Abstract
The study evaluates the effect of Artemisia dracunculus essential oil (EO) on two pathogenic bacteria Salmonella enterica serovar Typhimurium and Staphylococcus aureus and Vero cell line. To evaluating the anti-biofilm potential of the EO, a microtiter-plate test (MtP) and scanning electron microscopy (SEM) were performed. The quorum-sensing inhibitory properties were examined by QS-related gene expression at sub-MIC concentrations of Artemisia dracunculus EO. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a tetrazole) test was used to determine the cytotoxicity potential of the EO against the Vero cell line and finally, the major components of the EOs were determined using Gas chromatography–mass spectrometry (GC–MS) analysis. The minimum inhibitory concentration (MIC) of the tested EO against S. Typhimurium and S. aureus were 2.5 and 1.25 μl/ml, respectively. In addition, the minimum bactericidal concentration was 5 and 2.5 μl/ml for S. Typhimurium and S. aureus, respectively. Both MtP and SEM showed an acceptable inhibitory and disruption effect of the EO on the biofilm formation at Sub-MIC concentrations. Significant downregulation of luxS, pfs, and hld genes by treatment with MIC/2 concentration of A. dracunculus EO was observed. The IC50 value of A. dracunculus EO against Vero cells was 20 μl/ml. The main detected compound using GC–MS was estragole (methyl chavicol or tarragon) (64.94%). Anti-biofilm, QSI activity, and non-toxicity of A. dracunculus EO reported for the first time in this study propose the use of these plant compounds as alternatives to antibiotics and chemical additives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Herbal essential oils (EO) are also known as volatile oils and most of them well known for their antimicrobial activities. They contain many bioactive compounds with destructive effects on different parts of microbial pathogens as well as food spoiling bacteria. This property makes them attractive to be used for therapeutic and industrial purposes (Tongnuanchan and Benjakul 2014).
Plant essential oils also play a significant role in the control of bacterial biofilm production (Alni et al. 2020; Sharifi et al. 2018b) and cell–cell communication processes, commonly called quorum sensing (QS) (Sharifi et al. 2018a). Biofilms are specialized bacterial communities that create an extracellular matrix to adhere to biotic or abiotic surfaces and play a fundamental role in the survival of bacteria under adverse environmental conditions and pathogenesis of pathogenic bacteria (Aumeeruddy-Elalfi et al. 2018; O'Toole et al. 2000).
QS system, which operates by autoinducer (AI), modifies gene expression in response to population density. This system is associated with bacterial biofilm formation and antibiotic resistance, as well as bacterial proliferation in infectious tissue/catheters, and foods (Cortese et al. 2018). Therefore, QS inhibition (QSI) is considered a good strategy to control bacterial infection and ensure food safety. Besides, according to the relation between QS activity and biofilm formation, most anti-QS agents exhibit anti-biofilm properties (Hammer and Bassler 2003).
Lux and pfs are the two most important genes involved in the QS system in S. Typhimurium and other Gram-negative bacteria. Furthermore, previous studies have tested the expression level of these genes as an indicator of QS activity (Kim et al. 2016). Since the RNAIII transcript produced by the hld gene acts as a functional molecule in the S. aureus QS system (Yarwood and Schlievert 2003), previous studies such as Sharifi et al. (2018a, b) have used the expression level of this gene to study the QS system activity in S. aureus bacterium (Sharifi et al. 2018b).
Artemisia dracunculus L., known as Tarragon belongs to the Asteraceae family is an aromatic plant, spice, and natural food preservative. The A. dracunculus EO and its major components can be used as safe natural compounds in the food industry as a replacement for synthetic preservatives and additives (Obolskiy et al. 2011). Researches have shown that this plant has various pharmacological activity including carminative, digestive, anti-inflammatory, antioxidant, antipyretic, antiseptic, antispasmodic, antiparasitic, antimicrobial, anthelmintic, and fungicidal effects (Mumivand et al. 2017; Obolskiy et al. 2011).
Accordingly, the first aim of the present study was to determine the antimicrobial effect of A. dracunculus EO against S. Typhimurium and S. aureus as major human pathogens and cause of food poisoning with challenging treatment (Bintsis 2017; Taylor and Unakal 2019). Then, anti-biofilm and QSI activity of the plant EO were evaluated against both tested organisms by assessment of QS contributing genes. MTT assay was designed to check the cytotoxicity potential of the EO, and major bioactive components of the EO were determined to find the relation between observed effects and the EO compounds.
