Abstract
Environmental contamination by Te and Se oxyanions has become a serious concern, with the search for green, ecologically friendly methods for removal gaining ground. Bacteria capable of reducing these highly toxic compounds to a virtually non-toxic elemental form could provide a solution. In this study, four strains of bacteria with potential for bioremediation of Te and Se oxyanions were investigated. Under aerobic conditions over 48 h, Erythromicrobium ramosum, strain E5 removed 244 µg/ml tellurite and 98 µg/ml selenite, Erythromonas ursincola, KR99 203 µg/ml tellurite and 100 µg/ml selenite, AV-Te-18 98 µg/ml tellurite and 103 µg/ml selenite and ER-V-8 93 µg/ml tellurite and 103 µg/ml selenite. In the absence of oxygen, AV-Te-18 and ER-V-8 removed 10 µg/ml tellurite after 24 and 48 h, respectively and 46 and 25 µg/ml selenite, respectively, over 48 h. ER-V-8 removed 14 µg/ml selenate after 5 days. This highlights the great potential of these microbes for use in bioremediation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many microorganisms possess a wide range of extraordinary physiological abilities for production of bioactive molecules that show resistance against and transform highly toxic compounds (Bhatnagar and Kim 2010). Of great interest are bacteria, which can convert deleterious forms of metalloids from one oxidation state to another through reduction and/or oxidation (Yurkov et al. 1996; Li et al. 2009; Arenas et al. 2014; Bonificio and Clarke 2014). In recent years, there has been more interest in these microbes due to increased environmental contamination from industrial and agricultural activities (Fujii et al. 1988; Macy et al. 1993; Prakash et al. 2001; Li et al. 2009; Yang et al. 2014). Te is a metalloid element related to oxygen and sulfur in group 16 of the periodic table. It possesses stable oxidation states of VI (tellurate), IV (tellurite), 0 (elemental tellurium), and II (telluride). Se parallels Te in many instances. It also belongs to group 16 and is related to sulfur and oxygen with similar oxidation states of VI (selenate), IV (selenite), 0 (elemental selenium), and II (selenide). Reduction of tellurite, selenite and/or selenate, under both aerobic and anaerobic conditions, results in detoxification (Yurkov and Beatty 1998; Soudi et al. 2009; Li et al. 2014a, b; Javed et al. 2016); hence, heavy metalloid oxyanion reducers have an important role in nature. The removal of toxic metalloid contaminants from environments with elevated concentrations can allow many biological species to inhabit these locales (Yurkov et al. 1999; Rathgeber et al. 2002; Csotonyi et al. 2006; Bajaj and Winter 2014; Epelde et al. 2015; Maltman et al. 2015). The details of microbial interactions with very high levels of tellurite, selenite, and/or selenate are still not well–understood; however, some unique physiological properties possessed by microbes may provide a means for bioremediation (Soudi et al. 2009; Maltman and Yurkov 2014, 2015; Maltman et al. 2017a, b).
The increased environmental concentration of toxins has led to the search for removal methods, which will not in turn cause more pollution or other related consequences. Some chemicals and resins have been used to neutralize and/or remove oxyanions (Kim et al. 2004; Elwakeel et al. 2009). However, they can be expensive and their use may result in increased release of xenobiotic compounds, which is often problematic. More attraction to biological approaches of dealing with metalloids has arisen, as it would lead to a ‘greener’, environmentally friendlier clean-up of pollutants (Gadd 2010). Microbes reducing oxyanions from highly toxic oxidation states to less toxic elemental forms have been in the spotlight as a possible means to remediate contaminated locations. Bioremediation has been explored for removal of xenobiotics, metals, and radioactive compounds (Pieper and Reineke 2000; Ruggiero et al. 2005; Shah and Nongkynrih 2007; Gadd 2010; Jadhav et al. 2010; Yong and Zhong 2010; Dogan et al. 2011; Wasi et al. 2013), but much less attention has been given to tellurite, selenite, and/or selenate treatments. The use of Thauera selenatis for removal of selenite/selenate from drainage water (Macy et al. 1993; Cantafio et al. 1996), and tellurite/tellurate clean-up from waste using Pseudomonas mendocina, strain MCM B-180 (Rajwade and Paknikar 2003) and Pseudoalteromonas sp., EPR3 (Bonificio and Clarke 2014) and Bacillus STG-83 for removal of tellurite and selenite/selenate in the presence of nitrate (Soudi et al. 2009) have been studied, along with using mixed complex microbial communities for remediation (Luek et al. 2014; Ramon-Ruiz et al. 2016). While all these approaches did result in removal of the majority of contaminants, initial concentrations of the oxyanions were low, required substantial time before significant remediation occurred, and involved many other factors which needed to be adjusted and controlled, making the process either complicated or expensive (Cantafio et al. 1996; Hunter and Kuykendall 2005; Staicu et al. 2017). Another major issue, especially related to selenite/selenate remediation, is recovery of the elemental end-product (Staicu et al. 2017). Hence, currently used strains and technologies leave much room for optimization. Bacteria possessing greater resistance, faster rates of reduction, and the ability to internalize the elemental Se and Te, may prove to be more efficient and effective, allowing for feasible detoxification and possible recovery/recycling.
