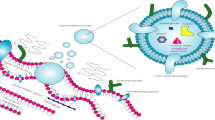
Abstract
Outer membrane vesicles (OMVs) are released from the outer membrane of Gram-negative bacteria. Moreover, Gram-positive bacteria also produce membrane-derived vesicles. As OMVs transport several bacterial components, especially from the cell envelope, their interaction with the host cell, with other bacteria or as immunogens, have been studied intensely. Several functions have been ascribed to OMVs, especially those related to the transport of virulence factors, antigenic protein composition, and development as acellular vaccines. In this work, we review some of the recent findings about OMVs produced by specific pathogenic bacterial species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The production of outer membrane vesicles (OMVs) from Gram-negative bacteria was reported 50 years ago (Bladen and Waters 1963; Bayer and Anderson 1965; Chatterjee and Das 1967). Moreover, in the last 15 years, much information related to these nanovesicles has been published (Beveridge 1999; Kuehn and Kesty 2005; Lee et al. 2008; Mashburn-Warren and Whiteley 2006). Many questions regarding them remain unanswered, including the molecular mechanism for the release of OMVs, or the sorting or regulated compositions of OMVs; in addition, new questions have arisen. This review will focus on the most recent information of OMVs from Gram-negative bacteria and the recently reported findings on vesicles from Gram-positive bacteria.
Neisseria meningitidis
N. Meningitidis is a Gram-negative diplococcus and an obligate human pathogen. Different research groups have been focused on the identification of meningococcus B antigens as the basis of a new vaccine. However, the high genetic variability of the proteins among different serotype B strains represents a challenge to obtain a broad multivalent effective N. meningitidis serotype B vaccine (Serruto et al. 2012). A vaccine based on OMVs of N. meningitidis is currently the only licensed vaccine against meningococcus serogroup B, and these have been used in Cuba, Chile, Norway, and New Zealand (Boslego et al. 1995; Fredriksen et al. 1991; Holst et al. 2009; Sierra et al. 1991). However, those vaccines required extraction with a detergent (deoxycholate) to decrease the amount of toxic LPS, resulting in partially intact and aggregated vesicles with an altered composition (Ferrari et al. 2006). There are two types of detergent-free OMVs. The first type is spontaneously released OMVs during in vitro growth and is without any treatments to enhance vesiculation (Ellis et al. 2010). The second type is obtained by treating cells with ethylene diamine tetraacetic acid (EDTA) to chelate calcium and magnesium ions (van de Waterbeemd et al. 2012). Such agents destabilize the outer membrane which enhances OMVs’ release and preserves the desired biochemical and immunological properties, even though these OMVs are not identical to naturally released OMVs (van de Waterbeemd et al. 2010; Haque and Russell 1974). Van der Ley et al. (2001) obtained a lpxL1 mutant of N. meningitidis that retained adjuvant activity similar to wild-type meningococcal LPS, but has reduced toxicity in vivo. This modified strain allowed generation of less toxic OMVs. Vaccines based on OMVs are able to protect against homologous strains; however, they cannot provide protection against heterologous strains. The explanation for this lack of a broad protection is because PorA, the major non-capsular protective antigen in N. meningitidis OMVs, is highly variable across different isolates of N. meningitidis. To deal with this variability, hexavalent and non-hexavalent engineered vaccines have been developed to over-express different PorA subtypes (Holst 2007). More recently, other highly immunogenic antigens have been combined with OMVs to allow the formulation of a multicomponent vaccine named 4CMenB (Serruto et al. 2012).
Escherichia coli
E. coli, one of the commensals in the human gut microbiota, inhabits the intestinal tract without causing injury to the host. However, several pathogenic E. coli strains express virulence factors and cause disease in humans. In 1976, Hoekstra et al. found membrane fragments (OMVs) in the culture supernatant of normally growing E. coli, consisting of practically unmodified outer membrane (Hoekstra et al. 1976). Later, the heat/labile enterotoxin (LT) activity was found in the newly synthesized OMVs released from E. coli (Gankema et al. 1980; Wai et al. 1995). In addition, Kollings and Matthews demonstrated the presence of DNA in OMVs purified from E. coli O157:H7, and using that DNA, they were able to identify the virulence genes eae, stx1, stx2, and uidA. Also, they confirmed that the Shiga toxins 1 and 2 were contained within the vesicles by western blot (Kolling and Matthews 1999).
Enterohemorragic E. coli (EHEC) release hemolysin toxin (EHEC-Hly) via OMVs (Aldick et al. 2009). EHEC-Hly is a typical RTX toxin that causes lysis of host cell by pore-forming action (Bielaszewska et al. 2013). The toxin exists as free EHEC-Hly and as EHEC-Hly associated with OMVs, both released by EHEC during growth. While the free toxin is lytic toward human endothelium, EHEC-Hly associated with OMVs does not lyse microvascular endothelial cells (HBMEC) and Caco-2 cells, but triggers their apoptosis (Bielaszewska et al. 2013). The OMVs-associated toxin is internalized by HBMEC and Caco-2 cells via dynamin-dependent endocytosis of OMVs and trafficked with OMVs into endo-lysosomal compartments. Upon endosome acidification and subsequent decrease in pH, EHEC-Hly is separated from OMVs, escapes from the lysosome, and targets mitochondria. Subsequent activation of caspase-9 and caspase-3 leads to apoptotic cell death. The OMVs-mediated intracellular delivery represents a newly recognized mechanism for a bacterial toxin to enter host cell in order to target mitochondria (Bielaszewska et al. 2013).
Cytolysin A (ClyA) is an α-pore-forming toxin secreted by pathogenic E. coli. ClyA monomer assembles into an oligomeric pre-pore structure independently of lipid membrane and detergent. ClyA cytotoxic protein was found in the OMVs of E. coli showed eightfold higher activity than the purified ClyA (Wai et al. 2003). Through an unknown mechanism, OMVs release ClyA, which then form a transmembrane pore in the host cell. OMVs may represent a non-classical pathway to attack eukaryotic host cells (Fahie et al. 2013).
Kim et al. demonstrated that OMVs derived from intestinal E. coli induced human endothelial cells to express functional cell adhesion molecules in vitro, and up-regulate vascular inflammation in vivo that is characterized by increased ICAM-1 expression in lung endothelial cells and neutrophil infiltration into the lung. The reduced neutrophil infiltration into the lung in ICAM-1−/− mice suggests that ICAM-1 regulates the OMVs-induced neutrophil infiltration into the lungs. Also, OMVs were more potent in inducing both ICAM-1 expression as well as leukocyte adhesion in vitro than free LPS (Kim et al. 2013).
Although nonpathogenic strains do not possess virulence factors, OMVs released from them have effects on the host cells. OMVs from pathogenic (enterotoxigenic E. coli: ETEC) and nonpathogenic E. coli (E. coli MK8A44: hypervesiculating mutant) were tested in human umbilical vein endothelial cells (HUVEC), and both increased expression of E-selectin, an intercellular adhesion molecule, IL-6, and NF-κB translocation to the nucleus. By eliciting an inflammatory response, OMVs could facilitate the transition from a localized infection to a systemic response and ultimately sepsis (Soult et al. 2013).
