Abstract
This work aims to characterize the arbuscular mycorrhizal association between maize genotypes and the effects of soil physical–chemical attributes on the symbiosis. A preliminary greenhouse assay evaluated five maize landraces and five conventional modern genotypes in non-sterile, low-P soil. Sixty days after sowing, we measured plant height, stem diameter, shoot and root dry biomass, root colonization structures, and shoot P concentration and total accumulation. In a second stage, a 2-year on-farm study evaluated how soil physical–chemical attributes in fields with three plant genotype groups affected the arbuscular mycorrhizal fungal symbiosis in a maize diversity microcenter in Southern Brazil. We collected soil and plant material in farms growing landrace, conventional modern genotypes, or genetically modified (GM) maize. There were five collection points at each group, and we measured mycorrhizal colonization, soil physicochemical attributes, and shoot phosphorus concentration. The greenhouse study showed that genotypes have different growth strategies for root production and shoot growth. No differences in mycorrhizal colonization rates occurred among landraces and modern maize genotypes in the low-P soil. The field study showed that soil and climate conditions had a more marked effect on mycorrhizal root colonization than plant genotype groups (landrace, conventional modern genotypes, or GM maize).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Maize (Zea mays L.) is one of the world’s most important food crops (Shiferaw et al. 2011). In Southern Brazil, the crop is grown mainly in smallholder farms (CONAB 2020), which conserve a high number of landraces in the region, a maize diversity micro center (Costa et al. 2017). Maize benefits from the association with arbuscular mycorrhizal fungi (AMF), and inoculation can double grain yield in low-P soils (Stoffel et al. 2020).
Plant breeding has resulted in maize varieties and hybrids that are highly productive, but require large amounts of fertilizers and agrochemicals (Machado et al. 2008; Brzozowski and Mazourek 2018). Landrace and modern hybrids, either genetically modified or non-modified, are grown with diverse management, input use, plant densities, as well as different methods of weed control, soil tillage, and fertilizer application. The association between plants and AMF can also be affected by genotypes and environmental factors, including temperature and humidity (Walter et al. 2016; Püschel et al. 2020), and soil attributes, such as aeration, organic matter, phosphorus (P), nitrogen (N), and pH (Mello et al. 2006; Moreira et al. 2007). Current management for high-yielding genotypes uses various pesticides, which can harm some groups of microorganisms that possess the product's target enzymes (Busse et al. 2001).
Some studies have shown that improved modern maize genotypes have lower AMF colonization rates than landrace genotypes (Aquino 2003; Morales-Londoño 2019). Kaeppler et al. (2000) found differences in response to AMF among maize genotypes in a soil with low P and responsiveness to AMF, suggesting that plant breeding may produce cultivars that are less responsive to association with those fungi. AMF fungi are dependent on the symbiont plant to function and reproduce (Liu 2010), and the effect of different maize genotypes on arbuscular mycorrhizal fungi (AMF) is still little understood (Cheeke et al. 2014), it is necessary to study the interaction of AMF and different maize genotypes.
We aimed to characterize the mycorrhizal association in the most widely used maize landraces and modern genotypes in a maize diversity micro center in Southern Brazil. The genotypes used in the experiment are the most widely used in the region, correspond to 22% of maize sown area of western Santa Catarina (Santa Catarina State Department of Agriculture and Fisheries, 2016; unpublished data). Landraces and modern genotypes were tested in a preliminary assay in greenhouse conditions and subsequently in maize fields for two consecutive years. We evaluated root mycorrhizal colonization and the effect of different plant and soil management procedures on the association.
Materials and methods
Greenhouse study
The experiment was carried out in a greenhouse in Florianópolis, Brazil (27°35′54.1"S 48°30′56.4"W) using an Aquic Quartzipsamment soil (Soil Survey Staff 1999), with the following attributes: organic matter 45 g dm−3, pH in water 4.73, resin-extracted P 6.8 mg kg−1, exchangeable K 16.0 mg kg−1 (Tedesco et al. 1995). The AMF community had 6124 spores 50 cm−3 (same soil used in Morales-Londoño et al. 2020). The soil was limed to reach pH 5.7 using 8.5 g limestone kg soil−1, homogenized, and placed in 5-L pots. Maize seeds were washed, disinfected with 70% alcohol (30 s), 2% sodium hypochlorite (2 min), and rinsed with sterile water (Sauer and Burroughs 1986). Three maize seeds were sown in each pot, and plants were thinned to one per pot one week after emergence.