Materials and methods
Bacterial strains and essential oil
Staphylococcus aureus ATCC 25923 and Salmonella enterica serovar Typhimurium ATCC 14028 were obtained from Persian Type Culture Collection (PTCC), Tehran, Iran. Iranian A. dracunculus was prepared from the Medicinal Plants and Drugs Research Institute (MPDR) of the Shahid Beheshti University of Iran. The essential oil extraction procedure was performed by a Clevenger apparatus for 4 h according to the standard protocol (El Gendy et al. 2015).
MIC and MBC determination
MIC and MBC for A. dracunculus EO were determined by the microdilution broth method as described previously (Duarte et al. 2015). Briefly, twofold serial dilutions of EO (from 20 to 0.15 μl/ml) prepared in 96-well plates in Müeller–Hinton broth (MHB) in 2% of Dimethyl sulfoxide (DMSO) were used to increase the solubility. Bacterial suspensions were prepared with a turbidity of 0.5 McFarland, diluted in MHB, and added to each well to yield a final concentration of 5 × 105 CFU/ml per well (MHB–DMSO (1%) was used as negative control). The plates were incubated at 37 °C for 48 h under aerobic conditions. After the incubation period, the growth was visually assessed. The MIC was defined as the lowest concentration of EO without visible growth. From the wells without visible growth, 10 μl was plated on the tryptic soy agar (TSA) and after incubation, the number of colonies was counted. The MBC was defined as the lowest compound concentration which caused the death of 99.9% of the bacterial inoculum. The tests were repeated three times independently.
Inhibition of biofilm formation
Inhibition and disruption effects of the A. dracunculus EO on S. Typhimurium and S. aureus biofilms were tested by MtP assay. In detail, for the assembly of the plates, sterile 96-well polystyrene plates were filled with 100 μl of MHB containing MIC/2, MIC/4, and MIC/8 concentrations of the EO (6 wells were considered for each concentration). Next, 100 μl of the bacterial culture with ~ 1.5 × 105 CFU/ml concentration (by three times 1/10 dilution preparation from turbidity equals 0.5 McFarland) was added to each well. The negative control contained medium-DMSO (1%) and bacteria and the positive controls contained medium-DMSO (1%) with bacteria and gentamycin (0.1 mg/ml). After 24 h incubation at 37 °C, the plates were washed with physiological saline three times (200 μl/well) to remove the loosely adhered and air-dried cells. After that, the adhered cells of the biofilm were fixed for 15 min by the addition of 200 μl of methanol. Next, methanol was removed and 200 μl of a 0.1% Safranin dye was added for 15 min. The plates were washed with physiological saline and air-dried again, after which 100 μl of ethanol (96%) was added to each well which was left shaking for 5 min, and optical density values (OD) were measured using a microplate reader (ELx808, BioTek, USA) at 490 nm. Each assay was repeated three times, and the data are presented as the mean ± SD (standard deviation). As a measure of efficacy, the mean ODs of treated wells were compared with those of negative control (without EO) (Sharifi et al. 2018b).
Disruption of the performed biofilm
MtP test was applied to study the effects of the EO on the performed biofilm or biofilm disruption. At first, 24 h biofilms were allowed to establish in 96-well microtiter plates as described above. After the incubation period, the supernatants (loosely adhered and air-dried cells) were discarded and replaced with 100 μl of MHB containing MIC/2, MIC/4, and MIC/8 concentrations of the A. dracunculus EO (six wells were allocated for each concentration). Negative control wells contained medium-DMSO with bacteria. After 24 h incubation at 37 °C, the plates were washed (three times) with physiological saline and air-dried. The adhered cells were fixed with methanol for 15 min, and then, the biofilm cells were treated with 200 μl Safranin. Next, the plates were filled with 100 μl ethanol (96%), the biofilm cells were disrupted for 5 min, and the OD of the plate was recorded at 490 nm. The biofilm disruption potential was measured by a comparison of the mean OD of the treated group versus the mean OD of the negative group (without EO) (Sharifi et al. 2018b).
