Abstract
Yersinia enterocolitica is an important zoonotic pathogen, which seriously endangers food-safety risk. In this study, the recombinant outer membrane protein OmpF and its antibody were prepared and coupled with immunomagnetic beads (IMBs) to capture Y. enterocolitica in food samples, combining the quantitative PCR detection with primers of virulence factor gene foxA for Yersinia enterocolitica contamination. The results showed that the capture efficiency of approximately 80% using anti-OmpF antibody-immunomagnetic beads and linearly dependent capture under 101–105 CFU/mL Y. enterocolitica compared with less than 10% capture of other bacteria. The detection limit of 64 CFU/mL was obtained based on foxA gene PCR detection combined with capture of the anti-OmpF antibody-immunomagnetic beads to detect Yersinia enterocolitica in artificially contaminated milk and pork samples. Compared to the culture method, the developed IMBs–qPCR method has higher consistency, was less time consuming, which taken together provides an effective alternative method for rapid detection of Y. enterocolitica in food.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Yersinia enterocolitica is a Gram-negative bacteria with a size of 0.5–1.3 μm × 1–3 μm, which can grow at 0–44 ℃, and the most suitable growth temperature is 20–28 ℃ (Saraka 2017). Y. enterocolitica is an important foodborne pathogen, which mainly infect its host through the digestive tract, such as eating contaminated pork and dairy products (Fabrega and Vila 2012). After infection with Y. enterocolitica, the main symptoms of the patient are self-limiting gastroenteritis, lymphadenitis, and terminal ileum inflammation, but a few patients may also have serious complications such as sepsis which may even lead to death (Fredriksson-Ahomaa et al. 2006; Rosner et al. 2010). Y. enterocolitica has outbreaks all around the world (Shayegani et al. 1983; Morse et al. 1984), and it is currently listed as a routine testing item for imported and exported foods in many countries.
Rapid detection of pathogenic bacteria in food is essential to reduce food safety risks. The culture detection method is the most common method to detect Y. enterocolitica. However, the detection method has many steps, which take at least 1 week to obtain the results (Thoerner 2003). Moreover, the specificity and accuracy of the culture detection are not high, which cannot fully meet the actual detection needs of the food industry (Rusak 2018). The outer membrane is a unique structure of the cell wall of Gram-negative bacteria, as it has important biological functions (Koebnik et al. 2000; Delcour 2009). Outer membrane protein (OMP) is a significant part of the outer membrane. Most of the OMPs are β-barrel structural proteins, composed of 8–24 β-sheets arranged in an anti-parallel pattern (Fairman et al. 2011). This special structure can maintain the good stability of OMPs and help bacteria to withstand complex and changeable external environments (Mikula et al. 2012). OMPs can not only exert their biological functions, but also have good immunogenicity, and are considered to be important candidate antigens for vaccines (Huang 2016; Zhang 2018). Immunomagnetic bead separation (IMS) technique is based on the specific reaction mechanism of antigen and antibody, which can achieve the specific capture of bacteria. In recent years, this technology is widely used to capture and enrich bacteria in food samples, combined with ELISA, real-time quantitative PCR (qPCR) and other technologies to specifically detect bacteria(Zhu 2011; Srisa-Art et al. 2018; Wang et al. 2018b). In this research, we aim to couple antibodies against the OMP of Yersinia on the surface of immunomagnetic beads to specifically capture Yersinia in food samples.
The outer membrane protein attachment invasion locus (Ail) is only found in pathogenic Yersinia and is considered to be an important virulence factor (Pierson and Falkow 1993; Tsang et al. 2013). Ail protein can promote the ability of Y. enterocolitica to attach and invade cells and help to improve the resistance of bacteria to serum (Bliska and Falkow 1992). Outer membrane protein F (OmpF) plays an important role in controlling the selective penetration of cell membranes (Shaban et al. 2017) and is also considered a candidate antigen for inhibiting Y. enterocolitica infection (Wang et al. 2018a). Both outer membrane proteins Ail and OmpF have strain-specificity and good conservation properties (Huang 2010; Stenkova et al. 2011). Therefore, we chose their antibodies as coupling proteins of immunomagnetic beads.
Many studies have shown that the quantitative real-time PCR method can be applied to the detection of foodborne pathogenic bacteria with good specificity and repeatability (Kasturi and Drgon 2017; Vital et al. 2017; Wang et al. 2018b). The ferrioxamine receptor gene (foxA) is located on the chromosome of Y. enterocolitica, which combines with ferrioxamin to take up ferric ion (Perry and Brubaker 1979). The gene foxA is stable and highly conserved in Y. enterocolitica with high species specificity (Huang et al. 2010; Wang 2014), hence, it is selected as the target gene of qPCR. In this research, we have established a detection method that combines immunomagnetic bead separation technology with qPCR technology, which can achieve rapid quantitative detection of bacteria in contaminated complex food matrices.
