Abstract
Microbial biofilm reveals a cluster of microbial population aggregated on a surface. Pseudomonas aeruginosa, a strong biofilm forming organism, often causes several human diseases. Microorganism-based diseases become more difficult to manage when the causative organism develops biofilm during the course of disease progression as the organism attains alarming drug resistance in biofilm form. Agents inhibiting microbial biofilm formation could be considered as a potential tool to weaken the extent of microbial pathogenesis. Tryptophan has already been reported as a promising agent against the biofilm development by P. aeruginosa. In the current study, we had focused on the underlying mechanism of microbial biofilm inhibition of P. aeruginosa under the influence of tryptophan. The expression level of the mRNA of the genes (lasR, lasB and lasI) associated with quorum sensing was compared between tryptophan treated and untreated cells under similar conditions using real time polymerase chain reaction (RT-PCR). The results showed that the tested concentrations of tryptophan considerably reduced the expression of those genes (lasR, lasB and lasI) that are required during the occurrence of quorum sensing in P. aeruginosa. Molecular docking also revealed that tryptophan can interact with the proteins responsible for the occurrence of quorum sensing in P. aeruginosa. The cytotoxicity assay was carried out wherein we observed that the tested concentration of tryptophan did not show any considerable cytotoxicity against the RAW 264.7 macrophage cell line. From this study, it may be concluded that the tryptophan-mediated inhibition of biofilm formation is associated with interference of quorum sensing in P. aeruginosa. Hence, tryptophan could be used as a potential agent against the microbial biofilm mediated pathogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biofilm includes an organized group of microorganisms living together over either biotic or abiotic surfaces (Hurlow et al. 2015). Microorganism prefers to live in environment in biofilm form (Parsek and Singh 2003). Both homogenous and heterogeneous communities of bacteria can develop microbial biofilm over the surface. Microbial biofilm get stabilized by the self-secreted extracellular polymeric substances (EPS) that form a scaffold and hold the biofilm efficiently. Though polysaccharides are found to be the most prevalent part of EPS, other bio-molecule like proteins, lipids and nucleic acids are also present in EPS (Cortes et al. 2011). It was noted that biofilms have severe harmful pathogenic manifestations (Gupta et al. 2016). Microorganisms in biofilm often exhibit antibiotic tolerance that makes the microorganism more competent in spreading diseases (Vasudevan 2014). The considerable presence of EPS retards the diffusion of antibiotics through the biofilm that leads to increases in drug resistance property among microbial population noticeably. Moreover, the formation of biofilm allows the component cells to avoid the host immune response during the infection process as biofilm cells frequently alter the surface receptors through rapid variation in gene expression (Crossley et al. 2009). Microbial biofilm often plays a determinant role in several microbial infections. It was reported that biofilm population covers almost 80% of the total microbial infection (Cortes et al. 2011). Pseudomonas aeruginosa happens to be a Gram-negative, rod-shaped, aerobic bacteria that contributes to the high rate of morbidity and mortality (Das et al. 2016). It causes several infectious diseases including urinary tract infections, gastro-intestinal infections, endo-carditis, septicemia, respiratory infections and so on, and in most of the cases it has been noticed that the infections caused by them are strongly linked to the biofilm (Gupta et al. 2016). P. aeruginosa, a Gram-negative human opportunistic pathogen, uses quorum sensing to coordinate the formation of biofilm, swarming motility, exo-polysaccharide production, virulence, and cell aggregation (Sauer et al. 2002; Adonizio et al. 2008; Nithya et al. 2010). Quorum sensing represents the regulation in gene expression in response to fluctuations in cell-population density (Miller and Bassler 2001). For the bacteria to use quorum sensing (QS), they must secrete a signaling molecule called autoinducer (Pan and Ren 2009). At low cell density, the autoinducer molecule may just diffuse away but at high cell density, the local concentration of signaling molecules may exceed its threshold level and trigger changes in gene expressions (Bassler 1999; Pan and Ren 2009). Inhibition of P. aeruginosa biofilm by tryptophan was reported in the literature (Brandenburg et al. 2013). However, the underlying mechanisms of biofilm inhibition by tryptophan are yet to be discovered. In the current study, we hypothesized that tryptophan interferes with the quorum sensing event of P. aeruginosa that might lead to the inhibition of microbial biofilm formation.
