Abstract
The present study evaluated the influence of the marine bacteria Bacillus cereus Mc-1 on the corrosion of 1020 carbon steel, 316L stainless steel, and copper alloy. The Mc-1 strain was grown in a modified ammoniacal citrate culture medium (CFA.ico-), CFA.ico- with sodium nitrate supplementation (NO3-), and CFA.ico- with sodium chloride supplementation (NaCl). The mass loss and corrosion rate were evaluated after the periods of 7, 15, and 30 days. The results showed that in CFA.ico- and CFA.ico- medium added NO3- the corrosion rates of carbon steel and copper alloy were high when compared to the control. Whereas the medium was supplemented with NaCl, despite the rates being above the averages of the control system, they were considerably below the previous results. In general, the corrosion rates induced by Mc-1 on 316L coupons were below the results compared to carbon steel and copper alloy. When analyzing the corrosion rate measurements, regardless of the culture medium, the corrosion levels decreased consistently after 15 days, being below the levels evaluated after 7 days of the experiment. Our analyses suggest that B. cereus Mc-1 has different influences on corrosion in different metals and environmental conditions, such as the presence of NO3- and NaCl. These results can help to better understand the influence of this bacteria genus on the corrosion of metals in marine environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metal alloys are materials commonly used in marine engineering and industrial installations, as well as in the construction of pier structures, ships, and bridges close to the marine environment. As marine environments are very conducive to corrosive processes, such as corrosion influenced by chemical and microbiological products, the safety and conservation of these structures are of great global concern (Dang and Lovell 2015). Metal corrosion can be characterized as a spontaneous process, in which the transfer of electrons from zero valence metal to a final acceptor occurs. For this process to happen, an electron donor site, or “anode site”, needs to transfer electrons to an “acceptor site” or “Catholic site” (Gadd 2004). This coupled reaction, dependent on each other, is also known as redox, and the transfer between the two sites is always from the most negative to the most positive potential. As a result, the transfer of electrons from one site to another will result in the dissolution of the anodic site or in the corrosion itself (Hamilton 2003; Watanabe et al. 2009). The deterioration of metallic and non-metallic materials by the corrosion process is a problem widely present in many sectors of the industry, as well as enormous impacts on public safety and the environment (Muyzer and Stams 2008; Hamilton 2003). Economic losses from corrosion are about US$ 2.5 trillion, equivalent to about 3.4% of the global Gross World Product (GDP) (Koch et al. 2016). The damages resulting from metallic corrosion affect several structures used in all productive sectors of the economy. However, the values associated with corrosion are not limited to damage directly caused by corrosion to the metal. Costs related to the prevention, an inspection of structures, loss of productivity due to interruption due to defects caused by corrosion also add to the total costs (Procópio 2020b).
Microbiologically influenced corrosion (MIC) is also a problem related to damage to the metallic infrastructure, especially in the marine environment, with losses that can reach billions of dollars (Koch et al. 2016). MIC on metallic surfaces is an interfacial process involving interactions between microbes, the environment, and metallic structures. Normally for this to occur, planktonic cells adhere to the metallic surfaces, called pine cells, then the process of biofilm formation begins, where there is the participation of different microbial species, which create a favorable environment for corrosion to occur (Procópio 2020b). The scientific literature describes corrosion as frequently associated with the activity of sulfate-reducing bacteria (SRB) and thiosulfate-reducing bacteria (TRB), which are present inside the biofilm, at the biofilm–metal interface, under anaerobic conditions (Boudaud et al. 2010). More recently, attention has been directed to corrosive processes, especially metal corrosion, due to the presence of oxidizing Fe lithotrophic bacteria (FeOB) and heterotrophic bacteria, such as strains of Bacillus and Pseudomonas (McBeth and Emerson 2016; Marty et al. 2014; Lee and Newman 2003; Maia et al. 2019).
