presence of terpenes, flavonoids, phenolics, etc. compounds as chemical entity (Bouyahya et al. 2017). In spite of their use as flavoring agents, plant essential oils have been utilized in the pharmaceutical industry. Murraya paniculata (L.) Jack is a potential medicinal plant, flowers of which have a demand in India as a source of flavor and perfumery (Semarayani et al. 2018). The plant belongs to Rutaceae family and is commonly known as orange jasmine in Asia and kamini kusum in Assam, India (Choudhury 2015). Although the plant was endemic to South-West Asia, now it is widely distributed from tropical to subtropical parts of the world including China, Nepal, India, Taiwan, Sri Lanka, North eastern Pakistan, Northern Australia and South eastern Asia (Dosoky et al. 2016). The leaves of M. paniculata are used as spice by the people of India, Pakistan and Malaysia in a variety of food preparation (Saqib et al. 2015; Ng et al. 2012). Moreover, in ancient India, China and Indonesia plant is used as phytomedicine for many healthcare purposes. In Chinese pharmacopeia, the plant is reported to possess analgesic property with potentiality to cure microbial infection and inflammatory diseases (Zhang et al. 2011). Studies revealed that M. paniculata is a natural source of antioxidant that may be useful for pharmaceutical industry or as a possible food supplement (Gautam et al. 2012).
Although, the chemical components of M. paniculata leaf essential oil were reported from Nepal (Dosoky et al. 2016), Brazil (Selestino Neta et al. 2016), China (Li et al. 1988), Nigeria (Olawore et al. 2004) and Bangladesh (Chowdhury et al. 2008), there is no report so far on the chemical composition or biological activities of the essential oil of M. paniculata leaf found in Assam, India. In continuation of our search for bioactive substances and molecules from the Indo-Burma Biodiversity mega center (Saikia et al. 2020; Neipihoi et al. 2020), we have studied the chemical composition of the essential oil of M. paniculata leaf from Assam, India and also detailed biological activities of the oil against a number of pathogenic micro-organisms and cancer cell lines. The chemical composition of the oil was found to be different from those reported and biological activities were found to be promising and are presented in this communication.
Material and methods
Plant materials
The leaves of Murraya paniculata (L.) Jack (Rutaceae) were collected in the month of March (2016) from the experimental farm of CSIR-NEIST, Jorhat, Assam, India. The plant samples were identified at CSIR-NEIST, Jorhat and voucher specimens [CSIR-NEIST Accession number 1830 (NPC/289)] were deposited at CSIR-NEIST, Jorhat 785006, Assam.
Antibiotics and chemicals
Isoniazid, rifampicin, amikacin, fluconazole, resazurin sodium, gentamycin, dimethyl sulfoxide (DMSO), brain heart infusion agar (BHIA) and broth (BHIB), Mueller Hinton agar (MHA) and broth (MHB), yeast malt agar (YMA) and potato dextrose broth (PDB) were obtained from HIMEDIA Laboratories, Mumbai, India. Anhydrous sodium sulfate, isopropanol, hydrochloric acid and acetone were procured from Sisco Research Laboratories (SRL) Pvt. Ltd. Maharashtra, India. Media for culture of cell lines viz., DMEM (Dulbecco’s Modified Eagle’s Medium), MEM (Minimum Essential Medium Eagle), FBS (Fetus Bovine Serum) were obtained from MERCK, Sigma-Aldrich Chemicals Private Limited, Bangalore, India. Distilled water was used for the preparation of agar and broth media.
Extraction of essential oil
The leaves of M. paniculata were extracted for essential oil by hydrodistillation method with the help of a Clevenger-type apparatus (Bouyahya et al. 2017) in which the condenser was connected with Buchi Recirculating Chiller F-305, circulating the mixer of ethylene glycol and water (1:1) at − 10 °C. The fresh leaves (300 g) of M. paniculata were taken in a round bottom flask separately and then distilled water (1 L) added to it. Then, the hydro distillation is continued for 7 h at 100 °C. The whole procedure was repeated for two times. After completion of the experiment, the essential oil was collected in glass bottles, dried over anhydrous sodium sulfate and then it was filtered to give 1.25 g (0.41% yield) of essential oil, which was stored at 4 °C for further experiments. The essential oil yield was calculated by using the following formula:
GC/MS analysis of essential oil
The investigation of chemical components present in the essential oil was conducted by Gas Chromatography–Mass Spectrometer (Clarus 680 GC & Clarus 600C MS, Perkin Elmer, USA, Liquid Autosampler, Library Software: Turbomass NIST 2008) coupled with FID detector for quantification. The GC settings were as follows: the initial oven temperature was maintained at 50 °C for 2 min and raised to maximum 260 °C, ramping temperature 5 °C/min hold time is 6 min. The injector port temperature was 280 °C, transfer and source temperature was maintained at 200 °C and 180 °C, respectively. The samples (1 µL) were injected with a split ratio of 50:1. The flow rate of Helium as a carrier gas was 1 mL/min. Oil sample was prepared in methanol and compounds were separated in a capillary column of 60.0 m × 250 µm thickness. The oil was analyzed by gas chromatography and gas chromatography–mass spectroscopy technique and quantification was done by external standard method along with the mass spectra of authentic samples available in NIST library data of the GC–MS system (Kovats et al. 1965; Adams et al. 1995). The retention indices (RI) of the identified compounds were calculated by Kovat’s method using n-alkanes as standard using the formula,
Antimicrobial activity
Bacterial and fungal strains
Bacterial strains used in this study were Mycobacterium smegmatis (ATCC®607™), Staphylococcus aureus (ATCC®11632™), Pseudomonas aeruginosa (ATCC®25619™) and fungal strain is Candida albicans (ATCC®90028™) procured from HIMEDIA. M. smegmatis was cultured on sterile Brain heart infusion (BHI) agar media. Sterile Nutrient agar (NA) was used for the culture of S. aureus and P. aeruginosa, and Yeast malt agar (YMA) media were used for C. albicans. Incubation temperature used for bacterial strains is 37 °C and for fungal strain is 30 °C. All the pathogens were incubated from 18 to 48 h. Stock cultures of the respected bacteria were maintained on Nutrient broth and Brain heart infusion broth, C. albicans was maintained on Potato dextrose broth. All the strains were stored at 4 °C for further use.