In this study, we set out to ascertain the bioremediation potential of several bacterial strains with the ability to reduce high levels of tellurite, selenite, and selenate to elemental form (Fig. 1) (Maltman and Yurkov 2014, 2015; Maltman et al. 2016). The elemental Te [~ 100–500 nm particles, comprising up to 20–30% of cell volume (Yurkov et al. 1996; Kim et al. 2012)] and Se [~ 100 nm particles (Rathgeber et al. 2002; Li et al. 2014a)] produced is contained within the cells, providing a possible means for removal, preventing re-release into the environment, possibly resulting in further issues, and recovery/recycling of these elements. Removal processes under both aerobic and anaerobic conditions were investigated to define their prospective for future remediation applications.
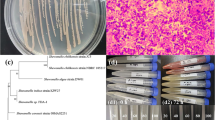
a Aerobic reduction resulting in visible reduction of TeO32− by strain KR99 (similar appearance for E5, ER-V-8, and AV-Te-18) and SeO32− by strain ER-V-8 (similar for E5, KR99, and AV-Te-18). b Anaerobic reduction resulting in visible reduction of TeO32− by strain AV-Te-18 (similar results for ER-V-8), SeO32− by strain ER-V-8 (similar for AV-Te-18), and SeO42− by strain ER-V-8. For TeO32− supplemented cultures, black coloration indicates reduction to elemental Te. Dissolved Se oxyanion color change from clear to red is due to reduction to elemental Se
Materials and methods
Strains, growth conditions and oxyanion reduction
Strains selected for study included the aerobic anoxygenic phototrophs Erythromonas ursincola, KR99 (Yurkov et al. 1997) and Erythromicrobium ramosum, E5 (Yurkov et al. 1994), which possess membrane-associated reduction (Maltman and Yurkov 2015) and the heterotrophic facultative anaerobes Pseudoalteromonas relative, AV-Te-18, and Shewanella relative, ER-V-8 (Maltman et al. 2016). For aerobic remediation, E5 and KR99 were grown under their optimal conditions, as published (Maltman and Yurkov 2015), in the presence of either 500 µg/ml tellurite or selenite. ER-V-8 and AV-Te-18 were grown in rich organic (RO) liquid medium containing 2% NaCl (Maltman and Yurkov 2014) on an incubator shaker at 200 rpm in the presence of 250 µg/ml (AV-Te-18) or 150 µg/ml (ER-V-8) tellurite or 250 µg/ml selenite, at 28 °C, pH 7.8, in the dark. For anaerobic experiments, AV-Te-18 and ER-V-8 were initially grown aerobically at 28 °C in the dark on RO NaCl agar plates, re-suspended, and used to inoculate 120 ml crimp-sealed anaerobic bottles, containing 100 ml of AMR medium (Maltman et al. 2015) amended with 100 µg/ml of tellurite, selenite, or selenate, under a headspace of N2. Samples were taken at various time intervals with the amount of protein and oxyanion monitored. Tellurite, selenite, and selenate concentrations were determined as previously described (Desai and Paul 1977; Molina et al. 2010) and protein by the Bradford assay (Bradford 1976). All experiments were performed in triplicate.
