Abstract
Strain WS9, a mutualistic-associated bacterium, was isolated from an unknown entomopathogenic Steinernema nematode, collected from a litchi orchard in Friedenheim, Mpumalanga, South Africa. Based on phenotypic and phylogenetic data of the 16S rRNA, gltX, recA, dnaN, gyrB and infB gene sequences, strain WS9 is identified as X. griffiniae. Strain WS9 has antibacterial activity against Gram-positive and Gram-negative bacteria. This is the first report of an association between X. griffiniae and an unknown Steinernema species from South Africa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Members of the genus Xenorhabdus are Gram-negative, asporogenic, fermentative, facultative anaerobic rods with a respiratory and fermentative metabolism, and belong to the family Enterobacteriaceae. They grow optimally at 28 °C, are oxidase and catalase negative and do not reduce nitrate to nitrite (Thomas and Poinar 1997; Akhurst and Boemare 2005). Acid is produced from the fermentation of glucose, but without the release of CO2. N-acetyl-glucosamine, glycerol, fructose and mannose are usually fermented (Akhurst and Boemare 2005). Xenorhabdus species are known to produce two phenotypic variants that differ in morphology and physiology. Phase I cells are larger than phase II cells, absorb certain dyes and produce proteases, lipases and antibiotics (Akhurst 1980; Boemare and Akhurst 1988).
Xenorhabdus spp. are closely associated with entomopathogenic nematodes of the family Steinernematidae that infects insects such as Lepidoptera, Diptera, Orthoptera, Coleoptera and Hymenoptera (Laumond et al. 1979; Poinar 1979). Although the association between Xenorhabdus spp. and nematodes is species-specific, a single species may infect different Steinernema spp. (Fischer-Le Saux et al. 1997; Lee and Stock 2010). More than 20 Xenorhabdus species have been characterised, with approximately 11 of these species being associated with more than one Steinernema nematode species (Tailliez et al. 2006; Stock 2015).
Steinernema dauer larvae or non-feeding infective juvenile (IJ) transport Xenorhabdus to the haemocoel by entering natural openings on the body of the insect. Once in the haemocoel, the IJ nematodes release an immunesuppressive agent that suppresses the activity of antimicrobial peptides produced by the insect (Götz et al. 1981). IJ develop into amphimictic females and males that feeds on the proliferating bacteria. Endo- and exotoxins (e.g., DNases, lipases and lecithinases) are released by the bacteria, killing the insect within 24–48 h. Colonization of the cadaver by other microorganisms is prevented by the release of antimicrobial compounds from the respective Xenorhabdus species. The bacteria and bio-converted host tissue supports the sexual reproduction of the nematodes as long as nutrients are available. The nematodes, colonized with a few cells of Xenorhabdus are then released into the environment to repeat the cycle of infection (Adams and Nguyen 2002).
In this study, the bacterial strain WS9 was isolated from an unknown entomopathogenic nematode Steinernema sp. WS9. The bacteria was identified using phenotypic and genotypic characteristics.
Materials and methods
Isolation of Xenorhabdus sp. and maintenance of cultures
Galleria mellonella L. (Lepidoptera: Pyralidae) larvae were exposed to IJs of Steinernema sp. WS9 (KP325086; MF443108) collected from a litchi orchard in Friedenheim (25°30.927′S 30°58.681′E), Mpumalanga, South Africa. After 18 h, the live Galleria larvae were dipped in 98% alcohol, bent and pricked close to a proleg, using a sterile syringe. A drop of haemolymph, was spread-plated onto Nutrient Agar (NA, Biolab, Biolab Diagnostics, Midrand, South Africa), supplemented with 0.025% (w/v) bromothymol blue and 0.004% (w/v) triphenyltetrazolium chloride (TTC), known as NBTA (Kaya and Stock 1997; Ferreira et al. 2013). The plates were incubated at 30 °C for 48 h. Colonies that absorbed bromothymol blue were regarded as Xenorhabdus spp. and streaked onto NBTA to obtain pure cultures.
