Abstract
Mycoplasma gallisepticum (MG) is a common and widespread cause of chronic respiratory disease in poultry. In this study, antigenic proteins were identified from MG membrane using two-dimensional gel electrophoresis (2-DE) analysis followed by Western blot and matrix-assisted desorption/ionization time-of-flight mass spectrometry (MALDI-TOF–MS), including translation elongation factor Tu, dihydrolipoamide acetyltransferase (E2) component of pyruvate dehydrogenase complex, trigger factor, chaperone protein DnaK, heat shock protein GroEL and so on. Furthermore, recombinant MG GroEL protein was successfully expressed in E. coli BL21 (DE3) with pET-28a (+) vector and found to possess ATPase activity and contributed to the refolding of recombinant MG PrpC protein. Complement-dependent bactericidal assay indicated that the rabbit antisera against MG rGroEL had satisfactory bactericidal effect, which is similar to the chicken antisera induced by MG-inactivated vaccine, suggesting MG GroEL is a protective antigen, could be used as a novel vaccine candidate. This study is the first report of the biological characterization of chaperone GroEL protein in MG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mycoplasma gallisepticum (MG) is a bacterium belonging to the class Mollicutes and the family Mycoplasmataceae. It is the causative agent of chronic respiratory disease (CRD) in chickens and infectious sinusitis in turkeys, chickens, pigeons and passerine birds of all ages (Hennigan et al. 2012). MG genome is extremely small, which contains only 996,442 bp nucleotides for R low strain. MG membrane proteins play important roles on Mycoplasma morphology, nutrient transport and colonization of the host (Cleavinger et al. 1994; Panicker et al. 2012). Many MG membrane proteins are shown to be components of solute transport systems or involved in antigenic variation and cytoadherence.
GroEL belongs to the heat shock family of proteins (HSPs), also known as Hsp60 or Group I chaperonin. Homologues of GroEL are present in almost all species of bacteria, and it is the only chaperone known to be essential for the growth of Escherichia coli (Fayet et al. 1989). The importance of homologues of GroEL has been demonstrated in Bacillus subtilis (Kobayashi et al. 2003), Riemerella anatipestifer (Han et al. 2012) and Mycobacterium tuberculosis (Hu et al. 2008). GroEL also serves as a moonlighting protein with pleiotropic functions (Lewthwaite et al. 2007). For example, GroEL assists in the formation of macromolecular complexes and acts in concert with its cofactor, GroES, to bring about the proper folding of non-natively folded substrates in an ATP-dependent manner (Chapman et al. 2006). Additionally, in both M. pneumoniae and M. genitalium infections, GroEL is associated with cytoadherence, which complements adhesin proteins such as HMW1 and P1.
Based on these concepts, the present study was undertaken by two-dimensional gel electrophoresis (2-DE), Western blot and matrix-assisted desorption/ionization time-of-flight mass spectrometry (MALDI-TOF–MS) to determine whether the MG GroEL is present on the membrane, and its biological characteristics such as ATPase and protein refolding functions were also clarified. Prokaryotic organism such as mycoplasma could utilize Ser/Thr protein kinase system to regulate critical biological activity of the protein. Sps and ptc gene of the MG encode PrkC (MGA_0459) and PrpC (MGA_0461) proteins; these two proteins are the most important Ser/Thr protein kinase system. Study on B. subtilis found that Ser46 of the HPr protein could be phosphorylation; the HPr kinase (also called PrkC) performed the catalytic action. The dephosphorylation of HPr Ser46 depended on the phosphatase PrpC which belongs to PPM phosphatase family (Obuchowski et al. 2000). Our previous experiments study on the PrkC and PrpC protein in the posttranslational modification and biological activity regulation. In this experiment, we utilize the natural PrpC protein possess the dephosphorylated characteristics to evaluate the denatured PrpC protein refolding rate with GroEL and GroES protein system.
The bactericidal assay was employed using rabbit antisera against recombinant MG GroEL protein to determine whether MG GroEL is a protective protein.