Recently, it has been proposed that intestinal bacteria may play a role in the etiology of gastric and colorectal cancer (Hasan et al. 2010). Tyrer et al. investigated whether OMVs from intestinal E. coli could be genotoxic. They found that E. coli-derived OMVs are readily internalized into Caco-2 cells, where they are potentially capable of inducing oxidative stress, leading to DNA damage, rereplication, and aneuploidy in susceptible cells. In addition, O157:H7-derived OMVs generated aneuploid cells typical of those with chromosomal instability (Tyrer et al. 2014).
Pseudomonas aeruginosa
P. aeruginosa is an opportunistic pathogen that colonizes a range of niches and causes a wide range of infections in humans which can vary from local to systemic, subacute to chronic, and superficial and self-limiting to life threatening (Ramos 2004). The release of vesicles from P. aeruginosa was observed after the treatment with gentamicin in 1993 (Kadurugamuwa et al. 1993). Later, peptidoglycan hydrolases were detected in P. aeruginosa OMVs, and those vesicles were able to lyse E. coli, and S. aureus cells (Kadurugamuwa and Beveridge 1996). Schooling and Beveridge observed that P. aeruginosa OMVs were a common biofilm constituents (Schooling and Beveridge 2006). Bomberger et al. in (2009) demonstrated that OMVs from P. aeruginosa deliver multiple virulence factors, including β-lactamase, alkaline phosphatase, hemolytic phospholipase C, and Cif toxin, directly into the host cytoplasm via fusion of OMVs with lipid rafts trough an actin-dependent pathway in the host plasma membrane or by endocytic pathway (Bomberger et al. 2009, 2011). The toxin Cif secreted into the OMVs of P. aeruginosa reduces cystic fibrosis transmembrane conductance regulator (CFTR)-mediated chloride secretion by human airway epithelial cells, a key driving force for mucociliary clearance. Later, Bomberger et al. found the mechanism of how a secreted P. aeruginosa virulence factor Cif disrupts mucociliary clearance in the lung by inactivating a host cell deubiquitinating enzyme (USP10), thereby facilitating the degradation of the CFTR secretory chloride channel, which reduces mucociliary clearance by human airway epithelial cells (Bomberger et al. 2011).
Interestingly, Kaparakis et al. reported that Gram-negative bacteria can deliver peptidoglycan to cytosolic nucleotide-binding oligomerization domain containing protein 1 (NOD1) in host cells via OMVs. Purified OMVs (containing peptidoglycan) from H. pylori, P. aeruginosa, and Neisseria gonorrhea up-regulated NF-κB and NOD1-dependent responses in vitro. Moreover, OMVs are delivered intragastrically to mice-induced innate and adaptive immune responses via NOD1-dependent but TLR-independent mechanism (Kaparakis et al. 2010).
Of the proteins identified in P. aeruginosa OMVs using LC–MS/MS, those with known functions include outer membrane proteins involved in antibiotic resistance, proteases, and proteins involved in host–bacteria interaction (Choi et al. 2011). In addition, Ellis et al. characterized the inflammatory responses caused by P. aeruginosa OMVs. They found that P. aeruginosa OMVs induce increased expression of MIP-2, TNF-α, IL-β, and IL-6 in RAW 264.7 macrophages compared with purified P. aeruginosa LPS. Both TLR ligands LPS and flagellin contributed to specific vesicle cytokine responses, whereas the CpG DNA content of vesicles did not. It is possible that a potent and distinct inflammatory response to vesicles is the cumulative effect of sensing vesicle-associated heterogeneous protein and LPS ligands by macrophages (Ellis et al. 2010).
Acinetobacter baumannii
A. baumannii has emerged as an opportunistic pathogen responsible for nosocomial infections with an extensive antibiotic resistance i.e., multi-drug resistance (MDR), extreme drug resistance (XDR), and the ultimate pan drug resistance (PDR). Some of its virulence factors are the biofilm formation, iron acquisition, phospholipase D, capsule, adherence, and ability to invade host cells, as well as mechanisms associated with antibiotic resistance (Eijkelkamp et al. 2011; Livermore 1995; Su et al. 2005). OmpA protein of E. coli facilitates adherence and invasion of host cells; its homologue in A. baumannii is a protein with a molecular mass of 38 kDa and plays a role in permeability of small solutes (Khan et al. 2003). Choi et al. (2008) demonstrated that OmpA of A. baumannii plays a major role in the interactions with epithelial cells contributing in the early stage of bacterial infection. Purified OMVs from a clinical isolate of A. baumannii were analyzed by proteomics, and among those proteins, OmpA was highly enriched and several putative virulence-associated proteins were also identified (Kwon et al. 2009). An isogenic OmpA mutant of A. baumannii allowed assessment of the influence of this protein in OMVs biogenesis as the OMVs production decreased significantly compared with the wild type (Moon et al. 2012). Previous studies suggested that OMVs may be involved in the transfer of genetic material among similar bacterial species i.e., N. gonorrheae (Dorward et al. 1989 ). Carbapenem resistance in A. baumannii is mainly due to the presence of β-lactamases as well as altered permeability and penicillin binding protein modification. It has been observed that clinical isolates of A. baumannii may release OMVs as a mechanism of horizontal gene transfer whereby carbapenem resistance genes are delivered to surrounding A. baumannii bacterial isolates (Rumbo et al. 2011). Recently, the elongation factor Tu (EF-Tu) was associated with A. baumannii OMVs; the EF-Tu contributes to mediate adhesion to cells through binding fibronectin on the host cell (Dallo et al. 2012). A. baumannii OMVs were used as a vaccine in a mouse model of disseminated sepsis. IgG1, IgG2c, and IgM immunoglobulins were observed in high levels while low levels of pro-inflammatory cytokines IL-6 and IL-1β were measured. Vaccination with OMVs provided protection to mice against the challenge with the reference strain A. baumannii ATCC 19606 (McConnell et al. 2011).
Vibrio spp.
Release of OMVs from V. cholerae and V. parahaemolyticus was reported in (1993) by Kondo et al. By fixing cells with the freeze-substitution technique, they were able to observe small membranous vesicles. They also found that anti-O (Inaba) serum reacted with the surface of the vesicles, while the inside of the vesicle appeared as an electro-dense mass (Kondo et al. 1993). V. cholerae is the etiologic agent of cholera with the cholera toxin (CT) acting as the major virulence factor of this pathogen. CT is found in the periplasm and also associated with the bacterial surface. Chatterjee and Chaudhuri demonstrated that the CT is not only secreted in a soluble form, but also it is associated with OMVs purified from V. cholerae strain O395. They also found using a CHO cell assay that OMVs were associated with a physiologically active CT, and OMVs could interact with intestinal epithelial cells via CT-receptor. This evidence identified V. cholerae OMVs as a vehicle for delivering CT to epithelial cells (Chatterjee and Chaudhuri 2011).