Materials and methods
Microbial strain, growth media and culture conditions
The organism P. aeruginosa MTCC (424) used in this current study was a kind gift to us from Dr. Surajit Bhattacharjee, Department of Molecular Biology and Bioinformatics, Tripura University, Agartala, India. The organism P. aeruginosa MTCC (424) was grown in Luria Broth (LB) medium (Himedia, India) at 37 °C for different lengths of time according to the need of the experiment.
Biofilm formation assessment by crystal violet assay
Crystal violet (CV) assay was followed to examine the biofilm formation ability of P. aeruginosa under different condition. Equal numbers of overnight saturated cultures of P. aeruginosa (~ 1 × 106 CFU ml−1) were separately inoculated in different sterile test tubes containing 5 ml of sterile LB media having varying concentrations of tryptophan (0, 5, 10, 25, 50 µg/ml). In the control set, the same microorganisms were not exposed to tryptophan. All test tubes were then incubated at 37 °C for 2 days. After the incubation, planktonic cells were separately discarded from all the test tubes. Sterile Milli Q water was used to wash each tube twice. Then, all the tubes were dried adequately. After that, to each tube, 5 ml of 0.4% CV was added and subsequently incubated at room temperature for 15 min. CV solution was then discarded off from each tube. All tubes were again washed with Milli Q water to remove any unabsorbed CV from the tubes. Afterward, 5 ml of 33% acetic acid was added to each tube to dissolve the CV adsorbed to bacterial biofilm. The intensity of color generated due to CV was then measured by measuring the optical density at 630 nm.
Growth pattern analysis
To examine the effect of different concentration of tryptophan on the growth profile of P. aeruginosa, equal numbers of the bacteria (~ 1 × 106 CFU ml−1) were separately inoculated in sterile LB media. After that, different concentrations of tryptophan (5, 10, 25, 50 µg/ml) were added into each growth media. In the control condition, the organism was grown in LB in the absence of tryptophan. All the experimental growth media were then incubated at 37 °C for 48 h. Equal volume of the culture broth was separately recovered from each growth media and absorbance was subsequently recorded at 600 nm in a colorimeter. To examine the viability of P. aeruginosa under the influence of tryptophan, we inoculated similar numbers of bacteria (~ 1 × 105 CFU ml−1) in different test tubes and treated with different concentrations of tryptophan. However, tryptophan was not given in the control set where the organism was grown in the absence of tryptophan. Then, all the tubes were incubated for 48 h at 37 °C. For colony forming unit (CFU) calculation between tryptophan treated and untreated condition, 1 ml of culture media was separately collected from each conditioned medium and dissolved in 9 ml of sterile saline solution and thereafter different dilutions were accordingly prepared. 100 µl of these diluted supernatants was plated onto solid LB agar plates. Thereafter, plates were incubated at 37 °C for 2 days and CFU was subsequently counted.
Gene expression measurement by real time PCR
The mRNA expression of the quorum sensing genes, namely lasB, lasI and lasR of P. aeruginosa was determined by real-time PCR. The bacteria (strain MTCC 424) was grown to mid-log phase and treated with different doses L-tryptophan followed by incubation at 37 °C for 48 h. Bacterial mRNA was extracted by Trizol reagent (Invitrogen). The purity and yield of the RNA were determined using spectrophotometer. Then, the total RNA from each sample was transcribed into first strand cDNA using reverse transcriptase M-MLV (Takara). Equal amount (1 mg) of cDNA was real-time amplified using the SYBR Premix kit (Applied Biosystems). A reaction mixture lacking cDNA was used as the negative control. The expression level of quorum sensing genes, namely lasR, lasB and lasI was calculated using the comparative threshold cycle method (\({{\text{2}}^{ - \Delta \Delta {{\text{C}}_t}}}\)) with 16S rRNA as the control gene. Primers used in the current study were procured from Xleris Genomics, India (Table 1).
