Abstract
The impact of agricultural land-use on soil microbial community composition and enzyme activity has not been extensively investigated in Ultisols. We investigated soil health parameters by analyzing phospholipid fatty acids (PLFAs), extracellular enzyme activity, C and N stocks, and soil structure. Four land uses were established in a tropical climate region of Brazil: native Cerrado (savanna), monoculture pasture [Urochloa brizantha (Hochst. Ex A. Rich.) R. Webster 'Marandu'], an integrated crop-livestock system (ICLS), and maize (Zea mays)-fallow in a no-tillage system. Soil microbial biomass was 40% higher in the native Cerrado than in the monoculture pasture, ICLS, and no-tillage maize. Soil organic carbon was positively correlated with microbial community composition (MB; gram–; AC; AMF; Fungi; F: B ratio) and enzyme activity (bG, AP, NAG). Large macroaggregates were positively correlated with bG, AP, and AMF. In summary, the native Cerrado had a higher level of carbon at the soil surface and greater soil structure with increased microbial biomass, gram+ bacteria, AMF, fungi, and F:B ratio in a tropical region of Brazil. However, bG and AP enzyme activities were lower in the ICLS and no-till maize at the soil surface (0–5 cm) compared to the native Cerrado. The conversion of native Cerrado to agricultural systems shifted the soil microbial community composition, enzyme activity, C and N, and soil structure of this sandy soil of the Brazilian Cerrado.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Land-use change in the tropical regions has led to shifts in soil microbial community composition and enzyme activity affecting soil carbon (C) and the potential to mitigate atmospheric CO2 (Scott et al. 2017; Li et al. 2018; Yang et al. 2019). Biological, chemical, and physical interactions resulting in C sequestration need to be evaluated (White and Rice 2009), and strategies that alter or enhance C sequestration must be identified (Sanz-Cobena et al. 2017; Leifeld and Menichetti 2018). Agricultural systems can affect the exchange of C between the soil and the atmosphere and represent an atmospheric C source or sink, depending on soil management (Powlson et al. 2016).
The dynamics and composition of microbial communities alter C sequestration and soil aggregation as arbuscular mycorrhizal fungi (AMF) and saprophytic fungi are positively associated with aggregation (Six et al. 2006). The soil microbial community and biomass are affected by land-use and soil depth (Lauber et al. 2008; Rousk et al. 2010), with intensification negatively affecting soil microbial community diversity (Culman et al. 2010). Crop diversification during changes in agricultural land use can affect microbial community composition and biomass mainly by changes in organic C and N inputs into the soil (Hansel et al. 2008; Garcia-Franco et al. 2015). Zhang et al. (2016) observed that all phospholipid fatty acids (PLFAs) decreased with soil depth for regardless of land use. Assessing the effects of land use on the soil microbial community composition can improve the understanding of human effects in the global C cycle (Zhang et al. 2016).
The activities of carbon-cycling enzymes, such as, β-glucosidase (bG), α-galactosidase, and β-glucosaminidase participate in the degradation of plant components (cellobiose, meliobiose, and chitin) and can reveal cropping system-induced differences in residue degradation and nutrient incorporation into the soil (Acosta-Martínez et al. 2007; Sotomayor-Ramírez et al. 2009). Soil microbial diversity and enzyme activity in pastures are related to the contents of soil C, nitrogen (N), phosphorus (P), and other nutrients (Lisboa et al. 2014; Waring et al. 2014). Pastures tend to have lower levels of SOC, microbial biomass, enzyme activity, and mycorrhizal diversity than native forests (Öpik et al. 2006; Eaton and Chassot 2012).
The Cerrado biome (the Brazilian savanna) covers 204 million hectares of Brazilian territory, 24% of the land area of Brazil (Araujo et al. 2012; Mendes et al. 2012; Anache et al. 2018). As one of the world's largest agricultural frontiers, the Brazilian Cerrado has become the most important area for agricultural production in the country. The edaphoclimatic conditions of the Cerrado have some parallels with the African savannas, and soil health studies are limited for both. The soil chemical properties of the Cerrado are characterized by high exchangeable aluminum (Al) concentration, low pH, and low available P concentration (Souza et al. 2016).
The integrated systems of grasses and crops, the purpose of which is to increase soil C and thus reestablish soil fertility, is increasingly implemented in the Brazilian Cerrado (Tonucci et al. 2017). These cropping systems are mainly used in sandy soils and are affected by dry conditions. There is little information on the biologically sensitive soil quality parameters [i.e., bacteria, fungi, AMF, actinomycetes (AC), bG, acid phosphatase (AP), and N-acetyl glucosidase (NAG)] (Acosta-Martínez et al. 2011). In this study, we hypothesized that land-use change and soil depth affect the soil microbial community composition and enzyme activity due to changes in C and N availability and soil physicochemical properties. Therefore, the present study measured selected soil health parameters including soil microbial biomass, microbial community composition, enzyme activity, C and N stocks, and soil aggregation in a monoculture pasture, ICLS, and no-tillage maize at depths of 0–5, 5–10, and 10–20 cm. This study also compared the data generated from the agriculturally managed Cerrado plots with those of long-term undisturbed soils in native Cerrado that have been under forest for 50 years.
Materials and methods
Site description
The study was located Votuporanga County, São Paulo State, Brazil (Figs. 1, 2), 20°28′ S and 50°04′ W, at an altitude of 450 m. The 15–ha area had been used as perennial monoculture pasture [Urochloa brizantha (Hochst. Ex A. Rich.) R. Webster ‘Marandu’] for ten years before the commencement of the experiment. According to Köppen, the climate was Cwa with dry winters and hot, rainy summers (Alvares et al. 2013). The average annual temperature was 24 °C, with a monthly average maximum of 30 °C and an average minimum of 18 °C. The annual average rainfall was 1300 mm. The mean temperatures and rainfall for the 8 years before sampling are provided in Fig. 3. The soil was a sandy Arenic Hapludult (Soil Survey Staff 2014) containing 81, 104, and 815 g kg−1 of clay, silt, and sand, respectively. Selected soil chemical (van Raij et al. 2001) and physical (Embrapa 1997) characteristics were determined (0–20 cm) at the beginning of the experiment in 2009 (Table 1).
Experimental design and treatments
The experiment was a completely randomized in a split-plot design, with four replications. The treatments included four land-use types [monoculture pasture, ICLS of maize integrated with Urochloa brizantha ‘Marandu’, maize grown under no-tillage, and native (Cerrado)] and three soil depths (0–5, 5–10, and 10–20 cm). Native (Cerrado) with the same soil type located 100 m from other land uses was used as a reference.