In addition to the V. cholerae O1 and O139 serogroups, other strains belonging to non-O1 and non-O139 V. cholerae serogroups (NOVC) have been implicated in gastroenteritis and extraintestinal infections in humans; however, little is known about the virulence of NOVC strains. Bielig and co-workers demonstrated that NOVC OMVs elicit immune response mediated by NOD1 and NOD2 proteins in mammalian host cells. Moreover, they provided evidence that quorum-sensing machinery plays an important regulatory role in the immune process by attenuating the inflammatory potential of OMVs under infective conditions (Bielig et al. 2011).
V. anguillarum O1, a major fish pathogen that causes vibriosis, also release OMVs. The purified OMVs from V. anguillarum contained major outer membrane proteins OmpU, hemolysin, and phospholipase. Flounders were immunized with purified OMVs and subsequent expression of TNF-α, IL-1β, and IL-6 in the spleens was observed by semi-quantitative RT-PCR (Hong et al. 2009).
The biofilm formation by V. fischeri depends on the presence of a polysaccharide gene cluster, syp, and a number of syp regulators, including the histidine sensor kinase RscS. By over-expressing the RscS sensor kinase, Shibata and Visick induced OMVs production in a manner that depends on the presence of the syp locus. These OMVs contained antigenically distinct molecules, possibly representing modified forms of lipopolysaccharide (LPS). Their findings support the correlation between OMVs production and biofilm formation (Shibata and Visick 2012).
OMVs from V. cholerae were used to immunize female mice by intranasal, intraperitoneal, and intragastric routes. Immunoglobulin A was induced to a significant level only by mucosal immunization, while the intranasal route induced the highest antibodies titers. The offspring of female mice immunized orally with V. cholerae OMVs were protected against the challenge with live V. cholerae (Schild et al. 2008). More recently, it has been observed that immunization using OMVs significantly reduced colonization of neonatal mice challenged with hyperinfectious V. cholerae strain (Bishop et al. 2012).
Brucella
Brucellosis is a debilitating illness in humans and causes abortion in livestock. B. melitensis, B. abortus, and B. suis are the most virulent species for humans and animals (Corbel 1997). Gamazo and Moriyon in (1987) reported for the first time the liberation of fragments from B. melitensis using differential ultracentrifugation. The free membranous material called “FMM” was separated by SDS-PAGE, and proteins with molecular masses of 84, 25–30, 22, and 18 kDa were observed. Two years later, Gamazo et al. obtained OMVs from B. ovis and B. melitensis. The electrophoretic profiles of purified OMVs were compared with hot saline extract of unlysed cells and showed proteins with similar masses in both species (Gamazo et al. 1989).
Later, Boigegrain et al. (2004) found OMVs while they were analyzing the soluble proteins content of supernatant of B. suis grown at acid pH. After ultracentrifugation of B. suis cell-free supernatant, OMVs were observed in the pellet. Both Omp25 and Omp31 were identified in the OMVs using monoclonal antibodies. More recently, we identified 29 proteins in OMVs purified from the reference strain B. melitensis 16 M by mass spectrometry analysis (Avila-Calderón et al. 2012); these included Omp25, SOD, IalB, and InvB. OMVs purified from B. melitensis 16 M and from VTRM1 (a rough mutant derived from strain 16 M) were used to vaccinate mice; live vaccine strain B. melitensis Rev1 was used as a positive control. Results showed no significant difference in the protection afforded to groups vaccinated with Brucella OMVs versus those vaccinated with the live vaccine B. melitensis Rev1. These results were notable as OMVs are acellular entities, while B. melitensis Rev1 is a live attenuated vaccine. The live vaccines used to control animal brucellosis have the ability to infect veterinarians, animal care workers, and slaughter house workers accidentally and cause low levels of abortions when given to pregnant cows.
Pluronics or polaxamers are nonionic block copolymers of polypropylene oxide and polyethylene oxide that have been shown to activate pro-inflammatory signaling pathways and immune responses (Batrakova and Kabanov 2008). Pluronic-85 is amphiphilic and has been shown to enhance the systemic and local expansion of antigen presenting cells and natural killer cells. We evaluated the efficacy of Pluronic-85 as an adjuvant to enhance OMVs protection against B. melitensis. Results showed that Pluronic-85 enhanced the secretion of TNF-α in macrophages after incubation with a mixture of OMVs and Pluronic-85. Also, a combination of OMVs and Pluronic-85 showed better protection in mice against B. melitensis challenge than OMVs alone (Jain-Gupta et al. 2012). Thus, OMVs could be the basis for the development of safer Brucella vaccine including a human vaccine that is currently not available.
Porphyromonas gingivalis
P. gingivalis, an anaerobic bacterium, has been found to be a predominant species in the advancing lesions of human periodontal disease. In the late 1980s, P. gingivalis was shown to release OMVs; these structures showed proteolytic activity and could attach to oral surfaces and co-aggregate to other bacteria (Grenier and Bélanger 1991; Mayrand and Grenier 1989). Also, it has been demonstrated that P. gingivalis OMVs strongly promote co-aggregation between Staphylococcus aureus and other oral flora (like Streptocooccus spp., Fusobacterium nucleatum, Actinomyces naeslundii, and Actinomyces viscosus). Kamaguchi et al. (2003) also found that strains of S. aureus, including methicillin resistant S. aureus (MRSA), could adhere to oral biofilms in dental plaque on the tooth surface or in the gingival crevice when P. gingivalis was present.
P. gingivalis OMVs are capable of inducing acute inflammation characterized by the accumulation of a large number of neutrophils in the connective tissue. This cellular response is due to the biosynthesis and expression of E-selectin and ICAM-1 by vascular endothelial cells with the inhibition of IFN-γ. This evidence indicates that P. gingivalis OMVs are capable of inducing and regulating the cellular response (Srisatjaluk et al. 1999).
P. gingivalis OMVs were used as a vaccine antigen for intranasal immunization of BALB/c mice (Nakao et al. 2011). Synthetic double-stranded RNA polyriboinosinic polyribocytycil [Poly (I:C)] was used as the mucosal adjuvant. In mice vaccinated with such a vaccine, high levels of P. gingivalis-specific IgA was found in nasal washes and saliva, while IgA and IgG antibodies were found in serum. They also found that the mutation of the galE gene in P. gingivalis altered the production of OMVs resulting in reduced OMVs per cell or no vesicles at all. Nakao et al. (2006) previously had shown that mutations in the galE gene in P. gingivalis caused accumulation of intracellular carbohydrates in the presence of 0.1 % of galactose, a shorter O-antigen chain, increasing of biofilm production, and significantly more susceptible to some antibiotics in comparison with the wild type. The absence of OMVs in galE mutant is noteworthy as very little information related to the mechanism of the production of vesicles is known.
