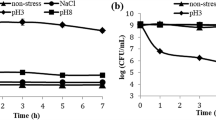
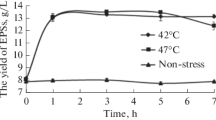
Abstract
Environmental factors can alter exopolysaccharide biosynthesis in lactic acid bacteria (LAB). To further clarify this potential relationship, the mRNA expression of genes involved in exopolysaccharide synthesis such as glmU, pgmB1, cps4E, cps4F, cps4J, and cps4H in Lactiplantibacillus plantarum VAL6 under different conditions including temperature, pH, sodium chloride (NaCl), and carbon dioxide (CO2) intensification culture was studied. The transcriptomic data revealed that the exposure of L. plantarum VAL6 at pH 3 increased the expression level of cps4H but decreased the expression levels of pgmB1 and cps4E. Under pH 8, cps4F and cps4E were significantly upregulated, whereas pgmB1 was downregulated. Similarly, the expression levels of cps4F, cps4E, and cps4J increased sharply under stresses at 42 or 47 °C. In the case of NaCl stress, glmU, pgmB1, cps4J, and cps4H were downregulated in exposure to NaCl at 7 and 10% concentrations while cps4E and cps4F were upregulated at 1 h of 10%-NaCl treatment and at 5 h of 4%-NaCl treatment. Remarkably, CO2 intensification culture stimulated the expression of all tested genes. In addition, simultaneous changes in expression of cps4E and cps4F under environmental challenges may elicit the possibility of an association between the two genes. These findings indicated that the expression level of eps genes is responsible for changes in the yield and monosaccharide composition of exopolysaccharides under environmental stresses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lactic acid bacteria (LAB) are ubiquitous appearance in food by their generally recognized as safe status (Ismail and Nampoothiri 2010). LAB are capable of producing exopolysaccharides (EPSs) which are widely used in various applications (Baruah et al. 2016). EPS production in LAB has been received a lot of attention in recent years due to the unique biological properties of these biopolymers (Silva et al. 2019). This is exemplified in Lactiplantibacillus plantarum, which is commonly utilized in the health and food areas owning to its ability to produce EPSs with specific functions regarding antitumor and antioxidant activities and prebiotic effects (Silva et al. 2019).
In nature as well as in industrial applications, LAB are often exposed to adverse environmental conditions (Le Marrec 2011). Indeed, LAB have various adaptive mechanisms to protect themselves from environmental stresses including changes related to the expression of genes and proteins (Mbye et al. 2020), to the cell's envelope with enhanced synthesis of EPSs (Huu Thanh et al. 2014). Many studies demonstrated that environmental stresses such as high temperature (Huu Thanh et al. 2014), acidic or alkaline pH, NaCl (Nguyen et al. 2021), CO2 (Ninomiya et al. 2009) among other factors can stimulate EPS synthesis.
In LAB, EPSs play an important role in protecting the cells from harsh environmental conditions (Nguyen et al. 2020). In addition, EPSs are also involved in the formation of biofilm and adhesion (Caggianiello et al. 2016) as well as in determining cell interaction characteristics (Lee et al. 2016). Nonetheless, the structure and biological function of EPSs may vary depending on environmental conditions (Vu et al. 2009) that related to the transcriptional levels of eps gene cluster (Boels et al. 2003).
EPS synthesis in LAB is a complex process involved in the regulation of expression of EPS-related genes (Zeidan et al. 2017). Furthermore, changing environmental conditions can alter the expression level of these genes (Wu and Shah 2018). Therefore, in this study, we evaluated the impact of various environmental challenges on the expression levels of genes involved in EPS synthesis in L. plantarum VAL6. The overall aim of the study was to clarify the potential relationships between these regulatory systems and environmental stresses.