Molecular Docking analysis of tryptophan with quorum sensing (QS) proteins of P. aeruginosa
In silico analysis was carried out to study the interactions of L-tryptophan with QS-related proteins from species P.aeruginosa. The crystallographic structures of proteins LasI (PDB ID: 1RO5), Las A (PDB ID: 3IT7) and LasR (PDB ID: 3JPU) used in the docking study were obtained from the RCSB protein data bank (Berman et al. 2000). The 3D structure files of ligand L-tryptophan (CID: 6305) were downloaded from the PubChem (Wang et al. 2009) in sdf format. UCSF Chimera (Pettersen et al. 2004) was used to convert the sdf files of ligands into pdb format for docking purpose. Prior to docking, both the proteins and ligand were energy minimized for obtaining the stable structures. The Autodock Tools (ADT) package was used for setting up all the input files of proteins and ligands for docking by adding polar hydrogens, merging non-polar hydrogens and adding Gastegier charges. After the preparation of protein and ligand files in ADT, molecular docking was performed using the AutoDock Vina program (Trott and Olson 2010) for the prediction of binding affinities of ligand with proteins. L-tryptophan was docked into the binding pocket of each protein separately. GROMACS package, version 4.5 was used to energy minimized the protein–ligand docked complexes to confirm their stability and binding analysis (Berendsen et al. 1995). LigPlot (Wallace et al. 1995) and PyMol (DeLano 2002) program was used to generate the schematic 2D diagram and cartoon structures of the final docked protein–ligand complexes.
Cell viability assay by MTT method
Cytotoxicity of L-tryptophan was performed with RAW 264.7 macrophage cell line with minor modifications. Cells (2 × 105) were seeded into each well of 96-well tissue culture plates (Eppendorf) and cultured in RPMI-1640 media supplemented with 10% FCS. Cells were treated with different doses (1–50 µg/ml) of L-tryptophan and incubated in presence of 5% CO2 at 37 °C for 48 h. Thereafter, the medium was replaced with fresh RPMI (without Phenol Red) containing 1 mg/ml MTT and incubated at 37 °C for 3 h. After that, the formed formazan crystals were dissolved in DMSO and incubated for 20 min at room temperature. The absorbance was then measured using a plate reader (Synergy H1 Hybrid) at a test wavelength of 590 nm.
Statistical analysis
Experimental results were statistically analyzed by one-way analysis of variance (ANOVA) using Minitab 16 software.
Results and discussion
Tryptophan does not inhibit microbial growth
To examine the effect of the different concentrations of tryptophan on the growth of P. aeruginosa, the organism was allowed to grow in Luria Broth (LB) with different concentrations (0, 5, 10, 25, 50 µg/ml) of tryptophan. Thereafter, we compared the microbial growth profile of the organism between tryptophan treated and untreated condition. All the conditioned media were incubated at 37 °C for 2 days. During the course of incubation, we separately recovered equal volumes of culture from both tryptophan-exposed and unexposed culture media at specific time intervals and measured the optical density (OD) at 600 nm. The results revealed that there is no considerable difference in microbial growth pattern between tryptophan-exposed and unexposed growth media (Fig. 1a). To gain further confidence, the viable microbial count was compared between tryptophan treated and untreated growth media. 100 µl of the bacterial cultures from both tryptophan-exposed and unexposed culture media was collected and subsequently serially diluted. Each dilution was plated on Luria Agar (LA) media and after the incubation at 37 °C for 2 days, we observed no significant difference in Colony Forming Unit (CFU) between tryptophan-exposed and unexposed growth media (Fig. 1B). Taken together, the results indicated that the different concentrations of tryptophan (0, 5, 10, 25, 50 µg/ml) do not exhibit microbial growth arresting properties.