Strain WS9, isolated from one colony, was cultured in Tryptone Soy Broth (TSB, Biolab) and stored at − 80 °C in cryotubes with 40% (v/v) glycerol. All experiments were performed with phase I cells, stored in glycerol, to prevent phase shifting. Phase I cells were differentiated from phase II cells by the absorption of bromothymol blue from NBTA plates. All assays were performed with cultures from glycerol to prevent phase shifting. Escherichia coli Xen 14 was cultured in Luria broth (LB, Biolab) and E. coli DH5α transformants in LB, supplemented with 100 µg ampicillin/ml. Bacillus subtilis subsp. subtilis BD170, Listeria monocytogenes EDGE, Staphylococcus aureus Xen 5, Pseudomonas aeruginosa Xen 5 and Salmonella typhimurium Xen 26 were cultured in brain heart infusion (BHI, Biolab). Stock cultures were prepared in 40% (v/v) glycerol and stored at − 80 °C.
Cell morphology and phenotypic characteristics
Strain WS9 was gram stained and the size of a single cell determined using a Leica DM2000 research microscope (Leica Microsystems), equipped with a camera, computer and digital image software [Leica Application Suite (LAS), version 3.5.0]. Oxidase activity was determined using Kovács oxidase reagent. Catalase activity was recorded by suspending a colony in a drop of 5% (v/v) H2O2. Optimal growth temperature was determined by growing cultures at 26, 30, 37 and 42 °C for 24 h in TSB. Changes in growth were monitored by recording optical density readings at 600 nm. Lecithinase activity was determined as described by Ferreira et al. (2013). Lipase activity was determined by streaking 48-h-old cultures onto media containing 10% (w/v) peptone, 5% (w/v) sodium chloride, 0.1% (w/v) CaCl2∙2H2O and either 10% (v/v) Tween 40, Tween 60 or Tween 80. All experiments were done in triplicate.
Carbohydrate fermentation and assimilation profiles were determined using API 50 CHE and 20 NE test strips, respectively (BioMérieux, Marcy I’Etoile, France), according to the instructions of the manufacturer. Results were recorded after 10 days of incubation at 30 °C.
Genotypic characterization and phylogenetic analysis
Genomic DNA was extracted using a modification of the method described by Crouse and Amorese (1987). The 16S rDNA and housekeeping genes glutamyl-tRNA synthetase catalytic subunit (gltX), recombinase A (recA) and DNA polymerase III beta chain (dnaN) were amplified using the primers listed in Table 1. 16S rDNA primers were from Inqaba Biotechnology (Pretoria, South Africa) and all other primers from Integrated DNA Technologies (Coralville, Iowa, USA). Amplifications were performed using Q5® High-Fidelity DNA Polymerase (Thermo Fisher Scientific, Waltham, Massachusetts, USA) or GoTaq® HotStart DNA Polymerase (Promega Corporation, Madison, Wisconsin, USA) and a SwiftMinipro thermal cycler (Esco Healthcare, Malaysia). Amplified fragments were ligated into plasmids pGEM®T Easy (Promega Corporation) or pJET1.2 (Thermo Fisher Scientific), according to instructions of the manufacturer. The plasmids were transformed to E. coli DH5α and cloned cells selected from colonies on LB agar supplemented with 100 µg/ml ampicillin. Positive clones were confirmed by sequencing. The sequencing facilities at the DNA sequencing unit, Central Analytical Facility, University of Stellenbosch, were used.
Whole genome sequencing (WGS) was used to determine the precise species of isolate WS9. Genomic DNA was sequenced on the Illumina Miniseq (Illumina, San Diego, California, USA) instrument. Before sequencing, genomic DNA was fragmented using the M220 Focused-Ultrasonicator (Thermo Fisher, Waltham, MA, USA). The NEBNext® Ultra™ DNA Library Prep Kit and Illumina® NEBNext® Multiplex Oligos (New England Biolabs Inc., Ipswich, MA, USA) were used for preparing sequencing libraries. This service was delivered by the Centre for Proteomic and Genomic Research (CPGR). The accession number for the WGS data of strain WS9 is SAMN07327420.
De novo assemblies of Illumina PE reads were constructed using SPAdes v3.10.1. Contigs containing the 16S rDNA and housekeeping genes, gltX, recA, dnaN, DNA gyrase B (gyrB) and initiation factor B (infB) were identified using BLASTn v2.6.0 + analysis and compared to respective gene reference sequences. MEGA6.0 (Tamura et al. 2013) was used to align sequences. The maximum likelihood method was used to construct phylogenetic trees from single gene sequences and concatenated sequences. The Kimura two-parameter model (Kimura 1980) was used to calculate the distance metrics for aligned sequences. The robustness of individual branches was determined by bootstrapping with 1000 replicates. Parameters were kept constant for the construction of all phylogenetic trees. Protein-coding sequences were compared to previously published sequences by performing translated BLAST searches (BLASTx, National Center for Biotechnology Information). Gene sequences of closely related species and outgroups were obtained from the National Center for Biotechnology Information (NCBI).