Materials and methods
Bacterial strain and culture conditions
L. plantarum VAL6 was obtained from Department of Biotechnology, An Giang University, Vietnam National University Ho Chi Minh City, Vietnam. To perform microbial cell culture for this study, L. plantarum VAL6 was grown in Man–Rogosa–Sharpe medium (MRS medium) (De Man et al. 1960). L. plantarum VAL6 was stored at −80 °C in solution containing 30% glycerol; it was rehydrated in MRS broth with 2% (v/v) inoculum, followed by incubating at 37 °C for 24 h, agitation rate was set up to 250 rpm under aerobic facultative condition.
Bioreactor operating conditions for stress treatments
All the cultures were carried out in 5-L bioreactors (BIOSTAT, Sartorius Stedim Biotech, Germany). Briefly, 5 L of MRS medium was inoculated with 100 mL of bacterial culture (L. plantarum VAL6 was cultivated in MRS medium and incubated at 37 °C for 24 h to obtain an optical density at 595 nm (OD 595) of 1.5 which corresponds to approximately 108 CFU/mL). The pH was maintained to 6.8 by regularly adding 10 M sodium hydroxide (NaOH), the temperature was kept at 37 °C, and agitation rate was set up to 250 rpm. After 24 h of culture, stress treatments were then performed independently:
For thermal stress, the culture was treated with high temperature either 42 and 47 °C for 7 h and the pH was maintained to 6.8. The time was calculated when the bioreactor reached the required stress temperature.
For pH stress, the culture was treated with pH conditions either pH 3 and 8 for 7 h by adding 10 M phosphoric acid (H3PO4) and the temperature was kept at 37 °C. The time was calculated when the bioreactor reached the required pH.
For NaCl stress, the culture was treated by adding NaCl at 4, 7, and 10% (w/v) concentrations for 7 h. The pH was maintained to 6.8 and the temperature was kept at 37 °C.
The non-stress control of these treatments was simultaneously carried out in another bioreactor, where the pH was maintained to 6.8, the temperature was maintained at 37 °C for the entire time.
In the case of CO2 treatment, right after inoculation, CO2 was continuously supplied at the rate 250 cm3/min for 4, 8, and 24 h. The pH was maintained to 6.8 and the temperature was kept at 37 °C. The non-stress control of this treatment was also carried out at the same time without CO2 supplement.
Extraction of total RNA and synthesis of first-strand cDNA
Total RNA of cells (For conditions of stress and non-stress, cells were collected at 1, 3, 5, and 7 h. For CO2 treatments, cells were collected at 24 h after inoculation) was extracted according to the instructions of TRIzol reagent (Invitrogen, UK). RNA was treated with RQ1 RNase-free DNase (Promega, USA) to remove contamination of chromosomal DNA. Qualitative test of RNA at 260 and 280 nm was found to be more than 1.8 using a NanoDrop DeNovix DS-11 Spectrophotometer (DeNovix, USA). The first-strand cDNA was synthesized according to the instructions of the GoScript Reverse Transcription System Kit (Promega, USA).
Design and synthesis of primers
The reference gene was selected from the housekeeping gene, i.e., 16S rRNA. Primers of the six target genes were designed for quantitative real-time polymerase chain reaction (qPCR) analysis based on the genome sequence of L. plantarum WCFS1 (Genbank: AL935263.2) using Primer-BLAST software (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). The primers were designed with sequence length from 18–23 nucleotides, coupling temperature (Tm) from 57–60 °C and GC rate not more than 70% (Table 1). The primers were synthesized by The Shanghai Brilliance Biotechnology Co. Ltd.
qPCR experiment
The qPCR experiment was performed on an Liberty16 mobile real-time PCR system (Ubiquitome Limited, New Zealand), according to the instructions of the SensiFAST SYBR No-Rox Kit (Bioline, Meridian Bioscience, USA). The reaction mixture contained 10 μL of SensiFAST SYBR No-Rox Solution (2×), 7.4 μL of cDNA template, 0.8 μL of forward primer (10 μM), 0.8 μL of reverse primer (10 μM), and 1 μL of molecular grade water. As a negative control for all qPCR experiments, cDNA was replaced with RNA (without the reverse transcription step as template). The reaction for each gene was carried out in triplicate in individual qPCR reactions. The PCR amplification conditions were denaturation at 95 °C for 3 min, followed by 40 cycles of amplification at 95 °C for 30 s, 58 °C for 30 s, and 72 °C for 30 s. Fluorescence performance was obtained at the end of each thermal cycle. The 2−ΔΔCt method was used to calculate the changes in gene expression (Livak and Schmittgen 2001). Expression values were obtained by log2 of the ratio between cells under stress and without stress (Luiz de Freitas et al. 2020).