Cell viability assays of P. aeruginosa under the influence of tryptophan. a Growth curve analysis. Bacteria were incubated in growth media mixed with varying concentrations of tryptophan. At regular time interval, the microbial culture media were taken from each media including control and subjected to the measurement of OD at 600 nm. Three replicates were used for each experimental set. Error bars indicate standard deviation (± SD). b Colony forming unit (CFU) assay. CFU was counted from both tryptophan-exposed and unexposed culture media after 48 h of incubation at 37 °C. Three replicates were performed for each experimental set. Error bars indicate standard deviation (± SD). Statistical significance between the groups was evaluated by ANOVA at 5% level. Mean values with the same letters are significantly similar among the treatments
Tryptophan inhibits the expression of genes associated with quorum sensing of P. aeruginosa
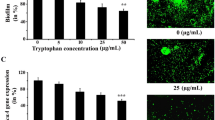
Pseudomonas aeruginosa had long been reported as a potential organism that can develop microbial biofilm (Gupta et al. 2016). It was also reported that tryptophan can efficiently inhibit the formation of microbial biofilm over the surface (Brandenburg et al. 2013). In the present study, we had also observed the inhibition of microbial biofilm formation using different doses of tryptophan (Supplementary Fig. 1). Thus, in the current work, we had focused on the exploration of underlying mechanism of microbial biofilm inhibition using tryptophan. Microorganisms use several signaling events to develop biofilm. Quorum sensing (QS) happens to be an important signaling event for the development of biofilm (Gupta et al. 2016). It is a process of bacterial communication where bacteria communicate to each other by the use of self-made autoinducers or pheromones (Gupta et al. 2016). Gram-negative bacteria produce N-acyl homoserine lactones as their autoinducer (Gupta et al. 2016). These autoinducers after reaching the threshold value can control the expression of various genes linked to virulence and biofilm formation (Bordi and de Bentzmann 2011). It was reported that the Las proteins play an important role in regulating QS-mediated biofilm formation in Pseudomonas aeruginosa (Zhu et al. 2004). The las system has a transcriptional activator LasR (Kiratisin et al. 2002). This LasR interacts with its cognate autoinducer (AI) molecule {N-3-oxo-dodecanoyl-l-homoserine lactone (3O-C12-HSL)} in order to get activated (Kiratisin et al. 2002). The LasR has two important binding sites. The N-terminal portion of LasR interacts with autoinducer while the carboxyl-terminal portion of the protein has a DNA-binding domain (Kiratisin et al. 2002). The activated LasR can upregulate the expression of several genes including lasB and lasI (Passador et al. 1993; Parsek and Greenberg 2000). Besides, lasB gene codes for elastase protein that helps the bacteria to evade host tissue during the course of pathogenesis. Activation of lasB gene expression requires a functional operator sequence (OP1) in the lasB promoter region (Anderson et al. 1999). Activated LasR (in association with AI) protein through its carboxyl terminal domain binds with the functional operator sequence (OP1) located upstream to the promoter of lasB gene (Anderson et al. 1999). This binding of LasR helps RNA polymerase to bind to the promoter of lasB gene and thereby upregulates the expression of lasB gene (Anderson et al. 1999). The lasI gene codes for acyl homoserine lactone synthase in P. aeruginosa. This enzyme (Las I protein) synthesizes 3OC12-HSL (N-3-[oxododecanoyl] homoserine lactone). The activated LasR (in association with 3OC12-HSL) then enhances the expression of lasI gene considerably (Parsek and Greenberg 2000). Thus, the activation of LasR depends on the availability of its cognate autoinducer molecule. Once LasR gets activated, it targets lasB and lasI gene that leads to the expression of LasB and LasI protein (Passador et al. 1993; Parsek and Greenberg 2000). Therefore, the above-mentioned relationship revealed that the expression of lasB and lasI is dependent on LasR activation while the activation of LasR depends on the expression of lasI gene. In the existing literature, it was documented that the compounds that can inhibit the formation of microbial biofilm could attenuate the expression of quorum sensing associated genes (Gupta et al. 2017). Thus, keeping this in mind, the variation in quorum sensing linked gene (lasB, lasI and lasR) expression was measured both in the presence and absence of tryptophan. The result indicated that the expression of quorum sensing associated genes got considerably reduced under the exposure of tryptophan (Fig. 2). To gain further confidence, the expression level of 16S rRNA gene which is not associated with quorum sensing property of the organism was also measured both in the presence and absence of tryptophan. The result showed that there is no considerable change in the expression level of 16S rRNA gene between tryptophan-treated and untreated microorganisms (Supplementary Fig. 2). Thus, the result demonstrated that tryptophan only reduces the expression of quorum sensing associated genes (lasB, lasI and lasR) but not the expression of unrelated gene (16S rRNA) in the control experiment. Since the results indicated that lasR gene expression got reduced by tryptophan, LasR can no longer activate the expression of lasB and lasI gene. However, since lasI gene expression also got reduced by tryptophan, it cannot help LasR protein to get activated.