Scanning electronic microscopy of biofilm cells
Electronic microscopy was also used to observe the effects of the EO on the bacterial biofilm. To do this, the biofilm of the tested bacteria was prepared in six-well plates (each well contained a glass coverslip at this time). The control wells contained medium-DMSO (1%) with bacteria, and the treated biofilm groups contained medium-DMSO (1%) with MIC/2 concentration (1.25 and 0.625 μl/ml for S. Typhimurium and S. aureus, respectively) of the EO and bacteria. After 24 h of incubation at 37 °C, the samples were fixed in 2.5% buffered glutaraldehyde for 3 h followed by dehydration in graded ethanol. The samples were then dried at room temperature and glued onto stubs. At last, the processed samples were sputter-coated with gold and examined in a JEOL JSM-840 SEM operating at an accelerating voltage of 15 kV.
Effect of the EO on the bacterial quorum sensing
The QSI activity of the EO was examined through the analysis of gene expression involved in the QS system of tested bacteria. Using quantitative real-time RT-PCR, the level expression of luxS and pfs for S. Typhimurium and hld for S. aureus in biofilm growth was evaluated and compared with negative control (bacterial culture without EO).
RNA extraction and cDNA synthesis
For total RNA extraction, the tested bacterial strains were grown with and without the EO in six-well polystyrene tissue culture plates containing MHB medium with DMSO and incubated at 37 °C for 24 h. Then the plates were washed with deionized water to remove the unattached cells and then the biofilm cells were scraped and immediately processed for RNA extraction using a commercial RNA extraction and purification kit (SinaClon, Iran) according to the manufacturer’s instructions.
The quality and quantity of the extracted RNA were cheeked by agarose gel electrophoresis and confirmed by measuring the absorbance at 260/280 nm using a Nanodrop spectrophotometer (ND-1000, Thermo Fisher Scientific, USA). The extracted RNAs were stored at − 70 °C until further experiments. After that, the purified RNAs were reverse transcribed to cDNA using a commercial cDNA synthesis kit according to the manufacturer’s instructions (Takara, Japan), and the obtained cDNA was stored at − 70 °C until used as DNA templates in the real-time RT-PCR reactions.
Quantitative real-time RT-PCR
The real-time PCR assay was carried out using a commercial SYBR Green master mix (Amplicon, Denmark) and previously described pairs of primers listed in Table 1. The reactions were conducted in a Corbett Life Science Rotor-Gene 6000 Cycler (Qiagen, Germany), and for both tested bacteria to normalize the expression of target genes, the 16S rRNA housekeeping gene was considered as an internal control. The efficacy of the real-time PCR was calculated by the following formula: E = 10(−1/slop) − 1. After the optimization and qualification of standards curves, the main reaction was performed and negative control was included in each run. In addition, the specificity of the real-time PCR was checked by gel electrophoresis for products as well as the post-PCR melting-curve analysis performed. All the samples were analyzed in triplicate and finally, relative gene expression was calculated by the 2−ΔΔCT method (Livak and Schmittgen 2001).
MTT assay
The toxicity of the tested EO against eukaryotic cells was measured using an MTT test which was based on the cleavage of the MTT, tetrazolium salt, by viable cells as reported previously (Quassinti et al. 2013). Briefly, the Vero cells (prepared from National Cell Bank of Iran (NCBI) in Pasteur Institute of Iran) were cultured in RPMI-1640 medium with 10% fetal bovine serum (FBS) and penicillin–streptomycin at 37 °C, in humidified air containing 5% CO2 (all materials prepared from Gibco, UK). Cells with a density of about 2 × 104–2 × 105 per well were plated in 96-well plates and cultured at 37 °C in the presence of 5% CO2. After 24 h incubation, samples in quadruplicate were exposed to different concentrations of A. dracunculus EO (0.16–40 μl/ml) with DMSO (1%). The plates were incubated for a further 24 h under the mentioned incubation conditions. Then, 10 μl of MTT dye solution (5 mg/ml in phosphate-buffered saline) was added to each well. The plates were incubated for an additional 4 h at 37 °C. After aspirating the medium, the formed formazan crystals were solubilized in 100 μl DMSO. The extent of MTT reduction was measured spectrophotometrically at 490 nm using a microplate reader (ELx808, BioTek, USA). This experiment was conducted in triplicate. The cytotoxicity potential was calculated by comparing the treated cells with the control group (only treated with 1% DMSO). The cytotoxicity effect was expressed as the concentration of compound inhibiting cell growth by 50% (IC50).