Results and discussion
Tellurite removal
Pseudomonas mendocina, strain MCM B-180, is currently considered the most effective bacterium for aerobic tellurite (TeO32−) bioremediation; however, optimal reduction takes place at a concentration of only 10 µg/ml and it takes 72 h to remove 100 µg/ml (1.4 mg/l/h) (Rajwade and Paknikar 2003). All strains in our study were able to remove similar levels of tellurite very quickly, requiring 6 h or less (Fig. 2a). Over 48 h, KR99 and E5 removed 203 and 244 µg/ml tellurite (4.2 and 5.1 mg/l/h), respectively, which is a significantly higher level than reported for P. mendocina (Table 1), with the elemental end-product contained within the cell (Yurkov et al. 1996). For AV-Te-18 and ER-V-8, removal of 98 and 93 µg/ml, (2.1 and 2.0 mg/l/h) respectively (Table 1), did require somewhat more time (48 h) than measured for KR99 and E5, but even with this increase, they were still much faster than P. mendocina (Fig. 2a). Anaerobic resistance and reduction of tellurite has been investigated, but not in terms of remediation potential. However, some future applications may require the absence of oxygen due to the logistics of aeration. Therefore, if a bacterium can detoxify oxyanions under both aerobic and anaerobic conditions, it would be of great benefit and advantage. To this end, strains ER-V-8 and AV-Te-18 are both capable of tellurite reduction under anoxic conditions (Fig. 3a). Amounts of converted TeO32− were lower compared to the aerobic experiments, but this is expected since toxicity is generally increased in the absence of oxygen (Moore and Kaplan 1992; Borghese et al. 2004; Csotonyi et al. 2006), confirmed by decreased tellurite minimum inhibitory concentration (MIC) values for both ER-V-8 (150 vs. 250 µg/ml) and AV-Te-18 (150 vs. 500 µg/ml). Nevertheless, both strains could remove 10 µg/ml tellurite, the same concentration P. mendocina functions optimally at aerobically, with ER-V-8 taking 48 h (0.2 mg/l/h) and AV-Te-18 only requiring 24 h (0.4 mg/l/h) (Table 1). For strain AV-Te-18, the total removal can be as high as 51 µg/ml after 5 days (0.4 mg/l/h) (Fig. 3a). Under both anaerobic and aerobic conditions, Pseudoalteromonas relative, AV-Te-18 and Shewanella relative ER-V-8 cells converted colorless tellurite into elemental Te internally, supported by a change in cell color into black (Fig. 1). The growth media remained clear after centrifugation, confirming Te is not released from the cells. This is similar to published results observed for related microorganisms (Rathgeber et al. 2002; Kim et al. 2014).
Specific removal rates varied depending on strain and conditions (Table 2). In the presence of oxygen, all four strains demonstrated different rates, with the most efficient detected for E5 (2.26 µg tellurite reduced/µg protein/day), followed by KR99 (1.45), AV-Te-18 (0.78), and ER-V-8 (0.65). Anaerobically, removal was constant at 0.81–0.82 µg tellurite reduced/µg protein/day, regardless of the strain (Table 2). Although AV-Te-18 removed approximately fivefold more tellurite than ER-V-8 over 5 days, the specific rate of reduction remained similar.
Selenite and selenate conversion
Removal of Se oxyanions has been attempted using a variety of approaches (Cantafio et al. 1996; Hunter and Kuykendall 2005; Staicu et al. 2017). While successful, there is still much room for improvement, as in almost all cases, the time needed for removal is significant, the concentrations involved are relatively low (ng/µl) (Cantafio et al. 1996), and the reclamation of the elemental Se remains a major concern (Staicu et al. 2017). Therefore, microbes capable of removing higher amounts in less time, while internalizing the Se0 produced, may prove to be beneficial. As with tellurite, strains in this study could detoxify large quantities of selenite aerobically, with E5 removing 98 (2.0 mg/l/h), KR99 100 (2.1 mg/l/h), AV-Te-18 103 (2.2 mg/l/h), and ER-V-8 103 µg/ml (2.2 mg/l/h) (Table 1), accumulating elemental Se internally, which is revealed by a cellular color change from the original appearance to deep red (Fig. 1) (Yurkov and Beatty 1998; Rathgeber et al. 2002; Li et al. 2014b). The growth media color remained clear. Under anaerobic conditions, selenite removal was quite significant, 46 µg/ml (1.0 mg/l/h) for AV-Te-18 and 25 µg/ml (0.5 mg/l/h) for ER-V-8 after 48 h (Table 1; Fig. 3b). Strain ER-V-8 was also capable of anaerobic selenate removal, with 14 µg/ml (0.12 mg/l/h) reduced after 5 days (Table 1; Fig. 3c). As one can see, the amount of Se oxyanions removed is orders of magnitude greater than the amounts reported in previous studies and within a much shorter time frame (Macy et al. 1993; Cantafio et al. 1996; Hunter and Kuykendall 2005; Luek et al. 2014). Of note, all Se0 produced from reduction was contained inside the cells, potentially providing a means of removal (Rathgeber et al. 2002; Li et al. 2014a, b).