Bordetella spp.
B. pertussis is a Gram-negative pathogen responsible for whooping cough, an acute respiratory disease. B. pertussis typically expresses a wide array of virulence factors which promote bacterial adhesion and invasion by altering the local environment, including pertussis toxin, tracheal cytotoxin, adenylate cyclase toxin, filamentous hemagglutinin, and the lipooligosaccharide. In 1999, for the first time, OMVs were isolated from B. pertussis Tohama strain CIP 8132. In those purified OMVs, the adenylate cyclase hemolysin (AC-Hly), pertussis toxin (Ptx), and filamentous hemagglutinin (FHA) were detected (Hozbor et al. 1999). Adenylate cyclase toxin (ACT) contained in OMVs (OMVs–ACT) was able to intoxicate murine macrophages and CHO-K1 cells independently of the toxin’s receptor CD11b/CD18 (Donato et al. 2012).
In 2008, Roberts et al. using mass spectrometry identified pertactin, adenylate cyclase hemolysin, pertussis toxin, and the lipooligosaccharide (LOS) in OMVs isolated from B. pertussis Tohama CIP 8132. Also, they tested the vesicles as a vaccine; results showed that mice immunized with B. pertussis OMVs were protected against the challenge with B. pertussis virulent strain (Roberts et al. 2008). With the goal to decrease the toxicity of B. pertussis OMVs, the pagL gene from B. bronchisptica, which encodes a lipid A 3-deacylase, was expressed in B. pertussis strain Tohama. The engineered OMVs, containing tetra-instead of penta-acylated LOS, pertactin and pertussis toxin, were interesting candidates to be considered for the development of novel multi-antigen vaccine. The engineered OMVs were used in murine B. pertussis intranasal challenge model. The protection experiments showed significant differences between immunized animals and the PBS control group. The recombinant vesicles showed lower endotoxic activity and maintained a protective response in mice (Asensio et al. 2011).
More recently, a proteomic analysis was performed on B. parapertussis OMVs, and results showed pertactin, outer membrane porin precursor and outer membrane OmpQ as major components. B. parapertussis OMVs used as a vaccine in intranasal challenge model showed that immunized mice were protected against B. parapertussis and also against B. pertussis challenge. These findings are interesting as the commercial vaccine used for B. pertussis offers little protection against B. parapertussis (Bottero et al. 2013).
Helicobacter pylori
H. pylori-induced inflammation is associated with the development of gastritis, peptic ulcer disease and gastric cancer in humans. Fiocca et al. purified H. pylori OMVs from strain 60190 and CCUG 17874. VacA toxin, one of the most important virulence factors produced by H. pylori, was immunolocalized in those vesicles. Moreover, the vesicles were internalized by MKN28 cells as well as detected in the gastric mucosa from H. pylori-infected humans. In such a scenario, OMVs may represent a mechanism, additional to secretory pathways, for the delivery of bacterial toxins and antigens to the gastric mucosa (Fiocca et al. 1999). More recently, a mass spectroscopy analysis revealed that the H. pylori OMVs contain the urease subunits, the cytotoxin VacA, CagA, GroEL, catalase, metabolic and ribosomal proteins, and the BabA and SabA adhesins as well as phosphatidylethanolamine and cardiolipin (Olofsson et al. 2010).
Different studies have used various genotypes of H. pylori for OMVs characterization, for example, cag PAI+ toxigenic (strain containing the complete pathogenicity island) and cag PAI− nontoxigenic strains. OMVs purified from cag PAI+ and cag PAI− increased the proliferation of human gastric adenocarcinoma AGS cells at low doses of OMVs, while at higher OMVs doses, a growth arrest, increased toxicity, and IL-8 production were observed (Ismail et al. 2003). In contrast, OMVs from H. pylori VacA− were able to induce apoptosis, independent of the mitochondrial pathway, in gastric epithelial cells with activation of caspase 8 and 3 (Ayala et al. 2006). This observation is interesting as the OMVs released from H. pylori VacA−, that is considered less pathogenic, also induced apoptosis in AGS cells.
Chitcholtan et al. analyzed the direct effect of H. pylori OMVs on cellular events associated with carcinogenesis. They found that the treatment of H. pylori OMVs (from toxigenic strain 60190) with AGS human gastric epithelial cells increased the micronuclei formation, while the OMVs purified from the deletion mutant vacA-did not. They concluded that OMVs delivery the vacuolating toxin VacA to the gastric epithelium that may represent a new mechanism for H. pylori to induce gastric carcinogenesis (Chitcholtan et al. 2008).
In a clinical isolate of H. pylori strain TK1402, biofilm formation was observed when grown in broth medium, and also OMVs were detected in the biofilm matrix. Recently, a 22 kDa protein was identified in OMVs attached to the biofilm, but the function or identity was not determined yet (Yonezawa et al. 2009, 2011).
Gram-positive bacteria also release membrane vesicles
Gram-positive bacteria such as Staphylococcus aureus (Lee et al. 2009), Mycobacterium ulcerans (Marsollier et al. 2007), Bacillus anthracis (Rivera et al. 2010), B. cereus, and B. subtilis (Dorward and Garon 1990) release membrane-derived vesicles to the extracellular milieu (MVs). MVs found in Gram-positives are spherical, bilayered structures with a diameter of approximately 20–150 nm and have been found to be released both in vitro and in vivo (Gurung et al. 2011; Rivera et al. 2010).
MVs were isolated from S. aureus and analyzed by mass spectrometry. Some of those identified proteins include the IgG-binding protein, ferritin, ferrichrome-binding lipoprotein precursor, ABC transporter extracellular binding protein, β-lactamase, and membrane protein oxaA. Also S. aureus MVs interacted with the plasma membrane of host cells via a cholesterol-rich membrane microdomain and delivered the protein A to host cell (Gurung et al. 2011; Rivera et al. 2010). This was the first evidence involving S. aureus MVs as a vehicle for delivery of bacterial effectors molecules to host cells. Recently, the α-toxin (α-hemolysin) was associated with S. aureus MVs. The α-toxin is a 33 kDa pore-forming protein able to lyse a wide range of human cells and induce apoptosis in T-lymphocytes. S. aureus MVs containing the α-toxin induced cytotoxicity in HeLa cells and also induced the erythrocyte lysis (Thay et al. 2013). Cysteine protease and α-hemolysin associated with MVs have been linked with atopic dermatitis, so S. aureus MVs have been explored as inducers of the host immune response. The topical application of S. aureus MVs increased the proinflammatory mediators by dermal fibroblasts (Thay et al. 2013). Also S. aureus MVs induced atopic dermatitis-like inflammation in the skin of mice. This evidence supports the implication of MVs in the pathogenesis of S. aureus (Hong et al. 2011).
