Abstract
Summary
Macro- and microarchitectural, bone material property, dynamic histomorphometric, and bone turnover marker data were studied in normal bone mineral density (BMD) post-menopausal women with fragility fracture. Women with fracture had thinner iliac cortices and more homogeneous bone material properties in cortical bone than age/BMD-matched non-fracture women. Low cortical thickness and bone tissue heterogeneity in normal BMD women are associated with prevalent fragility fracture.
Introduction
Bone mass (bone mineral density, (BMD)) of the spine and hip is today’s best single measurement for evaluating future fragility fracture risk. However, the majority of fragility fractures occur in women with BMD T-score above the WHO osteoporotic BMD threshold of − 2.5, indicating that non-BMD endpoints may play a role in their fragility fractures. We hypothesize that in non-osteoporotic women, bone micoarchitecture, bone material properties, dynamic histomorphometric endpoints, and bone turnover markers are related to fragility fracture.
Methods
Two groups (N = 60 each) of post-menopausal women with total hip BMD T-score ranging from + 0.3 to –2.49 were recruited: fragility fracture and age/BMD-matched, non-fragility fracture women. Normal (T-score > − 0.99) and osteopenic (T-score ≤ − 1.0) BMD cohorts were designated within both the fracture and non-fracture groups. Transiliac biopsy specimens were obtained to evaluate dynamic histomorphometric and microarchitectural endpoints and bone material properties by static and dynamic nanoindentation testing. All variables for fracture and non-fracture women within each BMD cohort were compared by the Wilcoxon signed-rank test (P < 0.01).
Results
Compared to non-fracture/normal BMD women, fracture/normal BMD women display lower iliac cortical thickness (− 12%, P = 0.0041) and lower heterogeneity of hardness (− 27%, P = 0.0068), elastic modulus (− 35%, P = 0.0009), and storage modulus (− 23%, P = 0.0054) in the cortical bone tissue, and lower heterogeneity of hardness (− 13%, P = 0.0088) in the trabecular bone tissue. Osteopenic women had no abnormalities related to fracture status.
Conclusion
Post-menopausal women with normal BMD and fragility fracture have low cortical thickness and heterogeneity of several bone material properties in cortical and trabecular mineralized bone tissue. These differences may explain a portion of the excess bone fragility in women with normal BMD and fragility fracture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a serious public health problem [1]. Currently approved medicinal treatments for osteoporosis reduce fracture risk in individuals with low total hip bone mineral density (BMD) [2]. Despite the availability of these treatments, the annual number of fragility fractures in the combined European Union and USA is projected to rise to 7.5 million by 2025, at a total direct cost of over $60 billion [1, 3].
A major reason for the high cost of osteoporosis is that the majority of fragility fractures occur in patients with total hip T-score above the WHO osteoporotic BMD threshold of − 2.5 [4,5,6]. This problem exists because, though the fragility fracture rate in osteoporotic women is higher than that in non-osteoporotic women (T-score > − 2.5), the non-osteoporotic population is larger, making the absolute number of non-osteoporotic women with fragility fractures greater [5, 6]. The efficacy of existing osteoporosis medications in most non-osteoporotic women is formally unproven, because only women with T-score < − 2.0 were enrolled in most Phase III trials [2]. Identifying non-osteoporotic women at risk for fragility fracture is difficult, because their fractures appear to be incompletely explained by BMD, marking an important fracture risk/BMD discrepancy.
Osteoporosis was once considered a disease characterized by normal mineralized bone tissue present in insufficient quantity [7], driving the validation of densitometric bone mass measurements [4]. The fracture risk/BMD discrepancy surrounding fragility fractures in non-osteoporotic women is one of six clinical examples of a fracture risk/BMD discrepancy. Prevalent fragility fracture predicts future fragility fracture risk much better than BMD [8]. Models that combine BMD with multiple clinical risk factors with no relationship to BMD predict future fragility fracture risk much better than BMD alone [9]. As fragility fracture risk in older women accelerates exponentially [10], the rate of loss in spine and hip BMD is constant [11]. Anti-resorptive therapy that increases total hip BMD ~ 3–6% and spine BMD ~ 6–10% after 3 years is far more effective in reducing hip (~ − 50%) and spine (~ − 70%) fracture risk [2] than corresponding increases in hip/spine BMD would suggest [12]. Glucocorticoid-treated and diabetic patients have higher fracture risk than is predicted by their T-score [13, 14]. Though DXA-based BMD by itself is a very good measure of future fracture risk, multiple examples of a fracture risk/BMD discrepancy exist. The most consequential for both patients and public health is that the majority of fragility fractures occur in women with non-osteoporotic BMD.
This fracture risk/BMD discrepancy has sharpened the bone field’s focus on whole bone strength. The factors that determine whole bone strength are as follows: mass, the amount of bone; material properties, the properties of the bone tissue itself; architecture, the shape and size of a bone; and microdamage, the amount of fatigue damage in a bone’s mineralized tissue [15]. Material properties, architecture, and microdamage are collectively recognized as bone quality, mass-independent bone properties that influence bone strength [16]. Bone quality can be assessed by imaging; static and dynamic histomorphometry; bone turnover markers; bone composition; collagen fiber orientation; degree of bone mineralization; collagen cross-linking and protein glycation; minimally invasive indentation; micropillar compression; and nanoindentation [16,17,18,19].
