Abstract
A Gram-positive, aerobic, endospore-forming, rod-shaped bacterial strain, CAU 1483 T, was isolated from tidal-flat mud in the Republic of Korea. It grew optimally at 30 °C, in a pH 7.0 medium with 2% (w/v) NaCl. Phylogenetic analysis based on the 16S rRNA gene sequence indicated that strain CAU 1483 T formed a separate clade within Paenibacillaceae together with members of the genus Cohnella. Strain CAU 1483 T exhibited the highest 16S rRNA gene sequence similarity (97.1%) to C. candidum 18JY8-7 T. Whole genome of strain CAU 1483 T was 4.29 Mb in size with a 53.7 mol% G + C content, and included 4046 coding sequences and included 4046 coding sequences, some of which associated with stress response. The average nucleotide identity and digital DNA–DNA hybridization similarity between strain CAU 1483 T and related members of the genus Cohnella were 71.8–74.9% and 22.6–33.9%, respectively. The major respiratory quinone present in this strain was menaquinone-7. Strain CAU 1483 T contained anteiso-C15:0 and iso-C16:0 as the major fatty acids, while its polar lipids consisted of phosphatidylglycerol, phosphatidylethanolamine, diphosphatidylglycerol, lysyl-phosphatidylglycerol, phosphatidylcholine, three unidentified aminophospholipids, two unidentified lipids and an unidentified phospholipid. Peptidoglycan type was A1γ meso-Dpm. On the basis of taxonomic characterization, strain CAU 1483 T constitutes a novel species, for which the name Cohnella pontilimi sp. nov. is proposed. The type strain of this novel species is CAU 1483 T (= KCTC 43047 T = NBRC 113953 T).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Cohnella, belonging to the family Paenibacillaceae together with the class Bacilli of the phylum Firmicutes, was proposed by Kämpfer et al. (2006) with the description of C. thermotolerans as the type species. At the time of writing, the genus Cohnella comprises 31 validly published members (https://www.bacterio.net/genus/cohnella), which have been isolated from a wide range of environmental habitats. Members of the genus Cohnella are gram-positive, aerobic, thermotolerant and rod-shaped, and can be motile or non-motile, and spore forming or endospore forming. During the investigation of novel bacteria, a bacterial isolate, designated CAU 1483 T, was retrieved from tidal-flat mud in Modo Island, Republic of Korea, and phylogenetically affiliated to members of the genus Cohnella. In the present study, the taxonomic position of strain CAU 1483 T was determined through a polyphasic approach, which included phylogenetic analysis based on the 16S rRNA gene sequences, as well as phenotypic and chemotaxonomic characterization.
Materials and methods
Bacterial strain isolation and culture conditions
A mud sample was collected from Modo Island (37º32′12.28′′ N, 126º24′51.47′′ E) in the Republic of Korea, serially diluted, spread on BD™ Difco™ R2A agar (MA; BD Difco, Sparks, MD, USA) according to Kim and Kim (2016), and incubated at 30 °C for 7 days. Isolates were randomly selected, grown at 30 °C on R2A and then subsequently purified on R2A medium. The obtained pure colonies were preserved in 25% (v/v) glycerol stocks at − 80 °C. Strain CAU 1483 T has been deposited in the Korean Collection for Type Cultures (KCTC; Jeongeup, Korea) and the National Institute of Technology and Evaluation Biological Resource Center (NBRC; Tokyo, Japan) as KCTC 43047 T and NBRC 113953 T, respectively. For comparative analysis, C. candidum KCTC 33969 T, C. thermotolerans DSM 17683 T and C. lubricantis LMG 29763 T were obtained from the Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures (DSMZ; Braunschweig, Germany), the KCTC and the Belgian Co-ordinated Collections of Micro-organisms (BCCM; Ghent, Belgium), respectively.