GC–MS analysis
The main components of the A. dracunculus EO were determined by gas chromatography–mass spectrometry (GC–MS) analysis according to the standard procedure (Hites 2016).
Statistical analysis
The statistical calculations were performed using GraphPad Prism 4 software (GraphPad Software, San Diego, CA, USA). All the experiments were performed in triplicate and repeated three times, and the data expressed as the mean ± SD. For evaluation of the anti-biofilm potential, a Student’s t test was used. A p value of less than 0.05 was statistically significant.
Results
Antibacterial activity
In vitro bacteriostatic and bactericidal properties of the EO were evaluated against S. Typhimurium and S. aureus bacteria. Results of MIC showed that the A. dracunculus EO could prevent the growth of tested bacteria. The MICs of the EO against planktonic cells of S. Typhimurium and S. aureus bacteria were 2.5 and 1.25 μl/ml, respectively. In addition, the A. dracunculus EO exhibited bactericidal properties, and the MBC values were 5 and 2.5 μl/ml for S. Typhimurium and S. aureus, respectively.
Anti-biofilm activity
MtP test
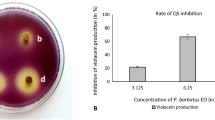
The results of the MtP assay showed that the EO of A. dracunculus significantly prevented the biofilm formation by S. Typhimurium (P < 0.001) in MIC/2 (1.25 μl/ml) and MIC/4 (0.625 μl/ml) concentration. Besides, in the presence of the EO, biofilm formation decreased in a dose-dependent manner. In addition, the A. dracunculus EO caused the significant disruption of the formed biofilm of S. Typhimurium at the MIC/2 and MIC/4 concentrations. In the case of S. aureus, the results indicated that the EO had good potential to inhibit biofilm formation and disrupt preformed biofilms of this pathogen. The EO was able to significantly inhibit the biofilm formation at MIC/2 (0.625 μl/ml) and MIC/4 (0.312 μl/ml) and disrupted the biofilm at MIC/2 concentrations (Fig. 1).
SEM observation
Microscopic picture analyses showed that the tested EO inhibited the biofilm formation for both tested bacteria, and by treatment with MIC/2 (1.25 and 0.625 μl/ml for S. Typhimurium and S. aureus, respectively) of A. dracunculus EO the concentration of attached cells was significantly reduced (Fig. 2).
QS inhibitory (QSI) activity
The effect of sub-MIC concentrations of A. dracunculus EO on the expression of QS-related genes was measured in biofilm form bacteria. Our results showed that the MIC/2 concentration of A. dracunculus EO caused a significant downregulation of three investigated genes (P < 0.05). Treatment of S. Typhimurium biofilm with MIC/2 concentration (1.25 μl/ml) of the EO resulted in a − 4.18- and − 3.12-fold decrease in the expression of luxS and pfs, respectively. Similarly, after treatment of the S. aureus biofilm with MIC/2 concentration of EO, the expression of the hld gene was significantly downregulated (− 5.35-fold) (P < 0.05) (Fig. 3).
Cytotoxicity assay
The results of the cytotoxicity assay showed no cytotoxic effect at MIC and MBC concentrations. Cell viability decreasing was a dose-dependent manner starting from 0.16 to 40 μl/ml. In addition, the IC50 value was 20 μl/ml (Fig. 4).
Chemical composition of the essential oil
In GC–MS analysis, 15 substances were detected. According to our results, estragole (64.94%), beta-cis-Ocimene (10.59%), trans-beta-Ocimene (10.21%), cinene (5.39%), alpha-pinene (2.74%), and methyl eugenol (2.42%) were the most detected compounds (Table 2).
Discussion
In our study, the antibacterial, anti-biofilm, and QSI properties of A. dracunculus EO were evaluated against two pathogens S. Typhimurium and S. aureus. Besides, the cytotoxicity and composition of A. dracunculus EO were determined. The results of MIC and MBC against S. Typhimurium were 2.5 and 5 μl/ml, respectively. Besides, the tested EO was effective on S. aureus and the MIC and MBC values for this bacterium were 1.25 and 2.5 μl/ml, respectively. In accordance with our results, in Raeisi et al. (2012) study, the antibacterial activity of A. dracunculus EO was evaluated against E. coli in Iranian white cheese and on culture media, and the possibility of using A. dracunculus EO as a natural preservative was proposed.