Despite the fact that Fe (II) is their primary energy source and they are often identified in corrosion biofilms, the role of the FeOB group in the MIC is poorly described and our understanding of the role of FeOB in the marine MIC is largely undervalued (Mumford et al. 2016). Studies have shown that FeOB is able to colonize and grow in steel coupons introduced into the environment (Dang and Lovell 2015; McBeth et al. 2011) or are present in corroded steel associated with ALWC structures (Marty et al. 2014). These microorganisms related to iron oxidation, accelerate the process through mechanisms such as pH change, acid production, secretion of corrosive metabolites, and extracellular enzymes (Little et al. 2007; Kato 2016). The basis of the aerobic corrosion reaction is characterized by the electrochemical coupling between the oxidation of iron (anodic reaction) and the reduction of oxygen (cathodic reaction) (Lee and Newman, 2003). The product of this reaction is ferrous iron Fe (II), which is later oxidized to ferric iron, Fe (III) (Enning and Garrelfs 2014). Fe (III) acts as a final electron acceptor in the electron transport chain, allowing the flow of electrons through the ATPase enzyme, generating electrochemical force for the synthesis of ATP, coupling the corrosion reactions with respiration bacteria of Fe (III) (Dawood & Brozel 1998).
Numerous environmental factors influence the corrosion capacity of microorganisms, including Bacillus species. For example, temperature gradients are commonly reported in studies on biocorrosion in marine environments, usually associated with oil pipelines (Conrad et al. 2009; Yang et al. 2016; Li et al. 2017). Differences in environmental pH also determine the speed of the MIC, directly affecting the adhesion between microorganisms and the metal surfaces (Sheng et al. 2008). In addition, interactions between different species present in the corrosive biofilm determine synergism or antagonism during the corrosion process (Suma et al. 2019; Kokilaramani et al. 2020). The concentration of NaCl and nitrate has also been reported as factors that influence MIC. In fact, a study shows that the concentration of NaCl affects the maintenance of biofilm structures (Dong et al. 2011). Although nitrate is directly associated with increased corrosion by nitrogen-reducing bacteria (NRB), in a study on the addition of nitrate and urea, corrosion by aerobic bacteria was inhibited (Pillay and Lin 2013 and 2014; Lin and Madida 2014). The involvement of heterotrophic bacteria of the genus Bacillus is widely described in marine corrosion studies, usually present in complex structures of microbial communities adhered to alloy surfaces (Selvaraj et al. 2014). B. cereus is a Gram-positive rod-forming bacteria that can grow in the presence or absence of oxygen and is widely distributed in many biotopes and in many different environments. The present work describes the B. cereus Mc-1 strain isolated from the marine environment close to the ship's maintenance area. DNA sequencing techniques were used to identify the isolate. Efforts were made to characterize the formation of biofilm in different cultivation conditions, to verify the most significant role in the induction/acceleration of corrosive processes in 1020 carbon steel, 316L stainless steel and copper coupons.
Materials and methods
Preparation of the specimens, bacteria, and culture medium
The B. cereus Mc-1 bacteria isolated in a previous study was grown in a nutrient broth medium up to absorbance of 1.0 at a wavelength of 600 nm. After satisfactory growth, the culture was centrifuged at 8,000 g, then the supernatant was discarded and the precipitate was washed with Phosphate Buffered Saline (PBS) (Merck, Germany). The centrifugation and washing processes were repeated twice. Then, the cell pellet was used for inoculation in 3 ml of broth medium for growth of iron precipitating bacteria ammonia ferric citrate medium (CFA), which was designed based on the chemical composition of 0.5 g/l (NH4) 2SO4, 0.2 g/l CaCl2.6 H2O, 0.5 g/l MgSO4.7 H2O, 0.5 g/l NO3- and 10.0 g/l ammonia ferric citrate, 0.5 g/l K2HPO4, and the pH adjusted for 6.6 using 6 N HCl. After 48 h the culture was centrifuged, the supernatant discarded and the precipitate was used to inoculate for the corrosion experiment (described below).