Treatments included ten maize genotypes: five landraces, and five conventional modern genotypes (four hybrids, and one open pollination variety) (Table 1). The experimental design was completely randomized with five replicates. Pots periodically received distilled, non-sterilized water to maintain 70–100% water retention capacity.
Each pot received 0.42 g of P and 2.83 g of K, split into two applications (at sowing and 30 days later), 0.45 g of N, split into three applications (at sowing and at 20 and 40 days), and a micronutrient solution (Hoagland and Arnon 1950) at sowing. The micronutrient solution contained 2.86 mg L−1 H3BO3, 1.81 mg L−1 MnCl2, 0.10 mg L−1 ZnCl2, 0.04 mg L−1 CuCl2, 0.02 mg L−1 H2MoO4.H2O, and Fe-EDTA (24.9 mg L−1 FeSO4.7H2O and 33.2 mg L−1 of EDTA).
At 60 days, plants were collected and we measured height, stem diameter, shoot and root biomass, mycorrhizal colonization (arbuscules, vesicles, and total), and soil AMF spore number. Root dry biomass was separated as structural roots (primary, nodal, and seminal roots) and lateral roots (York et al. 2013). Shoots and roots were dried at 60 °C until constant mass and weighted.
On-farm study location and data collection
Plant and soil were sampled in maize-producing farms in the western region of Santa Catarina State. The region has a humid subtropical climate (Cfa), according to Köeppen, with 18.1 °C mean annual temperature and 1959-mm annual rainfall, distributed throughout the year (Thomé et al. 1999). Meteorological stations in São Miguel do Oeste and São José do Cedro (Figure S1 and Table S1) provided temperature and rainfall data for the growing seasons.
Technicians from a local cooperative (OesteBio, São Miguel do Oeste) helped to select fifteen farms in six municipalities (Anchieta, Bandeirante, Barra Bonita, Palma Sola, Romelândia, and São Miguel do Oeste) (Figure S1). Five farms had landrace maize, five grew non-modified corn, and five had genetically modified (GM) maize.
Plant samples were taken in two consecutive years (February 2016 and January 2017) (Figure S1), at the reproductive stage (70–90 days after seeding (DAS)) (Tables S2 and S3). Soil samples for chemical analysis (about 500 g of soil) were taken at 0–20-cm depth in five random points from each maize field. At each point, an undisturbed sample (0–10-cm depth) was taken to assess apparent density, and we estimated mycorrhizal colonization in thin roots from each collected plant. The roots were placed in plastic bags, transported, and stored at 4 °C until processing. Two leaves (one above and one below the cob) from the corresponding maize plant were collected to assess phosphorus concentration.
Plant tissue analyses
Shoot and root samples were dried (60 ºC) and ground, submitted to sulfuric digestion (Tedesco et al. 1995), and P concentration was measured by colorimetry (Murphy and Riley 1962). P accumulated in plant tissues in the greenhouse experiment was calculated multiplying the concentration in shoots and roots by the respective dry mass.
AMF spore count and mycorrhizal colonization measurement
AMF spores were extracted from 50-cm3 soil samples by wet sieving, followed by centrifugation in a sucrose gradient (Gerdemann and Nicolson 1963) and counted with a stereomicroscope.
Fine-root samples (approximately 1 g) from each plant were washed in tap water, cleared in KOH 10% at 80 °C for 60 min, acidified in HCl 5.0%, and stained with trypan blue (Koske and Gemma 1989). Root colonization rates were quantified by slide intersection method (McGonigle et al. 1990) at 200× magnification. A total of 100 intersections per sample were examined, recording arbuscules, vesicles, and total colonization.
Determination of soil physical and chemical attributes
Soil pH (H2O), organic carbon (Walkley and Black 1934), and potassium and phosphorus (Mehlich-I) were quantified according to Tedesco et al. (1995) and Claessen (1997). P was also determined after extraction with anion-exchange resin (Tedesco et al. 1995). Exchangeable aluminum, calcium, and magnesium extracted with 1-mol L−1 KCl were determined by atomic spectrometry. Cation exchange capacity (CEC pH 7.0) was estimated as the sum of cations [Ca + Mg + (H + Al) + K]. Apparent density was determined by the cylinder method (Tedesco et al. 1995).
Statistical analyses
The Bartlett test analyzed data variance homogeneity. Since variances were homogeneous, we did analyses of variance (ANOVA), and when there were significant effects, we separated the means using the Skott–Knott test (p ≤ 0.05) for the greenhouse experiment and the confidence interval (p ≤ 0.05) for the field samples. Sigma-Plot v. 12.5 software (Systat Corp., San Jose, USA) generated the graphs with the mean standard error bars. Reference lines with the data of Betancur-Agudelo (2016) were added to the mycorrhizal colonization graphs.
