Abstract
The diversity of 43 bacterial strains isolated from Beninese entomopathogenic nematodes was investigated molecularly by analyzing the 16S rRNA, recA, and gyrB genes. Based on 16S rRNA sequence analysis, 15 bacterial strains were identified as Xenorhabdus sp., 27 strains as Photorhabdus sp., and one as Serratia sp. The Xenorhabdus strains were isolated from Steinernema nematodes and identified as Xenorhabdus indica based on 16S rRNA gene and concatenated recA and gyrB sequence analysis. However, analysis of 16S rRNA and concatenated recA and gyrB gene sequences of the Photorhabdus strains, all isolated from Heterorhabditis nematodes, resulted in two separate sub-clusters (A) and (B) within the Photorhabdus luminescens group, distinct from the existing subspecies. They share low sequence similarities with nearest phylogenetic neighbors Photorhabdus luminescens subsp. luminescens HbT, Photorhabdus luminescens subsp. caribbeanensis HG29T, and Photorhabdus luminescens subsp. noenieputensis AM7T.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Photorhabdus sp. and Xenorhabdus sp. are bacterial symbionts of entomopathogenic nematodes (EPNs) which are used around the world for biological control of insect pests (Ehlers 2001). The life cycle of EPNs is composed of a free living stage in soil and a parasitic stage inside an insect host. During the free stage, the Infective Juveniles (IJs), which represent the only stage of the nematode that is capable of infecting insects, live in a mutualistic relationship with symbiotic bacteria of the genera Photorhabdus and Xenorhabdus. These bacteria help the IJs once inside the insect host, to kill the latter by septicemia. The parasitic stage begins when the IJs enter the hemocoel of the insect host via body pores (Steinernema) or penetrate the cuticle (Heterorhabditis). There, they resume development and start feeding to become amphimictic adult males or females (Steinernema) or self-fertile hermaphrodites (Heterorhabditis) of the first generation. The subsequent generations of both Steinernema and Heterorhabditis are generally amphimictic. In most cases, 2–3 generations are completed inside the insect host before food is depleted (Emelianoff et al. 2007; Strauch and Ehlers 1998). In fact, the availability of food, mainly in the form of bacteria, is crucial for the EPNs’ development (Ehlers 2001).
Bacterial symbionts of EPNs have been described around the world, and so far, Photorhabdus (Boemare et al. 1993) and Xenorhabdus (Thomas and Poinar 1979) species have only been reported to be associated with EPNs belonging to the genera Heterorhabditis and Steinernema, respectively (Akhurst 1982; Boemare 2002; Torres-Barragan et al. 2011). These bacterial symbionts are carried by the IJs, within the whole intestine of Heterorhabditis or in a specialized vesicle of the intestine in Steinernema nematodes (Bird and Akhurst 1983; Martens and Goodrich-Blair 2005). Photorhabdus and Xenorhabdus bacterial symbionts play an important role in the infectivity of the associated EPNs to insect pests. For use in biological insect control, EPNs need to be grown at large scale under in vitro conditions, with bacteria as a main component of their diet (Ehlers 2001). Knowing the identity of the symbiotic bacteria may help in choosing the most suitable bacteria to maintain the natural association of nematode bacteria which is a requirement for a successful EPNs rearing and biocontrol.
EPNs have been retrieved from soil in many places around the world which suggests their worldwide presence. In general, local EPNs are considered more suitable for use as biological weapons against insect pests as they are better adapted to the indigenous environmental conditions than the exotic introduced EPNs (Grewal et al. 1994). In Benin, several surveys were carried out to evaluate the presence of EPNs in soil. Thirty-two nematode isolates were recovered from soil in the Southern part of the country including 29 strains identified as Heterorhabditis sonorensis and three as Heterorhabditis indica (Zadji et al. 2013). Several EPNs’ isolates were also retrieved from the Center and Northern regions of Benin, and so far, only few Steinernema strains have been reported without further characterization (Baimey et al. 2015; Zadji et al. 2013, 2014). These EPNs are currently being investigated for biological control study of mango fruit flies in Benin. In this work, we looked at the molecular diversity of symbiotic bacteria associated with nematodes collected in the Northern and Center Benin. To our knowledge, no published work has been carried out on the bacterial symbionts of Beninese EPNs despite their important role in the virulence process of the nematode against insect pests. Therefore, the main objective of this study was to investigate the biological diversity of Photorhabdus and Xenorhabdus bacteria associated with EPNs from Benin.
In the past, analysis of 16S rRNA gene sequences was traditionally used to characterize Photorhabdus and Xenorhabdus bacteria at subspecies and species level (Fischer-Le Saux et al. 1999; Liu et al. 2001; Rainey et al. 1995; Szállás et al. 1997). The inconsistent species-level grouping of some Photorhabdus strains (Akhurst et al. 2004) based on the 16S rRNA gene analysis, led to the use of more variable genes to provide complementary molecular information when evaluating bacterial phylogenies. Therefore, analysis of gyrB (Akhurst et al. 2004; Peat et al. 2010; Tóth and Lakatos 2008) and recA gene (Sergeant et al. 2006; Thanwisai et al. 2012) sequences have been used to complement 16S rRNA gene phylogeny, bacterial phenotypic, and DNA–DNA hybridization studies to better characterize new Photorhabdus and Xenorhabdus strains. Furthermore, potential lateral transfer of 16S rRNA genes was later demonstrated to exist in the Photorhabdus and Xenorhabdus clades (Tailliez et al. 2010) which may confound the classification of bacterial isolates, especially when only this gene is considered. In this study of new isolates from EPN from Benin, gyrB and recA gene sequences were, therefore, used in addition to 16S rRNA genes.