Antimicrobial activity test by well diffusion assay
Isolated leaf essential oil was dissolved in DMSO to prepare stock solution for assay. Antimicrobial activity of the leaf oil was evaluated by well diffusion method (CLSI 2012). Bacterial and fungal suspensions were homogeneously spread over MHA and YMA medium, respectively. Wells of 6 mm diameter were bored in petriplates containing previously inoculated strains. The inoculum size used has a final inoculum density of approximately 1 × 108 CFU/mL. 100 µL of stock solution was added to the wells and the plate containing fungal strain was incubated at 30 °C and plates containing bacterial strains were incubated at 37 °C for 18–48 h as per growth conditions of the selected pathogens. The experiments were carried out in triplicates. Antimicrobial activity was determined by measuring the inhibition zones in the test plates and results were presented as mean ± SD.
Determination of minimum inhibitory concentration
Resazurin assay was performed in 96-well microtiter plates (Nunc™, Thermo Fisher Scientific Inc) to determine minimum inhibitory concentration of the isolated oil using broth microdilution method with slight modification of Wiegand et al. 2008 (Wiegand et al. 2008; NCCLS 1999). Resazurin is an indicator to determine live and dead microbial cells. It is a non-toxic, blue to purple colored dye and converted to pink color in presence of live bacterial cells and become colorless in case of live Candida cells (Sarker et al. 2007). For bacteria 100 µL of MHB and for fungus 100 µL of PDB were added to the each well of microtiter plates. Stock concentration of antibiotics used is 24, 50 and 60 µg/mL for Rifampicin, Amikacin and Fluconazole, respectively. 100 µL from each stock solution of antibiotics and essential oil was added to the first well and twofold serial dilutions were carried out in MHB and PDB media (Magrys et al. 2021). Then, 20 µL of microbial suspension was added to each well to achieve a concentration of 5 × 105 CFU/mL. Finally 20 µL of resazurin indicator was added to each well. Plates were incubated at 30 °C (fungus) and 37 °C (bacteria) for 18–48 h. At the end of incubation period change of color was evaluated visually. In each plate the column C1 is for sterility check (C1 = broth + sample + resazurin), C5 and C6 are for negative control (C5 and C6 = broth + inoculum + resazurin), C7 and C8 are for positive control (C7 and C8 = broth + antibiotic + inoculum + resazurin) and in rest of the columns, test sample was added in triplicates. The color change from purple to pink or colorless indicates growth of microbes. MIC of test sample was interpreted as the lowest concentration of the essential oil that prevents a change in color of the resazurin (Sarker et al. 2007).
Minimum bactericidal and fungicidal (MBC and MFC) concentration
Minimum bactericidal activity was tested against all the microbial strains following NCCLS guideline (NCCLS 1999). After completion of MIC experiment, the concentration of test samples that did not show any visible growth of microbes in resazurin assay, was subcultured to determine bactericidal and fungicidal concentration. For MBC and MFC, 10 µL of the samples taken from the purple wells of the micro broth susceptibility studies and placed onto the surfaces of Mueller Hinton agar and Yeast malt agar plates. The MBC and MFC were defined as the concentration of test sample at which 99.9% of the test pathogens were killed compared to the test pathogens present in test wells at 0 h (NCCLS 1999).
Effect of essential oil on the cell surface of tested micro-organisms
At first, the test micro-organisms were cultured in respective broth media, i.e., nutrient broth for S. aureus and P. aeruginosa, Mueller Hinton broth for M. smegmatis and potato dextrose broth for C. albicans. After attaining mid log phase, the microbial cells were harvested by centrifugation at 1000g for 10 min. Then, cells were treated with phosphate buffered saline (PBS) (HIMEDIA, Mumbai, India) for two times. Two sets of each micro-organisms were taken, one set without treatment and another set was treated with essential oil at MIC concentration. Treated samples were incubated for 1 h from 30 to 37 °C. After removing from incubator, cells were washed thrice in PBS at 6000g for 5 min. Bacterial and fungal pellets were then fixed in 2.5% (v/v) glutaraldehyde (MERCK, Bangalore, India) at 4 °C overnight. Thereafter, the cell pellets were washed two times in PBS and dehydrated using a graded acetone (SRL, Maharashtra, India) series (30, 50, 70, 80, 90, 95 and 100%) for 15 min in each. At last, the cell pellets were immersed in TMS (MERCK, Bangalore, India) for 10 min at 4 °C and then brought to room temperature (25–26 °C) for drying. The samples prepared then subjected to SEM analysis (FESEM, Carl Zeiss Microscopy Ltd., Cambridge CB1 3JS, UK) (Saikia et al. 2020).