Coupon preparation and experimental setup
Three different metal alloys were employed. ASI-1020 carbon steel, which has a chemical composition of C = 16%, Mn = 0.63%, P = 1.2%, S = 3.1%, Si = 1.2%, Cu = 1%, Cr = 3% and Ni = 1%; 316L stainless steel with maximum chemical composition of 0.030% C, 2.00% Mn, 0.75% Si, 0.045% P, 0.030% S, from 16.00 to 18.00% Cr, from 10.00 to 14.00% Ni, from 2.00% to 3.00% Mo, and copper Cu = 99.9%, Sb = 0.002%, Bi = 0.001%, As = 0.002%, Fe = 0.005%, Pb = 0.005%, and S = 0.005%. The carbon steel coupons were cut to 3 cm2, whereas the 301L stainless steel and copper coupons were cut to 4 cm2. They were exposed to absolute ethanol to degrease the surface, washed with acetone to remove organic matter, dried in an oven 70 °C for 30 min, and kept in a desiccator. After the coupons were sterilized by autoclaving and then were cooled, identified, and weighed, to evaluate the weight loss by corrosion.
The determination of corrosion was done using the treated coupons placed in the bottom of the Erlenmeyer glass of 250 ml, and the growth medium was the CFA medium without the ammonia ferric citrate compound (CFA.Ico-). Initially, a pre-inoculum was prepared from an isolated colony of B. cereus Mc-1 strain in CFA medium for 72 h under the same conditions described above. After the growth reached the optical density of 0.50 at a wavelength of 600 nm, 100 µl were withdrawn and used to inoculate the CFA.Ico- medium with the coupons. Were analyzed the mass loss by microbial corrosion after 7 and 15 days of exposition to CFA.Ico-. All analysis was performed in triplicate. To analyze the influence of other nutrients in the corrosion rate by Mc-1 strain, additional coupons assays were performed followed by the same parameters described above with supplements of the individual compounds: 2.0 gL-1 NaCl and 2.0 gL-1 of NO3-.
Weight loss determination
After each determined period for analysis, three coupons from each system were retrieved for the measure of mass loss and corrosion rate. The rust deposited was scraped, and the coupons were immediately immersed in acid pickling (15% HCl) to remove all surface corrosion products according to ASTM G1 (Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens) (ASTM G1-03 2017). The acid reaction was stopped by the application of thiourea solution for 5 s, next the coupons were washed with distilled water, and then the reaction was neutralized with 10% NaOH for 5 s, and finally immersed in acetone for the same period. To determine the corrosion rate during the experimental period of incubation in all conditions, the weight-loss evaluation method was employed. The results of weight-loss measurement were utilized to calculate the value of corrosion rates (CR) following the equation:
where the W is the decrease in metal weight during the time period analyzed, K is the constant (3650), D the metal density in g/cm3, A the coupon area (mm2), and T the exposure time in days (NACE 2005). To evaluate the significant differences in the corrosion rates, an analytical analysis t test was employed.
Results
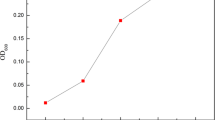
The strain B. cereus Mc-1 was previously isolated in a screening experiment to oxidize carbon steel bacteria. In the present study, the Mc-1 strain was analyzed if, under conditions of presence of NaCl and NO3, it favored its corrosive metabolism. Initially, B. cereus Ms-1 was grown in the CFA.Ico- medium in the presence of carbon steel coupons, for a period of up to 30 days. In the presence of the Mc-1 strain, weight loss was greater than in the control system, being 0.126 mg (± 0.03) after 7 days, 0.382 (± 0.13) in 15 days, and 0.517 (± 0.07) in 30 days cultivation (Fig. 1a and Table 1). The values of corrosion rates were also considerably higher, 279.58 (± 83.5) in 7 days, 394.61 (± 137.4) after 15 days, and 534.2 (± 82.2) after 30 days (Fig. 1a and Table 1).
When NaCl was added to the culture medium there was a decrease in the values of weight loss, 0.54 mg (± 0.01) in 7 days, 0.20 (± 0.05) in 15 days, and 0.26 (± 0.01) in 30 days of exposition, and in corrosion rates, 120.22 (± 40.8), 209.27 (± 59.7), and 275.08 (± 19.5) in the same period (Fig. 1b and Table 1). The addition of NO3- to the culture media showed an acceleration of mass losses, with values of 0.15 mg (± 0.01), 0.35 mg (± 0.04), 0.39 mg (± 0.08) in the three periods analyzed, and corrosion rates, 343.19 (± 32.6), 369.71 (± 42.6), and 402.83 (± 88.1) in the same incubation periods, concerning the previous experiment, as well as the control. (Fig. 1c and Table 1).