Materials and methods
Bacterial strains, culture conditions and animals
All Yersinia enterocolitica strains (Table 1) were cultured in modified phosphate buffer saline at 26 ℃, and other bacterial strains were cultured in Luria Broth (LB) broth at 37℃. All strains used in the experiment were stored in glycerol stocks in our laboratory (final concentration of 30%) in a refrigerator at −80 ℃. Two SPF adult rabbits were purchased from the Institute of Radiation Medicine of the Chinese Academy of Medical Sciences in Tianjin and handled in accordance with the guidelines for animal experiments of the University of Tianjin University and the Chinese Academy of Medical Sciences. The research protocol was approved by the Animal Ethical and Welfare Committee of Tianjin University (Approval No.TJUE-2021-051). The purified OmpF or Ail protein was subcutaneously injected into the rabbits at a concentration of 1 mg/mL each time. A total of four immunizations were performed with a week interval. The Blood was collected 5 days after the last immunization and the sera were separated to prepare the purified antibody by the method of precipitation with saturated ammonium sulfate (pH7.0).
Expression and purification of recombinant outer membrane protein
The ail and ompF gene were amplified and sub-cloned into the vector pET28a to generate the fusion plasmid of pET28a-ail and pET28a-ompF. The primers are listed in Table 2. The above sequences were verified by gene sequencing (Genewiz Corp, Beijing, China), and no mutations such as frameshift and gene deletion were detected. The expression and purification of recombinant Ail and OmpF using the process described in our previous report (Kang et al 2015). The protein samples were subjected to SDS-PAGE electrophoresis on 12% polyacrylamide gel using Mini-Protean (BioRad).
Preparation and purification of polyclonal antisera of recombinant outer membrane protein
The 5-month-old SPF rabbits were purchased from the Institute of Radiation Medicine Chinese Academy of Medical Sciences and were raised in specific pathogen-free facilities. We used 1 mg/mL purified recombinant outer membrane protein (Ail and OmpF) to generate polyclonal antisera. In the first immunization process, 1 mL of protein with a concentration of 1 mg/mL was mixed with an equal volume of Freund's complete adjuvant, and subcutaneous immunization was performed at multiple locations on the back of the rabbits. Freund's incomplete adjuvant was used for 2–4 immunizations. The immunization procedure is the same as the first immunization, with an interval of 10 days. Rabbit blood was taken 10 days after the fourth immunization, and the antibody was purified by the saturated ammonium sulfate method. Subsequently, the antibody concentration and titer were determined through the Bradford method and indirect ELISA method, respectively.
Western-blot analysis of polyclonal antibodies
Two strains of Y. enterocolitica (CMCC 55075, CMCC52217) single colonies were picked and cultured in 5 mL of modified phosphate buffer at 26 °C and 150 rpm shaker overnight. 20 μL bacterial solution was prepared from whole bacteria sample. In the prokaryotic expression and purification of OmpF and Ail proteins, whole bacteria samples were isolated by SDS-PAGE. Subsequently, the membrane was blocked with PBST and 5% horse serum (Gibco, Lot 26050–088) for 1 h. The membrane was stained with primary antibodies (rabbit anti-OMP antiserum diluted at 1:800) overnight at 4 °C, then it was washed with PBST and stained with secondary antibodies (HRP goat anti-rabbit IgG antibody diluted at 1:4000) for 1 h. The membrane was detected using a chemiluminescence detection kit (Thermo Scientific, Waltham, MA, USA) and observed through Gel Imaging System (BIO-RAD, USA).
Preparation of antibody-conjugated immunomagnetic beads
Carboxyl-modified Affimag PSC magnetic beads with mean diameter of 1.0 μm were purchased from Besile Technology (Tianjin, China). Unprocessed magnetic beads (2 mg) were washed three times with 800 µL 0.01 M phosphate buffer saline containing Tween 20 (PBST, pH 6.0). Next, magnetic beads were suspended with 600 µL 0.01 M PBST (pH 6.0), while 1–ethyl–3 carbodiimide (EDC) and N-Hydroxysuccinimide (NHS) were slowly added to a final concentration of 5 mg/mL. After complete shaking and mixing, the magnetic beads were activated in the shaker at room temperature for 30 min. After activation, the magnetic beads were washed three times with 800 µL 0.01 M PBST (pH 7.4) and resuspended with 600 µL 0.01 M PBST (pH 7.4). Purified antibodies were added slowly and placed in the shaker at room temperature for 4 h to ensure the antibody and magnetic beads are fully bound. The antibody-bound magnetic beads were washed three times with 800 µL 0.01 M PBST (pH 7.4) and then blocked with 600 µL 0.01 M PBST (pH 7.4) with 1% BSA in a shaker at room temperature for 30 min. After washing three times with 800 µL 0.01 M PBST (pH 7.4), the antibody-conjugated immunomagnetic beads (IMBs) were resuspend with 500 µL 0.01 M PBST (pH 7.4), 0.1% BSA and finally stored at 4 ℃ in the refrigerator.