As complementary for the previous studies in the second part of the present study, the anti-biofilm properties of the A. dracunculus EO at sub-MIC concentrations were studied against S. Typhimurium and S. aureus bacteria. As presented in Figs. 1 and 2, the EO inhibited the formation of biofilms and disrupted preformed biofilms of both tested bacteria. Based on previous reports the anti-biofilm agent may produce this effect in different ways including inhibition of bacterial growth, proliferation, and attachment (Roy et al. 2018), QSI activity (Koh et al. 2013), and the use of matrix-degrading materials (Kaplan 2010). However, the studied plant EO may have affected several bacterial pathways or components (Swamy et al. 2016).
The present study used an SEM to investigate the effect of plant EO on the attachment of bacteria to the surface and also the attachment of bacteria to each other. Using SEM images, we demonstrated that MIC/2 concentration of A. dracunculus EO could affect the S. Typhimurium and S. aureus biofilms and the formation of micro-colonies. As presented in Fig. 3, the bacterial cells grown in an EO-free medium were well connected to each other and have formed micro-colonies, but these structures were rarely seen in EO-treated cells. These observed anti-biofilm activates may be related to the anti-adhesive properties of the EO compounds (Nostro et al. 2007), inhibition of biofilm structural compounds such as exopolysaccharide (Swamy et al. 2016), or altering the expression of biofilm-associated genes (Kim et al. 2016). Considering the role of biofilm in infection progress and food spoilage, inhibition of biofilm formation by natural antimicrobial compounds such as EOs is expected to be an alternative to antibiotics and traditional sanitizers (Orhan-Yanıkan et al. 2019).
In another part of the study, the QSI properties of the A. dracunculus EO were investigated against S. Typhimurium and S. aureus. To do this, the expression QS-related genes (luxS, pfs for S. Typhimurium, and hld S. aureus) was evaluated in treatment with MIC/2 of the EO versus untreated cells. The results showed significant downregulation of luxS, pfs, and hld (− 4.18-, − 3.12- and − 5.35-fold, respectively) following treatment with MIC/2 concentration of EO. More importantly, it is shown that QSI agents do not impose any selection pressure; therefore, resistance to these compounds does not occur. According to this, QSI has been a novel strategy to control various bacterial infections (Koh et al. 2013). In this context, the QSI activity of some other plant species such as orange, garlic, tea tree, ginger, rosemary, and turmeric has been shown (Koh et al. 2013). The plant-derived compounds affect the bacterial QS system in three different ways, first block the signaling molecules, second degrading the signaling molecules, and third destroy the signal receptor (Koh et al. 2013).
Based on the important roles of QS in several virulence factors including bacterial toxin production and secretion, antibiotic resistance, and biofilm formation (Rutherford and Bassler 2012), A. dracunculus herbal EO can be used for modulation of these factors.
To evaluate the possibility of A. dracunculus EO compounds for human consumption, the cytotoxicity activity of the EO was evaluated against Vero cells. Our results showed the non-toxicity of the EO at sub-MIC and MBC concentrations. However, every component of the plant EO is suggested to be tested rigorously for toxicity before its use as a food additive.
The results of GC–MS showed that 64.94% of A. dracunculus EO was composed of estragole (methyl chavicol). It was reported that this compound possesses toxicity or mutagenic activity in rats and probably in humans (Paini et al. 2010; Phillips 1994). But according to the evidence, it is not the estragon but its metabolites activation in a dose-dependent reaction that with a very low probability can damage DNA. Therefore, the direct carcinogenic effect of the estragon was rejected (Paini et al. 2010; Rietjens et al. 2010). The results of the cytotoxicity assay on Vero cell lines showed no cytotoxic effect at MIC and MBC concentrations of the Tarragon EO. This results in parallel with reports of non-toxicity or no mutagenic activities on human make Tarragon a potential herbal drug or food additive candidate to be used against pathogenic bacteria.
According to the literature, there are variations in the concentration of chemical constituents of A. dracunculus EO based on harvest time, geographical situation, ground conditions, and genetic factors (Chaleshtori et al. 2013; Damjanović-Vratnica et al. 2011). This can be observed by comparison of the main compounds of A. dracunculus EO in this study with the results of studies from France and Georgia in which in accordance with our results estragole was the main compound of A. dracunculus EO and the reverse results from Russia and Canada which showed the low-percentage estragon in the Tarragon EO (Obolskiy et al. 2011).
