Abstract
A Cr(VI)-tolerant, Gram-staining-positive, rod-shaped, endospore-forming and facultative anaerobic bacterium, designated as GSS04T, was isolated from a heavy-metal-contaminated soil. Strain GSS04T was Cr(VI)-tolerant with a minimum inhibitory concentration of 600 mg l−1 and was capable of reducing Cr(VI) under both aerobic and anaerobic conditions. Growth occurred with presence of 0–3 % (w/v) NaCl (optimum 1 %), at pH 5.5–10.0 (optimum pH 7.0) and 15–50 °C (optimum 30–37 °C). The main respiratory quinone was MK-7 and the major fatty acids were anteiso-C15:0 and iso-C15:0. The DNA G+C content was 41.1 mol%. The predominant polar lipid was diphosphatidylglycerol. Based on 16S rRNA gene sequence similarity, the closest phylogenetic relative was Bacillus shackletonii DSM 18868T (97.6 %). The DNA–DNA hybridization between GSS04T and its closest relatives revealed low relatedness (<70 %). The results of phenotypic, chemotaxonomic and genotypic analyses clearly indicated that strain GSS04T represents a novel species of the genus Bacillus, for which the name Bacillus dabaoshanensis sp. nov. is proposed. The type strain is GSS04T (=CCTCC AB 2013260T = KCTC 33191T).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With rapid industrialization and urbanization, soil is more and more seriously polluted by heavy metals such as chromium (Cr). Cr can exist in several oxidation states, but in soil, the most stable and common species are Cr(III) and Cr(VI). Cr(VI) is considered highly toxic due to its mutagenicity, carcinogenicity and teratogenicity for humans and animals (Chen and Hao 1998). Cr(III) is less toxic because its spread and biological availability is restricted due to its low solubility (Gonzalez et al. 2003). Physical and chemical methods such as precipitation, ion exchange and electrodialysis are often used to detoxify Cr(VI) by reducing it to Cr(III). However, these methods are not only economically expensive but also have disadvantages such as high reagent consumption, energy requirements and incomplete metal removal. Therefore, detoxification of Cr(VI) by bioremediation strategy using microorganisms with low costs and reagent requirement is considered as a potential alternative method, and Cr(VI)-tolerant microorganisms play a key role in bioremediation.
A wide variety of bacteria including members of the genera Escherichia, Bacillus, Pseudomonas, Pantoea, Cellulomonas, Micrococcus, Staphylococcus, Achromobacter and Ochrobactrum has been reported to be able to reduce Cr(VI) to Cr(III) (Narayani and Shetty 2013). Gram-positive bacteria are predominant over Gram-negative bacteria, and the genus Bacillus is prominent among all Gram-positive bacteria for its Cr(VI) resistance. Most of Cr(VI)-tolerant bacteria are isolated from sewage and Cr-contaminated sites. Bacteria of the genus Bacillus are widespread in nature and can be found in some inhospitable places like deserts (Zhang et al. 2011), marine sediments (Zhang et al. 2010) or even in spacecraft assembly clean rooms (Vaishampayan et al. 2010).
In this study, a Cr(VI)-tolerant bacterium was isolated from a heavy-metal-contaminated soil and was characterized to represent a novel species of the genus Bacillus. The ability of the strain to reduce Cr(VI) was measured under aerobic and anaerobic conditions. The effects of temperature, pH and initial concentration of Cr(VI) on Cr(VI) reduction were investigated.
Materials and methods
Strain isolation
Samples were collected from a paddy soil amended with sludge compost in Dabaoshan Mine, Guangdong Province, China (113°24′2′′E, 24°19′12′′N), South China. The isolation was performed by using the method of Li et al. (2014). A colony of pale yellow color was isolated, purified and designated as strain GSS04T. The strain was stored at −80 °C in LB with 15 % (v/v) glycerol, and the strain has been conserved in the China Center for Type Culture Collection (CCTCC) and the Korean Collection for Type Cultures (KCTC) with the accession numbers of CCTCC AB 2013260T and KCTC 33191T, respectively.