Previous publications concerning the population of women described below reported low hardness and elastic modulus, and low heterogeneity of several bone material properties in cortical and trabecular bone of non-osteoporotic-by-BMD (+ 0.3 ≥ total hip T-score > − 2.5) women with fragility fracture [19], with no differences in degree of bone mineralization [20]. We hypothesize that in women with normal (total hip T-score ≥ − 0.99) or osteopenic BMD (− 1.00 ≥ total hip T-score > − 2.5), bone turnover markers, bone material properties, dynamic histomorphometric endpoints, bone micro- and macroarchitecture, and degree of mineralization of bone are associated with fragility fracture.
Materials and methods
Data covering five topics potentially relevant to fragility fracture are presented: bone turnover markers; bone material properties; dynamic histomorphometry; bone macro- and microarchitecture; and degree of bone mineralization. The bone turnover marker, dynamic histomorphometry, and macro- and microarchitecture data have not been published or analyzed previously. The data from bone material properties and degree of bone mineralization were previously published and analyzed only as “non-osteoporotic” patients (total hip T-score = + 0.3 to − 2.50, N = 120) in non-fracture and fracture subsets [19, 20]. The data from all five topics are presented here, separated for analysis into long-recognized BMD tiers [4], rather than as more heterogeneous-by-BMD groups of “non-osteoporotic” women: normal BMD (total hip T-score = + 0.3 to − 0.99) and osteopenic (T-score = − 1.00 to − 2.50). Thus, the data for bone material properties and degree of bone mineralization represent a new analysis of previously published data [19, 20]. Each BMD cohort was divided into non-fracture and fracture subsets, yielding non-fracture/normals, fracture/normals, non-fracture/osteopenics, and fracture/osteopenics.
Subjects
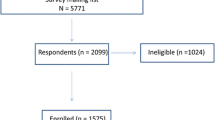
Two groups (N = 60 each) of healthy, non-osteoporotic women at least 4 years past their last menstrual period were recruited: fragility fracture women and BMD/age-matched women with no fragility fracture. The Creighton University Institutional Review Board approved the study. All subjects provided written consent prior to enrollment.
Fragility fracture subjects were women who had experienced a low trauma fracture during the previous 5 years and never taken any bone-active medications. “Low trauma” was defined as equal to or less than a fall to the ground from standing height. Fractures of digits, face, and skull were excluded. To limit morbidity of the transiliac biopsy procedure, subjects with body mass index (BMI) > 33 kg/m2 were excluded. All were first screened by DXA. Nine percent of those screened had total hip T-score < − 2.5 and were referred to their primary care provider for osteoporosis treatment. Those with total hip T-score ≥ − 2.5 were enrolled. Non-fracture women had never taken any bone-active medications, and had neither clinical history of fragility fracture nor vertebral fracture by lateral spine radiography. In all subjects, a complete medical history was obtained and then combined with routine clinical serum studies to exclude those with medical conditions known to cause secondary osteoporosis. Each fracture subject was matched to a non-fracture subject of similar age (± 2 years) and total hip BMD (± 10%). Previous descriptions of this population cite its total hip T-score range as − 1 to − 2.5 [19,20,21]. Re-inspection of the total hip T-scores revealed a range of + 0.3 to − 2.49.
Two 7.5 mm diameter transiliac biopsy specimens with intact cortices were obtained from about 2 cm posterior and inferior to the anterior–superior iliac spine of the right ilium of each subject. The minimum distance between the outside boundaries of the two specimens was 1.5 cm. In fracture subjects, the specimens were obtained a minimum of 11 months and a maximum of 5 years after fragility fracture. Both specimens were fixed in 70% ethanol for 48 h, and embedded without decalcification in methyl methacrylate (microCT and histomorphometry) or epoxy resin (nanoindentation).
Bone turnover markers
All bone turnover marker data here are presented for the first time. Fasting morning blood was drawn and analyzed for complete blood count, serum electrolytes, alkaline phosphatase, renal and hepatic function, bone-specific alkaline phosphatase (ELISA [Quidel; Santa Clara, CA]), PTH (RIA [Scantibodies; Santee, CA]), and 25OHD3 (RIA [DiaSorin; Stillwater, MN]). A 24 h urine was collected and analyzed for N-telopeptides (NTx) (ELISA [Inverness Medical; Princeton, NJ]).
Material properties of bone (nanoindentation)
The endpoints hardness, elastic modulus, storage modulus, loss modulus, and tan-delta were calculated from nanoindentation measurements presented previously [19], at which time patients were divided for analysis into non-fragility fracture and fragility fracture groups of “non-osteoporotic” women (T-score > − 2.50). In this manuscript, the same nanoindentation endpoint data from those patients are now grouped for analysis as traditional BMD-defined tiers [4] with normal (T-score > − 1.00) or osteopenic BMD (T-score = − 1.00 to − 2.50), rather than simply as “non-osteoporotic” women. Hardness is a surface property of a material that measures its resistance to localized plastic deformation. Elastic modulus reflects a material’s resistance to elastic deformation [22]. Storage and loss modulus describe viscoelastic properties of bone. The mechanical behavior of trabecular bone is similar to that of fluid-filled porous engineering materials [23].Viscoelastic properties of trabecular bone correlate with its strength and toughness [24]. Storage modulus, the endpoint most often correlated with the strength of a material, represents its capacity to store energy, and is routinely used to quantitate mechanical strength of hydrogels [25]. Loss modulus represents a material’s capacity to dissipate energy [25].