16S rRNA gene sequence analysis
The total genomic DNA of strain CAU 1483 T was extracted with a genomic DNA extraction kit (iNtRON, Seongnam, Korea). The 16S rRNA gene was amplified by PCR with universal primers (Lane 1991). The PCR products were then purified and directly sequenced by an automatic DNA sequencer (3730 DNA Analyzer; Applied Biosystems, Foster City, CA, USA). Multiple alignments between the sequence of CAU 1483 T and those of members of the genus Cohnella, retrieved from the EzBioCloud database (http://www.ezbiocloud.net/eztaxon), were generated through the Clustal X 2.1 software (Larkin et al. 2007) and the Basic Local Alignment Search Tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi.). Evolutionary distance matrices were calculated through the neighbour-joining method described by Jukes and Cantor (1969). Phylogenetic trees were constructed with MEGA 7 based on the neighbour-joining (Saitou and Nei 1987), maximum-likelihood (Felsenstein 1981) and maximum-parsimony (Fitch 1971) algorithms from the PHYLIP v.3.66 package (Felsenstein 1989). The topology was evaluated through a bootstrap resampling method based on 1000 replicates (Felsenstein 1985).
DNA G + C content and whole-genome sequencing analysis
The whole-genome sequence and the mol% G + C content of CAU 1483 T were examined using an Illumina HiSeq 2500 System (Illumina, San Diego, USA). De novo assembly of the sequencing data was performed with SPAdes v.3.13.0 (http://cab.spbu.ru/software/spades). The average nucleotide identity (ANI) between the genomic sequences of CAU 1483 T and those of closely related strains was calculated using the OrthoANI algorithm of the EzBioCloud web service (www.ezbiocloud.net/sw/oat) (Lee et al. 2016), whereas digital DNA–DNA hybridization (dDDH) values were calculated by the Genome-to-Genome Distance Calculator v.2.1 (http://ggdc.dsmz.de/ggdc.php) (Meier-Kolthoff et al. 2013). The genome of strain CAU 1483 T was annotated using the RAST Server (Brettin et al. 2015) and subsequently analysed in detail using the SEED database (Overbeek et al. 2014). Predicted bacterial species biosynthetic gene clusters were identified with antiSMASH v.5.1.0 (Blin et al. 2019). Proteome comparison between strain CAU 1483 T and its most closely related strain, C. candidum 18JY8-7 T, was carried out through the PATRIC website (www.patricbrc.org) (Wattam et al. 2017).
Phenotypic and biochemical analyses
For colony morphology characterization, the novel strain was cultured on R2A plates at 30 °C for 2 days. Motility was tested in cells grown in semisolid (0.3% agar) MA media (Bowman 2000). A Gram staining kit (bioMérieux, Craponne, France) was used to determine if bacteria were Gram positive or Gram negative. Cell morphology was observed with a DM1000 light microscope (Leica, Wetzlar, Germany) and a JEM-1010 transmission electron microscope (JEOL, Tokyo, Japan). The growth of strain CAU 1483 T under aerobic and anaerobic conditions was tested at various temperatures (4, 10, 20, 30, 37 and 45 °C). The pH of the medium was adjusted after sterilization to pH 4.5–11.0 (at 0.5 unit intervals) using sodium acetate/acetic acid, 1 M HCl for acid conditions and 1 M NaOH for alkaline conditions. Tolerance to NaCl concentrations of 0–15% (w/v, at 1% intervals) was investigated by observing growth in the R2A broth. Oxidase activity was determined with 0.1% tetramethyl-p-phenylenediamine while catalase activity was monitored by bubble production from cells placed in 3% (v/v) H2O2 (Cappuccino and Sherman 2005). Hydrolyses of urea, gelatin and starch were examined with standard methods (Smibert et al. 1994). Additional physiological, biochemical and enzymatic analyses were carried out using the commercial test kits API® 20 NE, API® 50 CH and API® ZYM (bioMérieux), according to the manufacturer’s instructions.