Materials and methods
Strains, cells and reagents
MG strain R low was provided by the Chinese Veterinary Culture Collection Center (CVCC, Beijing, China). The strain was cultured in complete media-243 (ATCC, Manassas, VA, USA) containing heart extract broth (BD, Franklin Lakes, NJ, USA), yeast extract solution, 10 % horse sera and 10 % swine sera (Gibco, Rockville, MD, USA). MG strain R low was cultured on solid media containing 1 % Noble agar (BD). E. coli strains DH5α and BL21 (DE3) (Invitrogen, CA, USA) were cultured in Luria–Bertani (LB) broth. PCR MIX was purchased from Dongsheng biotech (Guangzhou, China). Emulsified S6-inactivated MG was purchased from the Nanjing Tianbang Co. Inc. (Nanjing, China). Complement sera and pNPP substrate were purchased from Sigma-Aldrich (St. Louis, MO, USA). Micro-ATPase Assay Kit was purchased from Nanjing Jiancheng Research Institute, China.
Extraction of hydrophilic and hydrophobic membrane proteins of MG
The membrane proteins of MG strain R low (from the 21st passage) were extracted using ReadyPrep Protein Extraction Kit (Membrane I) according to the manufacturer’s instructions (Bio-Rad, Hercules, CA, USA). Briefly, in a microcentrifuge tube, MG cell pellets (200 mg) were added with 0.5 ml of buffer M1, sonicated on ice and added with an equal volume of chilled buffer M2. The tube was then vortexed for 4–5 times (60 s for each), then transferred to a 37 °C water bath for 30 min incubation with periodical shaking. After centrifuged at 16,000g for 5 min at room temperature, the top layer was transferred to a new tube and labeled as MG hydrophilic membrane proteins. The tube containing the bottom phase was added with 0.5 ml of prechilled buffer M2, vortexed and incubated again as described above. The bottom phase was transferred to a new tube and labeled as MG hydrophobic membrane proteins. The proteins were purified by acetone precipitation and suspended in 0.5 ml of re-swelling buffer, which was composed of 8 M urea, 2 % CHAPS, 2 % v/v immobilized pH gradient (IPG) buffer, 20 mM DTT and 0.002 % bromophenol blue. The purified proteins were quantified by RC DC protein assay kit (Bio-Rad, Hercules, CA, USA).
Two-dimensional gel electrophoresis (2-DE) and Western blot
2-DE was carried out in duplicate for Coomassie Brilliant Blue G-250 staining and Western blot procedures (Han et al. 2012). Briefly, the hydrophilic and hydrophobic membrane proteins (200 μg) were subjected, respectively, to isoelectric focusing (IEF) in the first dimension with IPG strips (11 cm, pH 4–7, pH 5–8) and a Protean IEF cell (Bio-Rad). After rehydration at 50 V for 12 h, the IEF procedure was conducted at a constant temperature of 20 °C at 250 V for 1 h, 500 V for 1 h, 4,000 V for 3 h, 8,000 V for 4 h and finally at 50,000 V/h. SDS polyacrylamide gel electrophoresis (SDS-PAGE) in the second dimension was completed with 12 % polyacrylamide gels. After 2-DE, the gel was stained with Coomassie Brilliant Blue G-250 and scanned with an Image scanner (Amersham Pharmacia Biotech) to visualize the 2-DE profiles. The other gel was used for Western blot with chicken anti-MG sera induced by MG-inactivated vaccine. The proteins that reacted with chicken anti-MG sera were selected for MALDI-TOF–MS analysis.
MALDI-TOF–MS analysis
The Coomassie-stained protein spots which corresponded to the spots on the Western blot membranes were manually excised from the gel and transferred to a V-bottomed 96-well plate for tryptic in-gel digestion. After trypsin digestion, the peptide mixtures were suspended in an ethanol–acetone solution containing 0.5 g/l alpha-cyano-4-hydroxycinnamic acid (at a ratio of 2:1, v/v), spotted on the MALDI plate and analyzed by a 4700 MALDI time-of-flight (MALDI-TOF) Proteomics Analyzer (Applied Biosystems, Carlsbad, CA, USA). Accuracy for peptide mass fingerprints (PMF) was searched using the online database tool, MASCOT (http://www.matrixscience.com). The theoretical isoelectric point (pI) and molecular weight (MW) for all confident spots were calculated using the Expasy Protparam tool (http://www.expasy.org/tools/). Protein scores surpassing 70 were selected. Lower scoring proteins were either verified manually or rejected from consideration. The corresponding DNA sequences were accessed from NCBI (http://www.ncbi.nlm.nih.gov/genome).