Materials and methods
Isolation and identification of entomopathogenic nematodes and their phylogenetic position
EPNs considered in this study were isolated from soil samples collected in the North and Center of Benin (Table 1) as previously described (Zadji et al. 2013) using the Galleria mellonella (Gm) baiting method (Bedding and Akhurst 1975). After isolation, EPNs were multiplied in the laboratory to have enough material for molecular identification and bacteria isolation. In a Petri dish, IJs were used to infect Gm larvae at the ratio 100IJs/Gm larva, at room temperature (22 °C). After approximately 7 days, dead Gm larvae were individually transferred onto a modified white trap (White 1927) to collect the new generation of IJs that would exit the Gm cadaver approximately 10 days later. Harvested IJs were used to infect new Gm or stored in the incubator (15 °C) for further use. To identify the nematodes, partial ITS regions were amplified and sequenced using primers proposed by Vrain et al. (1992).
Isolation of Photorhabdus and Xenorhabdus bacteria from nematodes
Mature Gm larvae were infected with IJs of pure culture of each nematode isolate at the rate of 100 IJs per Gm larva. After 48 h, a single moribund insect was washed 5–10 min in a glass staining block with 70% ethanol. A drop of the insect’s hemolymph was then streaked onto a Nutrient Bromothymol Agar (NBTA) plate containing 2,3,5 Triphenyltetrazolium chloride and Bromothylmol blue as described by Akhurst (1980) prior to incubation at 28 °C during 48 h. Bacterial isolates were purified by picking a single colony and plating it onto a successive new NBTA plate until homogenous colonies were observed.
Morphological examination of bacterial colonies
Visual characteristics such as colony diameter, shape, and color after 48 h of incubation at 28 °C on NBTA plates were recorded. In addition, the capacity for bioluminescence of some isolates was visually assessed in darkness (Kazimierczak et al. 2017).
Molecular characterization of symbiotic bacteria
Bacterial genomic DNA was extracted following the protocol of Pitcher et al. (1989) from a single colony of each bacterial isolate (Table 1).
The near-complete 16S rRNA gene was amplified using primers: forward 5′-AGAGTTTGATCCTGGCTCAG-3′ and reverse 5′-AAGGAGGTGATCCAGCCGCA-3 as previously described (Cleenwerck et al. 2002). PCR products were cleaned with Nucleofast 96 PCR membrane in the Tecan Genesis Workstation 200 machine. Partial 16S rRNA gene sequencing was achieved on all isolated bacterial strains using primer 5′-TATTACCGCGGCTGCTGGCA-3′ (Cleenwerck et al. 2007) producing a fragment of 427 nucleotides that covers V1–V2 variable regions. Sequencing was performed with the ABI Prism Big Dye Terminator cycle sequencing ready reaction kit and an Applied Biosystems 3130xl DNA sequencer, using the protocols of the manufacturer (Applied Biosystems).
Partial sequences were identified by blasting them in NCBI and based on their grouping, representatives for near-complete 16S rRNA gene sequencing were selected, so that different bacterial clusters and at least one bacterial strain from each nematode isolate were represented. Full sequencing of the 16S rRNA gene was performed on the selected strains using six sequencing primers described previously (Coenye et al. 1999). In addition, amplification and partial sequencing of two housekeeping genes, the recA and gyrB, were also achieved using primers and thermal cycling conditions as previously published (Tailliez et al. 2006).
Sequences were assembled in Bionumerics 7 (Applied Maths) and deposited in GenBank (accession numbers provided in Supplementary Table 1). Using Clustal W included in Mega 6.06 (Tamura et al. 2013), sequences of each gene were first aligned with reference type strains of Photorhabdus and Xenorhabdus retrieved from GenBank. Afterwards, non-overlapping reference sequences were removed and alignments were trimmed to the most common size of all sequences and visually inspected. Remaining sequences were realigned and used to construct neighbor-joining (Saitou and Nei 1987) phylogenetic trees, in Mega 6.06, using the Kimura two parameter model (Kimura 1980). Aligned recA and gyrB gene sequences were exported from Mega 6.06 and concatenated using an in-house Python script. Concatenated sequences were realigned and used to reconstruct a neighbor-joining phylogeny in Mega 6.06. Bootstrap percentages (1000 replicates) more than 50% were shown at the nodes of branches on the trees.