Quorum Sensing linked gene expression analysis. P. aeruginosa were grown both in the presence and absence of tryptophan for 2 days at 37 °C. After the incubation, RNA was isolated from both organisms and the expression of genes responsible for quorum sensing was carried out by real-time PCR analysis
To study the effects and interactions of amino acids, L-tryptophan over the QS-related proteins that are involved in biofilm formation in P. aeruginosa, the In silco binding analysis was performed. For docking purpose, two binding pockets were used for LasA (PDB ID: 3IT7) which was already occupied by tartaric acid and glycerol in the crystallographic structure of the protein. The protein LasI (PDB ID: 1RO5) was not available in the complex form with any ligand in PDB so the binding pocket used for the LasI protein was taken from its homologous protein acyl-homoserinelactone synthase (EsaI) (PDB ID: 1K4J) from Pantoea stewartii which was present as complex with perrhenate ions (O4Re) in protein data bank. Molecular docking of QS-associated proteins with L form of amino acid tryptophan showed that all the proteins LasI (Fig. 3a), LasA (Fig. 3b)(Fig. 3c), LasR (Fig. 3d) have high binding affinity towards tryptophan (Table 2). The amino acid L-tryptophan occupies the different binding pocket in LasI from the original one. A comparison between binding affinities of all the proteins showed that LasR protein exhibited the maximum binding affinity towards tryptophan among other proteins. To analyze the binding affinity and stability of docked complexes, further energy minimization was carried out which showed that LasR protein has the lowest potential energy in the docked state with L-tryptophan as compared to other docked complexes (Table 2). The binding sites used in this analysis are experimentally validated and hence we assume that the ligand tryptophan in reality may also bind there and may ultimately regulate their activities. Considering all the results, it appears that tryptophan hampers quorum sensing property of the organism as evident from gene expression and docking result analysis. Thus, the inhibition of microbial biofilm by tryptophan could be attributed to interfering in quorum sensing property of the organism.
Molecular docking and interaction analysis of L-tryptophan with quorum sensing-related proteins from species P. aeruginosa: a-i Cartoon structure of protein–ligand docked complex of LasI (PDB ID: 1RO5) and L-tryptophan, a-ii LigPlot interaction analysis of L-tryptophan with active residues of LasI; b-i Cartoon structure of protein–ligand docked complex of LasA (PDB ID: 3IT7) and L-tryptophan into glycine binding pocket, b-ii LigPlot interaction analysis of L-tryptophan with active residues of LasA; c-i Cartoon structure of protein–ligand docked complex of LasA (PDB ID: 3IT7) and L-tryptophan into tartaric acid, c-ii LigPlot interaction analysis of L-tryptophan with active residues of LasA; d-i Cartoon structure of protein–ligand docked complex of LasR (PDB ID: 3JPU) and L-tryptophan, d-ii LigPlot interaction analysis of L-tryptophan with active residues of LasR
The tested concentration of tryptophan does not show considerable cytotoxicity
The effect of L-tryptophan on cell viability was assessed in RAW 264.7 macrophage cell line. L-tryptophan showed a dose-dependent action on the viability of cell (Fig. 4). It was observed that the lowest dose (1 µg/mL) of L-tryptophan showed 99.8% cell viability, whereas cell viability was found to be decreased to ~ 88% at highest dose of L-tryptophan tested (50 µg/ml) (Fig. 4).
Cytotoxicity assay. The cytotoxicity effect of tryptophan on human cell line RAW was tested by following the MTT assay as described in “Materials and methods”
Conclusion
Exploring new agents that can target quorum sensing during biofilm formation may be considered as a potential tool for development of effective therapeutic strategies for infections with biofilm forming microbes such as P. aeruginosa. Our current findings indicate that tryptophan holds promise to interfere with quorum sensing event that leads to the inhibition of biofilm development. Thus, tryptophan could be used as an effective supplement to inhibit the microbial biofilm formation that might reduce the extent of pathogenicity caused by microorganisms.