Unlike for tellurite, specific removal rates do not vary as much between strains in the presence of oxygen (0.62–0.77 µg selenite reduced/µg protein/day) (Table 2). This is not surprising as all bacteria had removed similar amounts and possessed similar removal profiles (Fig. 2b). However, under anaerobic conditions, removal rates differed greatly between AV-Te-18 and ER-V-8 (0.33 and 0.72 µg selenite reduced/µg protein/day, respectively) (Table 2). As ER-V-8 was the only strain capable of anaerobic selenate reduction, it is premature to make any comparative conclusions about its effectiveness of removal (0.59 µg selenate reduced/µg protein/day) (Table 2). Clearly these bacteria are much more effective and efficient at removal of Se oxyanions than any others currently proposed (Macy et al. 1993; Cantafio et al. 1996; Hunter and Kuykendall 2005; Luek et al. 2014; Staicu et al. 2017).
Conclusion
With ‘green’ technologies becoming main stream, the attractiveness of using bacteria is increasing for development of future applications. Since tellurite, selenite, and selenate contamination is becoming of great concern, bioremediation has been increasing in popularity, but the main areas in which improvement is required must be actively addressed (Cantafio et al. 1996; Hunter and Kuykendall 2005; Gadd 2010; Bonificio and Clarke 2014; Staicu et al. 2017). The strains investigated in this work appear to have potential to improve upon all aspects of removal and are excellent candidates for the development of bioremediation processes and are promising as a means of biorecovery of the elements (Hunter and Kuykendall 2005; Gadd 2010; Bonificio and Clarke 2014; Maltman et al. 2016). Further research will undoubtedly fine tune and deliver a practical and economically viable process of pollutant bioremediation and Te and Se bioreclamation.
References
Arenas F, Pugin B, Henriquez N, Areanas-Salinas M, Diaz-Vasquez W, Pozo M, Munoz C, Chasteen T, Perez-Donoso J, Vasquez C (2014) Isolation, identification and characterization of highly tellurite-resistant, tellurite-reducing bacteria from Antartica. Polar Sci 8:40–52
Bajaj M, Winter J (2014) Se (IV) triggers faster Te (IV) reduction by soil isolates of heterotrophic aerobic bacteria: formation of extracellular SeTe nanospheres. Microb Cell Fact 13:168–178
Bhatnagar I, Kim S-K (2010) Immense essence of excellence: marine microbial bioactive compounds. Mar Drugs 8(10):2673–2701
Bonificio W, Clarke D (2014) Bacterial recovery and recycling of tellurium from tellurium-containing compounds by Pseudoalteromonas sp. EPR3. J Appl Microbiol 117(5):1293–1304
Borghese R, Borsetti F, Foladori P, Ziglio G, Zannoni D (2004) Effects of the metalloid oxyanion tellurite (TeO3 2–) on growth characteristics of the phototrophic bacterium Rhodobacter capsulatus. Appl Environ Microbiol 70(11):6595–6602
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cantafio A, Hagen K, Lewis G, Bledsoe T, Nunan K, Macy J (1996) Pilot-scale selenium bioremediation of San Joaquin drainage water with Thauera selenatis. Appl Environ Microbiol 62(9):3298–3303
Csotonyi J, Stackebrandt E, Yurkov V (2006) Anaerobic respiration on tellurate and other metalloids in bacteria from hydrothermal vent fields in the eastern Pacific Ocean. Appl Environ Microbiol 72(7):4950–4956
Desai G, Paul J (1977) Simultaneous spectrophotometric determination of tetravalent and hexavalent selenium. Microchem J 22(2):176–181
Dogan N, Kantar C, Gulcan S, Dodge C, Yilmaz B, Mazmanci M (2011) Chromium(VI) bioremoval by Pseudomonas bacteria: role of microbial exudates for natural attenuation and biotreatment of Cr(VI) contamination. Environ Sci Technol 45(6):2278–2285
Elwakeel K, Atia A, Donia A (2009) Removal of Mo(VI) as oxoanions from aqueous solutions using chemically modified magnetic chitosan resins. Hydrometallurgy 97(1–2):21–28