Culture of cell lines and cytotoxicity (MTT) assay
To study the cell toxicity activity, stock solution of the oil sample was prepared in DMSO at a concentration of 1 mg/mL. L6 (Normal Rat muscle), HeLa (Human cervix), MIAPaCa2 (Human Pancreatic) and PA1 (Human Ovary) cell lines used in this experiment were collected from National Centre for Cell Sciences (NCCS), Pune, India. The cell lines were cultured in respective complete media (DMEM for L6 and MIAPaCa2, MEM for PA1 and HeLa) supplemented with 10% FBS (Fetus Bovine Serum), 1% Gentamycin, 10% Penstrep and incubated at an optimum temperature of 37 °C with humidified 5% CO2 atmosphere. For MTT assay confluent cells (1 × 106 mL−1) were added to the complete medium in tissue culture grade 96-well plates (Nunc™, Thermo Fisher Scientific Inc) and incubated for 24 h. After incubation, the complete medium was removed and FBS free medium was poured on the plates, and then it was incubated overnight. Then, the cell lines were treated with different concentrations (0.5, 1, 2, 5, 10, 20, 50, 100, 150 µg/mL) of essential oil and incubated for 24 h. All the treatments were applied in triplicates. Cells maintained in culture medium without test sample served as control.
MTT (MERCK, Bangalore, India) assay was conducted as per guideline of manufacturer’s (Merck Millipore Corporation, Germany) for cytotoxicity evaluation of essential oil. After 24 h of incubation, 10 µL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (5 mg/mL) was added to each well, mixed gently and incubated for 4 h. After completion of incubation time, 96-well plates were examined under an inverted microscope and dark purple formazan crystals were found at the bottom of the wells. 100 µL of isopropanol and 0.04 N HCl mixture was added to the wells and thoroughly mixed by repeated pipetting. HCl can convert the phenol red of the culture media into yellow color which does not disturb MTT formazan measurement. The formazan is dissolved by isopropanol and we get a homogeneous blue solution suitable for absorbance measurement. With the help of ELISA plate reader (Filter Max F3 Multi Mode Microplate Readers, Molecular Devices) absorbance was measured with a test wavelength of 570 nm and a reference wavelength of 630 nm. The effect of oil on cell proliferation was expressed in percent of viable cells compared with the control, and it was calculated as absorbance of treated cells/absorbance of control cells × 100. Cytotoxic concentration (IC50) of the sample was determined by analysis of dose response curves (Saikia et al. 2020).
Statistical analysis
Zone of inhibition and IC50 values were determined in triplicates and data are presented as mean ± standard deviation (SD) (MS Excel 2007).
Results
Chemical composition of the essential oil
From leaf essential oil of M. paniculata, 29 compounds were identified through GC–MS analysis which represents about 97.62% of chemical components present in the oil (Table 1). Figure 1 shows the gas chromatogram of the essential oil. The oil is light yellowish in color with strong aroma; yield percentage after dehydration is about 0.41% (w/w on dry weight basis). Most of the identified compounds of M. paniculata leaf essential oil were sesquiterpene hydrocarbons (80%) and rest were belonged to oxygenated sesquiterpenes (12.26%), diterpenoids (1.5%), fatty acids (1.80%) and nitrogenous heterocyclic compounds (2.06%). Caryophyllene 5 (20.93%), a bicyclic sesquiterpene compound, is the most abundant component present in the oil. Apart from Caryophyllene, other major compounds of the oil are δ-Elemene 2, β-Elemene 4, α-Caryophyllene 7, β-Cubebene 9, Elixene 11 and δ-Cadinene 12. A few compounds were identified in trace amounts (˂ 1%) from the leaf oil viz., Isophytol 26, 3,3'-Bi-p-menthane 28 and Naphthalene, 1,2,3,4,4a,7-hexahydro-1,6-dimethyl-4-(1-methylethyl) 14. The leaf essential oil was analyzed through NMR spectroscopy (Fig. 2a, b) which validates the presence of all the identified compounds. Here, all the chemical shifts (δ) are presented in ppm. The signals appearing from δ 0.82 to δ 2.41 in 1H NMR indicates the higher percentage of compounds containing hydrocarbon or terpene types of analogs. Moreover, signals δ 3.91–δ 5.76 assign as proton under hydroxyl groups and C=C bonded moieties of the components of the essential oil (Fig. 2a). The 13C NMR spectrum also, indicated the same inferences (Fig. 2b). Since, essential oil contains many chemical constituents, so overlapping signals of chemical shifts were observed in the NMR spectrum.
Antimicrobial activity (MIC, MBC, MFC)
Agar well diffusion assay displayed that leaf essential oil of M. paniculata is active against all the test pathogens (Table 2; Fig. 3a). Minimum inhibitory concentration (MIC), bactericidal concentration (MBC) and fungicidal concentration (MFC) of the leaf essential oil were determined and presented in Table 2 (Fig. 3b, c). Antibacterial activity of the oil is first time reported against P. aeruginosa and M. smegmatis where MIC of the oil is close to standard drug rifampicin against M. smegmatis (Table 2). Here, the antimicrobial activity of the M. paniculata leaf essential oil is considered as susceptible for P. aeruginosa and M. smegmatis whereas, intermediate for C. albicans and S. aureus according to CLSI guideline (CLSI 2012).