Land uses
The experiment was initiated in the winter of 2009 in a degraded pasture of approximately 15 ha that had been planted for the previous 10 years. Initially, the land use treatments were plowed and disked. Limestone was applied to all land uses except the native Cerrado, at 2000 kg ha–1 (90% of the effective calcium carbonate equivalent) to raise the soil base saturation to 60%.
The monoculture pasture was established in 2009 with palisade grass [Urochloa brizantha (Hochst. Ex A. Rich.) R. Webster 'Marandu']. Nitrogen fertilization was applied annually on the pasture at a rate of 200 kg N ha–1 as urea at the beginning of the period with the highest precipitation (October). Beef cattle (mestizos) were introduced. The animals were 28-months old, weaned, and maintained in a continuous grazing system with a capacity of 2 animal units (AU) ha–1 until slaughter (Fig. 2a).
In the ICLS established in 2009, maize was sown at a depth of 8 cm below the soil surface using a no-till drill at a row spacing of 0.80 m, and a density of 60,000 seeds per ha. Fertilization in the sowing furrows consisted of 116 kg N ha–1, 40 kg P ha–1, and 70 kg K ha–1. Simultaneous with the sowing of maize, palisade grass was sown at a density of 7 kg ha–1 (pure live seed = 76%) at a depth of 8 cm below soil surface using a no-till drill at a row spacing of 0.34 m. After maize harvest, the palisade grass was left to grow and become pasture. Beef cattle were introduced 30 days after the maize harvest and maintained until September of each year; in October, the area was desiccated, and in November, the maize was sown again. The animals were 28-months old and weaned, with a capacity of 2 AU ha–1 (Fig. 2b).
The no-till system included the following crop sequence: Pearl millet (Pennisetum glaucum) was sown in September 2009, and in October 2009, was broadcast 20 kg P ha–1 using reactive rock phosphate. Sunn hemp (Crotalaria juncea) was grown in winter-spring of 2010 with a row spacing was 0.45 m with approximately 18 seeds m−1, and no fertilizer was applied. Maize was grown in association with palisade grass in the 2010/11 season. Maize (hybrid Pioneer P30F35) was sown (2011/2012 until 2016–2017) in rows 0.90 m apart at a depth of 5 cm, using 60,000 seeds per hectare. Fertilization in the sowing furrows consisted of 116 kg N ha–1, 40 kg P ha–1, and 70 kg K ha–1. After harvest, the area remained under fallow until the next growing season (Fig. 2c).
The native Cerrado (perennial for > 50 years) belongs to the intermediate Brazilian Cerrado; dominant tree species include Dalbergia spp. and Machaerium scleroxylon and Vateria spp., as described by Tonucci et al. (2017) (Fig. 2d).
Soil sampling
For each land use, four areas of 500 m2 were delineated. Soil samples were collected in February 2017 (rainy season) using a 1.4-m high steel cylinder with an internal diameter of 60 mm; each composite sample was represented by four samples within the four management systems for the 0–5, 5–10, and 10–20 cm soil layers. Samples were refrigerated at 4 °C and transported to the Soil Microbial Agroecology Lab in Manhattan, Kansas, USA. Each soil core was partitioned into three depth intervals, homogenized, and subsampled for each analysis. Subsamples for physical and chemical properties were air-dried, ground, and sieved through 2-mm mesh.
Soil SOC and TN
To determine SOC and TN, four soil cores per plot were taken at depths of 0–5, 5–10, and 10–15 cm to account for within-plot variability in February 2017. Samples were ground using a mortar and pestle and passed through a 250 μm sieve. All plant material was removed during the process. Samples were analyzed for SOC and TN by dry combustion using a C/N Elemental Analyzer gas chromatograph with a thermal conductivity detector (Flash EA 1112 Series Thermo Finnigan Italia S.p.A., MI, Italy).
Water-stable aggregates
Soil samples were taken at 0–5, 5–10, and 10–15 cm in February 2017 using a shovel to maintain soil structure. Water-stable aggregate (WSA) size distribution was determined using the wet-sieve method described by (Yoder 1936) with modifications by Mikha and Rice (2004). The soil was air-dried, carefully separated along natural breaks into large aggregates, and sieved through 4.0–8.0, 2.0–4.0, 0.5–2.0, and 0.5–0.25 mm diameter sieves. Briefly, 50 g air-dried soil was placed on top of a 4.0- to 8.0-mm mesh sieve. Sieves were then submersed in water for 10 min (slaking phase) and subjected to 10 min of 4 cm length oscillations at a frequency of 0.5 Hz. The soil remaining on the sieves at the conclusion of the oscillation cycle was collected, allowed to settle, and dried at 60 °C for 72 h. The soil that passed through both sieves was filtered through the 0.5–2.0, and 0.5–0.25 mm sieves. The dried soil was weighed and used to estimate % aggregate size fraction within the soil. A subsample was used to quantify the sand content of each fraction (Mikha and Rice 2004), and the aggregate weights were recorded to estimate the sand-free correction. Macroporosity, microporosity, total porosity, and soil bulk density were evaluated as described in Danielson and Sutherland (1986). Aggregate content greater than 2 mm and the mean weight diameter (MWD) were determined according to Kemper and Chepil (1965).
Enzyme activity
Soil enzyme activities of hydrolases were analyzed by a fluorometric method using the fluorometric substrate 4-methylumbelliferone (MUB) (Zeglin et al. 2013). Bravely, 1 g of soil was homogenized in 100 mL of 50 mM pH 5 acetate buffer. The soil slurry was added to a 96-well microplate with 200 μM fluorometric substrate analog specific to each enzyme. Six replicates were analyzed for each soil sample. Additionally, a buffer blank, soil blank, negative control, 4-methylumbelliferone reference standard, and quench control were measured for each sample to adjust the enzyme activity value. The specific incubation time was measured for each enzyme. The stop time was recorded as the time at which 0.5 N NaOH stop solution was added in hydrolase activity assays. Fluorescence/absorbance was determined by a Multi-Mode Microplate Reader (FilterMax F5, Molecular Devices, USA) at 365/450 nm excitation/emission for fluorescence for hydrolase activity. Potential enzyme activities were reported as nanomoles activity per gram of dry soil per hour.