Results
Phylogenetic assignment of nematodes
The phylogenetic analysis of the ITS fragments showed that collected nematode isolates (Table 1) were distributed within Heterorhabditis and Steinernema clusters. Ten nematode isolates shared 99.1–100% sequence similarity with Heterorhabditis sonorensis (junior synonym of Heterorhabditis taysearae) (Supplementary Fig. 1), while two isolates clustered with Heterorhabditis indica (99.6–99.8%). In addition, 4 isolates formed a separate cluster within the Steinernema group with Steinernema abbasi representing their closest phylogenetic relative (97.3–97.7%) with a 98% bootstrap value.
Morphological examination of bacterial colonies
In total, we obtained 43 bacterial strains from the 16 nematode isolates considered in this study (Table 1). These bacterial strains were assigned an R number (accession number) and were stored in the research collection of the Laboratory of Microbiology at Ghent University (LM-UGent) at − 80 °C. All bacterial strains were able to grow after 48 h with maximum colony diameter of 3 mm. Color of the colonies varied. They appeared on NBTA plate as either green or blue and sometimes reddish. Most of the bacterial strains were able to absorb dye on NBTA plates and bioluminescence was observed for some Photorhabdus strains and not for Xenorhabdus strains after 48 h of incubation (Table 1).
Molecular characterization of symbiotic bacteria
16S rRNA gene
Comparison of partial 16S rRNA gene sequences (414 bp) of the 43 isolated bacterial strains with the type strains of references species showed that 27 and 15 strains were, respectively, distributed within Photorhabdus and Xenorhabdus groups. One bacterial strain, isolated from Steinernema sp., formed a distinct cluster (D) together with Serratia nematodiphila (Supplementary Fig. 2). All bacterial strains isolated from Heterorhabditis nematodes, grouped with Photorhabdus reference strains and bacteria isolated from Steinernema nematodes clustered within Xenorhabdus species. The 27 Photorhabdus strains were all distributed within the Photorhabdus luminescens group with 24 of them forming a separate sub-cluster (A) (Supplementary Fig. 2). The 3 other strains grouped in a sub-cluster (B) together with P. luminescens subsp. akhurstii and P. luminescens subsp. noenieputensis. All Xenorhabdus strains shared identical partial 16S rRNA and were grouped together in cluster (C) with Xenorhabdus indica. Based on the partial 16S rRNA gene grouping, 7 and 18 representative strains from Xenorhabdus and Photorhabdus groups were selected for further molecular analysis.
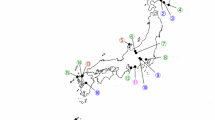
Analysis of full 16S rRNA gene sequences (1427 bp) revealed that the Xenorhabdus strains were highly similar (100% sequence similarity) and confirmed their highest sequences similarity (99.7%) with X. indica DSM 17382T (Fig. 1). In addition, as noted with the partial 16S rRNA gene analysis above, Photorhabdus strains formed two separate sub-clusters (A) and (B) (Fig. 2) based on the analysis of full sequences of 16S rRNA gene. Strains of sub-cluster (A) showed 7–14 nucleotide differences of the 16S rDNA (1349 positions compared) sequences with strains of sub-cluster (B). A cluster containing P. luminescens subsp. luminescens Hb and P. luminescens subsp. sonorensis Carbora and CH35 appeared to be a sister group of sub-cluster (A), and they all together formed a sister group of sub-cluster (B) (Fig. 2). P. luminescens subsp. luminescens Hb shared 98.7–99.3 and 98.7–98.8% sequence similarities with strains in sub-cluster (A) and (B), respectively, while P. luminescens subsp. sonorensis Carbora had 98.8–99.4 and 98.7% nucleotide identity with Beninese strains in sub-cluster (A) and (B), respectively. The Serratia strain R-52436 shared 99.7% 16S rRNA nucleotide identity with S. nematodiphila with high bootstrap value (100%).
Neighbor-joining tree based on 1,427 kb 16S rRNA sequences for 7 Xenorhabdus strains (indicated in bold), from entomopathogenic nematodes (EPN strains in brackets next to the bacterial strain) recovered from Beninese soils and reference strains. Bootstrap values above 50% based on 1000 replicates are indicated at the nodes of each branch. Numbers before reference strains name correspond to GenBank accession numbers. Proteus mirabilis and Serratia nematodiphila were used as outgroups. S, Steinernema strain
Neighbor-joining tree based on 1,349 kb 16S rRNA sequences for 18 Photorhabdus and 1 Serratia strain (indicated in bold), from entomopathogenic nematodes (EPN strain in brackets next to the bacterial strain) recovered in Beninese soils and reference strains. Bootstrap values above 50% based on 1000 replicates are indicated at the nodes of each branch. Numbers before reference strains name correspond to GenBank accession numbers. Proteus mirabilis was used as outgroup. H, Heterorhabditis strain, S, Steinernema strain
GyrB and recA genes
To increase the resolution of the molecular identification, gyrB and recA genes were explored. We were unable to sequence recA and gyrB gene sequences of the Serratia bacterial isolate (R-52436), because unexpectedly, the strain lost its viability and could not be recovered. The neighbor-joining trees reconstructed based on concatenated sequences of these two protein coding genes for Photorhabdus and Xenorhabdus strains are presented in Figs. 3 and 4. Sequences of individual genes were also used to build neighbor-joining phylogenetic trees which are presented in Supplementary Fig. 3 to 6.