B. anthracis is the spore producing bacillus that causes anthrax in a range of vertebrates. The release of vesicles has been reported in B. anthracis, and these vesicles had a double membrane structure and ranged from 50 to 300 nm in diameter. The components of the anthrax toxins: the protective antigen (PA), lethal factor (LF), and edema toxin (ET) as well as the anthrolysin were detected in B. anthracis MVs (Rivera et al. 2010). Mice immunized with B. anthracis MVs were protected against the challenge with B. anthracis (Rivera et al. 2010).
M. ulcerans is a slow-growing mycobacterium that infects the skin and subcutaneous tissues and is the etiologic agent of Buruli ulcer (George et al. 1999). A poliketide-derived macrolide designated mycolactone is the sole known virulence factor responsible for Buruli ulcer (George et al. 1999). M. ulcerans produces biofilm composed by an extracellular matrix that contains vesicles and also mycolactone. Vesicles recovered from M. ulcerans displayed cytotoxic activity in mouse macrophages that is thought to be due to the presence of mycolactone in the vesicles (Marsollier et al. 2007).
In (2011) Prados-Rosales et al. found that virulent and nonvirulent mycobacterial species such as Mycobacterium bovis BCG, M. tuberculosis H37Rv, M. tuberculosis H37Ra, M. kansasii, M. phlei, M. smegmatis, and M. avium released MVs. Proteomic analysis revealed that only MVs from M. bovis BCG and M. tuberculosis H37Rv were enriched in proteins associated with virulence, including a remarkably high content of putative TLR2 ligands (such lipoproteins 19 kDa, LprA, and LprG). The interaction of MVs with murine macrophages induced cytokines and chemokines in a TLR2-dependent fashion. This evidence showed that released mycobacterial vesicles are a delivery mechanism for immunologically active molecules that contribute to mycobacterial virulence (Prados-Rosales et al. 2011).
Recently, it was found that the protein VirR (encoded by the gene rv0431) in M. tuberculosis (Mtb) regulates the quantity of Mtb-derived membrane vesicles bearing Toll-like receptor 2 ligands, including the lipoproteins LpqH and SodC. Authors named the VirR protein as “vesiculogenesis and immune response regulator.” This evidence supports that vesiculogenesis in Mtb is subject to genetic regulation by an unknown mechanism (Rath et al. 2013).
The foodborne pathogen L. monocytogenes is able to resist stresses under different environmental conditions. Recently, the release of MVs was observed in L. monocytogenes. A mutant was analyzed to evaluate whether the general stress transcription factor σB affected the production of MVs. L. monocytogenes wild-type and sigB mutant produced double membrane spheres ranging from 20 to 100 nm in diameter. Purified MVs were analyzed by LC–ESI–MS/MS. A total of 130 proteins were identified in MVs of the wild type and 18 proteins are known as σB-dependent proteins in L. monocytogenes. InIB and listeriolysin O (LLO) were identified in MVs from the wild type and also in the mutant; InIB is necessary for entry of L. monocytogenes into host epithelial cells while LLO causes vacuolar lysis (Lee et al. 2013).
Conclusions
OMVs were observed for the first time about 50 years ago as acellular entities released from Gram-negative bacteria, and their presence in Gram-positive bacteria has also been documented. Moreover, their proposed functions have been shown to include alternative mechanism of protein release, toxin delivery, as well as modulating immune response in the host cells. The identification of the composition as well as the functions of these vesicles will contribute to the understanding of these fascinating biological nanostructures. Certainly, the ability of these vesicles as acellular vaccines is yet to be fully explored in veterinary and especially in human medicine.
References
Aldick T, Bielaszewska M, Uhlin BE, Humpf HU, Wai SN, Karch H (2009) Vesicular stabilization and activity augmentation of enterohaemorrhagic Escherichia coli haemolysin. Mol Microbiol 71:1496–1508. doi:10.1111/j.1365-2958.2009.06618.x
Asensio CJA, Gaillard ME, Moreno G, Bottero D, Zurita E, Rumbo M, Van der Ley P, Van der Ark A, Hozbor D (2011) Outer membrane vesicles obtained from Bordetella pertussis Tohama expressing the lipid A deacylase PagL as a novel acellular vaccine candidate. Vaccine 29:1649–1656. doi:10.1016/j.vaccine.2010.12.068
Avila-Calderón ED, Lopez-Merino A, Jain-Gupta N, Peralta H, López-Villegas EO, Sriranganathan N, Boyle SM, Witonsky S, Contreras-Rodríguez A (2012) Characterization of outer membrane vesicles from Brucella melitensis and protection induced in mice. Clin Dev Immunol 2012:352493. doi:10.1155/2012/352493
Ayala G, Torres L, Espinosa M, Fierros-Zarate G, Maldonado V, Meléndez-Zajgla J (2006) External membrane vesicles from Helicobacter pylori induce apoptosis in gastric epithelial cells. FEMS Microbiol Lett 260:178–185. doi:10.1111/j.1574-6968.2006.00305.x
Batrakova EV, Kabanov AV (2008) Pluronic block copolymers: evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J Control Release 130:98–106. doi:10.1016/j.jconrel.2008.04.013
Bayer ME, Anderson TF (1965) The surface structure of Escherichia coli. Proc Natl Acad Sci USA 54:1592–1599
Beveridge TJ (1999) Structures of gram-negative cell walls and their derived membrane vesicles. J Bacteriol 181:4725–4733
Bielaszewska M, Rüter C, Kunsmann L, Greune L, Bauwens A, Zhang W, Kuczius T, Kim KS, Mellmann A, Schmidt MA, Karch H (2013) Enterohemorrhagic Escherichia coli hemolysin employs outer membrane vesicles to target mitochondria and cause endothelial and epithelial apoptosis. PLoS Pathog 9:e1003797. doi:10.1371/journal.ppat.1003797
Bielig H, Dongre M, Zurek B, Wai SN, Kufer TA (2011) A role for quorum sensing in regulating innate immune responses mediated by Vibrio cholerae outer membrane vesicles (OMVs). Gut Microbes 2:274–279
Bishop AL, Tarique AA, Patimalla B, Calderwood SB, Qadri F, Camilli A (2012) Immunization of mice with Vibrio cholerae outer-membrane vesicles protects against hyperinfectious challenge and blocks transmission. J Infect Dis 205:412–421. doi:10.1093/infdis/jir756