Soil microbial community composition
Phospholipid fatty acid analysis (PLFA) was performed from the freeze-dried soil samples. Total lipids were extracted using a modification of the Bligh and Dyer (1959) extraction (White and Rice 2009). The PLFA were separated from the total lipid extract using silicic acid chromatography, the fatty acids were cleaved from the glycerol backbone using KOH saponification, and the harvested fatty acids were methylated to form fatty acid methyl esters (FAME). The resulting FAME was analyzed using a Thermo Scientific Trace GC-ISQ mass spectrometer (HP 6890, Agilent Incorporated, Palo Alto, CA, USA) equipped with a DB5-MS column (30 m × 250 μm i.d. × 0.25 μm film thickness; Agilent Technologies, Santa Clara, California, USA) was used with He as the carrier gas at 1 mL min–1. The FAME peaks were identified by comparison with the bacterial acid methyl esters mix (BAME; Matreya 1114; Matreya LLC, Pleasant Gap, Pennsylvania, USA). The PLFA were grouped into fungi (18:2ω6,9c and 18:1ω9c), AMF (16:1ω5), AC (10Me18:0 and 10Me17:0), gram+ bacteria (i15:0, a15:0, 10Me16:0, i17:0, and a17:0), and gram– bacteria (18:1 × 7c and cyclic 19:0) (Sarto et al. 2020b; Pires et al. 2020; Fabrizzi et al. 2009; McKinley et al. 2005). The fungal:bacterial ratios were calculated by dividing the sum of the fungal and AMF biomarkers by the sum of the gram-positive bacterial, gram-negative bacterial, and AC biomarkers. Total microbial biomass was estimated by PLFA as the sum of all biomarkers.
Statistical analysis
The results were tested for the assumptions of analysis of variance (normality and independence of the residues, homogeneity of variance). When these requirements were met, the data were submitted to ANOVA using the statistical software Sisvar® (Ferreira 2011).
The statistical model applied was Yijk = µ + Li + eaij + Dk + LDik + eijk, where Yijk = value observed in land uses i, at depth k, µ = overall mean; Li = effect of land uses i; eaij = random error of plot (error A); Pk = effect of depth k; LDik = effect of land uses and depth interaction; ebijk = random error of split-plot.
When differences were found, the means were compared using Fisher’s t test (LSD, P < 0.05). Simple correlation (Pearson) analysis was performed to determine the degree of association between variables (P < 0.05).
Results
SOC and NT
Soil OC was affected by land use and soil depth (P < 0.05). Soil OC in the native Cerrado (0.99 g kg–1) was higher than that in the ICLS (0.78 g kg–1) and no-tillage maize (0.77 g kg–1) and similar to that in the pasture (0.82 g kg–1) (Fig. 4a). Soil OC was higher (0.98 g kg–1) at the 0–5 cm depth than at the 5–10 cm (0.78 g kg–1) and 10–20 cm (0.75 g kg–1) depths (Fig. 4b). Total N was not affected by land use (Fig. 4c). Positive correlation was observed between SOC (P < 0.05) and bG (r = 0.57), AP (r = 0.53), NAG (r = 0.60), bG:AP (r = 0.48), MB (r = 0.78), gram+ (r = 0.75), gram– (r = 0.56), AC (r = 0.73), AMF (r = 0.76), fungi (r = 0.65), and F:B (r = 0.63) (Table 4).
Higher soil TN content (0.082 g kg–1) was observed at the 0–5 cm depth compared with the 5–10 cm (0.058 g kg–1) and 10–20 cm (0.054 g kg–1) depths (Fig. 4d). Total N was negative correlated (P < 0.05) with bG (r = − 0.53), AP (r = − 0.49), NAG (r = − 0.37), MB (r = − 0.54), gram+ (r = − 0.50), gram– (r = − 0.43), AC (r = − 0.60), AMF (r = − 0.63), fungi (r = − 0.38), and F:B (r = − 0.57) (Table 4).
Soil structure
Soil macroporosity, microporosity, and total porosity were higher and bulk density was lower in the native Cerrado at the 0–5 cm depth. At 5–10 and 10–20 cm, the Cerrado, pasture, ICLS, and no-tillage maize were similar in macroporosity, microporosity, total porosity, and soil bulk density (Table 2). The distribution of WSA and MWD were higher in the Cerrado than in the pasture, ICLS, and no-tillage maize at the 0–5 cm depth (Table 3). Compared with the Cerrado and pasture, the no-tillage maize had lower WSA at 5–10 cm and the no-tillage maize and ICLS had lower WSA and MWD at 10–20 cm. The proportion of large macroaggregates (4.0–8.0 mm) was 85.7% in the Cerrado, 65.4% in the pasture, 75.9% in the ICLS, and 62.9% in the no-tillage maize. The proportion of large macroaggregates (4.0–8.0 mm) was positive correlated (P < 0.05) with bG (r = 0.51), AP (r = 0.48), and AMF (r = 0.40) (Table 4).
Extracellular enzyme activity
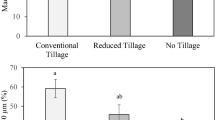
The enzyme activities of bG, AP, and NAG and the bG:NAG ratio were significantly affected (P < 0.05) by the interaction of land use and soil depth. Activity of bG was higher at the 0–5 cm depth in the ICLS (56.4 nmol h–1 g–1 soil) and no-tillage maize (58.7 nmol h–1 g–1 soil) (Fig. 5a).
bG: β-glucosidase (a); AP: acid phosphatase (b); NAG: N-acetyl glucosidase (c); bG:NAG: C-requiring to N-requiring enzyme activities (d). Values followed by a different lowercase letter are significant difference between land-use under same depth. Values followed by a different capital letter are significant difference among depth under same land-use (t test, P < 0.05)
In the 0–5 cm layer, AP activity was higher in the no-tillage maize system (374 nmol h–1 g–1 soil) than in the Cerrado (274 nmol h–1 g–1 soil) and pasture (200 nmol h–1 g–1 soil). In the pasture, soil AP activity was higher in the 10–20 cm layer (238 nmol h–1 g–1 soil) than the 5–10 cm layer (160 nmol h–1 g–1 soil). On the other hand, Cerrado and no-tillage maize AP activity was highest in the 0–5 cm layer (Fig. 5b).
In the Cerrado, NAG activity was higher in the 0–5 cm (81.7 nmol h–1 g–1 soil) and 5–10 cm (86.3 nmol h–1 g–1 soil) layers than in the 10–20 cm layer (58.5 nmol h–1 g–1 soil). In the pasture, ICLS and no-tillage maize system, NAG activity was higher in the 0–5 cm layer than in the 5–10 cm and 10–20 cm layers (Fig. 5c).
The bG:NAG ratio was lowest in the Cerrado at depths of 5–10 cm (0.93) and 10–20 cm (0.63) compared to all other layers and land uses. However, in the three layers of the Cerrado system, the ratios were less than one, indicating an N limited environment. In the ICLS, the bG:NAG enzyme ratio was not affected by depth and was higher than one, indicating C limitation in the soil (Fig. 5d).