Corrosion rates influenced by the B. cereus Mc-1 strain were also detected in 316L stainless steel coupons. In the CFA.Ico- medium, the weight loss results showed that there was a moderate increase in levels over the experimental period, with 0.006 mg (± 0.001) in 7 days, 0.011 mg (± 0.009) in 15 and 0.024 mg (± 0.003) after 30 days (Table 1). In corrosion rates, a similar pattern was described, with 0.011 (± 0.009) in 7 days, reaching 18.83 (± 2.8) after 30 days of growth (Fig. 2a and Table 1). The addition of NaCl to the culture medium did not show significant changes between the periods analyzed. Weight loss was 0.002 mg (± 0.001) in 7 days, followed by 0.007 mg ± 0.001) and 0.009 mg (± 0.002) after 15 and 30 days, respectively. The corrosion rates also showed little change, with values of 4.12 (± 3.2), 5.29 (± 0.4), and 7.3 (± 1.7) in the same periods analyzed (Fig. 2b and Table 1). The analyzes after NO3- supplementation showed measures similar to those described in the previous condition, with weight loss values between 0.005 mg (± 0.001) and 0.009 mg (± 0.001), and a corrosion rate of 8.53 (± 1.4) in 7 days, 7.07 (± 1.5) in 15 days, and 6.79 (± 1.4) in 30 days (Fig. 2c and Table 1).
The influence of B. cereus Mc-1 on the corrosion of copper coupons was also evaluated in this study. Under culture conditions in CFA.Ico medium, the loss of mass was constant throughout the experimental period, from 0.002 mg (± 0.001) in 7 days to 0.051 mg (± 0.008) after 30 days. The loss of mass was reflected in the corrosion rate, which was 2.9 (± 1.4) in 7 days, 8.6 (± 3.8) in 15 days, and 17.55 (± 2.8) after 30 days (Fig. 3a and Table 1). With the addition of NaCl to the culture medium, variations in the corrosion rate were negligible, e.g. 5.33 mg (± 0.83), 7.24 mg (± 2.57), and 8.82 mg (± 0, 9) throughout the experimental period (Fig. 3b and Table 1). Finally, in the cultivation with NO3- added, the values of mass loss rose from 0.002 mg (± 0.001) in 7 days to 0.053 mg (± 0.001) after 30 days, whereas the corrosion rates varied from 4.31 (± 3.56) to 7 days, 14.1 (± 0.6) in 15 days, and 18.21 (± 2.5) after 30 days of culture (Fig. 3c and Table 1).
Discussion
B. cereus is a typical heterotrophic and aerobic bacterium widely distributed in the environment, such as soil, fresh water, and marine environments (Capão et al. 2020). The Mc-1 strain was initially isolated from seawater in a previous study on the microbial diversity involved in the corrosion of carbon steel (Ribeiro et al. 2017). In this previous work, the Mc-1 strain was shown to be able to oxidize carbon steel at high levels and in a short period. In this study, the Mc-1 strain was evaluated for its ability to induce/accelerate the corrosion of two different metals, which are commonly used in offshore structures (Marconnet et al. 2008). Laboratory studies simulating corrosion processes influenced by microorganisms are a challenge. Although microcosms simulate the conditions found in the environment, several factors inherent to the corrosive process are difficult to be analyzed. However, controlled conditions in laboratories allow us to separately assess the main factors that influence corrosion of metals (Angell et al 1997; Mumford et al. 2016). Other studies have already reported the corrosive capacity of Bacillus species in different laboratory conditions (Guo et al. 2017; Wan et al. 2018; Yuan and Pehkonen 2007; Li et al. 2019; Abdoli et al. 2016). The presence of Bacillus species has been reported in previous studies of corrosion in marine environments (Bonifay et al. 2017; Moura et al. 2018; Procópio 2020a). In metagenomic approaches and culture of isolates grown on the surfaces of oil pipelines and immersed coupons in marine environments the Bacillus genus was widely detected (Rajasekar et al. 2010; Garcia and Procópio 2020). In a study on the biofilm of B. cereus in carbon steel immersed in seawater, this species demonstrated to adhere to the surface under aerobic conditions (Procópio 2020c). However, the results on corrosion rates indicated that the B. cereus biofilm played an inhibiting role in the corrosion of carbon steel (Aïmeur et al. 2015). In addition, another study on the corrosive action of Bacillus subtilis alongside Pseudoalteromonas lipolytica showed that B. subtilis had an inhibitory action on P. lipolytica-induced corrosion (Guo et al. 2017).The presence of other electron acceptors influences the corrosion rate of metals, including the presence of nitrate (Kato et al. 2015; Wan et al. 2018). An evaluation of the addition of nitrate to the culture medium showed that Bacillus licheniformis consistently induced pitting corrosion in C1018 carbon steel (Xu et al. 2013).