Bladen HA, Waters JF (1963) Electron microscopic study of some strains of Bacteroides. J Bacteriol 86:1339–1344
Boigegrain RA, Salhi I, Alvarez-Martinez MT, Machold J, Fedon Y, Arpagaus M, Weise C, Rittig M, Rouot B (2004) Release of periplasmic proteins of Brucella suis upon acidic shock involves the outer membrane protein Omp25. Infect Immun 72:5693–5703. doi:10.1128/IAI.72.10.5693
Bomberger JM, MacEachran DP, Coutermarsh BA, Ye S, O’Toole GA, Stanton BA (2009) Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog 5:e1000382. doi:10.1371/journal.ppat.1000382
Bomberger JM, Ye S, MacEachran DP, Koeppen K, Barnaby RL, O’Toole GA, Stanton BA (2011) A Pseudomonas aeruginosa toxin that hijacks the host ubiquitin proteolytic system. PLoS Pathog 7:e1001325. doi:10.1371/journal.ppat.1001325
Boslego J, Garcia J, Cruz C et al (1995) Efficacy, safety, and immunogenicity of a meningococcal group B (15:P1.3) outer membrane protein vaccine in Iquique, Chile. Vaccine 13:821–829
Bottero D, Gaillard ME, Errea A, Moreno G, Zurita E, Pianciola L, Rumbo M, Hozbor D (2013) Outer membrane vesicles derived from Bordetella parapertussis as an acellular vaccine against Bordetella parapertussis and Bordetella pertussis infection. Vaccine 31:5262–5268. doi:10.1016/j.vaccine.2013.08.059
Chatterjee D, Chaudhuri K (2011) Association of cholera toxin with Vibrio cholerae outer membrane vesicles which are internalized by human intestinal epithelial cells. FEBS Lett 585:1357–1362. doi:10.1016/j.febslet.2011.04.017
Chatterjee SN, Das J (1967) Electron microscopic observations on the excretion of cell-wall material by Vibrio cholerae. J Gen Microbiol 49:1–11
Chitcholtan K, Hampton MB, Keenan JI (2008) Outer membrane vesicles enhance the carcinogenic potential of Helicobacter pylori. Carcinogenesis 29:2400–2405. doi:10.1093/carcin/bgn218
Choi CH, Lee JS, Lee YC, Park TI, Lee JC (2008) Acinetobacter baumannii invades epithelial cells and outer membrane protein A mediates interactions with epithelial cells. BMC Microbiol 8:216–226. doi:10.1186/1471-2180-8-216
Choi DS, Kim DK, Choi SJ, Lee J, Choi JP, Rho S, Park SH, Kim YK, Hwang D, Gho YS (2011) Proteomic analysis of outer membrane vesicles derived from Pseudomonas aeruginosa. Proteomics 11:3424–3429. doi:10.1002/pmic.201000212
Corbel MJ (1997) Brucellosis: an overview. Emerg Infect Dis 3:213–221
Dallo SF, Zhang B, Denno J, Hong S, Tsai A, Haskins W, Ye JY, Weitao T (2012) Association of Acinetobacter baumannii EF-Tu with cell surface, outer membrane vesicles, and fibronectin. Sci World J 2012:128705. doi:10.1100/2012/128705
Donato GM, Goldsmith CS, Paddock CD, Eby JC, Gray MC, Hewlett EL (2012) Delivery of Bordetella pertussis adenylate cyclase toxin to target cells via outer membrane vesicles. FEBS Lett 586:459–465. doi:10.1016/j.febslet.2012.01.032
Dorward DW, Garon CF (1990) DNA is packaged within membrane-derived vesicles of gram-negative but not gram-positive bacteria. Appl Environ Microbiol 56:1960–1962
Dorward DW, Garon CF, Judd RC (1989) Export and intercellular transfer of DNA via membrane blebs of Neisseria gonorrhoeae. J Bacteriol 171:2499–2505
Eijkelkamp BA, Hassan KA, Paulsen IT, Brown MH (2011) Investigation of the human pathogen Acinetobacter baumannii under iron limiting conditions. BMC Genomics 12:126–139. doi:10.1186/1471-2164-12-126
Ellis TN, Leiman SA, Kuehn MJ (2010) Naturally produced outer membrane vesicles from Pseudomonas aeruginosa elicit a potent innate immune response via combined sensing of both lipopolysaccharide and protein components. Infect Immun 78:3822–3831. doi:10.1128/IAI.00433-10
Fahie M, Romano FB, Chisholm C, Heuck AP, Zbinden M, Chen M (2013) A non-classical assembly pathway of Escherichia coli pore-forming toxin cytolysin A. J Biol Chem 288:31042–31051. doi:10.1074/jbc.M113.475350
Ferrari G, Garaguso I, Adu-Bobie J et al (2006) Outer membrane vesicles from group B Neisseria meningitidis delta gna33 mutant: proteomic and immunological comparison with detergent-derived outer membrane vesicles. Proteomics 6:1856–1866. doi:10.1002/pmic.200500164
Fiocca R, Necchi V, Sommi P, Ricci V, Telford J, Cover TL, Solcia E (1999) Release of Helicobacter pylori vacuolating cytotoxin by both a specific secretion pathway and budding of outer membrane vesicles. Uptake of released toxin and vesicles by gastric epithelium. J Pathol 188:220–226
Fredriksen JH, Rosenqvist E, Wedege E, Bryn K, Bjune G, Frøholm LO, Lindbak AK, Møgster B, Namork E, Rye U et al (1991) Production, characterization and control of MenB-vaccine “Folkehelsa”: an outer membrane vesicle vaccine against group B meningococcal disease. NIPH Ann 14:67–80
Gamazo C, Moriyón I (1987) Release of outer membrane fragments by exponentially growing Brucella melitensis cells. Infect Immun 55:609–615
Gamazo C, Winter AJ, Moriyón I, Riezu-Boj JI, Blasco JM, Díaz R (1989) Comparative analyses of proteins extracted by hot saline or released spontaneously into outer membrane blebs from field strains of Brucella ovis and Brucella melitensis. Infect Immun 57:1419–1426
Gankema H, Wensink J, Guinée PAM, Jansen WH, Witholt B (1980) Some characteristics of the outer membrane material released by growing enterotoxigenic Escherichia coli. Infect Immun 29:704–713
George KM, Chatterjee D, Gunawardana G, Welty D, Hayman J, Lee R, Small PLC (1999) Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science 283:854–857. doi:10.1126/science.283.5403.854
Grenier D, Bélanger M (1991) Protective effect of Porphyromonas gingivalis outer membrane vesicles against bactericidal activity of human serum. Infect Immun 59:3004–3008
Gurung M, Moon DC, Choi CW, Lee JH, Bae YC, Kim J, Lee YC, Seol SY, Cho DT, Kim SI, Lee JC (2011) Staphylococcus aureus produces membrane-derived vesicles that induce host cell death. PLoS one 6:e27958. doi:10.1371/journal.pone.0027958