The bG:AP ratio was less than one for all land uses and soil depths, indicating a P-limited environment. In addition, the bG:AP ratio was lower in the Cerrado (0.59) and in the pasture (0.57) than in the ICLS (0.70) and no-tillage maize (0.69) (Fig. 6a). With respect to depth, the bG:AP enzyme ratio was significantly higher in the 0–5 cm layer than in the other layers (Fig. 6b).
Microbial biomass
Microbial biomass was significantly affected by the interaction of land use and soil depth (P < 0.05). Microbial biomass was similar at 0–5 cm in the Cerrado (32.3 nmol PLFA g–1 soil) and in the pasture (28.6 nmol PLFA g–1 soil). Microbial biomass was higher in the Cerrado at 5–10 cm and 10–20 cm compared to all other layers and land uses (Fig. 7a).
MB: microbial biomass (a); Gram–: gram–negative bacteria (b); AC: actinomycete (c). Values followed by a different lowercase letter are significant difference between land-use under same depth. Values followed by a different capital letter are significant difference among depth under same land-use (t test, P < 0.05)
The abundance of gram+ bacteria was higher in the Cerrado (66%) than in the other land-use types (Fig. 6c). The proportion of gram+ bacteria was higher at the 0–5 cm depth (50%) than at the 5–10 and 10–20 cm depths (Fig. 6d). The biomass of gram- bacteria and AC were significantly influenced (P < 0.05) by the interaction of land use and soil depth. In the ICLS, a greater concentration of gram- bacteria (5.1 nmol PLFA g–1 soil) occurred in the 0–5 cm layer. Gram- bacteria biomass was not affected by soil depth (Fig. 7b). The abundance of AC was higher in the Cerrado than in the no-tillage maize, regardless of depth. The abundance of AC was higher at the 0–5 cm depth than at the 10–20 cm depths for all land uses (Fig. 7c).
The abundance of AMF and fungi were higher (71%) in the Cerrado than in the other land-uses (Fig. 8a, c), and the biomasses of AMF and fungi were highest at the 0–5 cm depth (Fig. 8b, d). The F:B ratio in the Cerrado (0.097) was slightly higher than that in the ICLS (0.081 nmol PLFA g–1 soil) and no-tillage maize (0.080) and similar to that in the pasture (0.095) (Fig. 8e). At 0–5 cm soil depth, the F:B ratio was greater (0.106) than at the 5–10 cm (0.082) and 10–20 cm (0.076) soil depth (Fig. 8f).
Discussion
SOC and TN
Soil OC was highest in the Cerrado, as this area has never been tilled and features trees and shrubs with high rooting capacity (depth and mass). The accumulation of SOC was highest in the soil surface layer. Increased SOC in the soil surface layer has been reported as an important factor for soil quality biological activity (Ferreira et al. 2016), as verified in the present study with a positive correlation of the soil microbial community composition and enzyme activity and SOC (Table 4). The similar values of SOC at all soil layers in the Cerrado (Fig. 4b) can be explained in part by the deeper root systems of the plants (Jobbágy and Jackson 2000; Assmann et al. 2014).
Our results confirmed higher SOC in the pasture after 8 years as well as in the Cerrado at the 0–5 cm soil depth. The greater SOC observed under pasture in this study is attributable to a number of factors. Compared with forest biomass, grass biomass contains more labile compounds, which are associated with higher decomposition rates (Eclesia et al. 2012). The resulting high SOC and high root densities in the upper soil layers favor aggregate formation and soil C stabilization (Six et al. 2002). The palisade grass used in the study is known to have a large root system, which contributes to C accrual in soils. Fisher et al. (1994) reported that C sequestration by Urochloa species in South America ranges from 3.0 to 14.0 Mg ha−1 year−1.
Negative correlations of the TN and microbial community composition and enzyme activity were found in this study (Table 4). Adding nitrogen usually reduces soil microbial biomass in field and laboratory studies (Treseder 2008; Liu and Greaver 2010; Cusack et al. 2011). A recent meta-analysis showed that N additions decreased the F:B ratio in tropical/subtropical forests (Zhou et al. 2017). This may be directly related to increased N availability in the soil, possibly due to intense nitrogen applications. In addition, N deposition is increases rapidly in tropical regions (Galloway et al. 2004). Adding N can inhibit enzymes involved in decomposing recalcitrant soil C, which can reduce microbial activities (Gallo et al. 2004). Wang et al. (2018) found that changes in microbial abundance and composition after long-term soil N fertilization were accompanied by changes in extracellular enzyme activities, as observed in this study.
Soil structure
The MWD of WSA at the 0–5 cm depth was higher (18%) in the Cerrado than in the pasture, ICLS, and no-tillage maize (Table 2). MWD has been reported to be greater in forest sites (11–14%) than in cultivated land (Beheshti et al. 2012). MWD is related to many soil enzyme activities and the C and N fractions (Green et al. 2007). Previous studies have confirmed that soil microbial biomass is correlated with MWD (Kandeler and Murer 1993; Green et al. 2007). Soil disturbance by soil tillage increases the churning of aggregates and leads to lower soil biological activity (Raiesi and Beheshti 2014).
The proportion of large macroaggregates (4.0–8.0 mm) was higher at the 0–5 cm layer in the Cerrado. These results confirm that macroaggregates are dynamic and that the size distribution of macroaggregates is affected by land-use change and management. The relatively higher proportions of macroaggregates in the native Cerrado also suggest that the soil aggregates under forest were not affected by hydration or were more stable than in the cropped soil (Ashagrie et al. 2007). Low soil aggregation in cropland results in lower microbial biomass than in forest soils and may be related to other factors in addition to the lower concentration of C (Raiesi and Beheshti 2014). Macroaggregates provide a distinct and important microhabitat for soil microorganisms (Kandeler and Murer 1993).
Extracellular enzyme activity
This study demonstrated that changes in land-use impact the microbial community composition and enzyme activity under tropical conditions. The enzyme activities observed in the present study support the hypothesis that the addition of residues at the soil surface can lead to favorable conditions for balanced soil functional diversity (Zhang et al. 2016). The increased bG activity in the ICLS and no-tillage maize (Fig. 5a) was correlated with the composition and quality of plant residues, because bG is more active with less-complex residues (Lopes et al. 2013, 2015). This enzyme is linked to the final stage of the cellulose decomposition process, since it is responsible for the hydrolysis of cellobiose residues to form the simple sugar β-d-glucose (Tabatabai 1994). Celluloses are disaccharides that rapidly decompose in the soil, which explains the relationship between high bG activity and mineralizable C content in the ICLS and no-tillage maize in the present study (Fig. 5a). Numerous studies have reported beneficial impacts of conservation tillage on enzyme activities (Acosta-Martínez and Tabatabai 2001; Acosta-Martínez et al. 2003).