Corrosion reduction results were also obtained with the B. subtilis C2 strain after a period of initial corrosion (Wang et al. 2020). Other studies conducted with the genera Bacillus and Pseudomonas showed similar results (Xu et al. 2016; Li et al. 2019). The electrochemical analysis showed that Pseudomonas aeruginosa significantly reduced corrosion in duplex stainless steel (DSS) (Xu et al. 2016). Furthermore, a study on B. cereus grown in synthetic seawater showed that the presence of the biofilm had a protective action in the formation of pitting in the non-oxidizing action 316L (Li et al. 2019; Capão et al. 2020). Colonization of copper alloy surfaces by species of the Bacillus genus is common in marine environments under aerobic conditions (Guo et al. 2017; Zhang et al. 2019). Despite that the corrosive action of species of the genus Bacillus has been previously reported on copper surfaces (Hu et al. 2019), historically this genus has been described as an inhibitory role in the corrosion of these surfaces (Jayaraman et al. 1999; Guo et al. 2017; Moradi et al. 2019).
Conclusions
The bacterium B. cereus Mc-1 showed different corrosive behavior under different culture conditions and different metals evaluated. By analyzing the influence on corrosion of 1020 carbon steel and copper alloy, Mc-1 demonstrated to accelerate the corrosion process in the CFA.ico- and CFA.ico medium with NO3- supplementation. On the other hand, the addition of NaCl caused a deceleration of corrosion in carbon steel. When analyzing the measurements of the 316L coupons, the results showed that after a period of corrosion induced by Mc-1, there was a persistent decrease in metal corrosion in all cultivation conditions analyzed. These results bring new subsidies for the understanding of the biocorrosion by the bacterium B. cereus Mc-1 in different metallic surfaces and environmental conditions. These possible findings assist in mitigating bioccorsion influenced by microrganisms.
References
Abdoli L, Suo X, Li H (2016) Distinctive colonization of Bacillus sp. bacteria and the influence of the bacterial biofilm on electrochemical behaviors of aluminum coatings. Colloids Surf B Biointerfaces 145:688–694. https://doi.org/10.1016/j.colsurfb.2016.05.075
Aïmeur N, Houali K, Hamadou L, Benbrahim N, Kadri A (2015) Influence of strain Bacillus cereus bacterium on corrosion behaviour of carbon steel in natural sea water. Corr Eng Sci Tech 50:579–588. https://doi.org/10.1179/1743278215Y.0000000022
Angell P, Machowski WJ, Paul PP, Wall CM, Lyle FF Jr (1997) A multiple chemostat system for consortia studies on microbially influenced corrosion. J Microbiol Methods 30:173–178. https://doi.org/10.1016/S0167-701297.00057-2
ASTM G1-03 (2017) Standard practice for preparing, cleaning, and evaluating corrosion test specimens. ASTM International, West Conshohocken, PA
Bonifay V, Wawrik B, Sunner J, Snodgrass EC, Aydin E, Duncan KE, Callaghan AV, Oldham A, Liengen T, Beech I (2017) Metabolomic and metagenomic analysis of two crude oil production pipelines experiencing differential rates of corrosion. Front Microbiol 8:99. https://doi.org/10.3389/fmicb.2017.00099