Haque H, Russell AD (1974) Effect of chelating agents on the susceptibility of some strains of gram-negative bacteria to some antibacterial agents. Antimicrob Agents Chemother 6:200–206
Hasan N, Pollack A, Cho I (2010) Infectious causes of colorectal cancer. Infect Dis Clin N Am 24:1019–1039. doi:10.1016/j.idc.2010.07.009
Hoekstra D, van der Laan JW, de Leij L, Witholt B (1976) Release of outer membrane fragments from normally growing Escherichia coli. Biochim Biophys Acta Biomembr 455:889–899. doi:10.1016/0005-2736(76)90058-4
Holst J (2007) Strategies for development of universal vaccines against meningococcal serogroup B disease. Hum Vaccin 3:290–294
Holst J, Martin D, Arnold R, Campa-Huergo C, Oster P, O’Hallahan J, Rosenqvist E (2009) Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine 27S:B3–B12. doi:10.1016/j.vaccine.2009.04.071
Hong GE, Kim DG, Park EM, Nam BH, Kim YO, Kong IS (2009) Identification of Vibrio anguillarum outer membrane vesicles related to immunostimulation in the Japanese flounder, Paralichthys olivaceus. Biosci Biotechnol Biochem 73:437–439. doi:10.1271/bbb.80580
Hong SW, Kim MR, Lee EY, Kim JH, Kim YS, Jeon SG, Yang JM, Lee BJ, Pyun BY, Gho YS, Kim YK (2011) Extracellular vesicles derived from Staphylococcus aureus induce atopic dermatitis-like skin inflammation. Allergy 66:351–359. doi:10.1111/j.1398-9995.2010.02483.x
Hozbor D, Rodríguez ME, Fernández J, Lagares A, Guiso N, Yantorno O (1999) Release of outer membrane vesicles from Bordetella pertussis. Curr Microbiol 38:273–278
Ismail S, Hampton MB, Keenan JI (2003) Helicobacter pylori outer membrane vesicles modulate proliferation and interleukin-8 production by gastric epithelial cells. Infect Immun 71:5670–5675. doi:10.1128/IAI.71.10.5670-5675.2003
Jain-Gupta N, Contreras-Rodriguez A, Vemulapalli R, Witonsky SG, Boyle SM, Sriranganathan N (2012) Pluronic P85 enhances the efficacy of outer membrane vesicles as a subunit vaccine against Brucella melitensis challenge in mice. FEMS Immunol Med Microbiol 66:436–444. doi:10.1111/1574-695x.12010
Kadurugamuwa JL, Beveridge TJ (1996) Bacteriolytic effect of membrane vesicles from Pseudomonas aeruginosa on other bacteria including pathogens: conceptually new antibiotics. J Bacteriol 178:2767–2774
Kadurugamuwa JL, Clarke AJ, Beveridge TJ (1993) Surface action of gentamicin on Pseudomonas aeruginosa. J Bacteriol 175:5798–5805
Kamaguchi A, Nakayama K, Ichiyama S, Nakamura R, Watanabe T, Ohta M, Baba H, Ohyama T (2003) Effect of Porphyromonas gingivalis vesicles on coaggregation of Staphylococcus aureus to oral microorganisms. Curr Microbiol 47:485–491
Kaparakis M, Turnbull L, Carneiro L et al (2010) Bacterial membrane vesicles deliver peptidoglycan to NOD1 in epithelial cells. Cell Microbiol 12:372–385. doi:10.1111/j.1462-5822.2009.01404.x
Khan NA, Shin S, Chung JW, Kim KJ, Elliott S, Wang Y, Kim KS (2003) Outer membrane protein A and cytotoxic necrotizing factor-1 use diverse signaling mechanisms for Escherichia coli K1 invasion of human brain microvascular endothelial cells. Microb Pathog 35:35–42. doi:10.1016/S0882-4010(03)00090-1
Kim JH, Yoon YJ, Lee J, Choi EJ, Yi N, Park KS, Park J, Lötvall J, Kim YK, Gho YS (2013) Outer membrane vesicles derived from Escherichia coli up-regulate expression of endothelial cell adhesion molecules in vitro and in vivo. PLoS one 8:e59276. doi:10.1371/journal.pone.0059276
Kolling GL, Matthews KR (1999) Export of virulence genes and Shiga toxin by membrane vesicles of Escherichia coli O157:H7. Appl Environ Microbiol 65:1843–1848
Kondo K, Takade A, Amako K (1993) Release of the outer membrane vesicles from Vibrio cholerae and Vibrio parahaemolyticus. Microbiol Immunol 37:149–152
Kuehn MJ, Kesty NC (2005) Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev 19:2645–2655. doi:10.1101/gad.1299905
Kwon SO, Gho YS, Lee JC, Kim SI (2009) Proteome analysis of outer membrane vesicles from a clinical Acinetobacter baumannii isolate. FEMS Microbiol Lett 297:150–156. doi:10.1111/j.1574-6968.2009.01669.x
Lee EY, Choi DS, Kim KP, Gho YS (2008) Proteomics in gram-negative bacterial outer membrane vesicles. Mass Spectrom Rev 27:535–555. doi:10.1002/mas
Lee EY, Choi DY, Kim DK, Kim JW, Park JO, Kim S, Kim SH, Desiderio DM, Kim YK, Kim KP, Gho YS (2009) Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics 9:5425–5436. doi:10.1002/pmic.200900338
Lee JH, Choi CW, Lee T, Kim SI, Lee JC, Shin JH (2013) Transcription factor σB plays an important role in the production of extracellular membrane-derived vesicles in Listeria monocytogenes. PLoS one 8:e73196. doi:10.1371/journal.pone.0073196
Livermore DM (1995) Beta-lactamases in laboratory and clinical resistance. Clin Microbiol Rev 8:557–584
Marsollier L, Brodin P, Jackson M et al (2007) Impact of Mycobacterium ulcerans biofilm on transmissibility to ecological niches and Buruli ulcer pathogenesis. PLoS Pathog 3:e62. doi:10.1371/journal.ppat.0030062
Mashburn-Warren LM, Whiteley M (2006) Special delivery: vesicle trafficking in prokaryotes. Mol Microbiol 61:839–846. doi:10.1111/j.1365-2958.2006.05272.x
Mayrand D, Grenier D (1989) Biological activities of outer membrane vesicles. Can J Microbiol 35:607–613
McConnell MJ, Rumbo C, Bou G, Pachón J (2011) Outer membrane vesicles as an acellular vaccine against Acinetobacter baumannii. Vaccine 29:5705–5710. doi:10.1016/j.vaccine.2011.06.001
Moon DC, Choi CH, Lee JH, Choi CW, Kim HY, Park JS, Kim SI, Lee JC (2012) Acinetobacter baumannii outer membrane protein A modulates the biogenesis of outer membrane vesicles. J Microbiol 50:155–160. doi:10.1007/s12275-012-1589-4
Nakao R, Senpuku H, Watanabe H (2006) Porphyromonas gingivalis galE is involved in lipopolysaccharide O-antigen synthesis and biofilm formation. Infect Immun 74:6145–6153. doi:10.1128/IAI.00261-06
Nakao R, Hasegawa H, Ochiai K, Takashiba S, Ainai A, Ohnishi M, Watanabe H, Senpuku H (2011) Outer membrane vesicles of Porphyromonas gingivalis elicit a mucosal immune response. PLoS one 6:e26163. doi:10.1371/journal.pone.0026163