Optimization of preparation parameters of antibody-conjugated immunomagnetic beads
The dosage and coupling time of magnetic beads and antibodies were determined through preliminary experiments. Then we explored the capture efficiency of immunomagnetic beads coupled with OmpF or Ail antibodies of different qualities (0.005 mg, 0.01 mg, 0.02 mg, 0.05 mg, 0.1 mg, 0.2 mg, 0.25 mg) to 1 mL of Y. enterocolitica with a concentration of 104 CFU/mL–105 CFU/Ml. We compared and analyzed the difference between the capture efficiencies of the two antibody-conjugated immunomagnetic beads. After establishing the optimal antibody and conjugated immunomagnetic beads dosage, the capture efficiency of 1 mL series dilution concentration from 101 to 106 CFU/mL of Y. enterocolitica were further evaluated by PCR detection of the DNA extracted from the captured bacteria.
Specific analysis of immunomagnetic bead separation
Y. enterocolitica (CMCC 55075), Staphylococcus aureus (ATCC 25923), E. coli DH5α, Salmonella typhimurium (CMCC 50619) single colonies were picked and cultured in a culture medium overnight. The bacterial solution was gradient diluted with sterile PBS buffer, and the initial bacterial solution concentration was determined by colony counting method. Subsequently, we took 1 mL of bacteria with a concentration of 104–105 CFU/mL and added appropriate quantity of antibody-conjugated immunomagnetic beads, and vortex for 40 min at room temperature. Placed the immunomagnetic beads on a magnetic device (Besile Technology, Tianjin, China) and let it stand for 3 min to aspirate the supernatant. After the supernatant was diluted with sterile PBS buffer, the concentration of the captured bacteria in the supernatant was measured by the colony counting method. The capture efficiency of the antibody-conjugated immunomagnetic beads on different bacteria was also calculated and compared.
Scanning electron microscope observation of IMBs-Y. enterocolitica complex
A single colony of Y. enterocolitica (CMCC 55075) was picked and cultured in 5 mL of modified phosphate buffer at 26 °C and 150 rpm shaker overnight and diluted to 104 CFU/mL with sterile PBS. Bacterial colony was captured with 0.2 mg of immunomagnetic beads conjugated with OmpF and Ail antibodies. 800 μL of sterile water was added to wash the complex of antibody-conjugated immunomagnetic beads and bacteria twice and subsequently resuspended in 200 μL of sterile water. 10 μL of resuspended droplets was added onto the coverslip and placed in a 37 °C oven to remove water. After being completely air-dried, the samples were sprayed with gold and observed with a field emission scanning electron microscope (NOVA Nanosem 430, FEI, USA).
Quantification of Y. enterocolitica by qPCR
We used online software PrimerQuest (https://www.idtdna.com/Primerquest/), Primer-BLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast/), Beacon Free Edition (http://www.premierbiosoft.com/) to design the primers of the foxA gene (F: 5′–CATCCCTGGTGGTGTAGTA–3′, R: 5′–GTTCAGATATCGCATCGGTATAA–3′, amplified length:131 bp). This online software can ensure that the primers have good specificity and no obvious primer dimer formation. The entire reaction system was performed using a 20 μL volume containing 10 μL 2 × TransStart Top Green qPCR SuperMix (TransGen Biotech, China), 0.4 μL forward and reverse primers (10 μM), 2 μL DNA template, and 7.2 μL distilled water. A three-step method was adopted in this experiment. The qPCR conditions for foxA gene were as follows: initial denaturation at 95 °C for 5 min, followed by 40 cycles at 54 °C for 30 s for denaturation and 70 °C for 20 s for renaturation. The qPCR assay was carried out using the Roche LightCycler®96 qPCR instrument (ROCHE GROUP, Switzerland). Y. enterocolitica (CMCC 55,075) grown overnight was tenfold diluted with aseptic PBS buffer. Subsequently, we used bacterial genome extraction kit DNA (TianGen, China) to extract genomic DNA of 1 mL Y. enterocolitica at different dilutions, which was used as templates to establish the qPCR standard curve of foxA gene.