Testing for antibacterial activity
Antibacterial activity was determined by spotting 5 µl of a 12-h-old culture of WS9 onto TSB agar (TSA). The plates were incubated at 30 °C for 24, 48, 72 and 96 h, respectively, and then overlaid with 12-h-old cells of B. subtilis subsp. subtilis BD170 suspended in BHI agar (1%, w/v). Spot inoculated plates that were incubated for 96 h were overlaid with L. monocytogenes EDGE, S. aureus Xen 5, P. aeruginosa Xen 5 and S. typhimurium Xen 26, each suspended in BHI agar (1%, w/v), and E. coli suspended in LB agar (1%, w/v). Results were recorded after 24 h of incubation at 37 °C.
Results and discussion
Phenotypic and biochemical characteristics
Strain WS9 is Gram-negative, rod-shaped and 0.8–1.1 by 3.1–6.2 µm in size. Catalase and oxidase were not produced. All colonies absorbed bromothymol blue from NBTA plates, which is characteristic for Xenorhabdus spp. (Kaya and Stock 1997). Optimal growth was recorded at 26 and 30 °C, with slightly better growth at 30 °C. Strain WS9 does not produce lecithinase, and is negative for the hydrolysis of Tweens 60 and 80, but positive for Tween 40.
Strain WS9 produced acid from the fermentation of ribose, xylose, glucose, fructose, mannose, dulcitol, N-acetylglucosamine, maltose, melibiose, gentiobiose and fucose. Acid production from glycerol, arabinose, galactose, inositol, mannitol, esculin ferric citrate, trehalose, luxose, arabitol and potassium gluconate was weak. Strain WS9 assimilated glucose, mannose, N-acetyl-glucosamine, maltose, potassium gluconate, adipic acid, malic acid, trisodium citrate and phenylacetic acid. No other tested carbohydrates were metabolized. Nitrate was not reduced to nitrite.
Antibacterial activity
The antibacterial activity of strain WS9 reached maximum level after 72 h of growth. Colonies of strain WS9 inhibited the growth of B. subtilis subsp. subtilis BD170, L. monocytogenes EDGE, S. aureus Xen 5, E. coli Xen 5 and S. typhimurium Xen 26. No antibacterial activity was recorded against P. aeruginosa Xen 5, which is most likely due to a low secondary metabolite concentration. Most Xenorhabdus spp. produce one or more antimicrobial compounds (Webster et al. 2002). Further research is required to identify the antibacterial compounds produced by strain WS9.
Phylogenetic analysis of strain WS9
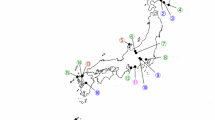
Strain WS9, grouped with the type strain of X. griffiniae when 16S rDNA, gltX, recA, dnaN, gyrB and infB sequences were concatenated (Fig. 1). Comparison of 16S rDNA, gltX, dnaN and gyrB sequences (Online Resources 1–4), separately, grouped strain WS9 with X. griffiniae, but with X. ehlersii and Xenorhabdus sp. TZ01 (JQ687358; JQ687369–JQ687373) when sequences of recA and infB (Online Resources 5, 6) were compared. This suggests that strain WS9 is phylogenetically related to X. griffiniae, and to a lesser extent to X. ehlersii and Xenorhabdus sp. TZ01. Nucleotide similarity searches produced lower identity percentages for X. ehlersii and Xenorhabdus sp. TZ01 compared to X. griffiniae, despite the fact that query coverages were similar, with exception of the recA gene (Online Resource 7). Protein-coding sequences of gltX, dnaN and gyrB obtained for isolate WS9 were similar, but not identical, to protein-coding sequences listed for X. griffiniae (Online Resource 7). However, corresponding to phylogenetic trees, protein-coding sequences for recA and infB were similar to those of X. ehlersii and Xenorhabdus sp. TZ01, respectively. Taking into consideration all the above mentioned, the bacterial isolate WS9 has been identified as a strain belonging to the species X. griffiniae. Closely related X. ehlersii has been isolated from Steinernema longicaudum Shen & Wang (syn. Steinernema serratum) (Lengyel et al. 2005; Tailliez et al. 2006), and Xenorhabdus sp. TZ01 from Steinernema pwaniensis (Půža et al. 2016). The Steinernema sp. strain WS9 is closely related to S. pwaniensis from Tanzania, and both belongs to the Karii-clade, while S. longicaudum belongs to the Longicaudum-clade (Spiridonov and Subbotin 2017).