The lower amount of C that is readily mineralizable in the native Cerrado might be due to the greater diversity of crop species and to the complexity of plant residues (roots, branches, leaves, flowers, fruits, and seeds) (Sarto et al. 2020a). However, bG activity was higher in the no-tillage maize and pasture. Increased bG activity is correlated to microbial function (Table 4). This can improve nutrient cycling, availability and root growth, in addition to plant–microbial interactions benefits, and increases the total soil C pool (Caldwell 2005; Sarto et al. 2020b).
The relatively high levels of AP activity observed in the ICLS and no-tillage maize might be due to plant and microbial responses to the severe P deficiency in these areas (Fig. 5b). P-deficient conditions stimulate phosphatase production, and the phosphatases subsequently exuded by plant roots and soil microorganisms enhance the release of inorganic P from organic P compounds (Sinsabaugh et al. 1993). However, high levels of inorganic P in the soil, inhibit the production of phosphatase, which demonstrates that this enzyme is important to mineralization of organic P in areas under native Cerrado, since organic carbon is the main source of nutrients for plant growth (Sarto et al. 2020b).
We also observed differences in NAG activity, which tended to be much higher in the Cerrado (Fig. 5c). This difference might be related to the higher accumulation of litter, which conserves soil moisture and creates a more favorable environment for microbial decomposition. Compared to soils from global surveys, ratios of enzyme activities in these soils indicated greater investment toward P acquisition relative to C acquisition (Fig. 6a), implying a primary microbial P limitation (Sinsabaugh et al. 2008). All land-uses were P-limited throughout the soil depths (Fig. 6b). Ratios of ln(bG):ln(NAG) were greater than one in surface soils but decreased rapidly with depth, indicating a relatively N-rich condition in the surface soil but N-limiting conditions to soil microbes below 5 cm (Fig. 5d).
Microbial biomass
The native Cerrado represented an undisturbed system with the highest soil microbial properties and enzyme activities compared with the agricultural systems. The Cerrado had higher microbial biomass and abundances of AC, gram+ bacteria, fungi, and AMF compared to the ICLS, no-tillage maize, and pasture (Fig. 7a, 8). Higher microbial community biomass in native forest soil has also been observed by McKinley et al. (2005). Several soil physicochemical properties that are affected by land management have been reported to affect soil microbial communities, including soil moisture, pH, C and N quality and quantity, bulk density, and porosity (Wakelin et al. 2008). In the present study, soil bulk density was lower in the Cerrado, and organic carbon, macroporosity, microporosity, and total porosity were greater in this land-use (Table 2). The combination of these properties might have favored a greater soil microbial community compared with the ICLS, no-tillage maize and pasture. High soil compaction severely alters the abundance of microbial groups due to less favorable conditions for their activities, including limited gas diffusion, nutrient availability, and water movement due to decreased porosity and macropore continuity (Pengthamkeerati et al. 2011).
The higher abundances of AMF and fungi observed in the native Cerrado might be associated with the high SOC and TN in the forest (Table 3), as fungi incorporate more soil C into biomass than bacteria and C turnover is slower in fungal-dominated ecosystems. Greater fungal C pool size is related to larger presumed biochemical recalcitrance of fungal versus bacterial byproducts (Six et al. 2006). The higher soil microbial populations and enzyme activities in the Cerrado compared with the agricultural systems were due to the higher SOC. It was found that the abundance of AMF increased macroaggregates (4–8 mm), which may contribute to the stabilization of the SOC (Six et al. 2002; Wilson et al. 2009; McGowan et al. 2019).
All PLFAs decreased with soil depth, and this decrease was mainly attributable to the decreases in soil C and N availability with increasing soil depth. Soil properties, including changes in soil pH, moisture, and temperature (Mueller et al. 2012; Araujo et al. 2012) can affect soil microbial community composition after land-use change. The microbial community (total PLFA and microbial types of PLFA) was positively correlated to soil moisture and negatively to soil pH (Zhang et al. 2016).
Higher abundances of gram + bacteria were found in the ICLS than in the pasture, no-tillage maize and Cerrado, especially in the 0–5 cm depth (Fig. 7b). Shifts in the composition of bacterial communities in response to management changes have been reported previously in different systems (Peixoto et al. 2010), highlighting the sensitivity of the microbial community as a soil quality indicator. Individual PLFA signatures might be sensitive indicators of improved soil abiotic conditions (Six et al. 2006).
The F:B ratio has been proposed as an indicator of soil microbial community responses to soil C and N dynamics and environmental change. Bacteria are relatively unaffected by cultivation compared to fungi (Kihara et al. 2012), and F:B ratios may be higher in native forest areas (Zhang et al. 2016). The increase in the F:B ratio in reforested lands compared to arable land may be due to poor quality litter input, as bacteria require more N per unit of biomass C accumulation than fungi (Fierer et al. 2009). Zhang et al. (2016) observed greater microbial biomass, greater abundances of bacteria, fungi, gram+ bacteria, gram– bacteria, AC, and AMF, and higher basal microbial respiration under forest soil. The higher F:B ratios in the Cerrado are probably attributable to the low soil temperature and high soil moisture.
Conclusion
Land-use change (native Cerrado to agricultural systems) impacts the microbial community, decreasing soil microbial biomass; gram–; AC; AMF; Fungi; F:B ratio, macroporosity, microporosity, and total porosity and increasing soil bulk density. Soil microbial biomass was 40% higher in the native Cerrado compared with agricultural systems.
The soil microbial community distribution and extracellular enzyme activity decreased with soil layers. Thus, depth plays an important role in the soil microbial community and enzyme activity in a sandy soil of the Brazilian Cerrado.
The native Cerrado had a higher levels of soil C on the surface and improved soil structure due to increases microbial biomass, gram+ bacteria, AMF, fungi, and the F:B ratio in this tropical climate region of Brazil. Soil enzyme activity (bG and AP) were increased by the addition of less-complex residues on the soil surface (0–5 cm) in the ICLS system and maize no-tillage compared with the native Cerrado.
SOC was positively correlated with microbial community composition (MB; gram–; AC; AMF; Fungi; F:B ratio) and enzyme activity (bG, AP, NAG). Large macroaggregates were positively correlated with bG, AP, and AMF.