The specificity of this method was verified by interference experiments. E. coli DH5α, Salmonella typhimurium (CMCC 50619), and Staphylococcus aureus (ATCC 25923) were selected as interference strains. Each strain was diluted after overnight cultivation, and sequentially added to Y. enterocolitica 102–106 CFU / mL in sequence. 1 mL of the mixed solution was taken to extract genomic DNA (100 μL). It was used as a template to perform qPCR detection according to the above reaction procedure and system to analyze the specificity of the detection method.
IMBs–qPCR detects Y. enterocolitica in food
Whole milk and pork were purchased from the local market, which was used to artificially simulate food contaminated by Y. enterocolitica. We used UV sterilization to ensure that bacteria in milk and pork were eliminated. 25 g test sample (milk/pork) was taken into a flask containing 250 mL of modified phosphate buffer. After fully shaking and mixing, Y. enterocolitica diluted with sterile PBS to the final concentration of about 10 CFU / mL was added and placed in a shaker at 26 ℃ for 5 h to enrich bacteria. 0.2 mg of antibody-conjugated immunomagnetic beads were mixed in 1 mL enriched food samples, and the mixture was shaken at room temperature for 40 min. After fully absorbing the antibody-conjugated immunomagnetic beads with a magnetic device, bacterial genomic DNA (100 μL) was extracted, and qPCR detection was performed according to the reaction procedure and system described previously. The enriched sample and supernatant after immunomagnetic bead capture were multiply diluted and subjected to colony count procedure. The results of the IMBs–qPCR method were compared with the results of the colony counting method, and the accuracy of the fluorescent quantitative PCR method was evaluated.
Statistical analysis
At least three independent repeated experiments were carried out for the above experiments. Statistical significance among different results was determined by GraphPad Prism software using t-test. P values less than 0.05 were considered statistically significant. (*p < 0.05; **p < 0.01; ***p < 0.001).
Results
Prokaryotic expression of recombinant outer membrane proteins have high concentration and purity
The distribution of ail and ompF genes in 13 Yersinia strains was evaluated by PCR analysis using specific primers. The results showed that the ail gene can be amplified only in Y. enterocolitica, while the ompF gene can be amplified in all 13 Yersinia strains (Table 1). The recombinant proteins of Ail and OmpF purified by nickel column affinity chromatography were analyzed by SDS-PAGE. The results show that the size of the purified protein was consistent with the predicted results. Moreover, no obvious mixed proteins were observed (Fig. 1). The concentrations of Ail and OmpF proteins were 5 mg/mL, which met the needs of subsequent immunization experiments.
SDS-PAGE (upper) and Western-blot (lower) analysis expression and purification of recombinant Ail a or OmpF b protein. Lane M, protein Marker (thermo scientific, USA); lane 1 and lane 2, total cellular protein before and after IPTG induction; lane 3 and lane 4, the supernatant and precipitation of ultrasound pyrolysis of the sample after IPTG induction, respectively; lane 5, the purified Ail or OmpF protein; YE1, YE2 represent extract protein from Y. enterocolitica CMCC 55075 and CMCC 52217, respectively
Polyclonal antibodies and titer determination and western blot analysis
The indirect ELISA method was used to determine the titers of purified Ail and OmpF antibodies, and the P/N ratio method was used to determine the ELISA results. The calculation formula is: P/N = test serum OD value / negative serum OD value. In this formula, P/N > = 2.1suggests that the tested serum is positive. The purified indirect ELISA results of Ail and OmpF antibodies are shown in Fig. 2. The antibody titers of Ail and OmpF are 1: 3200, which had relatively high titers.
Western blot analysis further verified the specificity of Ail and OmpF polyclonal antibodies and the results are shown in Fig. 2. The results showed that the two antibodies prepared by the experiment can not only bind to the recombinant protein expressed in prokaryotic cells but also specifically bind to the natural outer membrane proteins Ail and OmpF in Y. enterocolitica to meet the needs of subsequent experiments.
Capture efficiency analysis of IMBs
Through preliminary experiments, we determined that the amount of antibody that can be coupled to 1 mg of immunomagnetic beads is 112 μg, and the optimal time for coupling antibodies to immunomagnetic beads is 6 h. The capture efficiency of different qualities of IMBs (0.005 mg, 0.01 mg, 0.02 mg, 0.05 mg, 0.1 mg, 0.2 mg, 0.25 mg) coupled with OmpF and Ail antibodies against Y. enterocolitica (1.58 × 104 CFU/mL) is shown in Fig. 3. The platform period is basically reached when the amount of IMBs used is 0.2 mg. The capture efficiency of IMBs coupled with OmpF antibody is basically 80% (Fig. 3A), and the capture ability of IMBs coupled with Ail antibody is close to 70% (Fig. 3B). The difference in the capture capacity of the two antibodies may be related to the size of the antigen protein and the number of epitopes. The capture efficiency of the magnetic beads was not significantly improved when the amount of IMBs was increased from 0.2 to 0.25 mg. Considering the economic benefits, 0.2 mg of IMBs was selected as the prescribed amount for capturing per milliliter samples.
