Abstract
Summary
Atypical femoral fractures (AFFs) are categorized as low-energy fractures of the femoral shaft or subtrochanteric region. The use of computed tomography-based finite element analysis demonstrated that the femoral weakest point against tensile stress coincided with AFF location, which was determined by the lower limb axis and femoral bowing.
Introduction
This study aimed to assess the relationship between the femoral weakest point against tensile stress and the lower limb axis and geometry, including femoral bowing, using a computed tomography (CT)-based finite element analysis (FEA) model.
Methods
We retrospectively reviewed 19 patients with AFFs and analyzed their CT images of the contralateral intact femur. We performed FEA to find the maximum principal stress (MPS) and maximal tensile stress loading area (femoral weakest point, FWP) of each patient and matched the FWP with the real location of AFF. We applied mechanical axes differently, as neutral, varus, and valgus, in the FEA model, when we analyzed the change in MPS and FWP based on lower limb alignment. We compared the degree of agreement between the real fracture location and FWP before and after knee mechanical axis adjustment.
Results
The average participant age was 75.9 (range, 61–87) years, and all participants were women. In the 19 patients included, we observed 20 and 7 shaft and subtrochanteric AFFs, respectively. The average mechanical axis at the knee joint level was 22.6 mm (range, 0–70 mm) of the varus. All the patients showed an increasing trend of MPS and a distal movement of FWP when the mechanical axis of the knee was applied from the valgus to varus alignment. The root mean square errors between the FWP and real fracture location were 14.58% and 10.87% before and after adjustment, respectively, implying that the degree of agreement was better in patients who underwent mechanical adjustment.
Conclusion
The use of CT/FEA demonstrated that the FWP against tensile stress coincided with AFF location, which was determined by the lower limb axis and femoral bowing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Based on the recent report of the American Society for Bone and Mineral Research (ASBMR), an atypical femoral fracture (AFF) is defined as a complete or incomplete fracture which shows that the transverse fracture line originates at the lateral cortex of the femur, progresses medially across the femur as oblique, and is associated with minimal or no trauma, as in a fall from standing height or less [1]. Since 2005, when Odvina et al. first described a possible relationship between prolonged bisphosphonate (BP) use and AFF occurrence [2], a severe suppression of bone turnover following anti-resorptive therapy such as long-term use of BP or denosumab has been considered the main cause of AFF occurrence. Although the exact pathophysiology underlying AFF development remains unclear and is likely multifactorial, the potential role of lower limb geometry has been emerging recently. The role of lower limb geometry is being emphasized as a risk factor for AFF beyond long-term use of BP, because abnormal lower limb geometry may contribute to elevated stress within the lateral cortex of the femoral shaft and cause AFF by increasing mechanical fatigue [3].
The majority of AFF cases occur in the lateral aspect of the subtrochanteric or femur diaphysis due to the accumulation of tensile stress [4]. Recently, several studies have revealed that increased femoral bowing is associated with increased AFF risk and a more distal fracture location [5,6,7,8,9]. Several recent finite element simulation studies suggest that AFF risk is associated with the stress/strain environment of the femur [10,11,12]. The AFF site corresponds to the location of peak strain, and the bowing angle is related to the magnitude of peak strain. However, none of these simulation studies fully applied lower limb alignment or patient-specific gait.
We hypothesized that the lower limb axis would represent the patient-specific biomechanical factor of AFF occurrence. Thus, we developed a computed tomography (CT)–based finite element analysis (FEA) (CT/FEA) model to assess the femoral location that is the weakest point against the tensile stress related to lower limb geometry including the lower-extremity axis and femoral bowing.
Methods
Patient selection
This study was approved by the Institutional Review Board of the participating center (IRB No. 2019–1568). We retrospectively reviewed data of 19 patients with AFFs, as defined by the ASBMR in 2014 [1], including CT images of the intact femur and whole lower limb X-rays taken in the standing position. We excluded patients who had pathologic fractures with metastasis or metabolic bone disease such as Paget’s disease. Data were obtained from single centers between January 2015 and July 2020.