Antimicrobial activity of M. paniculata leaf essential oil. Photograph of a Zone of inhibition by leaf essential oil of M. paniculata against (A) C. albicans; (B) S. aureus; (C) P. aeruginosa and (D) M. smegmatis. b Inhibition of (A) C. albicans, (B) S. aureus, (C) P. aeruginosa and (D) M. smegmatis by M. paniculata leaf essential oil with minimum inhibitory concentrations (MIC). c A. Growth inhibition of C. albicans at MFC 8 µg/mL, A1.visible growth of C. albicans at less than MFC; B. growth inhibition of S. aureus at MBC 32 µg/mL, B1. visible growth of S. aureus at less than MBC; C. growth inhibition of P. aeruginosa at MBC 16 µg/mL, C1. visible growth of P. aeruginosa at less than MBC; D. growth inhibition of M. smegmatis at MBC 8 µg/mL, D1. visible growth of M. smegmatis at less than MBC
Antimicrobial efficacy of the oil was further analyzed by scanning electron microscopy (SEM). The differences in cellular morphology were distinctly observed on the tested microbial samples after application of M. paniculata leaf essential oil (Fig. 4).
Photomicrograph of C. albicans (A—control cells, A1 and A2—treated cells), S. aureus (B—control cells, B1 and B2—treated cells), P. aeruginosa (C—control cells, C1 and C2—treated cells) and M. smegmatis (A—control cells, A1 and A2—treated cells) under scanning electron microscope. M. paniculata leaf essential oil at MIC concentration were applied on the micro-organisms and some of the damaged cells were marked with arrows
Cytotoxicity assay
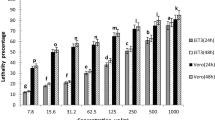
MTT assay of L6, HeLa, MIAPaCa2 and PA1 displayed that increase in the dose of leaf oil led to a decrease in the percentage viability of the cell lines (Fig. S1a). L6 cells showed 50% viability (IC50) at 13.62 ± 4.02 µg/mL which was close to 13.14 ± 1.56 µg/mL for essential oil-treated PA1 (Human Ovary) cells. IC50 value of 6.28 ± 1.82 µg/mL was seen for HeLa (Human cervix) cells implying that the oil is considerably toxic to it. However, oil is least toxic to MIAPaCa2 (human pancreatic) cell lines with IC50 value of 55.12 ± 0.77 µg/mL. The photomicrograph also supports the result where change in cell morphology of the treated cells is visible in comparison to control cells (Fig. S1b). The oil was previously reported with IC50 value 63.73 µg/mL against Hepa1c1c7 cell lines (Selestino Neta et al. 2016). We have first time reported the anticancer activity of M. paniculata leaf essential oil against human cervical (HeLa), pancreatic (MIAPaCa2) and ovary (PA1) cell lines with distinguished activity for cervical cancer cells. The compound Caryophyllene 5 is reported to have cytotoxicity against breast cancer cell lines (MDA-MB-231 and Hs 578 T), which may definitely impart toxic effect along with other compounds on three cancer cell lines, HeLa, MIAPaCa2 and PA1 in our experiment (Palazzo et al. 2009).
Discussion
Chemical constituents of medicinal plant M. paniculata leaf essential oil are first time reported from India, though a few countries have reported earlier (Selestino Neta et al. 2016; Li et al. 1988; Olawore et al. 2004). Due to the variation in geographical location, climatic factors and edaphic factors chemical composition and biological activity of the essential oils vary from country to country (Boira and Blanquer 1998). Our group at Jorhat, India and other groups reporting are in different geographical regions in different countries with the distance between the two collections sites is approximately 1000–14,000 km. This factor may be responsible for the variation in the phytochemical constituents (Akula et al. 2011; Verma et al. 2015). Here, we have found promising anticancer and antimicrobial activity of the oil for some of the tested pathogens and cancer cell lines that are reported first time. Leaf oil from China was characterized by 15 compounds with highest percentage of γ-Elemene (31.7%) (Li et al. 1988). Moreover, study of leaf essential oil from Bangladesh and Nepal reported with total 58 and 76 compounds, indicated the presence of Caryophyllene oxide (16.63%) and Methyl palmitate (11.05%) with highest percentage, respectively (Dosoky et al. 2016; Chowdhury et al. 2008). Among the similar compounds, the relative percentage of δ-Elemene 2 and Caryophyllene 5 is higher in the leaf oil representing India than the M. paniculata leaf oils of other countries (Dosoky et al. 2016; Chowdhury et al. 2008). A few compounds are first time reported from M. paniculata leaf essential oil are, 5-methyl thiazole 1, β-cadinene 8, viridiflorene 10, elixene 11, patchoulane 17, longifolene 18, guaiol 19, rosifoliol 20, dehydro-aromadendrene 22, γ-muurolene 23, isophytol 26, 4,4-dimethyl-3-(3-methylbut-3-enylidene)-2-methylenebicyclo[4.1.0]heptanes 13, naphthalene, 1,2,3,4,4a,7-hexahydro-1,6-dimethyl-4-(1-methylethyl) 14, N-hexadecanoic acid 27, 3,3'-Bi-p-menthane 28 and Bicyclo [5.3.0] decane, 2-methylene-5-(1-methylvinyl)-8-methyl 29 (Fig. 5).