0.2 mg IMBs coupled with OmpF antibody were used to capture 1 mL of Y. enterocolitica with different concentrations (6.31 × 101–6.31 × 106 CFU/mL). The capture efficiency is shown in Fig. 4: 69.12 ± 4.32%, 72.34 ± 3.33%, 75.12 ± 3.42%, 80.98 ± 3.10%, 63.66 ± 5.11%, 33.56 ± 4.10%, respectively. When the bacterial concentration was 6.31 × 101 CFU/mL-6.31 × 105 CFU/mL, the capture efficiency was relatively stable, close to 70%. The capture efficiency was the highest, almost 80%, at 6.31 × 104 CFU/mL.
Specific analysis and scanning electron microscope observation of IMBs
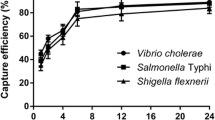
The capture efficiency of 0.2 mg IMBs for 104–105 CFU/mL other different bacteria was shown in Fig. 4. The capture efficiency of IMBs for Y. enterocolitica, E.coli, Staphylococcus aureus, and Salmonella typhimurium is 76.45 ± 1.5%, 9.11 ± 0.79%, 7.12 ± 1.06%, 4.90 ± 0.98%, respectively. Significantly, the results showed that the IMBs prepared in this experiment capture Y. enterocolitica more efficiently than other strains and have good species specificity.
The scanning electron microscope observation of Y. enterocolitica captured by IMBs is shown in Fig. 5. Figure 5A shows Y. enterocolitica CMCC 55075 and Fig. 5B shows IMBs after antibody coupling experiments. It can be observed that the surface of the IMBs is uneven due to antibody coating; Fig. 5C, D, respectively, display the complexes of OmpF and Ail antibody-conjugated magnetic beads to capture Y. enterocolitica CMCC 55075. Through scanning electron microscope (SEM) observation, it can be concluded that the IMBs prepared in this experiment can effectively capture Y. enterocolitica.
The scanning electron microscope photographs of anti-OmpF antibody-coupling immunomagnetic beads with Y. enterocolitica CMCC 55075. a Y. enterocolitica CMCC 55075; b anti-OmpF antibody-coupling immunomagnetic beads; c Y. enterocolitica CMCC 55075 was captured by anti-Ail antibody-coupling immunomagnetic beads; d Y. enterocolitica CMCC 55075 was captured by anti-OmpF antibody-coupling immunomagnetic beads
Determining the standard curve of qPCR and detection specificity
The Ct value of each template in qPCR is linearly related to the logarithm of the initial copy number of the template (Galluzzi et al. 2018), Ct = −klog X0 + b (X0 is the initial copy number of the template. The standard curve of foxA gene was established by Y. enterocolitica (CMCC 55075) with a concentration of 6.4 × 101–6.4 × 107 CFU/mL. For the standard curve y = −3.4601x + 41.01, the amplification efficiency is 95%, R2 = 0.997, and the detection limit is 64 CFU/mL.
The specificity of the detection method was verified by interference experiments using interference strains of E. coli DH5α, Salmonella typhimurium (CMCC 50619), and Staphylococcus aureus (ATCC 25923). The results (Fig. 6) showed that the amplification curve was not significantly affected after the interfering strains were added, indicating that the background strains did not affect the amplification of the foxA gene of Y. enterocolitica. Moreover, it proved that the foxA gene detection primer designed in this experiment had good specificity.
Detection and quantitative Y. enterocolitica in food samples by IMBs–qPCR
Whole milk and pork were inoculated with Y. enterocolitica to simulate contaminated food samples. The food inoculated with about 10 CFU/mL enterococci was enriched in a modified phosphate buffer at 26 °C for 6 h. Next, the anti-OmpF antibodies-conjugated immunomagnetic beads were captured and the bacterial genome was extracted to complete the qPCR detection. The results of the IMBs–qPCR method were compared with the results of the colony counting method.
The capture efficiency of Y. enterocolitica in whole milk and pork samples through the IMBs–qPCR method was 78.71% and 72.45%, respectively (Table 3). It could be concluded that the tissues present in the pork occupy part of the epitope of the immunomagnetic beads, resulting in reduced capture efficiency (Xiong and Cui 2014).