Embedding and preparation
Embedding and preparation of specimens were performed as described [19]. Bone marrow and residual water, but not tissue-bound water, were removed. The specimen was then embedded (EpoThin 2 Resin; Buehler; Lake Bluff, IL, USA) to provide mechanical support to the mineralized bone tissue during testing.
The embedded specimen was ground under irrigation with deionized water with a motorized polishing wheel (Ecomet-3 and Automet-2; Buehler; Lake Bluff, IL, USA) about 2 mm deep into the specimen, using silicon carbide discs of diminishing grit size (1200, 800, 600, 320 μm). This produced a flat face parallel to the long axis of the specimen that exposed its cortical and trabecular bone tissue. The face was polished with a sequential series of alumina powder slurries (1.0, 0.50, and 0.05 μm) (Buehler).
Nanoindentation procedure
Testing of these samples, described previously in detail [19], is summarized. An ATI 950 TriboIndenter (Hysitron, Bruker Corp.; Billerica, MA, USA) with a Berkovich tip (150 nm radius) was used. Before each quasi-static or dynamic test set, a series of indentations in the polished surface of a standard fused quartz material was used to calibrate the tip area function. Testing was performed on specimens presented in random order to operators (SV, AD) blinded to patient ID.
Quasi-static testing
Twenty-five indents per bone type were made at a constant loading rate of 50 nm/s [17, 19, 22]. Mean surface roughness and regions of interest for nanoindentation were determined using scanning probe microscopy (SPM). A nanoindentation target depth of 500 nm was used to be much larger than the mean roughness (50 nm) of the tested areas to avoid effects of topology on the measurements [17, 22]. Cortical bone indentation sites were always in interstitial bone along a line parallel to the periosteal surface [19]. Trabecular bone indentation sites were centered in trabeculae to avoid edges. Each indentation procedure included loading at a constant rate of 50 nm/s for 10 s, a 10-s holding period at maximum load, and unloading at the same rate for 10 s. The load–displacement data from each indentation were used to calculate bone tissue hardness and elastic modulus [22].
Dynamic testing
Twenty-five measurements per bone type were done in each specimen. All test sites were > 10 µm from any previous quasi-static or dynamic test site. The indenter tip was loaded at a constant rate of 50 nm/s and then held at a maximum load of 6 mN, comparable to the maximum load in quasi-static testing. A small sinusoidal force was superimposed onto the quasi-static force during the holding phase. Dynamic force amplitudes were prescribed in the range of 75–125 μN with load frequency incrementally increasing from 10 to 200 Hz in 32 equally spaced steps. Displacement amplitude and phase lag of the displacement response relative to the input forcing signal were measured at each frequency. The load–displacement data from each indentation were used to calculate storage modulus and loss modulus. Tan-delta for each indentation equals loss modulus divided by storage modulus [17]. The data are reported at the mid-range frequency (105 Hz).
Hardness, elastic modulus, storage modulus, loss modulus, and tan-delta for each type of bone for each sample were the mean of hardness, elastic modulus, storage modulus, loss modulus, and tan-delta values from the load–displacement curves of each indentation into each type of bone tissue. All measurements in each specimen were completed in one session before moving to the next specimen. The cortical bone quasi-static tests were followed by the trabecular bone quasi-static tests. Then, the cortical bone dynamic tests were followed by the trabecular bone dynamic tests. Hardness, elastic modulus, storage modulus, loss modulus, tan-delta, and intrasample variance in hardness, elastic modulus, storage modulus, and loss modulus for each type of mineralized bone tissue are reported. Intrasample variance (coefficient of variation) is equal to standard deviation/mean for the 25 nanoindentation measurements of each type (static and dynamic) in each type of tissue (cortical or trabecular) [26].
Microcomputed tomography (microCT) and degree of mineralization of bone
All microCT-based data here are presented for the first time. The degree of mineralization data were presented previously [20] with patients divided for analysis into non-fracture and fracture groups of “non-osteoporotic” women (T-score > − 2.50). In this manuscript, the same degree of mineralization data from the fracture and non-fracture patients are now grouped for analysis as traditional BMD-defined normal and osteopenic tiers [4]. For microCT, the entire methyl methacrylate–embedded bone biopsy was scanned using methods previously described [27]. A microfocus X-ray tube with a focal spot of 10 μm was used as a source and a standard bone phantom was scanned weekly. 3D isotropic slices of 30 μm voxel size were collected with an integration time of 250 ms, after which a standard procedure was used to reconstruct the 3D images, using a threshold of 275. A 5 mm diameter cylindrical volume of interest (VOI) in trabecular bone located at least 1 mm from the nearest endocortical surface that contained 110 slices (98 mm3) was designated for microarchitectural analyses. Both 2D and 3D microCT data are reported. The degree of mineralization of bone (DMB) data previously analyzed as non-fragility fracture and fragility fracture groups of non-osteoporotic-by-BMD women [20] are now analyzed as non-fragility fracture and fragility fracture groups within traditional BMD tiers [4] with normal BMD (T-score > − 1) and osteopenic BMD (T-score = − 1.00 to − 2.50).