Statistical analysis
The experiments were repeated three times. All the data were expressed as means ± standard deviations. Significance of difference was evaluated with one way ANOVA, followed by Tukey’ test procedure to identify statistically significant differences at the 95% confidence interval.
Results
The expression of genes involved in EPS synthesis under thermal stress
The effect of high-temperature stresses at 42 and 47 °C on the expression of genes involved in EPS synthesis in L. plantarum VAL6 was investigated via RNA sequencing. Through analysis and comparison of the gene expression profile of the stressed L. plantarum VAL6 and the control at 37 °C, we found that cps4E, cps4F, and cps4J genes were significantly (p < 0.05) upregulated in response to thermal stress. Also, the exposure time played an important role in the regulation of these genes (Fig. 1).
The expression of genes involved in EPS synthesis under stress at 42 and 47 °C: a glmU, b pgmB1, c cps4E, d cps4F, e cps4H, and f cps4J. Expression values were obtained by log2 of the ratio between cells under stress and without stress. Different superscript uppercase letters indicates statistically significant differences between treatments at the same sample collection time and different superscript lowercase letters indicate significant differences throughout time for each separate treatment by Tukey’ test (p < 0.05). Error bars indicate standard error (n = 3)
The expression level of cps4E (Fig. 1c) peaked at 5 h after thermal stress with log2 (Fold change) = 4.8 and 5 under 42 and 47 °C treatments, respectively. Similar to cps4E, the log2 (Fold change) at the same time of cps4F (Fig. 1d) was 4.5 under 42 °C and 5 under 47 °C. However, the expression levels of two genes decreased after exposure to thermal stress for 7 h. Meanwhile, the expression level of cps4J was steadily increased over time of stress and peaked after 7 h of treatment (log2 (Fold change) ~ 4.2) (Fig. 1f).
The expression of genes involved in EPS synthesis under acid and alkaline stress
In this work, the response of L. plantarum VAL6 to acid and alkaline stress via the transcriptional analysis of genes involved in EPS synthesis was also studied. The results disclosed that cps4H was significantly (p < 0.05) upregulated in exposure to acid at pH 3. Meanwhile, cps4E and cps4F were significantly (p < 0.05) upregulated in response to alkaline stress condition at pH 8 (Fig. 2).
The expression of genes involved in EPS synthesis under acid at pH 3 and alkaline at pH 8 stress: a glmU, b pgmB1, c cps4E, d cps4F, e cps4H, f cps4J. Expression values were obtained by log2 of the ratio between cells under stress and without stress. Different superscript uppercase letters indicates statistically significant differences between treatments at the same sample collection time and different superscript lowercase letters indicate significant differences throughout time for each separate treatment by Tukey’ test (p < 0.05). Error bars indicate standard error (n = 3)
Under stress at pH 3, there was a great increase in the expression level of cps4H (log2 (Fold change) ~ 2) (Fig. 2e), but the expression levels of glmU, pgmB1, and cps4E decreased (Fig. 2a–c). In the case of stress at pH 8, log2 (Fold change) of cps4F increased steadily from 1 at 1 h to 1.72 at 7 h (Fig. 2d). As a similar pattern with cps4F, the expression level of cps4E was also rose gradually during the time of stress, although this difference was not statistically significant (p > 0.05) (Fig. 2c).