Neighbor-joining tree based on concatenated recA and gyrB gene sequences showing the phylogenetic position of Beninese Photorhabdus strains (in bold) among type strains of described Photorhabdus species. Bootstrap values above 50% based on 1000 replicates are indicated at the nodes of each branch. Proteus mirabilis was used as outgroup
Neighbor-joining tree based on concatenated recA and gyrB gene sequences showing the phylogenetic position of Beninese Xenorhabdus strains (in bold) among type strains of described Xenorhabdus species. Bootstrap values above 50% based on 1000 replicates are indicated at the nodes of each branch. Proteus mirabilis was used as outgroup
For Photorhabdus, the two sub-clusters (A) and (B) observed in the 16S rRNA phylogeny were recovered in the concatenated phylogeny reconstructed based on recA and gyrB genes (Fig. 3) with high bootstrap value (100%). Strains in sub-cluster (B) shared a similar level of sequence similarity (94.8–96.1%) with the type strains of several subspecies of P. luminescens, including P. luminescens subsp. luminescens Hb, P. luminescens subsp. caribbeanensis HG29 and P. luminescens subsp. noenieputensis AM7 and P. luminescens subsp. sonorensis Caborca and CH35. For the strains of sub-cluster (B), concatenated recA and gyrB gene sequences showed a similar range of similarity (94.7–97.0%) to P. luminescens subsp. luminescens Hb, P. luminescens subsp. caribbeanensis HG29, P. luminescens subsp. noenieputensis AM7, and P. luminescens subsp. sonorensis Caborca and CH35. In the gyrB phylogeny, Photorhabdus strains were clearly separated in sub-clusters (A) and (B) and showed the closest similarity (95.9%) to P. luminescens subsp. noenieputensis (Supplementary Fig. 3). In the recA phylogeny, sub-cluster (B) was clearly delineated, but sub-cluster (A) strains were more dispersed and some grouped with different P. luminescens subspecies (Supplementary Fig. 4).
For the Xenorhabdus strains, comparison of concatenated sequences of recA and gyrB (Fig. 4) with the reference type strains in GenBank revealed that they shared 98.5% nucleotide identity with X. indica. Furthermore, analysis of gyrB (Supplementary Fig. 5) and recA (Supplementary Fig. 6) sequences confirmed their closest similarity with X. indica (98.3–98.4 and 98.8–98.9%, respectively).
Discussion
Bacterial symbionts of EPNs play an important role in the nematode’s virulence against insect pests (Liu et al. 2001).
The main objective of this study was to investigate the biological diversity of Photorhabdus and Xenorhabdus bacteria associated with EPNs from Benin.
Bacterial colonies isolated from Beninese Steinernema and Heterorhabditis nematodes, turned blue, dark-green, or red on NBTA plates after 48 h of incubation. This indicates that they are able to absorb the Bromothymol blue dye contained in the NBTA medium. The variation of colony color has been attributed to bacterial phase variation. Indeed, Xenorhabdus and Photorhabdus bacteria have been described as occurring in two forms: phase I, occurring in IJs, and phase II, occurring under in vitro conditions (Akhurst 1980; Smigielski et al. 1994). Phase II bacteria have been demonstrated to have less ability to absorb dye from NBTA medium compared to phase I bacteria (Boemare and Akhurst 1988). Some isolates identified as Photorhabdus species showed bioluminescence in the darkness which is a typical characteristic of Photorhabdus species (Forst and Nealson 1996).
Phylogenetic analysis of the 43 bacterial symbionts isolated from Beninese EPNs indicated that most bacterial symbionts isolated from Steinernema nematodes were identified as Xenorhabdus species, and Photorhabdus species were isolated from Heterorhabditis EPNs. This result confirmed the assumption established over the last 20 years that Xenorhabdus is present in Steinernema and Photorhabdus in Heterorhabditis (Forst et al. 1997). However, the bacterial strain (R-52436), identified as Serratia nematodiphila based on 16S rRNA gene analysis, was isolated from a nematode strain (138d) which appears to be a Steinernema species based on the ITS fragments analysis. Serratia nematodiphila has been isolated from Heterorhabditidoides chongmingensis (Zhang et al. 2008), a newly described nematode proposed to be part of the EPNs group. The present study is the first report of a Serratia strain isolated from G. mellonella larvae infected with Steinernema nematodes. However, despite the surface sterilization of the G. mellonella cadaver with 70% alcohol prior to bacterial isolation and the fact that we did not pick up members of other bacterial genera, it cannot be totally excluded that R-52436 might be a contaminant. In the previous studies, Steinernema have occasionally been found to be associated with bacteria other than Xenorhabdus (Aguillera et al. 1993; Elawad et al. 1999; Lysenko and Weiser 1974), although those bacteria were demonstrated to originate from the cuticle (Bonifassi et al. 1999). In addition, for EPN of Heterorhabditis indica, Babic et al. (2000) reported, in addition to the typical symbiotic bacteria (Photorhabdus luminescens subsp. akhurstii), the presence of Ochrobactrum spp. bacteria.