The Vegan package (Oksanen et al. 2013) was used for the Redundancy Analysis (RDA) using the mycorrhizal variables (colonization by arbuscules, vesicles, and total) and soil physical–chemical attributes. PERMANOVA (p ≤ 0.05) was used to verify the significance of the model RDA and the effect of soil physical–chemical variables on mycorrhizal variables. Log (x + 1) transformation was applied for data standardization before the RDA was performed. The RDA graph variables were selected using the “vif” command of the Vegan package (Oksanen et al. 2013).
Results
Greenhouse experiment
In the greenhouse experiment, two maize conventional modern genotypes and four landraces grew taller than the other genotypes (Table 2). Genotypes Morgan 20A55, SCS 155, Taquara, Pixurum 07, Língua-de-papagaio, and Amarelão had the highest height. Morgan 20A55, SHS 5050, SCS 155, Taquara, Pixurum 07, Língua-de-papagaio, and Amarelão had the highest shoot dry biomass (SDM). SHS 5050, Morgan 20A55, SHS 5070, SCS 155, and Língua-de-papagaio had higher root dry biomass (RDM) than the other treatments. Lateral roots were the most important contributors to root biomass, and total dry mass (TDM) followed the same group separation pattern as SDM (Table 2).
Phosphorus concentration in Morgan 20A55 genotype, which had the highest SDM, was lower than in all other genotypes (Table 3), but there were no differences among genotypes in total P accumulation. Mycorrhizal root colonization rates, as arbuscules, vesicles, and total colonization, did not differ among genotypes or maize genotype groups (Table 3), and there were no differences in AMF spore number.
On-farm study
The farms with genetically modified maize (GM) were concentrated around São Miguel do Oeste meteorological station, while those with conventional modern genotypes maize (CO) and landrace (LR) were near the São José do Cedro meteorological station (Figure S1). Accumulated rainfall in the first year (2015/16) differed between the meteorological stations; the São José do Cedro station recorded 41% more rainfall than the station at São Miguel do Oeste. In the second year (2016/17), rainfall was similar at both meteorological stations (Figure S1 and Table S1).
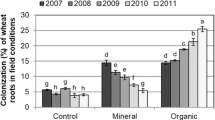
In both years, root colonization rates were highly variable within the genotype groups (GM, CO, or LR) (Fig. 1). In the first year, the percentages of arbuscules varied between 6 and 34%, while it ranged between 13 and 63% in the second year. Vesicle rates varied between 1 and 12% in the first year, and between 2 and 40% in the second year. Total colonization had its highest value in the GM2 field (67%) and the lowest in the GM5 field (27%) in the first year; in the second year, the highest rate occurred in the CO5 (87%) and the lowest in the LR5 (43%), with no difference among genotype groups.
Percentages of arbuscules, vesicles, total root colonization, in genetically modified (GM), conventional modern genotypes (CO), and landrace (LR) maize fields in two consecutive growing seasons: 2015/2016 (A, C, and E) and 2016/2017 (B, D, and F). Bars represent the standard error bar of the mean, n = 5. Horizontal lines represent means for maize fields (GM, CO, and LR) found by Betancur-Agudelo (2016), in the same study areas,
Root colonization in genotype groups had distinct patterns in each year. In the first year, mycorrhizal colonization rates were arranged, in descending order: CO > GM > LR for arbuscules, CO > LR > GM for vesicles, and CO > LR > GM (Fig. 1) for total colonization. In the second year, the descending order was CO > GM > LR for arbuscules, vesicles, and total colonization.
Shoot P concentration did not differ among maize genotypes in the first year, with a mean value of 1.97 ± 0.69. In the second year, shoot P concentrations in GM mean was 0.97 ± 0.47, 37% lower than the LR and CO genotypes (1.53 ± 0.49).
Soil pH, clay, exchangeable Al, H + Al, and AD did not differ among genotype groups in both years (Table 3). Organic matter was lower in CO, as compared with LR and GM in the first year, while in the second year, there were no differences among genotype groups. Resin-extracted P was higher in LR, intermediate in GM and lower in CO maize in the first year, while in the second year, GM cornfields had higher levels of resin-extracted P than the other two treatments. Resin-P, levels, as well as K, were higher in the second year than in the first year. In both years, exchangeable Ca was higher in CO than in GM fields, while LR fields had intermediate values, not differing from the other groups. CO fields had higher exchangeable Mg than LR and GM in both seasons. Cation exchange capacity (CEC) showed differences only in the second season, when CO fields had higher levels than GM, and LR had intermediate values, that did not differ from the other genotype groups (Table 3).