The expression of genes involved in EPS synthesis under NaCl stress
We also investigated the effect of the addition of NaCl at different concentrations on the expression of genes involved in EPS synthesis in L. plantarum VAL6. The overall results indicated that the expression of glmU, pgmB1, cps4H, and cps4J genes were significantly (p < 0.05) downregulated in exposure at 7 and 10% NaCl (Fig. 3).
The expression of genes involved in EPS synthesis under NaCl stress: a glmU, b pgmB1, c cps4E, d cps4F, e cps4H, and f cps4J. Different superscript uppercase letters indicates statistically significant differences between treatments at the same sample collection time and different superscript lowercase letters indicate significant differences throughout time for each separate treatment by Tukey’ test (p < 0.05). Error bars indicate standard error (n = 3)
In addition, an increase in the expression level of cps4E was detected at 1 h of 10%-NaCl treatment and at 5 h of 4%-NaCl treatment (Fig. 3c). Similarly, cps4F was upregulated at 1 h of 10%-NaCl treatment and at 5 h of 4%-NaCl treatment, respectively (Fig. 3d).
The expression of genes involved in EPS synthesis under CO 2 intensification culture
Unlike other stress treatments, CO2 intensification culture increased the expression of all tested genes. Furthermore, with the exception of cps4E, the other genes were upregulated with increasing time of CO2 supplementation (Fig. 4). The expression level of cps4E was highest upon exposure to 8-h CO2 treatment with log2 (Fold change) = 0.89, while its expression returned to log2 (Fold change) = 0.58 upon exposure to 24-h CO2 treatment (Fig. 4c).
The expression of genes involved in EPS synthesis under CO2 intensification culture for 4, 8, and 24 h: a glmU, b pgmB1, c cps4E, d cps4F, e cps4H, and f cps4J. Different letters indicate significant differences between treatments by Tukey’ test (p < 0.05). Error bars indicate standard error (n = 3)
Discussion
Changes in environmental conditions may alter extracellular polysaccharide production (Lloret et al. 1998) and induce the synthesis of new type of EPSs in bacteria (Nandal et al. 2005). In addition, the overexpression of a certain gene involved in EPS synthesis can increase or decrease the level of a specific sugar component in EPSs (Nguyen et al. 2021). Therefore, changes in expression level of eps gene under environmental stresses leads to differences in yield and monosaccharide composition in EPSs.
In this study, glmU was only upregulated under CO2 intensification culture but downregulated under exposure to pH 3 and NaCl concentrations at 7 and 10%. In comparison with a previous study in Lactobacillus vini, glmU was also downregulated under acid stress conditions (Mendonca et al. 2019). The enzymes encoded by glmU are glucosamine-1-phosphate N-acetyltransferase converting glucosamine-1-phosphate to N-acetylglucosamine-1-phosphate and N-acetylglucosamine-1-phosphate uridyltransferase catalyzing the formation of UDP-N-acetyl-d-glucosamine from N-acetylglucosamine-1-phosphate (Li et al. 2011). UDP-N-acetyl-d-glucosamine is one of the essential precursors of the peptidoglycan structure of cell wall (Chapot-Chartier and Kulakauskas 2014) and the synthesis of repeating units of EPSs (Zeidan et al. 2017). Altogether, we could conclude that exposing L. plantarum VAL6 to CO2 intensification culture impacts on the regulation of glmU, possibly leading to an increase in the synthesis of major component of bacterial cell wall and in the content of N-acetyl-d-glucosamine in EPSs.
The pgmB1 encodes for β-phosphoglucomutase which catalyzes the interconversion of d-glucose 6-phosphate and d-glucose 1-phosphate to form beta-d-glucose 1,6-(bis)phosphate. pgmB1 plays an important role in the formation of sugar nucleotides as UDP-glucose (Li et al. 2019). Thus, the overexpression of pgmB1 may result in increased glucose content in EPS composition. In our study, the expression of pgmB1 increased when L. plantarum VAL6 was exposed to CO2 intensification culture but decreased in exposure to stresses at pH 3 and at 7 and 10% NaCl. These results were inconsistent with the study of Li et al. (2019) in L. plantarum FS5-5, pgmB expression increased in response to high salt stress (> 6%) but remained unalterably in response to low salt stress (< 6%) (Li et al. 2019). The difference in the stress method could be the cause of this discrepancy. While Li et al. (2019) supplement NaCl right after inoculation, our study stressed after culture for 24 h.