Clinical and radiologic data
We collected demographic data, including sex, age, and T-score of bone mineral density (BMD). The fracture location was classified as subtrochanteric or femoral shaft fracture. A subtrochanteric fracture was defined as a fracture extending up to 5 cm below the lesser trochanter, while a shaft fracture was defined as a fracture extending from below the subtrochanteric region to the supracondylar metaphyseal flare. Moreover, we collected patient clinical data, including body mass index and BP history.
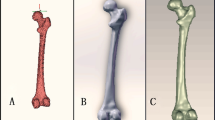
Femoral bowing was measured using a grading system that was previously reported by Park et al. which classifies the degree of bowing into four grades according to the position of the reference line with respect to the most curved portion of the medial cortex [13]. The reference line was drawn from the tip of the greater trochanter to the center of the intercondylar notch. When the reference line passed into the medial one-third of the medullary canal or more medially, the patient was classified into the bowing group, and the femur was graded as grade 0–3 femoral bowing according to its severity of bowing (Fig. 1).
Femoral bowing grades as classified by Park et al. [12]. (A) Grade 0: an almost straight femur with the reference line in the middle 1/3 of the medullary canal. (B) Grade 1: mild femoral bowing with the reference line in the medial 1/3 of the medullary canal. (C) Grade 2: moderate femoral bowing with the reference line of the medullary canal and in the medial cortex. (D) Grade 3: severe femoral bowing with the reference line of the medial cortex
Lower limb alignment was measured using the mechanical axis of the knee, which could assess the varus‐valgus alignment and capture anatomic variations in the proximal femur, femoral shaft, tibial shaft, and ankle (Fig. 2A). The mechanical axis was drawn from the femoral head center to the talar dome center. The knee joint center was defined as the center of the intercondylar notch. The degree of knee varus or valgus alignment was measured as the distance from the knee joint center to the mechanical axis line in the plane of the femoral condyle base. The distance was “0” mm, “ − ”, and “ + ” in the neutral, varus, and valgus positions, respectively.
Bone strength using CT/FEA
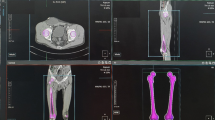
CT of the full length of both femurs was performed for each patient with a 2-mm slice thickness. CT Digital Imaging and Communication in Medicine (DICOM) data were obtained from the intact (contralateral) femur. A three-dimensional (3D) modeling software (AVIEW Modeler; Coreline Soft, Seoul, Republic of Korea) was used to produce 3D samplings of anatomical elements of the human femur with each DICOM dataset. With the use of reconstruction and parametrization on these datasets, structured data were segmented to form a 3D model representing both the bone surface and the cortico-cancellous interface. The established finite element models were mechanically reconstructed using Rapidform 2006 (INUS Technology, Inc., Seoul, Korea). The FEM used for validation was developed using a modified 10-node tetrahedron volume element (C3D10M). In all models, the maximum stress occurring in the AFF location was used to evaluate mesh quality. An appropriate mesh length was identified when the stress change due to the change in element size was less than 5% [14]. The maximum edge length was then adjusted to be less than 1 mm using 3-Matic 7.01 (Materialise, Leuven, Belgium). The mesh quality was checked whether the element had distorted angles.
As proposed by Heiner et al. [15], the elastic modulus, yield strength, and Poisson’s ratio were set as 17,000 MPa, 114 MPa, and 0.36 in cortical bone and 300 MPa, 15 MPa, and 0.3 in cancellous bone. In this study, standing configurations were designed for AFF evaluation. Load and boundary conditions were set as follows to analyze AFF location. For the load condition, the center of the femoral head was set as the point of application of the force vector. A concentrated force was applied at this point, and the force vector was directed to the heading point moved at the 10 mm posterior from the anterior border of the distal femur [16]. The loading stress was applied as the weight of each patient. A boundary condition was established to completely constrain 6 degrees of freedom at the lower end of the femur [17].