The leaf essential oil of M. paniculata showed significant anticandidal activity in our experiment with almost similar MIC and MFC value to standard drug Fluconazole (Table 2) whereas oil was inactive against C. albicans reported from Nepal (Dosoky et al. 2016). Literature depicted that, Gram-negative bacteria restricts accumulation of oil on cell wall and become resistant to the antimicrobial drugs (Oussalah et al. 2007). For this reason, in our experiment MBC is four times greater than the MIC of leaf essential oil against P. aeruginosa. Based on earlier studies, the anticancer activity and antimicrobial activity of the leaf essential oil can be attributed to the presence of various groups of chemical entities in the oil. Sesquiterpene hydrocarbons are abundantly present in the leaf essential oil of M. paniculata that may be the cause of potential bioactivity of the oil. M. paniculata leaf essential oil was active against Escherichia coli, S. aureus, Enterococcus faecalis and Salmonella typhimurium with MIC values 0.5–1 mg/mL (Selestino Neta et al. 2016). We have found better antibacterial activity against Gram-positive bacteria S. aureus (Table 2) than earlier report (MIC 1 mg/mL) by Selestino Neta et al. 2016. Previous studies also revealed that, the oil had antifungal activity against Aspergillus niger, A. fumigatus, A. parasiticum and Fusarium solani with MIC 0.1–0.2 mg/mL and Dosoky et al. (2016) reported that M. paniculata leaf oil is inactive against C. albicans and Bacillus cereus (Dosoky et al. 2016; Selestino Neta et al. 2016). In our experiment, M. paniculata leaf essential oil showed better antifungal activity (Table 2) than previous reports. Reports suggested that β-caryophyllene possesses bactericidal activity against Gram-positive bacteria Bacillus cereus, S. aureus, E. faecalis and Gram-negative bacteria E. coli, S. typhimurium (Moo et al. 2020; Selestino Neta et al. 2016). In another report, the compound was significantly able to inhibit the growth of bacterium P. aeruginosa and fungal cells Trichoderma reesei, Aspergillus spp. and F. solani (Dahham et al. 2015; Selestino Neta et al. 2016). β-Caryophyllene has the ability to damage the cell permeability of bacterial cells that was proved by various experiments on B. cereus cells. As a result of interaction between compound and cell membrane some non-selective pores were formed that results into leakage of intracellular materials of microbial cells (Moo et al. 2020). Thus, the cell integrity is disturbed and finally cells died. This mechanism is possible for other microbial cells also. Perez-Lopez reported that δ-cadinene is responsible for antibacterial activity against Streptococcus pneumoniae (Perez-Lopez et al. 2011). Cis and trans-nerolidol was also reported to possess antimicrobial activity against C. albicans, Salmonella enterica and S. aureus (Chan et al. 2016). According to Togashi et al., long-chain sesquiterpene alcohols possess bactericidal activity (Togashi et al. 2007). Nerolidol has potential activity against MSSA (methicillin-susceptible Staphylococcus aureus) and MRSA (Methicillin-resistant Staphylococcus aureus) strains that can interact with cell membrane and destroy the cell integrity by releasing K+ ions (Chan et al. 2016). Moreover, β-caryophyllene, δ-cadinene and nerolidol were reported to have anticancer activity. Nerolidol was effective against human cervical (HeLa) and breast cancer (BT 20) cell lines with IC50 values 3.02 and 2.96 µg/mL, respectively (Chan et al. 2016). Another terpenoid δ-cadinene showed anticancer activity against human ovarian cancer cell line OVCAR-3. The ovarian cancer cells after treatment with δ-cadinene became shrink, detached from the substratum and nuclear material get condensed (Hui et al. 2015). With increasing concentration of δ-cadinene cellular blebbing took place and finally apoptosis occurred to the cancerous cells. β-caryophyllene exhibited antiproliferative activity against human colon cancer (HCT 116, HT 29) and pancreatic cancer (PANC-1) cell lines. The HCT 116 cell lines treated with β-caryophyllene showed fragmented DNA and cause condensation of nuclear material (Dahham et al. 2015). So, in our experiment there are two possibilities related to bioactivity of the essential oil. The major compound caryophyllene alone is responsible or synergistic effect of active compounds like δ-cadinene, nerolidol, caryophyllene, etc. play major role to inhibit the growth of microbial cells and cancer cells.