In summary, the conversion of native Cerrado to agricultural systems shifted the soil microbial community composition, enzyme activity, C and N, and soil structure in a sandy soil of the Brazilian Cerrado.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Acosta-Martínez V, Tabatabai MA (2001) Tillage and residue management effects on arylamidase activity in soils. Biol Fertil Soils 34:21–24. https://doi.org/10.1007/s003740100349
Acosta-Martínez V, Zobeck TM, Gill TE, Kennedy AC (2003) Enzyme activities and microbial community structure in semiarid agricultural soils. Biol Fertil Soils 38:216–227. https://doi.org/10.1007/s00374-003-0626-1
Acosta-Martínez V, Mikha M, Vigil MF (2007) Microbial communities and enzyme activities in soils under alternative crop rotations compared to wheat–fallow for the Central Great Plains. Appl Soil Ecol 37:41–52. https://doi.org/10.1016/j.apsoil.2007.03.009
Acosta-Martínez V, Lascano R, Calderón F, Booker JD, Zobeck TM, Upchurch DR (2011) Dryland cropping systems influence the microbial biomass and enzyme activities in a semiarid sandy soil. Biol Fertil Soils 47:655–667. https://doi.org/10.1007/s00374-011-0565-1
Alvares CA, Stape JL, Sentelhas PC, Moraes G, Leonardo J, Sparovek G (2013) Köppen’s climate classification map for Brazil. Meteorol Z 22:711–728. https://doi.org/10.1127/0941-2948/2013/0507
Anache JA, Flanagan DC, Srivastava A, Wendland EC (2018) Land use and climate change impacts on runoff and soil erosion at the hillslope scale in the Brazilian Cerrado. Sci Total Environ 622:140–151. https://doi.org/10.1016/j.scitotenv.2017.11.257
Araujo JF, Castro AP, Costa MM, Togawa RC, Júnior GJP, Quirino BF, Bustamante MMC, Lynn W, Handelsman J, Krüger RH (2012) Characterizatio of soil bacterial assemblies in Brazilian savanna-like vegetation reveals acidobacteria dominance. Microb Ecol 64:760–770. https://doi.org/10.1007/s00248-012-0057-3
Ashagrie Y, Zech W, Guggenberger G, Mamo T (2007) Soil aggregation, and total and particulate organic matter following conversion of native forests to continuous cultivation in Ethiopia. Soil Tillage Res 94:101–108. https://doi.org/10.1016/j.still.2006.07.005
Assmann JM, Anghinoni I, Martins AP, Andrade SEVG, Cecagno D, Carlos FS, Faccio Carvalho PC (2014) Soil carbon and nitrogen stocks and fractions in a long-term integrated crop-livestock system under no-tillage in southern Brazil. Agric Ecosyst Environ 190:52–59. https://doi.org/10.1016/j.agee.2013.12.003
Beheshti A, Raiesi F, Golchin A (2012) Soil properties, C fractions and their dynamics in land use conversion from native forests to croplands in northern Iran. Agric Ecosyst Environ 148:121–133. https://doi.org/10.1016/j.agee.2011.12.001
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Phys 37:911–917. https://doi.org/10.1139/o59-099
Caldwell BA (2005) Enzyme activities as a component of soil biodiversity: a review. Pedobiologia 49:637–644. https://doi.org/10.1016/j.pedobi.2005.06.003
Culman SW, Young-Mathews A, Hollander AD, Ferris H, Sánchez-Moreno S, O’Geen AT, Jackson LE (2010) Biodiversity is associated with indicators of soil ecosystem functions over a landscape gradient of agricultural intensification. Landsc Ecol 25:1333–1348. https://doi.org/10.1007/s10980-010-9511-0
Cusack DF, Silver WL, Torn MS, Burton SD, Firestone MK (2011) Changes in microbial community characteristics and soil organic matter with nitrogen additions in two tropical forests. Ecology 92:621–632. https://doi.org/10.1890/10-0459.1
Danielson RE, Sutherland PL (1986) Porosity. In: Klute A (ed) Methods of soil analysis, physical and mineralogical methods (Part I). SSSA book series 5.1. Soil Science Society of America, Madison, pp 443–461
Eaton WD, Chassot O (2012) Characterization of soil ecosystems in Costa Rica using microbial community metrics. J Tropical Ecology 53:185–195
Eclesia RP, Jobbagy EG, Jackson RB, Biganzoli F, Piñeiro G (2012) Shifts in Soil organic carbon for plantation and pasture establishment in native forests and grasslands of South America. Glob Change Biol 18:3237–3251. https://doi.org/10.1111/j.1365-5412486.2012.02761.x
Embrapa (1997) Manual de métodos De Análises De Solo, 2nd edn. Centro Nacional de Pesquisa de Solos, Rio de Janeiro
Fabrizzi KP, Rice CW, Amado TJ, Fiorin J, Barbagelata P, Melchiori R (2009) Protection of soil organic C and N in temperate and tropical soils: effect of native and agroecosystems. Soil Biol Biochem 92:129–143. https://doi.org/10.1007/s10533-008-9261-0
Ferreira DF (2011) Sisvar: a computer statistical analysis system. Ciênc Agrotec 35:1039–1042. https://doi.org/10.1590/S1413-70542011000600001
Ferreira AO, Amado T, Rice CW, Diaz DAR, Keller C, Inagaki TM (2016) Can no–till grain production restore soil organic carbon to levels natural grass in a subtropical Oxisol? Agric Ecosyst Environ 229:13–20. https://doi.org/10.1016/j.agee.2016.05.016
Fierer N, Strickland MS, Liptzin D, Bradford MA, Cleveland CC (2009) Global patterns in belowground communities. Ecol Lett 12:1238–1249. https://doi.org/10.1111/j.1461-0248.2009.01360.x(Epub 2009 Aug 11)
Fisher MJ, Rao IM, Ayarza MA, Lascano CE, Sanz JI, Thomas RJ, Vera RR (1994) Carbon storage by introduced deep-rooted grasses in the South American savannas. Nature 371:236–238. https://doi.org/10.1038/371236a0
Gallo M, Amonette R, Lauber C, Sinsabaugh RL, Zak DR (2004) Microbial community structure and oxidative enzyme activity in nitrogen-amended north temperate forest soils. Microb Ecol 48:218–229. https://doi.org/10.1007/s00248-003-9001-x
Galloway JN, Dentener FJ, Capone DG et al (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 70:153–226. https://doi.org/10.1007/s10533-004-0370-0
Garcia-Franco N, Martínez-Mena M, Goberna M, Albaladejo J (2015) Changes in soil aggregation and microbial community structure control carbon sequestration after afforestation of semiarid shrublands. Soil Biol Biochem 87:110–121. https://doi.org/10.1016/j.soilbio.2015.04.012
Green VS, Stott DE, Cruz JC, Curi N (2007) Tillage impacts on soil biological activity and aggregation in a Brazilian Cerrado Oxisol. Soil Tillage Res 92:114–121. https://doi.org/10.1016/j.still.2006.01.004
Hansel CM, Fendorf S, Jardine PM (2008) Changes in bacterial and archaeal community structure and functional diversity along a geochemically variable soil profle. Appl Environ Microbiol 74:1620–1633. https://doi.org/10.1128/AEM.01787-07
Jobbágy EG, Jackson RB (2000) The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol Appl 10:423–436. https://doi.org/10.1890/1051-0761(2000)010[0423:TVDOSO]2.0.CO;2
Kandeler E, Murer E (1993) Aggregate stability and soil microbial processes in a soil with different cultivation. Geoderma 56:503–513. https://doi.org/10.1016/B978-0-444-81490-6.50040-6