Histomorphometry
All histomorphometric data here are presented for the first time. Each subject received oral in vivo double fluorochrome labeling for 3 days ON, 14 days OFF, and 3 days ON. The biopsy procedure was done 5–14 days after the second ON period [28, 29]. After microCT scanning, the block was ground to form a face parallel to the long axis of the specimen about 250 µm deep into the specimen. The block was leveled and sections were obtained from two portions of the specimen, using previously established methods and adhering to more recently established guidelines [29,30,31]. Representative sections from each portion were used for measurements. The measured and calculated variables are fully described [28, 29]. Cortical thickness was measured on one section from each specimen portion, being the mean of ~ 48 periosteal-to-endocortical surface measurements (24 from each cortex) with an intersite distance of ~ 0.5 mm, which were taken perpendicular to the periosteal surface of each cortex.
Statistical analysis
Each BMD cohort was divided into non-fracture and fracture subsets, yielding non-fracture/normals (N = 35), fracture/normals (N = 31), non-fracture/osteopenics (N = 25), and fracture/osteopenics (N = 29). Shapiro–Wilk testing revealed that the data for the majority of variables had a non-normal distribution. Thus, values for all variables for the non-fracture and fracture subsets within each BMD cohort are presented as medians and interquartile range and are compared by the Wilcoxon signed-rank test (Tables 1, 2, and 3 and Supplemental Tables S1–S3). Demographic and anthropometric endpoints and spine/total hip BMD and T-score of the normal and osteopenic cohorts in the non-fracture and fracture subsets were compared by the Wilcoxon signed-rank test. In consideration of the number of variables tested, a P value ≤ 0.01 was considered significant.
Results
Demographic, anthropometric, BMD, and bone turnover marker data
The fracture and non-fracture subsets of the normal BMD cohort did not differ for any demographic or anthropometric variable. The fracture and non-fracture subsets of the osteopenic cohort had lower total hip BMD and T-score than the fracture and non-fracture subsets of the normal BMD cohort. The fracture subset of the osteopenic cohort had lower spine BMD and T-score than the fracture group of the normal BMD cohort (Table 1). The fracture and non-fracture subsets of the normal BMD cohort did not differ for any bone biochemical marker of turnover (Table S1).
Material properties of mineralized bone
In cortical bone of the normal BMD cohort, there were no significant differences between fracture and non-fracture women in absolute values for any endpoint (Table S2). Variance of hardness, elastic modulus, and storage modulus was lower in fracture subjects than in non-fracture women, respectively, by 27%, 35%, and 23% (all at least P < 0.007) (Table 2).
In trabecular bone of the normal BMD cohort, there were no significant differences between fracture and non-fracture women in absolute values for any endpoint (Table S2). Variance of hardness was 13% lower in fracture than in non-fracture women (P < 0.009) (Table 2).
Dynamic bone histomorphometric data (Table 3) and microarchitecture and degree of mineralization of bone (Tables 2 and S3)
In the normal BMD cohort, cortical thickness was 12% lower in fracture than in non-fracture women (Table 2) (P < 0.005). The fracture and non-fracture subsets of the normal BMD cohort did not differ for any other endpoints (Tables 3 and S3).
Osteopenic cohort
There were no significant differences between fracture and non-fracture women for any endpoints (Tables 1, 2, and 3; Tables S1–S3).
Discussion
We report data from four fracture/BMD subsets of post-menopausal women: non-fracture/normal BMD; fracture/normal BMD; non-fracture/osteopenic; and fracture/osteopenic [4]. The following low trauma fractures (number in parenthesis) were present in the fracture groups: vertebral (23), wrist (20), ankle (16), humerus (7), patella (4), shoulder (3), elbow (2), hip (2), and others (6) (fibula, foot, knee, lower leg, pelvis, and wrist/elbow). Some patients had multiple fractures. All vertebral fractures were considered “low trauma,” because they were identified on lateral spine films, rather than by medical history [19]. The fracture and non-fracture subsets of the two BMD cohorts were matched for age, total hip BMD, and body habitus (Table 1), all proven determinants of fragility fracture risk [9, 10]. Fracture/normal BMD women had thinner iliac cortices than non-fracture/normal BMD women. Fracture/normal BMD women also had lower variance of hardness, elastic modulus, and storage modulus in cortical mineralized bone tissue and lower variance of hardness in trabecular mineralized bone tissue of the ilium, than non-fracture/normal BMD women. Thus, the portion of our hypothesis stating that a bone architectural property, iliac cortical thickness, and, in this case, the heterogeneity of the bone material properties, hardness, elastic modulus, and storage modulus are associated with fragility fracture was supported for normal BMD women.
Iliac cortical thickness has been informative previously. In this normal BMD cohort, iliac cortical thickness was 12% lower in fracture than in non-fracture women. Iliac cortical thickness in normal BMD subjects declines 6% transmenopausally [31]. Thinner iliac cortices in osteoporotic subjects with fragility fracture, with the percentage difference from non-fracture subjects in cortical thickness generally being greater than the percentage difference from non-fracture subjects for total hip BMD, have been reported [30, 32,33,34]. The new bone architectural information here is that iliac cortical thickness is lower in age/BMD-matched post-menopausal women with normal BMD and fragility fracture than in similar women without fragility fracture.
To pursue this finding, it would be ideal to measure cortical thickness non-invasively at fragility fracture sites, just as BMD is measured non-invasively by DXA. Measuring cortical thickness on a transiliac biopsy specimen requires an invasive procedure that is unsuitable for large population-based studies and community practice. The general idea of measuring cortical thickness non-invasively at fragility fracture sites is promising because though cortical thickness of the contralateral hip by radiogrammetry was low in all women with hip fragility fractures, 21% of hip fracture women had non-osteoporotic total hip BMD, indicating that cortical thickness at the fracture site may be a quantitative marker of fragility fracture which is partially independent of BMD [35]. However, though computerized tomography (CT)–measured proximal femur cortical thickness also appears lower in hip fracture than in non-fracture women [36], obtaining adequate resolution to consistently do CT-based measurement of cortical thickness at the proximal femur requires an unacceptable radiation dose [37].