Chemotaxonomic characterization
For chemotaxonomic characterization, strain CAU 1483 T and its closely related reference strains C. candidum 18JY8-7 T, C. thermotolerans CCUG 47242 T and C. lubricantis KSS-154-50 T were harvested after growth in R2A at 30 ℃ for 2 days. Fatty acid methyl esters were extracted according to the standard protocol of the Sherlock Microbial Identification System v.6.2B. Cellular fatty acids were separated through a gas chromatography system (6890 N GC System with 7683 Autosampler; Agilent Technologies, Carpinteria, CA, USA) as described previously (Sasser 1990). The cell wall peptidoglycan of strain CAU 1483 T was analysed by the Identification Service of the DSMZ (Braunschweig, Germany) as described by Schumann (2011). The polar lipids of strain CAU 1483 T were identified by two-dimensional thin-layer chromatography (Minnikin et al. 1980). The plate was sprayed with an ethanolic solution of 10% molybdatophosphoric acid, ninhydrin, α-naphthol/sulfuric acid, molybdenum blue and Dragendorff’s reagent (Sigma-Aldrich, St. Louis, MI, USA) for separation of total lipids, amino lipids, glycolipids, phospholipids and phosphatidylcholine, respectively. Respiratory quinones were extracted as described by Collins and Jones (1981) and analyzed by reversed-phase high-performance lipid chromatography (Kroppenstedt 1982).
Nucleotide sequence and whole-genome shotgun project accession number
The GenBank/EMBL/DDBJ accession number for the 16S rRNA gene sequence of strain CAU 1483 T is MH892071. The whole-genome sequence has been deposited in GenBank/EMBL/DDBJ under the accession number SUPK00000000.
Results and discussion
Phylogenetic characterization
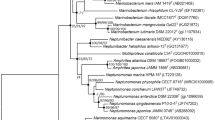
The nearly complete sequence of the 16S rRNA gene of strain CAU 1483 T (1520 bp) was obtained and compared with the corresponding sequences of the most closely related strains, retrieved from the GenBank database. According to 16S rRNA gene sequence similarity, strain CAU 1483 T was most closely related to C. candidum 18JY8-7 T (97.1%), C. thermotolerans CCUG 47242 T (95.1%) and C. lubricantis KSS-154-50 T (95.0%). The neighbour-joining tree indicated that that CAU 1483 T formed a distinct lineage within the family Paenibacillaceae and clustered with the members of the genus Cohnella (Fig. 1). The trees obtained by applying the maximum-likelihood and maximum-parsimony algorithms showed the same topology.
Phylogenetic tree constructed by neighbour-joining based on nearly complete 16S rRNA gene sequences showing the relationships between strain CAU 1483 T and its closely related species of the genus Cohnella. Bootstrap values refer to neighbour-joining, maximum-likelihood and maximum-parsimony analyses (NJ/ML/MP) of 1000 resampled datasets; only values > 70% are given. The sequence determined in this study is shown in bold. Bar, 0.02 nucleotide substitutions per position. Erythrobacter longus OChIOIT is used as an outgroup organism
Morphological, physiological and biochemical analyses
Detailed comparisons of physiological and biochemical characteristics between strain CAU 1483 T and the reference species are shown in Table 1. Cells of strain CAU 1483 T were gram-negative, non-motile, spore-forming and rod shaped, with a size of 1.5–2.6 × 0.5–0.8 µm (Supplementary Fig. S1). Colonies were white, round, smooth, raised and with entire margins after incubation for 2 days at 30 °C in R2A. Strain CAU 1483 T could grow at 25–30 °C (optimum, 30 °C), at pH 5.0–9.5 (optimum, pH 6.5–7.5) and at a 0–4% (w/v) NaCl concentration (optimum, 2%). The details of morphological, physiological and biochemical results are shown in Table 1. Strain CAU 1483 T had many same features with the three reference strains. For example, all strains were positive for C4 esterase, C8 esterase lipase, naphthol-AS-BI-phosphohydrolase and acid phosphatase activities, and could hydrolyze esculin, starch, D-mannitol and inositol. However, strain CAU 1483 T differed from C. candidum 18JY8-7 T, C. thermotolerans CCUG 47242 T and C. lubricantis KSS-154-50 T by its inability to display α-galactosidase and β-glucosidase activities. In addition, strain CAU 1483 T could also be distinguished from the reference strains since it scored positive for the hydrolysis of methyl-α-D-mannopyranoside. The list of all negative traits from commercial kits between CAU 1483 T and reference strains are shown in Supplementary Table S2.