Mechanism of antimicrobial effect of the M. paniculata leaf essential oil on microbial cells was analyzed by scanning electron microscopic (SEM) study. The cellular morphology of the tested micro-organisms observed under SEM, depicted that cells lost their normal structure after the application of leaf essential oil at minimum inhibitory concentrations (Fig. 4). P. aeruginosa is a Gram-negative bacterium consists of thick cell wall with outer lipopolysaccharide and inner peptidoglycan layer whereas, Gram-positive bacteria S. aureus consist of outer thick layer of only peptidoglycan (Selvamani et al. 2020). Oil-treated cells of P. aeruginosa and M. smegmatis showed porous structure through which intracellular materials are moved outside (Fig. 4). Untreated cells of acid-fast bacteria M. smegmatis showed distinct rod-shaped structure under SEM, whereas oil-treated M. smegmatis cells undergone structural deformation. Cell wall is composed of mycolic acid that provide waxy texture to M. smegmatis, after oil treatment we noticed that cells are attached with each other losing their original structure and finally got lysed (Fig. 4). On the other hand, oil-treated S. aureus and C. albicans cells noticeably showed rough surface and cell shrinkage (Fig. 4). Whereas normal cells were observed with smooth surface. The structural rigidity of C. albicans cells is provided by its cell wall which is formed of β-glucan–chitin skeleton (Garcia Rubio et al. 2020). Oil-treated C. albicans cells became flattened with ridges like appearance. Generally, bacterial cells are surrounded by a layer of polysaccharide molecule called peptidoglycan that provides cell shape and structural rigidity by maintaining the turgor pressure. When antimicrobial agents are applied this peptidoglycan biosynthesis is blocked that results into cell wall disorganization and finally the cells die (Abrahams and Besra 2018; Neu and Gootz 1996). M. paniculata leaf essential oil mostly contains sesquiterpene compounds which are lipophilic in nature (Chadwick et al. 2013) and act as antimicrobials on tested pathogens. Due to the presence of sesquiterpene compounds, oil can easily interact with the phospholipid bilayer and damage the outer cover of bacterial cells. As a result, physical integrity of the cells is lost and affects the cellular mechanism (Sikkema et al. 1995). One-third of the cellular proteins of bacteria are embedded in cell membrane that is essential site for cell functions. When lipophilic compounds are accumulated on cell membrane they disturb the function of proteins as well as enzymes attached on the cell membrane (Sikkema et al. 1995). Therefore, it can be suggested that damage of bacterial cells by M. paniculata leaf essential oil occur mainly due to sesquiterpene-lipid interaction as well as sesquiterpene-protein interaction. In case of yeast cells, the mechanical strength is achieved through a network of carbohydrate molecules viz., chitin, mannan and β-glucan present as cell wall components (Torey et al. 2016). Cell wall is the first target of any antimicrobials, when sesquiterpene rich essential oil interacts with the yeast cell wall components normal function of the cells disrupted.
Conclusions
The chemical composition of the leaf essential oil of M. paniculata of Assam, India was found to be different from those reported from Nepal, Bangladesh, China, Brazil and Nigeria. Most of the compounds identified in the oil belonged to the sesquiterpenes class. A few compounds of this oil viz., 5-methyl thiazole, β-cadinene, viridiflorene, elixene, nerolidol, patchoulane, longifolene, guaiol, rosifoliol, dehydro-aromadendrene, γ-muurolene, 3,3'-bi-p-menthane, isophytol and N-hexadecanoic acid, etc. were reported for the first time from this region. It was found for the first time that the leaf essential oil possesses very good antibacterial activity against Mycobacterium smegmatis (MIC 4 µg/mL) and Pseudomonas aeruginosa (MIC 4 µg/mL). The oil also showed excellent activities against S. aureus and C. albicans as compared to the earlier report. Moreover, anticancer activity of the oil was also studied for the first time against human carcinoma cell lines, HeLa (cervix), MIAPaCa2 (pancreatic) and PA1 (ovarian) using MTT assay. The best anticancer activity was observed for HeLa cells (IC50 = 6.28 μg/mL). The antimicrobial and anticancer activity of the oil can be attributed to the most abundant compound caryophyllene 5 (20.93%) and synergistic effect of bioactive compounds identified in the leaf essential oil. Further, scanning electron microscopic studies revealed that the oil kills micro-organisms with the deformation of cellular morphology of the microbial cells. Drug resistance in microbial pathogens and cancer causes severe health issues in human being all over the world. Thus, the essential oil of M. paniculata leaf from Assam, India can be an excellent alternative source of new antimicrobials and anticancer agents for the pharmaceutical industries.