Non-invasively measured cortical thickness at surrogate sites, including the second metacarpal, distal radius, and distal tibia, has already produced data relevant to fragility fracture. Metacarpal cortical thickness by radiogrammetry predicts fragility fracture risk [38]. Similar to proximal femur radiogrammetry, second metacarpal cortical thickness by radiogrammetry correctly identified all fragility fracture women, but 25% of fracture women had non-osteoporotic BMD, again suggesting that cortical thickness is a quantitative indicator of fragility fracture which is partially independent of BMD [35, 38]. Thus, metacarpal and ilium data agree that surrogate site cortical thickness is related to fragility fracture. It is also crucial to note here that the metacarpal and proximal femur agree that cortical thickness at both a surrogate fracture site and a fragility fracture site is related to fragility fracture [35, 38].
High-resolution peripheral quantitative CT (HRpQCT; 82 µm voxel size) non-invasively evaluates cortical thickness of the distal radius and tibia. Since iliac and metacarpal data support the utility of measuring cortical thickness at surrogate sites, multiple HRpQCT studies report that cortical thickness at the distal radius and tibia in osteopenic and osteoporotic women is related to fracture in a manner which is partially independent of BMD [39, 40]. However, a similar number of HRpQCT studies report that the relationship of cortical thickness to fragility fracture risk is BMD-dependent [41]. Published data thus suggest that cortical thickness at the distal radius and tibia is a non-invasive measurement that should be tested as a prospective means to identify normal BMD women with elevated risk of fragility fracture [5, 6]. This could be efficiently investigated in population-based studies of normal BMD women in which baseline non-invasive measurement of cortical thickness and BMD was done and subsequent fragility fracture is being followed.
We found significantly lower variance in hardness, elastic modulus, and storage modulus of mineralized cortical bone tissue and lower variance in hardness of trabecular bone tissue in the fracture/normal BMD group than in the non-fracture/normal BMD group. These data resemble studies in which low heterogeneity in mineral-to-matrix ratio and carbonate-to-mineral ratio (C/P) in trabecular bone of the femoral neck occurred in hip fracture cases [42]. Evaluation of material heterogeneity is a well-recognized approach to understanding strength of materials [26]. Higher heterogeneity within a material offers resistance to microcrack propagation, defining a material with greater resistance to fracture [25, 26]. Therefore, lower heterogeneity of hardness, elastic modulus, and storage modulus in cortical bone tissue and of hardness of trabecular bone tissue of normal BMD women may contribute to increased fracture risk due to its relative inability to prevent microcrack propagation [26, 43,44,45]. Interindividual variation in femoral lamellar properties has been observed, leading to the idea that tissue heterogeneity may influence bone tissue strength via strain concentration, damage accumulation, and crack propagation [43]. Micro- and nanoscale measurements confirm that greater heterogeneity of tissue is directly related to increased strength [44, 45]. The abnormally homogeneous mineralized cortical bone tissue and to some extent trabecular bone tissue in normal BMD women with fragility fracture may partially explain their increased risk of fracture.
Unlike normal BMD patients, osteopenic patients with fragility fracture had no macro-architectural or material property abnormalities in either cortical or trabecular mineralized bone tissue. Osteopenic fragility fracture patients may be characterized by other non-quantitative traits from FRAX and similar models that predict fracture risk [9].
These findings in normal BMD women are important because the majority of fragility fractures occur in women with non-osteoporotic BMD [5, 6]. The care of such individuals currently presents a conundrum. While one might embrace the general idea that increasing BMD reduces fracture risk, and hope that treating them with an osteoporosis medication that increases BMD would reduce fracture risk, there is no evidence that this would be true in normal BMD women, because most osteoporosis medications were formally tested in women with total hip T-score < − 2.0 [2]. New trials are needed in which normal BMD women with prevalent fragility fracture, or potentially only low cortical thickness, are given current osteoporosis medications and monitored for incident fracture to establish whether these women, despite their already normal BMD, experience increased BMD and reduced fracture risk.
The general goal of this type of work is to interrogate bone material property endpoints that correlate to strength of mineralized bone tissue from non-osteoporotic humans with fragility fracture, rather than endpoints that may indirectly reflect bone strength and fracture risk. It is appropriate to consider additional potentially fracture-related data that might be obtained from these specimens. For example, we report here that the degree of mineralization of cortical and trabecular bone was not related to fracture in either the normal BMD or the osteopenic cohorts [20]. Nanoindentation is a relatively non-destructive technique, in the sense that each indent reaches a depth of only 0.5 µm, perhaps damaging tissue ~ 50 µm deeper in the sample. One can easily polish 100 µm from the face of each specimen to create a new face with undamaged tissue very near previously tested tissue. A reasonable next goal would be to test the material properties of mineralized bone tissue in these specimens by atomic force microscopy (AFM), another commonly used testing method [25] that like nanoindentation determines viscoelastic properties of hard tissues. Since nanoindentation cannot be used to measure compressive strength, a second goal would be to use micropillar compression to determine compressive strength [18, 44, 46]. In view of the importance of hydration state to nanoindentation testing [47, 48], a third goal would be to do additional nanoindentation tests on these specimens after rehydration. A fourth goal would be to measure endpoints from bone composition tests on these specimens that correlate to hardness, elastic modulus, and storage modulus. Measuring bone composition by FTIR [21, 49] or Raman spectroscopy [50] could also be useful. The ultimate goal is to find a non-invasive test that identifies strength deficits in mineralized bone tissue, much as DXA non-invasively identifies the insufficient quantity of bone in half of fragility fracture women. Quantitative 3D magnetic resonance elastography, which measures soft tissue stiffness in vivo by using shear waves from a mechanical vibrating device, may be such a technique [51].