Discussion
The immunomagnetic separation (IMS) technology has been widely used in the detection of pathogenic bacteria in food (Lim 2016; Chen and Park 2018; Song et al. 2018). Outer membrane protein (OMP) is a significant component of Gram-negative bacteria, which has important biological functions and immunogenicity (Wen, 2016; Chen et al. 2017; Schrammel et al. 2018). In our research, based on the detection of virulence genes of several strains of Y. enterocolitica and the bioinformatics analysis of Ail and OmpF bioinformatics of specific outer membrane protein genes of Enterococcus. The recombinant outer membrane proteins Ail and OmpF at a concentration of 5 mg/mL were purified, and polyclonal antibodies were prepared by immunizing rabbits. ELISA and Western blot assays revealed that the polyclonal antibodies prepared from recombinant outer membrane protein have high titer and specificity.
The parameters of immunomagnetic beads preparation were optimized through experiments, and the optimal time for magnetic beads coupling was determined to be 6 h. For every 1 mg of immunomagnetic beads coupled, 200 μg of antibody was added. 0.2 mg IMBs coupled with OmpF antibody were used to capture 1 mL of Y. enterocolitica with different concentrations samples. Therefore, the platform period is basically reached when t0.2 mg of IMBs were used. The immunomagnetic beads prepared in this experiment have good specificity. When the bacterial concentration is 104–105 CFU/mL, the capture efficiency of IMBs for Y. enterocolitica is higher than 80%, while the capture efficiency for other strains is less than 10%. Furthermore, we found that the maximum capture efficiency (80%) of the IMBs prepared with OmpF polyclonal antibody was higher than the IMBs prepared with Ail polyclonal antibody (70%). The difference in capture efficiency between the two polyclonal antibodies may be mainly due to the molecular weight difference between OmpF (42 kDa) and Ail (20 kDa), and that more antigenic epitopes existed in OmpF protein compared to of Ail protein. The increase of antigen epitopes can improve the contact site of immunomagnetic beads and bacterial surface and promote the binding of bacteria and antibody. Moreover, the expression of Ail protein in Y. enterocolitica is greatly impacted by the ambient temperature. As it was observed that the protein expression level at room temperature is low, which is not conducive to the capture of IMBs (Bliska and Falkow 1992).
The real-time quantitative PCR (qPCR) for specific detection of bacteria has received widespread attention (Garrido-Maestu et al. 2018; Agrimonti et al. 2019). The ferrioxamine receptor gene (foxA) is relatively conserved in Y. enterocolitica and has a high bacterial species specificity (Kornreich-Leshem 2005; Huang et al. 2010). Therefore, to improve the sensitivity of IBM-qPCR, foxA was selected as the fluorescent quantitative PCR detection gene. Based on online software, the qPCR primers for foxA gene with good specificity and non-specific amplification were designed with a detection limit of 64 CFU/mL.
The IMBs–qPCR method was used to detect Y. enterocolitica in artificially contaminated milk and pork, and the capture rate was greater than 70% when the bacterial concentration was 103–104 CFU/mL. Compared to the cultivation method, the IMBs–qPCR method has higher consistency and the whole process only takes 12 h, which greatly shortens the detection time. In summary, we have established a detection method based on the IMBs–qPCR method, which can rapidly and accurately detect Y. enterocolitica in food and can be applied to emergency food safety incidents.