Closely related type strains Bacillus shackletonii DSM 18868T (97.6 %), Bacillus gottheilii DSM 23668T (97.5 %), Bacillus horneckiae DSM 23495T (97.2 %), Bacillus firmus JCM 2512T (97.2 %), Bacillu acidicola DSM 14745T (97.1 %) and Bacillus oceanisediminis CGMCC1.10115T (97.1 %) were selected as reference strains. Strain JCM 2512T was purchased from the Japan Collection of Microorganisms (JCM), strains DSM 18868T, DSM 23668T, DSM 23495T and DSM 14745T were purchased from the German Collection of Microorganisms and Cell Cultures (DSMZ), and strain of CGMCC1.10115T was purchased from the China General Microbiological Culture Collection Center (CGMCC).
Morphological and physiological characteristics
Cell morphology was observed using a JEM 1400 (JEO) transmission electron microscope after incubation on LB agar for 48 h. Endospores were observed with a transmission electron microscope after cells were grown for 4 days according to Vaz-Moreira et al. (2012). Anaerobic growth was assayed in 10-ml rubber-stopperd, screw-capped tubes containing 9 ml LB medium under an atmosphere of 100 % N2. DNase activity was assessed on DNase agar plates (42 g l−1, Merck). Growth on MacConkey agar was tested at 30 °C for 1 week. Gram reaction, motility, oxidase activity, catalase activity and hydrolysis of Tween 20, Tween 80, casein and starch were tested as described by Dong and Cai (2001). Growth at different temperatures (0, 4, 10, 15, 20, 25, 30, 37, 40, 45, 50, 55 and 60 °C) was investigated, and salt tolerance (0–14.0 % (w/v) NaCl, with increments of 0.5 %) was tested for up to 1 week. pH range (4–11) for growth was determined in trypticase soy broth (TSB) using the following buffer systems: pH 4.0–5.0, 0.1 M citric acid/0.1 M sodium citrate; pH 6.0–8.0, 0.1 M KH2PO4/0.1 M NaOH; pH 9.0–10.0, 0.1 M NaHCO3/0.1 M Na2CO3; and pH 11.0, 0.05 M Na2HPO4/0.1 M NaOH (Han et al. 2013). Other biochemical properties were determined using API 20E, API 20NE, API ID 32GN and API 50CH galleries (bioMérieux) according to the manufacturer’s instructions.
GC content, PCR amplification, sequencing and phylogenetic analysis
DNA G+C content was measured using the high-performance liquid chromatography (HPLC) (Mesbah et al. 1989). Genomic DNA of strain GSS04T was extracted using a commercial DNA extraction kit (Aidlab Biotechnologies Co., Ltd.). PCR amplification of the 16S rRNA gene was performed using the bacterial universal primer pair 27F and 1492R (Weisburg et al. 1991). The PCR product was purified using a gel extraction kit D2500-01 (Omega Bio-tek) and sequenced by BGI (Shenzhen, China). Calculation of pairwise sequence similarity was achieved using the EzTaxon-e server (http://eztaxon-e.ezbiocloud.net/) (Kim et al. 2012). The multiple alignments of the sequence data was performed using multiple alignment software CLUSTAL_X (Thompson et al. 1997), and phylogenetic trees were constructed using MEGA 5.0 program with the neighbor-joining method and the minimum-evolution method (Tamura et al. 2011). Evolutionary distances were calculated using Kimura’s two-parameter model (Kimura 1980). Bootstrap analysis with 1,000 replicates was conducted in order to obtain confidence levels for the branches (Felsenstein 1985).