Boudaud N, Coton M, Coton E, Pineau S, Travert J, Amiel C (2010) Biodiversity analysis by polyphasic study of marine bacteria associated with biocorrosion phenomena. J Appl Microbiol 109:166–179
Capão A, Moreira-Filho P, Garcia M, Bitati S, Procópio L (2020) Marine bacterial community analysis on 316L stainless steel coupons by Illumina MiSeq sequencing. Biotechnol Lett 42:1431–1448. https://doi.org/10.1007/s10529-020-02927-9
Conrad R, Klose M, Noll M (2009) Functional and structural response of the methanogenic microbial community in rice field soil to temperature change. Environ Microbiol 11(7):1844–1853. https://doi.org/10.1111/j.1462-2920.2009.01909.x
Dang H, Lovell CR (2015) Microbial surface colonization and biofilm development in marine environments. Microbiol Mol Biol Rev 80(1):91–138. https://doi.org/10.1128/MMBR.00037-15
Dawood Z, Brozel VS (1998) Corrosion-enhancing potential of Shewanella putrefaciens isolated from industrial cooling waters. J Appl Microbiol 84:929–936
Dong ZH, Liu T, Liu HF (2011) Influence of EPS isolated from thermophilic sulphate-reducing bacteria on carbon steel corrosion. Biofouling 27:487–495. https://doi.org/10.1080/08927014.2011.584369
Enning D, Garrelfs J (2014) Corrosion of iron by sulfate-reducing bacteria: new views of an old problem. Appl Environ Microbiol 80:1226–1236
Gadd GM (2004) Microbial influence on metal mobility and application for bioremediation. Geoderma 122:109–119. https://doi.org/10.1016/j.geoderma.2004.01.002
Garcia M, Procópio L (2020) Distinct profiles in microbial diversity on carbon steel and different welds in simulated marine microcosm. Curr Microbiol 77:967–978. https://doi.org/10.1007/s00284-020-01898-4
Guo Z, Liu T, Cheng YF, Guo N, Yin Y (2017) Adhesion of Bacillus subtilis and Pseudoalteromonas lipolytica to steel in a seawater environment and their effects on corrosion. Colloids Surf B Biointerfaces 157:157–165. https://doi.org/10.1016/j.colsurfb.2017.05.045
Hamilton WA (2003) Microbially influenced corrosion as a model system for the study of metal microbe interactions: a unifying electron transfer hypothesis. Biofouling 19:65–76. https://doi.org/10.1080/0892701021000041078
Hu Y, Xiao K, Zhang D, Yi P, Xiong R, Dong C, Wu J, Li X (2019) Corrosion acceleration of printed circuit boards with an immersion silver layer exposed to Bacillus cereus in an aerobic medium. Front Microbiol 10:1493. https://doi.org/10.3389/fmicb.2019.01493
Jayaraman A, Ornek D, Duarte DA, Lee CC, Mansfeld FB, Wood TK (1999) Axenic aerobic biofilms inhibit corrosion of copper and aluminum. Appl Microbiol Biotechnol 52:787–790. https://doi.org/10.1007/s002530051592
Kato S (2016) Microbial extracellular electron transfer and its relevance to iron corrosion. Microb Biotechnol 9:141–148. https://doi.org/10.1111/1751-7915.12340
Kato S, Yumoto I, Kamagata Y (2015) Isolation of acetogenic bacteria that induce biocorrosion by utilizing metallic iron as the sole electron donor. Appl Environ Microbiol 81:67–73. https://doi.org/10.1128/AEM.02767-14
Koch GH, Varney J, Thompson NO, Moghissi O, Gould M, Payer JH (2016) NACE International IMPACT report 2016.