Finite element models were assessed using Abaqus v6.13–1 (Dassault Systemes Simulia Corp., Providence, USA). We performed FEA to find the maximum principal stress (MPS) and the maximum tensile stress loading area (femoral weakest point, FWP) of intact, contralateral femurs in all 19 patients. We compared the abovementioned parameters with those of the true AFF location, with an adjustment of the whole lower limb axis for each patient. In addition, we modified the mechanical axis as neutral, varus (− 5 mm, − 10 mm, − 15 mm, − 20 mm, − 25 mm, and − 30 mm), and valgus (5 mm, 10 mm, 15 mm, 20 mm, 25 mm, and 30 mm) (Supplement 1), and analyzed the change of the MPS and FWP according to lower limb alignment. The location of the FWP was represented as the ratio of length from the fracture point to the base of the femoral condyle divided by the femoral length (from the tip of the femoral head to the base of the femoral condyle). The real fracture site was represented as a ratio using the abovementioned method (Fig. 3).
Statistical analysis
We investigated the MPS based on the femoral bowing grade. We drew line graphs of MPS and FWP for each patient based on the change of the mechanical alignment of the knee to investigate the relationship between MPS, FWP, and the mechanical alignment of the knee. The real AFF site was compared with the FWP using CT/FEA with and without adjustment by the knee mechanical axis adjustment; the degree of agreement between the two sites was evaluated. The root mean square error (RMSE) [18] was calculated to compare the degree of agreement between the real fracture site and the FWP location, before and after adjustment by the mechanical axis of the knee. Moreover, scatter plots were drawn to compare between groups. We also investigated the correlation between the duration of BP use and other variables including MPS before/after axis adjustment, FWP before/after axis adjustment, and real AFF site using Spearman’s correlation analysis. P values less than 0.05 were considered statistically significant. All of the statistical analyses were performed using SPSS version 26.0.
Results
All patients were women with an average age of 75.9 years (range, 61–87 years). Among 19 patients, 16 had osteoporosis (T-score ≤ − 2.5) and 3 had osteopenia (mean T-score, − 3.2 [range, − 2.0 to − 4.7]). Eight patients (42.1%) had bilateral AFFs, seven of whom had AFFs in the same location of AFF and one patient had right shaft AFF and left subtrochanteric AFF (Fig. 2B). In the 19 patients, we observed 20 and 7 shaft and subtrochanteric AFFs, respectively. Eighteen patients (94.7%) had a history of BP use for an average of 76.5 months (range, 20–175 months). All of the included patients used BP as the only anti-osteoporotic agent.
Concerning the grade of femoral bowing, we found 6, 3, 4, and 6 cases of grades 0, 1, 2, and 3, respectively. Supplement 2 shows the distribution of MPS according to femoral bowing grade. MPS tended to increase with the increase in the grade.
The average mechanical axis at the knee joint level was 22.6 mm (range, 0–70 mm) of varus. All 19 patients showed an increasing trend of MPS as the mechanical axis of the knee moved from the valgus to the varus alignment. The trend line of MPS showed a positive linear relationship with the change in knee mechanical alignment (Fig. 4A), which implies that a varus change in the knee joint weakens the femur against tensile stress. Contrarily, cases of FWP showed a negative linear relationship at the trend line, as all 19 patients showed decreasing FWP trend (Fig. 4B), implying that a varus change in the knee joint moves the FWP distally.
Comparing the degree of agreement of real fracture location and FWP before and after adjustment by the knee mechanical axis, the RMSEs resulting from the comparison were 14.58% and 10.87% before and after adjustment, respectively. This signified that the degree of agreement was better in patients who underwent mechanical axis adjustment. The details are shown with scatter plots in Fig. 5.
The duration of BP use showed no significant correlation with FWP, MPS, or fracture site. The correlation coefficient and significance level are shown in Table 1.