Phylogenetic relationship of strain WS9 with known Xenorhabdus spp. The maximum likelihood tree was constructed using concatenated sequences of six genes (16S rDNA, gltX, recA, dnaN, gyrB and infB). Three Photorhabdus spp. were used as outgroups. Type strains are indicated by a superscript T. Bootstrap values of more than 70% are shown at branch points. The bar indicates a sequence divergence of 2%
Xenorhabdus griffiniae has previously been isolated from the nematode Steinernema hermaphroditum Stock, Griffin & Chaerani (Nguyen and Hunt 2007) and more recently from the nematode Steinernema khoisanae strain BMMCB (Mothupi et al. 2015). Based on the internal transcribed spacer (ITS) region (KT027382), S. khoisanae BMMCB is 91% similar to the type species, S. khoisanae SF80 (DQ314287), and 92% to Steinernema sp. strain WS9 (KP325086). According to Nguyen and Hunt (2007), a sequence similarity in the ITS region of 95% or less to the most closely related species indicate a possible new Steinernema species. This suggests that strain BMMCB could be neither S. khoisanae nor Steinernema sp. WS9, but appears to be a new Steinernema species.
Previously, Spiridonov et al. (2004) group all three nematode species associated with Xenorhabdus griffiniae in one clade (clade V). According to recent molecular information (Spiridonov and Subbotin 2017), the three nematode species associated with X. griffiniae belong to different clades; S. khoisanae SF80 belongs to the Khoisanae-clade, Steinernema sp. WS9 to the Karii-clade and S. hermaphroditum to the Longicaudum-clade. Host switches of the bacterial symbionts are generally observed between nematodes belonging to the same clade, however, increasing reports have shown that host switches can occur between nematodes from different clades, which is also the case for this study (Lee and Stock 2010; Çimen et al. 2016; Dreyer et al. 2017).
This study reports, for the first time, an association between X. griffiniae and an unknown locally isolated Steinernema sp. WS9. Accession numbers for the PCR obtained sequences 16S rDNA, gltX, recA and dnaN are KT954035, KT954028, KT954030 and KT954033, respectively.
References
Adams BJ, Nguyen KB (2002) Taxonomy and systematics. In: Gaugler R (ed) Entomopathogenic nematology. CAB International, London, pp 1–33
Akhurst RJ (1980) Morphological and functional dimorphism in Xenorhabdus spp., bacteria symbiotically associated with the insect pathogenic nematodes Neoplectana and Heterorhabditis. J Gen Microbiol 212:303–309
Akhurst RJ, Boemare NE (2005) Xenorhabdus. In: Brenner DJ, Krieg NR, Staley JT, Garrity GM (eds) Bergey’s manual of systematic bacteriology, vol 2b, 2nd edn. Springer, New York, pp 831–838
Boemare NE, Akhurst RJ (1988) Biochemical and physiological characterization of colony form variants in Xenorhabdus spp. (Enterobacteriaceae). J Gen Microbiol 134:751–761
Çimen H, Půža V, Nermut J, Hatting J, Ramakuwela T, Faktorová L, Hazir S (2016) Steinernema beitlechemi n. sp., a new entomopathogenic nematode (Nematoda: Steinernematidae) from South Africa. Nematology 18(4):439–453
Crouse J, Amorese D (1987) Ethanol precipitation: ammonium acetate as an alternative to sodium acetate. Focus (Madison) 9:3–5
Dreyer J, Malan AP, Dicks LMT (2017) Three novel Xenorhabdus–Steinernema associations and evidence of strains of X. khoisanae switching between different clades. Curr Microbiol 74(8):938–942
Ferreira T, van Reenen C, Pagès S, Tailliez P, Malan AP, Dicks LMT (2013) Photorhabdus luminescens subsp. noenieputensis subsp. nov., a symbiotic bacterium associated with a novel Heterorhabditis species related to Heterorhabditis indica. Int J Syst Evol Microbiol 63:1853–1858
Fischer-Le Saux M, Maule´on H, Constant P, Brunel B, Boemare N (1997) PCR-ribotyping of Xenorhabdus and Photorhabdus isolates from the Caribbean region to the taxonomy and geographic distribution of their nematode hosts. Appl Environ Microbiol 64:4246–4254