Kokilaramani S, AlSalhi MS, Devanesan S, Narenkumar J, Rajasekar A, Govarthanan M (2020) Bacillus megaterium-induced biocorrosion on mild steel and the effect of Artemisia pallens methanolic extract as a natural corrosion inhibitor. Arch Microbiol 202(8):2311–2321. https://doi.org/10.1007/s00203-020-01951-7
Lee AK, Newman DK (2003) Microbial iron respiration: impacts on corrosion processes. Appl Microbiol Biotechnol 62:134–139. https://doi.org/10.1007/s00253-003-1314-7
Li X-X, Liu J-F, Zhou L, Mbadinga SM, Yang S-Z, Gu J-D, Mu B-Z (2017) Diversity and composition of sulfate-reducing microbial communities based on genomic DNA and RNA transcription in production water of high temperature and corrosive oil reservoir. Front Microbiol 8:1011. https://doi.org/10.3389/fmicb.2017.01011
Li S, Li L, Qu Q, Kang Y, Zhu B, Yu D, Huang R (2019) Extracellular electron transfer of Bacillus cereus biofilm and its effect on the corrosion behaviour of 316L stainless steel. Colloids Surf B Biointerfaces 173:139–147. https://doi.org/10.1016/j.colsurfb.2018.09.059
Little B, Lee J, Ray R (2007) A review of “green” strategies to prevent or mitigate microbiologically influenced corrosion. Biofouling 23:87–97. https://doi.org/10.1080/08927010601151782
Maia M, Capão A, Procópio L (2019) Biosurfactant produced by oil-degrading Pseudomonas putida AM-b1 strain with potential for microbial enhanced oil recovery. Bioremed J 23:302–310. https://doi.org/10.1080/10889868.2019.1669527
Marconnet C, Dagbert C, Roy M, Féron D (2008) Stainless steel ennoblement in freshwater: from exposure tests to mechanism. Corr Sci 50:2342–2352. https://doi.org/10.1016/j.corsci.2008.05.007
Marty F, Gueuné H, Malard E, Sánchez-Amaya JM, Sjögren L, Abbas B, Quillet L, van Loosdrecht MC, Muyzer G (2014) Identification of key factors in accelerated low water corrosion through experimental simulation of tidal conditions: influence of stimulated indigenous microbiota. Biofouling 30:281–297. https://doi.org/10.1080/08927014.2013.864758
McBeth JM, Emerson D (2016) In situ microbial community succession on mild steel in estuarine and marine environments: exploring the role of iron-oxidizing bacteria. Front Microbiol 7:767. https://doi.org/10.3389/fmicb.2016.00767
McBeth JM, Little BJ, Ray RI, Farrar KM, Emerson D (2011) Neutrophilic iron-oxidizing “Zetaproteobacteria” and mild steel corrosion in nearshore marine environments. Appl Environ Microbiol 77:1405–1412. https://doi.org/10.1128/AEM.02095-10
Moradi M, Sun Z, Song Z, Hu H (2019) Effect of proteases secreted from a marine isolated bacterium Bacillus vietnamensis on the corrosion behaviour of different alloys. Bioelectrochemistry 126:64–71. https://doi.org/10.1016/j.bioelechem.2018.08.003
Moura V, Ribeiro I, Moriggi P, Capão A, Salles C, Bitati S, Procópio L (2018) The influence of surface microbial diversity and succession on microbiologically influenced corrosion of steel in a simulated marine environment. Arch Microbiol 200:1447–1456. https://doi.org/10.1007/s00203-018-1559-2
Mumford AC, Adaktylou IJ, Emerson D (2016) Peeking under the iron curtain: development of a microcosm for imaging the colonization of steel surfaces by Mariprofundus sp. strain DIS-1, an oxygen-tolerant fe-oxidizing bacterium. Appl Environ Microbiol 82(22):6799–6807
Muyzer G, Stams AJ (2008) The ecology and biotechnology of sulphate-reducing bacteria. Nat Rev Microbiol 6:441–454. https://doi.org/10.1038/nrmicro1892
NACE RP-07-75 (2005) Standard recommended practice, preparation, installation, analysis and interpretation of corrosion coupons in oilfield operations. NACE International, Houston
Procópio L (2020a) Changes in microbial community in the presence of oil and chemical dispersant and their effects on the corrosion of API 5L steel coupons in a marine-simulated microcosm. Appl Microbiol Biotechnol 104:6397–6411. https://doi.org/10.1007/s00253-020-10688-8