Chemotaxonomy characteristics
To investigate the respiratory quinones, cells of strain GSS04T was cultured in LB at 37 °C till the late exponential growth phase, harvested by centrifugation, washed with distilled water and freeze-dried. Respiratory quinones were extracted according to Collins et al. (1977) and analyzed with HPLC as described by Tamaoka et al. (1983). In preparation for cellular fatty acid profile analysis, cells of strain GSS04T and the reference strains were grown in LB at 37 °C for 24 h. Cellular fatty acid methyl esters from cells were separated and identified using the Sherlock Microbial Identification System (MIDI Corporation) and the microbial identification software package according to the manufacturer’s instructions (Sasser 1990). Polar lipids of cells were extracted, separated by two-dimensional thin-layer chromatography (TLC) and identified by spraying individual plates with appropriate detection reagents according to Minnikin et al. (1984): molybdophosphate for total lipids, molybdenum blue for phospholipids, ninhydrin reagent for amino-containing lipids and alpha-naphthol reagent for glycolipids.
DNA–DNA hybridization
DNA–DNA hybridization between strain GSS04T and the reference strains were carried out with photobiotin-labeled probes in microplate wells as described Ezaki et al. (1989). Hybridization values are means of results from at least two hybridization experiments (reciprocal and non-reciprocal values).
Minimum inhibitory concentration (MIC)
MIC is defined as the minimum concentration of Cr(VI) completely inhibiting bacterial growth, and the MIC for GSS04T was determined using the plate dilution method (Aleem et al. 2003). One hundred microliters of seed inoculum was transferred to 10 ml LB medium in a glass tube supplemented with 50–1,000 mg l−1 Cr(VI) in the form of K2Cr2O7 and incubated aerobically for 50 h at 35 °C. Cell optical density of the samples was observed with a spectrophotometer (TU-1901) at 600 nm. The minimum concentration of Cr(VI) without growth was identified as the MIC for GSS04T.
Optimization of Cr(VI) reduction
To investigate the effect of oxygen on Cr(VI) reduction by strain GSS04T, Cr(VI) reduction was performed under both anaerobic and aerobic conditions. For aerobic experiment, 100 μl (OD600 ≈ 1.0, 6 × 107 cells/ml) seed inoculum was transferred to 10 ml LB medium in a glass tube supplemented with 50 mg l−1 Cr(VI) in the form of K2Cr2O7 and incubated aerobically at 35 °C. In anaerobic experiment, the glass tubes were bubbled with 100 % N2, rubber-stopperd and screw-capped. Samples were drawn out at regular time intervals and analyzed for Cr(VI) concentration and cell counts. Cell counts were measured using the optical density at 600 nm (OD600), and Cr(VI) concentration was determined using diphenylcarbazide (DPC) reagent as described by Ishibashi et al. (1990).
The effects of temperature (25, 30, 35, 40 °C), pH (6, 7, 8, 9) and initial Cr(VI) concentration (50, 75, 100, 125, 150 mg l−1) on Cr(VI) reduction were investigated. The tubes with 10 ml LB medium and 100 μl seed inoculum were incubated for 0, 24, 48, 72, 96, 120 or 144 h. All the experiments were performed in triplicate. All the statistical analyses were carried out using origin 8.0.
Results and discussion
Strain GSS04T was found to be Gram-staining-positive, facultative anaerobic and motile. Cells were rod-shaped with peritrichous flagella, approximately 2.6–3.2 μm in length and 0.8 μm in width, and were capable of forming ellipsoidal endospores lying paracentrally or subterminally in sporangia that were slightly swollen (Supplementary Fig. S1). Colonies of this strain were pale yellow, convex, circular with regular margins and 1–1.2 mm in diameter. The strain grew at 15–50 °C (optimum 30–37 °C) and pH 5.5–10.0 (optimum pH 7.0). The NaCl concentration range for growth was 0–3.0 % (w/v) (optimum 1 % NaCl), which was specific among reference strains. All the data gave a hint that strain GSS04T was a novel species. The physiological and biochemical characteristics for strain GSS04T are presented in Table 1 and Supplementary Table S1 and in the species description.