The multivariate analysis showed that some patterns in mycorrhiza-related traits varied according to environmental conditions. The axes explain 26% of the total variation, with the RDA1 axis explaining 20.5% while the RDA2 axis explains 5.5% (Fig. 2). On the other hand, PERMANOVA, which tested the effect of environmental variables on mycorrhizae, showed no significance at a 5% probability of error.
Redundancy analysis (RDA) of mycorrhizal colonization rates and soil physical–chemical attributes in maize fields in two growing seasons. AD Apparent density; OM = Organic matter; Resin-P Resin-extracted phosphorus; pH pH in H2O; K exchangeable potassium; Mg exchangeable magnesium; Al exchangeable aluminum; H + Al Potential acidity; Coln Total colonization; Arbu Arbuscule colonization; Vesi Vesicle colonization. Legend: LR landrace; CO conventional modern genotypes maize; GM genetically modified maize
When the mycorrhizal variables were compared between years, there were significant differences, in higher total colonization and arbuscule and vesicle frequencies in the second year, and those differences appear in the RDA graph. Analyses of differences in soil and climate variables between years show less rainfall, with a more uniform distribution in the second year, as compared with the first year, at both weather stations (Figure S2; Table S1). There was also P and K higher concentration f in the soil in the second year than in the first growth season (Table 3).
Discussion
The genotypes showed different growth strategies with low soil P. The first experiment showed that maize genotypes differ in shoot and root biomass ratio, and in tissue P concentration. Increased root production by some genotypes may be a strategy towards better use of soil resources, by increasing uptake of nutrients, such as phosphorus. The Morgan 20A55 genotype is an example, as it accumulated biomass with an 18% lower P concentration than the other maize genotypes, which indicates higher efficiency in the use of P (Table 4).
Mycorrhiza-related traits did not differ among genotypes nor genotype groups in the low-P soil used in the greenhouse experiment. Mycorrhizal colonization rates are more responsive to soil P levels than to plant genotypes (Kaeppler et al. 2000). Lehmann et al. (2012) performed a meta-analysis, encompassing 320 different crop plant genotypes, including maize. Although modern genotypes showed lower colonization, there was no evidence that modern genotypes have lost their ability to respond to arbuscular mycorrhizae. On the other hand, Cobb et al. (2016) evaluated two landraces and two modern sorghum hybrids and found modern hybrids less responsive to mycorrhization and more responsive to mineral fertilizer application. They argue that plant breeding seems to have selected plants that are less responsive to mycorrhizas. However, as they analyzed a limited number of genotypes, it is not possible to infer a generalized pattern from their work. A previous study in the same region in which we worked (Betancur-Agudelo 2016) found 50% higher arbuscular colonization and 37% higher total colonization in landrace than in GM maize, a pattern that did not occur in the present study. That suggests that changes in climate conditions affect mycorrhizal colonization to a significant extent.
In our on-farm assay, mycorrhizal root colonization in maize is within the range found in previous works, such as those by Miranda et al. (2005), with 84%, and Barboza (2016), with 69% colonization rates (ranging from 20 to 100%) in various soil P levels. Those results are possibly due to soil and plant management, including fertilizer and agrochemical applications, weeding, presence and diversity of spontaneous plants, and the use of tillage or no-tillage systems. Such procedures may be more important for AMF root colonization than plant genotype (Carrenho et al. 2010).
Some factors related to management may have affected the results of our study. In the previous year (Betancur-Agudelo 2016) and in the first year of our study, farmers growing landrace maize used primarily organic fertilizers (animal manure and green manure), while in the second growth season, they changed to chemical fertilizers (Table S2 and S3). The immediate availability of nutrients from mineral fertilizers affects the mycorrhizal symbiosis in a different way from organic fertilizers (Smith and Read 2008; Moura 2015), which release nutrients gradually as materials decompose (Busato et al. 2009).