Among the upregulated genes, cps4E and cps4F encode for glycosyltransferases which transport sugar nucleotides to a lipid carrier to form repeating units (Zeidan et al. 2017). In Streptococcus thermophilus Sfi6, epsE (cps4E) encodes for a priming galactosyl-1-phosphate transferase and epsF (cps4F) encodes for a galactosyltransferase which adds the branching α-1,6-galactose (Stingele et al. 1996). In Lactobacillus johnsonii FI9785, epsE (cps4E) encodes for a glycosyltransferase which adds galactose-1-phosphate to the lipid carrier (Dertli et al. 2013). In this study, with the exception of stress at pH 3, other treatment conditions could enhance the expression of these two genes. Taken together, it was suggested that EPSs isolated from L. plantarum VAL6 under environmental stress may be rich in galactose. In addition, simultaneous changes in expression of cps4E and cps4F under environmental challenges may elicit the possibility of an association between the two genes.
The cps4J encoding for flippases which are responsible for taking repeating units and transporting them across the cytoplasmic membrane (Zeidan et al. 2017) was upregulated under stresses of temperature at 42 and 47 °C, and CO2 intensification culture. Meanwhile, the overexpression of cps4H encoding for enzymes which polymerize repeating units (Zeidan et al. 2017) was detected under stress at pH 3 and CO2 intensification culture. Thus, the enzymes encoded by cps4J and cps4H are exclusively involved in the secretion of EPSs, which demonstrated that the increase in the expression levels of two genes do not change the sugar composition of the obtained EPSs, but it was related to the resulting EPS yield.
Conclusion
To survive and adapt to environmental challenges, microorganisms have developed complex mechanisms at physiological and molecular levels (Guan and Liu 2020). Our results revealed that different environmental conditions can alter the expression level of genes involved in EPS synthesis including glmU, pgmB1, cps4E, cps4F, cps4H, and cps4J. Based on achieved results, we propose a profile for the changes in the expression of these genes in L. plantarum VAL6 by applying environmental challenges (Table 2). The expression of these genes may lead to changes in the monosaccharide composition of EPSs (Fig. 5). However, it is necessary to further study the expression levels of the respective proteins and the analysis of EPS composition, such as mass spectrometry and other techniques, to identify monosaccharides to better understand the potential relationships between these regulatory systems and environmental stresses.
Expression of genes involved in EPS synthesis in response to environmental stresses. The reactions are catalyzed by enzymes encoded by the genes in italic: Phosphoglucomutase (galU), Glucosamine-1-phosphate N-acetyltransferase (pgmB1), Glycosyltransferase (cps4E, cps4F), Flippase (cps4J), Polymerase (cps4H). Glu-6-P, glucose-6-phosphate; Glu-1-P, glucose-1-phosphate; UDP-Glu, uridine diphosphate glucose; UDP-Gal, uridine diphosphate galactose; dTDP-Rha, thymidine diphosphate rhamnose; UDP-GlcNAc, uridine diphosphate N-acetyl-glucosamine
References
Baruah R, Das D, Goyal A (2016) Heteropolysaccharides from lactic acid bacteria: current trends and applications. J Probiot Health 4:2. https://doi.org/10.4172/2329-8901.1000141
Boels IC, Van Kranenburg R, Kanning MW, Chong BF, De Vos WM, Kleerebezem M (2003) Increased exopolysaccharide production in Lactococcus lactis due to increased levels of expression of the NIZO B40 eps gene cluster. Appl Environ Microbiol 69:5029–5031. https://doi.org/10.1128/aem.69.8.5029-5031.2003