Discussion
The present CT/FEA study revealed that the FWP against the tensile stress was associated with the lower limb axis and femoral bowing grade. First, the FWP was located at the subtrochanteric and shaft areas in the straight (bowing grade 0) and bowed (bowing grade 1–3) femurs, respectively. These results were comparable to those of previous clinical and FEA studies. Numerous clinical studies have identified an association between femoral curvature and fracture location, and found that increased femoral bowing was associated with a more distal fracture location [7, 19,20,21]. Hitherto, the largest study on the abovementioned association has been conducted by Kim et al. [19], who in a cross-sectional study of 147 patients with AFF reported greater anterior and lateral bowing angles in patients with diaphyseal AFF compared to those in patients with subtrochanteric AFF. Several FEA studies also suggested that increased bowing was associated with a greater magnitude of tensile stress and a peak strain location closer to the midshaft than the proximal femur [10,11,12]. Oh et al. presented that both lateral and anterior bowing were significantly greater in the mid-shaft AFF group by Mann–Whitney U test analysis of 22 patients in a multicenter study in Japan between 2015 and 2017 [11]. They also showed a significantly higher lateral bowing angle and anterior radius of curvature of the femur in shaft AFF cases with a higher maximum MPS in a prospective study of 18 patients conducted between 2012 and 2014 [12]. Haider et al. showed a comparable result using 2-level full factorial analysis of an FEA of 10 patients in 2018 [10]. Herein, we also found that the FWP location moved from the proximal femur to the midshaft based on the femoral bowing grade.
In addition, we had an important finding; the lower limb geometry changed the peak strain location and magnitude. The MPS was increased from the valgus, via neutral, to varus alignment, and the FWP location moved to the distal femur based on varus alignment. These findings suggested that patients with lower limb varus alignment (for instance, a varus change of the knee resulting from osteoarthritis) would be prone to developing AFF, as a kind of stress (insufficiency) fracture. Previous study findings on the relationship between lower limb axis and AFF location were limited and debatable. Morin et al. [7] failed to reveal a significant relationship between lower extremity alignment and AFF location, after comparing a small number of patients (5 and 11 patients with subtrochanteric and diaphyseal AFFs, respectively). Contrarily, Saita et al. [22] reported that the femoral-tibial angle explained 67% of the variance in AFF location, with greater angles associated with distal AFF locations; however, they observed no relationship between a variable (femoral-tibial angle) and patients with non-AFF. The previous study limitations included a small sample size and the non-consideration of femoral bowing.
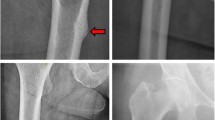
Our results demonstrated that the potential AFF location should be evaluated based on a combination of femoral bowing and lower limb geometry. Moreover, we found that the real fracture location was better correlated with FWP location adjusted with lower limb mechanical axis than that with femur geometry. In the current study, before lower limb axis adjustment, not all patients with neutral axes showed the same location of the FWP in the ipsilateral femur and the actual location of AFF in the injured femur. However, after varus axis adjustment for each patient using whole lower limb X-ray, the FWP was well correlated with the fracture site in all included patients (Fig. 6). Figure 2 presents a representative case showing the important role of the mechanical axis of the knee joint; the FWP moved from the proximal femur to the midshaft could be different from the lower limb mechanical axis.
The change of maximal stress location according to the varus deformity of the knee. (A) The femoral weakest point (FWP) in a neutral position. (B) The mechanical axis of the knee. (C) The location of the incomplete atypical femoral fracture. (D) The FWP adjusted with the varus and a 37-mm medial translation
Since 2005, when Odvina et al. [2] described AFF, the severe suppression of bone turnover after a long-term use of BPs has been considered the main cause of AFF. Although AFFs are considered an adverse effect of long‐term BP use, the exact pathophysiology underlying AFF development remains unclear, and AFFs are reported in BP‐naïve patients as well. The second ASBMR report emphasized the role of lower limb geometry as an AFF risk factor [1]. Considering our findings that the AFF location of each patient was consistent with the FWP after adjustment following the whole lower limb axis, we can conclude that an AFF is a kind of insufficiency fracture that is initiated by a force greater than the MPS of the femur. A recent study by Jung et al. [23] revealed that femurs of Asian (Korean) elderly women were more bowed than those of young women or elderly men. In our opinion, in connection with the abovementioned findings that a more bowed femur has a higher MPS (weaker against tensile stress), the femoral geometric characteristic of the Asian population could be a possible reason why more AFFs (especially diaphyseal AFF) occurred in Asians, even in BP‐naïve patients.