Though dynamic histomorphometric endpoints (Table 2) did not help define fracture patients, they further define the normal range in non-osteoporotic fracturing and non-fracturing post-menopausal women. These data are similar to published data from healthy post-menopausal women [31, 52].
Adding CRFs that tend not to reflect BMD to total hip T-score significantly improves the prediction of a patient’s fragility fracture risk [9]. As cortical thickness is related to fragility fracture but at most only partially related to BMD, it may represent a quantitative expression of fracture risk embodied in one or more CRFs. Since cortical thickness has potential to be a relevant non-invasive quantitative endpoint in large populations, should its data permit, consideration could be giving to adding it to these algorithms.
Cortical thickness and heterogeneity of bone material property endpoints appear to be endpoints that contribute to fragility fracture risk in women whose fracture risk is BMD independent. This may also be the case for two disease groups, glucocorticoid-treated [13] and diabetic patients [14], both of which have higher fracture risk than predicted by their T-score. Cortical thickness and mineralized bone tissue abnormalities as described here could explain a portion of the excess fracture risk in glucocorticoid and diabetic patients.
Some specific weaknesses and strengths of this work should be noted. These specimens came from healthy post-menopausal white females. The data may not apply to bone of males, pre-menopausal women, or persons of other ethnicities or races. In fracture subjects, the biopsy specimens were obtained 0.9–5 years after fragility fracture. Therefore, they may not represent the status of the skeleton at the time of fracture, in particular whether low cortical thickness and/or heterogeneity of bone material properties was present before fragility fracture. Thirteen percent of patients contacted for enrollment declined to join because they did not wish to have a transiliac biopsy procedure. It is not known whether biopsy-refusing subjects differed from those who enrolled, because no measurements of them were taken. Though the ilium is neither a direct load bearing bone nor a site of fragility fracture, its data showing a relationship to fragility fracture are supported by cortical thickness data from other surrogate sites including the metacarpal, the distal radius, and the distal tibia. Studies of bone material properties in fragility fracture sites such as the proximal femur and vertebral body could reveal whether similar abnormalities are present in sites of fragility fracture. The nanoindentation tests made here were done on bone tissue in a dehydrated state. Tests on rehydrated specimens would add additional clarity [47, 48]. Our data indicate that when investigating endpoints that characterize fragility fracture risk in non-osteoporotic women [5, 6], separating subjects into long-recognized normal and osteopenic BMD tiers can be revealing [4].
Conclusions
We studied age/BMD-matched normal BMD and osteopenic BMD women with and without fragility fracture. We found low cortical thickness and low heterogeneity of hardness, elastic modulus, and storage modulus in cortical bone tissue, and low heterogeneity of hardness in trabecular bone tissue of normal BMD women with fragility fracture. These data suggest additional studies that can refine these findings, potentially leading to additional non-invasive, non-BMD measurements that predict fragility fracture in normal BMD women.
References
Burge R, Dawson-Hughes B et al (2007) Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 22(3):465–475. https://doi.org/10.1359/jbmr.061113
Black DM, Delmas PD, HORIZONPivotalFractureTrial et al (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. New Engl J Med. 356:1809–1822. https://doi.org/10.1056/NEJMoa067312
Hernlund E, Svedbom A, Ivergard M et al (2013) Osteoporosis in the European Union: medical management, epidemiology and economic burden. Arch Osteoporos 8(1–2):136. https://doi.org/10.1007/s11657-013-0136-1
Kanis JA, Melton LJ, Christiansen C et al (1994) The diagnosis of osteoporosis. J Bone Min Res 9:1137–1141. https://doi.org/10.1002/jbmr.5650090802
Siris ES, Chen YT, Abbott TA et al (2004) BMD thresholds for pharmacological intervention to prevent fractures. Arch Intern Med 164(10):1108–1112. https://doi.org/10.1001/archinte.164.10.1108
Pasco JA, Seeman E, Henry MJ et al (2006) The population burden of fractures originates in women with osteopenia, not osteoporosis. Osteoporos Int 17(9):1404–1409. https://doi.org/10.1007/s00198-006-0135-9
Nordin BEC (1961) The pathogenesis of osteoporosis. Lancet 1(7185):1011–1015. https://doi.org/10.1016/s0140-6736(61)91827-x
Ross PD, Genant HK, Davis JW et al (1993) Predicting vertebral fracture incidence from prevalent fractures and BMD among non-black, osteoporotic women. Osteo Int 3:120–126. https://doi.org/10.1007/BF01623272