Strain GSS04T contained menaquinone 7 (MK-7) as the predominant respiratory quinone. As shown in Table 2, the major fatty acids of strain GSS04T were iso-C15:0 (42.9 %) and anteiso-C15:0 (24.2 %). The property of strain GSS04T having high content (70 %) of iso- and anteiso-branched fatty acids is in line with that of members of the Genus Bacillus (Kämpfer 1994). The DNA G+C content was 41.1 mol%. As shown in supplementary Fig. S3, the polar lipid pattern of strain GSS04T consisted of a predominant component of diphosphatidylglycerol (DPG) and moderate to trace components of phosphatidylmonomethylethanolamine (PME), phosphatidylglycerol (PG), phosphatidylethanolamine (PE), three unidentified phospholipids and two unknown lipids.
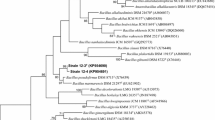
A total of 1,476 bp was determined for the 16S rRNA gene sequence of strain GSS04T. The obtained 16S rRNA nucleotide sequence has been deposited in the Genbank database (http://www.ncbi.nlm.nih.gov/nuccore/) with the accession number of KJ818278. The phylogenetic trees (Fig. 1, supplementary Fig. S2) showed that strain GSS04T formed a separate lineage within the genus Bacillus and is distantly related to other members of the genus. Besides the DNA–DNA hybridization experiment between strains GSS04T and DSM 18868T, DSM 23668T, DSM 23495T, JCM 2512T, DSM 14745T and CGMCC1.10115T revealed a relatedness of 12.9, 19.4, 14.7, 21.8, 15.1 and 12.2 %, respectively, well below the 70 % cutoff point for species classification, indicating that strain GSS04T represents a novel species of the genus Bacillus.
Neighbour-joining tree based on 16S rRNA gene sequences showing the phylogenetic position of strain GSS04T and representatives of some other related taxa. Bootstrap values (expressed as percentages of 1,000 replications) >50 % are shown at the branch points. Bar 0.005 substitutions per nucleotide position
The MIC of Cr(VI) for GSS04T was 600 mg l−1 which is close to 500 mg l−1 for Bacillus licheniformis (Narayani and Shetty 2013). Cr(VI) reduction under both aerobic and anaerobic conditions is shown in Fig. 2. Under aerobic condition, the value of OD600 increased steadily from 0.05 to approximately 2 and then dropped a little, while Cr(VI) concentration decreased rapidly from 50 to 0 mg l−1 within 50 h. Under anaerobic conditions, OD600 increased very slowly to approximately 0.3 and Cr(VI) concentration dropped from 50 to approximately 25 mg l−1. Many bacteria can reduce Cr(VI) under anaerobic or aerobic conditions but few under both conditions. The results showed that GSS04T has the ability of Cr(VI) reduction under both aerobic and anaerobic conditions, but it grows and reduces Cr(VI) much more rapidly when oxygen is present, which is also the case with P. putida PRS2000 (Ishibashi et al. 1990).
In the present study, bacterial reduction of Cr(VI) was studied under four different temperatures (25, 30, 35 and 40 °C). The results showed that 35 °C is the optimum temperature for Cr(VI) reduction. Complete reduction of 100 mg l−1 Cr(VI) was achieved after 120 h at 35 °C (Fig. 3a). The optimum temperature for Cr(VI) reduction was reported to be 35 °C for B. firmus (Sau et al. 2010), but a higher temperature of 50 °C for B. thermoamylovoras SKC1 (Slobodkina et al. 2007). Our study and other reports support that the optimum temperature for Cr(VI) reduction depends mainly on optimum temperature for cells growth.
The pH can affect microbial growth rate and the activities of enzymes significantly and can also influence chemical speciation, solubility and bioavailability of Cr(VI) (Adriano 2001). In the present study, Cr(VI) reduction was studied in the pH range of 6, 7, 8 and 9. The results (Fig. 3b) showed an increasing Cr(VI) reduction with pH increasing from 6 to 7. However, Cr(VI) reduction declined with pH increasing from 7 to 9. Therefore, at pH 7, the strain showed the highest Cr(VI) reduction ability. Optimum Cr(VI) reduction by Bacillus cereus SJ1 (He et al. 2010) and Bacillus sp. CSB-4 (Dhal et al. 2010) was also found at pH 7. pH ranging from 6 to 8.5 was found to be optimum for Cr(VI) reduction by most bacterial strains (Narayani and Shetty 2013).