Epelde L, Lanzen A, Blanco F, Urich T, Garbisu C (2015) Adaptation of soil microbial community structure and function to chronic metal contamination at an abandoned Pb-Zn mine. FEMS Microbiol Ecol 91(1):1–11
Fujii R, Deverel S, Hatfield D (1988) Distribution of selenium in soils of agricultural fields, western San Joaquin Valley, California. Soil Sci Soc Am J 52(5):1274–1283
Gadd G (2010) Metals, minerals and microbes: geomicrobiology and bioremediation. Microbiology 156:609–643
Hunter W, Kuykendall D (2005) Removing selenite from groundwater with an in situ biobarrier: laboratory studies. Curr Microbiol 50:145–150
Jadhav J, Kalyani D, Telke A, Phugare S, Govindwar S (2010) Evaluation of the efficacy of a bacterial consortium for the removal of color, reduction of heavy metals, and toxicity from textile dye effluent. Bioresour Technol 101(1):165–173
Javed S, Sarwar A, Tassawar M (2016) Conversion of selenite to elemental selenium by indigenous bacteria isolated from polluted areas. Chem Speciat Bioavailab 27(4):162–168
Kim Y, Kim C, Choi I, Rengaraj S, Yi J (2004) Arsenic removal using mesoporous alumina prepared via a templating method. Environ Sci Tech 38:924–931
Kim D-H, Kanaly R, Hur H-G (2012) Biological accumulation of tellurium nanorod structures via reduction of tellurite by Shewanella oneidensis MR-1. Bioresour Technol 125:127–131
Li H, Feng Y, Zou X, Luo X (2009) Study on microbial reduction of vanadium metallurgical waste water. Hydrometallurgy 99:13–17
Li B, Liu N, Li Y, Jing W, Fan J, Li D, Zhang L, Zhang X, Zhang Z, Wang L (2014a) Reduction of selenite to red elemental selenium by Rhodopseudomonas palustris strain N. PLoS One 9(4):e95955
Li D-B, Cheng Y-Y, Wu C, Li W-W, Li N, Yang Z-C, Tong Z-H, Yu H-Q (2014b) Selenite reduction by Shewanella oneidensis MR-1 is mediated by fumarate reductase in periplasm. Sci Rep 4:3735. https://doi.org/10.1038/srep03735
Luek A, Brock C, Rowan D, Rasmussen J (2014) A simplified anaerobic bioreactor for the treatment of selenium-laden discharges from non-acidic, end-pit lakes. Mine Water Environ 33:295–306
Macy J, Lawson S, DeMoll-Decker H (1993) Bioremediation of selenium oxyanions in San Joaquin drainage water using Thauera selenatis in a biological reactor system. Appl Microbiol Biotechnol 40:588–594
Maltman C, Yurkov V (2014) The impact of tellurite on highly resistant marine bacteria and strategies for its reduction. Int J Environ Eng Nat Resour 1(3):109–119
Maltman C, Yurkov V (2015) The effect of tellurite on highly resistant freshwater aerobic anoxygenic phototrophs and their strategies for reduction. Microorganisms 3(4):826–838
Maltman C, Piercey-Normore M, Yurkov V (2015) Tellurite-, tellurate-, and selenite-based anaerobic respiration by strain CM-3 isolated from gold mine tailings. Extremophiles 19(5):1013–1019
Maltman C, Walter G, Yurkov V (2016) A diverse community of metal(loid) oxide respiring bacteria are associated with tube worms in the vicinity of the Juan de Fuca Ridge black smoker field. PLoS One 11(2):e0149812
Maltman C, Donald L, Yurkov V (2017a) Two distinct periplasmic enzymes are responsible for tellurite/tellurate and selenite reduction by strain ER-Te-48 associated with the deep sea hydrothermal vent tube worms at the Juan de Fuca Ridge black smokers. Arch Microbiol 199(8):1113–1120
Maltman C, Donald L, Yurkov V (2017b) Tellurite and tellurate reduction by the aerobic anoxygenic phototroph Erythromonas ursincola, strain KR99 is carried out by a novel membrane associated enzyme. Microorganisms 5(20):https://doi.org/10.3390/microorganisms5020020
Molina R, Burra R, Perez-Donoso J, Elias A, Munoz C, Montes R, Chasteen T, Vasquez C (2010) Simple, fast, and sensitive method for quantification of tellurite in culture media. Appl Environ Microbiol 76(14):4901–4904
Moore M, Kaplan S (1992) Identification of intrinsic high-level resistance to rare-earth oxides and oxyanions in members of the class Proteobacteria: characterization of tellurite, selenite, and rhodium sesquioxide reduction in Rhodobacter sphaeroides. J Bacteriol 174(5):1505–1514