The 16S rRNA gene sequence analysis for the Xenorhabdus strains showed their high similarity (99.7%) with X. indica DSM 17382T. In addition, concatenated recA and gyrB sequences confirmed their identification as X. indica (98.5% similarity with strain DSM 17382T). This species has been reported for the first time by Somvanshi et al. (2006) and found to be associated with Steinernema thermophilum, junior synonym of S. abbasi (Hunt and Subbotin 2016; Nguyen and Hunt 2007; Tailliez et al. 2006). In our case, these bacterial isolates were, indeed, isolated from nematodes clustering with S. abbasi based on ITS regions sequences analysis (Supplementary Fig. 1). Recently, X. indica has also been reported to be associated to Steinernema yirgalemense (Ferreira et al. 2016). It can, therefore, be assumed that X. indica may not be specifically associated with a single nematode species, although S. yirgalemense forms a sister clade to S. abbasi/S. thermophilum based on the ITS phylogeny (Supplementary Fig. 1). The association of a single bacterial species to different nematode species within and in between clades is increasingly reported in recent years. More than 17 host switches have been reported by Lee and Stock (2010). Furthermore, Dreyer et al. (2017) have recently demonstrated three new Xenorhabdus–Steinernema associations with X. khoisanae found in association with Steinernema jeffreyense and Steinernema sacchari which belong to distantly related EPN clades V an III, respectively. The same authors reported the association of Steinernema nguyeni with X. bovieni, initially reported (Stock 2015) to be associated with nematodes in the Affine clade such as Steinernema affine and Steinernema intermedium on one hand and with nematodes in Feltiae clade such as Steinernema feltiae on another hand.
Analysis of 16S rRNA, gyrB, and recA genes sequences of Photorhabdus strains demonstrated that they belong to the P. luminescens cluster. They constitute two separate sub-clusters (A) and (B) within the P. luminescens group in the phylogenetic trees of 16S rRNA and concatenated gyrB and recA genes (Figs. 2, 3). Based on the concatenated recA and gyrB sequence analysis, the two sub-clusters (A) and (B) show similar levels of sequence similarity (approx. 94 to 97%) to several other P. luminescens subspecies. These relatively low similarity values indicate that the two sub-clusters (A) and (B) probably represent two different new subspecies within P. luminescens group.
Strains contained in sub-cluster (A) were isolated from nematodes identified, based on ITS region analysis (Supplementary Fig. 1), as H. sonorensis, a junior synonym of H. taysearae (Hunt and Subbotin 2016). Bacterial strains in sub-cluster (B) were isolated from nematode isolates grouping with H. indica (Supplementary Fig. 1). H. taysearae was described as having P. luminescens subsp. sonorensis as bacterial symbiont (Orozco et al. 2013), while H. indica has been reported to live in association with P. luminescens subsp. akhurstii (Fischer-Le Saux et al. 1999). In our study, we found H. taysearae and H. indica to be in association with new sub-clusters (A) and (B), respectively. Both Heterorhabditis species belong to the H. indica clade with each of them belonging to one of two proposed sub-clades (Spiridonov and Subbotin 2016).
In this study, in addition to the conserved 16S rRNA genes, also the more variable housekeeping genes gyrB and recA were sequenced to assess for the first time the diversity of Photorhabdus and Xenorhabdus isolates from EPN recovered from soil from Benin. Some or all three of these genes have been used in recent years to characterize EPN bacteria (Cimen et al. 2016; Fukruksa et al. 2017; Muangpat et al. 2017; Thanwisai et al. 2012). However, for more comprehensive taxonomic characterization and definition of new groups, a multigene approach involving more housekeeping genes (recA, gyrB, gltX, dnaN, and infB) has recently been proposed (Tailliez et al. 2010, 2012) to increase the robustness of the phylogeny of Photorhabdus and Xenorhabdus bacteria. A threshold of 97% nucleotide identity has been proposed (Tailliez et al. 2010) to distinguish species in Xenorhabdus and subspecies in Photorhabdus groups. In our study, in accordance with 16S rRNA gene analysis, concatenated recA and gyrB sequence phylogeny clearly supported the clustering of Beninese Photorhabdus strains in separate sub-clusters (A) and (B). Nevertheless, further molecular information based on gltX, dnaN and infB genes and some phenotypic studies are needed to fully clarify the status of sub-clusters (A) and (B) strains in the P. luminescens group as potential new subspecies of Photorhabdus luminescens.
Overall, this molecular characterization study of symbiotic bacteria of Beninese EPNs allowed us to find two new groups of Photorhabdus luminescens strains associated with H. taysearae and H. indica. In addition, Xenorhabdus indica was identified in association with EPNs that cluster with Steinernema abbasi based on ITS region analysis. These bacteria will be used in large-scale multiplication of the associated Beninese EPNs, as they constitute their major food source, for insect pest biological control purposes.