Cloning and protein expression of rGroEL, rGroES and rPrpC
MG strain R low (21st passage) was cultured to the mid-logarithmic phase of growth. Genomic DNA was extracted from MG cultures and purified as described (Boguslavsky et al. 2000). Primers for amplification of GroEL, GroES and PrpC protein were designed according to the genomic sequence of MG R low strain (GenBank accession: NC_004829.2). Due to the specific codon of the MG, we used the overlap PCR to change the PrpC codons.
The groEL gene was amplified with the following gene-specific primers: groELF (CAAGAGCTCATGGCAAAAGAATTAAC) and groELR (CAACTCGAGT TATAGGTGATTTAAGCT). The groES gene was amplified with the following primers: groESF (CAAGAGCTCATGAATATTAAA CCACTACACG) and groESR (CAACTCGAGTTATCTAATTACCCCAATGA). PrpC protein encoded by the ptc gene which was amplified by two pairs of primers ptc1 and ptc2 through point mutation of the nucleotides TGA to TGG: ptc1F (CAAGGATCCATGAAAAAGATATATGCAA GTTCGAC) and ptc1R (AGTCTCCCACCCAGAACGTGT); ptc2F (ACACGTTCTGGGTGGGAGACT) and ptc2R (CAAGAATTCTTAGCCATTAATAACCTCAGTTAG).
The full length of ptc gene was amplified by the primers ptc1F and ptc2R, using the previous PCR products as the template. The PCR was completed under the following conditions: 94 °C for 3 min and 30 cycles of 94 °C for 30 s, 50 °C for 30 s, 72 °C for 1 min 30 s, and then the final elongation reaction at 72 °C for 10 min. The PCR products were purified, digested and inserted into a pET-28a (+) vector, and the recombinant plasmids were designated as pET-GroEL, pET-GroES and pET-PrpC, respectively.
E. coli BL21 was transformed with the pET-GroEL, pET-GroES and pET-PrpC plasmids, respectively, and the recombinant GroEL, GroES and PrpC proteins were induced by 1 mM isopropyl-β-D-thiogalactopyranoside (IPTG) and analyzed by SDS-PAGE and Western blot as described above. The rGroEL, rGroES and rPrpC proteins were further purified using Ni-band Resin kit (Merck, Madison, WI, USA) according to manufacturer’s protocol. Fractions were collected every 1 ml, dialyzed overnight at 4 °C against PBS and the protein quantified by the RC DC protein assays kit.
Now, GroEL protein has identified existence in many bacteria; we download the representative GroEL protein sequences such as E. coli [WP_003969669.1], Salmonella enterica [WP_000729126.1], Staphylococcus carnosus [WP_015900785.1], Clostridium perfringens [WP_003462314.1] and MG from the NCBI website (http://www.ncbi.nlm.nih.gov/protein). The homology of GroEL protein derived from different bacteria was analyzed by Cluster X method with DNA Lagergene software MegAlign module (DNA Star Inc. Madison, WI, USA).
Determination of rGroEL ATPase activity
Total ATPase activity of rGroEL protein was measured with Micro-ATPase Assay Kit and expressed as μmol inorganic phosphate (Pi) per mg protein per hour (μmolPi/mg/h) according to the manufacturer’s instructions (Jiancheng Bioengineering Institute, Nanjing, China). Briefly, rGroEL protein was added to the enzymatic reaction TEA buffer (50 mM triethanolamine, pH 7.5, 50 mM KCl, and 20 mM MgCl2) to a final concentration of 84 ng/μl. The solution was incubated at 25, 35, 45, 50, 55, 60 or 70 °C for 10 min, respectively, and then reaction mixture was centrifuged at 2,000g for 10 min. A standard sample (0.02 μmol/ml Pi), a negative sample control and a blank sample were provided in the Kit and used as controls. Supernatants of rGroEL and negative samples were collected and added with the chromogenic agent buffer for two minutes reaction at room temperature, respectively. The Pi released from rGroEL ATP hydrolysis or from standard sample reacted with the chromogenic agent and caused a color change. The absorbance of the sample solutions was measured at OD636 nm with a spectrophotometer (Bio-Rad, Hercules, CA, USA). The trials were repeated three times, and the mean OD636 was calculated and presented as mean ± SD (Huang et al. 2006). The ATPase activity (μmolPi/mg/h) is calculated with formula described below:
Phosphatase PrpC refolding by rGroEL protein