Kemper WD, Chepil WS (1965) Size distribution of aggregates. In: Blake CA, Evans DD, White JL, Ensminger LE, Clark FE (eds) Methods of soil analysis: physical and mineralogical properties, including statistics of measurement and sampling. American Society of Agronomy, Madison, pp 499–510
Kihara J, Martius C, Bationo A, Thuita M, Lesueur D, Herrmann L, Amelung W, Vlek PLG (2012) Soil aggregation and total diversity of bacteria and fungi in various tillage systems of sub-humid and semi-arid Kenya. Appl Soil Ecol 58:12–20. https://doi.org/10.1016/j.apsoil.2012.03.004
Lauber CL, Strickland MS, Bradford MA (2008) The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol Biochem 40:2407–2415. https://doi.org/10.1016/j.soilbio.2008.05.021
Leifeld J, Menichetti L (2018) The underappreciated potential of peatlands in global climate change mitigation strategies. Nat Commun 9:1–7. https://doi.org/10.1038/s41467-018-03406-6
Li N, Shao T, Zhu T, Long X, Gao X, Liu Z, Shao H, Rengel Z (2018) Vegetation succession influences soil carbon sequestration in coastal alkali-saline soils in southeast China. Sci Rep 8:1–12. https://doi.org/10.1038/s41598-018-28054-0
Lisboa FJG, Chaer G, Fernandes MF, Berbara RLL, Madari BE (2014) The match between microbial community structure and soil properties is modulated by land use types and sample origin within an integrated agroecosystem. Soil Biol Biochem 78:97–108. https://doi.org/10.1016/j.soilbio.2014.07.017
Liu L, Greaver TL (2010) A global perspective on belowground carbon dynamics under nitrogen enrichment. Ecol Lett 13:819–828
Lopes AAC, Sousa DMG, Chaer GM, Berbara RLL, Madari BE (2013) Interpretation of microbial soil indicators as a function of crop yield and organic carbon. Soil Sci Soc Am J 77:461–472. https://doi.org/10.2136/sssaj2012.0191
Lopes AAC, Sousa DMG, Reis Junior FB, Mendes IC (2015) Air-drying and long-term storage effects on β-glucosidase, acid phosphatase and arylsulfatase activities in a tropical savannah oxisol. Appl Soil Ecol 93:68–77. https://doi.org/10.1016/j.apsoil.2015.04.001
McGowan AR, Nicoloso RS, Diop HE, Roozeboom KL, Rice CW (2019) Soil organic carbon, aggregation, and microbial community structure in annual and perennial biofuel crops. Agron J 111:128–142. https://doi.org/10.2134/agronj2018.04.0284
McKinley VL, Peacock AD, White DC (2005) Microbial community PLFA and PHB responses to ecosystem restoration in tallgrass prairie soils. Soil Biol Biochem 37:1946–1958. https://doi.org/10.1016/j.soilbio.2005.02.033
Mendes IC, Fernandes MF, Chaer GM, Reis Junior FB (2012) Biological functioning of Brazilian Cerrado soils under different vegetation types. Plant Soil 359:183–195. https://doi.org/10.1007/s11104-012-1195-6
Mikha MM, Rice CW (2004) Tillage and manure effects on soil and aggregate-associated carbon and nitrogen. Soil Sci Soc Am J 68:809–816. https://doi.org/10.1016/10.2136/sssaj2004.0809
Mueller KE, Eissenstat DM, Hobbie SE, Oleksyn J, Jagodzinski AM, Reich PB, Chadwick OA, Chorover J (2012) Tree species effects on coupled cycles of carbon, nitrogen, and acidity in mineral soils at a common garden experiment. Biogeochemistry 111:601–614. https://doi.org/10.1007/s10533-011-9695-7
Öpik M, Moora M, Liira J, Zobel M (2006) Composition of root-colonizing arbuscular mycorrhizal fungal communities in different ecosystems around the globe. J Ecol 94:778–790. https://doi.org/10.1111/j.1365-2745.2006.01136.x
Peixoto R, Chaer G, Franco N, Junior FR, Mendes IC, Rosado AS (2010) A decade of land use contributes to changes in the chemistry, biochemistry and bacterial community structures of soils in the Cerrado. Antonie Leeuwenhoek 98:403–413. https://doi.org/10.1007/s10482-010-9454-0
Pengthamkeerati P, Motavalli PP, Kremer RJ (2011) Soil microbial activity and functional diversity changed by compaction, poultry litter and cropping in a claypan soil. Appl Soil Ecol 48:71–80. https://doi.org/10.1016/j.apsoil.2011.01.005
Pires CAB, Amado TJC, Reimche G, Schwalbert R, Sarto MVM, Nicoloso RS, Fiorin JE, Rice CW (2020) Diversified crop rotation with no-till changes microbial distribution with depth and enhances activity in a subtropical Oxisol. Eur J Soil Sci. https://doi.org/10.1111/ejss.12981
Powlson DS, Stirling CM, Thierfelder C, White RP, Jat ML (2016) Does conservation agriculture deliver climate change mitigation through soil carbon sequestration in tropical agro-ecosystems? Agric Ecosyst Environ 220:164–174. https://doi.org/10.1016/j.agee.2016.01.005
Raiesi F, Beheshti A (2014) Soil specific enzyme activity shows more clearly soil responses to paddy rice cultivation than absolute enzyme activity in primary forests of northwest Iran. Appl Soil Ecol 75:63–70. https://doi.org/10.1016/j.apsoil.2013.10.012
Rousk J, Brookes PC, Bååth E (2010) Investigating the mechanisms for the opposing pH relationships of fungal and bacterial growth in soil. Soil Biol Biochem 42:926–934. https://doi.org/10.1016/j.soilbio.2010.02.009
Sanz-Cobena A, Lassaletta L, Aguilera E et al (2017) Strategies for greenhouse gas emissions mitigation in Mediterranean agriculture: a review. Agric Ecosyst Environ 238:5–24. https://doi.org/10.1016/j.agee.2016.09.038
Sarto MV, Borges WL, Sarto JR, Rice CW, Rosolem CA (2020a) Root and shoot interactions in a tropical integrated crop–livestock–forest system. Agric Syst 181:1–11. https://doi.org/10.1016/j.agsy.2020.102796
Sarto MVM, Borges WLB, Sarto JRW, Pires CAB, Rice CW, Rosolem CA (2020b) Soil microbial community and activity in a tropical integrated crop–livestock system. Appl Soil Ecol 145:103350. https://doi.org/10.1016/j.apsoil.2019.08.012
Scott DA, Baer SG, Blair JM (2017) Recovery and relative influence of root, microbial, and structural properties of soil on physically sequestered carbon stocks in restored grassland. Soil Sci Soc Am J 81:50–60. https://doi.org/10.2136/sssaj2016.05.0158
Sinsabaugh RL, Antibus R, Linkins A, McClaugherty CA, Rayburn L, Repert D, Weiland T (1993) Wood decomposition: nitrogen and phosphorus dynamics in relation to extracellular enzyme activity. Ecology 74:1586–1593. https://doi.org/10.2307/1940086
Sinsabaugh RL, Lauber CL, Weintraub MN et al (2008) Stoichiometry of soil enzyme activity at global scale. Ecol Lett 11:1252–1264. https://doi.org/10.1111/j.1461-0248.2008.01245.x
Six J, Conant RT, Paul EA, Paustian K (2002) Stabilization mechanisms of soil organic carbon: implications for C-saturation of soils. Plant Soil 241:155–176. https://doi.org/10.1023/A:1016125726789
Six J, Frey SD, Thiet RK, Batten KM (2006) Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci Soc Am J 70:555–569. https://doi.org/10.2136/sssaj2004.0347
Soil Survey Staff (2014) Keys to soil taxonomy, 12th edn. USDA-Natural 695 Resources Conservation Service, Washington, DC.