References
Aguillera MM, Hodge NC, Stall RE, Smart GC (1993) Bacterial symbionts of Steinernema scapterisci. J Invertebr Pathol 62:68–72
Akhurst R (1980) Morphological and functional dimorphism in Xenorhabdus spp., bacteria symbiotically associated with the insect pathogenic nematodes Neoaplectana and Heterorhabditis. J Gen Microbiol 121:303–309
Akhurst R (1982) Antibiotic activity of Xenorhabdus spp., bacteria symbiotically associated with insect pathogenic nematodes of the families Heterorhabditidae and Steinernematidae. J Gen Microbiol 128:3061–3065
Akhurst R, Boemare N, Janssen P, Peel M, Alfredson D, Beard C (2004) Taxonomy of Australian clinical isolates of the genus Photorhabdus and proposal of Photorhabdus asymbiotica subsp. asymbiotica subsp. nov. and P. asymbiotica subsp. australis subsp. nov. Int J Syst Evol Microbiol 54:1301–1310
Babic I, Fischer-Le Saux M, Giraud E, Boemare N (2000) Occurrence of natural dixenic associations between the symbiont Photorhabdus luminescens and bacteria related to Ochrobactrum spp. in tropical entomopathogenic Heterorhabditis spp.(Nematoda, Rhabditida). Microbiology 146:709–718
Baimey H, Zadji L, Afouda L, Moens M, Decraemer W (2015) Influence of pesticides, soil temperature and moisture on entomopathogenic nematodes from southern Benin and control of underground termite nest populations. Nematology 17:1057–1069
Bedding R, Akhurst R (1975) A simple technique for the detection of insect paristic rhabditid nematodes in soil. Nematologica 21:109–110
Bird A, Akhurst R (1983) The nature of the intestinal vesicle in nematodes of the family Steinernematidae. Int J Parasitol 13:599–606
Boemare N (2002) Biology, taxonomy and systematics of Photorhabdus and Xenorhabdus. In: Gaugler R (ed) Entomopathogenic nematology. CABI Publishing, New York, pp 35-56
Boemare N, Akhurst R (1988) Biochemical and physiological characterization of colony form variants in Xenorhabdus spp. (Enterobacteriaceae). J Gen Microbiol 134:751–761
Boemare N, Akhurst R, Mourant R (1993) DNA relatedness between Xenorhabdus spp. (Enterobacteriaceae), symbiotic bacteria of entomopathogenic nematodes, and a proposal to transfer Xenorhabdus luminescens to a new genus, Photorhabdus gen. nov. Int J Syst Bacteriol 43:249–255
Bonifassi E, Fischer-Le Saux M, Boemare N, Lanois A, Laumond C, Smart G (1999) Gnotobiological study of infective juveniles and symbionts of Steinernema scapterisci: a model to clarify the concept of the natural occurrence of monoxenic associations in entomopathogenic nematodes. J Invertebr Pathol 74:164–172
Cimen H, Půža V, Nermuť J, Hatting J, Ramakuwela T, Faktorova L, Hazir S (2016) Steinernema beitlechemi n. sp., a new entomopathogenic nematode (Nematoda: Steinernematidae) from South Africa. Nematology 18:439–453
Cleenwerck I, Vandemeulebroecke K, Janssens D, Swings J (2002) Re-examination of the genus Acetobacter, with descriptions of Acetobacter cerevisiae sp. nov. and Acetobacter malorum sp. nov. Int J Syst Evol Microbiol 52:1551–1558
Cleenwerck I, Camu N, Engelbeen K, De Winter T, Vandemeulebroecke K, De Vos P, De Vuyst L (2007) Acetobacter ghanensis sp. nov., a novel acetic acid bacterium isolated from traditional heap fermentations of Ghanaian cocoa beans. Int J Syst Evol Microbiol 57:1647–1652
Coenye T, Falsen E, Vancanneyt M, Hoste B, Govan JR, Kersters K, Vandamme P (1999) Classification of Alcaligenes faecalis-like isolates from the environment and human clinical samples as Ralstonia gilardii sp. nov. Int J Syst Evol Microbiol 49:405–413
Dreyer J, Malan AP, Dicks LMT (2017) Three novel Xenorhabdus–Steinernema associations and evidence of strains of X. khoisanae switching between different clades. Curr Microbiol 74:938–942
Ehlers R-U (2001) Mass production of entomopathogenic nematodes for plant protection. Appl Microbiol Biotechnol 56:623–633
Elawad S, Robson R, Hague N (1999) Observations on the bacterial symbiont associated with the nematode, Steinernema abbasi (Steinernematidae: Nematoda). In: COST, pp 105–111
Emelianoff V, Sicard M, Le Brun N, Moulia C, Ferdy J-B (2007) Effect of bacterial symbionts Xenorhabdus on mortality of infective juveniles of two Steinernema species. Parasitol Res 100:657–659
Ferreira T, Van Reenen C, Tailliez P, Pagès S, Malan A, Dicks L (2016) First report of the symbiotic bacterium Xenorhabdus indica associated with the entomopathogenic nematode Steinernema yirgalemense. J Helminthol 90:108–112