Root colonization rates occurred in the second growing season were 22% higher than in the first year, when there were lower P and K and higher rainfall. Since abiotic conditions such as water availability affect the mycorrhizal association, the higher rainfall in the first growth season in the CO and LR fields than in the GM areas, may explain their higher vesicle production in the first year than in the second year (Fig. 1). Vesicle formation increases under stressful environmental conditions (Cooke et al. 1993; Smith and Read 2008), and differently from the first year, vesicle intensity rates in the second season were similar among genotype groups, as were rainfall values. There are other possible reasons for the differences, such as a change in the pattern of AMF species that predominate in different climate conditions, or AMF which produce more vesicles (Pereira 2013) or do not produce vesicles (Morton and Benny 1990). Some management procedures do not favor formation of large spores (Varela-Cervero et al. 2015), as observed with AMF in the Gigasporaceae family (Douds et al. 1993; Cuenca et al. 1998; Picone 2000).
The low tissue P concentration found in GM genotypes may result from a strategy to increase maize yield. As genotypes are selected for higher yields, soil P (Table 3) may not have been sufficient to supply the entire plant. Phosphorus is translocated from older leaves to plant organs with higher demand, such as grains (Raghothama 1999), which results in plants having lower foliar P while maintaining growth and yield.
None of the soil attributes correlated with mycorrhiza-related variables, although studies show that soil characteristics affect mycorrhiza establishment. According to Joner and Jakobsen (1995), higher organic matter levels increase soil porosity and facilitate hyphal growth, affecting fungal structures and root colonization. Vieira et al. (2018) showed that soil pH, MO, Al, Mg, and S differently affect the abundance of several AMF species in dry and rainy seasons. However, as stated by Stürmer and Siqueira (2008), it is difficult to establish a clear relationship of AMF occurrence with soil and climate variables.
Phosphorus is the best-known modulator of mycorrhizal symbiosis. Availability of this nutrient significantly affects mycorrhizal root colonization, as low P levels stimulate the symbiosis, while high levels tend to impair it (Smith and Read 2008). Studies with different P doses and inoculation with phosphate-solubilizing bacteria and AMF have shown that controlled reductions in P fertilization favor the association, and in general, plant yield is equivalent to plants receiving full P fertilizer application (Bressan and Vansconcellos 2002; Bressan et al. 2001; Suri et al. 2011; Pereira et al. 2014). Since symbioses are seldom considered in soil fertility management, many farmers add high P doses, thus reducing the potential for association of plants with beneficial microorganisms. A promising strategy would be establishing adequate fertilizer doses for each type of soil, aiming at high crop yield and better use of benefits promoted by soil microorganisms (Suri et al. 2006). Improved soil management, selection of plant species and variety, crop rotation, and adjusted fertilization (Bonfim et al. 2010) may promote AMF growth. That would avoid the negative influence on the AMF caused by application of high amounts of chemical fertilizers and other associated practices (Verbruggen et al. 2012; Roy et al. 2017). The use of highly soluble fertilizers and agrochemicals on crops leads to strong ecological and evolutionary selection in agroecosystems (Verbruggen and Toby-Kiers 2010). Plant genotypes, and specifically maize varieties and hybrids, need to be investigated in a broader way, aiming to better understand plant-microbial symbioses.
Conclusions
Maize genotypes—landrace and conventional modern genotypes—have diverse strategies for shoot and root growth at low soil P, and the Morgan 20A55 genotype can yield high plant dry matter under these conditions.
There are no differences in mycorrhizal root colonization and spore production among landrace maize and modern maize genotypes in low-P soils.
Soil and climate conditions have a stronger effect on mycorrhizal root colonization than maize genotype groups.
Data availability
All collected and analyzed data is presented in the paper.
Code availability
Not applicable.