Comparative genomic analysis
The genome of strain CAU 1483 T consisted of 24 contigs with an N50 of 430 044 bp and a k-mer coverage of 370 × . The draft genome of CAU 1483 T was 4,289,335 bp long and contained 4046 total coding sequences, including genes coding for 20 rRNAs (six 5S rRNAs, five 16S rRNAs and nine 23S rRNAs) and 54 tRNAs. According to whole-genome sequencing, the DNA G + C content of CAU 1483 T was 53.7 mol%. The whole-genome sequence of strain CAU 1483 T has been deposited in GenBank under the accession number SUPK00000000. The genome of strain CAU 1483 T displayed 1397 predicted gene functions. In particular, SEED subsystem analysis highlighted a total of 37 genes associated with stress response, specifically involved in osmotic stress, oxidative stress, detoxification and periplasmic stress (Supplementary Fig. S2). The antiSMASH analysis indicated that the genome of strain CAU 1483 T includes five biosynthetic gene clusters encoding a type III polyketide synthase and enzymes for bacteriocin, terpene, phosphonate and linear azol(in)e-containing peptide synthesis. The values of ANI between CAU 1483 T and its most closely related strains, C. candidum 18JY8-7 T and C. thermotolerans CCUG 47242 T, were 74.9 and 72.4%, respectively. Both values were significantly lower than the threshold of 95% above which two strains can be assigned to the same species (Kim et al. 2014). The ANI values relative to the comparison of CAU 1483 T with other members of the genus Cohnella are shown in Supplementary Fig. S3, Supplementary data. Furthermore, according to digital DNA–DNA hybridization (dDDH), the similarity between strain CAU 1483 T and other members of the genus Cohnella was 18.0–33.9% (Supplementary Table S1), which is significantly lower than the threshold of 70% above which two strains can be attributed to the same species (Goris et al. 2007). Finally, the genomic map of CAU 1483 T based on protein sequences revealed that strain CAU 1483 T displays 10 − 80% identity with the C. candidum 18JY8-7 T strain (Supplementary Fig. S4). Altogether, these results suggested that strain CAU 1483 T represents a distinct species of the genus Cohnella.
Chemotaxonomic analysis
The most abundant quinone in strain CAU 1483 T was menaquinone-7, which is in line with the genus Cohnella. Moreover, the predominant fatty acids of strain CAU 1483 T were anteiso-C15:0 (55.6%), iso-C16:0 (18.9%) and iso-C14:0 (7.8%). The overall fatty acid profile of strain CAU 1483 T was significantly similar to those of the closely related strains C. candidum KCTC 33969 T, C. thermotolerans DSM 17683 T and C. lubricantis LMG 29763 T. Detailed cellular fatty acid profiles of strain CAU 1483 T and its closely related strains are shown in Table 2. In contrast, the polar lipid profile of strain CAU 1483 T consisted of phosphatidylglycerol, phosphatidylethanolamine, diphosphatidylglycerol, three unidentified aminophospholipids, lysyl-phosphatidylglycerol, phosphatidylcholine, two unidentified lipids and an unidentified phospholipid. The polar lipid profile of strain CAU 1483 T was significantly similar to those of other members of the genus Cohnella, but CAU 1483 T differed from the three reference strains owing to the absence of two unidentified phospholipids (Supplementary Fig. S5). The cell wall peptidoglycan of strain CAU 1483 T contained diaminopimelic acid (Dpm), alanine (Ala) and glutamic acid (Glu) in a molar ratio of 1.4: 1.0:1.0. The particle hydrolysis detected peptides M-Ala, Ala-Glu, Ala-Dpm, Ala-Glu-Dp, Dpm-Ala-Dpm, Glu-Dpm-Ala-Dpm and Ala-Glu-Dpm-Ala. These data suggested that the composition of major of peptidoglycan type of the strain CAU 1483 T was A1γ meso-Dpm.
Taxonomic conclusion
Phylogenetic analyses, conducted through the neighbour-joining, maximum-likelihood and maximum-parsimony algorithms, based on the 16S rRNA sequences showed that strain CAU 1483 T and C. candidum 18JY8-7 T form a separate branch within the genus Cohnella. Moreover, ANI and dDDH analyses, together with morphological, physiological, biochemical and chemotaxonomic characterization, suggested that strain CAU 1483 T represents a novel species of the genus Cohnella, for which the name Cohnella pontilimi sp. nov. is proposed.