Caggianiello G, Kleerebezem M, Spano G (2016) Exopolysaccharides produced by lactic acid bacteria: from health-promoting benefits to stress tolerance mechanisms. Appl Microbiol Biotechnol 100:3877–3886. https://doi.org/10.1007/s00253-016-7471-2
Chapot-Chartier M-P, Kulakauskas S (2014) Cell wall structure and function in lactic acid bacteria. Microb Cell Fact 13:S9. https://doi.org/10.1186/1475-2859-13-S1-S9
De Man JC, Rogosa M, Sharpe ME (1960) A medium for the cultivation of lactobacilli. J Appl Bacteriol 23:130–135. https://doi.org/10.1111/j.1365-2672.1960.tb00188.x
Dertli E et al (2013) Structure and biosynthesis of two exopolysaccharides produced by Lactobacillus johnsonii FI9785. J Biol Chem. https://doi.org/10.1074/jbc.M113.507418
Guan N, Liu L (2020) Microbial response to acid stress: mechanisms and applications. Appl Microbiol Biotechnol 104:51–65. https://doi.org/10.1007/s00253-019-10226-1
Huu Thanh N, Razafindralambo H, Blecker C, Yapo NC, Thonart P, Delvigne F (2014) Stochastic exposure to sub-lethal high temperature enhances exopolysaccharides (EPS) excretion and improves Bifidobacterium bifidum cell survival to freeze-drying. Biochem Eng J 88:85–94. https://doi.org/10.1016/j.bej.2014.04.005
Ismail B, Nampoothiri KM (2010) Production, purification and structural characterization of an exopolysaccharide produced by a probiotic Lactobacillus plantarum MTCC 9510. Arch Microbiol 192:1049–1057. https://doi.org/10.1007/s00203-010-0636-y
Le Marrec C (2011) Responses of lactic acid bacteria to osmotic stress. In: Tsakalidou E, Papadimitriou K (eds) Stress responses of lactic acid bacteria. Food Microbiology and Food Safety. Springer, Boston, MA. https://doi.org/10.1007/978-0-387-92771-8_4
Lee IC et al. (2016) Strain-specific features of extracellular polysaccharides and their impact on host interactions of Lactobacillus plantarum. Appl Environ Microbiol 82:AEM.00306-00316. https://doi.org/10.1128/AEM.00306-16
Li Y, Zhou Y, Ma Y, Li X (2011) Design and synthesis of novel cell wall inhibitors of Mycobacterium tuberculosis GlmM and GlmU. Carbohyd Res 346:1714–1720. https://doi.org/10.1016/j.carres.2011.05.024
Li M, Wang Q, Song X, Guo J, Wu J, Wu R (2019) iTRAQ-based proteomic analysis of responses of Lactobacillus plantarum FS5-5 to salt tolerance. Ann Microbiol 69:377–394. https://doi.org/10.1007/s13213-018-1425-0
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Lloret J, Wulff B, Rubio J, Downie J, Bonilla I, Rivilla R (1998) Exopolysaccharide II production is regulated by salt in the Halotolerant strain Rhizobium meliloti EFB1. Appl Environ Microbiol 64:1024–1028. https://doi.org/10.1128/AEM.64.3.1024-1028.1998
Luiz de Freitas L, Aparecida Dos Santos CI, Carneiro DG, Dantas Vanetti MC (2020) Nisin and acid resistance in Salmonella is enhanced by N-dodecanoyl-homoserine lactone. Microb Pathog 147:104320. https://doi.org/10.1016/j.micpath.2020.104320
Mbye M et al (2020) Updates on understanding of probiotic lactic acid bacteria responses to environmental stresses and highlights on proteomic analyses. Compr Rev Food Sci Food Saf 19:1110–1124. https://doi.org/10.1111/1541-4337.12554
Mendonca AA et al (2019) Lactobacillus vini: mechanistic response to stress by medium acidification. Microbiology (reading) 165:26–36. https://doi.org/10.1099/mic.0.000738