References
Aquino SS (2003) Associação micorrízica arbuscular com genótipos de milho. Universidade Estadual Paulista
Barboza EA (2016) Variabilidade espacial micorrízica, teor de fósforo no solo e produtividade da cultura do milho (Zea mays) Dissertação (Mestrado em Agricultura de Precisão). Universidade Federal de Santa Maria
Betancur-Agudelo M (2016) Ocorrência de fungos micorrízicos arbusculares e caracterização da simbiose em milho crioulo, híbrido convencional e transgênico no oeste de Santa Catarina. Dissertação (Mestrado em Recursos Genéticos Vegetais), Universidade Federal de Santa Catarina
Bonfim JA, Matsumoto SN, Lima JM, César FRCF, Santos MAF (2010) Arbuscular mycorrhizal fungi and physiological aspects of coffee conducted in agroflorestal system and at full sun. Bragantia 69:201–206. https://doi.org/10.1590/S0006-87052010000100025
Bressan W, Vasconcellos CA (2002) Morphological alterations on root system of maize induced by mycorrhizal fungi and phosphorus. Pesq Agropec Bras 37:509–517. https://doi.org/10.1590/S0100-204X2002000400013
Bressan W, Siqueira JO, Vasconcellos CA, Purcino AAC (2001) Mycorhizal fungi and phosphorus on growth, yield and nutrition of intercropped grain sorghum and soybean. Pesq Agropec Bras 36:315–323. https://doi.org/10.1590/S0100-204X2001000200015
Brzozowski L, Mazourek M (2018) A sustainable agricultural future relies on the transition to organic agroecological pest management. Sustainability 10:1–25. https://doi.org/10.3390/su10062023
Busato JG, Canellas LP, Dobbss LB, Baldoto MA, Aguiar NO, Rosa RCC, Schiavo JA, Marciano CR, Olivares FL (2009) Guia para adubação orgânica baseado na experiência com solos e resíduos do Norte Fluminense. Secretaria de Agricultura do Estado do Rio de Janeiro
Busse MD, Ratcliff AW, Shestak CJ, Powers RF (2001) Glyphosate toxicity and the effects of long-term vegetation control on soil microbial communities. Soil Biol Biochem 33:1777–1789. https://doi.org/10.1016/s0038-0717(01)00103-1
Carrenho R, Gomes-da-Costa SM, Balota EL, Colozza-Filho A (2010) Fungos micorrízicos arbusculares em agrossistemas brasileiros. In: Siqueira JO, Souza FA, Cardoso EJBN, Tsai SM (eds) Micorrizas 30 anos de pesquisa no Brasil. Universidade Federal de Lavras, pp 154–214
Cheeke TE, Darby H, Rosenstiel TN, Bever JD, Cruzan MB (2014) Effect of Bacillus thuringiensis (Bt) maize cultivation history on arbuscular mycorrhizal fungal colonization, spore abundance and diversity, and plant growth. Agric Ecosyst Environ 195:29–35. https://doi.org/10.1016/j.agee.2014.05.019
Claessen MEC (1997) Manual de métodos de análise de solo, 2nd edn. EMBRAPA-CNPS
Cobb AB, Wilson GW, Goad CL, Bean SR, Kaufman RC, Herald TJ, Wilson JD (2016) The role of arbuscular mycorrhizal fungi in grain production and nutrition of sorghum genotypes: enhancing sustainability through plant-microbial partnership. Agric Ecosyst Environ 233:432–440. https://doi.org/10.1016/j.agee.2016.09.024
CONAB (2020) Perspectivas para a agropecuária. Safra 2019/2020. Companhia Nacional de Abastecimento
Cooke MA, Widden P, O’Halloran I (1993) Development of vesicular–arbuscular mycorrhizae in sugar maple (Acer saccharum) and effects of base-cation ammendment on vesicle and arbuscule formation. Can J Bot 71:1421–1426. https://doi.org/10.1139/b93-171
Costa FM, de Silva NCA, Ogliari JB (2017) Maize diversity in southern Brazil: indication of a microcenter of Zea mays L. Genet Resour Crop Evol 64:681–700. https://doi.org/10.1007/s10722-016-0391-2
Cuenca G, De Andrade Z, Escalante G (1998) Diversity of glomalean spores from natural, disturbed and revegetated communities growing on nutrient-poor tropical soils. Soil Biol Biochem 30:711–719. https://doi.org/10.1016/S0038-0717(97)00191-0
de Miranda JCC, Vilela L, de Miranda LN (2005) Dynamics and contribution of arbuscular mycorrhiza in culture systems with crop rotation. Pesq Agropec Bras 40:1005–1014. https://doi.org/10.1590/S0100-204X2005001000009
Douds DD, Janke RR, Peters SE (1993) VAM fungus spore populations and colonization of roots of maize and soybean under conventional and low-input sustainable agriculture. Agric Ecosyst Environ 43:325–335. https://doi.org/10.1016/0167-8809(93)90095-7
Gerdemann JW, Nicolson TH (1963) Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans Br Mycol Soc 46:235–244. https://doi.org/10.1016/S0007-1536(63)80079-0