PrpC is a dephosphorylation enzyme. Its substrate is pNPP which is achromatic and changes to orange as a result of dephosphorylation. To determine whether rGroEL protein helps the refolding of denatured phosphatase rPrpC, 8 reaction systems were designed: pNPP substrate+rGroEL+rGroES+ATP; pNPP substrate+rGroEL+ATP; pNPP substrate+rGroES+ATP; PNPP substrate+ATP; pNPP substrate+rGroEL; pNPP substrate+rGroES; pNPP substrate+denatured rPrpC protein (negative control); and pNPP substrate+undenatured rPrpC protein (positive control). rPrpC protein (80 μg/ml) was unfolded in a 52 °C water bath and incubated for 5 min. Denatured rPrpC was diluted to 3.2 μg/ml in a buffer containing the substrate pNPP and rGroEL (2.5 μM) at 37 °C. GroES (2.5 μM) and/or 2 mM ATP was added to the mixture and incubated for 10 min. Substrate dephosphorylation caused a color change, and absorbance was determined at a wavelength of 405 nm. The absorbance of un-denatured rPrpC protein with pNPP substrate was measured at OD405 nm and defined as 100 % refolding rate. The refolding rate was calculated with the formula described below:
Bactericidal assay
Bactericidal activity of the rabbit anti-rGroEL sera was tested by a modified microbactericidal assay (Martin et al. 2005). Briefly, 60 μl rabbit anti-rGroEL sera, chicken anti-MG sera (positive control) and normal rabbit sera (negative control) were inactivated at 56 °C for 30 min. Then, 180 μl of MG suspension (at a density of 104 CFU/ml in PBS) and 60 μl of rabbit complement (1:500 in DPBS) were added. After incubation at 37 °C for 60 min, 50 μl of the mixture was applied to solid medium (ATCC 243 broth medium with agar). The plates were incubated at 37 °C with 5 % CO2 for 4 days, and the colonies were counted. The bactericidal activity was calculated by the geometric mean of [1−(CFU from anti-sera/CFU from negative sera)] × 100 % from three independent trials (Ayalew et al. 2012).
Statistical analysis
ATPase activity of the rGroEL, rGroES and rGroEL+rGroES protein was measured by three independent reactions and were determined statistical differences using the two-way ANOVA. Refolding rate and bactericidal activity were expressed as the mean ± SD (standard deviation) of three replicates. The statistical differences between mean values were determined using the one-way ANOVA by GraphPad 5.0 software. P < 0.05 is regarded as significant.
Results
Identification of immunogenic MG membrane proteins
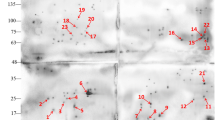
We performed 2-DE to separate hydrophilic and hydrophobic MG membrane proteins, respectively. Coomassie blue staining showed that dozens of spots were present on the gel (Fig. 1a, c). The immunogenic proteins were identified by Western blot with chicken anti-MG sera and DAB staining (Fig. 1b, d), excised and analyzed by MALDI-TOF–MS. In total, eight hydrophilic proteins and eight hydrophobic proteins were identified. Of them, five proteins were presented in both hydrophilic and hydrophobic fractions of MG membrane proteins, including translation elongation factor Tu (EF-Tu), dihydrolipoamide acetyltransferase (E2) component of pyruvate dehydrogenase complex, trigger factor, chaperone protein DnaK and heat shock protein GroEL. The detailed information for all membrane proteins identified was listed in Tables 1 and 2.
2-DE and Western blot analyses of MG membrane proteins. a Separation of hydrophilic membrane proteins was shown on Coomassie-stained 2-DE gel over the pH range 4–7. Excised spots indicated by arrows, marked as 1–8. b Western blot analysis of the hydrophilic membrane proteins with chicken anti-MG sera and visualized with DAB staining. c Separation of hydrophobic membrane proteins was shown on Coomassie-stained 2-DE gel over the pH range 5–8. Excised spots indicated by arrows, marked as 1-8. d Western blot analysis of the hydrophobic membrane proteins with chicken anti-MG sera and visualized with DAB staining
Expression and purification of rGroEL, rGroES and rPrpC proteins
To characterize the biological function of MG GroEL, groEL, groES and prpC genes were amplified from R low genomic DNA using specific primers and cloned to pET-28a to construct recombinant plasmids pET-rGroEL, pET-rGroES and pET-rPrpC, respectively. These recombinant plasmids were transformed into E. coli BL21 (DE3) competent cells, in which the protein expression was induced by IPTG. The proteins were then purified with Ni–NTA His Bind resin kit. SDS-PAGE showed a 14, 40 and 62 kDa purified protein bands for rGroES, rPrpC and rGroEL, respectively (Fig. 2). Each 200 μg recombinant protein was prepared for biological function assays.