Kanis JA, McCloskey EV, Johansson H et al (2010) Development and use of FRAX in osteoporosis. Osteoporos Int 21(Suppl 2):S407–S413. https://doi.org/10.1007/s00198-010-1253-y
Melton LJ 3rd (1996) Epidemiology of hip fractures: implications of the exponential increase with age. Bone 18(3 Suppl):121S-125S. https://doi.org/10.1016/8756-3282(95)00492-0
Riggs BL, Wahner HW, Dunn WL et al (1981) Differential changes in BMD of the appendicular and axial skeleton with aging: relationship to spinal osteoporosis. J Clin Invest 67(2):328–335. https://doi.org/10.1172/JCI110039
Delmas PD, Seeman E (2004) Changes in BMD explain little of the reduction in vertebral or nonvertebral fracture risk with anti-resorptive therapy. Bone 34:599–604. https://doi.org/10.1016/j.bone.2003.12.022
Van Staa TP, Laan RF, Barton IP et al (2003) BMD threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum 48(11):3224–3229. https://doi.org/10.1002/art.11283
Sellmeyer DE, Civitelli R, Hofbauer LC et al (2016) Skeletal metabolism, fracture risk, and fracture outcomes in Type 1 and Type 2 diabetes. Diabetes 65(7):1757–1766. https://doi.org/10.2337/db16-0063
Frost HM (2002) Emerging views about osteoporosis, bone health, strength, fragility, and their determinants. J Bone Miner Metab 20(6):319–325. https://doi.org/10.1007/s007740200046
Seeman E (2008) Bone quality: the material and structural basis of bone strength. J Bone Miner Metab 26(1):1–8. https://doi.org/10.1007/s00774-007-0793-5
Polly BJ, Yuya PA, Akhter MP et al (2012) Intrinsic material properties of trabecular bone by nanoindentation testing of biopsies taken from healthy women before and after menopause. Calcif Tissue Int 90(4):286–293. https://doi.org/10.1007/s00223-012-9575-8
Uchic MD, Dimiduk DM, Florando JN, Nix WD (2004) Sample dimensions influence strength and crystal plasticity. Science 305(5686):986–989. https://doi.org/10.1126/science.1098993
Vennin S, Desyatova A, Turner JA et al (2017) Intrinsic material property differences in bone tissue from patients suffering low-trauma osteoporotic fractures, compared to matched non-fracturing women. Bone 97:233–242. https://doi.org/10.1016/j.bone.2017.01.031
Rizzo S, Farlay D, Akhter M et al (2018) Variables reflecting the mineralization of bone tissue from fracturing versus non-fracturing post-menopausal nonosteoporotic women. JBMR Plus 2(6):323–327. https://doi.org/10.1002/jbm4.10062
Boskey AL, Donnelly E, Boskey E et al (2015) Examining the relationships between bone tissue composition, compositional heterogeneity and fragility fracture: a matched case controlled FTIRI study. J Bone Miner Res 31(5):1070–1081. https://doi.org/10.1002/jbmr.2759
Oliver WC, Pharr GM (1992) An improved technique for determining hardness and elastic modulus using load displacement sensing indentation experiments. J Mater Res 7(6):1564–1583
Carter DR, Hayes WC (1977) The compressive behavior of bone as a two-phase porous structure. J Bone Joint Surg Am 59(7):954–962
Currey JD (1988) Strain rate and mineral content in fracture models of bone. J Orthop Res 6(1):32–38. https://doi.org/10.1002/jor.1100060105
Cohen SR, Kalfon-Cohen E (2013) Dynamic nanoindentation by instrumented nanoindentation and force microscopy: a comparative review. Beilstein J Nanotechnol 4:815–833. https://doi.org/10.3762/bjnano.4.93
Tai K, Dao M, Suresh S et al (2007) Nanoscale heterogeneity promotes energy dissipation in bone. Nat Mater 6(6):454–462. https://doi.org/10.1038/nmat1911
Akhter MP, Lappe JM, Davies KM, Recker RR (2007) Transmenopausal changes in the trabecular bone structure. Bone 41:111–116. https://doi.org/10.1016/j.bone.2007.03.019
Parfitt AM, Drezner MK, Glorieux FH et al (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR histomorphometry nomenclature committee. J Bone Miner Res 2(6):595–610. https://doi.org/10.1002/jbmr.5650020617
Dempster DW, Compston JE, Drezner MK et al (2013) Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 28(1):1–16. https://doi.org/10.1002/jbmr.1805
Kimmel DB, Recker RR, Gallagher JC et al (1990) A comparison of iliac bone histomorphometric data in post-menopausal osteoporotic and normal subjects. Bone Miner 11(2):217–235. https://doi.org/10.1016/0169-6009(90)90061-j
Recker RR, Lappe JM, Davies M, Kimmel D (2018) Perimenopausal bone histomorphometry before and after menopause. Bone 108:55–61. https://doi.org/10.1016/j.bone.2017.12.016
Oleksik A, Ott SM, Vedi S et al (2000) Bone structure in patients with low BMD with or without vertebral fractures. J Bone Miner Res 15(7):1368–1375. https://doi.org/10.1359/jbmr.2000.15.7.1368
Foldes J, Parfitt AM, Shih MS et al (1991) Structural and geometric changes in iliac bone: relationship to normal aging and osteoporosis. J Bone Miner Res 6(7):759–766. https://doi.org/10.1002/jbmr.5650060714
Uitewaal PJM, Lips P, Netelenbos JC (1987) Analysis of bone structure in patients with hip fracture. Bone Mineral 3:63–73
Tarantino U, Rao C, Tempesta V, Gasbarra E, Feola M (2016) Hip fractures in the elderly: The role of cortical bone. Injury 47(Suppl 4):S107–S111. https://doi.org/10.1016/j.injury.2016.07.058