Fischer-Le Saux M, Viallard V, Brunel B, Normand P, Boemare NE (1999) Polyphasic classification of the genus Photorhabdus and proposal of new taxa: P. luminescens subsp. luminescens subsp. nov. P. luminescens subsp. akhurstii subsp. nov., P. luminescens subsp. laumondii subsp. nov., P. temperata sp. nov., P. temperata subsp. temperata subsp. nov. and P. asymbiotica sp. nov. Int J Syst Evol Microbiol 49:1645–1656
Forst S, Nealson K (1996) Molecular biology of the symbiotic-pathogenic bacteria Xenorhabdus spp. and Photorhabdus spp. Microbiol Rev 60:21–43
Forst S, Dowds B, Boemare N, Stackebrandt E (1997) Xenorhabdus and Photorhabdus spp.: bugs that kill bugs. Annu Rev Microbiol 51:47–72
Fukruksa C, Yimthin T, Suwannaroj M, Muangpat P, Tandhavanant S, Thanwisai A, Vitta A (2017) Isolation and identification of Xenorhabdus and Photorhabdus bacteria associated with entomopathogenic nematodes and their larvicidal activity against Aedes aegypti. Parasites Vectors 10:440
Grewal PS, Selvan S, Gaugler R (1994) Thermal adaptation of entomopathogenic nematodes: niche breadth for infection, establishment, and reproduction. J Therm Biol 19:245–253
Hunt DJ, Subbotin SA (2016) Taxonomy and systematics. In: Hunt DJ, Nguyen KB (eds) Advances in Taxonomy and Phylogeny of Entomopathogenic Nematodes of the Steinernematidae and Heterorhabditidae, vol 12. Koninklijke Brill NV, Leiden, pp 13–58
Kazimierczak W, Skrzypek H, Sajnaga E, Skowronek M, Waśko A, Kreft A (2017) Strains of Photorhabdus spp. associated with polish Heterorhabditis isolates: their molecular and phenotypic characterization and symbiont exchange. Arch Microbiol 199:979–989
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Lee M-M, Stock SP (2010) A multigene approach for assessing evolutionary relationships of Xenorhabdus spp.(γ-Proteobacteria), the bacterial symbionts of entomopathogenic Steinernema nematodes. J Invertebr Pathol 104:67–74
Liu J, Berry RE, Blouin MS (2001) Identification of symbiotic bacteria (Photorhabdus and Xenorhabdus) from the entomopathogenic nematodes Heterorhabditis marelatus and Steinernema oregonense based on 16S rDNA sequence. J Invertebr Pathol 77:87–91
Lysenko O, Weiser J (1974) Bacteria associated with the nematode Neoaplectana carpocapsae and the pathogenicity of this complex for Galleria mellonella larvae. J Invertebr Pathol 24:332–336
Martens EC, Goodrich-Blair H (2005) The Steinernema carpocapsae intestinal vesicle contains a subcellular structure with which Xenorhabdus nematophila associates during colonization initiation. Cell Microbiol 7:1723–1735
Muangpat P, Yooyangket T, Fukruksa C, Suwannaroj M, Yimthin T, Sitthisak S, Chantratita N, Vitta A, Tobias NJ, Bode HB (2017) Screening of the antimicrobial activity against drug resistant bacteria of Photorhabdus and Xenorhabdus associated with entomopathogenic nematodes from Mae Wong National Park, Thailand. Front Microbiol 8:1142
Nguyen K, Hunt D (2007) Entomopathogenic nematodes: systematics, phylogeny and bacterial symbionts vol 5. Nematology monographs and perspectives. Brill, Leiden-Boston
Orozco RA, Hill T, Stock SP (2013) Characterization and Phylogenetic Relationships of Photorhabdus luminescens subsp. sonorensis (γ-Proteobacteria: Enterobacteriaceae), the Bacterial Symbiont of the Entomopathogenic Nematode Heterorhabditis sonorensis (Nematoda: Heterorhabditidae). Curr Microbiol 66:30–39
Peat SM, Waterfield NR, Marokházi J, Fodor A, Adams BJ (2010) A robust phylogenetic framework for the bacterial genus Photorhabdus and its use in studying the evolution and maintenance of bioluminescence: a case for 16S, gyrB, and glnA. Mol Phylogenet Evol 57:728–740
Pitcher D, Saunders N, Owen R (1989) Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett Appl Microbiol 8:151–156
Rainey F, Ehlers R-U, Stackebrandt E (1995) Inability of the polyphasic approach to systematics to determine the relatedness of the genera Xenorhabdus and Photorhabdus. Int J Syst Evol Microbiol 45:379–381
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sergeant M, Baxter L, Jarrett P, Shaw E, Ousley M, Winstanley C, Morgan JAW (2006) Identification, typing, and insecticidal activity of Xenorhabdus isolates from entomopathogenic nematodes in United Kingdom soil and characterization of the xpt toxin loci. Appl Environ Microbiol 72:5895–5907
Smigielski AJ, Akhurst RJ, Boemare NE (1994) Phase variation in Xenorhabdus nematophilus and Photorhabdus luminescens: differences in respiratory activity and membrane energization. Appl Environ Microbiol 60:120–125