The homology of GroEL protein difference among the E. coli, S. enterica, S. carnosus, C. perfringens and MG is shown in Fig. 3. The homology is only 42.6 % between the E. coli and MG, which suggests that the function of the MG GroEL protein may be altered compare to E. coli.
The homology comparison of GroEL proteins. a The homology of GroEL protein of E. coli, S. enterica, S. carnosus, C. perfringens and MG calculated with DNA Star software MegAlign module. The black block indicated the sequence difference from the mycoplasma to other bacteria. b The results of the percent identity are shown, among these bacteria, the MG GroEL proteins are only 42.6 % homologous to the corresponding E. coli GroEL protein
ATPase activity of rGroEL protein
ATPase activity was calculated based on the detection of absorbance at OD636 nm. The optimal ATPase activity of the rGroEL protein was observed at 50 °C, and rGroEL+rGroES protein complex showed 1.5-fold greater activity than that of the rGroEL protein alone (Fig. 4). The result is different from the previous report (Gray and Fersht 1991), in which the GroEL+GroES complex showed a decreased ATPase activity, compared with GroEL only. The amino acid sequence of the MG GroEL protein that has dramatic difference with E. coli GroEL may cause the changes of the spatial conformation of these two proteins, which probably alter the protein function. Consequently, the ATPase hydrolysis activity of MG rGroEL+rGroES complex is higher than that of rGroEL or rGroES protein alone.
ATPase activity of the rGroEL protein. ATPase activities of the rGroEL, rGroEL+rGroES and rGroES measured at different temperatures from 25 to 70 °C and expressed as μmolPi/mg/h. Three independent reactions were measured and the bars represent standard deviations. The statistical significance of the differences between rGroEL, rGroEL+rGroES and rGroES groups determined using the two-way ANOVA. The ATPase activity of rGroEL is significantly higher than that of rGroES, but lower than that of rGroEL+rGroES from 25 to 60 °C (P < 0.05)
rGroEL protein functions on phosphatase PrpC refolding
Recombinant MG PrpC protein was denatured at 52 °C and rGroEL protein was added to promote refolding activity. We defined the refolding rate of the positive control (un-denatured rPrpC protein) as 100 %. The data showed that rGroEL protein only, with rGroES, or with rGroES plus ATP could refold the denatured rPrpC protein efficiently, compared to that of the rGroES or rGroES plus ATP, demonstrating that rGroEL protein assists in rPrpC refolding. rGroEL combined with rGroES+ATP reached highest refolding rate of 38 % for denatured MG rPrpC protein. rGroES only showed a refolding rate of 2.03 % (Fig. 5).
Effect of the rGroEL protein on activation of PrpC protein refolding. Colume 1: Denatured PrpC protein serves as a negative control, refolding rate was measured as 2.53 %. Colume 2: rGroEL with rGroES and ATP produced a 38 % refolding rate; Colume 3: rGroEL with ATP produced a 31.67 % refolding rate; Colume 4: rGroES with ATP produced a 4.06 % refolding rate; Colume 5: ATP only showed a 0.83 % refolding rate; Colume 6: rGroEL only produced a 29.33 % refolding rate; Colume 7: rGroES only showed a 2.03 % refolding rate. Colume 8: Non-denatured PrpC protein serves as a positive control, the refolding rate was set as 100 %
Complement-dependent bactericidal assay
The bactericidal activity was calculated according to the formula described above. Chicken antiserum induced by inactivated MG vaccine served as a positive control; PBS and normal rabbit sera were used as negative controls. As the results, the rabbit anti-rGroEL sera and chicken anti-MG sera showed the bactericidal activity of 58.9 % ± 2.62 and 67.7 % ± 3.25, respectively. There is no significant difference between them; however, both were significantly higher than those of PBS and negative sera (P < 0.01), indicating MG GroEL protein may be used as a potential vaccine candidate. The assay was performed three times independently, and the data are presented as the mean ± SD in Table 3.