Olofsson A, Vallström A, Petzold K, Tegtmeyer N, Schleucher J, Carlsson S, Haas R, Backert S, Wai SN, Gröbner G, Arnqvist A (2010) Biochemical and functional characterization of Helicobacter pylori vesicles. Mol Microbiol 77:1539–1555. doi:10.1111/j.1365-2958.2010.07307.x
Prados-Rosales R, Baena A, Martinez LR et al (2011) Mycobacteria release active membrane vesicles that modulate immune responses in a TLR2-dependent manner in mice. J Clin Invest 121:1471–1483. doi:10.1172/JCI44261
Ramos J (2004) Pseudomonas. Kluwer Academic/Plenum Publishers, New York
Rath P, Huang C, Wang T, Wang T, Li H, Prados-Rosales R, Elemento O, Casadevall A, Nathan CF (2013) Genetic regulation of vesiculogenesis and immunomodulation in Mycobacterium tuberculosis. Proc Natl Acad Sci USA 110:E4790–E4797. doi:10.1073/pnas.1320118110
Rivera J, Cordero RJB, Nakouzi AS, Frases S, Nicola A, Casadevall A (2010) Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins. PNAS 107:19002–19007. doi:10.1073/pnas.1008843107
Roberts R, Moreno G, Bottero D, Gaillard ME, Fingermann M, Graieb A, Rumbo M, Hozbor D (2008) Outer membrane vesicles as acellular vaccine against pertussis. Vaccine 26:4639–4646. doi:10.1016/j.vaccine.2008.07.004
Rumbo C, Fernández-Moreira E, Merino M, Poza M, Méndez JA, Soares NC, Mosquera A, Chaves F, Bou G (2011) Horizontal transfer of the OXA-24 carbapenemase gene via outer membrane vesicles: a new mechanism of dissemination of carbapenem resistance genes in Acinetobacter baumannii. Antimicrob Agents Chemother 55:3084–3090. doi:10.1128/AAC.00929-10
Schild S, Nelson EJ, Camilli A (2008) Immunization with Vibrio cholerae outer membrane vesicles induces protective immunity in mice. Infect Immun 76:4554–4563. doi:10.1128/IAI.00532-08
Schooling SR, Beveridge TJ (2006) Membrane vesicles: an overlooked component of the matrices of biofilms. J Bacteriol 188:5945–5957. doi:10.1128/JB.00257-06
Serruto D, Matthew JB, Sanjay R, Marzia MG, Rino R (2012) The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: immunological, functional and structural characterization of the antigens. Vaccine 30:B87–B97. doi:10.1016/j.vaccine.2012.01.033
Shibata S, Visick KL (2012) Sensor kinase RscS induces the production of antigenically distinct outer membrane vesicles that depend on the symbiosis polysaccharide locus in Vibrio fischeri. J Bacteriol 194:185–194. doi:10.1128/JB.05926-11
Sierra GV, Campa HC, Varcacel NM, Garcia IL, Izquierdo PL, Sotolongo PF, Casanueva GV, Rico CO, Rodriguez CR, Terry MH (1991) Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann 14:195–210
Soult MC, Lonergan NE, Shah B, Kim WK, Britt LD, Sullivan CJ (2013) Outer membrane vesicles from pathogenic bacteria initiate an inflammatory response in human endothelial cells. J Surg Res 184:458–466
Srisatjaluk R, Doyle RJ, Justus DE (1999) Outer membrane vesicles of Porphyromonas gingivalis inhibit IFN-γ -mediated MHC class II expression by human vascular endothelial cells. Microb Pathog 27:81–91
Su XZ, Chen J, Mizushima T, Kuroda T, Tsuchiya T (2005) AbeM, an H + -coupled Acinetobacter baumannii multidrug efflux pump belonging to the MATE family of transporters. Antimicrob Agents Chemother 49:4362–4364. doi:10.1128/AAC.49.10.4362-4364.2005
Thay B, Wai SN, Oscarsson J (2013) Staphylococcus aureus α-toxin-dependent induction of host cell death by membrane-derived vesicles. PLoS one 8:e54661. doi:10.1371/journal.pone.0054661
Tyrer PC, Frizelle FA, Keenan JI (2014) Escherichia coli-derived outer membrane vesicles are genotoxic to human enterocyte-like cells. Infect Agent Cancer 9:1–14. doi:10.1186/1750-9378-9-2
van de Waterbeemd B, Streefland M, van der Ley P, Zomer B, van Dijken H et al (2010) Improved OMV vaccine against Neisseria meningitidis using genetically engineered strains and a detergent-free purification process. Vaccine 28:4810–4816
van de Waterbeemd B, Streefland M, van Keulen L, van den IJssel J, De Haan A et al (2012) Identification and optimization of critical process parameters for the production of NOMV vaccine against Neisseria meningitidis. Vaccine 30:3683–3690
van der Ley P, Steeghs L, Hamstra HJ et al (2001) Modification of lipid A biosynthesis in Neisseria meningitidis lpxL mutants: influence on lipopolysaccharide structure, toxicity, and adjuvant activity. Infect Immun 69:5981–5990. doi:10.1128/IAI.69.10.5981
Wai SN, Takade A, Amako K (1995) The release of outer membrane vesicles from strains of enterotoxigenic Escherichia coli. Microbiol Immunol 39:451–456
Wai SN, Lindmark B, Söderblom T, Takade A, Westermark M, Oscarsson J, Jass J, Richter-Dahlfors A, Mizunoe Y, Uhlin BE (2003) Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell 115:25–35
Yonezawa H, Osaki T, Kurata S, Fukuda M, Kawakami H, Ochiai K, Hanawa T, Kamiya S (2009) Outer membrane vesicles of Helicobacter pylori TK1402 are involved in biofilm formation. BMC Microbiol 9:197–208. doi:10.1186/1471-2180-9-197
Yonezawa H, Osaki T, Woo T, Kurata S, Zaman C, Hojo F, Hanawa T, Kato S, Kamiya S (2011) Analysis of outer membrane vesicle protein involved in biofilm formation of Helicobacter pylori. Anaerobe 17:388–390. doi:10.1016/j.anaerobe.2011.03.020
Acknowledgments
This work was funded by CONACYT CB-2011-01 No. 169259, SIP-IPN 20131610, SIP-IPN 20144471, and Proyecto de investigacion en apoyo a la consolidacion de profesores del Instituto Politecnico Nacional, con nivel de candidato a investigador Nacional ICYT-DF/IPN. EDAC and MGAV were supported by CONACYT and PIFI-IPN scholarships. ACR, JCCD and EOLV were supported by fellowships from COFAA, SIP-EDI, and SNI-CONACYT. ACR was supported by semestre sabatico agosto-enero 2013-2014 ENCB-IPN.
Conflict of interest
The authors declare that they have no competing interests in relation to this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Erko Stackebrandt.
Rights and permissions
About this article
Cite this article
Avila-Calderón, E.D., Araiza-Villanueva, M.G., Cancino-Diaz, J.C. et al. Roles of bacterial membrane vesicles. Arch Microbiol 197, 1–10 (2015). https://doi.org/10.1007/s00203-014-1042-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-014-1042-7