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. California Agricultural Experiment Station
Joner EJ, Jakobsen I (1995) Growth and extracellular phosphatase activity of arbuscular mycorrhizal hyphae as influenced by soil organic matter. Soil Biol Biochem 27:1153–1159. https://doi.org/10.1016/0038-0717(95)00047-I
Kaeppler SM, Parke JL, Mueller SM, Senior L, Stuber C, Tracy WF (2000) Variation among maize inbred lines and detection of quantitative trait loci for growth at low P and responsiveness to arbuscular mycorrhizal fungi. Crop Sci 40:358–364. https://doi.org/10.2135/cropsci2000.402358x
Koske RE, Gemma JN (1989) A modified procedure for staining roots to detect VA mycorrhizas. Mycol Res 92:486–488. https://doi.org/10.1016/S0953-7562(89)80195-9
Lehmann A, Barto EK, Powell JR, Rillig MC (2012) Mycorrhizal responsiveness trends in annual crop plants and their wild relatives—a meta-analysis on studies from 1981 to 2010. Plant Soil 355:231–250
Machado AT, Santilli J, Magalhães R (2008) A agrobiodiversidade com enfoque agroecológico: implicações conceituais e jurídicas. Embrapa Cerrados-Livro científico (ALICE)
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:495–501. https://doi.org/10.1111/j.1469-8137.1990.tb00476.x
Mello AH, Antoniolli ZI, Kaminski J, Souza EL, Oliveira VL (2006) Arbuscular and ectomycorrhizal fungi in eucalypt cultivation and grassland sandy soil. Ciênc Florestal 16:293–301. https://doi.org/10.5902/198050981909
Morales-Londoño DM, Meyer E, González D, Hernández AG, Soares CRFS, Lovato PE (2019) Landrace maize varieties differ from conventional and genetically modified hybrid maize in response to inoculation with arbuscular mycorrhizal fungi. Mycorrhiza 29:237–249. https://doi.org/10.1007/s00572-019-00883-5
Morales-Londoño DM, Meyer E, Silva KJ, Hernández AG, Armas RD, Soares LM, Stürmer SL, Nodari RO, Soares CRFS (2020) Root colonization and arbuscular mycorrhizal fungal community composition in a genetically modified maize, its non-modified isoline, and a landrace. Mycorrhiza. https://doi.org/10.1007/s00572-020-00969-5
Moreira M, Nogueira MA, Tsai SM, Gomes-da-Costa SM, Cardoso EJBN (2007) Sporulation and diversity of arbuscular mycorrhizal fungi in Brazil Pine in the field and in the greenhouse. Mycorrhiza 17:519–526. https://doi.org/10.1007/s00572-007-0124-7
Morton JB, West VU, Benny GL (1990) Revised classification of arbuscular mycorrhizal fungi (Zygomycetes): a new order, Glomales, two new suborders, Glomineae and Gigasporineae, and two new families, Acaulosporaceae and Gigasporaceae, with an emendation of Glomaceae. Mycotaxon (USA) 37:471–491
Moura JB (2015) Diversidade e colonização micorrízica em diferentes usos do solo no cerrado. Tese de Doutorado (Pós-Graduação em Agronomia). Universidade de Brasília
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36. https://doi.org/10.1016/S0003-2670(00)88444-5
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Wagner H (2013) Package ‘vegan.’ R Packag 254:8–20
Pereira CMR (2013) Diversidade de fungos micorrízicos arbusculares em área de Mata. Dissertação de mestrado (Pós-graduação em Biologia de Fungos). Universidade Federal de Pernambuco
Pereira SIA, Castro PML (2014) Phosphate-solubilizing rhizobacteria enhance Zea mays growth in agricultural P-deficient soils. Ecol Eng 73:526–535. https://doi.org/10.1016/j.ecoleng.2014.09.060
Picone C (2000) Diversity and abundance of arbuscular-mycorrhizal fungus spores in tropical forest and pasture. Biotropica 32:734–750. https://doi.org/10.1111/j.1744-7429.2000.tb00522.x
Püschel D, Bitterlich M, Rydlová J, Jansa J (2020) Facilitation of plant water uptake by an arbuscular mycorrhizal fungus: a Gordian knot of roots and hyphae. Mycorrhiza 30:299–313. https://doi.org/10.1007/s00572-020-00949-9
Raghothama KG (1999) Phosphate acquisition. Annu Rev Plant Physiol Plant Mol Biol 50:665–693. https://doi.org/10.1146/annurev.arplant.50.1.665
Roy J, Reichel R, Brüggemann N, Hempel S, Rillig MC (2017) Succession of arbuscular mycorrhizal fungi along a 52-years agricultural recultivation chronosequence. FEMS Microbiol Ecol 93:1–13
Sauer DB, Burroughs R (1986) Disinfection of seed surfaces with sodium hypochlorite. Phytopathology 76:745–749
Shiferaw B, Prasanna BM, Hellin J, Bänziger M (2011) Crops that feed the world 6. Past successes and future challenges to the role played by maize in global food security. Food Sec 3:307–327. https://doi.org/10.1007/s12571-011-0140-5