References
Abrahams KA, Besra GS (2018) Mycobacterial cell wall biosynthesis: a multifaceted antibiotic target. Parasitology 145(2):116–133. https://doi.org/10.1017/S0031182016002377
Abreu AC, Mc Bain AJ, Simoes M (2012) Plants as source of new antimicrobials and resistance-modifying agents. Nat Prod Rep 29(9):1007–1021. https://doi.org/10.1039/C2NP20035J
Adams RP (1995) Identification of essential oil components by gas chromatography/mass spectrometry. Allured Publishing Corporation, Carol Stream
Akula R, Ravishankar GA (2011) Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav 6(11):1720–1731. https://doi.org/10.4161/psb.6.11.17613
Boira H, Blanquer A (1998) Environmental factors affecting chemical variability of essential oils in Thymus piperella L. Biochem Syst Ecol 26(8):811–822. https://doi.org/10.1016/S0305-1978(98)00047-7
Bouyahya A, Dakka N, Talbaoui A, Et-Touys A, El-Boury H, Bakri AJ, Y, (2017) Correlation between phenological changes, chemical composition and biological activities of the essential oil from Moroccan endemic Oregano (Origanum compactum Benth). Ind Crops Prod 108:729–737. https://doi.org/10.1016/j.indcrop.2017.07.033
CDC, Antibiotic resistance threats in the United States, Atlanta (GA): U.S. Department of Health and Human Services (2019). https://www.cdc.gov/drugresistance/Biggest-Threats.html. Accessed on 3 Nov 2020
Chadwick M, Trewin H, Gawthrop F, Wagstaff C (2013) Sesquiterpenoids lactones: benefits to plants and people. Int J Mol Sci 14(6):12780–12805. https://doi.org/10.3390/ijms140612780
Chan WK, Tan LT, Chan KG, Lee LH, Goh BH (2016) Nerolidol: a sesquiterpene alcohol with multi-faceted pharmacological and biological activities. Molecules 21(5):529. https://doi.org/10.3390/molecules21050529
Chassagne F, Samarakoon T, Porras G, Lyles JT, Dettweiler M, Marquez L, Salam AM, Shabih S, Farrokhi DR, Quave CL (2021) A systematic review of plants with antibacterial activities: a taxonomic and phylogenetic perspective. Front Pharmacol 11:586548. https://doi.org/10.3389/fphar.2020.586548
Choudhury M (2015) Distribution and ex-situ conservation of plants, few observations from Assam state zoo, Guwahati, Assam. J Sci 5:361–365
Chowdhury JU, Bhuiyan MNI, Yusuf M (2008) Chemical composition of the leaf essential oils of Murraya koenigii (L) Spreng and Murraya paniculata (L) Jack, Bangladesh. J Pharmacol 3(2):59–63. https://doi.org/10.3329/bjp.v3i2.841
Clinical and Laboratory Standards Institutes (CLSI) (2012) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, Approved Standard-Ninth Edition; M07-A9.
Dahham SS, Tabana YM, Iqbal MA, Ahamed MB, Ezzat MO, Majid AS, Majid AM (2015) The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 20(7):11808–11829. https://doi.org/10.3390/molecules200711808
Dosoky NS, Satyal P, Gautam TP, Setzer WN (2016) Composition and biological activities of Murraya paniculata (L) Jack essential oil from Nepal. Medicines (basel) 3(1):7. https://doi.org/10.3390/medicines3010007
Garcia-Rubio R, de Oliveira HC, Rivera J, Trevijano-Contador N (2020) The fungal cell wall: Candida, Cryptococcus, and Aspergillus species. Front Microbiol 10:2993. https://doi.org/10.3389/fmicb.2019.02993
Gautam MK, Gangwar M, Nath G, Rao CV, Goel RK (2012) In-vitro antibacterial activity on human pathogens and total phenolic, flavonoid contents of Murraya paniculata Linn. leaves. Asian Pac J Trop Biomed 2(3):S1660–S1663. https://doi.org/10.1016/S2221-1691(12)60472-9
Hui LM, Zhao GD, Zhao JJ (2015) δ-Cadinene inhibits the growth of ovarian cancer cells via caspase-dependent apoptosis and cell cycle arrest. Int J Clin Exp Pathol 8(6):6046–6056
Kovats E (1965) Gas chromatographic characterization of organic substances in the retention index system. Adv Chromatogr 1:229–247
Li Q, Zhu LF, But PPH, Kong YC, Chang HT, Waterman PG (1988) Monoterpene and sesquiterpene rich oils from the leaves of Murraya species: chemotaxonomic significance. Biochem Syst Ecol 16(5):491–494. https://doi.org/10.1016/0305-1978(88)90050-6
Magrys A, Olender A, Tchorzewska D (2021) Antibacterial properties of Allium sativum L. against the most emerging multidrug-resistant bacteria and its synergy with antibiotics. Arch Microbiol. https://doi.org/10.1007/s00203-021-02248-z
Moo CL, Yang SK, Osman MA et al (2020) Antibacterial activity and mode of action of β-caryophyllene on Bacillus cereus. Pol J Microbiol 69(1):1–6. https://doi.org/10.33073/pjm-2020-007
National Committee for Clinical Laboratory Standards (NCCLS) (1999) Performance standards for antimicrobial susceptibility testing M100-S9
Neipihoi NB, Saikia S, Saikia S, Tamuli KJ, Bordoloi M (2020) Anticancer compounds from Croton caudatus Giesel and Eurya acuminata DC: traditional edible medicinal plants of Kuki Tribe. Nat Prod Res. https://doi.org/10.1080/14786419.2020.1815737
Neu HC, Gootz TD (1996) Antimicrobial chemotherapy, medical microbiology, 4th edn. University of Texas Medical Branch at Galveston, Galveston, pp 1–15
Ng MK, Abdulhadi-Noaman Y, Cheah YK, Yeap SK, Alitheen NB (2012) Bioactivity studies and chemical constituents of Murraya paniculata (Linn.) Jack. Int Food Res J 19(4):1307–1312
Olawore NO, Ogunwande IA, Ekundayo O, Adeleke KA (2004) Chemical composition of the leaf and fruit essential oils of Murraya paniculata (L.) Jack (Syn Murraya exotica Linn.). Flavour Fragr J 20(1):54–56. https://doi.org/10.1002/ffj.1365