Procópio L (2020b) The era of ‘omics’ technologies in the study of microbiologically influenced corrosion. Biotechnol Lett 42:341–356. https://doi.org/10.1007/s10529-019-02789-w
Procópio L (2020c) Microbial community profiles grown on 1020 carbon steel surfaces in seawater-isolated microcosm. Ann Microbiol 70:13. https://doi.org/10.1186/s13213-020-01547-y
Rajasekar A, Anandkumar B, Maruthamuthu S, Ting YP, Rahman PK (2010) Characterization of corrosive bacterial consortia isolated from petroleum-product-transporting pipelines. Appl Microbiol Biotechnol 85:1175–1188. https://doi.org/10.1007/s00253-009-2289-9
Ribeiro I, Moura V, Moriggi P, Pereira S, Procopio L (2017) Steel corrosion by iron oxidant bacteria isolated from sea water. Inter J Biosci 11:240–246. https://doi.org/10.12692/ijb/11.3.240-246
Selvaraj C, Sivakamavalli J, Vaseeharan B, Singh P, Singh SK (2014) Examine the characterization of biofilm formation and inhibition by targeting SrtA mechanism in Bacillus subtilis: a combined experimental and theoretical study. J Mol Model 20:2364. https://doi.org/10.1007/s00894-014-2364-8
Sheng X, Ting YP, Pehkonen SO (2008) The influence of ionic strength nutrients and pH on bacterial adhesion to metals. J Colloid Interface Sci 321(2):256–264. https://doi.org/10.1016/j.jcis.2008.02.038
Suma MS, Basheer R, Sreelekshmy BR, Riyas AH, Bhagya TC, Ameen Sha M, Shibli SMA (2019) Synergistic action of Bacillus subtilis, Escherichia coli and Shewanella putrefaciens along with Pseudomonas putida on inhibiting mild steel against oxygen corrosion. Appl Microbiol Biotechnol 103(14):5891–5905. https://doi.org/10.1007/s00253-019-09866-0
Wan H, Song D, Zhang D, Du C, Xu D, Liu Z, Ding D, Li X (2018) Corrosion effect of Bacillus cereus on X80 pipeline steel in a Beijing soil environment. Bioelectrochemistry 121:18–26. https://doi.org/10.1016/j.bioelechem.2017.12.011
Wang YS, Liu L, Fu Q, Sun S, An ZY, Ding R, Li Y, Zhao XD (2020) Effect of Bacillus subtilis on corrosion behavior of 10 MnNiCrCu steel in marine environment. Sci Rep 10:5744
Watanabe K, Manefield M, Lee M, Kouzuma A (2009) Electron shuttle in biotechnology. Curr Opin Biotechnol 20:633–641. https://doi.org/10.1016/j.copbio.2009.09.006
Xu D, Li Y, Song F, Gu T (2013) Laboratory investigation of microbiologically influenced corrosion of C1018 carbon steel by nitrate reducing bacterium Bacillus licheniformis. Corr Sci 77:385–390. https://doi.org/10.1016/j.corsci.2013.07.044
Xu D, Li Y, Gu T (2016) Mechanistic modeling of biocorrosion caused by biofilms of sulfate reducing bacteria and acid producing bacteria. Bioelectrochemistry 110:52–58. https://doi.org/10.1016/j.bioelechem.2016.03.003
Yang G-C, Zhou L, Mbadinga SM, Liu J-F, Yang S-Z, Gu J-D, Mu B-Z (2016) Formate-dependent microbial conversion of CO2 and the dominant pathways of methanogenesis in production water of high-temperature oil reservoirs amended with bicarbonate. Front Microbiol 7:365. https://doi.org/10.3389/fmicb.2016.00365
Yuan SJ, Pehkonen SO (2007) Microbiologically influenced corrosion of 304 stainless steel by aerobic Pseudomonas NCIMB 2021 bacteria: AFM and XPS study. Colloids Surf B Biointerfaces 59:87–99
Zhang Y, Ma Y, Duan J, Li X, Wang J, Hou B (2019) Analysis of marine microbial communities colonizing various metallic materials and rust layers. Biofouling 35(4):429–442. https://doi.org/10.1080/08927014.2019.1610881
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moreira-Filho, P., de Paula da Silva Figueiredo, P., Capão, A. et al. The influence of the marine Bacillus cereus over carbon steel, stainless corrosion, and copper coupons. Arch Microbiol 204, 9 (2022). https://doi.org/10.1007/s00203-021-02607-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-021-02607-w