References
Agrimonti C, Bottari B, Sardaro MLS, Marmiroli N (2019) Application of real-time PCR (qPCR) for characterization of microbial populations and type of milk in dairy food products. Crit Rev Food Sci Nutr 59:423–442. https://doi.org/10.1080/10408398.2017.1375893
Bliska JB, Falkow S (1992) Bacterial resistance to complement killing mediated by the Ail protein of Yersinia enterocolitica. Proc Natl Acad Sci U S A 89:3561–3565. https://doi.org/10.1073/pnas.89.8.3561
Chen J, Park B (2018) Effect of immunomagnetic bead size on recovery of foodborne pathogenic bacteria. Int J Food Microbiol 267:1–8. https://doi.org/10.1016/j.ijfoodmicro.2017.11.022
Chen Q, Li Y, Tao T, Bie X, Lu F, Lu Z (2017) Development and application of a sensitive, rapid, and reliable immunomagnetic separation-PCR detection method for Cronobacter spp. J Dairy Sci 100:961–969. https://doi.org/10.3168/jds.2016-11087
Delcour AH (2009) Outer membrane permeability and antibiotic resistance. Biochim Biophys Acta 1794:808–816. https://doi.org/10.1016/j.bbapap.2008.11.005
Fabrega A, Vila J (2012) Yersinia enterocolitica: pathogenesis, virulence and antimicrobial resistance. Enferm Infecc Microbiol Clin 30:24–32. https://doi.org/10.1016/j.eimc.2011.07.017
Fairman JW, Noinaj N, Buchanan SK (2011) The structural biology of beta-barrel membrane proteins: a summary of recent reports. Curr Opin Struct Biol 21:523–531. https://doi.org/10.1016/j.sbi.2011.05.005
Fredriksson-Ahomaa M, Stolle A, Korkeala H (2006) Molecular epidemiology of Yersinia enterocolitica infections. FEMS Immunol Med Microbiol 47:315–329. https://doi.org/10.1111/j.1574-695X.2006.00095.x
Galluzzi L, Ceccarelli M, Diotallevi A, Menotta M, Magnani M (2018) Real-time PCR applications for diagnosis of leishmaniasis. Parasit Vectors 11:273. https://doi.org/10.1186/s13071-018-2859-8
Garrido-Maestu A, Azinheiro S, Carvalho J, Prado M (2018) Rapid and sensitive detection of viable Listeria monocytogenes in food products by a filtration-based protocol and qPCR. Food Microbiol 73:254–263. https://doi.org/10.1016/j.fm.2018.02.004
Huang Y et al (2010) Possible use of ail and foxA polymorphisms for detecting pathogenic Yersinia enterocolitica. BMC Microbiol 10:211. https://doi.org/10.1186/1471-2180-10-211
Huang W et al (2016) Immunization with a 22-kDa outer membrane protein elicits protective immunity to multidrug-resistant Acinetobacter baumannii. Sci Rep 6:20724. https://doi.org/10.1038/srep20724
Kang HZDH, Shen ML et al (2015) Superantigenicity analysis of staphylococcal enterotoxins SElK and SElQ in a mouse model. RSC Adv 5:29684–29692
Kasturi KN, Drgon T (2017) Real-time PCR method for detection of salmonella spp in environmental samples. Appl Environ Microbiol. https://doi.org/10.1128/aem.00644-17
Koebnik R, Locher KP, Van Gelder P (2000) Structure and function of bacterial outer membrane proteins: barrels in a nutshell. Mol Microbiol 37:239–253. https://doi.org/10.1046/j.1365-2958.2000.01983.x
Kornreich-Leshem H et al (2005) Ferrioxamine B analogues: targeting the FoxA uptake system in the pathogenic Yersinia enterocolitica. J Am Chem Soc 127:1137–1145. https://doi.org/10.1021/ja035182m
Lim MC et al (2016) Biological preparation of highly effective immunomagnetic beads for the separation, concentration, and detection of pathogenic bacteria in milk. Colloids Surf B Biointerfaces 145:854–861. https://doi.org/10.1016/j.colsurfb.2016.05.077
Mikula KM, Kolodziejczyk R, Goldman A (2012) Yersinia infection tools-characterization of structure and function of adhesins. Front Cell Infect Microbiol 2:169. https://doi.org/10.3389/fcimb.2012.00169
Morse DL, Shayegani M, Gallo RJ (1984) Epidemiologic investigation of a Yersinia camp outbreak linked to a food handler. Am J Public Health 74:589–592. https://doi.org/10.2105/ajph.74.6.589
Perry RD, Brubaker RR (1979) Accumulation of iron by yersiniae. J Bacteriol 137:1290–1298
Pierson DE, Falkow S (1993) The ail gene of Yersinia enterocolitica has a role in the ability of the organism to survive serum killing. Infect Immun 61:1846–1852
Rosner BM, Stark K, Werber D (2010) Epidemiology of reported Yersinia enterocolitica infections in Germany, 2001–2008. BMC Public Health 10:337. https://doi.org/10.1186/1471-2458-10-337
Rusak LA et al (2018) Rapid detection of Yersinia enterocolitica serotype O:3 using a duplex PCR assay. J Microbiol Methods 154:107–111. https://doi.org/10.1016/j.mimet.2018.10.014
Saraka D et al (2017) Yersinia enterocolitica, a neglected cause of human enteric infections in Cote d’Ivoire. PLoS Negl Trop Dis 11:e0005216. https://doi.org/10.1371/journal.pntd.0005216