Oussalah M, Caillet S, Saucier L, Lacroix M (2007) Inhibitory effects of selected plant essential oils on the growth of four pathogenic bacteria: E. coli O157:H7, Salmonella typhimurium, Staphylococcus aureus and Listeria monocytogenes. Food Control 18(5):414–420. https://doi.org/10.1016/j.foodcont.2005.11.009
Palazzo MC, Agius BR, Wright BS, Haber WA, Moriarity DM, Setzer WN (2009) Chemical composition and cytotoxic activities of leaf essential oils of four Lauraceae tree species from Monteverde. Costa Rica Rec Nat Prod 3(1):32–37
Perez-Lopez A, Cirio AT, Rivas-Galindo VM, Aranda RS, de Torres NW (2011) Activity against Streptococcus pneumoniae of the essential oil and δ-cadinene isolated from Schinus molle fruit. J Essent Oil Res 23(5):25–28. https://doi.org/10.1080/10412905.2011.9700477
Russo R, Corasaniti MT, Bagetta G, Morrone LA (2015) Exploitation of cytotoxicity of some essential oils for translation in cancer therapy. Evid Based Complement Alternat Med 2015:397821. https://doi.org/10.1155/2015/397821
Saikia S, Tamuli KJ, Narzary B, Banik D, Bordoloi M (2020) Chemical characterization, antimicrobial activity, and cytotoxic activity of Mikania micrantha Kunth flower essential oil from North East India. Chem Pap 74(8):2515–2528. https://doi.org/10.1007/s11696-020-01077-6
Saqib F, Ahmed MG, Janbaz KH, Dewanjee S, Jaafar HZE, Zia-Ul-Haq M (2015) Validation of ethnopharmacological uses of Murraya paniculata in disorders of diarrhea, asthma and hypertension. BMC Complement Altern Med 15:319. https://doi.org/10.1186/s12906-015-0837-7
Sarker SD, Nahar L, Kumarasamy Y (2007) Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 42(4):321–324. https://doi.org/10.1016/j.ymeth.2007.01.006
Selestino Neta MC, Vittorazzi C, Guimaraes AC, Lopes Martins JD, Fronza M, Endringer DC, Scherer R (2016) Effects of beta-caryophyllene and Murraya paniculata essential oil in the murine hepatoma cells and in the bacteria and fungi 24-h time-kill curve studies. Pharm Biol 55(1):190–197. https://doi.org/10.1080/13880209.2016.1254251
Selvamani V, Zareei A, Elkashif A, Maruthamuthu MK, Chittiboyina S, Delisi DA, Li Z, Cai L, Pol V, Seleem M, Rahimi R (2020) Hierarchical micro/mesoporous copper structure with enhanced antimicrobial property via laser surface texturing. Adv Mater Interfaces 7(7):1901890. https://doi.org/10.1002/admi.201901890
Semarayani CIM, Aziz SA, Melati M (2018) Essential oil production of Murraya paniculata (L.) Jack at different harvest times. Adv Hortic Sci 32(4):471–477. https://doi.org/10.13128/ahs-21989
Sikkema J, De Bont JAM, Poolman B (1995) Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev 59(2):201–222. https://doi.org/10.1128/mr.59.2.201-222.1995
Togashi N, Shiraishi A, Nishizaka M, Matsuoka K, Endo K, Hamashima H, Inoue Y (2007) Antibacterial activity of long-chain fatty alcohols against Staphylococcus aureus. Molecules 12(2):139–148. https://doi.org/10.3390/12020139
Torey A, Vijayarathna S, Jothy SL, Gothai S, Chen Y, Latha LY, Kanwar JR, Dharmaraj S, Sasidharan S (2016) Exploration of the anticandidal mechanism of Cassia spectabilis in debilitating candidiasis. J Trad Complement Med 6(1):97–104. https://doi.org/10.1016/j.jtcme.2014.11.017
Verma N, Shukla S (2015) Impact of various factors responsible for fluctuation in plant secondary metabolites. J Appl Res Med Aromat Plants 2(4):105–113. https://doi.org/10.1016/j.jarmap.2015.09.002
WHO report on cancer: setting priorities, investing wisely and providing care for all Geneva: World Health Organization (2020) Licence: CC BY-NC-SA 3.0 IGO
WHO (2017) http://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf. Accessed 3 Nov 2020
Wiegand I, Hilpert K, Hancock REW (2008) Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 3:163–175. https://doi.org/10.1038/nprot.2007.521
Zhang JY, Li N, Che YY, Zhang Y, Liang SX, Zhao MB, Jiang Y, Tu PF (2011) Characterization of seventy polymethoxylated flavonoids (PMFs) in the leaves of Murraya paniculata by on-line high-performance liquid chromatography coupled to photodiode array detection and electrospray tandem mass spectrometry. J Pharm Biomed Anal 56(5):950–961. https://doi.org/10.1016/j.jpba.2011.08.019
Acknowledgements
We thank the Director, CSIR-NEIST, Jorhat, Assam for providing laboratory facilities. The work was supported by CSIR-New Delhi for CSC-0130/207 and DBT, India for BT/PR25189/NER/95/1067/2017.
Funding
This article was funded by Council of Scientific and Industrial Research, India (CSC-130/207), Department of Biotechnology, Ministry of Science and Technology (BT/PR25189/NER/95/1067/2017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interests to declare.
Additional information
Communicated by Yusuf Akhter.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Saikia, S., Tamuli, K.J., Narzary, B. et al. Chemical composition, antimicrobial activity and cytotoxicity of Murraya paniculata (L.) Jack leaf essential oil from Assam, India: the effect of oil on cellular morphology of micro-organisms. Arch Microbiol 204, 99 (2022). https://doi.org/10.1007/s00203-021-02665-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-021-02665-0