Götz P, Boman A, Boman HG (1981) Interactions between insect immunity and an insect-pathogenic nematode with symbiotic bacteria. Proc R Soc Lond 212:333–350
Griffin CT, Chaerani R, Fallon D, Reid AP, Downes MJ (2000) Occurrence and distribution of the entomopathogenic nematodes Steinernema spp. and Heterorhabditis indica in Indonesia. J Helminth 74:143–150
Kaya HK, Stock SP (1997) Techniques in insect nematology. In: Lawrence L (ed) Manual of techniques in insect pathology. Academic Press, London, pp 281–322
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Laumond C, Maule´on H, Kermarrec A (1979) Donne´es nouvelles sur le spectre d’hoˆ tes et le parasitisme du ne´matode entomophage Neoplectana carpocapsae. Entomophaga 24:13–27
Lee MM, Stock SP (2010) A multigene approach for assessing evolutionary relationships of Xenorhabdus spp. (γ-Proteobacteria), the bacterial symbionts of entomopathogenic Steinernema nematodes. J Invertebr Pathol 104:67–74
Lengyel K, Lang E, Fodor A, Szállás E, Schumann P, Stackebrandt E (2005) Description of four novel species of Xenorhabdus, family Enterobacteriaceae: Xenorhabdus budapestensis sp. nov., Xenorhabdus ehlersii sp. nov., Xenorhabdus innexi sp. nov., and Xenorhabdus szentirmaii sp. nov. Syst Appl Microbiol 28:115–122
Mothupi B, Featherston J, Gray V (2015) Draft whole-genome sequence and annotation of Xenorhabdus griffiniae strain BMMCB associated with the South African Entomopathogenic nematode Steinernema khoisanae strain BMMCB. Genome Announc 3(4):e00785–e00715
Nguyen B, Hunt D (2007) Entomopathogenic nematodes: systematics, phylogeny and bacterial symbionts. Brill, The Netherlands
Poinar GO (1979) Nematodes for biological control of insects. CRC Press Inc., Boca Raton
Půža V, Nermut J, Mráček Z, Gengler S, Haukeland S (2016) Steinernema pwaniensis n. sp., a new entomopathogenic nematode (Nematoda: Steinernematidae) from Tanzania. J Helminth 91:20–34
Spiridonov SE, Subbotin SA (2017) Phylogeography of Heterorhabditis and Steinernema. In: Hunt DJ, Nguyen KB (eds) Advances in entomopathogenic nematode taxonomy and phylogeny. Brill, The Netherlands, pp 413–427
Spiridonov SE, Reid AP, Podrucka K, Subbotin SA, Moens M (2004) Phylogenetic relationships within the genus Steinernema (Nematoda: Rhabditida) as inferred from analysis of sequences of the ITS-5.8S-ITS2 region of rDNA and morphological features. Nematology 6:547–566
Stock SP (2015) Diversity, biology and evolutionary relationships. In: Campos-Herrera (ed) Nematode pathogenesis of insects and other pests. Springer, Switzerland, pp 3–27
Tailliez P, Pagès S, Ginibre N, Boemare N (2006) New insight into diversity in the genus Xenorhabdus, including the description of ten novel species. Int J Syst Evol Microbiol 56:2805–2818
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Thomas GM, Poinar JR (1979) Xenorhabdus gen. nov., a genus of entomopathogenic, nematophilic bacteria of the family Enterobacteriaceae. Int J Syst Bacteriol 29(4):352–360
Webster JM, Chen G, Hu K, Li J (2002) Bacterial metabolites. In: Gaugler R (ed) Entomopathogenic nematology. CAB International, New York, pp 99–114
Acknowledgements
The National Research Foundation (NRF, South Africa) for funding and Willem Steyn (Agricultural Research Council) for the collection of the nematode sample. Finally, this study would not have been possible without the help of my colleague, Riaan de Witt, with processing of WGS data.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Erko Stackebrandt.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dreyer, J., Malan, A.P. & Dicks, L.M.T. First report of a symbiotic relationship between Xenorhabdus griffiniae and an unknown Steinernema from South Africa. Arch Microbiol 200, 349–353 (2018). https://doi.org/10.1007/s00203-017-1452-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-017-1452-4