The current study has several limitations. First, this study did not investigate bone turnover status. However, our study findings are meaningful given that the FWP was well correlated with the fracture site in all included patients with AFF. Thus, both the geometric factor and bone turnover status are important in AFF development. Second, this study simulated various lower limb axes; however, no patient-specific factors, such as activity volume, subject-specific gait, or postural loading scenarios, were evaluated. In the future, FEA models that apply more patient-specific information will be needed.
Compared to incomplete AFFs, the treatment of complete AFFs is more difficult and has poorer outcomes [24, 25]. Our findings can be applied clinically in high-risk patients to assess potential AFF locations for early fracture detection using the complete lower limb axis. Imaging modalities, such as dual-energy X-ray absorptiometry with lower limb geometry, could be used in the early prediction of AFF occurrence; this could achieve a more successful AFF treatment in clinical practice. Furthermore, we suggest that an extended femur scan should be performed on the entire femur [26], including the femoral condyle, to evaluate the presence of anterolateral bowing, and estimate the apex of the bowed femur using a reference line.
Conclusion
This CT/FEA study demonstrated that the weakest location of the femur against tensile stress was associated with the lower limb axis and femoral bowing. Lower limb varus alignment or femoral bowing moved the weakest point distally, and the MPS increased related to varus alignment. The weakest point, adjusted for the mechanical axis of the lower limb using FEA, was well correlated with the real AFF location based on clinical data.
Data availability
Data cannot be shared publicly because it contains potentially identifying information of each patient. Data are available from the Asan Medical Center Institutional Data Access/Ethics Committee (contact via Asan Medical Center Institutional Review Board, Convergence Innovation Bldg. 88, Olympic-ro 43-gil, Songpa-gu, Seoul, Republic of Korea. Website link, http://eirb.amc.seoul.kr/; E-mail, irb@amc.seoul.kr; Phone, + 82–2-3010–7165) for researchers who meet the criteria for access to confidential data.
Code availability
Not applicable.
References
Shane E, Burr D, Abrahamsen B et al (2014) Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 29:1–23
Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY (2005) Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab 90:1294–1301
Haider IT, Schneider PS, Edwards WB (2019) The role of lower-limb geometry in the pathophysiology of atypical femoral fracture. Curr Osteoporos Rep 17:281–290
Kim K-k, Park Y-w, Kim T-h, Seo K-d (2020) Atypical femoral neck fracture after prolonged bisphosphonate therapy. J Pathol Translat Med 54:346–350
Buitendijk SKC, van de Laarschot DM, Smits AAA, Koromani F, Rivadeneira F, Beck TJ, Zillikens MC (2019) Trabecular bone score and hip structural analysis in patients with atypical femur fractures. J Clin Densitom 22:257–265
Lim SJ, Yeo I, Yoon PW, Yoo JJ, Rhyu KH, Han SB, Lee WS, Song JH, Min BW, Park YS (2018) Incidence, risk factors, and fracture healing of atypical femoral fractures: a multicenter case-control study. Osteoporos Int 29:2427–2435
Morin SN, Wall M, Belzile EL, Godbout B, Moser TP, Michou L, Ste-Marie LG, de Guise JA, Rahme E, Brown JP (2016) Assessment of femur geometrical parameters using EOS imaging technology in patients with atypical femur fractures; preliminary results. Bone 83:184–189
Oh Y, Wakabayashi Y, Kurosa Y, Ishizuki M, Okawa A (2014) Stress fracture of the bowed femoral shaft is another cause of atypical femoral fracture in elderly Japanese: a case series. J Orthop Sci 19:579–586
Sasaki S, Miyakoshi N, Hongo M, Kasukawa Y, Shimada Y (2012) Low-energy diaphyseal femoral fractures associated with bisphosphonate use and severe curved femur: a case series. J Bone Miner Metab 30:561–567
Haider IT, Schneider P, Michalski A, Edwards WB (2018) Influence of geometry on proximal femoral shaft strains: implications for atypical femoral fracture. Bone 110:295–303