The effect of initial Cr concentration (50–150 mg l−1) on Cr(VI) reduction was determined (Fig. 3c). Complete reduction of Cr(VI) was observed at low Cr(VI) initial concentrations of 50, 75 and 100 mg l−1 after 48, 72 and 96 h, respectively. However, at higher initial concentrations of 125 and 150 mg l−1, Cr(VI) reduction rates of 25.7 and 85.0 % over 144 h, respectively, were recorded. The reduction rates for the first 24 h of Cr(VI) reduction were 1.60, 1.78, 1.85, 1.05 and 0.17 mg Cr(VI) l−1 h−1 when the initial Cr(VI) concentrations were 50, 75, 100, 125 and 150 mg l−1, respectively. The initial rate of Cr(VI) reduction increased with the initial Cr(VI) concentration increasing from 50 to 100 mg l−1. This increase in the initial rate can be explained by that with the initial Cr(VI) concentration increasing, the number of metal ions in the media increases, which ultimately enhances the collision rate of metal ions onto the active sites on the cell surface. However, when the initial concentration exceeds a limit (i.e., >100 mg l−1), initial reduction rate would start to decrease due to toxicity (Desai et al. 2008).
In conclusion, phylogenetic analysis based on the 16S rRNA gene grouped strain GSS04T in the genus Bacillus, most closely related to B. shackletonii among all species with validly published names. Some physiological and biochemical characters (Gram-staining positive, endospore-forming, catalase positive, etc.) and chemotaxonomic characters (MK-7 as the major quinone, large amounts of iso- and anteiso-branched fatty acids and DPG as the predominant polar lipid) supported strain GSS04T as a member of the genus Bacillus as well. However, strain GSS04T can tolerate lower NaCl than all the reference strains except for B. acidicola. Besides, strain GSS04T can be separated from B. shackletonii and B. horneckiae by characters such as positive activity of arginine dihydrolase and β-Galactosidase, from B. gottheilii by characters such as positive nitrate reduction, from B. firmus by characters such as hydrolyzing esculin or gelatin, from B. acidicola by characters such as higher maximum pH, positive activity of arginine dihydrolase and β-Galactosidase, positive nitrate reduction and hydrolyzing gelatin, and from B. oceanisediminis by characters such as lower DNA G+C content and hydrolyzing gelatin. On the basis of the above characteristics, strain GSS04T can be regarded as a novel species of the genus Bacillus, and for this novel species, the name Bacillus dabaoshanensis sp. nov. is proposed.
Description of Bacillus dabaoshanensis sp. nov
Bacillus dabaoshanensis (dabao.shan.en’sis. N.L. masc. adj. dabaoshanensis pertaining to a heavy metal mine area in Guangdong Province, China, the source of the isolated).
Cells are Gram-staining positive, facultative anaerobic and motile. Cells were rod-shaped with peritrichous flagella, approximately 2.6–3.2 μm in length and 0.8 μm in width. Ellipsoidal endospores are observed to be lying paracentrally or subterminally in sporangia that were slightly swollen. Colonies of this strain were pale yellow, convex, circular with regular margins and 1–1.2 mm in diameter after incubation at 50 °C for 1 day on LB. Growth occurs at 15–50 °C (optimum 30–37 °C), at pH 5.5–10.0 (optimum pH 7.0) and in 0–3.0 % (w/v) NaCl (optimum 1 % NaCl). Cells cannot grow on MacConkey agar. Hydrolysis of starch, casein, and gelatin was positive, but hydrolysis of cellulose was negative. Cells were tested to be positive for catalase, β-galactosidase, esculin and reduction of nitrates to nitrites, but negative for lecithin, oxidase, tryptophane deaminase, indole production, reduction of nitrates to nitrogen, lysine decarboxylase and H2S production. Acid was produced from l-arabinose, d-mannitol, arginine dihydrolase, trisodium citrate, d-glucose, sodium malonate, sucrose, salicin, sodium acetate and histidine, but not from potassium 2-keto-gluconate, d-maltose and malate. d-galactose, d-mannose, d-fructose, d-raffinose, d-xylose, l-rhamnose, glycerol, methyl-α-D-glucopyranoside, salicin, glycogen and arbutin were utilized as sole carbon sources, but d-arabinose was not. The predominant quinone system is MK-7. The major fatty acids were anteiso-C15:0 and iso-C15:0. The major polar lipids are determined to be DPG, PME, PG and PE. The DNA G+C content was 41.1 mol%.