Somvanshi VS, Lang E, Ganguly S, Swiderski J, Saxena AK, Stackebrandt E (2006) A novel species of Xenorhabdus, family Enterobacteriaceae: Xenorhabdus indica sp. nov., symbiotically associated with entomopathogenic nematode Steinernema thermophilum Ganguly and Singh 2000. Syst Appl Microbiol 29:519–525
Spiridonov SE, Subbotin SA (2016) Phylogeny and phylogeography of Heterorhabditis and Steinernema. Adv Entomopathog Nematode Taxon Phylogeny 12:413–427
Stock SP (2015) Diversity, biology and evolutionary relationships. In: R C-H (ed) Nematode pathogenesis of insects and other pests. Springer, Berlin, pp 3–27
Strauch O, Ehlers R-U (1998) Food signal production of Photorhabdus luminescens inducing the recovery of entomopathogenic nematodes Heterorhabditis spp. in liquid culture. Appl Microbiol Biotechnol 50:369–374
Szállás E, Koch C, Fodor A, Burghardt J, Buss O, Szentirmai A, Nealson KH, Stackebrandt E (1997) Phylogenetic evidence for the taxonomic heterogeneity of Photorhabdus luminescens. Int J Syst Evol Microbiol 47:402–407
Tailliez P, Pages S, Ginibre N, Boemare N (2006) New insight into diversity in the genus Xenorhabdus, including the description of ten novel species. Int J Syst Evol Microbiol 56:2805–2818
Tailliez P, Laroui C, Ginibre N, Paule A, Pagès S, Boemare N (2010) Phylogeny of Photorhabdus and Xenorhabdus based on universally conserved protein-coding sequences and implications for the taxonomy of these two genera. Proposal of new taxa: X. vietnamensis sp. nov. P. luminescens subsp. caribbeanensis subsp. nov., P. luminescens subsp. hainanensis subsp. nov., P. temperata subsp. khanii subsp. nov., P. temperata subsp. tasmaniensis subsp. nov., and the reclassification of P. luminescens subsp. thracensis as P. temperata subsp. thracensis comb. nov. Int J Syst Evol Microbiol 60:1921–1937
Tailliez P, Pagès S, Edgington S, Tymo LM, Buddie AG (2012) Description of Xenorhabdus magdalenensis sp. nov., the symbiotic bacterium associated with Steinernema australe. Int J Syst Evol Microbiol 62:1761–1765
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Thanwisai A, Tandhavanant S, Saiprom N, Waterfield NR, Long PK, Bode HB, Peacock SJ, Chantratita N (2012) Diversity of Xenorhabdus and Photorhabdus spp. and their symbiotic entomopathogenic nematodes from Thailand. PLoS One 7:e43835
Thomas GM, Poinar GO (1979) Xenorhabdus gen. nov., a genus of entomopathogenic, nematophilic bacteria of the family Enterobacteriaceae. Int J Syst Bacteriol 29:352–360
Torres-Barragan A, Suazo A, Buhler WG, Cardoza YJ (2011) Studies on the entomopathogenicity and bacterial associates of the nematode Oscheius carolinensis. Biol Control 59:123–129
Tóth T, Lakatos T (2008) Photorhabdus temperata subsp. cinerea subsp. nov., isolated from Heterorhabditis nematodes. Int J Syst Evol Microbiol 58:2579–2581
Vrain T, Wakarchuk D, Levesque A, Hamilton R (1992) Intraspecific rDNA restriction fragment length polymorphism in the Xiphinema americanum group. Fund Appl Nematol 15:563–573
White G (1927) A method for obtaining infective nematode larvae from cultures. Science 66:302–303
Zadji L, Baimey H, Afouda L, Houssou FG, Waeyenberge L, de Sutter N, Moens M, Decraemer W (2013) First record on the distribution of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) in Southern Benin. Russ J Nematol 21:117–130
Zadji L, Baimey H, Afouda L, Moens M, Decraemer W (2014) Comparative susceptibility of Macrotermes bellicosus and Trinervitermes occidentalis (Isoptera: Termitidae) to entomopathogenic nematodes from Benin. Nematology 16:719–727
Zhang C, Liu J, Xu M, Sun J, Yang S, An X, Gao G, Lin M, Lai R, He Z (2008) Heterorhabditidoides chongmingensis gen. nov., sp. nov.(Rhabditida: Rhabditidae), a novel member of the entomopathogenic nematodes. J Invertebr Pathol 98:153–168
Acknowledgements
This research was supported by the Special Research Fund (BOF) of Ghent University (Belgium), grant 01W00713. The authors would like to thank Pia Clercx and Andy Vierstraete for the excellent technical assistance and Nancy de Sutter for maintaining the nematode cultures at Flanders Research Institute for Agriculture, Fisheries and Food (ILVO).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Erko Stackebrandt.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Godjo, A., Afouda, L., Baimey, H. et al. Molecular diversity of Photorhabdus and Xenorhabdus bacteria, symbionts of Heterorhabditis and Steinernema nematodes retrieved from soil in Benin. Arch Microbiol 200, 589–601 (2018). https://doi.org/10.1007/s00203-017-1470-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-017-1470-2