Oh Y, Fujita K, Wakabayashi Y, Kurosa Y, Okawa A (2017) Location of atypical femoral fracture can be determined by tensile stress distribution influenced by femoral bowing and neck-shaft angle: a CT-based nonlinear finite element analysis model for the assessment of femoral shaft loading stress. Injury 48:2736–2743
Oh Y, Wakabayashi Y, Kurosa Y, Fujita K, Okawa A (2014) Potential pathogenic mechanism for stress fractures of the bowed femoral shaft in the elderly: mechanical analysis by the CT-based finite element method. Injury 45:1764–1771
Park YC, Song HK, Zheng XL, Yang KH (2017) Intramedullary nailing for atypical femoral fracture with excessive anterolateral bowing. J Bone Joint Surg Am 99:726–735
Mosleh H, Rouhi G, Ghouchani A, Bagheri N (2020) Prediction of fracture risk of a distal femur reconstructed with bone cement: QCSRA, FEA, and in-vitro cadaver tests. Phys Engineer Sci Med 43:269–277
Heiner AD (2008) Structural properties of fourth-generation composite femurs and tibias. J Biomech 41:3282–3284
Lee S-J, Lee H-J, Kim J-I, Oh K-J (2011) Measurement of the weight-bearing standing coronal and sagittal axial alignment of lower extremity in young Korean adults. J Korean Orthop Assoc 46:191–199
Polgár K, Gill HS, Viceconti M, Murray DW, O’Connor JJ (2003) Strain distribution within the human femur due to physiological and simplified loading: finite element analysis using the muscle standardized femur model. Proc Inst Mech Engineer H 217:173–189
Ha S, Lee JJ (2018) A comparison of the accuracy of different single plasma sample methods for measuring glomerular filtration rate using 51Cr-EDTA in children. Nucl Med Molec Imag 52:293–302
Kim JW, Kim JJ, Byun YS, Shon OJ, Oh HK, Park KC, Kim JW, Oh CW (2017) Factors affecting fracture location in atypical femoral fractures: a cross-sectional study with 147 patients. Injury 48:1570–1574
Hyodo K, Nishino T, Kamada H, Nozawa D, Mishima H, Yamazaki M (2017) Location of fractures and the characteristics of patients with atypical femoral fractures: analyses of 38 Japanese cases. J Bone Miner Metab 35:209–214
Chen LP, Chang TK, Huang TY, Kwok TG, Lu YC (2014) The correlation between lateral bowing angle of the femur and the location of atypical femur fractures. Calcif Tissue Int 95:240–247
Saita Y, Ishijima M, Mogami A et al (2014) The fracture sites of atypical femoral fractures are associated with the weight-bearing lower limb alignment. Bone 66:105–110
Jung IJ, Choi EJ, Lee BG, Kim JW (2021) Population-based, three-dimensional analysis of age- and sex-related femur shaft geometry differences. Osteopor Intl. Online ahead of print.
Kim JW, Kim H, Oh CW, et al. (2017) Surgical outcomes of intramedullary nailing for diaphyseal atypical femur fractures: is it safe to modify a nail entry in bowed femur? Arch Orthop Trauma Surg
Shon OJ, Yoon JY, Kim JW (2020) Clinical outcomes of using contralateral-side laterally bent intramedullary nails in atypical femur fractures with femoral bowing. Arch Orthop Trauma Surg 141:1291–1296
McKenna MJ, McKiernan FE, McGowan B, Silke C, Bennett K, van der Kamp S, Ward P, Hurson C, Heffernan E (2017) Identifying incomplete atypical femoral fractures with single-energy absorptiometry: declining prevalence. J Endocr Soc 1:211–220
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2021R1A2C1012972).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Institutional Review Board of Asan Medical Center and waiver was received for the need to provide written informed consent (IRB No. 2019–1568).
Consent to participate
For this type of study, formal consent is not required.
Consent for publication
Not applicable.
Statement of human rights
The manuscript does not contain clinical studies or patient data. For this type of study, formal consent is not required.
Conflicts of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hwang, D., Kim, CH., Lee, Y. et al. Association of atypical femoral fracture location and lower limb mechanical axis: a computed tomography-based finite element analysis. Osteoporos Int 33, 1285–1293 (2022). https://doi.org/10.1007/s00198-021-06173-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-021-06173-1