Pieper D, Reineke W (2000) Engineering bacteria for bioremediation. Curr Opin Biotechnol 11:262–270
Prakash V, Rao N, Bhatnagar A (2001) Linear optical properties of niobium-based tellurite glasses. Solid State Commun 119:39–44
Rajwade J, Paknikar K (2003) Bioreduction of tellurite to elemental tellurium by Pseudomonas mendocina MCM B-180 and its practical application. Hydrometallurgy 71:243–248
Ramon-Ruiz A, Field J, Wilkening J (2016) Recovery of elemental tellurium nanoparticles by the reduction of tellurium oxyanions in a methanogenic microbial consortium. Environ Sci Technol 50(3):1492–1500
Rathgeber C, Yurkova N, Stackebrandt E, Beatty T, Yurkov V (2002) Isolation of tellurite- and selenite-resistant bacteria from hydrothermal vents of the Juan de Fuca Ridge in the Pacific ocean. Appl Environ Microbiol 68(9):4613–4622
Ruggiero C, Boukhalfa H, Forsythe J, Lack J, Hersman L, Nue M (2005) Actinide and metal toxicity to prospective bioremediation bacteria. Environ Microbiol 7(1):88–97
Shah K, Nongkynrih J (2007) Metal hyperaccumulation and bioremediation. Biol Plant 51(4):618–634
Soudi M, Ghazvini P, Khajeh K, Gharavi S (2009) Bioprocessing of seleno-oxyanions and tellurite in a novel Bacillus sp. strain STG-83: a solution to removal of toxic oxyanions in presence of nitrate. J Hazard Mater 165:71–77
Staicu L, van Hullecusch E, Lens P (2017) Industrial selenium pollution: wastewaters and physical-chemical treatment technologies. In: van Hullebusch E (ed) Bioremediation of selenium contaminated wastewater. Springer, Cham
Wasi S, Tabrez S, Ahmad M (2013) Use of Pseudomonas spp. for the bioremediation of environmental pollutants: a review. Environ Monit Assess 185(10):8147–8155
Yang J, Tang Y, Yang K, Rouff A, Elzinga E, Hyang J (2014) Leaching characteristics of vanadium in mine tailings and soils near a vanadium titanomagnetite mining site. J Hazard Mater 264:498–504
Yong Y-C, Zhong J-J (2010) Recent advances in biodegradation in China: new microorganisms and pathways, biodegradation engineering, and bioenergy from pollutant biodegradation. Process Biochem 45(12):1937–1943
Yurkov V, Beatty T (1998) Aerobic anoxygenic phototrophic bacteria. Microbiol Mol Biol Rev 62(3):695–724
Yurkov V, Stackebrandt E, Holmes A, Fuerst J, Hugenholtz P, Golecki J, Gad’on N, Gorlenko V, Kompantseva E, Drews G (1994) Phylogenetic positions of novel aerobic bacteriochlorophyll a-containing bacteria and description of Roseococcus thiosulfatophilus gen. nov., sp. nov., Erythromicrobium ramosum gen. nov., sp. nov., and Erythrobacter litoralis sp. nov. Int J Syst Bacteriol 44:427–434
Yurkov V, Jappe J, Vermeglio A (1996) Tellurite resistance and reduction by obligately aerobic photosynthetic bacteria. Appl Environ Microbiol 62(11):4195–4198
Yurkov V, Stackebrandt E, Buss O, Vermeglio A, Gorlenko V, Beatty T (1997) Reorganization of the genus Erythromicrobium: description of “Erythromicrobium sibiricum” as Sandaracinobacter sibiricus gen. nov., sp. Nov., and of “Erythromicrobium ursincola” as Erythromonas ursincola gen. nov., sp. nov. Int J Syst Bacteriol 47(4):1172–1178
Yurkov V, Krieger S, Stackebrandt E, Beatty T (1999) Citromicrobium bathyomarinum, a novel aerobic bacterium isolated from deep-sea hydrothermal vent plume waters that contains photosynthetic pigment–protein complexes. J Bacteriol 181(15):4517–4525
Acknowledgements
This work was supported by a National Science and Engineering Research Council of Canada Discovery grant held by V. Yurkov.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Erko Stackebrandt.
Rights and permissions
About this article
Cite this article
Maltman, C., Yurkov, V. Bioremediation potential of bacteria able to reduce high levels of selenium and tellurium oxyanions. Arch Microbiol 200, 1411–1417 (2018). https://doi.org/10.1007/s00203-018-1555-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-018-1555-6