Description of Cohnella pontilimi sp. nov.
Cohnella pontilimi sp. nov. (pon.ti.li’mi L. masc. n. pontus, sea; L. masc. n. limus, mud; N.L. gen. n. pontilimi, of mud of the sea, where the type strain was isolated).
Cells are Gram-stain positive, aerobic, rod shaped, approximately 1.2–2.3 × 0.4–0.7 µm in size, non-motile and spore forming. Colonies appearing on R2A after 2 days at 30 °C are white, smooth and shiny. Optimum growth occurs at 30 °C on R2A at pH 6.5–7.5 with 2% (w/v) NaCl. On API® 20 NE strips, this strain is positive for β-galactosidase activity and citrate hydrolysis, but negative for arginine dihydrolase and urease activities, as well as for nitrate reduction, H2S production, indole production and the Voges–Proskauer reaction. On API® 50 CH strips, cells are positive for D-mannitol, methyl-α-D-mannopyranoside, esculin, D-maltose, D-lactose, starch and potassium 5-ketogluconate hydrolysis. In the API® ZYM system, strain CAU 1483 T scores positive for C4 esterase, C8 esterase lipase, acid phosphatase and naphthol-AS-BI-phosphohydrolase activities. The major cellular fatty acids are anteiso-C15:0, iso-C16:0 and iso-C14:0. Moreover, the most abundant polar lipids of strain CAU 1483 T consist of phosphatidylglycerol, phosphatidylethanolamine, diphosphatidylglycerol, three unidentified aminophospholipids, lysyl-phosphatidylglycerol, phosphatidylcholine, two unidentified lipids and an unidentified phospholipid. The peptidoglycan type is A1γ meso-Dpm. The DNA G + C content of this strain is 53.7 mol%. The type strain, CAU 1483 T (= KCTC 49037 T = NBRC 113953 T), was isolated from a soil sample in Modo Island, Republic of South Korea.
References
Bowman JP (2000) Description of Cellulophaga algicola sp. nov., isolated from the surfaces of Antarctic algae, and reclassification of Cytophaga uliginosa (ZoBell and Upham 1944) Reichenbach 1989 as Cellulophaga uliginosa comb. nov. Int J Syst Evol Microbiol 50:1861–1868
Brettin T, Davis JJ, Disz T, Edwards RA, Gerdes S, Olsen GJ, Olsen R, Overbeek R, Parrello B, Pusch GD, Shukla M, Thomason JA III, Stevens R, Vonstein V, Wattam AR, Xia F (2015) RASTtk: a modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci Rep 5:8365
Blin K, Shaw S, Villebro SK, R, Zeimert N, Lee SY, Medema M, Weber T, (2019) Antismash 5.0: updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res 47:W81–W87
Cai F, Wang Y, Qi H, Dai J, Yu B, An H, Rahman E, Fang C (2010) Cohnella luojiensis sp. nov., isolated from soil of a Euphrates poplar forest. Int J Syst Evol Microbiol 60:1605–1608
Cappuccino JG, Sherman N (2005) Microbiology: a laboratory manual. Pearson/Benjamin Cummings, San Francisco, p 507
Cho EA, Lee JS, Lee KC, Jung HC, Pan JG, Pyun YR (2007) Cohnella laeviribosi sp. nov., isolated from a volcanic pond. Int J Syst Evol Microbiol 57:2902–2907
Choi JH, Seok JH, Jang HJ, Cha JH, Cha CJ (2016) Cohnella saccharovorans sp. nov., isolated from ginseng soil. Int J Syst Evol Microbiol 66:1713–1717
Collins MD, Jones D (1981) A note on the separation of natural mixtures of bacterial ubiquinones using reverse-phase partition thin layer chromatography and high performance liquid chromatography. J Appl Bacteriol 51:129–134
FelsensteinJ, (1989) PHYLIP – phylogeny inference package (version3.2). Cladistics 5:164–166
Felsenstein J (1985) Confidence limits on phylogenies: an approachusing the bootstrap. Evolution 39:783–791