Bousson VD, Adams J, Engelke K et al (2011) In vivo discrimination of hip fracture with quantitative computed tomography: Results from the prospective European Femur Fracture Study (EFFECT). J Bone Miner Res 26(4):881–893. https://doi.org/10.1002/jbmr.270
Yang L, Udall WJ, McCloskey EV, Eastell R (2014) Distribution of bone density and cortical thickness in the proximal femur and their association with hip fracture in postmenopausal women: a quantitative computed tomography study. Osteoporos Int 25(1):251–263. https://doi.org/10.1007/s00198-013-2401-y
Meema HE, Meindok H (1992) Advantages of peripheral radiogrammetry over dual-photon absorptiometry of the spine in the assessment of prevalence of osteoporotic vertebral fractures in women. J Bone Miner Res 7(8):897–903. https://doi.org/10.1002/jbmr.5650070806
Boutroy S, Bouxsein ML, Munoz F, Delmas PD (2005) In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab 90(12):6508–6515. https://doi.org/10.1210/jc.2005-1258
Kral R, Osima M, Borgen TT et al (2017) Increased cortical porosity and reduced cortical thickness of the proximal femur are associated with nonvertebral fracture independent of FRAX and Garvan estimates in postmenopausal women. PLoS ONE 12(9):e0185363. https://doi.org/10.1371/journal.pone.0185363
Sornay-Rendu E, Boutroy S, Duboeuf F, Chapurlat RD (2017) Bone microarchitecture assessed by HR- pQCT as predictor of fracture risk in postmenopausal women: The OFELY Study. J Bone Miner Res 32(6):1243–1251. https://doi.org/10.1002/jbmr.3105
Gourion-Arsiquaud S, Lukashova L, Power J et al (2013) Fourier transform infrared imaging of femoral neck bone: reduced heterogeneity of mineral-to-matrix and carbonate-to-phosphate and more variable crystallinity in treatment-naive fracture cases compared with fracture-free controls. J Bone Miner Res 28(1):150–161. https://doi.org/10.1002/jbmr.1724
Zysset PK, Guo XE, Hoffler CE, Moore KE, Goldstein SA (1999) Elastic modulus and hardness of cortical and trabecular bone lamellae measured by nanoindentation in the human femur. J Biomech 32(10):1005–1012. https://doi.org/10.1016/s0021-9290(99)00111-6
Groetsch A, Gourrier A, Schwiedrzik J et al (2019) Compressive behaviour of uniaxially aligned individual mineralised collagen fibres at the micro- and nanoscale. Acta Biomater 89:313–329. https://doi.org/10.1016/j.actbio.2019.02.053
Tertuliano OA, Greer JR (2016) The nanocomposite nature of bone drives its strength and damage resistance. Nat Mater 15(11):1195–1202. https://doi.org/10.1038/nmat4719
Schwiedrzik J, Raghavan R, Bürki A et al (2014) In situ micropillar compression reveals superior strength and ductility but an absence of damage in lamellar bone. Nat Mater 13(7):740–747. https://doi.org/10.1038/nmat3959
Granke M, Does MD, Nyman JS (2015) The role of water compartments in the material properties of cortical bone. Calcif Tissue Int 97(3):292–307. https://doi.org/10.1007/s00223-015-9977-5
Wolfram U, Wilke HJ, Zysset PK (2010) Rehydration of vertebral trabecular bone: influences on its anisotropy, its stiffness and the indentation work with a view to age, gender and vertebral level. Bone 46(2):348–354. https://doi.org/10.1016/j.bone.2009.09.035
Rokidi S, Bravenboer N, Gamsjaeger S et al (2020) Impact microindentation measurements correlate with cortical bone material properties measured by Fourier transform infrared imaging in humans. Bone 137:115437. https://doi.org/10.1016/j.bone.2020.115437
Mandair GS, Morris MD (2015) Contributions of Raman spectroscopy to the understanding of bone strength. BoneKey 4:1–8. https://doi.org/10.1038/bonekey.2014.115
Arunachalam SP, Rossman PJ, Arani A et al (2017) Quantitative 3D magnetic resonance elastography: Comparison with dynamic mechanical analysis. Magn Reson Med. 77(3):1184–1192. https://doi.org/10.1002/rnrm.26207
Vedi S, Compston JE, Webb A, Tighe JR (1982) Histomorphometric analysis of bone biopsies from the iliac crest of normal British subjects. Metab Bone Dis Relat Res. 4(4):231–6. https://doi.org/10.1016/0221-8747(82)90032-7
Acknowledgements
We acknowledge Susan Bare who collected the histomorphometry data.
Funding
This work was funded by National Institutes of Health Grant # R01 AR054496-01A1 to RRR.
Author information
Authors and Affiliations
Contributions
The following individuals contributed respectively to study design/planning (RRR, MPA, JML, JAT); experimental conduct (RRR, JML); data collection (RRR, JML, SV, AD, MPA); data analysis (DBK, RRR, AD, JAT); data interpretation (all); original draft (DBK, RRR); and revised draft (all).
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kimmel, D.B., Vennin, S., Desyatova, A. et al. Bone architecture, bone material properties, and bone turnover in non-osteoporotic post-menopausal women with fragility fracture. Osteoporos Int 33, 1125–1136 (2022). https://doi.org/10.1007/s00198-022-06308-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-022-06308-y