Discussion and conclusions
In this study, eight proteins were identified from hydrophilic and hydrophobic fractions of MG membrane each using 2-DE analysis followed by Western blot and MALDI-TOF–MS. Of them, translation EF-Tu, dihydrolipoamide acetyltransferase (E2) component of pyruvate dehydrogenase complex, trigger factor, chaperone protein DnaK and GroEL protein were identified from both hydrophilic and hydrophobic fractions of MG membrane proteins. EF-Tu functions on helping the aminoacyl-tRNA moves onto a free site on the ribosome. In the cytoplasm, EF-Tu binds an aminoacylated, or charged, tRNA molecule. This complex enters the ribosome. Pyruvate dehydrogenase E2 component was also termed aceF or pdhC, which is the second catalytic component enzyme of PDC (pyruvate dehydrogenase complex). The trigger factor is an ATP-independent chaperone and displays chaperone and peptidyl-prolyl-cis–trans-isomerase (PPIase) activities in vitro. DnaK chaperone protein involved in the stabilization of newly synthesized proteins is crucial for the reversion of MG to a vegetative form (Demina et al. 2010). The GroEL protein belongs to HSPs, which is highly conserved among prokaryotic and eukaryotic cells.
It has been documented that some HSPs located on the cell surface facilitating pathogen adherence to host cells and therefore play a key role in virulence (Ensgraber and Loos 1992). Some HSPs that function as chaperones may help in transporting proteins across cell membranes or in protein folding (Goulhen et al. 1998). However, its exact function in MG is unknown. Our results showed that MG GroEL has the ATPase hydrolysis activity; moreover, MG GroES enhanced the activity which is different from the previously published studies on E. coli, in which GroES decreased the ATP hydrolysis of GroEL (Gray and Fersht 1991; Mendoza et al. 1996). This is probably duo to the amino acid difference in MG GroEL and GroES compared with the E. coli. The amino acid homology of the GroEL protein between the E. coli and MG is 42.6 %. Moreover, the E. coli GroES residues Ile-25 and Leu-27 play critical role on the GroEL–GroES interaction, and residue Val-26 plays a minor role in the complex formation (Illingworth et al. 2011). The corresponding residues in MG GroES were Ile-25, Ile-26 and Thr-27, respectively, indicating amino acid alterations may induce the protein functional variation. The precise mechanism of the MG GroEL and GroES enhanced the GroEL ATPase activity that need to be further elucidated. The current study also demonstrated that MG rGroEL functions on the rPrpC protein refolding; however, MG rGroES plays litter role on the protein refolding function. Our results confirmed that MG GroEL functions as a molecular chaperone which is consistent with previously published studies (Sielaff et al. 2011; Fujimoto et al. 2012).
MG GroEL protein was shown to be a good protective immunogenic protein by the complement-dependent bactericidal assay. Rabbit antisera against MG rGroEL presented similar bactericidal activity to chicken anti-MG sera induced by inactivated vaccine (P > 0.05). The immunogenicity of GroEL has been reported in several experimental animal models (Leclerq et al. 2002; Amemiya et al. 2007; Bansal et al. 2010). GroEL protein from several pathogenic microorganisms is antigenic and often immunodominant in cellular and humoral immune responses. The mechanism(s) involved in host immunity to microbial infection is incompletely understood, but GroEL proteins appear to be important antigens in host defense. GroEL derived from Bacillus anthracis provided total protection to BALB/c mice against B. anthracis challenge (Sinha and Bhatnagar 2010). GroEL from Salmonella immunized BALB/c mice, produced significantly increased antibody titers and protected 50 % of the mice against lethal Salmonella challenge (Bansal et al. 2010). Other reports regarding GroEL of Streptococcus pneumoniae, Burkholderia pseudomallei, R. anatipestifer and Francisella tularensis also demonstrate increased immunogenicity and immune protection (Woo et al. 2001; Hartley et al. 2004; Khan et al. 2009; Han et al. 2012).
Above all, it is the first report that MG GroEL functions on ATPase activity and protein refolding. Rabbit antisera against MG GroEL possess a good bactericidal efficacy, which indicated the GroEL protein may be a potential candidate for MG vaccine. These observations provided us deeper understood on MG GroEL protein.