Felsenstein J (1981) Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evolution 17:368–376
Fitch WM (1971) Toward defining the course of evolution: minimum change for a specific tree topology. Syst Zool 20:406–416
Flores-Félix JD, Carro L, Ramírez-Bahena MH, Tejedor C, Igual JM, Peix A, Velazquez E (2014) Cohnella lupini sp. nov., an endophytic bacterium isolated from root nodules of Lupinus albus. Int J Syst Evol Microbiol 64:83–87
Garcia-Fraile P, Velazquez E, Mateos PF, Martínez-Molina E, Rivas R (2008) Cohnella phaseoli sp. nov., isolated from root nodules of Phaseolus coccineus in Spain, and emended description of the genus Cohnella. Int J Syst Evol Microbiol 58:1855–1859
Goris J, Konstantinidis KT, Klappenbach JA, Coenye T, Vandamme P, Tiedje JM (2007) DNA–DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol 57:81–91
Hameed A, Hung MH, Lin SY, Hsu YH, Liu YC, Shahina M, Lai WA, Huang HC, Young LS, Young CC (2013) Cohnella formosensis sp. nov., a xylanolytic bacterium isolated from the rhizosphere of Medicago sativa L. Int J Syst Evol Microbiol 63:2806–2812
Huang Z, Yu YJ, Bao YY, Xia L, Sheng XF, He LY (2014) Cohnella nanjingensis sp. nov., an extracellular polysaccharide-producing bacterium isolated from soil. Int J Syst Evol Microbiol 64:3320–3324
Jiang F, Dai J, Wang Y, Xue X, Xu M, Li W, Fang C, Peng F (2012) Cohnella arctica sp. nov., isolated from Arctic tundra soil. Int J Syst Evol Microbiol 62:817–821
Jukes TH, Cantor CR (1969) Evolution of protein molecules. Mammalian protein metabolism 3:132
Kämpfer P, Rossello-Mora R, Falsen E, Busse HJ, Tindall BJ (2006) Cohnella thermotolerans gen. nov., sp. nov., and classification of ‘Paenibacillus hongkongensis’ as Cohnella hongkongensis sp. nov. Int J Syst Evol Microbiol 56:781–786
Kämpfer P, Glaeser SP, McInroy JA, Busse HJ (2014) Cohnella rhizosphaerae sp. nov., isolated from the rhizosphere environment of Zea mays. Int J Syst Evol Microbiol 64:1811–1816
Kämpfer P, Glaeser SP, Busse HJ (2017) Cohnella lubricantis sp. nov., isolated from a coolant lubricant solution. Int J Syst Evol Microbiol 67:466–471
Khianngam S, Tanasupawat S, Akaracharanya A, Kim KK, Lee KC, Lee JS (2010a) Cohnella thailandensis sp. nov., a xylanolytic bacterium from Thai soil. Int J Syst Evol Microbiol 60:2284–2287
Khianngam S, Tanasupawat S, Akaracharanya A, Kim KK, Lee KC, Lee JS (2010b) Cohnella xylanilytica sp. nov. and Cohnella terrae sp. nov., xylanolytic bacteria from soil. Int J Syst Evol Microbiol 60:2913–2917
Khianngam S, Tanasupawat S, Akaracharanya A, Kim KK, Lee KC, Lee JS (2012) Cohnella cellulosilytica sp. nov., isolated from buffalo faeces. Int J Syst Evol Microbiol 62:1921–1925
Kim JH, Kim W (2016) Tumebacillus soli sp. nov., isolated from non-rhizosphere soil. Int J Syst Evol Microbiol 66:2192–2197
Kim M, Oh HS, Park SC, Chun J (2014) Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int J Syst Evol Microbiol 64:346–351
Kim SJ, Weon HY, Kim YS, Anandham R, Jeon YA, Hong SB, Kwon SW (2010) Cohnella yongneupensis sp. nov. and Cohnella ginsengisoli sp. nov., isolated from two different soils. Int J Syst Evol Microbiol 60:526–530
Kim SJ, Weon HY, Kim YS, Kwon SW (2011) Cohnella soli sp. nov. and Cohnella suwonensis sp. nov. isolated from soil samples in Korea. J Microbiol 49:1033–1038
Kudryashova EB, Karlyshev AV, Ariskina EV, Streshinskaya GM, Vinokurova NG, Kopitsyn DS, Evtushenko LI (2018) Cohnella kolymensis sp. nov., a novel bacillus isolated from Siberian permafrost. Int J Syst Evol Microbiol 68:2912–2917