The type strain GSS04T (= CCTCC AB 2013260T = KCTC 33191T) was isolated from a heavy-metal-contaminated soil in South China. The GenBank accession number for the 16S rRNA gene sequence of GSS04T is KJ818278.
References
Adriano DC (2001) Trace elements in terrestrial environments: biogeochemistry, bioavailability, and risks of metals. Springer, New York
Albert RA, Archambault J, Rossello-Mora R, Tindall BJ, Matheny M (2005) Bacillus acidicola sp. nov., a novel mesophilic, acidophilic species isolated from acidic Sphagnum peat bogs in Wisconsin. Int J Syst Evol Microbiol 55:2125–2130
Aleem A, Isar J, Malik A (2003) Impact of long-term application of industrial wastewater on the emergence of resistance traits in Azotobacter chroococcum isolated from rhizosphere soil. Bioresour Technol 86:7–13
Chen JM, Hao OJ (1998) Microbial chromium (VI) reduction. Crit Rev Environ Sci Technol 28:219–251
Collins M, Pirouz T, Goodfellow M, Minnikin D (1977) Distribution of menaquinones in actinomycetes and corynebacteria. J Gen Microbiol 100:221–230
Desai C, Jain K, Madamwar D (2008) Hexavalent chromate reductase activity in cytosolic fractions of Pseudomonas sp. G1DM21 isolated from Cr(VI) contaminated industrial landfill. Process Biochem 43:713–721
Dhal B, Thatoi H, Das N, Pandey BD (2010) Reduction of hexavalent chromium by Bacillus sp. isolated from chromite mine soils and characterization of reduced product. J Chem Technol Biotechnol 85:1471–1479
Dong X, Cai M (2001) Manual of systematic and determinative bacteriology. Science Press, Beijing
Ezaki T, Hashimoto Y, Yabuuchi E (1989) Fluorometric deoxyribonucleic acid deoxyribonucleic acid hybridization in microdilution wells as an alternative to membrane-filter hybridization in which radioisotopes are used to determine genetic relatedness among bacterial strains. Int J Syst Bacteriol 39:224–229
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Gonzalez C, Ackerley D, Park C, Matin A (2003) A soluble flavoprotein contributes to chromate reduction and tolerance by Pseudomonas putida. Acta Biotechnol 23:233–239
Han L, Yang G, Zhou X, Yang D, Hu P, Lu Q, Zhou S (2013) Bacillus thermocopriae sp. nov., isolated from a compost. Int J Syst Evol Microbiol 63:3024–3029
He M, Li X, Guo L, Susan JM, Christopher R, Wang G (2010) Characterization and genomic analysis of chromate resistant and reducing Bacillus cereus strain SJ1. BMC Microbiol 10:221
Ishibashi Y, Cervantes C, Silver S (1990) Chromium reduction in Pseudomonas putida. Appl Environ Microbiol 56:2268–2270
Kämpfer P (1994) Limits and possibilities of total fatty acid analysis for classification and identification of Bacillus species. Syst Appl Microbiol 17:86–98
Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, Park SC, Jeom YS, Lee JH, Yi H, Won S, Chun H (2012) Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol 62:716–721
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Li J, Yang G, Wu M, Zhao Y, Zhou S (2014) Bacillus huizhouensis sp. nov., isolated from a paddy field soil. Anton Leeuw Int JG 106:357–363
Logan NA, Lebbe L, Verhelst A, Goris J, Forsyth G, Rodríguez-Díaz M, Heyndrickx M, Vos PD (2004) Bacillus shackletonii sp. nov., from volcanic soil on Candlemas Island, South Sandwich archipelago. Int J Syst Evol Microbiol 54:373–376