Abbreviations
- MG:
-
Mycoplasma gallisepticum
- 2-DE:
-
Two-dimensional gel electrophoresis
- MALDI-TOF–MS:
-
Matrix-assisted desorption/ionization time-of-flight mass spectrometry
- CRD:
-
Chronic respiratory disease
- HSPs:
-
Heat shock family of proteins
- PMF:
-
Peptide mass fingerprints
- MW:
-
Molecular weight
- Pi:
-
Inorganic phosphate
- IPTG:
-
Isopropyl-β-D-thiogalactopyranoside
References
Amemiya K, Meyers JL, Deshazer D, Riggins RN, Halasohoris S, England M, Ribot W, Norris SL, Wagg DM (2007) Detection of the host immune response to Burkholderia mallei heat-shock proteins GroEL and DnaK in a glanders patient and infected mice. Diagn Microbiol Infect Dis 59:137–147
Ayalew S, Confer AW, Shrestha B, Payton ME (2012) A rapid microtiter plate serum bactericidal assay method for determining serum complement-mediated killing of Mannheimia haemolytica. J Microbiol Methods 89:99–101
Bansal A, Paliwal PK, Sagi SS, Sairam M (2010) Effect of adjuvants on immune response and protective immunity elicited by recombinant Hsp60 (GroEL) of Salmonella typhi against S. typhi infection. Mol Cell Biochem 337:213–221
Boguslavsky S, Menaker D, Lysnyansky I, Liu T, Levisohn S, Rosengarten R, García M, Yogev D (2000) Molecular characterization of the Mycoplasma gallisepticum pvpA gene which encodes a putative variable cytadhesin protein. Infect Immun 68:3956–3964
Chapman E, Farr GW, Usaite R, Furtak K, Fenton WA, Chaudhuri TK, Hondorp ER, Matthews RG, Wolf SG, Yates JR, Pypaert M, Horwich AL (2006) Global aggregation of newly translated proteins in an Escherichia coli strain deficient of the chaperonin GroEL. Proc Natl Acad Sci USA 103:15800–15805
Cleavinger CM, Kim MF, Wise KS (1994) Processing and surface presentation of the Mycoplasma hyorhinis variant lipoprotein VlpC. J Bacteriol 176:2463–2467
Demina IA, Serebryakova MV, Ladygina VG, Rogova MA, Kondratov IG, Renteeva AN, Govorun VM (2010) Proteomic characterization of Mycoplasma gallisepticum nanoforming. Biochemistry 75:1252–1257
Ensgraber M, Loos M (1992) A 66-kilodalton heat shock protein of Salmonella typhimurium is responsible for binding of the bacterium to intestinal mucus. Infect Immun 60:3072–3078
Fayet O, Ziegelhoffer T, Georgopoulos C (1989) The groES and groEL heat shock gene products of Escherichia coli are essential for bacterial growth at all temperatures. J Bacteriol 171:1379–1385
Fujimoto A, Kosaka N, Hasegawa H, Suzuki H, Sugano S, Chiba J (2012) Enhancement of antibody responses to native G protein-coupled receptors using E. coli GroEL as a molecular adjuvant in DNA immunization. J Immunol Methods 375:243–251
Goulhen F, Hafezi A, Uitto VJ, Hinode D, Nakamura R, Grenier D, Mayrand D (1998) Subcellular localization and cytotoxic activity of the GroEL-like protein isolated from Actinobacillus actinomycetemcomitans. Infect Immun 66:5307–5313
Gray TE, Fersht AR (1991) Cooperativity in ATP hydrolysis by GroEL is increased by GroES. FEBS Lett 292:254–258
Han X, Hu Q, Ding S, Chen W, Ding C, He L, Wang X, Ding J, Yu S (2012) Identification and immunological characteristics of chaperonin GroEL in Riemerella anatipestifer. Appl Microbiol Biotechnol 93:1197–1205
Hartley MG, Green M, Choules G, Rogers D, Rees DG, Newstead S, Sjostedt A, Titball RW (2004) Protection afforded by heat shock protein 60 from Francisella tularensis is due to copurified lipopolysaccharide. Infect Immun 72:4109–4113
Hennigan SL, Driskell JD, Ferguson-Noel N, Dluhy RA, Zhao Y, Tripp RA, Krause DC (2012) Detection and differentiation of avian mycoplasmas by surface-enhanced Raman spectroscopy based on a silver nanorod array. Appl Environ Microbiol 78:1930–1935