Lane DJ (1991) 16S/23S rRNA sequencing In Nucleic Acid Techniques in Bacterial Systematics. pp. 115–175. Edited by E. Stackebrandt & M. Goodfellow. London: John Wiley & Sons Ltd
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Lee KC, Kim KK, Kim JS, Kim DS, Ko SH, Yang SH, Lee JS (2015) Cohnella collisoli sp. nov., isolated from lava forest soil. Int J Syst Evol Microbiol 65:3125–3130
Lee I, Kim YO, Park SC, Chun J (2016) OrthoANI: an improved algorithm and software for calculating average nucleotide identity. Int J Syst Evol Microbiol 66:1100–1103
Lin SY, Chen WM, Hameed A, Huang GH, Hung MH, Young CC (2020) Cohnella fermenti sp. nov., isolated from a fermentation process. Int J Syst Evol Microbiol 70:2602–2610
Maeng S, Kim MK, Jang JH, Yi H, Subramani G (2019) Cohnella candidum sp. nov., radiation-resistant bacterium from soil. Antonie Van Leeuwenhoek 112:1029–1037
Meier-Kolthoff JP, Auch AF, Klenk H-P, Göker M (2013) Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics 14:60
Minnikin DE, Hutchinson IG, Caldicott AB, Goodfellow M (1980) Thin-layer chromatography of methanolysates of mycolic acid-containing bacteria. J Chromatogr A 188:221–233
Overbeek R, Olson R, Pusch GD, Olsen GJ, Davis JJ, Disz T, Edwards RA, Gerdes S, Parrella B, Shukla M, Vonstein V, Wattam AR, Xia F, Stevens R (2014) The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res 42:D206–D214
Richter M, Rosselló-Móra R (2009) Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci USA 106:19126–19131
Sasser M (1990) Identification of bacteria by gas chromatography of cellular fatty acids.
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Schumann P (2011) Peptidoglycan structure Method Microbiol 38:01–129
Smibert RM, Krieg NR, Gerhardt P (1994) Methods for general and molecular bacteriology. American Society for Microbiology, Washington, DC, pp 607–654
Wattam AR, Davis JJ, Assaf R, Boisvert S, Brettin T, Bun C, Conrad N, Dietrich EM, Disz T, Gabbard JL, Gerdes S, Henry CS, Kenyon RW, Machi D, Mao C, Nordberg EK, Olsen GJ, Murphy-Olson DE, Olson R, Overbeek R, Parrello B, Pusch GD, Shukla M, Vonstein V, Warren A, Xia F, Yoo H, Stevens RL (2017) Improvements to PATRIC, the all-bacterial bioinformatics database and analysis resource center. Nucleic Acids Res 45:D535–D542
Yoon JH, Jung YT (2012) Cohnella boryungensis sp. nov., isolated from soil. Antonie Van Leeuwenhoek 101:769–775
Zhu HZ, Liu XD, Jiang CY, Liu SJ (2019) Cohnella faecalis sp. nov., isolated from animal faeces in a karst cave. Int J Syst Evol Microbiol 69:572–577
Kroppenstedt RM (1982) Separation of bacterial menaquinones by HPLC using reverse phase (RP18) and a silver loaded ion exchanger as stationary phases. J Liq Chromatogr 5:2359–2367
Acknowledgement
This work was supported by a grant from the National Institute of Biological Resources (NIBR), funded by the Ministry of Environment (MOE) of the Republic of Korea (NIBR201902203) and the Chung-Ang University Research Grants in 2019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no competing interests.
Ethics approval
Not applicable.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Baek, J., Kim, JH., Yoon, JH. et al. Cohnella pontilimi sp. nov., isolated from tidal-flat mud. Arch Microbiol 203, 2445–2451 (2021). https://doi.org/10.1007/s00203-021-02266-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-021-02266-x