Nandal K, Sehrawat AR, Yadav AS, Vashishat RK, Boora KS (2005) High temperature-induced changes in exopolysaccharides, lipopolysaccharides and protein profile of heat-resistant mutants of Rhizobium sp. (Cajanus). Microbiol Res 160:367–373. https://doi.org/10.1016/j.micres.2005.02.011
Nguyen P-T, Nguyen T-T, Bui D-C, Hong P-T, Hoang Q-K, Nguyen H-T (2020) Exopolysaccharide production by lactic acid bacteria: the manipulation of environmental stresses for industrial applications. AIMS Microbiol 6(4):451–469
Nguyen P-T, Nguyen T-T, Vo T-N-T, Nguyen T-T-X, Hoang Q-K, Huu Thanh N (2021) Response of Lactobacillus plantarum VAL6 to challenges of pH and sodium chloride stresses. Sci Rep. https://doi.org/10.1038/s41598-020-80634-1
Ninomiya K et al (2009) Effect of CO2 concentration on the growth and exopolysaccharide production of Bifidobacterium longum cultivated under anaerobic conditions. J Biosci Bioeng 107:535–537. https://doi.org/10.1016/j.jbiosc.2008.12.015
Silva LA, Lopes Neto JHP, Cardarelli HR (2019) Exopolysaccharides produced by Lactobacillus plantarum: technological properties, biological activity, and potential application in the food industry. Ann Microbiol 69:321–328. https://doi.org/10.1007/s13213-019-01456-9
Stingele F, Neeser JR, Mollet B (1996) Identification and characterization of the eps (Exopolysaccharide) gene cluster from Streptococcus thermophilus SFi6. J Bacteriol 178:1680–1690. https://doi.org/10.1128/jb.178.6.1680-1690.1996
Vu B, Chen M, Crawford RJ, Ivanova EP (2009) Bacterial extracellular polysaccharides involved in biofilm formation. Molecules (basel, Switzerland) 14:2535–2554. https://doi.org/10.3390/molecules14072535
Wu Q, Shah NP (2018) Comparative mRNA-Seq analysis reveals the improved EPS production machinery in Streptococcus thermophilus ASCC 1275 during optimized milk fermentation. Front Microbiol 9:445. https://doi.org/10.3389/fmicb.2018.00445
Zeidan AA et al (2017) Polysaccharide production by lactic acid bacteria: from genes to industrial applications. FEMS Microbiol Rev 41:S168–S200. https://doi.org/10.1093/femsre/fux017
Acknowledgements
P.-T.N is a graduate student researcher of Graduate University of Sciences and Technology, Vietnam Academy of Science and Technology, Vietnam. The authors would like to acknowledge the Science and Technology Program for the Southwestern Sustainable Development, Vietnam National University Ho Chi Minh City for the financial support.
Funding
This study was supported by the Science and Technology Program for the Southwestern Sustainable Development, Vietnam National University Ho Chi Minh City (Project/grant number KHCN-TNB/14-19/C32), Vietnam.
Author information
Authors and Affiliations
Contributions
Conceptualization, T.-S.L, P.-T.N., T.-T.N., and H.-T.N.; methodology, T.-S.L, P.-T.N., and T.-T.N.; investigation, T.-S.L, P.-T.N., S.-H.N.-H., T.-P.N., M.-N.T., and T.-T.N.; data curation, T.-S.L, P.-T.N., T.-U.N.-T., M.-C.N., Q.-K.H., and H.-T.N.; writing—original draft preparation, T.-S.L, P.-T.N., and H.-T.N.; writing—review and editing, T.-S.L, P.-T.N., M.-C.N., Q.-K.H., and H.-T.N.; supervision, M.-C.N., Q.-K.H., and H.-T.N.; funding acquisition, H.-T.N. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no financial conflicts of interest to declare.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Le, TS., Nguyen, PT., Nguyen-Ho, SH. et al. Expression of genes involved in exopolysaccharide synthesis in Lactiplantibacillus plantarum VAL6 under environmental stresses. Arch Microbiol 203, 4941–4950 (2021). https://doi.org/10.1007/s00203-021-02479-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-021-02479-0