References
Adonizio A, Kong KF, Mathee K (2008) Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa by South Florida plant extracts. Antimicrob Agents Chemother 52(1):198–203
Anderson RM, Zimprich CA, Rust L (1999) A second operator is involved in Pseudomonas aeruginosa elastase (lasB) activation. J Bacteriol 181(20):6264–6270
Bassler BL (1999) How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr Opin Microbiol 2(6):582–587
Berendsen HJC, Van der Spoel D, Van Drunen R (1995) GROMACS: A message-passing parallel molecular dynamics implementation. Comput Phys Commun 91:43–56
Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE (2000) The protein data bank. Nucleic Acids Res 28(1):235–242
Bordi C, de Bentzmann S (2011) Hacking into bacterial biofilms: a new therapeutic challenge. Ann Intensive Care 1(1):19
Brandenburg KS, Rodriguez KJ, McAnulty JF, Murphy CJ, Abbott NL, Schurr MJ, Czuprynski CJ (2013) Tryptophan inhibits biofilm formation by Pseudomonas aeruginosa.. Antimicrob Agents Chemother 57(4):1921–1925
Cortes ME, Consuegra J, Sinisterra RD (2011) Biofilm formation, control and novel strategies for eradication. Sci Against Microbial Pathog Commun Curr Res Technol Adv 2:896–905
Crossley KB, Jefferson KK, Archer GL, Fowler VG (2009) Staphylococci in human disease, 2nd edn. Blackwell, West Sussex
Das MC, Paul S, Gupta P, Tribedi P, Sarkar S, Manna D, Bhattacharjee S (2016) 3-Amino-4-aminoximidofurazan derivatives: small molecules possessing antimicrobial and antibiofilm activity against Staphylococcus aureus and Pseudomonas aeruginosa. J Appl Microbiol 120(4):842–859
DeLano WL (2002) The PyMOL User’s Manual. DeLano Scientific, San Carlos
Gupta P, Sarkar S, Das B, Bhattacharjee S, Tribedi P (2016) Biofilm, pathogenesis and prevention-a journey to break the wall: a review. Arch Microbiol 198(1):1–15
Gupta P, Sarkar A, Sandhu P, Daware A, Das MC, Akhter Y, Bhattacharjee S (2017) Potentiation of antibiotic against Pseudomonas aeruginosa biofilm: A study with plumbagin and gentamicin. J Appl Microbiol 123(1):246–261
Hurlow J, Couch K, Laforet K, Bolton L, Metcalf D, Bowler P (2015) Clinical biofilms: a challenging frontier in wound care. Adv Wound Care 4(5):295–301
Kiratisin P, Tucker KD, Passador L (2002) LasR, a transcriptional activator of Pseudomonas aeruginosa virulence genes, functions as a multimer. J Bacteriol 184(17):4912–4919
Miller MB, Bassler BL (2001) Quorum sensing in bacteria. Annu Rev Microbiol 55(1):165–199
Nithya C, Aravindraja C, Pandian SK (2010) Bacillus pumilus of Palk Bay origin inhibits quorum-sensing-mediated virulence factors in Gram-negative bacteria. Res Microbiol 161(4):293–304
Pan J, Ren D (2009) Quorum sensing inhibitors: a patent overview. Expert Opin Ther Pat 19(11):1581–1601
Parsek MR, Greenberg EP (2000) Acyl-homoserine lactone quorum sensing in gram-negative bacteria: a signaling mechanism involved in associations with higher organisms. Proc Natl Acad Sci USA 97(16):8789–8793
Parsek MR, Singh PK (2003) Bacterial biofilms: an emerging link to disease pathogenesis. Annu Rev Microbiol 57(1):677–701
Passador L, Cook JM, Gambello MJ, Rust L, Iglewski BH (1993) Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science 260(5111):1127–1130
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera-a visualization system for exploratory research and analysis. J Comput Chem 25(13):1605–1612
Sauer K, Camper AK, Ehrlich GD, Costerton JW, Davies DG (2002) Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J Bacteriol 184(4):1140–1154
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31(2):455–461
Vasudevan R (2014) Biofilms: microbial cities of scientific significance. J Microbiol Exp 1(3):00014
Wallace AC, Laskowski RA, Thornton JM (1995) LIGPLOT: a program to generate schematic diagrams of protein–ligand interactions. Protein Eng 8(2):127–134
Wang Y, Xiao J, Suzek TO, Zhang J, Wang J, Bryant SH (2009) PubChem: a public information system for analyzing bioactivities of small molecules. Nucleic Acids Res 37(2):W623–W633
Zhu H, Bandara R, Conibear TC, Thuruthyil SJ, Rice SA, Kjelleberg S, Givskov M, Willcox MD (2004) Pseudomonas aeruginosa with lasI quorum-sensing deficiency during corneal infection. Invest Ophthalmol Vis Sci 45(6):1897–1903
Acknowledgements
The authors would like to thank Dr. Subhasis Sarkar for his valuable contributions for the improvement of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflict of interest.
Additional information
Communicated by Erko Stackebrandt.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chakraborty, P., Daware, A.V., Kumari, M. et al. Free tryptophan residues inhibit quorum sensing of Pseudomonas aeruginosa: a potential approach to inhibit the development of microbial biofilm. Arch Microbiol 200, 1419–1425 (2018). https://doi.org/10.1007/s00203-018-1557-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-018-1557-4