Hu Y, Henderson B, Lund PA, Tormay P, Ahmed MT, Gurcha SS, Besra GS, Coates AR (2008) A Mycobacterium tuberculosis mutant lacking the groEL homologue cpn60.1 is viable but fails to induce an inflammatory response in animal models of infection. Infect Immun 76:1535–1546
Huang YY, Deng JY, Gu J, Zhang ZP, Maxwell A, Bi LJ, Chen YY, Zhou YF, Yu ZN, Zhang XE (2006) The key DNA-binding residues in the C-terminal domain of Mycobacterium tuberculosis DNA gyrase A subunit (GyrA). Nucleic Acids Res 34:5650–5659
Illingworth M, Ramsey A, Zheng Z, Chen L (2011) Stimulating the substrate folding activity of a single ring GroEL variant by modulating the cochaperonin GroES. J Biol Chem 286:30401–30408
Khan MN, Shukla D, Bansal A, Mustoori S, Ilavazhagan G (2009) Immunogenicity and protective efficacy of GroEL (hsp60) of Streptococcus pneumoniae against lethal infection in mice. FEMS Immunol Med Microbiol 56:56–62
Kobayashi K, Ehrlich SD, Albertini A, Amati G, Andersen KK, Arnaud M, Asai K, Ashikaga S, Aymerich S, O N (2003) Essential Bacillus subtilis genes. Proc Natl Acad Sci USA 100:4678–4683
Leclerq S, Harms JS, Rosinha GM, Azevedo V, Oliveira SC (2002) Induction of a th1-type of immune response but not protective immunity by intramuscular DNA immunisation with Brucella abortus GroEL heat-shock gene. J Med Microbiol 51:20–26
Lewthwaite JC, Clarkin CE, Coates AR, Poole S, Lawrence RA, Wheeler-Jones CP, Pitsillides AA, Singh M, Henderson B (2007) Highly homologous Mycobacterium tuberculosis chaperonin 60 proteins with differential CD14 dependencies stimulate cytokine production by human monocytes through cooperative activation of p38 and ERK1/2 mitogen-activated protein kinases. Int Immunopharmacol 7:230–240
Martin D, McCallum L, Glennie A, Ruijne N, Blatchford P, O’Hallahan J, Oster P (2005) Validation of the serum bactericidal assay for measurement of functional antibodies against group B meningococci associated with vaccine trials. Vaccine 23:2218–2221
Mendoza JA, Warren T, Dulin P (1996) The ATPase activity of chaperonin GroEL is highly stimulated at elevated temperatures. Biochem Biophys Res Commun 229:271–274
Obuchowski M, Madec E, Delattre D, Boël G, Iwanicki A, Foulger D, Seror SJ (2000) Characterization of PrpC from Bacillus subtilis, a member of the PPM phosphatase family. J Bacteriol 182:5634–5638
Panicker IS, Kanci A, Chiu CJ, Veith PD, Glew MD, Browning GF, Markham PF (2012) A novel transposon construct expressing PhoA with potential for studying protein expression and translocation in Mycoplasma gallisepticum. BMC Microbiol 12:138
Sielaff B, Lee KS, Tsai FT (2011) Structural and functional conservation of Mycobacterium tuberculosis GroEL paralogs suggests that GroEL1 Is a chaperonin. J Mol Biol 405:831–839
Sinha K, Bhatnagar R (2010) GroEL provides protection against Bacillus anthracis infection in BALB/c mice. Mol Immunol 48:264–271
Woo PC, Leung PK, Wong SS, Ho PL, Yuen KY (2001) groEL encodes a highly antigenic protein in Burkholderia pseudomallei. Clin Diagn Lab Immunol 8:832–836
Acknowledgments
This research was sponsored by the Special Fund for Agro-scientific Research in the Public Interest (Grant Nos. 201303044 and 201303038). We would like to thank Prof. Howard Barry Gelberg for reading of this manuscript and professional language editing.
Conflict of interest
The authors have not declared any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Erko Stackebrandt.
Lei Tan and Meirong Hu have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Tan, L., Hu, M., Yu, S. et al. Characterization of the chaperonin GroEL in Mycoplasma gallisepticum . Arch Microbiol 197, 235–244 (2015). https://doi.org/10.1007/s00203-014-1047-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-014-1047-2