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd eds. Elsevier/Academic Press
Soil Survey Staff (1999) Soil taxonomy: a basic system of soil classification for making and interpreting soil surveys, 2nd eds. Natural Resources Conservation Service
Stoffel SCG, Soares CRFS, Meyer E, Lovato PE, Giachini AJ (2020) Yield increase of corn inoculated with a commercial arbuscular mycorrhizal inoculant in Brazil. Ciênc Rural 50:e20200109. https://doi.org/10.1590/0103-8478cr20200109
Stürmer SL, Siqueira JO (2008) Diversidade de fungos micorrizicos arbusculares em ecossistemas brasileiro. In: Moreira FMS, Siqueira JO, Brussard L (eds) Biodiversidade do solo em ecossistemas brasileiros. Universidade Federal de Lavras, pp 537–584
Suri V, Choudhary A, Chander G, Verma T (2006) Studies on VAM fungi as a potential biofertilizer in an acid Alfisol of north-western Himalayas. In: 18th WCSS congress abstracts. International Union of Soil Science, Vienna
Suri VK, Choudhary AK, Chander G, Verma TS, Gupta MK, Dutt N (2011) Improving phosphorus use through co-inoculation of vesicular arbuscular mycorrhizal fungi and phosphate-solubilizing bacteria in maize in an acidic Alfisol. Commun Soil Sci Plant Anal 42:2265–2273. https://doi.org/10.1080/00103624.2011.602451
Tedesco MJ, Gianello C, Bissani CA, Bohnen H, Volkweiss SJ (1995) Análises de solo, plantas e outros materiais. UFRGS
Thomé VMR, Zampieri S, Braga HJ, Pandolfo C, Silva-Júnior VP, Bacic I, Laus-Neto J, Soldateli D, Gebler E, Ore JD, Echeverria L, Mattos M, Suski PP (1999) Zoneamento agroecológico e socioeconômico de Santa Catarina. Epagri
Varela-Cervero S, Vasar M, Davison J, Barea JM, Öpik M, Azcón-Aguilar C (2015) The composition of arbuscular mycorrhizal fungal communities differs among the roots, spores and extraradical mycelia associated with five Mediterranean plant species: AMF community composition of mycorrhizal propagules. Environ Microbiol 17:2882–2895. https://doi.org/10.1111/1462-2920.12810
Verbruggen E, Toby-Kiers E (2010) Evolutionary ecology of mycorrhizal functional diversity in agricultural systems: AMF in agriculture. Evol Appl 3:547–560. https://doi.org/10.1111/j.1752-4571.2010.00145.x
Verbruggen E, van de Heijden MGA, Weedon JT, Kowalchuk GA, Roling WFM (2012) Community assembly, species richness and nestedness of arbuscular mycorrhizal fungi in agricultural soils: community assembly of mycorrhizal fungi. Mol Ecol 21:2341–2353. https://doi.org/10.1111/j.1365-294X.2012.05534.x
Vieira CK, Marascalchi MN, Rodrigues AV, Armas RD, Stürmer SL (2018) Morphological and molecular diversity of arbuscular mycorrhizal fungi in revegetated iron-mining site has the same magnitude of adjacent pristine ecosystems. J Environ Sci 67:330–343. https://doi.org/10.1016/j.jes.2017.08.019
Walkley A, black IA, (1934) An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci 37:29–38. https://doi.org/10.1097/00010694-193401000-00003
Walter J, Kreyling J, Singh BK, Jentsch A (2016) Effects of extreme weather events and legume presence on mycorrhization of Plantago lanceolata and Holcus lanatus in the field. Plant Biol J 18:262–270. https://doi.org/10.1111/plb.12379
York LM, Nord EA, Lynch JP (2013) Integration of root phenes for soil resource acquisition. Front Plant Sci 4:1–15. https://doi.org/10.3389/fpls.2013.00355
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001, and CNPq.
Author information
Authors and Affiliations
Contributions
EM designed the study, performed research, analyzed data, and wrote the first draft of the paper. MBA, BSV, and CRFSS performed research, analyzed data, and revised the manuscript. KGA, JAS, ASV, LM, SCGS, and AM research and revised the manuscript. PEL supervised the work, contributed to the study design and revised all drafts of the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
This publication has the consent of all participants.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Meyer, E., Betancur-Agudelo, M., Ventura, B.S. et al. Mycorrhizal root colonization in maize fields is more affected by soil management and climate conditions than by plant genotype. Arch Microbiol 203, 4609–4618 (2021). https://doi.org/10.1007/s00203-021-02429-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-021-02429-w