Mesbah M, Premachandran U, Whitman WB (1989) Precise measurement of the G+C content of deoxyribonucleic acid by high-performance liquid chromatography. Int J Syst Bacteriol 39:159–167
Minnikin DE, O’Donnell AG, Goodfellow M, Alderson G, Athalye M, Schaal A, Parlett JH (1984) An integrated procedure for the extraction of bacterial isoprenoid quinones and polar lipids. J Microbiol Methods 2:233–241
Narayani M, Shetty KV (2013) Chromium-resistant bacteria and their environmental condition for hexavalent chromium removal: a review. Crit Rev Environ Sci Technol 43:955–1009
Sasser M (1990) Identification of bacteria by gas chromatography of cellular fatty acids. MIDI Technical note 101. MIDI Inc, newark
Sau GB, Chatterjee S, Mukherjee SK (2010) Chromate reduction by cell-free extract of Bacillus firmus KUCr1. Pol J Microbiol 59:185–190
Seiler H, Wenning M, Schmidt V, Scherer S (2013) Bacillus gottheilii sp. nov., isolated from a pharmaceutical manufacturing site. Int J Syst Evol Microbiol 63:867–872
Slobodkina G, Bonch-Osmolovskaya E, Slobodkin A (2007) Reduction of chromate, selenite, tellurite, and iron (III) by the moderately thermophilic bacterium Bacillus thermoamylovorans SKC1. Microbiol 76:530–534
Tamaoka J, Katayama-Fujimura Y, Kuraishi H (1983) Analysis of bacterial menaquinone mixtures by high performance liquid chromatography. J Appl Microbiol 54:31–36
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetic analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Vaishampayan P, Probst A, Krishnamurthi S, Ghosh S, Osman S, McDowall A, Ruckmani A, Mayilraj S, Venkateswaran K (2010) Bacillus horneckiae sp. nov., isolated from a spacecraft-assembly clean room. Int J Syst Evol Microbiol 60:1031–1037
Vaz-Moreira I, Figueira V, Lopes AR, Lobo-da-Cunha A, Spröer C, Schumann P, Nunes OC, Manaia CM (2012) Bacillus purgationiresistans sp. nov., isolated from a drinking-water treatment plant. Int J Syst Evol Microbiol 62:71–77
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Zhang Jianli, Wang Jiewei, Fang Caiyuan, Song Fei, Xin Yuhua, Qu Lei, Ding Kai (2010) Bacillus oceanisediminis sp. nov., isolated from marine sediment. Int J Syst Evol Microbiol 60:2924–2929
Zhang L, Wu GL, Wang Y, Dai J, Fang CX (2011) Bacillus deserti sp. nov., a novel bacterium isolated from the desert of Xinjiang China. Anton Leeuw Int JG 99:221–229
Acknowledgments
This study was funded by the National Natural Science Foundation of China (41203078, 31100353) and the Guangdong Natural Science Funds for Distinguished Young Scholar (S20120011151).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by Erko Stackebrandt.
The GenBank accession numbers for the 16S rRNA gene sequences of strain GSS04T is KJ818278.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cui, X., Wang, Y., Liu, J. et al. Bacillus dabaoshanensis sp. nov., a Cr(VI)-tolerant bacterium isolated from heavy-metal-contaminated soil. Arch Microbiol 197, 513–520 (2015). https://doi.org/10.1007/s00203-015-1082-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-015-1082-7