Sotomayor-Ramírez D, Espinoza Y, Acosta-Martínez V (2009) Land use effects on microbial biomass C, β-glucosidase and β-glucosaminidase activities, and availability, storage, and age of organic C in soil. Biol Fertil Soils 45:487–497. https://doi.org/10.1007/s00374-009-0359-x
Souza RC, Mendes IC, Reis-Junior FB, Carvalho FM, Nogueira MA, Vasconcelos ATR, Vicente VA, Hungria M (2016) Shifts in taxonomic and functional microbial diversity with agriculture: How fragile is the Brazilian Cerrado? BMC Microbiol 16:1–15. https://doi.org/10.1186/s12866-016-0657-z
Tabatabai MA (1994) Soil enzymes. In: Weaver RW, Scott A, Bottomeley PJ (eds) Methods of soil analysis: microbiological and biochemical properties. Soil Science Society of America, Madison, pp 778–835
Tonucci RG, Nair VD, Nair PKR, Garcia R (2017) Grass vs. tree origin of soil organic carbon under different land-use systems in the Brazilian Cerrado. Plant Soil 419:281–292. https://doi.org/10.1007/s11104-017-3347-1
Treseder KK (2008) Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies. Ecol Lett 11:1111–1120. https://doi.org/10.1111/j.1461-0248.2008.01230.x
van Raij B, Andrade JC, Cantarella H, Quaggio JA (2001) Análise química para avaliação da fertilidade de solos tropicais. Instituto Agronômico, Campinas
Wakelin SA, Macdonald LM, Rogers SL (2008) Habitat selective factors influencing the structural composition and functional capacity of microbial communities in agricultural soils. Soil Biol Biochem 40:803–813. https://doi.org/10.1016/j.soilbio.2005.02.033
Wang C, Lu X, Mori T, Mao Q, Zhou K, Zhou G, Nie Y, Mo J (2018) Responses of soil microbial community to continuous experimental nitrogen additions for 13 years in a nitrogen-rich tropical forest. Soil Biol Biochem 121:103–112. https://doi.org/10.1016/j.soilbio.2018.03.009
Waring BG, Weintraub SR, Sinsabaugh RL (2014) Ecoenzymatic stoichiometry of microbial nutrient acquisition in tropical soils. Biogeochemistry 117:101–113. https://doi.org/10.1007/s10533-013-9849-x
White PM, Rice CW (2009) Tillage effects on microbial and carbon dynamics during plant residue decomposition. Soil Sci Soc Am J 73:1–8. https://doi.org/10.2136/sssaj2007.0384
Wilson GWT, Rice CW, Rillig MC, Springer A, Hartnett DC (2009) Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: results from long-term field experiments. Ecol Lett 12:452–461. https://doi.org/10.1111/j.1461-0248.2009.01303.x
Yang Y, Tilman D, Furey G, Lehman C (2019) Soil carbon sequestration accelerated by restoration of grassland biodiversity. Nat Commun 10:1–7. https://doi.org/10.1038/s41467-019-08636-w
Yoder RE (1936) A direct method of aggregate analysis of soil and a study of the physical nature of soil erosion losses. Soil Sci Soc Am J 28:337–351. https://doi.org/10.2134/agronj1936.00021962002800050001x
Zeglin LH, Bottomley PJ, Jumpponen A, Rice CW, Arango M, Lindsley A, McGowan A, Mfombep P, Myrold DD (2013) Altered precipitation regime affects the function and composition of soil microbial communities on multiple time scales. Ecology 94:2334–2345. https://doi.org/10.1890/12-2018.1
Zhang Q, Wu J, Yang F (2016) Alterations in soil microbial community composition and biomass following agricultural land use change. Sci Rep 6:1–10. https://doi.org/10.1038/srep36587
Zhou Z, Wang C, Zheng M, Jiang L, Luo Y (2017) Patterns and mechanisms of responses by soil microbial communities to nitrogen addition. Soil Biol Biochem 115:433–441. https://doi.org/10.1016/j.soilbio.2017.09.015
Acknowledgements
To Coordination for the Improvement of Higher Education Personnel (CAPES). To FAPESP (São Paulo Research Foundation) for financial support of this research (Registry numbers: 2014/10656-3 and 2016/14323-4) and CNPq (Brazilian National Council for Scientific and Technological Development).
Funding
Coordination for the Improvement of Higher Education Personnel (CAPES). To FAPESP (São Paulo Research Foundation) for financial support of this research (Registry numbers: 2014/10656-3 and 2016/14323-4) and CNPq (Brazilian National Council for Scientific and Technological Development).
Author information
Authors and Affiliations
Contributions
Not applicable.
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sarto, M.V.M., Borges, W.L.B., Bassegio, D. et al. Soil microbial community, enzyme activity, C and N stocks and soil aggregation as affected by land use and soil depth in a tropical climate region of Brazil. Arch Microbiol 202, 2809–2824 (2020). https://doi.org/10.1007/s00203-020-01996-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-020-01996-8