Conclusion
Based on the results that are reported for the first time in this study, anti-biofilm and anti-QSI activities of the A. dracunculus show the probable possibility of plant compounds penetration to the bacterial biofilm. This property alongside the non-toxicity of the EO makes the plant compounds potential candidates for drug or food additive development against strong biofilm-producing bacteria.
References
Alni RH, Ghorban K, Dadmanesh M (2020) Combined effects of Allium sativum and Cuminum cyminum essential oils on planktonic and biofilm forms of Salmonella typhimurium isolates. 3 Biotech 10(7):1–10
Aumeeruddy-Elalfi Z, Ismaël IS, Hosenally M, Zengin G, Mahomoodally MF (2018) Essential oils from tropical medicinal herbs and food plants inhibit biofilm formation in vitro and are non-cytotoxic to human cells. 3 Biotech 8(9):395
Bintsis T (2017) Foodborne pathogens. AIMS Microbiol 3(3):529–563
Brunelle BW, Bearson BL, Bearson S (2015) Chloramphenicol and tetracycline decrease motility and increase invasion and attachment gene expression in specific isolates of multidrug-resistant Salmonella enterica serovar Typhimurium. Front Microb 5:801
Chaleshtori RS, Rokni N, Razavilar V, Kopaei MR (2013) The evaluation of the antibacterial and antioxidant activity of Tarragon (Artemisia dracunculus L) essential oil and its chemical composition. Jundishapur J Microbiol 6:e7877
Cortese YJ, Wagner VE, Tierney M, Devine D, Fogarty A (2018) Review of catheter-associated urinary tract infections and in vitro urinary tract models. J Healthc Eng 3:1–16
Damjanović-Vratnica B, Perović A, Šuković D, Perović S (2011) Effect of vegetation cycle on chemical content and antibacterial activity of Satureja montana L. Arch Biol Sci 63:1173–1179
Duarte A, Alves AC, Ferreira S, Silva F, Domingues FC (2015) Resveratrol inclusion complexes: antibacterial and anti-biofilm activity against Campylobacter spp. and Arcobacter butzleri. Food Res Int 77:244–250
El Gendy AN, Leonardi M, Mugnaini L, Bertelloni F, Ebani VV, Nardoni S, Mancianti F, Hendawy S, Omer E, Pistelli L (2015) Chemical composition and antimicrobial activity of essential oil of wild and cultivated Origanum syriacum plants grown in Sinai, Egypt. Ind Crops Prod 67:201–207
Hammer BK, Bassler BL (2003) Quorum sensing controls biofilm formation in Vibrio cholerae. Mol Microbiol 50:101–104
Hites RA (2016) Development of gas chromatographic mass spectrometry. Anal Chem 88(14):6955–6961
Kaplan JA (2010) Biofilm dispersal: mechanisms, clinical implications, and potential therapeutic uses. J Dent Res 89:205–218
Kim Y-G, Lee J-H, Gwon G, Kim S-I, Park JG, Lee J (2016) Essential oils and eugenols inhibit biofilm formation and the virulence of Escherichia coli O157: H7. Sci Rep 6:36377
Koh C-L, Sam C-K, Yin W-F, Tan L, Krishnan T, Chong Y, Chan K-G (2013) Plant-derived natural products as sources of anti-quorum sensing compounds. Sensors 13:6217–6228
Kolar SL et al (2013) Extracellular proteases are key mediators of Staphylococcus aureus virulence via the global modulation of virulence-determinant stability. Microbiologyopen 2:18–34
Koprivnjak T, Mlakar V, Swanson L, Fournier B, Peschel A, Weiss JP (2006) Cation-induced transcriptional regulation of the dlt operon of Staphylococcus aureus. J Bacteriol 188:3622–3630
Li X-H, Lee J-H (2017) Antibiofilm agents: a new perspective for antimicrobial strategy. J Microbiol 55:753–766
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25:402–408
Mumivand H, Babalar M, Tabrizi L, Craker LE, Shokrpour M, Hadian J (2017) Antioxidant properties and principal phenolic phytochemicals of Iranian tarragon (Artemisia dracunculus L.) accessions. Hortic Environ Biotechnol 58:414–422
Nostro A et al (2007) Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J Med Microbiol 56:519–523