Schrammel B, Petzold M, Cervero-Arago S, Sommer R, Luck C, Kirschner A (2018) Persistent presence of outer membrane epitopes during short- and long-term starvation of five Legionella pneumophila strains. BMC Microbiol 18:75. https://doi.org/10.1186/s12866-018-1220-x
Shaban H, Na I, Kislichkina AA, Dentovskaya SV, Anisimov AP, Uversky VN (2017) Effect of natural polymorphism on structure and function of the Yersinia pestis outer membrane porin F (OmpF protein): a computational study. J Biomol Struct Dyn 35:2588–2603. https://doi.org/10.1080/07391102.2016.1224734
Shayegani M, Morse D, DeForge I, Root T, Parsons LM, Maupin PS (1983) Microbiology of a major foodborne outbreak of gastroenteritis caused by Yersinia enterocolitica serogroup O:8. J Clin Microbiol 17:35–40
Song X, Shukla S, Kim M (2018) Detection of Cronobacter species in powdered infant formula using immunoliposome-based immunomagnetic concentration and separation assay. Food Microbiol 72:23–30. https://doi.org/10.1016/j.fm.2017.11.002
Srisa-Art M, Boehle KE, Geiss BJ, Henry CS (2018) Highly sensitive detection of salmonella typhimurium using a colorimetric paper-based analytical device coupled with Immunomagnetic Separation. Anal Chem 90:1035–1043. https://doi.org/10.1021/acs.analchem.7b04628
Stenkova AM, Isaeva MP, Shubin FN, Rasskazov VA, Rakin AV (2011) Trends of the major porin gene (ompF) evolution: insight from the genus Yersinia. PLoS ONE 6:e20546. https://doi.org/10.1371/journal.pone.0020546
Thoerner P et al (2003) PCR detection of virulence genes in Yersinia enterocolitica and Yersinia pseudotuberculosis and investigation of virulence gene distribution. Appl Environ Microbiol 69:1810–1816. https://doi.org/10.1128/aem.69.3.1810-1816.2003
Tsang TM, Wiese JS, Felek S, Kronshage M, Krukonis ES (2013) Ail proteins of Yersinia pestis and Y. pseudotuberculosis have different cell binding and invasion activities. PLoS ONE 8:e83621. https://doi.org/10.1371/journal.pone.0083621
Vital PG, Van Ha NT, Tuyet LT, Widmer KW (2017) Application of quantitative real-time PCR compared to filtration methods for the enumeration of Escherichia coli in surface waters within Vietnam. J Water Health 15:155–162. https://doi.org/10.2166/wh.2016.173
Wang JZ et al (2014) Real-time TaqMan PCR for Yersinia enterocolitica detection based on the ail and foxA genes. J Clin Microbiol 52:4443–4444. https://doi.org/10.1128/jcm.02528-14
Wang E et al (2018a) Molecular characterization, phylogenetic, expression, and protective immunity analysis of OmpF, a promising candidate immunogen against yersinia ruckeri infection in channel catfish. Front Immunol 9:2003. https://doi.org/10.3389/fimmu.2018.02003
Wang J et al (2018b) Rapid detection of food-borne Salmonella contamination using IMBs–qPCR method based on pagC gene. Braz J Microbiol 49:320–328. https://doi.org/10.1016/j.bjm.2017.09.001
Wen Z et al (2016) Recombinant expression of Chlamydia trachomatis major outer membrane protein in E. Coli outer membrane as a substrate for vaccine research. BMC Microbiol 16:165. https://doi.org/10.1186/s12866-016-0787-3
Xiong Q, Cui X (2014) Development of an immunomagnetic separation method for efficient enrichment of Escherichia coli O157:H7. Food Control 37:41–45
Zhang X et al (2018) Immunization with pseudomonas aeruginosa outer membrane vesicles stimulates protective immunity in mice. Vaccine 36:1047–1054. https://doi.org/10.1016/j.vaccine.2018.01.034
Zhu P et al (2011) Detection of E. coli O157:H7 by immunomagnetic separation coupled with fluorescence immunoassay. Biosens Bioelectron 30:337–341. https://doi.org/10.1016/j.bios.2011.09.029
Acknowledgements
This project was supported by National Key Research and Development Program of China (2018YFD0500501), and Key Projects of Science and Technology Support Grant of Tianjin in China (20YFZCSN00340).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: JHH. Performed the experiments: JXS, HC, APC, YNS, MZ, LLZ and FZX. Analyzed the data: APC and JHH. Contributed reagents/materials /analysis tools: JHH. Wrote the paper: JXS, HC, and JHH.
Corresponding authors
Ethics declarations
Conflict of interest
We declare that we have no competing interests.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shi, J., Chi, H., Cao, A. et al. Development of IMBs–qPCR detection method for Yersinia enterocolitica based on the foxA gene. Arch Microbiol 203, 4653–4662 (2021). https://doi.org/10.1007/s00203-021-02459-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-021-02459-4