Obolskiy D, Pischel I, Feistel B, Glotov N, Heinrich M (2011) Artemisia dracunculus L. (tarragon): a critical review of its traditional use, chemical composition, pharmacology, and safety. J Agric Food Chem 59:11367–11384
Orhan-Yanıkan E, da Silva-Janeiro S, Ruiz-Rico M, Jiménez-Belenguer AI, Ayhan K, Barat JM (2019) Essential oils compounds as antimicrobial and antibiofilm agents against strains present in the meat industry. Food Control 101:29–38
O’Toole G, Kaplan HB, Kolter R (2000) Biofilm formation as microbial development. Annu Rev Microbiol 54:49–79
Paini A, Punt A, Viton F, Scholz G, Delatour T, Marin-Kuan M, Schilter B, van Bladeren PJ, Rietjens IM (2010) A physiologically based biodynamic (PBBD) model for estragole DNA binding in rat liver based on in vitro kinetic data and estragole DNA adduct formation in primary hepatocytes. Toxicol Appl Pharm 245:57–66
Phillips D (1994) DNA adducts derived from safrole, estragole and related compounds, and from benzene and its metabolites. IARC Sci Publisher, Lyon, p 131
Quassinti L et al (2013) Antioxidant and antiproliferative activity of Hypericum hircinum L. subsp. majus (Aiton) N. Robson essential oil. Nat Prod Res 27:862–868
Raeisi M, Tajik H, Razavi RS, Maham M, Moradi M, Hajimohammadi B, Naghili H, Hashemi M, Mehdizadeh T (2012) Essential oil of tarragon (Artemisia dracunculus) antibacterial activity on Staphylococcus aureus and Escherichia coli in culture media and Iranian white cheese. Iran J Microbiol 4:30
Rietjens IM, Punt A, Schilter B, Scholz G, Delatour T, van Bladeren PJ (2010) In silico methods for physiologically based biokinetic models describing bioactivation and detoxification of coumarin and estragole: implications for risk assessment. Mol Nutr Food Res 54:195–207
Roy R, Tiwari M, Donelli G, Tiwari V (2018) Strategies for combating bacterial biofilms: a focus on anti-biofilm agents and their mechanisms of action. Virulence 9:522–554
Rutherford ST, Bassler BL (2012) Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb Perspect 2:a012427
Sharifi A, Ahmadi A, Mohammadzadeh A (2018a) Streptococcus pneumoniae quorum sensing and biofilm formation are affected by Thymus daenensis, Satureja hortensis, and Origanum vulgare essential oils. Acta Microbiol Immunol Hung 65:345–359
Sharifi A, Mohammadzadeh A, Zahraei Salehi T, Mahmoodi P (2018b) Antibacterial, antibiofilm and antiquorum sensing effects of Thymus daenensis and Satureja hortensis essential oils against Staphylococcus aureus isolates. J Appl Microbiol 124:379–388
Swamy MK, Akhtar MS, Sinniah UR (2016) Antimicrobial properties of plant essential oils against human pathogens and their mode of action: an updated review. Evid Based Complement Altern Med 2016:1–21
Taylor TA, Unakal CG (2019) Staphylococcus aureus. StatPearls Publishing, Treasure Island
Tongnuanchan P, Benjakul S (2014) Essential oils: extraction, bioactivities, and their uses for food preservation. J Food Sci 79:1231–1249
Yarwood JM, Schlievert PM (2003) Quorum sensing in Staphylococcus infections. J Clin Invest 112:1620–1625
Funding
None.
Author information
Authors and Affiliations
Contributions
Conceptualization: LB, Methodology: LB, MG, SHM, and LK, Investigation: LB and MG. Writing original draft preparation: SHM, Writing—review and editing: LB. Supervision: LB. Project administration: LB, MG, SHM, and LK.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohammadi Pelarti, S., Karimi Zarehshuran, L., Babaeekhou, L. et al. Antibacterial, anti-biofilm and anti-quorum sensing activities of Artemisia dracunculus essential oil (EO): a study against Salmonella enterica serovar Typhimurium and Staphylococcus aureus. Arch Microbiol 203, 1529–1537 (2021). https://doi.org/10.1007/s00203-020-02138-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-020-02138-w